Modification of Density Dependence and Habitat Filtering on Seedling Survival of Different Mycorrhizal-Type Tree Species in Temperate Forests
Abstract
:1. Introduction
2. Methods
2.1. Study Site and Data Collection
2.2. Seedling Census Data and Mycorrhizal Types of Tree Species
2.3. Neighborhood Variables
2.4. Abiotic Factors
2.4.1. Soil Characteristics
2.4.2. Light Availability
2.5. Data Analyses
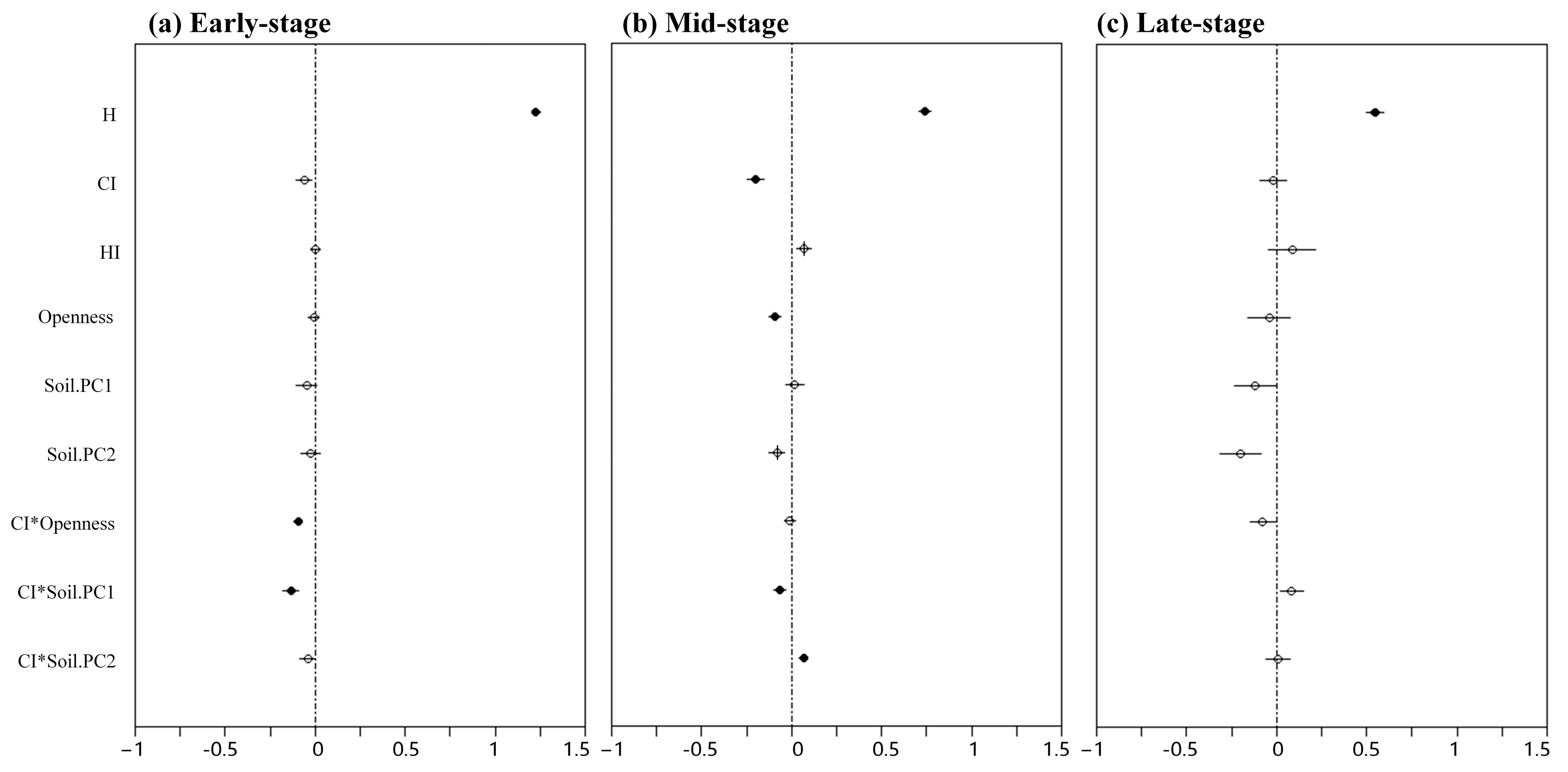
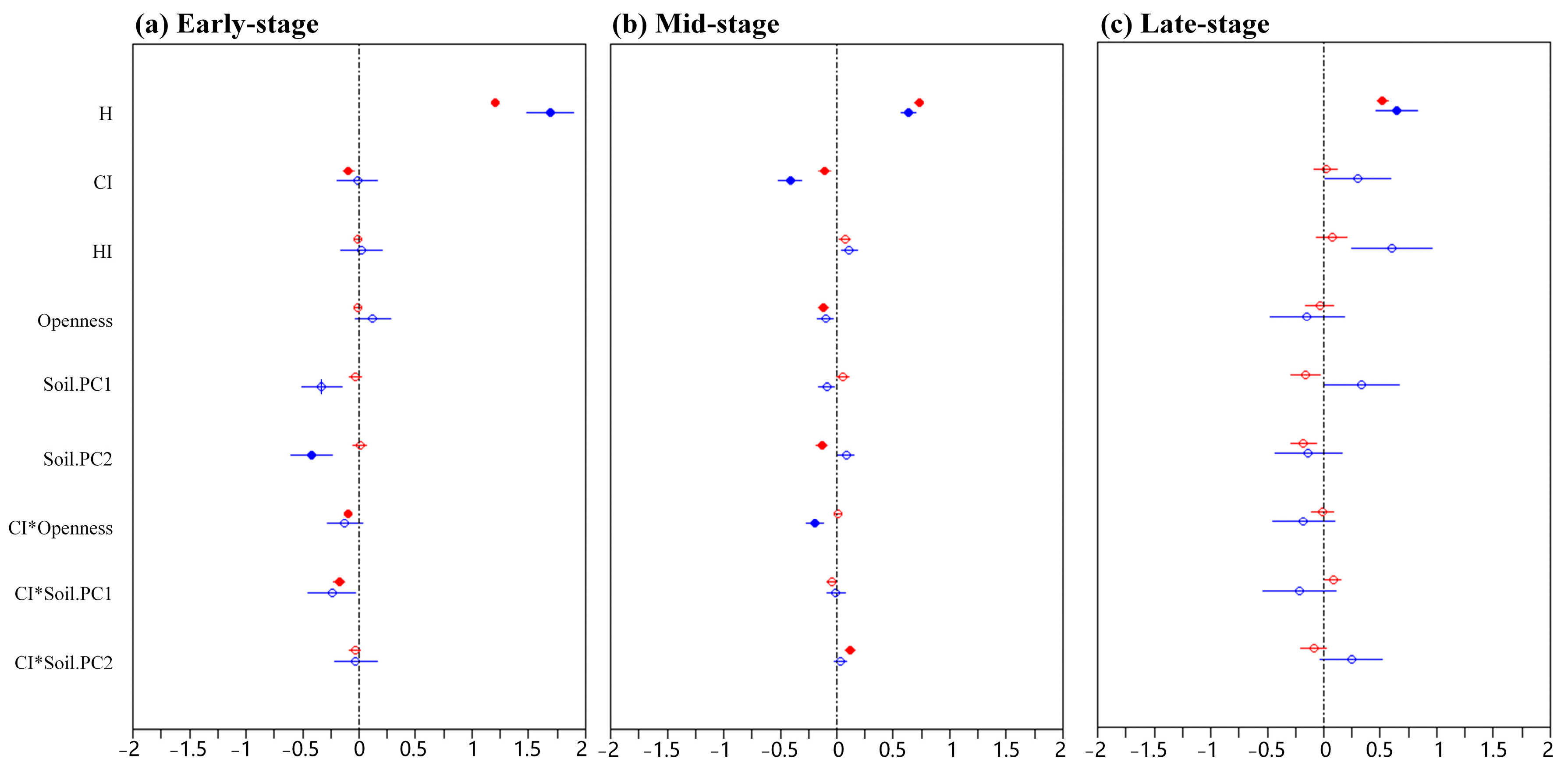
3. Results
4. Discussion
4.1. Effects of Variables on Seedling Survival at the Community Level
4.2. Effects of Variables on Survival of Different Types of Mycorrhizal Seedlings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bagchi, R.; Henrys, P.A.; Brown, P.E.; Burslem, D.F.R.P.; Diggle, P.J.; Gunatilleke, C.V.S.; Gunatilleke, I.A.U.N.; Kassim, A.R.; Law, R.; Noor, S.; et al. Spatial Patterns Reveal Negative Density Dependence and Habitat Associations in Tropical Trees. Ecology 2011, 92, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Comita, L.S.; Queenborough, S.A.; Murphy, S.J.; Eck, J.L.; Xu, K.; Krishnadas, M.; Beckman, N.; Zhu, Y. Testing Predictions of the Janzen–Connell Hypothesis: A Meta-analysis of Experimental Evidence for Distance- and Density-dependent Seed and Seedling Survival. J. Ecol. 2014, 102, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.J.; Condit, R.; Hubbell, S.P.; Comita, L.S. Abiotic Niche Partitioning and Negative Density Dependence Drive Tree Seedling Survival in a Tropical Forest. Proc. R. Soc. B 2017, 284, 20172210. [Google Scholar] [CrossRef] [PubMed]
- LaManna, J.A.; Mangan, S.A.; Alonso, A.; Bourg, N.A.; Brockelman, W.Y.; Bunyavejchewin, S.; Chang, L.-W.; Chiang, J.-M.; Chuyong, G.B.; Clay, K.; et al. Plant Diversity Increases with the Strength of Negative Density Dependence at the Global Scale. Science 2017, 356, 1389–1392. [Google Scholar] [CrossRef]
- Connell, J.H. On the Role of Natural Enemies in Preventing Competitive Exclusion in Some Marine Animals and in Rain Forest Trees. Dyn. Popul. 1971, 298, 298–313. [Google Scholar]
- Janzen, D.H. Herbivores and the Number of Tree Species in Tropical Forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Klironomos, J.N. Feedback with Soil Biota Contributes to Plant Rarity and Invasiveness in Communities. Nature 2002, 417, 67–70. [Google Scholar] [CrossRef]
- Mangan, S.A.; Schnitzer, S.A.; Herre, E.A.; Mack, K.M.L.; Valencia, M.C.; Sanchez, E.I.; Bever, J.D. Negative Plant–Soil Feedback Predicts Tree-Species Relative Abundance in a Tropical Forest. Nature 2010, 466, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010; ISBN 0-08-055934-4. [Google Scholar]
- Bennett, J.A.; Maherali, H.; Reinhart, K.O.; Lekberg, Y.; Hart, M.M.; Klironomos, J. Plant-Soil Feedbacks and Mycorrhizal Type Influence Temperate Forest Population Dynamics. Science 2017, 355, 181–184. [Google Scholar] [CrossRef]
- Brown, A.J.; Payne, C.J.; White, P.S.; Peet, R.K. Shade Tolerance and Mycorrhizal Type May Influence Sapling Susceptibility to Conspecific Negative Density Dependence. J. Ecol. 2020, 108, 325–336. [Google Scholar] [CrossRef]
- Jiang, F.; Lutz, J.A.; Guo, Q.; Hao, Z.; Wang, X.; Gilbert, G.S.; Mao, Z.; Orwig, D.A.; Parker, G.G.; Sang, W.; et al. Mycorrhizal Type Influences Plant Density Dependence and Species Richness across 15 Temperate Forests. Ecology 2021, 102, e03259. [Google Scholar] [CrossRef]
- Jia, S.; Wang, X.; Yuan, Z.; Lin, F.; Ye, J.; Lin, G.; Hao, Z.; Bagchi, R. Tree Species Traits Affect Which Natural Enemies Drive the Janzen-Connell Effect in a Temperate Forest. Nat. Commun. 2020, 11, 286. [Google Scholar] [CrossRef]
- Jevon, F.V.; De La Cruz, D.; LaManna, J.A.; Lang, A.K.; Orwig, D.A.; Record, S.; Kouba, P.V.; Ayres, M.P.; Matthes, J.H. Experimental and Observational Evidence of Negative Conspecific Density Dependence in Temperate Ectomycorrhizal Trees. Ecology 2022, 103, e3808. [Google Scholar] [CrossRef] [PubMed]
- Dickie, I.A.; Schnitzer, S.A.; Reich, P.B.; Hobbie, S.E. Spatially Disjunct Effects of Co-Occurring Competition and Facilitation: Co-Occurring Competition and Facilitation. Ecol. Lett. 2005, 8, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Holík, J.; Janík, D.; Adam, D. Light Can Modify Density-dependent Seedling Mortality in a Temperate Forest. J. Veg. Sci. 2021, 32, e12992. [Google Scholar] [CrossRef]
- LaManna, J.A.; Walton, M.L.; Turner, B.L.; Myers, J.A. Negative Density Dependence Is Stronger in Resource-rich Environments and Diversifies Communities When Stronger for Common but Not Rare Species. Ecol. Lett. 2016, 19, 657–667. [Google Scholar] [CrossRef]
- Johnson, D.J.; Beaulieu, W.T.; Bever, J.D.; Clay, K. Conspecific Negative Density Dependence and Forest Diversity. Science 2012, 336, 904–907. [Google Scholar] [CrossRef]
- Bruelheide, H.; Böhnke, M.; Both, S.; Fang, T.; Assmann, T.; Baruffol, M.; Bauhus, J.; Buscot, F.; Chen, X.-Y.; Ding, B.-Y.; et al. Community Assembly during Secondary Forest Succession in a Chinese Subtropical Forest. Ecol. Monogr. 2011, 81, 25–41. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, X.; Zhang, C.; Zhao, X.; Von Gadow, K. Effects of Density Dependence in a Temperate Forest in Northeastern China. Sci. Rep. 2016, 6, 32844. [Google Scholar] [CrossRef]
- Burton, J.I.; Zenner, E.K.; Frelich, L.E.; Cornett, M.W. Patterns of Plant Community Structure within and among Primary and Second-Growth Northern Hardwood Forest Stands. For. Ecol. Manag. 2009, 258, 2556–2568. [Google Scholar] [CrossRef]
- Lin, F.; Comita, L.S.; Wang, X.; Bai, X.; Yuan, Z.; Xing, D.; Hao, Z. The Contribution of Understory Light Availability and Biotic Neighborhood to Seedling Survival in Secondary versus Old-Growth Temperate Forest. Plant. Ecol. 2014, 215, 795–807. [Google Scholar] [CrossRef]
- Kalacska, M.; Sanchez-Azofeifa, G.A.; Calvo-Alvarado, J.C.; Quesada, M.; Rivard, B.; Janzen, D.H. Species Composition, Similarity and Diversity in Three Successional Stages of a Seasonally Dry Tropical Forest. For. Ecol. Manag. 2004, 200, 227–247. [Google Scholar] [CrossRef]
- Jiang, S.; Xing, Y.; Liu, G.; Hu, C.; Wang, X.; Yan, G.; Wang, Q. Changes in Soil Bacterial and Fungal Community Composition and Functional Groups during the Succession of Boreal Forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Sheng, L.; Ma, L.; Liu, X. 2020 Study on the Characteristics of Climate Change in Changbai Mountain National. Arab. J. Geosci. 2020, 13, 777. [Google Scholar] [CrossRef]
- Yu, D.; Han, S. Ecosystem Service Status and Changes of Degraded Natural Reserves—A Study from the Changbai Mountain Natural Reserve, China. Ecosyst. Serv. 2016, 20, 56–65. [Google Scholar] [CrossRef]
- Yue, Q.; Hao, M.; Li, X.; Zhang, C.; Gadow, K.; Zhao, X. Assessing Biotic and Abiotic Effects on Forest Productivity in Three Temperate Forests. Ecol. Evol. 2020, 10, 7887–7900. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, C.; Zhao, B.; Li, X.; Hou, M.; Zhao, X. Seedling Density Dependence Regulated by Population Density and Habitat Filtering: Evidence from a Mixed Primary Broad-Leaved Korean Pine Forest in Northeastern China. Ann. For. Sci. 2018, 75, 25. [Google Scholar] [CrossRef]
- Liu, S.; Liao, J.-X.; Xiao, C.; Fan, X.-H. Effects of biotic neighbors and habitat heterogeneity on tree seedling survival in a secondary mixed conifer and broad-leaved forest in Changbai Mountain. Chin. J. Plant Ecol. 2016, 40, 711–722. [Google Scholar]
- Miao, W.; Qiu-rong, L.; Dong-mei, X.; Bai-li, D. Effects of Soil Temperature and Soil Water Content on Soil Respiration in Three Forest Types in Changbai Mountain. J. For. Res. 2004, 15, 113–118. [Google Scholar] [CrossRef]
- Soudzilovskaia, N.A.; Vaessen, S.; Barcelo, M.; He, J.; Rahimlou, S.; Abarenkov, K.; Brundrett, M.C.; Gomes, S.I.F.; Merckx, V.; Tedersoo, L. FungalRoot: Global Online Database of Plant Mycorrhizal Associations. New Phytol. 2020, 227, 955–966. [Google Scholar] [CrossRef]
- Qin, J.; Geng, Y.; Li, X.; Zhang, C.; Zhao, X.; von Gadow, K. Mycorrhizal Type and Soil Pathogenic Fungi Mediate Tree Survival and Density Dependence in a Temperate Forest. For. Ecol. Manag. 2021, 496, 119459. [Google Scholar] [CrossRef]
- Jiang, F.; Zhu, K.; Cadotte, M.W.; Jin, G. Tree Mycorrhizal Type Mediates the Strength of Negative Density Dependence in Temperate Forests. J. Ecol. 2020, 108, 2601–2610. [Google Scholar] [CrossRef]
- Armand, N.; Shirvany, A.; Matinizadeh, M.; Khoshnevis, M. Seasonal changes in abundance of arbuscular mycorrhiza fungi of Cerasus mahaleb (L.) Mill. and their correlation with activity of some rhizosphere enzymes (case study: Chahartagh-e-Ardal). Iran. J. For. 2018, 10, Pe1–Pe12. [Google Scholar]
- Yan, Y.; Zhang, C.; Wang, Y.; Zhao, X.; Gadow, K. Drivers of Seedling Survival in a Temperate Forest and Their Relative Importance at Three Stages of Succession. Ecol. Evol. 2015, 5, 4287–4299. [Google Scholar] [CrossRef]
- Pu, X.; Jin, G. Conspecific and Phylogenetic Density-Dependent Survival Differs across Life Stages in Two Temperate Old-Growth Forests in Northeast China. For. Ecol. Manag. 2018, 424, 95–104. [Google Scholar] [CrossRef]
- Pu, X.; Umaña, M.N.; Jin, G. Trait-Mediated Neighbor Effects on Plant Survival Depend on Life Stages and Stage-Specific Traits in a Temperate Forest. For. Ecol. Manag. 2020, 472, 118250. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 13 June 2023).
- Comita, L.S.; Muller-Landau, H.C.; Aguilar, S.; Hubbell, S.P. Asymmetric Density Dependence Shapes Species Abundances in a Tropical Tree Community. Science 2010, 329, 330–332. [Google Scholar] [CrossRef]
- Laliberté, E.; Lambers, H.; Burgess, T.I.; Wright, S.J. Phosphorus Limitation, Soil-borne Pathogens and the Coexistence of Plant Species in Hyperdiverse Forests and Shrublands. New Phytol. 2015, 206, 507–521. [Google Scholar] [CrossRef]
- Lambers, H.; Albornoz, F.; Kotula, L.; Laliberté, E.; Ranathunge, K.; Teste, F.P.; Zemunik, G. How Belowground Interactions Contribute to the Coexistence of Mycorrhizal and Non-Mycorrhizal Species in Severely Phosphorus-Impoverished Hyperdiverse Ecosystems. Plant Soil 2018, 424, 11–33. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How Mycorrhizal Associations Drive Plant Population and Community Biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef] [PubMed]
- Benavent-González, A.; Raggio, J.; Villagra, J.; Blanquer, J.M.; Pintado, A.; Rozzi, R.; Green, T.G.A.; Sancho, L.G. High Nitrogen Contribution by Gunnera Magellanica and Nitrogen Transfer by Mycorrhizas Drive an Extraordinarily Fast Primary Succession in Sub-Antarctic Chile. New Phytol. 2019, 223, 661–674. [Google Scholar] [CrossRef] [PubMed]
- De Kroon, H.; Hendriks, M.; Van Ruijven, J.; Ravenek, J.; Padilla, F.M.; Jongejans, E.; Visser, E.J.W.; Mommer, L. Root Responses to Nutrients and Soil Biota: Drivers of Species Coexistence and Ecosystem Productivity: Root Responses and Ecosystem Productivity. J. Ecol. 2012, 100, 6–15. [Google Scholar] [CrossRef]
- Liese, R.; Leuschner, C.; Meier, I.C. The Effect of Drought and Season on Root Life Span in Temperate Arbuscular Mycorrhizal and Ectomycorrhizal Tree Species. J. Ecol. 2019, 107, 2226–2239. [Google Scholar] [CrossRef]
- Liu, X.; Burslem, D.F.R.P.; Taylor, J.D.; Taylor, A.F.S.; Khoo, E.; Majalap-Lee, N.; Helgason, T.; Johnson, D. Partitioning of Soil Phosphorus among Arbuscular and Ectomycorrhizal Trees in Tropical and Subtropical Forests. Ecol. Lett. 2018, 21, 713–723. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The Diversity and Biogeography of Soil Bacterial Communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef]
- Lin, G.; Yuan, Z.; Zhang, Y.; Zeng, D.-H.; Wang, X. Dominant Tree Mycorrhizal Associations Affect Soil Nitrogen Transformation Rates by Mediating Microbial Abundances in a Temperate Forest. Biogeochemistry 2022, 158, 405–421. [Google Scholar] [CrossRef]
- Iversen, C.M.; Powell, A.S.; McCormack, M.L.; Blackwood, C.B.; Freschet, G.T.; Kattge, J.; Roumet, C.; Stover, D.B.; Soudzilovskaia, N.A.; Valverde-Barrantes, O.J.; et al. Fine-Root Ecology Database (FRED): A Global Collection of Root Trait Data with Coincident Site, Vegetation, Edaphic, and Climatic Data, Version 2. Oak Ridge National Laboratory, TES SFA, U.S. Department of Energy, Oak Ridge, Tennessee, U.S.A. FRED 3.0: The Fine-Root Ecology Database 3.0. 2018. Available online: https://roots.ornl.gov/ (accessed on 13 June 2023). [CrossRef]
- Ren, J.; Fang, S.; Lin, G.; Lin, F.; Yuan, Z.; Ye, J.; Wang, X.; Hao, Z.; Fortunel, C. Tree Growth Response to Soil Nutrients and Neighborhood Crowding Varies between Mycorrhizal Types in an Old-Growth Temperate Forest. Oecologia 2021, 197, 523–535. [Google Scholar] [CrossRef]

| Early Stage | Mid Stage | Late Stage | |
|---|---|---|---|
| Land-use history | Clear-cut in 1930s | Selected-cut during 1930s–1940s | Undisturbed at least 200 years |
| Coordinates | 42°19′10″ N, 128°07′50″ E | 42°21′0″ N, 128°08′0″ E | 42°20′1″ N, 127°54′47″ E |
| Elevation (m) [min–max] | 878 [873–887] | 811 [807–817] | 979 [958–998] |
| Plot size (ha) [dimensions, m] | 5.2 [260 × 200] | 5.2 [260 × 200] | 40 [800 × 500] |
| Establishment time | 2005 | 2006 | 2014 |
| Stem density (N·ha−1) | 4450 | 2447 | 2509 |
| DBH (cm, mean ± SE) | 5.64 ± 0.05 | 8.45 ± 0.09 | 7.05 ± 0.04 |
| Mean height (m, mean ± SE) | 5.60 ± 0.03 | 7.20 ± 0.05 | 6.44 ± 0.02 |
| Dominant species (sorted by BA) | Betula platyphylla, Tilia amurensis, Fraxinus mandschurica, Populus davidiana | Tilia amurensis, Betula platyphylla, Abies nephrolepis, Quercus mongolica | Pinus koraiensis, Quercus mongolica, Tilia amurensis, Fraxinus mandschurica |
| Soil Variables | Early Stage | Mid Stage | Late Stage | |||
|---|---|---|---|---|---|---|
| PC1 | PC2 | PC1 | PC2 | PC1 | PC2 | |
| Organic matter (%) | 0.586 | −0.129 | 0.525 | - | 0.530 | 0.245 |
| Total nitrogen (%) | 0.488 | 0.311 | 0.462 | 0.108 | 0.567 | - |
| Total phosphorus (%) | 0.541 | 0.214 | 0.516 | 0.141 | 0.402 | −0.438 |
| Total potassium (%) | −0.355 | 0.538 | −0.475 | −0.114 | −0.485 | −0.131 |
| pH | - | −0.742 | 0.139 | −0.974 | - | −0.855 |
| Cumulative proportion (%) | 36.0 | 58.7 | 48.0 | 67.8 | 57.4 | 80.5 |
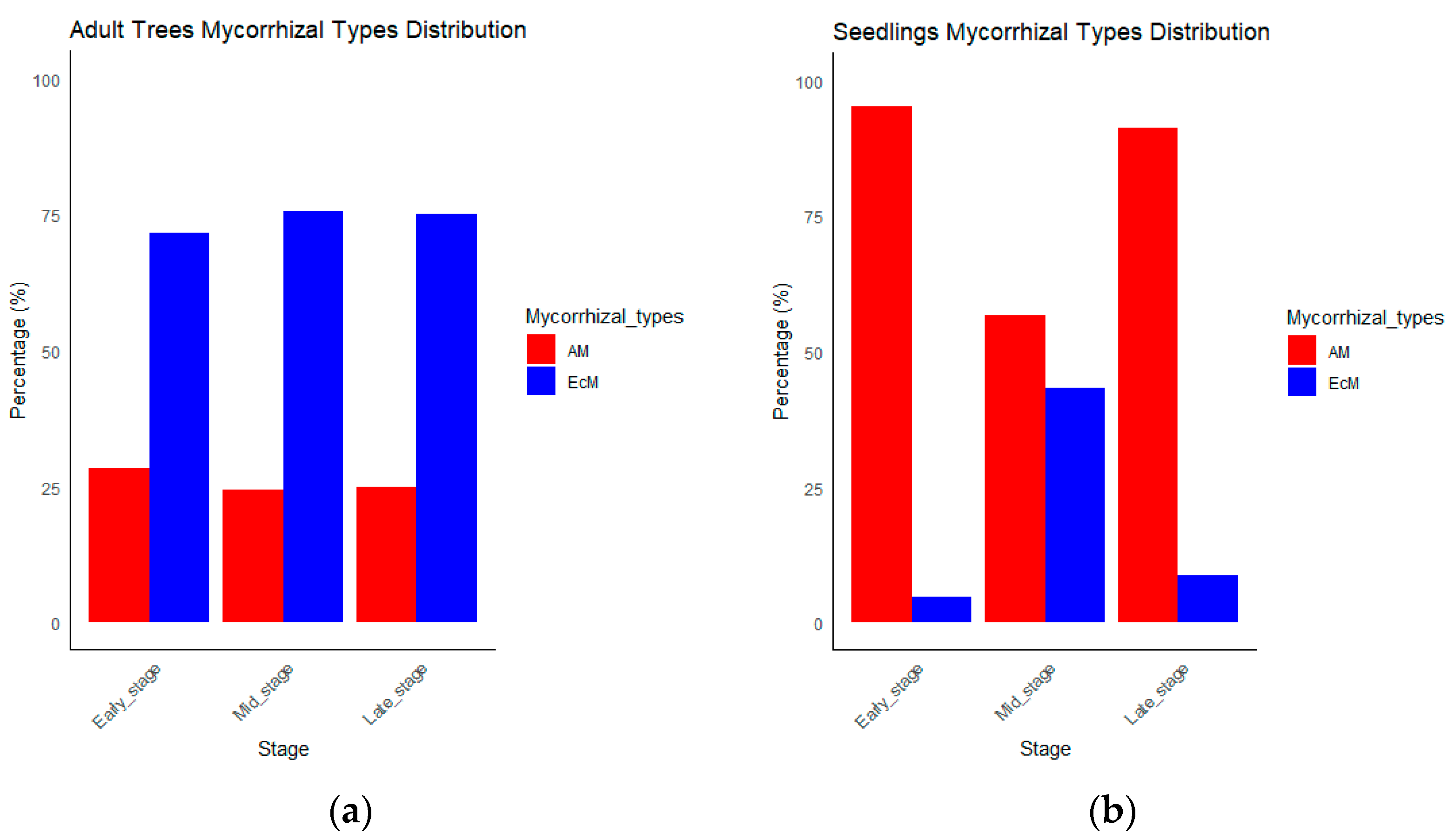
| Growth Stage | Mycorrhizal Types | Early Stage | Mid Stage | Late Stage |
|---|---|---|---|---|
| Adult trees | AM | 8.70 | 8.61 | 11.07 |
| EcM | 22.05 | 26.54 | 33.21 | |
| Total | 30.75 | 35.15 | 44.28 | |
| Seedlings | AM | 28,097 | 6130 | 3885 |
| EcM | 1429 | 4658 | 369 | |
| Total | 29,526 | 10,788 | 4254 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhao, X. Modification of Density Dependence and Habitat Filtering on Seedling Survival of Different Mycorrhizal-Type Tree Species in Temperate Forests. Forests 2023, 14, 1919. https://doi.org/10.3390/f14091919
Li J, Zhao X. Modification of Density Dependence and Habitat Filtering on Seedling Survival of Different Mycorrhizal-Type Tree Species in Temperate Forests. Forests. 2023; 14(9):1919. https://doi.org/10.3390/f14091919
Chicago/Turabian StyleLi, Jian, and Xiuhai Zhao. 2023. "Modification of Density Dependence and Habitat Filtering on Seedling Survival of Different Mycorrhizal-Type Tree Species in Temperate Forests" Forests 14, no. 9: 1919. https://doi.org/10.3390/f14091919
APA StyleLi, J., & Zhao, X. (2023). Modification of Density Dependence and Habitat Filtering on Seedling Survival of Different Mycorrhizal-Type Tree Species in Temperate Forests. Forests, 14(9), 1919. https://doi.org/10.3390/f14091919





