Abstract
A protocol for the cultivation of Larix sibirica Ledeb. by somatic embryogenesis was developed (RF Patent No. 2456344, 2012). The L. sibirica collection consisted of 22 actively proliferating cell lines (CLs) obtained from immature zygotic embryos. The age of CLs ranged from 1 to 14 years. CLs differed in their growth intensity, embryonic productivity, hormonal balance, and genetic stability, as well as in their regenerative ability. In most proliferating CLs, the formation of globular somatic embryos continued for 2–4 years. Here, a number of CLs actively proliferated for 9–14 years or more. The formation of embryogenic cultures in L. sibirica is associated with the content of phytohormones and their localization in embryo cells. The cytogenetic studies revealed the genetic stability of young CLs (up to 1 year), in which the karyotype consisted of the diploid number of chromosomes (2n = 24). Genomic mutations were observed in the long-term proliferation of CLs. Individual CLs can maintain cytogenetic stability for many years; such CLs can successfully be used to preserve germplasm, obtain planting material, and for plantation reforestation.
1. Introduction
Over the past 30 years, the most promising direction in microclonal propagation of conifers has been the application of somatic embryogenesis technology in in vitro cultures. Using the technology of somatic embryogenesis, it is possible to perform not only the vast propagation of coniferous species with breeding-significant traits but also fundamental research in the field of morphogenetic programs and physiological and genetic-molecular studies in early ontogenesis [1,2,3].
Somatic embryogenesis is a unique method of asexual vegetative propagation based on the totipotency of plant cells [4,5,6]. Currently, somatic embryogenesis is intensively investigated. Certain factual data have been accumulated on the study of morphological, physiological–biochemical, histological, and molecular genetic features of somatic embryo formation in gymnosperms [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. The use of somatic embryogenesis technology in combination with genomic selection and cryopreservation is the basis for multi-variety plantation reforestation (Multi-Varietal Forestry program, MVF) [23,24], aimed at growing economically valuable genetically tested clones and elite genotypes [23,25].
Species of the genus Larix have extremely high plasticity of vegetative and generative organs, which distinguishes larch from other representatives of the Pinaceae family [26]. Due to the plasticity, Larix species are well introduced into cultures in vitro. Somatic embryogenesis was obtained and comprehensively studied in such Larix species as L. decidua [27,28,29], L. leptolepis [27,30,31], L. occidentalis [28,32], L. Principis-Rupprechtii [33], L. olgensis [34] and hybrids such as Larix × eurolepis (L. decidua × L. kaempferi) and Larix × marschlinsii (L. kaempferi × L. decidua) [9], L. kaempferi × L. gmelinii [35], etc.
A protocol for the cultivation of Larix sibirica by somatic embryogenesis was first developed at the V.N. Sukachev Institute of Forest SB RAS (Krasnoyarsk, Russia) (RF Patent No. 2456344, 2012). A collection of long-term proliferating cell lines was created, which has remained active for 14 years and is intensively producing somatic embryos. In this paper, the structural–functional and molecular genetic studies of the development of somatic embryos are carried out on the cell lines of the collection, and the possibility of using the protocol for the cultivation of L. sibirica by somatic embryogenesis for larch forest plantations is also considered.
2. Materials and Methods
The studied materials (zygotic embryos) originated from the L. sibirica genotypes A4 (without galls by the larch bud gall midge) and 10 grown in the arboretum of the V.N. Sukachev Institute of Forest (55°59′ N 92°45′ E). In addition, eleven-year-old genetically identical emblings of the clone obtained from CL6 derived from the A4 donor tree growing at the experimental station of the Institute of Forest SB RAS (56°22′ N 92°57′ E) were included in the study.Controlled pollination was performed between the donor trees and the emblings of the clone.
Immature seeds at the cotyledon initiation stage obtained from open and controlled pollination were collected from the donor and emblings of the clone. The explants were zygotic embryos at the cotyledon initiation stage collected in the second half of July. The embryos were sterilized and introduced into in vitro culture in an AI medium according to the method developed earlier for larch (RF Patent No. 2456344, 2012).
The preparation of the laboratory samples of cloned Siberian larch seedlings from embryonal-suspensor masses (ESMs), ready for planting in a greenhouse, took 4–9 months: stage 1—ESMs initiation lasted for 30–45 days (AI medium with 2,4-dichlorophenoxyacetic acid (2,4-D) and 6-benzylaminopurine (6-BA)); stage 2—proliferation proceeded for 1–2 months, the ability to proliferate preserved in individual cell lines for 14 or more years with regular subcultivation (AI medium with 2.4-D and 6-BA); stage 3—maturation of the somatic embryos for 20–60 days (AI medium with abscisic acid (ABA) and indole-3-butyric acid (IBA)); stage 4—germination of the somatic embryos took 5–8 weeks (½AI medium without hormones and vitamins); stage 5—adaptation of the seedlings in sterile soil in a growth chamber for 3 months. The cloned plantlets, having reached heights of 2–3 cm with well-developed roots and epicotyl, were ready for planting in a greenhouse. In the greenhouse, the seedlings were kept for 1 year, subject to regular care (watering and weed control), and then planted in the soil of the forest nursery.
2.1. Productivity of the CLs
The productivity of each cell line was determined by counting the number of globular somatic embryos (SEs) per 1 g fresh ESMs a week after subculturing. The staining was carried out by a 2% aqueous solution of safranin for the globular SEs, and by an 1% solution of acetocarmine and a 0.5% Evans blue solution for the precotyledons SEs.The samples were examined on, MICMED-6 and BLM M-1 microscopes (both LOMO, St. Petersburg, Russia).
The mature somatic embryos were counted on the 45th day of cultivation. The frequency of the mature embryos was calculated by the proportion in which the number of globular embryos per 1 g of ESMs fresh weight was taken as 100%.
2.2. Purification of Plant Hormones and Their Enzyme Immunoassay
The material for the study of hormone content in the proliferating embryogenic cultures was selected from collection bank CLs: CL4, initiated in 2009, open pollination; CL5, initiated in 2009, controlled pollination trees A4 × 1; CL6, initiated in 2011, open pollination; and CL19.20.2, initiated in 2019, controlled pollination trees 1(35) × A4. Hormone levels were assessed twice in 2016 and 2022. Data for CL5 in 2022 and CL19.20.2 in 2016 are missing due to the fact that CL5 was necrotized in 2018, and CL19.20.2 was obtained only in 2019.
Extraction of IAA and ABA was performed according to a modified scheme, as described [36]. Cytokinins (zeatin and its riboside and nucleotide) from an aliquot of the aqueous residue were purified and separated by thin-layer chromatography as described [37]. Hormone assay was performed as described [36,37].
2.3. Cytogenetic Analysis
For cytogenetic studies, the proliferating embryogenic cell lines of different ages were selected from the collection bank of embryogenic cultures of Siberian larch: CL4 and CL6 [38] described above in the Section 2.2.; CL21.20.1, initiated in 2021, open pollination; CL22.5.1, initiated in 2022, open pollination; CL22.47.1, initiated in 2022, controlled pollination of trees A4 × 10. The cytogenetic study of the CL4 and CL6 was repeated [39].
Cytogenetic analysis was carried out on the globular SEs at the stage of proliferation. Different treatment methods were used for metaphase arrest and chromosome contraction. The pretreatment of the material with 0.2% colchicine for 18–20 h at room temperature provided the best metaphase yield for most lines. The material was fixed in ethanol–acetic acid (3:1) for 24 h. The material was stained in a 1% aceto-hematoxylin solution at 50 °C for 10–15 min. Further, a few SEs were dissected using a fine needle and forceps and then placed in a drop of a saturated chloral hydrate solution. The embryos were macerated with a razor blade for 2–3 min until a homogeneous suspension was obtained. The preparation was covered with a coverslip, drained with filter paper, and squashed. The chromosome number was assessed in 100 metaphase cells per each CL. The slides were examined using a MICMED-6 microscope (LOMO, St. Petersburg, Russia) equipped with a MS-12 digital camera (LOMO, St. Petersburg, Russia).
2.4. Characteristics of the Clone Growth and Seed Production
The assessment of the condition of eleven-year-old clones obtained from CL6 was performed based on the results of the growth of vegetative and generative organs. The annual growth of shoots, the appearance of generative organs, and the seed productivity of female cones were estimated.
Data were expressed as means ± S.E., which were calculated in all variants using MS Excel. Significant differences between means were analyzed by a t-test, one-way analysis of variance (ANOVA), and a least significant difference (LSD) test to discriminate means.
3. Results
3.1. Initiation and Proliferation of Embryogenic Cultures
Initiation of an embryogenic culture (EC) in Larix sibirica begins with polarization, elongation of somatic cells, and localization of IAA at one end of an elongated cell [16]. Elongated cells divide asymmetrically to form a small embryonic cell (the initials) and an elongated tube cell. A globule of a somatic embryo is formed from the embryonic cell. From the basal cells of the globule, suspensor tube cells are formed [16]. Globular embryos always have a clear polarization: the globule occupies the apical end, and the suspensor adjoins the basal part of the globule. In the globular embryos, there is a clear distribution of hormones: IAA, cytokinins, and ABA are localized in the cells of the globule, being completely absent in the cells of the embryonic tubes of the suspensor.
Further, a well-developed embryogenic tissue is formed, i.e., the ESMs, which consists of the globular embryos and suspensors (Figure 1a). The morphological markers of the embryogenic CLs are cream, white, or light yellow colors of the culture and its friable structure. Active ESMs proliferation begins at the age of three months and persists for up to 14 years or more.
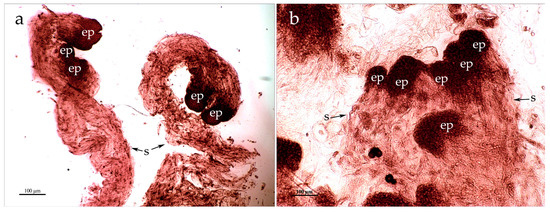
Figure 1.
Histology of the embryonal-suspensor masses of Siberian larch. (a) Globular somatic embryos. (b) Polyembryonic complexes. The scale is 100 μm. The embryo proper (ep) and the suspensors (s) are indicated with arrows.
During the study (2008–2022), 54 proliferating embryogenic cell lines (CLs) were obtained as a result of open and controlled pollination in L. sibirica. Currently, the collection of proliferating ECs of Siberian larch consists of 22 CLs of different ages (from 1 year up to 14 years or more). For most proliferating cell lines, the ESMs can maintain the capability to form globular somatic embryos within 2–4 years after their creation. Then, the proliferation rate slowed down, and the cell lines died. At the same time, some cell lines, for example, CL4, CL6, CL5, and CL12, actively proliferated for 14, 11, 9, and 5 years, respectively. Significant differences were observed in CLs in the number of globular somatic embryos and their ability to mature and germinate.
The smallest somatic embryos with a short suspensor were formed in the long-term proliferating CL5 (the globule diameter being 90 ± 18 µm with the suspensor length of 126.4 ± 37.53 µm), while the largest globules were observed in the long-term proliferating CL6 (with the globule diameter of 282 ± 27.5 and the suspensor length of 1124.05 ± 27.53 µm) [15] and CL19.20.2 (the globule diameter being 450.00 ± 33.64 µm with the suspensor length of 1900.1 ± 31.27 µm).
The globules of the somatic embryos were localized on the surface of the embryonic culture, and the suspensors were closely adjacent to each other. Polyembryonic complexes were formed. These complexes included 2–8 or even more globular embryos (Figure 1b).
Multiplication of the globular somatic embryos occurred through cleavage, the formation of adventitious buds on the suspensor, and cleavage of its cells, followed by their asymmetric division and the formation of embryo globules [15].
Thus, the proliferating larch cell lines significantly differ in the number of globular embryos. As is typical for somatic embryogenesis, the globules with a diameter of about 200 μm and the long suspensor have a clear “globule-suspensor” polarization. Such embryos actively multiply. The number of somatic embryos per 1 g fresh weight of ESMs and globule diameter, polarization, and length of the suspensor are the markers of somatic embryogenesis.
3.2. Hormonal Balance of the Proliferating CLs
For study content, hormones in proliferating CL4, CL5, CL6, CL 19.20.2 were selected. In addition, we compared hormone balance in the long-term proliferation of CL4 and CL6, which were cultivated for 14 and 11 years, respectively. The data of the enzyme immunoassay of ESMs showed that the proliferating CLs contain a large amount of IAA (Figure 2). The level of the accumulation of IAAs after 11 (CL6) and 14 years (CL4) of cultivation increased (p < 0.01). There was also a statistically significant (p < 0.01) increase in the content of IAA in CL4, having proliferated for 14 years.
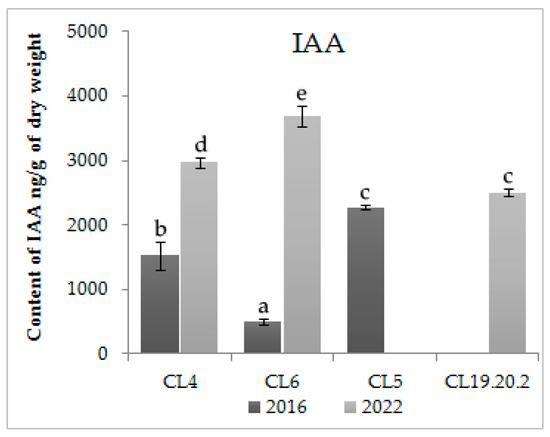
Figure 2.
Content of IAA in proliferative embryogenic cultures of Siberian larch. Means ± SE are presented. Statistically different means are indicated by different letters.
The content of IAA in the three-year CL 19.20.2 is also high; the level of accumulation of cytokinins is significantly lower than auxins (Figure 2 and Figure 3). In long-term cultured CL6 and CL4, the zeatin content did not change statistically significantly (p < 0.05). The contents of zeatin riboside in CL6 and zeatin nucleotide in both CLs were increased statistically significantly (p < 0.05) with the time of proliferation. The level of cytokinins in CL6 was increased by four times with an increase in the proliferation time (Figure 3).
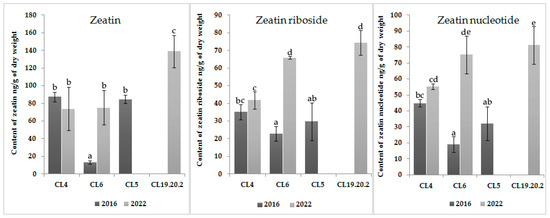
Figure 3.
Content of zeatin, zeatin riboside, and zeatin nucleotide in proliferative embryogenic cultures of Siberian larch. Means ± SE are presented. Statistically different means are indicated by different letters.
The level of ABA in proliferating CLs is varied. In cells actively proliferating for three–seven years, the ABA content is very low—9–24 ng/g dry weight. In 11–14-year-old proliferating cultures, the content of ABA increased statistically significantly (p < 0.05) to 970 ng/g dry weight (Figure 4). Somatic embryos of CL6, after 11 years of cultivation, lost the ability to differentiate and did not form plantlets. For CL4, actively proliferating for 14 years, plantlets had problems with rooting (callus instead of the root or on the root neck). In three-year-old CL19.20.2, the ABA content was very high and had no statistically significant differences (p > 0.05) from CL6 proliferating for 11 years (Figure 4). In this CL, somatic embryos did not mature.
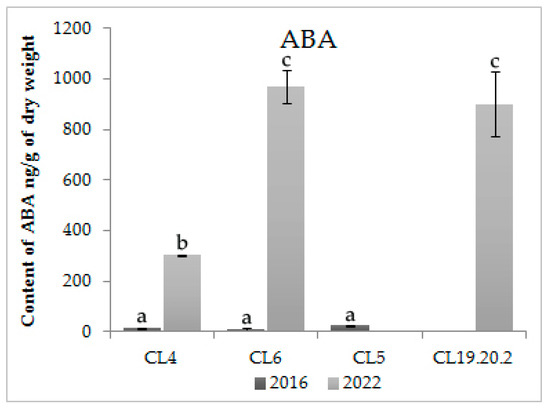
Figure 4.
Content of ABA in proliferative embryogenic cultures of Siberian larch. Means ± SE are presented. Statistically different means are indicated by different letters.
It can be concluded that the formation of embryogenic cultures in Siberian larch was associated with the content of phytohormones and their localization in cells. The most informative was the IAA. At the same time, actively proliferating embryogenic cultures were characterized by a high accumulation of IAA and a low content of cytokinins and ABA. The high level of IAA and ABA in proliferative cell lines is due to the inability of somatic embryos to mature and form viable plantlets. This was especially evident during long-term proliferation, during which the ability of cultures to regenerate is lost. Thus, the high content of IAA and ABA in proliferating cells of L. sibirica are criteria according to which it is possible to conclude the regenerative ability of L. sibirica CLs.
3.3. Genetic Stability of the Proliferating CLs
The cytogenetic studies revealed the genetic stability of young CLs (up to 1 year), whose karyotype consisted of the diploid number of chromosomes (2n = 24). The genomic mutations that may have been present in the initial material introduced into the culture or that may have appeared during the cultivation period were observed in some young CLs studied. A change in the number of chromosomes (2n = 25; 2n = 26) was revealed in the long-term proliferating CLs (seven years of cultivation and more), which retained the ability to form somatic embryos. The morphological analysis of the karyotype and selection of pairs of homologous chromosomes allowed us to determine that the most common type of genomic disorder in the embryogenic cultures of the Siberian larch is aneuploidy, namely, trisomy for one or several pairs of chromosomes.
CL4 is of great interest. It is cytogenetically stable; most metaphase plates contain a constant number of chromosomes, but it differs from the normal number both for this species and for the genus Larix as a whole. In 2016–2017 (seven years of cultivation), the majority of cells in this cell line contained 25 chromosomes in the karyotype: 12 long metacentrics and 13 short submetacentrics (2n = 12 m + 13 sm). A repeat experiment in 2022 (14 years of cultivation) revealed 26 chromosomes: 13 long metacentrics and 13 short submetacentrics (2n = 13 m + 13 sm) (Figure 5a). The morphological analysis of chromosomes and selection of the pairs of homologs allowed us to determine the type of genomic disorders in CL4 as trisomy for two pairs of chromosomes. At the same time, individual embryogenic cell lines (CL6) can maintain cytogenetic stability for 11 years and contain the normal number of chromosomes in the karyotype (2n = 24, Figure 5b).
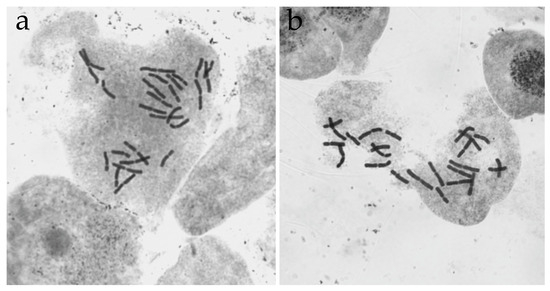
Figure 5.
Metaphase cells of L. sibirica CLs with different numbers of chromosomes stained in aceto-hematoxylin. (a) CL4 (2n = 26, 13-year-old). (b) CL6 (2n = 24, 11-year-old).
3.4. Maturation and Germination of the Somatic Embryos
To stimulate the maturation of mitosis arrest in globular somatic embryos, embryogenic cultures are transferred to a hormone-free AI medium supplemented with activated carbon for a week and then to a medium with the same mineral composition but supplemented with ABA, IBA, and PEG 8000, where differentiation and maturation processes occur. The somatic embryos matured and germinated in 16 collection CLs. The number of mature embryos ranged from 9 per 1 g fresh weight of ESMs (CL16.19, self-pollination) to 1221 (CL4) (Table 1). The somatic embryos of 14-year-old CL4 are still able to mature (Figure 6a) and actively germinate (Figure 6b); however, the plantlets often had calluses, especially on the primary root. The small somatic embryos of CL5 and 19.20.2 with short suspensors actively proliferated but did not mature. In vitro-derived plantlets and clones were obtained from the somatic embryos of CL6. A young CL22.27.1 obtained from clone No. 8 originating from CL6 and pollinated by the pollen of the A4 donor tree showed an active ability to mature, but the maturation process in this experiment is still ongoing (Figure 6c,d).

Table 1.
Productivity of the mature somatic embryos of the Larix sibirica collection cell lines.
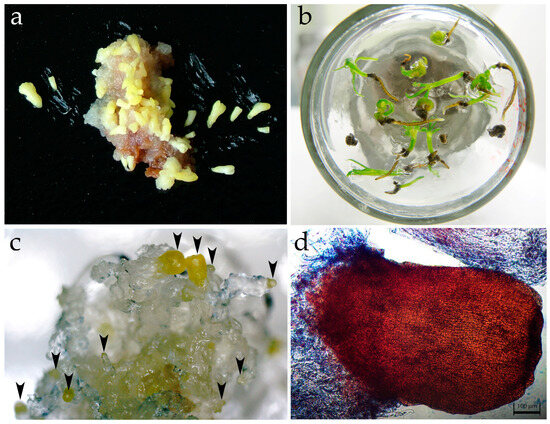
Figure 6.
Late stages of somatic embryogenesis and conversion into plantlets in Larix sibirica. (a) Mature somatic embryos at the cotyledon stage (CL4). (b) Plantlets of the CL4. (c) Somatic embryos in the process of maturation at the globular and precotyledonary stage indicated with arrows (CL22.27.1). (d) Somatic embryo at the precotyledonary stage after growth for 38 days on maturation medium (CL22.27.1). The scale is 100 μm.
3.5. Features of the Clone Growth
The cloned Larix sibirica trees obtained as a result of the somatic embryogenesis successfully grew for eleven years in the soil of the forest nursery (Figure 7a). The trees show no signs of damage from the larch bud gall. Their growth rate is 1.4 times higher than that of the trees obtained from seeds (Figure 7b).
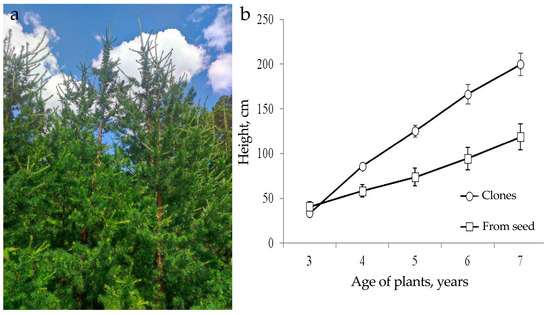
Figure 7.
Clones of the Siberian larch at the “Pogorelsky Bor” station, Institute of Forests, Siberian Branch, Russian Academy of Sciences. (a) Vegetation of the clones (summer). (b) Comparative dynamics of the growth of the emblings of the clone (CL6) and trees from seeds.
Generative organ buds were formed in tree clones at the age of seven years. In the subsequent spring–summer period, the process of micro- and macrosporogenesis was completed, and the clones produced male and female cones (micro- and megastrobilus) containing pollen and seeds, respectively (Figure 8a–c). The clones obtained from the cell cultures formed larger cones compared to the donor trees (Table 2). In terms of the seed productivity of female cones, the clones did not differ from the donor trees. The controlled pollination experiments were conducted on the larch clones in 2022. Hybrid embryos were introduced into the in vitro culture.
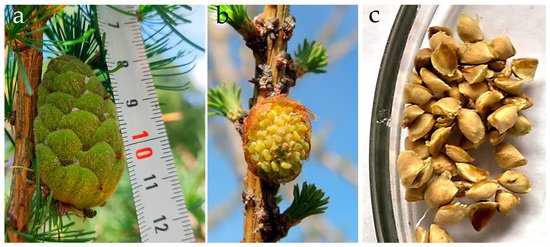
Figure 8.
Generative structures of the clones of the Siberian larch at the “Pogorelsky Bor”. (a) Female cone. (b) Male cone. (c) Seeds obtained from the clone No. 8 (from CL6).

Table 2.
Structure of the female cone yield of the clones and trees from the seeds of Larix sibirica.
3.6. OP * Open Pollination
The earlier genotyping of nine microsatellite loci from the needles of the cloned larch trees showed that all of them were identical to CL6 from which they were obtained. The clones obtained from this cell line were homozygous with one allele identical to the maternal genotype No. A4 [40].
4. Discussion
The proliferating larch cell lines we obtained can remain active for 14 years or more. At the same time, the embryonic productivity of the proliferating CLs is extremely high—up to 11,103 globular embryos per 1 g of ESMs (CL10) [15]. A total of 3.5 kg of embryogenic callus can be obtained per year from one CL [41]. The formation of the somatic embryos in a long-term proliferating embryogenic culture was shown to occur in L. × eurolepis and L. × marschlinsii for 10 years [9] and in L. decudua for 17 years. In Pinus pinaster, the proliferating cell lines remain active for about a year and then degrade [42].
The cell lines of Larix sibirica differed in the duration of cultivation, intensity of growth and embryonic productivity, hormonal balance, and ploidy. For most proliferating cell lines, the ESMs can maintain the capability to form globular somatic embryos within 2–4 years after their creation. Then, the proliferation rate slowed down, and the cell lines died. At the same time, a number of cell lines such as CL4, CL5, and CL6 actively multiplied for 14, 9, and 11 years, respectively. CL5 developed numerous small embryos with a short suspensor that were unable to mature. CL4 intensively produces somatic embryos that actively germinate. In CL6, large somatic embryos were formed, which successfully passed differentiation and formed seedlings. The emblings of the clone were obtained from CL6 [40,43,44]. The emblings of the clone are characterized by intensive growth and early development of generative organs, and they are not damaged by the larch bud gall midge, as well as the donor tree
The proliferating larch CLs are characterized by a high content of hormones. Such CLs have a high content of IAA and lower levels of cytokinins and especially ABA [44]. In the long-term proliferating CLs (9–14 years), the level of IAA and especially ABA increases, which can lead to a decrease in the regenerative ability of the cultures.
As a rule, long-term proliferating embryogenic cultures are characterized by genetic instability. Polyploid cells were described in ESMs of Pinus nigra [45], L. decidua, L. leptolepis, L. marschlinsii [46], and P. radiata [47]. Mutations at microsatellite loci were detected in embryogenic CLs of Pinus sylvestris [48,49] and Pinus pinaster embryogenic CLs [50,51]. However, out of 23 cell lines of P. pinaster, one cell line was genetically stable [52]. A single proliferative cell line, Pinus abies, also showed genetic stability [53]. Our study of the ploidy of the long-term proliferating Larix sibirica cultures showed the genetic stability of a single CL, namely CL6. This stability was revealed both by the number of chromosomes and the microsatellite loci.
The microsatellite analysis performed earlier on ESMs of L. sibirica embryogenic cultures showed a weak allelic variability between CLs and the donor tree [40]. These data were consistent with the literature data, indicating the genetic stability of embryogenic cultures of some Picea and Pinus species [48,54,55,56,57,58]. In our experiments with the Siberian larch, it was shown that the ploidy of the embryogenic CLs did not change within one to two years. Mutations began in the embryogenic CLs at the age of three years. Their number after 5–7 years of chromosome cultivation (CL5) was 27–28. In CL4 (after seven years of cultivation), the majority of cells contained 25 chromosomes in the karyotype [39]. The repeat experiment in 2022 (14 years of cultivation) revealed 26 chromosomes; CL4 is characterized by high embryonic productivity of ESMs and a large number of mature somatic embryos that actively germinate. However, when regenerants germinate, they form swells in the root area.
However, only CL6 retained cytogenetic stability. Thus, during 11 years of cultivation, CL6 contained in the karyotype the normal diploid number of chromosomes for this species (2n = 24). Such cell lines can be successfully used for the preservation of germplasm, the production of planting material, and plantation reforestation.
5. Conclusions
Among the Siberian larch cell lines analyzed, only one, namely CL6, had a high regenerative ability. The ESMs in this CL were genetically stable, somatic embryos germinated successfully, and plantlets grew in the greenhouse and further in the nursery soil. The trees of the clone were genetically stable and fully corresponded to CL6, from which they were obtained. In the clonal trees, the female and male cones were produced at the age of seven years. That is, we conducted a long-term experiment “From seed-to-seed” via somatic embryogenesis. Thus, improving the protocols for obtaining somatic embryos and selecting genetically stable larch cell lines with a long lifetime in vitro is a promising direction for the preservation of germplasm and the creation of highly productive pathogen-resistant clone plantations of larch in Siberia.
Author Contributions
I.N.T. and M.E.P. designed the study, analyzed the results, and wrote the manuscript. M.E.P. conducted the investigation, collected plant materials, obtained cell lines of Larix sibirica, prepared SE samples for analysis, and visualized the results. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Russian Science Foundation and the Krasnoyarsk Regional Fund for Support of Scientific and Technical Activities, grant number 22-14-20008.
Data Availability Statement
The data is available on request from the corresponding author.
Acknowledgments
The authors sincerely thank O.V. Goryachkina for the consultation on cytogenetic research, as well as the reviewers and the editor for valuable comments on the early version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lelu, M.A.; Bastien, C.; Klimaszewska, K.; Ward, C.; Charest, P.J. An improved method for somatic plantlet production in hybrid larch (Larix × leptoeuropaea): Part 1. Somatic embryo maturation. Plant Cell Tissue Organ Cult. 1994, 36, 107–115. [Google Scholar] [CrossRef]
- Von Arnold, S.; Sabala, I.; Bozhkov, P.; Dyachok, J.; Filonova, L. Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult. 2002, 69, 233–249. [Google Scholar] [CrossRef]
- Park, Y.-S.; Bonga, J.; McCartney, A.; Adams, G. Integration of tree biotechnologies into multivarietal forestry. In Proceedings of the Third International Conference of the IUFRO Unit 2.09. 02: Somatic Embryogenesis and Other Vegetative Propagation Technologies, Vitoria-Gasteiz, Spain, 8–12 September 2014; pp. 95–97. [Google Scholar]
- Batygina, T.B. Genetic heterogeneity of seeds. Acta Biol. Crac. Ser. Bot. 1999, 41, 39–50. [Google Scholar]
- Batygina, T.B. Biologiya Razvitiya Rastenii (Biology of Plant Development); DEAN: St. Petersburg, FL, USA, 2014. (In Russian) [Google Scholar]
- Tretyakova, I.N.; Mineev, V.V. Reproductive potential of conifers, somatic embryogenesis and apomixes. Russ. J. Dev. Biol. 2021, 52, 75–86. [Google Scholar] [CrossRef]
- Lelu, M.-A.; Bastien, C.; Klimaszewska, K.; Charest, P.J. An improved method for somatic plantlet production in hybrid larch (Larix × leptoeuropaea): Part 2. Control for germination and plantlet development. Plant Cell Tissue Organ Cult. 1994, 36, 117–127. [Google Scholar] [CrossRef]
- Lelu-Walter, M.-A.; Bernier-Cardou, M.; Klimaszewska, K. Clonal Plant Production from self- and cross-pollinated seed families of Pinus sylvestris (L.) through somatic embryogenesis. Plant Cell Tissue Organ Cult. 2008, 92, 31–45. [Google Scholar] [CrossRef]
- Lelu-Walter, M.-A.; Pâques, L.E. Simplified and improved somatic embryogenesis of hybrid larches (Larix × eurolepis and Larix × marschlinsii). Perspectives for breeding. Ann. For. Sci. 2009, 66, 1–10. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Bernier-Cardou, M.; Cyr, D.R.; Sutton, B.C.S. Influence of gelling agents on culture medium gel strength, water availability, tissue water potential, and maturation response in embryogenic cultures of Pinus strobus L. Vitr. Cell. Dev. Biol.-Plant 2000, 36, 279–286. [Google Scholar] [CrossRef]
- Cairney, J.; Pullman, G. The cellular and molecular biology of conifer embryogenesis. New Phytol. 2007, 176, 511–536. [Google Scholar] [CrossRef]
- Belorusova, A.S.; Tretyakova, I.N. Osobennosti formirovaniya somaticheskih zarodyshej u listvennicy sibirskoj: Embriologicheskie aspekty (Features of the formation of somatic embryos in Siberian larch: Embryological aspects). Russ. J. Dev. Biol. 2008, 39, 1–10. (In Russian) [Google Scholar]
- Pullman, G.S.; Bucalo, K. Pine somatic embryogenesis using zygotic embryos as explants. In Plant Embryo Culture: Methods and Protocols, Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 710, pp. 267–291. [Google Scholar] [CrossRef]
- Tret’yakova, I.N.; Barsukova, A.V. Somatic embryogenesis in in vitro culture of three larch species. Russ. J. Dev. Biol. 2012, 43, 353–361. [Google Scholar] [CrossRef]
- Tret’yakova, I.N.; Park, M.E. Somatic Polyembriogenesis of Larix sibirica in Embryogenic in vitro Culture. Russ. J. Dev. Biol. 2018, 49, 222–233. [Google Scholar] [CrossRef]
- Tret’yakova, I.N.; Shuklina, A.S.; Park, M.E.; Yang, L.; Akhiyarova, G.R.; Kudoyarova, G.R. The role of phytohormones in the induction of somatic embryogenesis in Pinus sibirica and Larix sibirica. Cytologia 2021, 86, 55–60. [Google Scholar] [CrossRef]
- Vondrakova, Z.; Eliašova, K.; Fischerov, L.; Vagner, M. The role of auxins in somatic embryogenesis of Abies alba. Cent. Eur. J. Biol. 2011, 6, 587–596. [Google Scholar] [CrossRef]
- Vondrakova, Z.; Dobrev, P.I.; Pesek, B.; Fischerova, L.; Vagner, M.; Motyka, V. Profiles of endogenous phytohormones over the course of Norway spruce somatic embryogenesis. Front. Plant Sci. 2018, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Pullman, G.S. Embryogenic tissue initiation in Loblolly pine (Pinus taeda L.). In Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants, 2nd ed.; Jain, S.M., Gupta, P., Eds.; Springer: Cham, Switzerland, 2018; pp. 13–31. [Google Scholar] [CrossRef]
- Peng, C.; Gao, F.; Wang, H.; Tretyakova, I.N.; Nosov, A.M.; Shen, H.; Yang, L. Morphological and physiological indicators for screening cell lines with high potential for somatic embryo maturation at an early stage of somatic embryogenesis in Pinus koraiensis. Plants 2022, 11, 1867. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cui, Y.; Zhao, R.; Chen, X.; Zhang, J.; Zhao, J.; Kong, L. Cryo-treatment enhances the embryogenicity of mature somatic embryos via the lncRNA–miRNA–mRNA Network in White Spruce. Int. J. Mol. Sci. 2022, 23, 1111. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Hargreaves, C.; Lelu-Walter, A.M.; Trontin, J.F. Advances in conifer somatic embryogenesis since year 2000. In In Vitro Embryogenesis in Higher Plants, Methods in Molecular Biology; Germanà, M.A., Lambardi, M., Eds.; Springer Science + Business Media: New York, NY, USA, 2016; Volume 1359, pp. 131–166. [Google Scholar] [CrossRef]
- Park, Y.S.; Beaulieu, J.; Bousquet, J. Multi-varietal forestry integrating genomic selection and somatic embryogenesis. In Vegetative Propagation of Forest Trees; National Institute of Forest Sciences (NIFoS): Seoul, Republic of Korea, 2016; pp. 302–322. [Google Scholar]
- Bonga, J.; Park, Y.S.; Ding, C. What technical improvements are needed to achieve industrial application of conifer somatic embryogenesis? In Proceedings of the Clonal Trees in the Bioeconomy Age: Opportunities and Challenges, Coimbra, Portugal, 10–15 September 2018; pp. 14–24. [Google Scholar]
- Ding, C.; Park, Y.S.; Bonga, J.; Bartlett, B.; Li, Y.; Raley, F. A brief review of combining genomic selection and somatic embryogenesis for tree improvement. In Proceedings of the Clonal Trees in the Bioeconomy Age: Opportunities and Challenges, Coimbra, Portugal, 10–15 September 2018; pp. 55–69. [Google Scholar]
- Tretyakova, I.N.; Baranchikov, Y.N.; Buglova, L.V.; Belorussova, A.S.; Romanova, L.I. Osobennosti formirovaniya generativnykh organov listvennitsy sibirskoy i ikh morfogeneticheskiy potentsial (Peculiarities of forming siberian larch generative organs and their morphogenetic potential). Biol. Bull. Rev. 2006, 126, 472–481. (In Russian) [Google Scholar]
- von Aderkas, P.; Klimaszewska, K.; Bonga, J.M. Diploid and haploid embryogenesis in Larix leptolepis, L. decidua, and their reciprocal hybrids. Can. J. For. Res. 1990, 20, 9–14. [Google Scholar] [CrossRef]
- von Aderkas, P.; Bonga, J.; Klimaszewska, K.; Owens, J. Comparison of Larch Embryogeny In Vivo and In Vitro. In Woody Plant Biotechnology; NATO ASI Series; Ahuja, M.R., Ed.; Springer: New York, NY, USA, 1991; Volume 210. [Google Scholar] [CrossRef]
- Rupps, A.; Raschke, J.; Rümmler, M.; Linke, B.; Zoglauer, K. Identification of putative homologs of Larix decidua to Baby Boom (BBM), Leafy Cotyledon1 (LEC1), Wuschel-related Homeobox2 (WOX2) and Somatic Embryogenesis Receptor-like Kinase (SERK) during somatic embryogenesis. Planta 2016, 243, 473–488. [Google Scholar] [CrossRef]
- Kim, Y.W.; Moon, H.K. Enhancement of somatic embryogenesis and plant regeneration in Japanese larch (Larix leptolepis). Plant Cell Tissue Organ Cult. 2007, 88, 241–245. [Google Scholar] [CrossRef]
- Li, W.F.; Zhang, S.G.; Han, S.Y.; Wu, T.; Zhang, J.H.; Qi, L.W. Regulation of LaMYB33 by miR159 during maintenance of embryogenic potential and somatic embryo maturation in Larix kaempferi (Lamb.) Carr. Plant Cell Tissue Organ Cult. 2013, 113, 131–136. [Google Scholar] [CrossRef]
- Thompson, R.G.; von Aderkas, P. Somatic embryogenesis and plant regeneration from immature embryos of western larch. Plant Cell Rep. 1992, 11, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, B.; Wang, X.; Zhang, Y.; Dong, M.; Zhang, J. iTRAQ-based comparative proteomic analysis of embryogenic and non-embryogenic tissues of Prince Rupprecht’s larch (Larix principis-rupprechtii Mayr). Plant Cell Tissue Organ Cult. 2015, 120, 655–669. [Google Scholar] [CrossRef]
- Song, Y.; Li, S.; Bai, X.; Zhang, H. Screening and verification of the factors influencing somatic embryo maturation of Larix olgensis. J. For. Res. 2018, 29, 1581–1589. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, S.; An, P.; Cao, Q.; Wang, C.; Wang, J.; Zhang, H.; Zhang, L. Embryogenic callus induction from immature zygotic embryos and genetic transformation of Larix kaempferi 3× Larix gmelinii 9. PLoS ONE 2021, 16, e0258654. [Google Scholar] [CrossRef]
- Veselov, S.U.; Kudoyarova, G.R.; Egutkin, N.L.; Gyuli-Zade, V.G.; Mustafina, A.R.; Kof, E.K. Modified solvent partitioning scheme providing increased specificity and rapidity of immunoassay for indole 3-acetic acid. Physiol. Plant 1992, 86, 93–96. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Korobova, A.V.; Akhiyarova, G.R.; Arkhipova, T.N.; Zaytsev, D.Y.; Prinsen, E.; Egutkin, N.L.; Medvedev, S.S.; Veselov, S.Y. Accumulation of cytokinins in roots and their export to the shoots of durum wheat plants treated with the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP). J. Exp. Bot. 2014, 65, 2287–2294. [Google Scholar] [CrossRef]
- Pak, M.E.; Ivanitskaya, A.S.; Dvoynina, L.M.; Tret’yakova, I.N. The embryogenic potential of long-term proliferation cell lines of Larix sibirica in vitro. Sib. Lesn. Zurnal (Sib. J. For. Sci.) 2016, 1, 27–38, (In Russian with English abstract). [Google Scholar] [CrossRef]
- Goryachkina, O.V.; Park, M.E.; Tretyakova, I.N.; Badaeva, E.D.; Muratova, E.N. Cytogenetic stability of young and long-term embryogenic cultures of Larix sibirica. Cytologia 2018, 83, 323–329. [Google Scholar] [CrossRef]
- Tret’yakova, I.N.; Park, M.E.; Oreshkova, N.V.; Padutov, V.E. The regenerative capacity of Siberian larch cell lines in vitro. Biol. Bull. Russ. Acad. Sci. 2022, 49, 609–619. [Google Scholar] [CrossRef]
- Tretiakova, I.N. Embryogenic cell lines and somatic embryogenesis in in vitro culture of Siberian larch. Dokl. Biol. Sci. 2013, 450, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Klimaszewska, K.; Noceda, C.; Pelletier, G.; Label, P.; Rodriguez, R.; Lelu-Walter, M.-A. Biological characterization of young and aged embryogenic cultures of Pinus pinaster (Ait.). Vitr. Cell. Dev. Biol.-Plant 2009, 45, 20–33. [Google Scholar] [CrossRef]
- Tretyakova, I.N.; Park, M.E.; Ivanitskaya, A.S.; Oreshkova, N.V. Peculiarities of somatic embryogenesis of long-term proliferating embryogenic cell lines of Larix sibirica in vitro. Russ. J. Plant Physiol. 2016, 63, 800–810. [Google Scholar] [CrossRef]
- Tret’yakova, I.N.; Kudoyarova, G.R.; Park, M.E.; Kazachenko, A.S.; Shuklina, A.S.; Akhiyarova, G.R.; Korobova, A.V.; Veselov, S.U. Content and immunohistochemical localization of hormones during in vitro somatic embryogenesis in long-term proliferating Larix sibirica cultures. Plant Cell Tissue Organ Cult. 2019, 136, 511–522. [Google Scholar] [CrossRef]
- Salajova, T.; Salaj, J. Somatic embryogenesis in European black pine (Pinus nigra Arn.). Biol. Plant 1992, 4, 213–218. [Google Scholar] [CrossRef]
- Nkongolo, K.K.; Klimaszewska, K. Cytological and molecular relationships between Larix decidua, L. leptolepis and Larix × eurolepis: Identification of species-specific chromosomes and synchronization of mitotic cell. Theor. Appl. Genet. 1995, 90, 827–834. [Google Scholar] [CrossRef]
- O’Brien, E.W.; Smith, D.R.; Gardner, R.C.; Murray, B.G. Flow cytometric determination of genome size in Pinus. Plant Sci. 1996, 115, 91–99. [Google Scholar] [CrossRef]
- Fourre, J.L.; Berger, P.; Niquet, L.; André, P. Somatic embryogenesis and somaclonal variation in Norway spruce: Morphogenetic, cytogenetic and molecular approaches. Theor. Appl. Genet. 1997, 94, 159–169. [Google Scholar] [CrossRef]
- De Verno, L.L.; Park, Y.S.; Bonga, J.M.; Barrett, J.D. Somaclonal variation in cryopreserved embryogenic clones of white spruce (Picea glauca (Moench) Voss.). Plant Cell Rep. 1999, 18, 948–953. [Google Scholar] [CrossRef]
- Marum, L.; Rocheta, M.; Maroco, J.; Oliveira, M.; Miguel, C. Analysis of genetic stability at SSR loci during somatic embryogenesis in maritime pine (Pinus pinaster). Plant Cell Rep. 2009, 28, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Miguel, C.; Marum, L. An epigenetic view of plant cells cultured in vitro: Somaclonal variation and beyond. J. Exp. Bot. 2011, 62, 3713–3725. [Google Scholar] [CrossRef] [PubMed]
- Arrillaga, I.; Guevara, M.A.; Muñoz-Bertomeu, J.; Lazaro-Gimeno, D.; Sáez-Laguna, E.; Diaz, L.M.; Torralba, L.; Mendoza-Poudereux, I.; Segura, J.; Cervera, M.T. Selection of haploid cell lines from megagametophyte cultures of maritime pine as a DNA source for massive sequencing of the species. Plant Cell Tissue Organ Cult. 2014, 118, 147–155. [Google Scholar] [CrossRef]
- Hazubska-Przybył, T.; Chmielarz, P.; Michalak, M.; Dering, M.; Bojarczuk, K. Survival and genetic stability of Picea abies embryogenic cultures after cryopreservation using a pregrowth-dehydration method. Plant Cell Tissue Organ Cult. 2013, 113, 303–313. [Google Scholar] [CrossRef]
- Mo, L.M.; von Arnold, S.; Lagererantz, U. Morphogenic and genetic stability in long term embryogenic cultures and somatic embryos of Norway spruce (Picea abies [L.] Karst.). Plant Cell Rep. 1989, 8, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Isabel, N.; Tremblay, L.; Michaud, M.; Tremblay, F.M.; Bousquet, J. RAPDs as an aid to evaluate the genetic integrity of somatic embryogenesis-derived populations of Picea mariana (Mill.) BSP. Theor. Appl. Genet. 1993, 86, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Harvengt, L.; Trontin, J.F.; Reymond, I.; Canlet, F.; Pâques, M. Molecular evidence of true-to-type propagation of a 3-year-old Norway spruce through somatic embryogenesis. Planta 2001, 213, 828–832. [Google Scholar] [CrossRef]
- Helmersson, A.; von Arnold, S.; Burg, K.; Bozhkov, P.V. High stability of nuclear microsatellite loci during the early stages of somatic embryogenesis in Norway spruce. Tree Physiol. 2004, 24, 1181–1186. [Google Scholar] [CrossRef]
- Nunes, S.; Marum, L.; Farinha, N.; Pereira, V.T.; Almeida, T.; Sousa, D.; Mano, N.; Figueiredo, J.; Dias, M.C.; Santos, C. Somatic embryogenesis of hybrid Pinus elliottii var. elliottii × P. caribaea var. hondurensis and ploidy assessment of somatic plants. Plant Cell Tissue Organ Cult. 2018, 132, 71–84. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).