Abstract
Soil degradation and poor fertility severely constrain vegetation growth in urban ecosystems, particularly in compacted and nutrient-depleted tree pits. Mulching has emerged as an effective strategy to improve soil quality and regulate soil–microbe–plant interactions, yet the combined use of organic and inorganic mulching in urban landscapes remains underexplored. In this study, a one-year field experiment was conducted to evaluate the effects of four mulching treatments on soil bacterial community diversity and functional potential. Four treatments were applied green waste compost + wood chips (GW), green waste compost + wood chips + volcanic rocks (GWV), green waste compost + wood chips + pebbles (GWP), and a non-mulched control (CK). Organic mulching (GW) effectively reduced bulk density, enhanced cellulase and protease activities, increased bacterial community richness and balance, and enriched microbial genes associated with carbon and nitrogen metabolism, while organic–inorganic mulching further promoted soil nutrition and reshaped bacterial community structure. Soil pH, nitrogen content, and protease activity served as key drivers of bacterial community structure and function. These findings demonstrate that different mulching practices provide distinct ecological advantages, and together highlight the role of mulching in regulating soil–microbe–plant interactions and improving urban tree pit management.
1. Introduction
Soil degradation and poor fertility are common challenges in both natural and urban ecosystems, often constraining vegetation establishment and long-term stability [1,2]. In urban landscapes, bare tree pits are widespread, characterized by soil compaction, nutrient depletion, and unstable microenvironments, such as rapid fluctuations in moisture and temperature irregular nutrient availability, and frequent disturbance, all of which hinder tree growth and ecosystem functions [3,4]. However, the extent and manifestation of these problems are not uniform: urban soils differ considerably across climatic zones, soil parent materials, and management histories, leading to diverse constraints on tree growth. Effective soil management practices are therefore urgently required to improve soil quality, promote plant performance, and enhance the resilience of urban green spaces.
Among the numerous approaches available, mulching has gained considerable attention as a practical and multifunctional strategy widely applied in agriculture, forestry, and landscaping [5,6]. Its effectiveness lies in its ability to improve soil physical conditions, enhance organic matter content, regulate soil temperature and water fluxes, and stimulate nutrient cycling [7,8]. Organic mulches, such as compost and wood chips, primarily function as nutrient inputs that enrich soil organic matter pools and provide carbon and nitrogen substrates for microbial metabolism [9]. By stimulating microbial colonization and extracellular enzyme activities, mulches enhance nutrient mineralization and humus formation, thereby improving soil fertility and aggregate stability. The efficiency of these processes is largely determined by the biochemical traits of the mulching materials, such as their C:N ratio and lignin content, which regulate decomposition dynamics and nutrient release, and are strongly influenced by substrate quality, environment conditions, and initial microbial community composition, all of which can vary across different locations and treatments [10]. Beyond these direct benefits, organic mulches derived from landscaping residues also offer a sustainable pathway for urban green waste recycling, thereby linking soil management practices with broader sustainability goals [11,12].
In contrast, inorganic mulches such as pebbles, gravels, and volcanic rocks primarily function through physical regulation: they reduce soil evaporation, buffer temperature extremes, and stabilize the soil surface microenvironment [13,14]. Such modifications to the hydrothermal regime can indirectly influence microbial activity, enzymatic turnover, and organic matter decomposition [15]. While the functions of organic and inorganic mulches are often considered separately, their combined application has the potential to generate synergistic outcomes. Organic materials supply readily decomposable substrates and nutrients, whereas inorganic layers protect the soil against environmental fluctuations, creating more favorable conditions for microbial-mediated processes and nutrient retention [16]. However, several studies have highlighted that inorganic mulching alone may exert neutral or even negative impacts. For example, gravel-sand mulching was reported to alter bacterial communities without significantly enhancing microbial diversity or soil fertility [17]. and prolonged use of mineral mulches has been linked to increased soil bulk density and restricted root penetration, thereby offsetting potential benefits [18,19].
Despite these recognized benefits, much of the existing research on mulching has been conducted within agricultural soils or arid and semi-arid regions [20,21]. These studies primarily emphasize water conservation, temperature regulation, and crop productivity, reflecting the pressing concerns of those systems. However, soil conditions in afforestation and urban landscaping contexts are highly heterogeneous: some sites are severely compacted and nutrient-limited, while others may retain relatively better fertility due to differences in previous management practices [22]. These variations make research in such settings particularly challenging. Existing research indicates that studies on the synergistic effects of organic and inorganic mulching are relatively limited [23].
The interactions between organic–inorganic mulching regimes and soil processes under these urban conditions remain poorly understood. Specifically, there is limited knowledge on how mulching influences the integration of soil physicochemical properties, enzyme activities, and microbial community composition, which together determine soil functional capacity. Furthermore, the temporal dimension of mulching effects is under explored: while short-term improvements in soil quality are frequently reported, whether these benefits persist, diminish, or even shift in character over longer periods remains uncertain. This knowledge gap restricts the broader application of mulching as a scientifically grounded soil restoration strategy for urban ecosystems, where maintaining soil health, microbial diversity, and vegetation stability is critical for long-term ecological sustainability.
To address these gaps, we conducted a one-year field mulching experiment in a nursery, using triploid Populus tomentosa as the test species. This fast-growing, highly adaptable tree is commonly used in northern China for windbreaks, sand stabilization, and carbon sequestration, making it ideal for urban and afforestation applications. Four mulching treatments were applied: green waste compost + wood chips (GW), green waste compost + wood chips + volcanic rocks (GWV), green waste compost + wood chips + pebbles (GWP), and a non-mulched control (CK). We aimed (i) to assess short-term impacts of organic–inorganic mulches on soil quality and tree growth, specifically hypothesizing that the synergistic combination of organic and inorganic materials will more effectively promote the above-mentioned improvements compared to organic mulch; and (ii) to elucidate bacterial community responses to different mulching strategies, with the hypothesis that certain mulch practices will promote the growth of beneficial microbial communities that enhance soil nutrient cycling. This study provides scientific guidance for mulch management and highlights the potential of landscaping waste-derived mulches for sustainable urban soil improvement.
2. Materials and Methods
2.1. Study Site
The field experiments were conducted in October 2023 at Huangfa Nursery, Daxing District, Beijing (116°19′48″ E, 39°34′28″ N). The region belongs to temperate monsoon climate, characterized by hot and rainy summers and cold and dry winters. The mean annual temperature is 11.6 °C, and mean annual precipitation is 656 mm, mostly concentrated in summer. The mean altitude is 32.5 m. The soil at the site is classified as alluvial sandy loam, with a pH value of 8.40, bulk density (BD) of 1.29 g cm−3, soil organic matter (SOM) of 8.96 g kg−1, total nitrogen (TN) of 0.60 g kg−1, available nitrogen (AN) of 40.26 mg kg−1, available phosphorus (AP) of 13.03 mg kg−1 and available potassium (AK) of 86.51 mg kg−1.
2.2. Materials and Treatments
The mulching materials include green waste compost (G), wood chips (W), volcanic rocks (V), and pebbles (P). Four treatments were set up in the experiment (Figure 1): CK, no mulching; GW, green waste compost + wood chips; GWV, green waste compost + wood chips + volcanic rocks; GWP, green waste compost + wood chips + pebbles. In the GW treatment, a 2 cm layer of green waste compost was applied at the bottom and covered with a 3 cm layer of wood chips. In GWV and GWP treatments, an additional 3 cm layer of volcanic rocks or pebbles was applied on top of the GW layer. Treatments were randomly assigned in a completely randomized design (CRD) to 2 m diameter pits of 2-year-old triploid Populus tomentosa planted at 3 m × 2 m spacing, with 20 replicates per treatment. Basic physicochemical properties of organic mulching materials were shown in Table 1. During the experimental, irrigation and other management practices were kept consistent across all treatments to minimize confounding effects. The total mulch thickness in our design (5–8 cm) is within the recommended range for tree mulching (generally 5–10 cm), which has been suggested to optimize soil moisture conservation, nutrient supply, and root growth without causing excessive soil compaction or oxygen limitation [24,25].
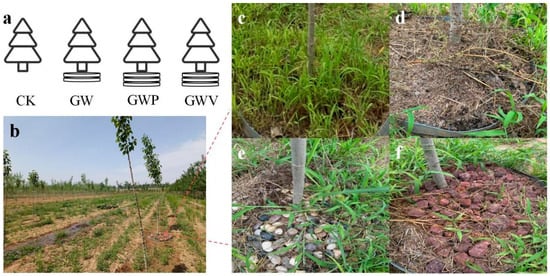
Figure 1.
(a) Schematic diagram of layered mulching; (b) The field-controlled experiment in Huangfa Nursery; (c–f) photographs of mulching treatments (CK, GW, GWP, and GWV, respectively).

Table 1.
Basic physicochemical properties of mulching materials.
2.3. Methods
2.3.1. Soil and Plant Sampling
After one year of mulching, 10 trees were randomly selected from 20 replicates per treatment. Tree height (TH) was determined using a Blume-Leiss hypsometer, and crown width (CW) was calculated as the average of the maximum and perpendicular crown diameters [26]. Diameter at breast height (DBH) was recorded at 1.3 m above ground level using a digital caliper. The selected trees were destructively sampled to determine biomass-related traits. Above-ground dry weight (ADW) and below-ground dry weight (BDW) were obtained by oven-dried at 65 °C until constant weight [27]. Root volume (RV) was measured by the water displacement method [28], and total biomass (TB) was calculated as the sum of ADW and BDW. For soil sample, surface mulching materials were carefully removed from each tree pit. Soil samples were collected using a soil drill with a diameter of 5 cm. For each tree pit, three soil cores (0–10 cm) were randomly taken at approximately 20 cm from the trunk, avoiding visible roots and then mixed into a composite sample. The composite sample was subsequently divided into three portions [29]: (1) soil bulk density (BD) analysis, stored in a ring knife; (2) soil physicochemical properties, after air-drying, sieving through a 2 mm sieve using a ball mill; (3) microbial community analysis, stored at −80 °C and sent to Shanghai Meiji Biotechnology Co., Ltd. (Shanghai Meiji Biotechnology Co., Ltd., Shanghai, China) [30,31]; and (4) analysis of cellulase (CEL) and protease (PR) activity, stored at 4 °C.
2.3.2. Soil and Test Materials’ Physicochemical Property Measurement
Soil pH was measured using a pH meter (Mettler Toledo, Beijing, China) in a 1:2.5 soil to water suspension. Soil bulk density (BD) was determined using the ring knife method. Soil organic matter (SOM) was determined by the potassium dichromate external heating method. Total nitrogen (TN) and available phosphorus (AP) were determined using the Kjeldahl method and Olsen methods, respectively [32,33]. Available nitrogen (AN) was determined using the alkali diffusion method [34] and available potassium (AK) was measured by the NH4OAc extraction-flame photometry method [35]. Soil cellulase activity was determined by the 3,5-dinitrosalicylic acid (DNS) colorimetric method using sodium carboxymethyl cellulose (CMCNa) as the substrate, and soil protease activity was determined by the Folin–Ciocalteu colorimetric method using casein as the substrate [36,37]. Green waste compost (G) and wood chip (W) samples were air-dried, ground, and sieved (<1 mm) prior to analysis, and their physicochemical properties were measured following the same procedures applied for soil samples. For all analyses, three replicates were prepared for each sample. Quality assurance was ensured by including reagent blanks and standard reference materials, and instruments were calibrated.
2.3.3. Soil Bacterial Community
Total genomic DNA was extracted from soil samples using the Mag-Bind® Soil DNA Kit (Omega Bio-tek, Norcross, GA, USA). Concentration and purity of extracted DNA was determined with TBS-380 and NanoDrop2000 (Thermo Fisher Scientific, Waltham, MA, USA), respectively. DNA extract quality was checked on 1% agarose gel. DNA extract was fragmented to an average size of about 400 bp using Covaris M220 (Gene Company Limited, HK, China) for paired-end library construction. Paired-end library was constructed using NEXTFLEX Rapid DNA-Seq (Bioo Scientific, Austin, TX, USA). Adapters containing the full complement of sequencing primer hybridization sites were ligated to the blunt end of fragments. Paired-end sequencing was performed on Illumina NovaSeq (Illumina Inc., San Diego, CA, USA) at Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China) using NovaSeq 6000 S4 Reagent Kit v1.5 (300 cycles) (Illumina, San Diego, CA, USA.). The paired-end Illumina reads were trimmed of adaptors, and low-quality reads (length < 50 bp or with a quality value < 20 or having N bases) were removed by fastp (v 0.20.0) [38]. Metagenomics data were assembled using MEGAHIT (v 1.1.2) [39]. Contigs with a length ≥ 300 bp were selected as the final assembling result. Open reading frames (ORFs) from each assembled contig were predicted using MetaGene (https://www.metagene.de/) [40]. The predicted ORFs with a length ≥ 100 bp were retrieved and translated into amino acid sequences using the NCBI translation table. A non-redundant gene catalog was constructed using CD-HIT (v 4.6.1) with 90% sequence identity and 90% coverage [41]. High-quality reads were aligned to the non-redundant gene catalogs to calculate gene abundance with 95% identity using SOAPaligner (v 2.21) [42]. Taxonomic annotation was performed by aligning representative sequences from the non-redundant gene catalog to the NR database (Non-Redundant Protein Sequence Database) using Diamond (v 0.8.35; e-value ≤ 1 × 10−5). Functional annotation of KEGG pathways was conducted by aligning the same sequences to the KEGG database under identical parameters.
2.4. Analysis
Excel and SPSS 23.0 were used for data processing and statistical analysis. Origin 2023 and R 4.4.3 were employed to create visualization charts. The least significant difference (LSD) test was conducted to verify the significance of differences at the p < 0.05 level. Soil metagenomic analyses and data visualization were conducted on the Majorbio Cloud Platform (www.majorbio.com, accessed on 12 February 2025) according to the platform’s standard protocols. Correlation analyses were performed to examine the relationships between the relative abundances of dominant soil bacteria (at the phylum and family levels) and environmental factors. Mantel tests were conducted to evaluate the associations between soil bacterial community composition and environmental variables. Additionally, the relative abundances of KEGG functional pathways in soil bacterial communities under different treatments were quantified and visualized.
3. Results
3.1. Response of Soil Physicochemical Properties and Test Plant Growth to Mulching Practices
Significant differences in soil characteristics were observed among the mulching treatments (Table 2). BD was the highest under GWP, significantly (p < 0.05) higher than that under GW. Soil pH reached its maximum in GW and was the lowest in GWV. Regarding nutrients, GWV showed the greatest SOM and TN, while GWP had the highest AP and AK. In terms of enzyme activities, CEL and PR were most pronounced in GW, significantly (p < 0.05) surpassing the other treatments.

Table 2.
Means (±SE, n = 3) of soil physicochemical properties characteristics under different treatments.
Compared with CK, mulching significantly promoted the growth of triploid Populus tomentosa, with growth parameters being improved to varying degrees (Table 3). All mulching treatments (GW, GWP, GWV) showed significantly (p < 0.05) higher TH, CW, DBH, and ADW than CK. Among them, GWP exhibited the greatest TH, CW, DBH, and BDW, whereas GWV showed the highest ADW, RV, and TB.

Table 3.
Means (±SE, n = 10) of test plant index under different treatments.
3.2. Response of Soil Bacterial Diversity and Community Structure Response to Mulching Practices
All measured alpha diversity indices responded significantly to mulching treatments (Table 4). GW and GWP exhibited the highest Chao index (225), significantly higher than CK (223) and GWV (221). GWV had the highest Shannon and Pielou indices (2.68 and 0.4971, respectively), significantly exceeding those of other treatments. GW ranked second (2.66 and 0.4912, respectively), whereas GWP had the lowest values (2.62 and 0.4835, respectively), significantly lower than all other treatments. GW showed the highest Simpson index (0.1351), significantly exceeding CK (0.1303) and GWV (0.1199) but did not differing from GWP (0.1319).

Table 4.
Means (±SE, n = 3) of soil bacterial alpha diversity to index under different treatments (Class level).
Venn diagram illustrated the shared and unique bacterial genera under different mulching treatments (Figure 2). A total of 3759 genera were common across all treatments. Among the unique taxa, CK, GW, GWP, and GWV harbored 30, 28, 4, and 11 exclusive genera, respectively. The number of genera shared by three treatments ranged from 4 to 63, with the highest overlap observed between CK and GW (63 genera). Overall, mulching treatments (GW, GWP, GWV) increased the number of shared bacterial genera compared with CK, indicating that mulching not only shaped distinct community structures but also promoted greater microbial overlap.
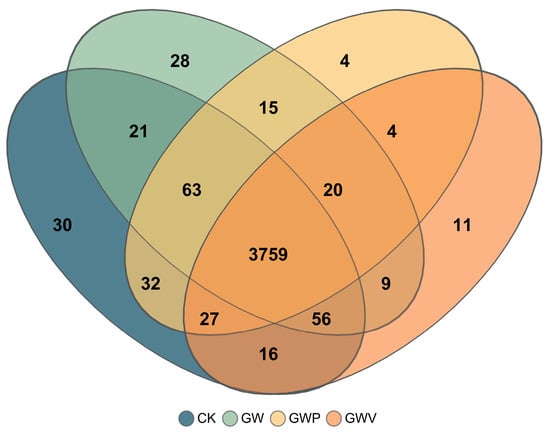
Figure 2.
Venn diagram of soil bacterial communities under different treatments (genus level).
Principal coordinate analysis (PCoA) further revealed clear differences in bacterial community composition among treatments (Figure 3). The first two axes explained 31.41% (PC1) and 20.52% (PC2) of the total variation. Mulching samples (GW, GWP, GWV) were clearly separated from the control (CK), with GWV showing divergence along PC1, whereas CK exhibited higher intra-group variation. Permutational multivariate analysis of variance (PERMANOVA) confirmed that mulching significantly altered bacterial community structures (R2 = 0.441, p = 0.009).
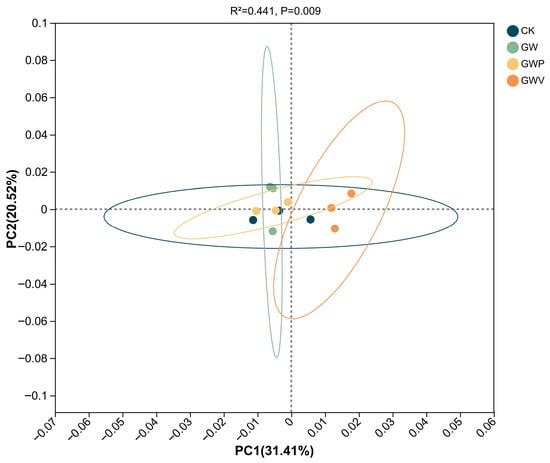
Figure 3.
Principal coordinate analysis (PCoA) of soil bacterial community structures under different treatments. The analysis was based on Bray–Curtis distance matrices. Ellipses indicate 95% confidence intervals.
3.3. Soil Bacterial Community Composition and Environmental Drivers
At the phylum level (Figure 4a), Pseudomonadota, Actinomycetota, and Acidobacteriota were dominant, together accounting for 60.66–63.13% of the total bacterial community. Pseudomonadota exhibited the highest abundance, followed by Actinomycetota and Acidobacteriota, whereas Chloroflexota, Rokubacteria, Bacteroidota, and Gemmatimonadota were less abundant. The relative abundances of Pseudomonadota and Acidobacteriota were significantly (p < 0.05) higher in GW than in other treatments, while Actinomycetota was significantly (p < 0.05) lower. Compared with CK, mulching significantly reduced Chloroflexota. Notably, Candidatus Rokubacteria reached its highest proportion in GWV (6.36%), whereas Thermomicrobiota peaked in GWP (4.18%). At the family level (Figure 4b), Vichamilbaetcracae were most abundant (5.91–8.80%), followed by Candidatus Rokubacteria (2.64–6.35%), Nocardioidaceae (1.85–3.91%) and Anaerolineaceae (2.22–3.29%). The relative abundances of Nocardioidaceae and Gemmatimonadaceae were significantly (p < 0.05) higher in CK and GWP than in other treatments, whereas Sphingomonadaceae was significantly (p < 0.05) lower in GWP.
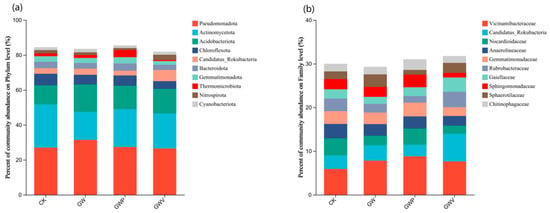
Figure 4.
The relative abundances of dominant phylum (a) and family (b) for soil bacterial community under different treatments (top 10).
Pearson’s correlation analysis revealed significant associations between dominant bacterial taxa and soil properties (Figure 5). At the phylum level (Top 7), the relative abundance of Pseudomonadota was positively correlated with pH (R2 = 0.552, p < 0.05). Actinomycetota showed strong negative correlations with pH, AN, PR, and ALP (R2 = −0.771 to −0.851, p < 0.01), but was positively correlated with TN (R2 = 0.548, p < 0.05). Acidobacteriota also showed a positive relationship with pH (R2 = 0.538, p < 0.05), while Bacteroidota correlated with BD (R2 = 0.523, p < 0.05), and Candidatus Rokubacteria with TN (R2 = 0.515, p < 0.05). In addition, Gemmatimonadota displayed significant positive correlations with AP (R2 = 0.522) and AK (R2 = 0.554) (p < 0.05). At the family level (Top 5), Vicinamibacteraceae showed strong positive correlations with SOM, TN, AN, CEL, and PR (R2 = 0.673 to 0.895, p < 0.01). Candidatus Rokubacteraceae correlated positively with AP (R2 = 0.786, p < 0.05). In contrast, Gemmatimonadaceae and Anaerolineaceae were negatively related to AP (R2 = −0.624 and −0.594; p < 0.05), with Anaerolineaceae also showing a negative correlation with SOM (R2 = −0.601, p < 0.05).
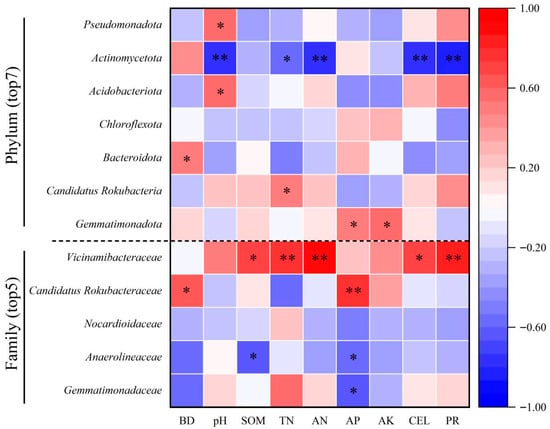
Figure 5.
Correlation analysis between soil dominant bacterial relative abundance (at phylum and family level) and environmental factors. * p < 0.05, ** p < 0.01.
Mantel test analysis demonstrated that soil bacterial community composition was significantly correlated with environmental factors (Figure 6). The results showed that the soil bacterial community was significantly (p < 0.05) correlated with PR and highly significant (p < 0.01) correlation with pH. These results indicated that nutrient availability and enzyme activities are the primary drivers shaping bacterial community structure under different mulching treatments.
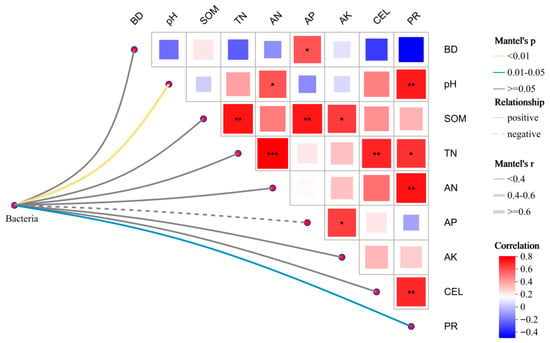
Figure 6.
Correlations between soil bacterial community and environmental factors analyzed by Mantel test. * p < 0.1, ** p < 0.05, *** p < 0.01.
3.4. Response of Soil Bacterial Community Functional Gene Abundance to Mulching Practices
KEGG functional annotation demonstrated that the functional gene repertoire of the soil bacterial community was predominantly assigned to metabolism-related pathways, followed by those associated with environmental information processing and cellular processes (Figure 7). Significant variation was detected in the relative abundance of several core metabolic pathways across the mulching treatments. In particular, the GW treatment exhibited markedly higher gene abundances in most metabolic pathways, with especially pronounced enrichment in carbohydrate metabolism, amino acid metabolism, and energy metabolism (p < 0.05). These results indicate that mulching substantially influences the functional potential of soil bacteria, although no significant differences were observed among treatments other than GW.
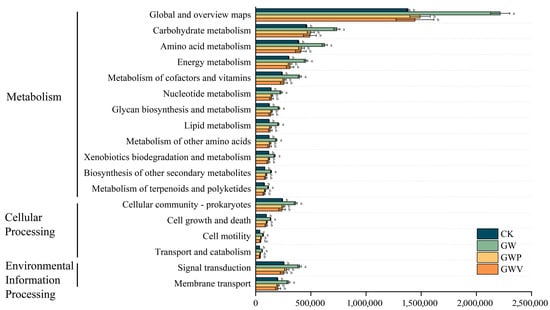
Figure 7.
Relative abundance of KEGG functional pathway in soil bacterial communities under different treatments. Different letters in the same column indicate statistically significant differences (p < 0.05).
4. Discussion
4.1. Response of Soil Physicochemical and Test Tree Growth Composition to Mulching Practices
After one year of mulching, soil physicochemical properties and enzymatic activities were improved to varying degrees, demonstrating the effectiveness of mulching in soil amelioration. This aligns with previous studies reporting that organic mulching enhances soil fertility by improving nutrient retention and stimulating enzymatic activities [9,43]. Among the treatments, GW was particularly effective in reducing soil BD and increasing CEL and PR activities, which can be attributed to the combined input of green waste compost and wood chips. This is consistent with previous findings that green waste compost mulched increase soil porosity and stimulate microbial enzyme activity [44]. These materials provide organic substrates for soil fauna and microorganisms, promoting microbial metabolism, soil aggregation, and organic matter transformation, similar to organic inputs in natural ecosystems.
The addition of pebbles and volcanic rocks produced significant and divergent effects on soil physicochemical properties, mainly reflecting the synergistic enhancement between organic mulch (as a carbon and nitrogen source) and mineral mulch, which can regulate soil microenvironments. GWV showed the best performance in increasing SOM and TN, while maintaining high levels of AN, making it the most favorable strategy for enhancing nitrogen accumulation and organic matter stabilization. Previous studies have suggested that gravel or mineral mulches can indirectly regulate the transformation and decomposition of organic matter by altering soil microclimate conditions, thereby influencing microbial activity [45]. The superior performance of GWV in this study further supports this mechanism. In contrast, pebbles mulching (GWP) exhibited a remarkable advantage in rapidly and substantially increasing AP and AK. This may be associated with the dissolution of mineral components and the release of specific ions [46,47]. Indeed, previous studies have reported that mineral mulches can enhance the dissolution and release of AK under wet–dry cycles [48], and that nutrient release from rock weathering may alleviate soil nutrient depletion induced by crop growth [49]. However, studies have also reported that certain types of inorganic mulching, particularly pebble mulches, may increase soil compaction, reduce infiltration, or suppress root activity over time, thereby offsetting their short-term nutrient benefits [18,19]. These contradictory findings suggest that the positive effects of mineral mulching may be context-dependent, influenced by soil type, rainfall regime, and duration of application.
The improvement of soil conditions significantly promoted the growth of test trees. These results suggest that organic and inorganic mulches create a favorable soil environment for root development and nutrient uptake, thereby accelerating biomass accumulation both above and below ground. Although the differences among mulching treatments were relatively small, all mulched plots outperformed the control, indicating that mulching can provide notable benefits for early tree establishment. These findings align with previous studies, for instance, Machado et al. [50] reported that organic mulching improved seedling survival and enhanced height and diameter growth in restoration plantings. On the other hand, some studies on urban tree pits and arid regions have found neutral or even negative effects of inorganic mulching on tree performance, due to restricted gas exchange or soil hardening [18,19]. Therefore, while our results highlight clear benefits within the first year, longer-term evaluations are necessary to determine whether such positive effects persist.
4.2. Response of Soil Bacterial Diversity and Community Composition to Mulching Practices
As decomposers in ecosystems, soil microorganisms play pivotal roles in nutrient cycling and energy flow [51]. Mulching not only enhances soil nutrient content but also regulates soil moisture and temperature, thereby creating favorable habitats for microbial communities [9]. Consistent with earlier studies [52,53,54], our results showed that mulching significantly reshaped the bacterial community.
Analysis of α-diversity revealed that GW had higher richness and diversity, indicating a tendency toward a more diverse and balanced bacterial community. In particular, the enrichment of Pseudomonadota and Actinomycetota may reflect microbial interactions such as nutrient exchange or chemical signaling that support their proliferation [55,56]. The widespread dominance of these phyla across ecosystems underscores their strong ecological adaptability [57,58]. For instance, Pseudomonadota are known for their ability to degrade a variety of organic compounds, promoting nutrient cycling in mulched soil [59]. At the β-diversity level, PCoA based on Bray–Curtis distances clearly separated mulched soils from CK, indicating that mulching altered bacterial community assembly, which is consistent with findings from previous studies [10,12,15]. Nevertheless, other reports have found that inorganic mulching alone exerts neutral or limited effects on microbial diversity, suggesting that organic inputs may be the dominant factor driving microbial community restructuring [17].
Notably, the GWP treatment markedly enriched the thermophilic phylum Thermomicrobiota, characterized by optimal growth at 45–65 °C and typically associated with compost and thermally altered soils [60]. This group was favored by the unique microenvironment created by pebbles mulching. Enhanced thermal absorption elevated soil temperatures, while organic inputs supplied lignocellulosic and other organic carbon sources, together forming a warm, carbon-rich niche that facilitated the expansion of Thermomicrobiota into otherwise less favorable environments [61].
4.3. Relationships Between Soil Properties, Bacterial Communities and Functional Potential
Correlation analyses revealed strong associations between soil factors and microbial community composition. Correlations with Mantel r ≥ 0.4 and p < 0.05 were considered statistically significant. Soil pH, nutrient availability, and enzyme activities emerged as key drivers of microbial community structure. Nutrient inputs from green waste compost mulching have been widely reported to alter microbial community structures [62], and our findings are consistent with this pattern. In particular, the abundance of Actinomycetota and Acidobacteriota was significantly correlated with soil nitrogen and pH, in line with their known ecological preferences [63]. Previous studies also demonstrated that soil physicochemical properties, especially soil organic carbon and nitrogen contents, are critical determinants of bacterial diversity and composition [64,65].
At finer taxonomic levels, Vicinamibacteraceae exhibited strong positive correlations with SOM, TN, AN, and enzymatic activities, suggesting that copiotrophic taxa are more likely to dominate under nutrient-rich and biologically active conditions, a trend also observed in straw mulching systems where copiotrophic Proteobacteria and Actinobacteria are enriched [66]. In contrast, Anaerolineaceae and Gemmatimonadaceae showed negative associations with AP and SOM, indicating that oligotrophic or stress-tolerant taxa tend to persist in less favorable environments. These patterns highlight the divergent ecological strategies among bacterial groups in response to mulching-induced changes in soil chemistry, properties, and fertility. Such findings support the broader view that soil bacterial assemblages are shaped by complex interactions between soil chemistry and microbial metabolic traits, and that microbial functional potential encompasses not only enzymatic activities measured in soils but also the presence and diversity of genes associated with key biogeochemical processes [63,67].
Mantel test analysis further confirmed that pH and protease activity were the dominant drivers of bacterial community variation, emphasizing the dual importance of soil chemistry and enzymatic processes in shaping microbial assembly. This is consistent with previous reports that soil pH acts as a primary environmental filter in structuring bacterial communities across ecosystems [68]. These results suggest that mulching regulates microbial ecology by altering soil physicochemical properties and enzyme activities, which in turn influence microbial community composition and functional roles in key biochemical processes such as carbon and nitrogen cycling. Since the effects of soil factors on microbial communities may vary seasonally due to temperature and moisture fluctuations, long-term studies are needed to evaluate the temporal stability of mulching impacts.
4.4. The Role of Mulching Practices in Shaping Soil Bacterial Communities and Functional Genes
The PICRUSt functional prediction approach can be used to assess differences in bacterial metabolic pathways under different mulching and provides phylogenetic insights relevant to soil bioremediation [69]. KEGG functional annotation demonstrated that mulching significantly influenced the metabolic potential of soil bacterial communities, with the GW treatment showing the most pronounced effects. GW markedly enhanced genes associated with carbohydrate, amino acid, and energy metabolism, indicating that organic mulching significantly stimulated microbial metabolic processes such as carbon substrate decomposition, nitrogen turnover, and energy production. This enhancement is also linked to the increased enzymatic activities observed in the soils under this treatment (see Table 2), suggesting a more active microbial community capable of breaking down complex organic compounds. These enhancements likely reflect the combined effects of organic inputs providing labile carbon sources and mineral mulching modifying soil microenvironments, thereby stimulating microbial activity. Core metabolic pathways such as carbohydrate and amino acid metabolism were particularly enriched, indicating an improved potential for both carbon utilization and protein/nitrogen recycling. Such shifts are consistent with previous findings that organic matter amendments promote microbial metabolism and nutrient cycling [9,70].
By contrast, the GWP and GWV treatment did not exhibit significant functional enhancement, suggesting that pebbles and volcanic rocks mulches may lack sufficient labile substrates to support microbial metabolism, particularly in energy and nitrogen metabolism, which are highly dependent on the availability of readily decomposable organic materials. Furthermore, the organic–inorganic mulch treatment did not exhibit a synergistic effect. These results highlight that mulching strategies differ not only in their effects on community composition but also in their regulation of microbial functional diversity, consistent with previous findings [70,71]. Overall, mulching treatments increased the abundance of most metabolism-related genes compared with CK, indicating enhanced bacterial metabolic activity. Meanwhile, the functional enrichment observed under GWP and GWV implies that combined organic–inorganic mulching can reinforce soil nutrient cycling processes, thereby contributing to improved soil fertility and plant growth. As Cheng et al. [72] emphasized, soil nutrient status strongly influences bacterial communities by shaping resource acquisition and utilization; thus, mulching practices may affect microbial communities both directly and indirectly through improvements in soil physicochemical properties, ultimately enhancing soil quality.
In brief, mulching underscores the positive impacts of organic amendments on soil bacterial communities. Reasonable mulching practices can enhance bacterial biomass turnover and nutrient cycling, thereby improving soil quality, promoting plant growth, and providing practical guidance for sustainable forest and landscape management. However, this study has certain limitations. Firstly, the prolonged impacts and underlying mechanisms of both organic and inorganic mulching on soil microbial communities remain unclear, indicating the need to establish long-term monitoring systems to evaluate their ecological consequences in urban landscaping. Additionally, it focused on bacterial communities, and future research will explore the role of fungal communities to provide a more complete understanding of microbial dynamics.
5. Conclusions
Different mulching practices exert additive effects on soil fertility, promoting plant biomass accumulation, while simultaneously reshaping bacterial diversity, community composition, and functional potential. Organic mulching (GW) reduced soil BD and enhanced enzymatic activity, and notably enriched microbial genes associated with carbon and nitrogen metabolism. In contrast, combined organic–inorganic mulching (GWV and GWP) further enhanced soil nutrient accumulation and reshaped bacterial community structure. Mantel test analyses identified that soil pH, nitrogen, and PR as the primary drivers of bacterial community variation. Overall, these findings suggest that different mulching practices provide distinct ecological benefits, and that their selection should be guided by specific management objectives. Nevertheless, the temporal dynamics and seasonal effects on mulching effectiveness, along with the long-term sustainability of these effects, remain uncertain, highlighting the need for extended field studies and continuous monitoring to optimize mulching strategies for sustainable urban forestry and landscape management.
Author Contributions
Conceptualization, writing, and methodology, Y.Z.; Visualization and resources, J.C. and Y.W. (Ying Wang); Data curation, Y.W. (Yafen Wei); Investigation, Y.T.; Project administration and supervision, Y.W. (Yanchun Wang). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Beijing Garden and Greening Youth Innovation Talent Support Program—Research on Physical Control Technology for Plant-Derived Pollutants, grant number KJCXQT202418.
Data Availability Statement
Due to issues related to data copyright, the authors will make this material available upon request to interested researchers.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Layman, R.M.; Day, S.D.; Mitchell, D.K.; Chen, Y.; Harris, J.R.; Daniels, W.L. Below ground matters: Urban soil rehabilitation increases tree canopy and speeds establishment. Urban For. Urban Green. 2016, 16, 25–35. [Google Scholar] [CrossRef]
- Murata, T.; Kawai, N. Degradation of the urban ecosystem function due to soil sealing: Involvement in the heat island phenomenon and hydrologic cycle in the Tokyo metropolitan area. Soil Sci. Plant Nutr. 2018, 64, 145–155. [Google Scholar] [CrossRef]
- Jim, C.Y. Soil compaction as a constraint to tree growth in tropical & subtropical urban habitats. Environ. Conserv. 1993, 20, 35–49. [Google Scholar] [CrossRef]
- Kargar, M.; Jutras, P.; Clark, O.G.; Hendershot, W.H.; Prasher, S.O. Macro-nutrient availability in surface soil of urban tree pits influenced by land use, soil age, and soil organic matter content. Urban Ecosyst. 2015, 18, 921–936. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Turner, N.C.; Gong, Y.H.; Li, F.M.; Fang, C.; Ge, L.J. Benefits and limitations to straw-and plastic-film mulch on maize yield and water use efficiency: A meta-analysis across hydrothermal gradients. Eur. J. Agron. 2018, 99, 138–147. [Google Scholar] [CrossRef]
- Tang, M.; Gao, X.; Wu, P.; Li, H.; Zhang, C. Effects of living mulch and branches mulching on soil moisture, temperature and growth of rain-fed jujube trees. Plants 2022, 11, 2654. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Basit, A.; Mohamed, H.I.; Ali, I.; Ullah, S.; Kamel, E.A.R.; Shalaby, T.A.; Ramadan, K.M.A.; Alkhateeb, A.A.; Ghazzawy, H.S. Mulching as a sustainable water and soil saving practice in agriculture: A review. Agronomy 2022, 12, 1881. [Google Scholar] [CrossRef]
- Ramos, T.B.; Darouich, H.; Pereira, L.S. Mulching effects on soil evaporation, crop evapotranspiration and crop coefficients: A review aimed at improved irrigation management. Irrig. Sci. 2024, 42, 525–539. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, X.; Li, S.; Qu, B.; Zhang, J. How organic mulching influences the soil bacterial community structure and function in urban forests. Microorganisms 2024, 12, 520. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, Y.-H.; Zhang, Z.-T.; Huang, W.-L.; Li, L.-P.; Li, J.-T.; Liu, J.-S.; Zheng, Y.; Mo, J.-M.; Zhang, W.; et al. Dissolved organic matter characteristics in soils of tropical legume and non-legume tree plantations. Soil Biol. Biochem. 2020, 148, 107880. [Google Scholar] [CrossRef]
- Marble, S.C.; Steed, S.T.; Saha, D.; Khamare, Y. On-farm evaluations of wood-derived, waste paper, and plastic mulch materials for weed control in Florida container nurseries. HortTechnology 2019, 29, 866–873. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, X.; Li, S.; Du, T.; Zheng, Y.; Fan, Z. Effects of organic mulching on soil aggregate stability and aggregate binding agents in an urban forest in Beijing, China. J. For. Res. 2022, 33, 1083–1094. [Google Scholar] [CrossRef]
- Dlamini, P.; Ukoh, I.B.; van Rensburg, L.D.; du Preez, C.C. Reduction of evaporation from bare soil using plastic and gravel mulches and assessment of gravel mulch for partitioning evapotranspiration under irrigated canola. Soil Res. 2016, 55, 222–233. [Google Scholar] [CrossRef]
- Al-Zboon, K.K.; Al-Tabbal, J.A.; Al-Kharabsheh, N.M.; Al-Mefleh, N.K. Natural volcanic tuff as a soil mulching: Effect on plant growth and soil chemistry under water stress. Appl. Water Sci. 2019, 9, 123. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, J.; Li, Q.; Liang, F.; Mu, X.; Pei, D.; Jia, H.; Wang, Z. Effects of film mulching on soil microbial diversity and community structure in the maize root zone under drip irrigation in northwest China. Agronomy 2024, 14, 1139. [Google Scholar] [CrossRef]
- He, X.; Wu, Y.; Liu, K.; Ji, J.; Wu, C.; Li, J.; Song, H.; Hu, D.; Zhou, C. The combined application of inorganic and organic materials over two years improves soil pH, slightly increases soil organic carbon, and enhances crop yields in severely acidic red Soil. Agronomy 2025, 15, 498. [Google Scholar] [CrossRef]
- Hao, H.; Zhao, X.; Wang, Y.; Zhang, Y.; Xie, Z.; Guo, Z.; Wang, R. Effects of gravel-sand mulching on soil bacterial community and metabolic capability in the semi-arid Loess Plateau, China. World J. Microbiol. Biotechnol. 2017, 33, 209. [Google Scholar] [CrossRef]
- Czarnecki, S.; Düring, R.A. Influence of long-term mineral fertilization on metal contents and properties of soil samples taken from different locations in Hesse, Germany. Soil Discuss. 2014, 1, 239–265. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, R.; Blagodatskaya, E.; Blagodatsky, S.; Liu, D.; Yu, Y.; Zhu, X.; Feng, Y. Long-term application of mineral fertilizer weakens the stability of microbial n-transforming functions via the decrease of soil microbial diversity. J. Sustain. Agric. Environ. 2024, 3, e70014. [Google Scholar] [CrossRef]
- Kader, M.A.; Singha, A.; Begum, M.A.; Jewel, A.; Khan, F.H.; Khan, N.I. Mulching as water-saving technique in dryland agriculture: Review article. Bull. Natl. Res. Cent. 2019, 43, 147. [Google Scholar] [CrossRef]
- Khan, S.U.; Wang, X.; Mehmood, T.; Latıf, S.; Khan, S.U.; Fiaz, S.; Qayyum, A. Comparison of organic and inorganic mulching for weed suppression in wheat under rain-Fed conditions of Haripur, Pakistan. Agronomy 2021, 11, 1131. [Google Scholar] [CrossRef]
- Yang, J.; Mcbride, J.; Zhou, J.; Sun, Z. The urban forest in beijing and its role in air pollution reduction. Urban For. Urban Green. 2005, 3, 65–78. [Google Scholar] [CrossRef]
- Dhiman, D.; Vishvamitera, S.; Baghla, S.; Singh, S.; Kumar, D.; Kumar, A.; Chauhan, R. Synergistic effect of mulch and nitrogen management on growth and essential oil yield of Salvia sclarea L. Sci. Rep. 2024, 14, 32075. [Google Scholar] [CrossRef]
- Greenly, K.M.; Rakow, D.A. The effect of wood mulch type and depth on weed and tree growth and certain soil parameters. Arboric. Urban For. 1995, 21, 225–232. [Google Scholar] [CrossRef]
- Maggard, A.O.; Will, R.E.; Hennessey, T.C.; McKinley, C.R.; Cole, J.C. Tree-based mulches influence soil properties and plant growth. HortTechnology 2012, 22, 353–361. [Google Scholar] [CrossRef]
- Jenkins, J.C.; Chojnacky, D.C.; Heath, L.S.; Birdsey, R.A. National-Scale Biomass Estimators for United States Tree Species. For. Sci. 2003, 49, 12–35. [Google Scholar] [CrossRef]
- Catchpole, W.R.; Wheeler, C.J. Estimating plant biomass: A review of techniques. Austral Ecol. 1992, 17, 121–131. [Google Scholar] [CrossRef]
- Burdett, A.N. A nondestructive method for measuring the volume of intact plant parts. Can. J. For. Res. 1979, 9, 120–122. [Google Scholar] [CrossRef]
- Vogt, K.A.; Persson, H. Measuring growth and development of roots. In Techniques and Approaches in Forest Tree Ecophysiology; CRC Press: Boca Raton, FL, USA, 1991; pp. 477–501. [Google Scholar]
- Angel, R.; Soares, M.I.M.; Ungar, E.D.; Gillor, O. Biogeography of soil archaea and bacteria along a steep precipitation gradient. ISME J. 2010, 4, 553–563. [Google Scholar] [CrossRef]
- Pellissier, L.; Niculita-Hirzel, H.; Dubuis, A.; Pagni, M.; Guex, N.; Ndiribe, C.; Salamin, N.; Xenarios, I.; Goudet, J.; Sanders, I.R.; et al. Soil fungal communities of grasslands are environmentally structured at a regional scale in the Alps. Mol. Ecol. 2014, 23, 4274–4290. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.M. Nitrogen-Total. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Keeney, D.R.; Nelson, D.W. Nitrogen-Inorganic Forms. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Knudsen, D.; Peterson, G.A.; Pratt, P.F. Potassium. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1982; pp. 225–246. [Google Scholar]
- Böhme, L.; Langer, U.; Böhme, F. Microbial biomass, enzyme activities and microbial community structure in two European long-term field experiments. Agric. Ecosyst. Environ. 2005, 109, 141–152. [Google Scholar] [CrossRef]
- DeForest, J.L. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and l-DOPA. Soil Biol. Biochem. 2009, 41, 1180–1186. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Noguchi, H.; Taniguchi, T.; Itoh, T. MetaGene: Prokaryotic gene finding from environmental genome shotgun sequences. Nucleic Acids Res. 2006, 34, 5623–5630. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Li, R.; Yu, C.; Li, Y.; Lam, T.W.; Yiu, S.M.; Kristiansen, K.; Wang, J. SOAP2: An improved ultrafast tool for short read alignment. Bioinformatics 2009, 25, 1966–1967. [Google Scholar] [CrossRef]
- Kumar, R.; Sood, S.; Sharma, S.; Kasana, R.C.; Pathania, V.L.; Singh, B.; Singh, R.D. Effect of plant spacing and organic mulch on growth, yield and quality of natural sweetener plant Stevia and soil fertility in Western Himalayas. Int. J. Plant Prod. 2014, 8, 311–334. [Google Scholar] [CrossRef]
- Sun, X.; Wang, G.; Ma, Q.; Liao, J.; Wang, D.; Guan, Q.; Jones, D.L. Organic mulching promotes soil organic carbon accumulation to deep soil layer in an urban plantation forest. For. Ecosyst. 2021, 8, 2. [Google Scholar] [CrossRef]
- Pavl, L.; Kodeová, R.; Fér, M.; Nikodem, A.; Proke, R. The impact of various mulch types on soil properties controlling water regime of the Haplic Fluvisol. Soil Tillage Res. 2021, 205, 104748. [Google Scholar] [CrossRef]
- Basak, B.B.; Sarkar, B.; Naidu, R. Environmentally safe release of plant available potassium and micronutrients from organically amended rock mineral powder. Environ. Geochem. Health 2021, 43, 3273–3286. [Google Scholar] [CrossRef]
- Abou-El-Seoud, I.I.; Abdel-Megeed, A. Impact of rock materials and biofertilizations on P and K availability for maize (Zea maize) under calcareous soil conditions. Saudi J. Biol. Sci. 2012, 19, 55–63. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Li, W.; Su, Q.; Ma, B.; Mu, M.; Jia, Z.; Zhao, G. Effect of the release of gravel elements on soil nutrients and jujube fruit yield under wet-and-dry cycles. Agronomy 2022, 12, 2881. [Google Scholar] [CrossRef]
- Ramos, C.G.; Querol, X.; Oliveira, M.L.S.; Pires, K.; Kautzmann, R.M.; Oliveira, L.F.S. A preliminary evaluation of volcanic rock powder for application in agriculture as soil a remineralizer. Sci. Total Environ. 2015, 512, 371–380. [Google Scholar] [CrossRef]
- Machado, D.L.; Dourado, M.N.; de Freitas, M.S.; de Souza, L.M.; da Silva, E.M.; Podadera, D.S.; Andrade, C.R.; Ferreira, W.C.; Guilherme, F.A.G. Organic mulching alters the soil microclimate, increases survival and growth of tree seedlings in restoration planting. Forests 2024, 15, 1777. [Google Scholar] [CrossRef]
- Degens, B.P.; Schipper, L.A.; Sparling, G.P.; Vojvodic-Vukovic, M. Decreases in organic C reserves in soils can reduce the catabolic diversity of soil microbial communities. Soil Biol. Biochem. 2000, 32, 189–196. [Google Scholar] [CrossRef]
- Nair, A.; Ngouajio, M. Soil microbial biomass, functional microbial diversity, and nematode community structure as affected by cover crops and compost in an organic vegetable production system. Appl. Soil Ecol. 2012, 58, 45–55. [Google Scholar] [CrossRef]
- Detheridge, A.P.; Brand, G.; Fychan, R.; Crotty, F.V.; Sanderson, R.; Griffith, G.W.; Marley, C.L. The legacy effect of cover crops on soil fungal populations in a cereal rotation. Agric. Ecosyst. Environ. 2016, 228, 49–61. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Luo, Y.; Awasthi, M.; Yang, J.; Duan, Y.; Li, H.; Zhao, Z. Mulching practices alter the bacterial-fungal community and network in favor of soil quality in a semiarid orchard system. Sci. Total Environ. 2020, 725, 138527. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Ren, C.; Wang, D.; Wu, R.; Wang, Y.; Li, Z.; Huang, D.; Qi, H. Microbial community assembly and its influencing factors of secondary forests in Qinling Mountains. Soil Biol. Biochem. 2023, 184, 109075. [Google Scholar] [CrossRef]
- Wang, M.; Shao, Y.; Zhang, W.; Yu, B.; Shen, Z.; Fan, Z.; Zu, W.; Dai, G.; Fu, S. Secondary succession increases diversity and network complexity of soil microbial communities in subtropical and temperate forests. Catena 2024, 249, 108662. [Google Scholar] [CrossRef]
- Giguere, A.T.; Eichorst, S.A.; Meier, D.V.; Herbold, C.W.; Richter, A.; Greening, C.; Woebken, D. Acidobacteria are active and abundant members of diverse atmospheric H2-oxidizing communities detected in temperate soils. ISME J. 2021, 15, 363–376. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, S.H.; Jo, H.Y.; Finneran, K.T.; Kwon, M.J. Diversity and composition of soil Acidobacteria and Proteobacteria communities as a bacterial indicator of past land-use change from forest to farmland. Sci. Total Environ. 2021, 797, 148944. [Google Scholar] [CrossRef]
- Hu, J.; Wei, Z.; Weidner, S.; Friman, V.-P.; Xu, Y.-C.; Shen, Q.-R.; Jousset, A. Probiotic Pseudomonas communities enhance plant growth and nutrient assimilation via diversity-mediated ecosystem functioning. Soil Biol. Biochem. 2017, 113, 122–129. [Google Scholar] [CrossRef]
- Miyatake, F.; Iwabuchi, K. Effect of high compost temperature on enzymatic activity and species diversity of culturable bacteria in cattle manure compost. Bioresour. Technol. 2005, 96, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Blumer-Schuette, S.E.; Brown, S.D.; Sander, K.B.; Bayer, E.A.; Kataeva, I.; Zurawski, J.V.; Conway, J.M.; Adams, M.W.W.; Kelly, R.M. Thermophilic lignocellulose deconstruction. FEMS Microbiol. Rev. 2014, 38, 393–448. [Google Scholar] [CrossRef]
- Chi, Z.; Ju, S.; Li, H.; Li, J.; Wu, H.; Yan, B. Deciphering edaphic bacterial community and function potential in a Chinese delta under exogenous nutrient input and salinity stress. Catena 2021, 201, 105212. [Google Scholar] [CrossRef]
- Mu, X.; Wang, J.; Qin, H.; Ding, J.; Mou, X.; Liu, S.; Wang, L.; Zhang, S.; Zhang, J.; Wang, P. Analyses of Rhizosphere Soil Physicochemical Properties and Microbial Community Structure in Cerasus humilis Orchards with Different Planting Years. Horticulturae 2024, 10, 1102. [Google Scholar] [CrossRef]
- Gui, H.; Fan, L.; Wang, D.; Yan, P.; Li, X.; Zhang, L.; Han, W. Organic management practices shape the structure and associations of soil bacterial communities in tea plantations. Appl. Soil Ecol. 2021, 163, 103975. [Google Scholar] [CrossRef]
- Shen, F.; Fei, L.; Tuo, Y.; Peng, Y.; Yang, Q.; Zheng, R.; Wang, Q.; Liu, N.; Fan, Q. Effects of water and fertilizer regulation on soil physicochemical properties, bacterial diversity and community structure of Panax notoginseng. Sci. Hortic. 2024, 326, 112777. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Z.; Chen, K.; Chen, Z.; Zeng, X.; Yu, H.; Guo, S.; Shangguan, Y.; Chen, Q.; Fan, H.; et al. Changes in soil physicochemical properties and bacterial communities at different soil depths after long-term straw mulching under a no-till system. Soil 2021, 7, 595–609. [Google Scholar] [CrossRef]
- Ridl, J.; Kolar, M.; Strejcek, M.; Strnad, H.; Stursa, P.; Paces, J.; Macek, T.; Uhlik, O. Plants rather than mineral fertilization shape microbial community structure and functional potential in legacy contaminated Soil. Front. Microbiol. 2016, 7, 995. [Google Scholar] [CrossRef]
- Liu, H.Q.; Li, S.C.; Li, H.J.; Peng, Z.C. Soil pH Determining the assembly processes of abundant and rare bacterial communities in response to cultivation modes in lemon farmlands. Plants 2025, 14, 1852. [Google Scholar] [CrossRef]
- Ren, K.; Song, W.; Wei, Z.; Song, L.; Liu, M.; Zhou, Y.; Zhen, Y.; Wu, X.; Gu, K.; Simarani, K.; et al. Organic material mulching regulated core microbial groups to promote soil carbon and nitrogen cycling and improve faba bean productivity under a triple-cropping system in purple soil hilly region of southwest China. Front. Microbiol. 2025, 16, 1602633. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, L.; Xie, J.; Coulter, J.A.; Zhang, R.; Luo, Z.; Cai, L.; Wang, L.; Gopalakrishnan, S. Soil bacterial diversity and potential functions are regulated by long-term conservation tillage and straw mulching. Microorganisms 2020, 8, 836. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Q.; Liu, C.; Ding, Y.; Liu, L.; Tian, Y.; Wu, X.; Li, H.; Awasthi, M.K.; Zhao, Z. Mulching practices alter soil microbial functional diversity and benefit soil quality in orchards on the Loess Plateau. Sci. Total Environ. 2020, 271, 138527. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, B.; Wei, M.; Wang, S.; Rong, X.; Du, D.; Wang, C. Changes in community structure and metabolic function of soil bacteria depending on the type restoration processing in the degraded alpine grassland ecosystems in Northern Tibet. Sci. Total Environ. 2021, 755, 142619. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).