Abstract
Emerald ash borer (EAB, Agrilus planipennis Fairmaire) is an Asian woodborer that is a destructive pest of ash (Fraxinus spp.) trees throughout North America and in parts of Asia and Europe. It has killed hundreds of millions of ash trees worldwide in the past two decades and has shown some ability to infest novel hosts, most notably white fringetree (Chionanthus virginicus L.). Here, I review the evidence that this beetle can succeed on cultivated olive (Olea europaea L), a close relative of white fringetree. Studies and observations thus far indicate that, while young trees are largely resistant to EAB larval development, adults will oviposit on olive trees, can feed on their foliage and produce viable eggs, and that larger, older and possibly stressed trees have the potential to support larval development to the adult stage in the field. Emerald ash borer will soon interact with cultivated olive trees in the wild in parts of the U.S. and in Europe, and the realized risk to olives by this beetle will be revealed.
1. Hosts and Impacts of Emerald Ash Borer in Its Native and Invasive Ranges
Emerald ash borer (EAB, Agrilus planipennis Fairmaire) is an Asian woodborer in the family Buprestidae that specializes on ash trees (Fraxinus spp., Oleaceae). In its native range in northeastern Asia, this beetle infests species including Manchurian ash (Fraxinus mandshurica Rupr.) and Chinese ash (Fraxinus chinensis Roxb.), being mostly a secondary pest of stressed trees [1]. Since its detection in Michigan in the United States in 2002, EAB has killed hundreds of millions of ash trees across United States and Canada, with a particularly strong impact on important hardwood and landscape species like white (Fraxinus americana L.), green (Fraxinus pennsylvanica Marshall) and black ash (Fraxinus nigra Marshall) [2]. While all North American ash species tested to date are susceptible to the beetle [2], there is variation in the degree of susceptibility among species, with blue ash (Fraxinus quadrangulata Michx.) surviving at notably high rates in EAB-aftermath forests [3]. Emerald ash borer was detected on the west coast of the United States for the first time in 2022 in Oregon, where it has quickly become a pest of native Oregon ash (Fraxinus latifolia Benth.) as well as ornamentally planted ashes [4]. Emerald ash borer was detected in Russia in the Moscow area in 2003, and has since spread south and west as far as Ukraine and is poised to enter other nearby countries [5]. There, it infests species such as the widespread common ash (Fraxinus excelsior L.) as well as ornamentally planted or invasive North American ash species, especially green ash. As the beetle continues to move south and west, it will encounter other ash species native to southern Europe and the Mediterranean basin known to be highly susceptible to EAB, including narrow-leaved ash (Fraxinus angustifolia Vahl) and manna ash (Fraxinus ornus L.) [5].
2. Alternate Host Use by Emerald Ash Borer
Upon its discovery in North America, attention was quickly given to the potential host range of EAB beyond the Fraxinus genus. Early host range tests done largely by inoculating cut stems or young trees with EAB eggs showed no significant vulnerability of several non-native ornamental and native North American shrub and tree species in the Oleaceae, as well as some species in closely related families, like the Juglandaceae [6]. Limited evidence for alternate host use of this beetle in Asia was also largely dismissed [2]. In 2014, Cipollini [7] showed that white fringetree (Chionanthus virginicus L., Oleaceae), a small multi-stemmed tree native to the southeastern United States and planted ornamentally, was being attacked naturally by EAB in the field in Ohio. This finding confirmed white fringetree as the first non-ash host for this beetle. In a series of studies, this species proved to be an acceptable host for EAB larvae both in growing plants in the field and in cut stems or potted plants in the laboratory, albeit less susceptible and more tolerant to EAB than most North American ash species [8,9,10]. Natural infestation rates of white fringetrees in the field were highly variable among and within locations, but infested trees were detected in several midwestern and northeastern states in both cultivated and wild situations, and factors such as tree size (larger) and health (poorer) were shown to affect the susceptibility of this tree (Figure 1A) [9]. Controlled laboratory tests revealed that the foliage of white fringetree is highly suitable for adult maturation feeding prior to mating and oviposition [11], and adults clearly oviposit readily on it in the field. In managed plantings, dendrochronological studies showed that white fringetrees start to get infested around the same time as nearby ash trees [12], but infestation rates decline and tree health generally improves in low density plantings once beetle populations decline with the loss of nearby ash trees [13]. However, surveys in 2024 of a small unmanaged wild population of this tree in Ohio showed that, after the first infestation was detected in 2016 [14], 85 percent of the 30 trees monitored in this population had been attacked at some point, and 20 percent had been killed or showed severe stem mortality (D. Cipollini, personal observation). Currently infested trees were still detected in this population 8 years after the first infestation, suggesting that white fringetree may serve as a refuge for EAB after highly susceptible ash trees fall to low densities.
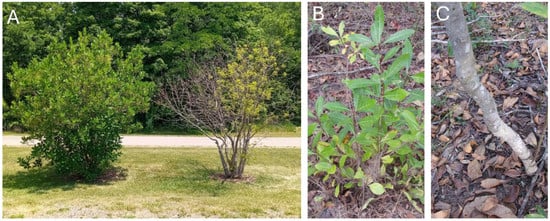
Figure 1.
White and pygmy fringetree in cultivated and wild situations. (A) Healthy (left) and emerald ash borer-infested (right) white fringetrees planted along a bike trail in Yellow Springs, Ohio, as observed in 2024. These trees are located within 200 m of where the initial attack of emerald ash borer on white fringetree was discovered in 2014. (B) Pygmy fringetree growing wild in the sandhills of central Florida. (C) A smaller relative of white fringetree, pygmy fringetree produces stems large enough (over 2.5 cm in diameter) to be attacked by emerald ash borer.
3. Cultivated Olive as a Potential Host for Emerald Ash Borer
After the discovery of white fringetree as a host, attention quickly turned to other closely related species. The closely related, and federally endangered, pygmy fringetree (Chionanthus pygmaeus Small) native to a few locations in central Florida, showed similar vulnerability to EAB as white fringetree in limited laboratory tests with cut stems (Figure 1B–D. Cipollini, unpublished data). In contrast, Chinese fringetree (Chionanthus retusus L), an ornamental tree whose native range overlaps that of EAB in China, proved to be completely resistant to EAB in laboratory tests, and no infested trees have ever been found in the field [8]. Devilwood (Cartrema americana (L.) G.L. Nesom), a close relative of the fringetrees native to the far southeastern United States, also proved to be a poor host for EAB in laboratory tests of cut stems, albeit a better host than Chinese fringetree [8]. Cultivated olive (Olea europaea L.) is closely related to white fringetree based on DNA sequence information, and is much more closely related to the fringetrees than the fringetrees are to Fraxinus species [15]. Using relatively large cut stems from field-grown trees of the cultivar Manzanilla, Cipollini et al. [16] showed that EAB was capable of developing from egg to adult in laboratory tests at a moderately high rate. While a poorer host to EAB than susceptible North American ash species, larval success was better on olive in this study than that typically observed in healthy Manchurian ash, the native host of EAB [17]. Adults reared from olive in this study also fed normally on foliage of susceptible ash species, but no further work was done with them. While larval development from egg to the pre-pupal stage has been observed in young, potted olive trees of the cultivar Arbequina in the greenhouse (Figure 2B,C), young trees appear to resist larval development better than stems of older trees [18]. Trees where significant larval development was seen showed significant branch dieback and epicormic sprouting of stems (Figure 2A). Tree stress in the form of mechanical bark wounding enhanced the establishment of EAB larval feeding galleries in the widely-planted cultivar Arbequina, but young trees of at least this cultivar are still poor hosts [18]. Olive tree foliage produces several potential volatile oviposition attractants in common with ash tree and white fringetree foliage [19], and adults have been observed to oviposit naturally on young olive trees in field studies of potted trees in Ohio and in planted trees in Oregon [20,21]. While adults generally survived poorly on the tough xerophilous foliage of olive in controlled laboratory tests, a small percentage were shown to live long enough to mate and produce viable eggs yielding live larvae after feeding solely on olive foliage (Figure 2D) [11]. Variation in adult and larval performance likely exists among the hundreds of olive cultivars that are grown around the world, but only a limited number of cultivars have been studied so far. Overall, these results suggest that, while young trees are largely resistant to EAB larval development, adults will oviposit on olive trees, can feed on their foliage and produce viable eggs, and that larger, older and possibly stressed trees have the potential to support larval development to the adult stage in the field.

Figure 2.
Performance of emerald ash borer on cultivated olive. (A) Young potted olive tree (cv. Arbequina) showing significant dieback of branches inoculated with emerald ash borer eggs (center-left). (B) Feeding gallery of fourth instar larvae of emerald ash borer in inoculated branch of the tree in (A). (C) “J-shaped” pre-pupal emerald ash borer larva in inoculated branch of this tree. (D) Adult emerald ash borer mating after feeding solely on olive foliage for several weeks. Viable eggs were produced from this mating.
4. Potential Interactions of EAB and Olive in the Field
While there have been no reports of EAB infesting olive trees in commercial orchards, ornamental plantings or in the wild, EAB has had little to no contact with this tree as of yet. While olive is cultivated in western and central China and in some small parts of South Korea and Japan, the range of olive cultivation generally does not overlap the distribution of EAB and there have been no reports of EAB as a pest of olive in this region [22]. That situation will likely change soon in Oregon, where EAB infestations of Oregon ash in the vicinity of Forest Grove occur within 20 km of some small commercial olive orchards and within 40 km of a larger commercial olive orchard with an olive mill near Dayton [23]. Olive trees are also grown ornamentally in the nearby Portland metropolitan area (Figure 3B), as they are in other parts of the world where olives are also grown commercially (Figure 3). The distribution of the highly susceptible Oregon ash extends from the states of Washington through Oregon and deep into olive growing regions of central California [24], which is the center of olive production in the U.S. Emerald ash borer is also encroaching on some commercial olive orchards in the state of Georgia and some other southern states in the U.S. [25], and olive is grown as an occasional potted or planted ornamental in suitable habitats throughout the U.S. where EAB is encroaching (Figure 3). In Europe, the first encounters of EAB with olive trees in the wild will likely occur in Crimea, where some old olive plantations exist along the Black Sea [5], and its movement will accelerate once it reaches habitats where narrow-leaved ash is common. This highly susceptible species will likely facilitate the dispersal of EAB to olive growing areas around the Mediterranean, the Middle East, and in southern Europe, as its distribution overlaps almost entirely with the distribution of olive cultivation in this region. Olive is also planted as an ornamental in many European countries where it is not grown commercially, either in the ground or in pots (Figure 3A and Figure 4). Olives are grown commercially in other continents of the world, including Australia and South America, which have yet to record the presence of EAB. Importantly, in most places where olive trees are grown commercially, even outside of their native range, they often escape and grow wild, sometimes reaching the status of invasive species [26,27]. There they may intermingle with susceptible ash species either native to the area or planted ornamentally and escaped, including the highly susceptible green ash that is present and naturalized throughout Europe [28].

Figure 3.
Olives grown ornamentally in the ground in suitable habitats. (A) In a courtyard of The Vatican in Rome, Italy. (B) In a bed in a yard in Portland, Oregon. (C) In a yard in Phoenix, Arizona. (D) In a bed in a yard in Melbourne, Florida.

Figure 4.
Potted olives grown ornamentally in areas less suitable to growth in the ground. (A) Potted olive trees are a common street tree in Amsterdam, The Netherlands. (B) Potted olive tree in Leipzig, Germany, where they are used frequently as decorative accents for restaurants.
5. Potential Versus Realized Risk to Olives
Studies thus far indicate that young, healthy olive trees are a sub-par host for EAB, but larger and older trees, which are also more likely to show signs of stress, could be suitable hosts for this beetle. Access to nearby ash trees would increase the risk to olives, as they could help subsidize adult feeding needs and boost adult numbers. While young trees appear to be highly resistant, a variety of stresses, ranging from abiotic stress due to climate change, to various diseases that affect olive, such as olive quick decline [29], could enhance the susceptibility of olive trees to EAB, as these sorts of stress factors do for resistant ash trees [30]. The fact that EAB will oviposit on even small olive trees suggests that dispersal of EAB could be facilitated by the transport of olive trees or olive wood, even if larvae do poorly on the trees. While EAB is manageable with pesticides, the most effective pesticides for it are systemically introduced [2], which would seemingly be complicated for a fruit-bearing tree. Removal of ash trees in the vicinity of olive groves, alleviation of abiotic stress (e.g., via irrigation) and other cultural interventions could be employed in cultivated situations, but olive trees grow wild throughout the world in unmanaged populations where these interventions will not be employed. If EAB were able to establish a foothold in such areas, perhaps subsidized by co-occurring ash tree species, selection could lead to strains of EAB that are better capable of feeding on this species, which could lead to the evolution of a new pest of even healthy olive trees [20]. This ecological and evolutionary scenario will begin to play out over the next decade in North America and southern Europe, and the world will be watching.
Funding
This research received no external funding.
Data Availability Statement
No new data were produced or analyzed in the writing of this manuscript. Data sharing is not applicable.
Acknowledgments
The author thanks Kendra Cipollini for editorial assistance.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EAB | Emerald ash borer |
References
- Orlova-Bienkowskaja, M.J.; Volkovitsh, M.G. Are native ranges of the most destructive invasive pests well known? A case study of the native range of the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). Biol. Invasions 2018, 20, 1275–1286. [Google Scholar] [CrossRef]
- Herms, D.A.; McCullough, D.G. Emerald ash borer invasion of North America: History, biology, ecology, impacts, and management. Annu. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, D.; Morton, E. The persistence of blue ash in the aftermath of emerald ash borer may be due to adult oviposition preferences and reduced larval performance. Agric. For. Entomol. 2023, 25, 584–589. [Google Scholar] [CrossRef]
- Emerald Ash Borer Resources. Oregon State University Extension. Available online: https://extension.oregonstate.edu/collection/emerald-ash-borer-resources (accessed on 2 January 2025).
- Orlova-Bienkowskaja, M.J.; Bieńkowski, A.O. Southern range expansion of the emerald ash borer, Agrilus planipennis, in Russia threatens ash and olive trees in the Middle East and Southern Europe. Forests 2022, 13, 541. [Google Scholar] [CrossRef]
- Anulewicz, A.C.; McCullough, D.G.; Cappaert, D.L.; Poland, T.M. Host range of the emerald ash borer (Agrilus planipennis Fairmaire) (Coleoptera: Buprestidae) in North America: Results of multiple-choice field experiments. Environ. Entomol. 2008, 37, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, D. White fringetree as a novel larval host for emerald ash borer. J. Econ. Entomol. 2015, 108, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, D.; Rigsby, C.M. Incidence of infestation and larval success of emerald ash borer (Agrilus planipennis) on white fringetree (Chionanthus virginicus), Chinese fringetree (Chionanthus retusus), and devilwood (Osmanthus americanus). Environ. Entomol. 2015, 44, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.L.; Cipollini, D. Distribution, predictors, and impacts of emerald ash borer (Agrilus planipennis) (Coleoptera: Buprestidae) infestation of white fringetree (Chionanthus virginicus). Environ. Entomol. 2017, 46, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, C.E.; Arango-Velez, A. Larval survival and growth of emerald ash borer (Coleoptera: Buprestidae) on white ash and white fringetree saplings under well-watered and water-deficit conditions. Environ. Entomol. 2017, 46, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.L.; Slager, B.; Anulewicz, A.C.; Cipollini, D. Feeding, survival, and fecundity of adult emerald ash borer (Coleoptera: Buprestidae) on foliage of two novel hosts and implications for host range expansion. Environ. Entomol. 2020, 49, 709–716. [Google Scholar] [CrossRef]
- Thiemann, D.; Lopez, V.; Ray, A.M.; Cipollini, D. The history of attack and success of emerald ash borer (Coleoptera: Buprestidae) on white fringetree in Southwestern Ohio. Environ. Entomol. 2016, 45, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Ellison, E.A.; Peterson, D.L.; Cipollini, D. The fate of ornamental white fringetree through the invasion wave of emerald ash borer and implications for novel host use by this beetle. Environ. Entomol. 2020, 49, 489–495. [Google Scholar] [CrossRef]
- Peterson, D.L.; Cipollini, D. Attack dynamics and impacts of emerald ash borer on wild white fringetree populations. Biol. Invasions 2022, 24, 9–15. [Google Scholar] [CrossRef]
- Dong, W.; Li, E.; Liu, Y.; Xu, C.; Wang, Y.; Liu, K.; Cui, X.; Sun, J.; Suo, Z.; Zhang, Z.; et al. Phylogenomic approaches untangle early divergences and complex diversifications of the olive plant family. BMC Biol. 2022, 20, 92. [Google Scholar] [CrossRef]
- Cipollini, D.; Rigsby, C.M.; Peterson, D.L. Feeding and development of emerald ash borer (Coleoptera: Buprestidae) on cultivated olive, Olea europaea. J. Econ. Entomol. 2017, 110, 1935–1937. [Google Scholar] [CrossRef] [PubMed]
- Rigsby, C.M.; Villari, C.; Peterson, D.L.; Herms, D.A.; Bonello, P.; Cipollini, D. Girdling increases survival and growth of emerald ash borer larvae on Manchurian ash. Agric. For. Ent. 2018, 21, 130–135. [Google Scholar] [CrossRef]
- Peterson, D.L.; Cipollini, D. Larval performance of a major forest pest on novel hosts and the effect of stressors. Environ. Entomol. 2020, 49, 482–488. [Google Scholar] [CrossRef]
- Peterson, D.L.; Böröczky, K.; Tumlinson, J.; Cipollini, D. Ecological fitting: Chemical profiles of plant hosts provide insights on selection cues and preferences for a major buprestid pest. Phytochemistry 2020, 176, 112397. [Google Scholar] [CrossRef]
- Cipollini, D.; Peterson, D.L. The potential for host switching via ecological fitting in the emerald ash borer-host plant system. Oecologia 2018, 187, 507–519. [Google Scholar] [CrossRef]
- 2023 Research and Field Observations on Olive as a Viable Host for Emerald Ash Borer in Oregon. Oregon Department of Agriculture. Available online: https://cdn.ymaws.com/www.oan.org/resource/resmgr/gr23/ODAOliveEABResearchStatement.pdf (accessed on 2 January 2025).
- Su, C.; Sun, J.; Zhu, W.; Peng, L. History, distribution, and potential of the olive industry in China: A review. Sustainability 2018, 10, 1426. [Google Scholar] [CrossRef]
- Newberry, K. Oregon Olive Mill: From Tree to Table. Portrait Magazine. Available online: https://www.portraitmagazine.com/oregon-olive-mill (accessed on 2 January 2025).
- Smither-Kopperl, M.; Bartow, A. Plant Guide for Oregon Ash (Fraxinus latifolia); USDA-Natural Resources Conservation Service, Lockeford Plant Materials Center: Lockeford, CA, USA, 2020. Available online: https://plants.sc.egov.usda.gov/DocumentLibrary/plantguide/pdf/pg_frla.pdf (accessed on 6 January 2025).
- Douglas, Z. Who Knew? Georgia Is Producing Some of the Country’s Best Olive Oil. Feast and Field. 2021. Available online: https://feastandfield.net/read/condiments-and-sauces/who-knew-georgia-is-producing-some-of-the-country-s-best-olive-oils/article_bc84912c-10d0-11ec-83be-bfc2abb80175.html (accessed on 5 January 2025).
- Calflora: Information on California Plants for Education, Research and Conservation, with Data Contributed by Public and Private Institutions and Individuals. Berkeley, California: The Calflora Database. 2024. Available online: https://www.calflora.org/ (accessed on 5 January 2025).
- Government of South Australia. Declared Plant Policy, Wilding Olives (Olea europaea). South Australia. 2021. Available online: https://pir.sa.gov.au/ (accessed on 6 January 2025).
- Orlova-Bienkowskaja, M.J.; Drogvalenko, A.N.; Zabaluev, I.A.; Sazhnev, A.S.; Peregudova, E.Y.; Mazurov, S.G.; Komarov, E.V.; Struchaev, V.V.; Martynov, V.V.; Nikulina, T.V.; et al. Current range of Agrilus planipennis Fairmaire, an alien pest of ash trees, in European Russia and Ukraine. Ann. For. Sci. 2020, 77, 29. [Google Scholar] [CrossRef]
- Morelli, M.; García-Madero, J.M.; Jos, Á.; Saldarelli, P.; Dongiovanni, C.; Kovacova, M.; Saponari, M.; Baños Arjona, A.; Hackl, E.; Webb, S.; et al. Xylella fastidiosa in olive: A review of control attempts and current management. Microorganisms 2021, 9, 1771. [Google Scholar] [CrossRef]
- Chakraborty, S.; Whitehill, J.G.; Hill, A.L.; Opiyo, S.O.; Cipollini, D.; Herms, D.A.; Bonello, P. Effects of water availability on emerald ash borer larval performance and phloem phenolics of Manchurian and black ash. Plant Cell Environ. 2014, 37, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).