Abstract
The recent Glasgow Climate Pact has recognized the contribution of ecosystems as sinks and reservoirs of greenhouse gases and their importance to achieve the objective of a maximum temperature increase of 1.5 °C. Thus, the knowledge of the long-term storage capacity of the soil organic carbon (C) in forest soils, and the driving factors, are considered of great importance for the mitigation of global climate changes. A database of published data in a ‘grey’ Greek bibliography, concerning the long-term storage of soil organic C in soil profiles for Greek forests, was compiled, including 307 full soil profiles, distributed between 21 types of forest ecosystem throughout the country (Greece). The data collected concerned the amount of long-term stored carbon in the full soil profile, per soil horizon, up to the uncracked bedrock. These also contained information on the sampling location, the type of forest ecosystem, the soil depth, the type of land management, the forest origin, the floristic zone, the altitude, and the climate type. According to the results analysis, the average soil organic C stored was 108.19 Mg ha−1, and ranged greatly between 11.49 and 409.26 Mg ha−1. The type of forest ecosystem, soil depth, land management practices, forest origin, floristic zone, and climate type played an important role in the carbon sequestration process, greatly influencing the long-term amount of stored carbon. Under the demands for mitigating climate change and reducing the rates of global warming, data evaluation indicates the directions to be followed for increasing the long-term storage of carbon, named systematic forest management, and the exclusion of the drivers responsible for the low carbon storage of soil, such as human pressure and overgrazing. Restoration actions such as reforestation and rehabilitation of the degraded forest ecosystems, which were found to store low carbon amounts, can be also considered as effective tools for increasing the long-term carbon storage in forest ecosystems.
1. Introduction
In recent years, under great demands for the mitigation of observed climate changes, global society has tried to find effective ways to increase carbon storage in pools other than air, removing it from the atmosphere, since the atmospheric CO2 is considered as the main cause for the greenhouse phenomenon and global warming on a planetary scale. These efforts to be effective should focus on long-term carbon pools, where carbon is stored in a relatively stable form, such as soil carbon. The long-term storage (over 100 years) of carbon in terrestrial ecosystems mainly concerns the carbon amount stored in the past. Soil carbon is considered one of the largest terrestrial carbon pools [1,2,3] and plays an important role in the Earth’s carbon cycling. In relation to land uses, approximately 40–90% of the global soil carbon resides in forest ecosystems [4,5].
Soil organic carbon (SOC) holds a very important role in the global C cycle, as it is the largest terrestrial C pool [4,6,7]. However, soil can be a source as well as a sink of greenhouse gases (CO2, CH4, and N2O) depending on the land use and management [8]. Nearly all recent models of global climate change forecast a loss of carbon storage in soils as a result of global warming [9,10]. Particularly in Europe, the Mediterranean region is considered as a hotspot for soil erosion and land degradation due to a combination of climate, soil conditions, geomorphology, and long-term human pressure [11]. Grilli et al. [12] reported a critical limit of 20 g SOC kg−1 for an adequate soil quality in southern Europe lands. Thus, the evaluation of the amount of soil carbon in different land uses, and especially in forests that cover extensive areas, could provide basic information regarding the multiple ecosystem services, nutrient cycling, and the climate change effect, besides soil conservation [13,14]. The Intergovernmental Panel on Climate Change (IPCC) (2014) estimated the total soil C pool in the top 1 m at 2011 Pg C, while according to Lal [8], the estimate of the soil C pool was 2300 Pg C, which is about 4.1 times the biotic pool, and about three times the atmospheric C pool.
Forests play an important role in the global C cycle by sequestering large amounts of atmospheric C, and thus can significantly offer a mitigation strategy for reducing global warming [10,15]. In forest land, the major sources of soil organic C are forest aboveground and root biomass, which form the organic inputs to the soil [16]. Depending on the photosynthesis rates, the forest soil and litter accumulate large carbon amounts depending on several factors, mainly those affecting biomass production in forest ecosystems [17,18]. However, there are further factors that contribute to the carbon sequestration process, such as the rate of litter decomposition [19], the type of dead organic material (e.g., leaf decomposition in deciduous species is faster than conifer needles), the site conditions, in terms of soil characteristics, the underlying bedrock, the amount of precipitation, soil, and atmospheric temperature, etc. Among them, the most important are the land use history, the dominant forest tree species, the site conditions, and the forest management system [15,20,21,22].
Generally, for forest land, it has been concluded that the multi millennial forest cover is the main soil forming factor that determines the carbon stock in soils, since edaphogenesis is a very low continuous biological process that happens as a result of the synergetic function of plant roots, soil fauna, and microfauna, under the specific ecological conditions prevailing in a specific site [23]. This happens naturally, but a human presence and intervention greatly influences this process on a long-term basis [24]. Livestock grazing, for example, removes a great amount of plant material, thus reducing the available amount of litter for decomposition and decreasing the level of organic inputs in the soil. Wood harvesting also removes wood materials, altering the type and the amount of available organic sources for soil fauna and decomposers, resulting in a different decomposition process and rates, which form the final carbon sequestration and the stored soil organic carbon [25].
Concerning the forest ecosystems, a long-term forest management practice remains the determining factor [26], while short-term use is not usually able to considerably alter the amount of long-term carbon storage. For example, recently applied stand thinning cannot affect the carbon stock in soils [23], contrary to a long forest cover history, where pedogenesis and long-term land use have determined the soil profile. In addition, Deng et al. [27] reported that the age of restoration (the time since the restoration action was taken) of degraded land to forest was the main factor affecting the soil carbon stock change; they reported that soil C sequestration significantly increased with the time over the long-term land-use change. On the other hand, the conversion of natural vegetation, and especially forests to cropland, during the past two centuries has greatly contributed to the increased atmospheric carbon dioxide [28,29,30]. Thus, afforestation and reforestation have been proposed as effective strategies for mitigating climate change [31,32,33], since it is widely accepted that they lead to an increase in carbon in ecosystems pools [6,27].
Soil carbon estimates are generally seldom for Greek forests, except those reported in a ‘grey’ bibliography (data produced outside of traditional publishing and distribution channels, which is often not well represented in indexing databases). In addition, there is no National Forest Soil Survey. In a recent study, Ganatsas et al. [34] estimated an amount of ca 44 Mg C per hectare in a secondary degraded oak forest ecosystem under conversion, while Ganatsas and Papaioannou [35] reported much higher values for spruce and beech forest ecosystems in the Rhodope mountains, northern Greece. The present study aims to summarize the estimations of the total soil organic C accumulated in the whole soil depth, up to the stable bedrock, in a wide range of forest type ecosystems in Greece. The estimation was based on using soil organic C densities of collected data from various forest types. We, furthermore, analyzed the effect of the type of forest ecosystem, soil depth, type of land management, forest origin, floristic zone, and climate type, which were expected to have played an important role in the carbon sequestration process, and influenced the long-term amount of stored organic carbon in forest pools.
2. Materials and Methods
2.1. Study Area
The study concerns the whole country of Greece, which is part of southeastern Europe. It lies to the North of the equator and the latitude ranges from 35° N to 42° N and its longitude from 19° E to 28° E. Due to its physical geography, topography, and sea influence, the country presents a considerable climatic variation. According to the Hellenic National Meteorological Service (H.N.M.S), the Mediterranean climate (Köppen climate classification: Csa) is the predominant climate found in Greece (Attica, Central Greece, Crete, Epirus, Ionian Islands, Mount Athos, Northern Aegean, Southern Aegean, Thessaly). Other climate types less distributed are the type Csb with Mediterranean, warm summer (Eastern Macedonia and Thrace, Peloponnese, Western Greece), the type Cfa Humid subtropical, no dry season (Central Macedonia), and the type Cfb Marine west coast, warm summer (Western Macedonia), Humid continental (Dsb in the high altitudes of the mountains, and Dfb in the high altitudes of the northern part of the country).
2.2. Methods
A database of published Greek studies was compiled, in total 24 studies, which included 21 PhD theses (in Greek), and 3 scientific papers. These dissertations were carried out during the period 1990 to 2006, in Greek universities. In total these contained 307 full soil profiles, with data concerning, inter alia, the sampling location, forest type, soil type, the depth of soil profile, organic C percent, soil texture (sand and clay percent), and in some cases the soil bulk density. All the studies used the same methodology for measuring the soil organic carbon. Analytically, the sampling design was carried out following stratified random sampling, where the strata were: (1) the research area (location), (2) the type of forest ecosystem. At least five full soil profiles were taken for each stratum. Field sampling was carried out following similar guidelines and instructions, supervised by Greek professors, which followed the same scientific approach. Soil analyses were performed in the soil laboratories of the Greek universities, mainly at the Aristotle University of Thessaloniki. It can be pointed out that most of the referred scientists (those who made the soil sampling and analyses in their PhD dissertations) have undertaken the position of professor at a Greek university (e.g., Zagas, T., Tsitsoni T., Seilopoulos D., Theodoropoulos C., Ganatsas P., Papaioannou A., Radoglou K., Tantos V., Goudelis G., Stampoulidis A., Aslanidou M., Pipinis E.) or senior researchers at the Greek Forest Research Institute (Spanos I., Konstantinidis P.).
Where the organic matter (percentage) was reported, a fraction of 0.58 (organic matter (%) = total organic carbon (%) × 1.72) was taken as the soil organic C percent. In cases where the soil bulk density was not reported, this was estimated from the reported soil texture characteristics using the following equation suggested by Tomasella and Hodnett [36], which according to Martín et al. [37] is the most accurate:
where ρb is the estimation of soil bulk density (g/cm3), OC is the percentage of organic matter (by weight), and silt and clay values in percentage.
ρb = 1.578 − 0.054∙OC − 0.006∙silt − 0.004∙clay
The studied soil profiles were classified according to the dominant tree species (the type of forest ecosystem), soil depth, the floristic zone they belonged to, the applied management type, and the forest origin. We also analyzed any differentiation in relation to the climate type (Koppen classification).
The amount of total soil organic C stock was calculated as follows [7]. Initially, the soil organic C density (Mg ha−1) was computed for each soil horizon in each soil profile, following the distinction made in the analyzed studies. By multiplying the organic C percent, the soil bulk density (g cm−3), and thickness of the horizon (cm), the carbon storage in each soil horizon was computed for all soil profiles. Then, by adding the amount of carbon for each horizon, we computed the total amount of soil organic C per soil profile, in terms of Mg per hectare.
2.3. Statistical Analysis
The amount of stored carbon was analyzed in relation to the effect of the type of forest ecosystem, soil depth, type of management, forest origin, floristic zone, climate type, which all were expected to have played an important role in the carbon sequestration process, greatly influencing the long-term amount of stored carbon. The collected data of carbon stored were then statistically analyzed following several analyses (e.g., multivariate ANOVA, regression analysis, discriminant function analysis, Chi-square automatic interaction detection (CHAID) analysis). After testing, CHAID analysis was selected for the three factors (type of forest ecosystem, type of land management, floristic zone) to build a predictive tree determining how each of the above-mentioned variables best merged to explain the outcome in C storage (the dependent variable), as it gave a clear picture of the factor effect, and by considering that the method is usually used for summarizing the data as the relationships between variables can be easily visualized. For classification problems, it relies on the Chi-squared test to determine the best split; the algorithm used searches for the split point with the smallest adjusted p-value (the probability value that can be related to significance, see Figure 1, Figure 2 and Figure 3). The categories of each tested variable were analyzed to determine which ones can be merged safely to reduce the number of categories. In our case, based on the results of the CHAID analysis, three groups were revealed, and consequently, the soil profiles were grouped according to each of the following parameters: forest types, management type, and floristic zone. The effect of soil depth and type of climate were tested based on a regression analysis and ANOVA, respectively, since these analyses gave a clearer picture of the factor effect. All statistical analyses were performed with IBM SPSS Statistics 28.0 software package.
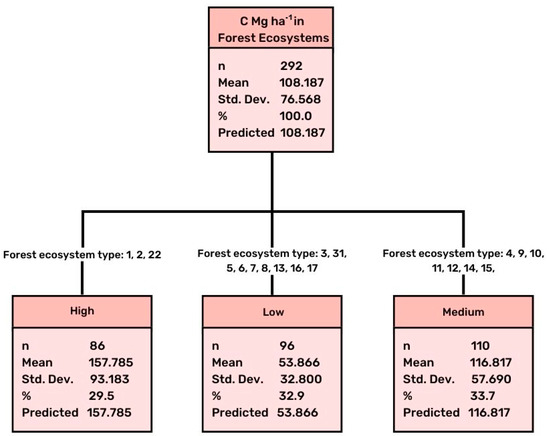
Figure 1.
Results of CHAID analysis concerning differentiation of stored C in relation to the type of forest ecosystem. Adj p-value = 0.000, F = 60.420, Risk estimate 4119.937, Std error 508.099.
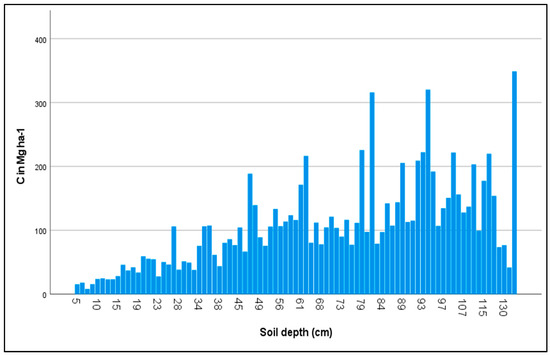
Figure 2.
Concentration of organic carbon stored in soils of different depths. Values are C Mg ha−1.
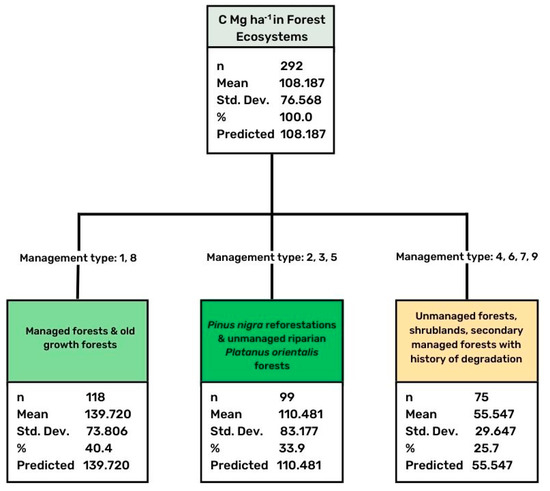
Figure 3.
Results of CHAID analysis concerning differentiation of stored C in relation to the type of land management. Adj p-value = 0.000, F = 34.092, Risk estimate 4727.310, Std error 589.422.
3. Results
Based on the analysis of 307 cases of full soil profiles, 21 different types of forest ecosystems were included in the study (Table 1) [38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59]. However, the final statistical analysis concerned 19 forest types, because two types of forests were excluded due to the low number (fewer than five) of observations. The performed statistical analysis of the collected data revealed great differences in C soil pools, while the average value of C storage, averaged across all types, was found to be 108.19 Mg ha−1. The observed differences were distributed in a wide range of forest ecosystems, including natural forests, forests originating from reforestation, degraded forests, shrublands, and phryganic ecosystems. These differences are analytically presented and analyzed in the following subsections.

Table 1.
Characteristics of the studied forest ecosystems.
3.1. Differentiation of Soil C according to the Type (Dominant Tree Species) of Forest Ecosystem
The ecosystems included in the study were dominated by the following species (Table 1): the conifer species Picea abies, Pinus sylvestris, P. nigra, P. halepensis, P. brutia, P. pinea, P. pinaster, Abies borisii regis, and A. cephalonica. The deciduous broadleaved species Fagus sylvatica, Quercus petraea, Q. frainetto, Q. pubescens, and the riparian forests of Platanus orientalis; the evergreen oak species Quercus coccifera and Q. ilex, the mixed evergreen forests and shrublands of lower altitude, dominated by Quercus ilex, Q. coccifera, Pistacia lentiscus, Arbutus unedo, Phillyrea latifolia, Erica manipuliflora, and highly degraded forest areas, dominated by phryganic species, mainly of the following genera: Cistus, Dorycnium, Hypericum, Micromeria, etc.
According to the data analysis, a great differentiation in the soil carbon between the studied ecosystems (Table 2) was observed. The performed CHAID analysis revealed the existence of three groups of forest ecosystem (Figure 1). The first group included only three types of forest ecosystems that were characterized by a high amount of carbon: the natural forest ecosystems of Picea abies, the natural forest ecosystems of Pinus nigra of northern Greece and in relatively high altitude (over 1000 m asl), and the old growth forests of Fagus sylvatica with Ilex aquifolium. All these forests have found to store a great amount of carbon in the soil, presenting high values of over 150 Mg per hectare (mean value 157.79 Mg ha−1), similar to those reported for other temperate high productive, high forest ecosystems [60].

Table 2.
Concentration of carbon soil in the studied forest ecosystems.
The second group included several types of forest ecosystems, such as the natural forest ecosystems of Pinus sylvestris, P. halepensis, P. brutia, Quercus petraea, Q. frainetto, Fagus sylvatica, Castanea sativa, and Platanus orientalis, which were found to store medium levels of carbon in soil, with values ranging between 55 and 150 Mg per hectare (mean value 116.82 Mg ha−1). These ecosystems had been generally subjected to great pressures in the past (e.g., overgrazing, repeated fires, wood overexploitation), but during the last decades were under the management of the Forest Service, which had the responsibility for forest management at local scale.
Finally, the third group included generally degraded forest ecosystems, and showed a low carbon storage, approximately 50 Mg per hectare (mean value 53.87 Mg ha−1). The included degraded ecosystems were dominated by the evergreen and deciduous oak species of a lower altitude, such as Quercus coccifera, Q. ilex, and Q. pubescens, as well as ecosystems dominated by the evergreen broadleaved species, such as Pistacia lentiscus, Phillyrea latifolia, Arbutus unedo, and Erica arborea mixed with the abovementioned two evergreen oak species. The phryganic ecosystems dominated by plants of a low height also stored a low amount of carbon, such as the values recorded in the evergreen broadleaved species.
3.2. Effect of Soil Depth on Stored C
Soil depth seems to be the main factor affecting carbon storage, regardless of the type of forest ecosystem (Figure 2). According to regression analysis, there is a strong linear relationship between the soil depth and the amount of carbon stored. This strong relationship observed in all studied forest types, both when data were analyzed separately and when summarized, indicates that the soil depth was the most crucial parameter forming the capacity of an ecosystem to store long-term carbon, even within the same type of forest ecosystem.
3.3. Effect of Land Management Practices
Forests that were under the systematical management by the Greek State Forest Service for the last seven decades were characterized by a significantly higher amount of soil carbon compared to grazed forests or those that were unmanaged (Table 3). In addition, old growth forests that are isolated from human pressure were found to store a high amount of carbon. On the contrary, recently burned forests did not differ compared with the unburned ones, indicating that wildfire consumed only the above ground and litter carbon, and did not influence soil carbon. The performed CHAID statistical analysis revealed three groups of different land management types (Figure 3). The first included the systemically managed forests and the old growth forest, presenting a mean value of 139.72 Mg ha−1, representing a total number of 118 soil profiles. The second group included the forests that originated from old reforestations of Pinus nigra, and the unmanaged riparian forests of Platanus orientalis, while the third group included unmanaged forests or shrubland at lower and medium altitudes, and secondary managed forests (mainly oak dominated forests) that were highly degraded in the past, but have been subjected to systematic management during the last decades. The third category was found to store a low amount of carbon in the soil (mean value 55.55 Mg ha−1), as a result of the long-term adverse impacts of human pressure.

Table 3.
Concentration of carbon soil in the different types of land management.
3.4. Differentiation of Soil Carbon in Relation to Floristic Zone
A great differentiation was observed in carbon storage in relation to the floristic zone to which each ecosystem type belonged (Table 4). The CHAID analysis also revealed three groups with great differences between them in soil carbon (Figure 4). The first group included the forests belonging to the Vaccinio-picetalia floristic zone and Quercetum montanum alliance, both distributed at the higher altitudes of northern Greece, and presented high values of stored soil carbon (mean value 166.06 Mg ha−1). The second group followed with relatively high values (mean value 123.03 Mg ha−1), and included forests belonging to the Fagetalia zone and the Tilio castanetum alliance, as well as the azonal forests of the Platanion orientalis alliance. The third group with low carbon storage (mean value 68.63 Mg ha−1) included the forests or shrublands belonging to the floristic zone of Quercetalia ilicis. Here, the phryganic ecosystems, dominated by the Cistus species and other low scrub species, were also included. However, in this group, even in the same floristic zone, there was observed a secondary differentiation depending on the type of ecosystem. The stored carbon greatly differed in forest areas dominated by the Mediterranean pines (e.g., P. halepensis) compared to the areas occupied by evergreen broadleaves, presenting mean values 108.58 and 62.29 Mg ha−1, respectively (Table 2). This indicates the great importance of forest function that accelerates the soil biological process and accumulates carbon in the long-term pools, such as in soil [61].

Table 4.
Concentration of carbon soil in the different floristic zones.
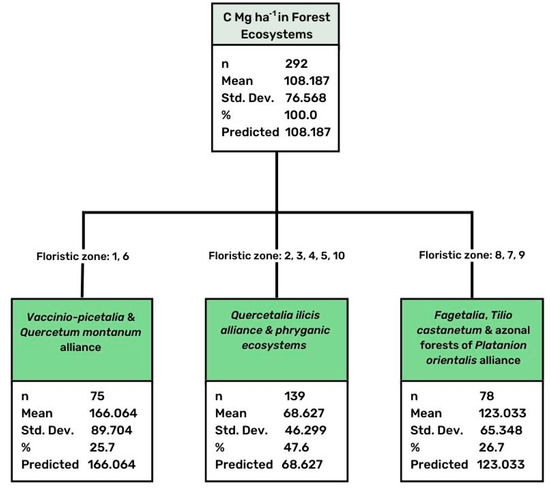
Figure 4.
Results of CHAID analysis concerning differentiation of stored C in relation to the floristic zones. Adj p-value = 0.000, F = 57.552, Risk estimate 4178.419, Std error 487.583.
3.5. Differentiation According to the Climate Type
According to the Koppen classification, the studied forest ecosystems belong to five types of climate types: Csa, Csb, Cfa, Dsb, and Dfb. The forest ecosystems of the first three climate types were found to have accumulated significantly lower quantities of carbon compared to the other two types, which presented higher carbon values (Table 5). This means that forest ecosystems distributed in areas belonging to the climate types Dsb and Dfb, climate types that occur in the high altitudes of the Greek mountains and are characterized either as continental with dry summer (type Dsb) or humid temperate (type Dfb), stored significantly higher amounts of carbon in soils than the forest ecosystems in areas of the other climate types, which are characterized by low precipitation and a summer dry period.

Table 5.
ANOVA for the effect of climate type on soil carbon. The mean values followed by different letters are significantly different (Waller–Duncan, a = 0.05).
4. Discussion
Data analysis showed that the recorded values of stored carbon in Greek forest soils ranged greatly from 11.49 to 409.26 Mg ha−1, indicating the importance of the analyzed drivers. According to the bibliography, the ambient organic amount of C in the soil is determined by a wide range of factors, such as the land use history, the dominant forest tree species, site conditions, and the forest management system [17,18,21,22], including those analyzed in this study. Chhabra et al. [7] reported for Indian forests that the mean soil C densities in the top 1 m of soil were in the range of 69.9–161.9 Mg ha−1, depending on several factors such as the type of forest ecosystem and land management. However, our study reveals an even greater differentiation of stored carbon in Greek forests. Chiti et al. [15] found that climate and forest cover are the principal factors in determining the amount of SOC stored in Spanish forests.
4.1. Main Drivers Forming the Long-Term Ecosystem Storage Capacity
4.1.1. The Type of Forest Ecosystem (Dominant Tree Species)
The studied forest ecosystems were found to have stored different amounts of carbon in the soil, which depended on several factors that acted synergistically, and formed the final soil capacity for carbon storage. The first, and probably the most important, driver forming the storage capacity was the type of forest ecosystem—the dominant tree species, which has been also reported in other studies (e.g., [2,7,60]). The 19 analyzed types of forest ecosystems distributed throughout the country (Greece) were grouped into three main categories according to their carbon storage capacity. The first group, which presented a high carbon storage value, over 150 Mg per hectare, included only three types of forest ecosystems, the natural forest ecosystems of Picea abies, the natural forest ecosystems of Pinus nigra of northern Greece and in relatively high altitude (over 1000 m asl), and the old growth forests of Fagus sylvatica with Ilex aquifolium. These ecosystems are dominated by tree species that, when mature, acquire large dimensions (above and below ground), and thus they can produce a large amount of biomass. These types of forest, due to their isolated location at a long distance from any human influence, are hypothesized to have been continuously covered by trees for many centuries, even millennia, that gradually created a high capacity for soils to store carbon (the multi millennial forest cover theory). Similar results were reported for other temperate high productive forest ecosystems [60].
The second group was found to have stored a medium amount of carbon in soil, with a mean value of 116.82 Mg ha−1. This group includes a wide range of high forest ecosystems, such as the natural forest ecosystems of Pinus silvestris, P. halepensis, P. brutia, Quercus petraea, Q. frainetto, F. sylvatica, Castanea sativa, and Platanus orientalis. These ecosystems had been generally subjected to some human pressure in the past, such as overgrazing, fires, irrational cutting, but during the last decades were under the systematic management and protection of the Greek State Forest Service. These ecosystems were characterized by a low soil depth, an irregular stand structure, and a low stem quality and wood volume. However, they gradually recovered with the help of the applied species-specific silvicultural treatments during the last seven decades, which aimed at stand rehabilitation and conversion to well-structured productive forests.
The third category was characterized by low carbon storage, approximately 50 Mg per hectare (mean value 53.87 Mg ha−1), and included generally degraded forest ecosystems, with a low soil depth. These are dominated by the evergreen and deciduous oak species of the lower altitude, such as Quercus coccifera, Q. ilex, and Q. pubescens, as well as ecosystems dominated by the evergreen broadleaved species, such as Pistacia lentiscus, Phillyrea latifolia, Arbutus unedo, and Erica arborea mixed with the abovementioned two evergreen oak species. In addition, the phryganic ecosystems belonged to this group, presented carbon values such as those of the evergreen broadleaved species. A similar low amount of soil organic carbon was reported for the evergreen broadleaf Mediterranean forests (65.0 C Mg ha−1) by Chiti et al. [15] in Spain.
Similar to the results of our study, Schulp et al. [22] report that SOC stocks differed between several forest types in the Netherlands, ranging between 53.3 Mg C ha−1 (beech) and 97.1 Mg C ha−1 (larch). Chiti et al. [15] also report for Spanish forests that the SOC stocks of conifers was much greater (100.0 C Mg ha−1) compared to the evergreen broadleaf forests (65.0 C Mg ha−1) in the whole soil profile.
4.1.2. Land Management Practices
The type of land use and management was also found to have strongly influenced the amount of organic C in soil. The long-term land management practice was one of the main factors determining soil carbon [26]; they concluded that the multi millennial forest cover was the main soil forming factor that determined the carbon stock in soils [22]. Several land uses lead to significant declines in soil organic carbon. Soil C is rapidly reduced due to the conversion from forest vegetation to agricultural uses [7], mainly because of the reduced production of detritus, the reduction in biochemical cycles, and the decomposition of soil organic matter by oxidation, while increasing erosion rates and the uptake of nutrients [62]. Thus, the conversion of forest land to cropland or other uses contributes to the increased atmospheric carbon dioxide [28,29,30], while afforestation and reforestation lead to an increase in carbon in ecosystem pools [14], resulting in a decrease in the atmospheric carbon dioxide. Accordingly, afforestation and reforestation are included in the framework of effective strategies for mitigating climate change [31,32,33]. The analysis of Muñoz-Rojas et al. [63] concluded that afforestation increases soil organic C mostly in the topsoil, while deforestation processes lead to important C losses, particularly in Cambisols, Luvisols, and Vertisols. On the contrary, recently applied stand thinning cannot affect the long-term carbon stock in soils [23].
Our data analysis shows that systematically managed forests were characterized by a significantly higher amount of soil carbon compared to grazed forests or those that were unmanaged (Figure 3). In addition, the isolated old growth forests with no human pressure were found to have stored a high amount of carbon. It is interesting that a freshly burnt forest did not differ from the unburned ones, concluding that wildfire consumes only above-ground and litter carbon, and does not influence soil carbon. Unmanaged forests or shrubland at lower and medium altitudes, and secondary managed forests (mainly oak dominated forests) that were highly degraded in the past but have been subjected to systematic management during the last decades, stored a low amount of carbon, indicating the strong negative of long-term impacts of human pressure on these ecosystems. Similar results were reported by many authors throughout the world (e.g., [15,21,22,23] for several types of forest ecosystems.
The conversion of closed forests to open woodland may also reduce the soil organic carbon; Chhabra et al. [7] reported that forests with a crown density over 40% sequestrated higher than open forests (crown density of 10% to less than 40%). Gong et al. [24] reported that forest thinning increased the soil carbon stocks in the forests of China. However, our study does not include such information, while our estimates were based on forest types and the silviculture of forests. Schulp et al. [22] also reported for the Netherlands that at managed locations the carbon stocks were lower than at unmanaged locations, indicating that multiple factors should be considered in explaining the drivers affecting carbon sequestration in forest soils.
4.1.3. Soil Depth
The depth of soil profile greatly differed throughout the country, leading to the sequestration of different amounts of carbon (Figure 2). More specifically, it was found to range from 5 cm to approximately 150 cm, which in turn resulted in great differences in stored carbon. However, the majority of carbon was found concentrated in the upper surface horizon up to the depth of ca 30 cm, as was reported in other studies (e.g., [22,34]. Recently, Balesdent et al. [64] concluded through a meta-analysis that SOC dynamics and its responses to climatic changes or land use are strongly dependent on the soil depth. Similar differentiation in relation to the soil depth was reported by Chhabra et al. [7] for Indian forests. However, the forest soil depth presents a high variability depending on several factors such as the bedrock type [65,66] and land use history and management [67]. This suggests that an appropriate systematic land management can increase long-term soil carbon storage [32].
4.1.4. Floristic Zone
Vegetation distribution depends on several ecological factors, such as latitude, altitude, climate, bedrock and soil conditions, and land topography, as well as human influence [68]. As a result, a great variation of different vegetation types is observed in Greece [69]. Accordingly, soil C was found to be strongly differentiated in relation to the floristic zone, revealing that the zones where a human presence had a long history, such as the lower altitudinal zones Quercetalia ilicis, Ostryo carpinion, and phyganic vegetation, stored a low amount of carbon in soils mainly due to the low soil depth recorded in these vegetation zones of the Mediterranean region. The long-term effects of human activities such as repeated fires, wood overexploitation, and livestock overgrazing are considered important land degradation drivers [70].
4.1.5. Climate Type
Soil carbon was also significantly differentiated in relation to the climate type in which each forest ecosystem occurred, as previously reported by others (e.g., [71]). Forest ecosystems in areas of climate types prevailing in the upper part of the Greek mountains, which are characterized by high precipitation and no appearance of a dry period (climate types Dsb and Dfb), stored a significantly higher amount of carbon compared to those appearing in climate types with low precipitation and a summer dry period (climate types Csa, Csb, and Cfa). Similar trends for soil carbon storage were reported by Chhabra et al. [7] for Indian forests, by Grace et al. [72] for tropical savannas, as well as by Becknell et al. [73] for above ground biomass in mature and secondary seasonally dry tropical forests.
4.1.6. Analysis of the Combined Impacts
The observed high differentiation of stored carbon in soil of Greek forests can be attributed to the combined effect of many factors during a long period process (over millennium), since edaphogenesis and organic C sequestration in soils is a very slow, continuous biological process, which happens under a synergetic function of plant roots, soil fauna, and microfauna, and the specific ecological conditions prevailing in a specific site [23]. This happens naturally, but the presence of humans and their intervention greatly influences this process on a long-term basis [24]. Thus, long-term land management practices determine soil carbon [26]. The historic loss of forest soil organic C in the topsoil due to a long human presence in the lower altitudes of the country, since ancient times, perhaps is the basis on which all the other factors acted together. Human civilization has developed mainly at low altitude areas, which belong to the Quercetalia ilicis zone, dominated by evergreen broadleaved species, phryganic ecosystems, and are generally characterized as long degraded forest lands. There are probably a few additional factors (not included in the analysis of the current study) such as forest density and altitude that can also be important. For example, Chhabra et al. [7] reported that these two factors influence the stored C in forest soils of India, while Massaccesi et al. [74] reported that the soil organic matter is significantly positively correlated with altitude in Apennine Forest Soils (Italy).
Data provided by the current study can be used to estimate the net C release due to reforestation, contributing to estimating the C loss per hectare, as well in the National Inventory Reports that each country must annually submit in accordance with international climate change conventions, such as the Kyoto Protocol and the United Nations Framework Convention on Climate Change. These data, combined with those regarding the national forest distribution, can greatly help in a well-documented National Inventory Report [75], since they provide accurate estimations of stored amount of C in each forest ecosystem type. In addition, they can be used in forming the appropriate guidelines for land management, which are greatly requested for many countries. It is estimated that 60–75% of soil organic C lost can be re-sequestered through the adoption of sound land uses and recommended agricultural practices over a 25–50-year period [32]. In addition, the development of separate layers in the Geographic Information Database, incorporating all these factors, including the climate, soil type, altitude, and forest density, etc. that influence the soil organic C density, could help in improving the soil C pool assessments at a country or biogeographical level.
5. Conclusions
The recorded values of soil carbon ranged from 11.49 to 409.26 Mg C per hectare, indicating the importance of the analyzed drivers. Under the demands for climate change mitigation and lowering the rates of global warming, the current data evaluation shows the directions to be taken to increase the long-term storage of carbon, namely systematic forest management, and cessation of the drivers responsible for the low carbon storage in soil, such as human pressure and overgrazing. Restoration actions can also be considered as an effective tool for increasing soil carbon storage for degraded forest ecosystems, which were found in the present study to store low carbon amounts. In any case, a systematic inventory system could improve restoration success by determining the areas that need specific land management.
Author Contributions
Conceptualization, P.G. and M.T.; methodology, P.G. and M.T.; software, L.-M.P.; validation, M.T.; investigation, L.-M.P.; data curation, M.T. and L.-M.P.; writing—original draft preparation, P.G. and M.T.; writing—review and editing, M.T. and L.-M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Köhl, M.; Lasco, R.; Cifuentes, M.; Jonsson, Ö.; Korhonen, K.T.; Mundhenk, P.; de Jesus Navar, J.; Stinson, G. Changes in forest production, biomass and carbon: Results from the 2015 UN FAO Global Forest Resource Assessment. For. Ecol. Manag. 2015, 352, 21–34. [Google Scholar] [CrossRef]
- Bellassen, V.; Luyssaert, S. Carbon sequestration: Managing forests in uncertain times. Nature 2014, 506, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Ameray, A.; Bergeron, Y.; Valeria, O.; Montoro Girona, M.; Cavard, X. Forest Carbon Management: A Review of Silvicultural Practices and Management Strategies Across Boreal, Temperate and Tropical Forests. Curr. For. Rep. 2021, 7, 245–266. [Google Scholar] [CrossRef]
- Dixon, R.K.; Brown, S.; Houghton, R.A.; Solomon, A.M.; Trexler, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef]
- Sedjo, R.A. The carbon cycle and global forest ecosystem. Water Air Soil Pollut. 1993, 70, 295–307. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Chhabra, A.; Palria, S.S.; Dadhwal, V.K. Soil organic carbon pool in Indian forests. For. Ecol. Manag. 2003, 173, 187–199. [Google Scholar] [CrossRef]
- Lal, R. Soil management and restoration for C sequestration to mitigate the accelerated greenhouse effect. Prog. Environ. Sci. 1999, 1, 307–326. [Google Scholar]
- Joos, F.; Prentice, I.C.; Sitch, S.; Meyer, R.; Hooss, G.; Plattner, G.-K.; Gerber, S.; Hasselmann, K. Global warming feedbacks on terrestrial carbon uptake under the Intergovernmental Panel on Climate Change (IPCC) Emission Scenarios. Glob. Biogeochem. Cycles 2001, 15, 891–907. [Google Scholar] [CrossRef]
- Schimel, D.S.; House, J.I.; Hibbard, K.A.; Bousquet, P.; Ciais, P.; Peylin, P.; Braswell, B.H.; Apps, M.J.; Baker, D.; Bondeau, A.; et al. Recent patterns and mechanisms of carbon exchange by terrestrial ecosystems. Nature 2001, 414, 169–172. [Google Scholar] [CrossRef]
- Stolte, J.; Tesfai, M.; Øygarden, L.; Kværnø, S.; Keizer, S.J.; Verheijen, F.; Panagos, P.; Ballabio, C.; Hessel, R. Soil Threats in Europe; EUR 27607 EN; European Union: Luxembourg, 2016. [Google Scholar] [CrossRef]
- Grilli, E.; Carvalho, S.C.P.; Chiti, T.; Coppola, E.; D’Ascoli, R.; La Mantia, T.; Marzaioli; Mastrocicco, M.; Pulido, F.; Rutigliano, F.A.; et al. Critical range of soil organic carbon in southern Europe lands under desertification risk. J. Environ. Manag. 2021, 287, 112285. [Google Scholar] [CrossRef]
- Paul, K.I.; Polglase, P.J.; Nyakuengama, J.G.; Khanna, P.K. Change in soil carbon following afforestation. For. Ecol. Manag. 2002, 168, 241–257. [Google Scholar] [CrossRef]
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Chiti, T.; Díaz-Pinés, E.; Rubio, A. Soil organic carbon stocks of conifers, broadleaf and evergreen broadleaf forests of Spain. Biol. Fertil. Soils 2012, 48, 817–826. [Google Scholar] [CrossRef]
- Schlesinger, W.H. Biogeochemistry: An Analysis of Global Change, 2nd ed.; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Hyvönen, R.; Ågren, G.I.; Linder, S.; Persson, T.; Cotrufo, M.F.; Ekblad, A.; Freeman, M.; Grelle, A.; Janssens, I.A.; Jarvis, P.G.; et al. The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: A literature review. New Phytol. 2007, 173, 463–480. [Google Scholar] [CrossRef]
- Landsberg, J.; Sands, P. Physiological Ecology of Forest Production Principles, Processes and Models; Elsevier: Oxford, UK, 2011. [Google Scholar]
- Çömez, A.; Güner, Ş.T.; Tolunay, D. The effect of stand structure on litter decomposition in Pinus sylvestris L. stands in Turkey. Ann. For. Sci. 2021, 78, 19. [Google Scholar] [CrossRef]
- Whittaker, R.H.; Likens, G.E. Primary production: The biosphere and man. Hum. Ecol. 1973, 1, 357–369. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Schulp, C.J.E.; Nabuurs, G.-J.; Verburg, P.H.; de Waal, R.W. Effect of tree species on carbon stocks in forest floor and mineral soil and implications for soil carbon inventories. For. Ecol. Manag. 2008, 256, 482–490. [Google Scholar] [CrossRef]
- Camponi, L.; Cardelli, V.; Cocco, S.; Serrani, D.; Salvucci, A.; Cutini, A.; Agnelli, A.; Fabbio, G.; Bertini, G.; Roggero, P.P. Effect of coppice conversion into high forest on soil organic C and nutrients stock in a Turkey oak (Quercus cerris L.) forest in Italy. J. Environ. Manag. 2022, 312, 114935. [Google Scholar] [CrossRef]
- Gong, C.; Tan, O.; Liu, G.; Xu, M. Forest thinning increases soil carbon stocks in China. For. Ecol. Manag. 2021, 482, 118812. [Google Scholar] [CrossRef]
- Currie, W.S.; Yanai, R.D.; Piatek, K.B.; Prescott, C.E.; Goodale, C.L. Processes Affecting Carbon Storage in the Forest Floor and in Downed Woody Debris. In The Potential of U.S. Forest Soils to Sequester Carbon and Mitigate the Greenhouse Effect; CRC Press: Boca Raton, FL, USA, 2002; p. 23. ISBN 9780429137020. [Google Scholar]
- Borys, A.; Suckow, F.; Reyer, C.; Gutsch, M.; Lasch-Born, P. The impact of climate change under different thinning regimes on carbon sequestration in a German forest district. Mitig. Adapt Strateg. Glob. Chang. 2016, 21, 861–881. [Google Scholar] [CrossRef]
- Deng, L.; Liu, G.B.; Shangguan, Z.P. Land-use conversion and changing soil carbon stocks in China’s ‘Grain-for-Green’ Program: A synthesis. Glob. Chang. Biol. 2014, 20, 3544–3556. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Wei, X.; Shao, M.; Gale, W.; Li, L. Global pattern of soil carbon losses due to the conversion of forests to agricultural land. Sci. Rep. 2014, 4, 4062. [Google Scholar] [CrossRef]
- Le Quéré, C.; Raupach, M.R.; Canadell, J.G.; Marland, G.; Bopp, L.; Ciais, P.; Conway, T.J.; Doney, S.C.; Feely, R.A.; Foster, P.; et al. Trends in the sources and sinks of carbon dioxide. Nat. Geosci. 2009, 2, 831–836. [Google Scholar] [CrossRef]
- United Nations Framework Convention on Climate Change (UNFCCC). Decision 5/CMP.1. Modalities and Procedures for Afforestation and Reforestation Project Activities under the Clean Development Mechanism in the First Commitment Period of the Kyoto Protocol; FCCC/KP/CMP/2005/8/Add.1. United Nations: New York, NY, USA, 2005; p. 61. Available online: http://cdm.unfccc.int/Reference/COPMOP/08a01.pdf#page=61 (accessed on 30 April 2023).
- IPCC. Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Heimann, M.; Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef]
- Ganatsas, P.; Tsakaldimi, M.; Karydopoulos, T.; Petaloudi, L.-M.; Papaemmanouil, A.; Papadopoulos, S.; Gerochristou, S. Carbon Pools in a 77 Year-Old Oak Forest under Conversion from Coppice to High Forest. Sustainability 2022, 14, 13764. [Google Scholar] [CrossRef]
- Ganatsas, P.; Papaioannou, A. Estimation of accumulation of organic matter and nutrients in forest floor and forest soils of spruce and beech ecosystems in Elatia Drama, northern Greece. In Scientific Annals of the Department of Forestry& Natural Environment; AUTH: Thessaloniki, Greece, 1996; Volume 39/2. (In Greek) [Google Scholar]
- Tomasella, J.; Hodnett, M.G. Estimating soil water retention characteristics from limited data in Brazilian Amazonia. Soil Sci. 1998, 163, 190–202. [Google Scholar] [CrossRef]
- Martín, M.A.; Reyes, M.; Taguas, F.J. Estimating soil bulk density with information metrics of soil texture. Geoderma 2017, 287, 66–70. [Google Scholar] [CrossRef]
- Ganatsas, P. Stand Structure and Natural Regeneration of Spruce Forest in Elatia, Drama. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1993. (In Greek). [Google Scholar] [CrossRef]
- Zagas, T. Conditions of Natural Reforestation of Scotch Pine in an Area of Rodopi. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1990. (In Greek). [Google Scholar] [CrossRef]
- Papaioannou, A. Relations between Site Productivity and Humus Characteristics in Black Pine and Beach Forests in Northern Greece. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1993. (In Greek). [Google Scholar] [CrossRef]
- Mantzavelas, A. Typologie des Stations: Un Outil Traitement Statistique des Données Phytoécologiques et d’ Aide à la Decision en Aménagement Forestier. Application à la Foret Domaniale de Kerdylio (Grèce). Ph.D. Thesis, Universite Henri Poincaré, Nancy, France, 1994. [Google Scholar]
- Stamatopoulos, E. The Regeneration of Abies cephalonica in the Park of Parnitha. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1995. (In Greek). [Google Scholar] [CrossRef]
- Spanos, I. Analysis of Stand Structure and Natural Regeneration of Pinus brutia Forest in Thasos Island, Greece. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1993; p. 170. (In Greek). [Google Scholar]
- Pentarakis, K. Analysis of Stand Structure and Natural Regeneration of Pinus brutia Forest in Crete, Greece. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1993. (In Greek). [Google Scholar]
- Tsitsoni, T. Stand Structure and Conditions Determining Natural Regeneration after Fire in the Aleppo Pine Forest of Kassandra Peninsula (Chalkidiki, Greece). Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1991. (In Greek). [Google Scholar] [CrossRef]
- Theodoropoulos, K. Definition and Classification of Plantsociological Units in University Forest Taxiarchis, Chalkidiki. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1991. (In Greek). [Google Scholar] [CrossRef]
- Gouvas, M. The Plant Communities of the Hymettus Mountain. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2001. (In Greek). [Google Scholar] [CrossRef]
- Kostantinidou, E. Dynamic Evolution of the Form and Biomass of the Root System of Aleppo pine in the Area of Kassandra, Chakidiki. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1998. (In Greek). [Google Scholar] [CrossRef]
- Aslanidou, M. Evaluation of Ecological Factors that Affect the Appearance and Growth of the Forest Species in N. E. Chalkidiki. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2000. (In Greek). [Google Scholar] [CrossRef]
- Pipinis, E. Analysis of Stand Structure, Site Requirements and Potential Utilization of Platanus orientalis. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2003. (In Greek). [Google Scholar] [CrossRef]
- Kostantinidis, P. Investigation of the Relation between Physiographic Units in Forest of Aleppo Pine (Pinus halepensis Mil.) and the Plant Communities Which Have Occurred in Them. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1990. (In Greek). [Google Scholar] [CrossRef]
- Katsaros, L. The Importance of Ilex aquifolium from the Forest Policy, Ecological and Forest Botany Point of View. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1991. (In Greek) [Google Scholar] [CrossRef]
- Stampoulidis, A. Analysis of Beech (Fagus sylvatica L. s.l.) Natural Regeneration in the Late Successional Stands of the Species on the Grammos Mountain. Ph.D. Dissertation, Democritus University of Thrace, Orestiada, Greece, 2016. (In Greek). [Google Scholar] [CrossRef]
- Tantos, V. Nutrients Cycling in an Abies borisii regis Ecosystem. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1997. [Google Scholar] [CrossRef]
- Thanasis, G. Research on the Pinus nigra Reforestations in the Area of Mount Olympus. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2004. (In Greek). [Google Scholar] [CrossRef]
- Radoglou, K. Site Influence on the Success of Reforestation and on the Ecophysiological Condition of Trees of the Kedrinos Hill. Thessaloniki. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1987. (In Greek). [Google Scholar] [CrossRef]
- Goudelis, G. Comparative Research on Reforestations of Experimental University Forest of Taxiarchis-Chalkidiki (Behaviour of Artificial Stands). Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1992. (In Greek). [Google Scholar] [CrossRef]
- Mela, F.; Ganatsas, P. Effect of land preparation methods on restoration success of degraded oak forest ecosystems. Dendrobiology 2023, 89, 56–64. [Google Scholar] [CrossRef]
- Seilopoulos, D. Influence of Forest Fires on Soil Properties. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1991. (In Greek). [Google Scholar] [CrossRef]
- Jandl, R.; Ledermann, T.; Kindermann, G.; Weiss, P. Soil Organic Carbon Stocks in Mixed-Deciduous and Coniferous Forests in Austria. Front. For. Glob. Chang. 2021, 4, 688851. [Google Scholar] [CrossRef]
- Panchal, P.; Preece, C.; Peñuelas, J.; Giri, J. Soil carbon sequestration by root exudates. Trends Plant Sci. 2022, 27, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Prescott, C.E.; Abaker, W.E.A.; Augusto, L.; Cécillon, L.; Ferreira, G.W.D.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.-P.; et al. Tamm Review: Influence of forest management activities on soil organic carbon stocks: A knowledge synthesis. For. Ecol. Manag. 2020, 466, 118127. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Jordán, A.; Zavala, L.M.; De la Rosa, D.; Abd-Elmabod, S.K.; Anaya-Romero, M. Impact of Land Use and Land Cover Changes on Organic Carbon Stocks in Mediterranean Soils (1956–2007). Land Degrad. Dev. 2015, 26, 168–179. [Google Scholar] [CrossRef]
- Balesdent, J.; Basile-Doelsch, I.; Chadoeuf, J.; Cornu, S.; Derrien, D.; Fekiacova, Z.; Hatté, C. Atmosphere–soil carbon transfer as a function of soil depth. Nature 2018, 559, 599–602. [Google Scholar] [CrossRef]
- Eimil-Fraga, C.; Rodríguez-Soalleiro, R.; Sánchez-Rodríguez, F.; Pérez-Cruzado, C.; Álvarez-Rodríguez, E. Significance of bedrock as a site factor determining nutritional status and growth of maritime pine. For. Ecol. Manag. 2014, 331, 19–24. [Google Scholar] [CrossRef]
- Cardelli, V.; Cocco, S.; Agnelli, A.; Nardi, S.; Pizzeghello, D.; Fernández-Sanjurjo, M.J.; Corti, G. Chemical and Biochemical Properties of Soils Developed from Different Lithologies in Northwestern Spain (Galicia). Forests 2017, 8, 135. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, M.; Zhu, Y.; Liu, Z. Impacts of land use and plant characteristics on dried soil layers in different climatic regions on the Loess Plateau of China. Agric. For. Meteorol. 2011, 151, 437–448. [Google Scholar] [CrossRef]
- Chauvier, Y.; Thuiller, W.; Brun, P.; Lavergne, S.; Descombes, P.; Karger, D.N.; Renaud, J.; Zimmermann, N.E. Influence of climate, soil, and land cover on plant species distribution in the European Alps. Ecol. Monogr. 2021, 91, e01433. [Google Scholar] [CrossRef]
- Dafis, S. Classification of forest vegetation of Greece. Sch. Agric. For. Sci. Yearb. 1973, 15, 75–90. (In Greek) [Google Scholar]
- Thirwood, J.V. Man and the Mediterranean Forest; Academic Press: Cambridge, MA, USA, 1981. [Google Scholar]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Grace, J.; José, J.S.; Meir, P.; Miranda, H.S.; Montes, R.A. Productivity and carbon fluxes of tropical savannas. J. Biogeogr. 2006, 33, 387–400. [Google Scholar] [CrossRef]
- Becknell, J.M.; Kucek, L.K.; Powers, J.S. Aboveground biomass in mature and secondary seasonally dry tropical forests: A literature review and global synthesis. For. Ecol. Manag. 2012, 276, 88–95. [Google Scholar] [CrossRef]
- Massaccesi, L.; De Feudis, M.; Leccese, A.; Agnelli, A. Altitude and Vegetation Affect Soil Organic Carbon, Basal Respiration and Microbial Biomass in Apennine Forest Soils. Forests 2020, 11, 710. [Google Scholar] [CrossRef]
- Adger, W.N. Adaptation to climate change in the developing world. Prog. Dev. Stud. 2003, 3, 179–195. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).