Integrative Analysis of the Identified Transcriptome and Proteome Major Metabolism Pathways Involved in the Development of Grafted Apricot Hybrids
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Total RNA Extraction, cDNA Library Construction, Sequencing and Analysis
2.3. Protein Extraction, TMT Labeling and Quantification
2.4. Conjoint Analysis of Transcriptome and Proteome Profile
2.5. Quantitative Real-Time PCR (qRT-PCR) Analysis
3. Results
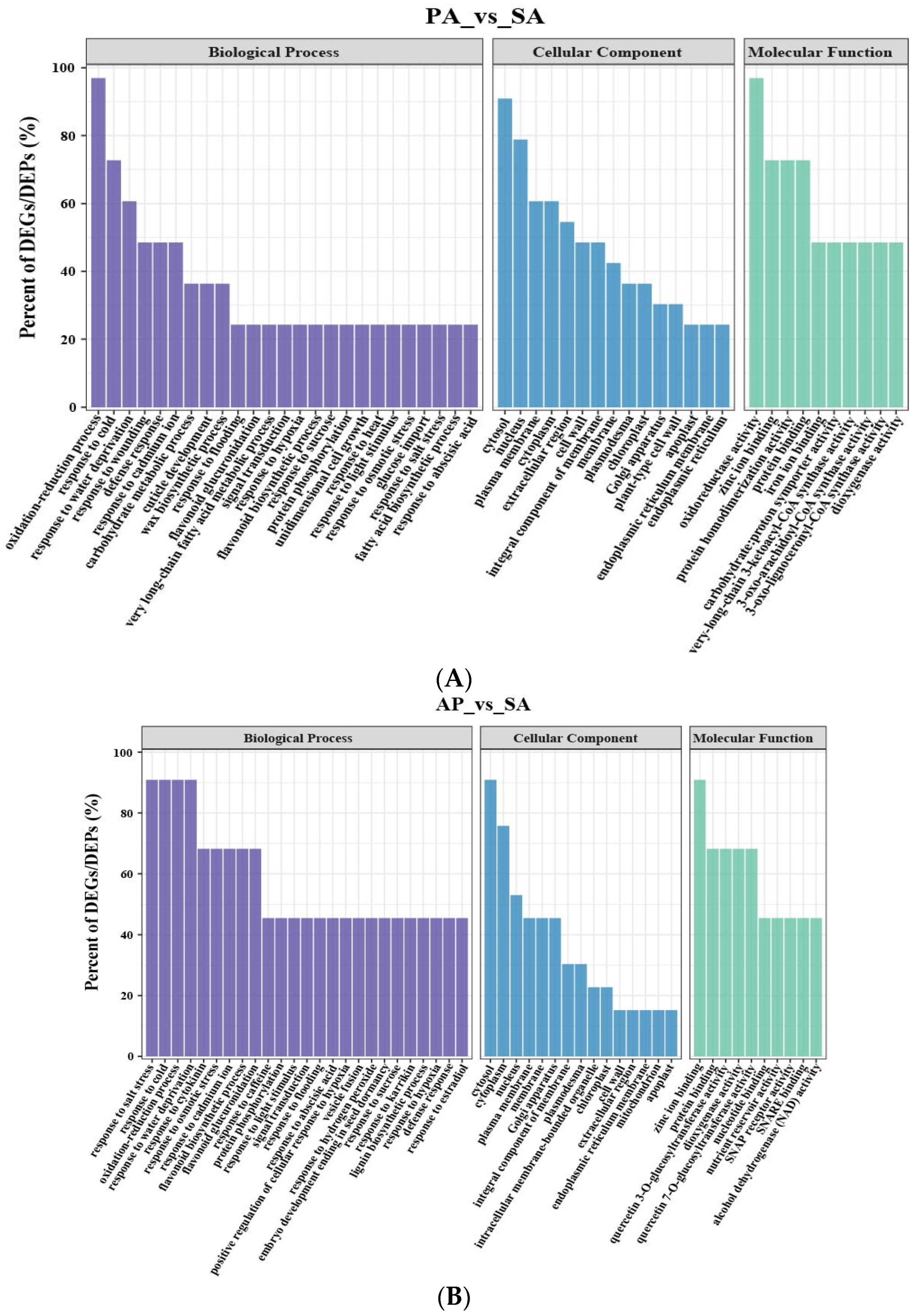
3.1. Identification and Function Study of the Differentially Expressed Genes in Apricot Grafted Hybrids
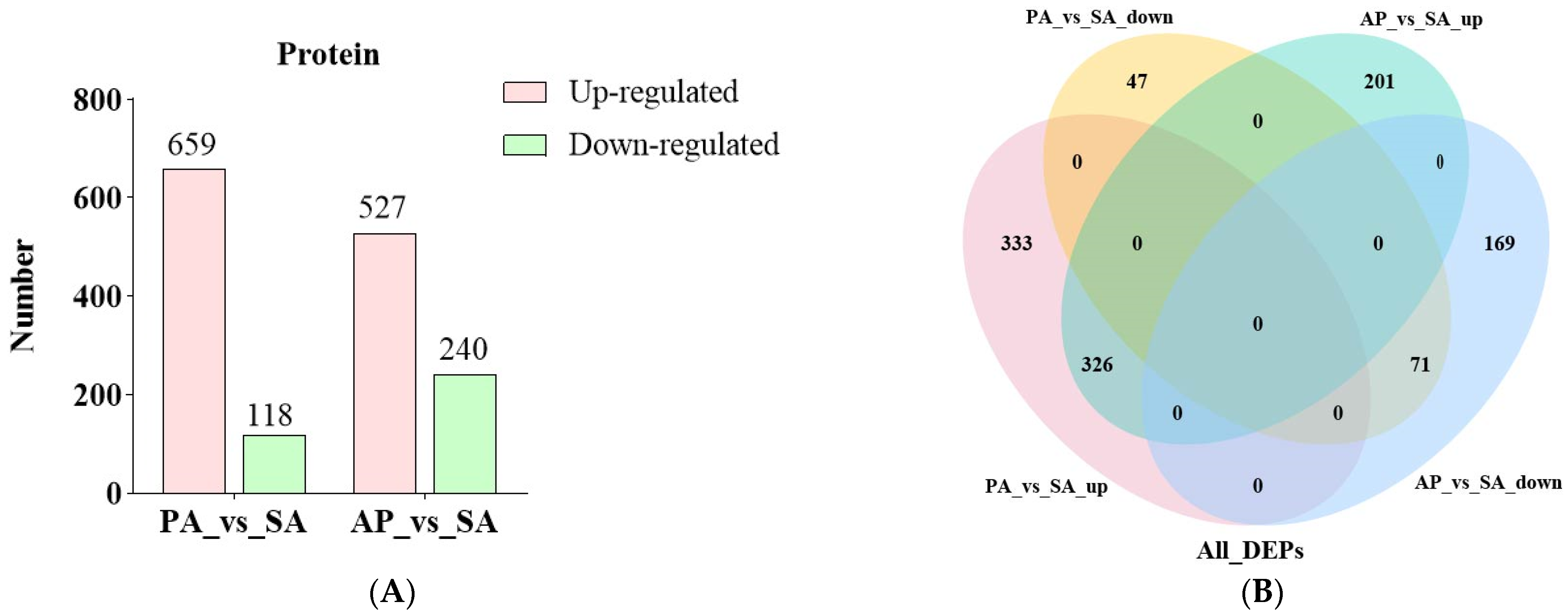
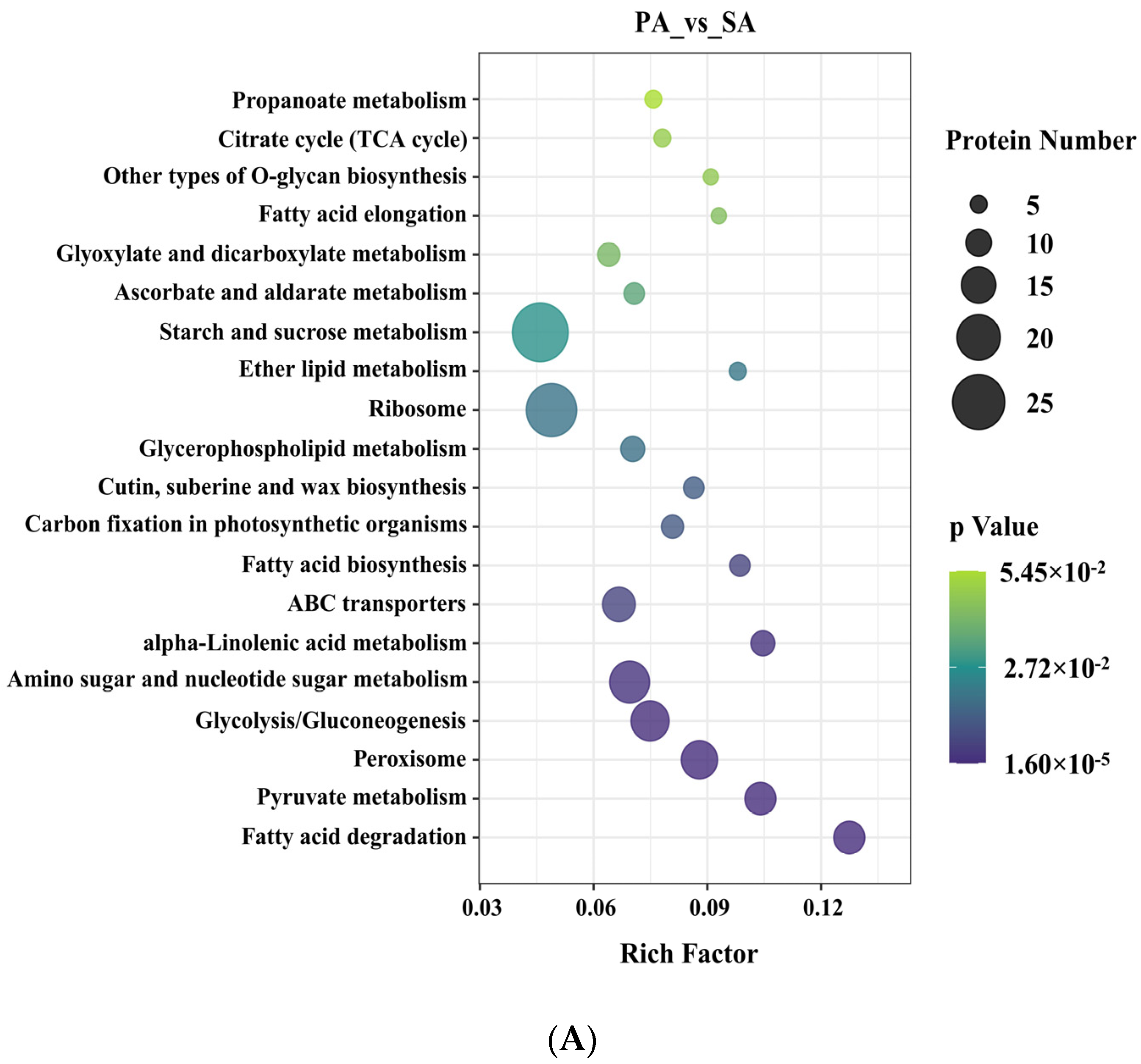
3.2. Identification and Function Study of the Differentially Expressed Proteins in Apricot Grafted Hybrids
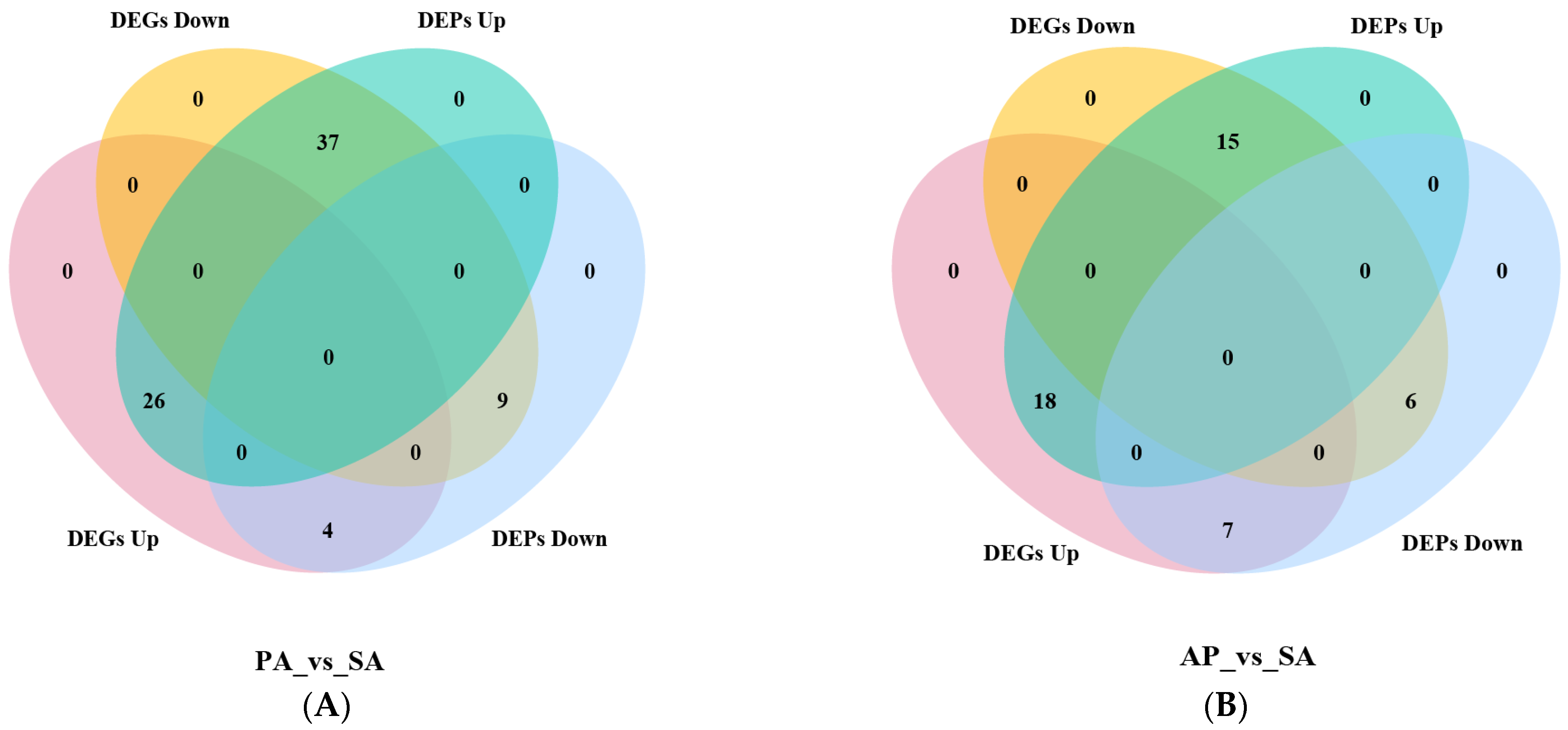
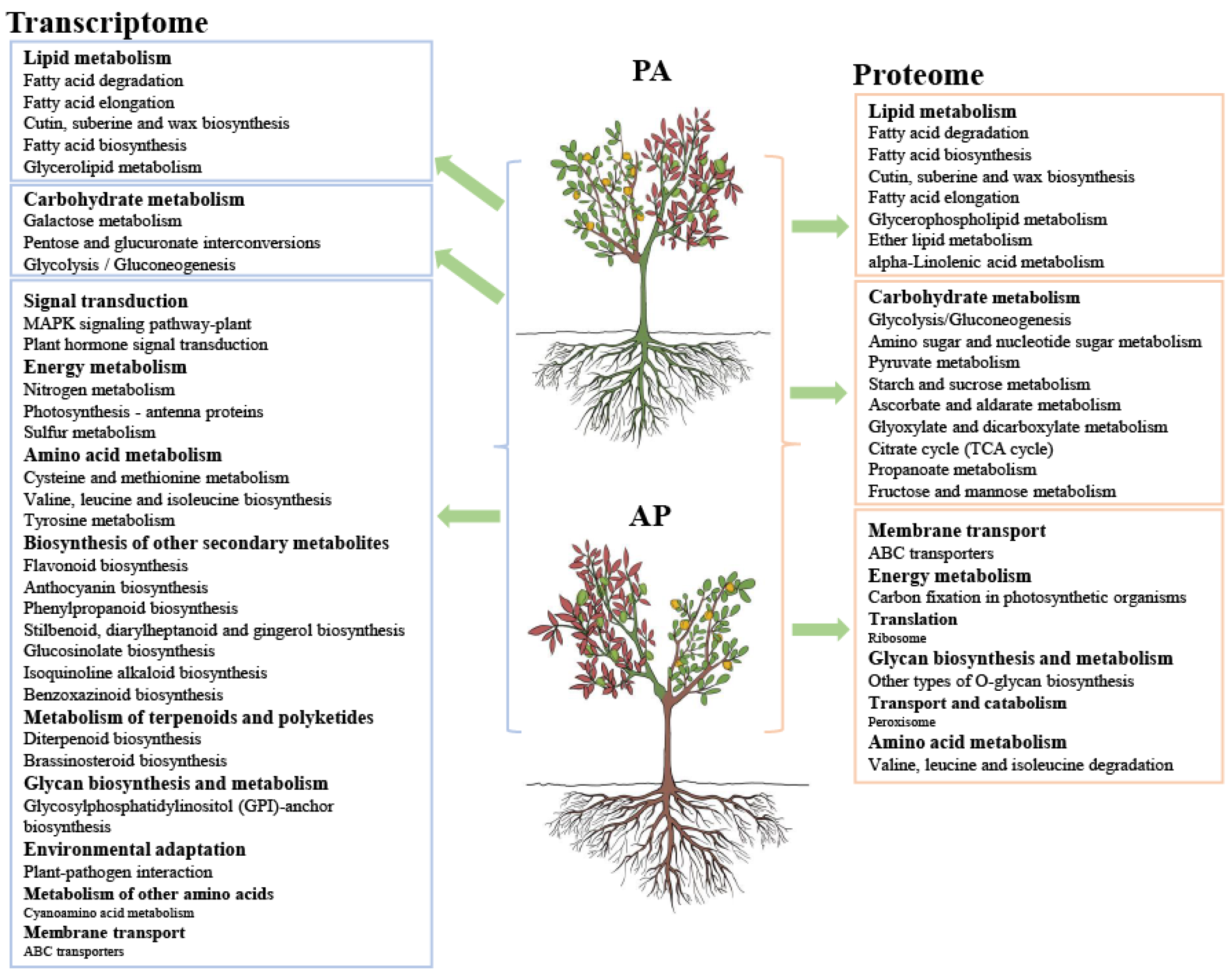
3.3. Joint Transcriptomic and Proteomic Analysis in Apricot Hybrids
3.4. Analysis of DEGs/DEPs Involved in Lipid Metabolism and Carbohydrate Metabolism in Grafted Apricot Hybrids
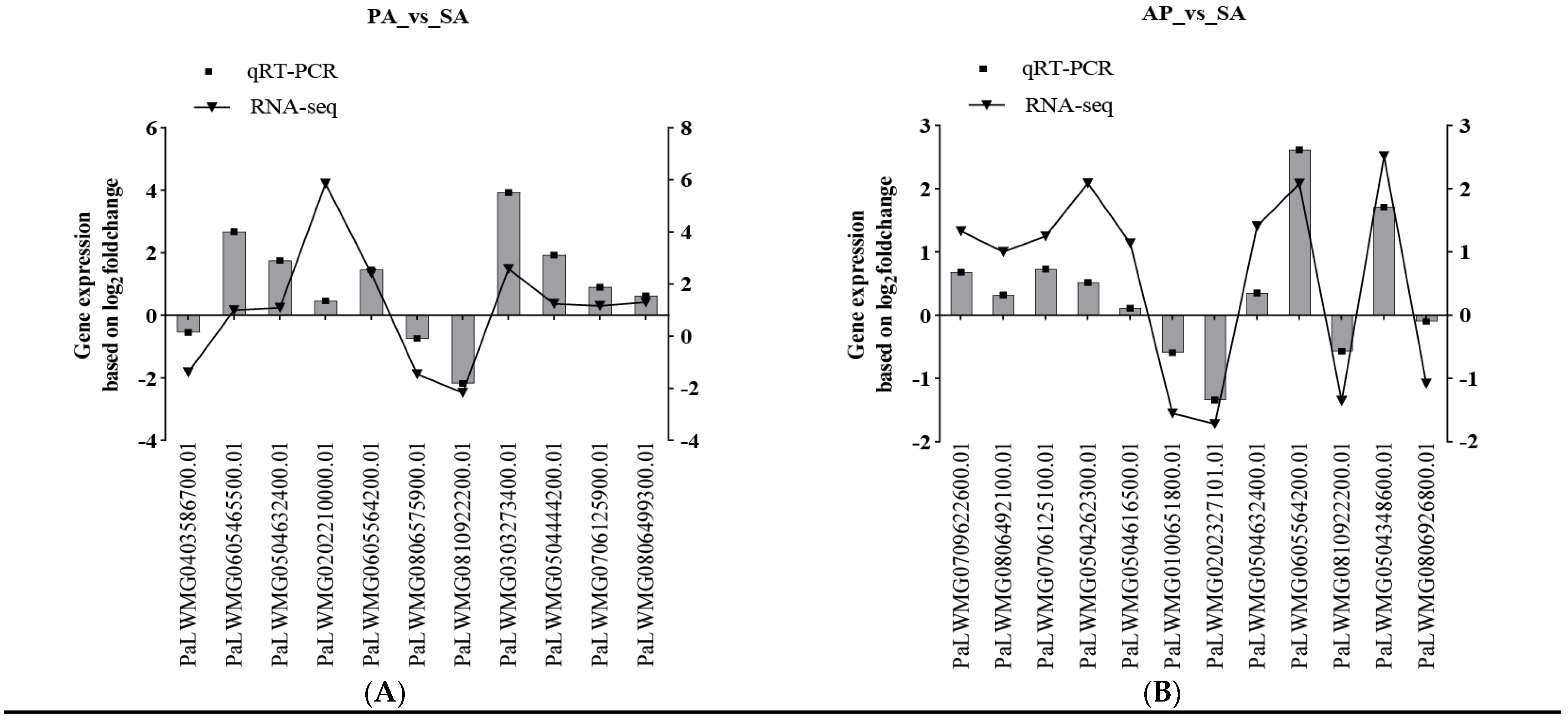
3.5. Validation of qRT-PCR of DEG Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khadivi-Khub, A.; Anjam, K. Prunus scoparia, a suitable rootstock for almond (Prunus dulcis) under drought condition based on vegetative and fruit characteristics. Sci. Hortic. 2016, 210, 220–226. [Google Scholar] [CrossRef]
- Xanthopoulou, A.; Tsaballa, A.; Ganopoulos, I.; Kapazoglou, A.; Avramidou, E.; Aravanopoulos, F.A.; Moysiadis, T.; Osathanunkul, M.; Tsaftaris, A.; Doulis, A.G.; et al. Ιntra-species grafting induces epigenetic and metabolic changes accom-panied by alterations in fruit size and shape of Cucurbita pepo L. Plant Growth Regul. 2019, 87, 93–108. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Sánchez-Romera, B.; Aroca, R.; Asins, M.J.; Declerck, S.; Dodd, I.C.; Martínez-Andújar, C.; Albacete, A.; Ruiz-Lozano, J.M. Exploring the use of recombinant inbred lines in combination with beneficial microbial inoculants (AM fungus and PGPR) to improve drought stress tolerance in tomato. Environ. Exp. Bot. 2016, 131, 47–57. [Google Scholar] [CrossRef]
- Huang, W.; Liao, S.; Lv, H.; Khaldun, A.; Wang, Y. Characterization of the growth and fruit quality of tomato grafted on a woody medicinal plant, Lycium chinense. Sci. Hortic. 2015, 197, 447–453. [Google Scholar] [CrossRef]
- Irisarri, P.; Errea, P.; Pina, A. Physiological and Molecular Characterization of New Apricot Cultivars Grafted on Different Prunus Rootstocks. Agronomy 2021, 11, 1464. [Google Scholar] [CrossRef]
- Tang, M.; Bai, X.; Wang, J.; Chen, T.; Meng, X.; Deng, H.; Li, C.; Xu, Z.-F. Efficiency of graft-transmitted JcFT for floral induction in woody perennial species of the Jatropha genus depends on transport distance. Tree Physiol. 2022, 42, 189–201. [Google Scholar] [CrossRef]
- Habibi, F.; Liu, T.; Folta, K.; Sarkhosh, A. Physiological, biochemical, and molecular aspects of grafting in fruit trees. Hortic. Res. 2022, 9, uhac032. [Google Scholar] [CrossRef]
- Stegemann, S.; Bock, R. Exchange of Genetic Material Between Cells in Plant Tissue Grafts. Science 2009, 324, 649–651. [Google Scholar] [CrossRef]
- Li, W.; Chen, S.; Liu, Y.; Wang, L.; Jiang, J.; Zhao, S.; Fang, W.; Chen, F.; Guan, Z. Long-distance transport RNAs between rootstocks and scions and graft hybridization. Planta 2022, 255, 96. [Google Scholar] [CrossRef]
- Zhao, H.; Diao, S.F.; Liu, P.; Luo, Y.; Wuyun, T.N.; Zhu, G.P. The communication of endogenous biomolecules (RNA, DNA, protein, hormone) via graft union might play key roles in the new traits formation of graft hybrids. Pak. J. Bot. 2018, 50, 717–726. [Google Scholar]
- Deng, Z.; Wu, H.; Li, D.; Li, L.; Wang, Z.; Yuan, W.; Xing, Y.; Li, C.; Liang, D. Root-to-Shoot Long-Distance Mobile miRNAs Identified from Nicotiana Rootstocks. Int. J. Mol. Sci. 2021, 22, 12821. [Google Scholar] [CrossRef]
- Xu, F.; Chen, S.; Zhou, S.; Yue, C.; Yang, X.; Zhang, X.; Zhan, K.; He, D. Genome-wide association, RNA-seq and iTRAQ analyses identify candidate genes controlling radicle length of wheat. Front. Plant Sci. 2022, 13, 939544. [Google Scholar] [CrossRef]
- Chin, E.L.; Ramsey, J.S.; Mishchuk, D.O.; Saha, S.; Foster, E.; Chavez, J.D.; Howe, K.; Zhong, X.F.; Polek, M.; Godfrey, K.E.; et al. Longitudinal transcriptomic, proteomic, and metabolomic analyses of Citrus sinensis (L.) osbeck graft-inoculated with “Candidatus Liberibacter asiaticus”. J. Proteome Res. 2020, 19, 719–732. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Zhao, Y.; Li, G.; Zhang, W.; Wu, Y.; Huang, L. iTRAQ and RNA-Seq analyses revealed the effects of grafting on fruit development and ripening of oriental melon (Cucumis melo L. var. makuwa). Gene 2021, 766, 145142. [Google Scholar] [CrossRef]
- Ji, P.Y.; Liang, C.L.; Yang, Y.J.; Wang, R.; Wang, Y.; Yuan, M.T.; Qiu, Z.Y.; Cheng, Y.Y.; Liu, J.L.; Li, D.L. Comparisons of ana-tomical characteristics and transcriptomic differences between heterografts and homografts in Pyrus L. Plants 2022, 11, 580. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Q.; Chen, W.; Guo, Q.; Xia, Y.; Wang, S.; Jing, D.; Liang, G. Physiological and transcription analyses reveal the regulatory mechanism of melatonin in inducing drought resistance in loquat (Eriobotrya japonica Lindl.) seedlings. Environ. Exp. Bot. 2020, 181, 104291. [Google Scholar] [CrossRef]
- Sun, J.S.; Hu, R.Y.; Lv, F.L.; Yang, Y.F.; Tang, Z.M.; Zheng, G.S.; Li, J.B.; Tian, H.; Xu, Y.; Li, S.F. Comparative transcriptome analysis reveals stem secondary growth of grafted Rosa rugosa ‘Rosea’ scion and R. multiflora ‘Innermis’ rootstock. Genes 2020, 11, 228. [Google Scholar] [CrossRef]
- Xu, D.; Yuan, H.; Tong, Y.; Zhao, L.; Qiu, L.; Guo, W.; Shen, C.; Liu, H.; Yan, D.; Zheng, B. Comparative Proteomic Analysis of the Graft Unions in Hickory (Carya cathayensis) Provides Insights into Response Mechanisms to Grafting Process. Front. Plant Sci. 2017, 8, 676. [Google Scholar] [CrossRef]
- Yao, L.; Yu, Q.; Huang, M.; Hung, W.; Grosser, J.; Chen, S.; Wang, Y.; Gmitter, F.G. Proteomic and metabolomic analyses provide insight into the off-flavour of fruits from citrus trees infected with ‘Candidatus Liberibacter asiaticus’. Hortic. Res. 2019, 6, 31. [Google Scholar] [CrossRef]
- Kain, S.R. Methods and protocols. Methods Biochem. Anal. 2006, 47, 407–421. [Google Scholar]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Bo, C.; Zhang, J.; Sai, L.; Du, Z.; Yu, G.; Li, C.; Li, M.; Peng, C.; Jia, Q.; Shao, H. Integrative transcriptomic and proteomic analysis reveals mechanisms of silica-induced pulmonary fibrosis in rats. BMC Pulm. Med. 2022, 22, 13. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Götz, S. Blast2GO: A Comprehensive Suite for Functional Analysis in Plant Genomics. Int. J. Plant Genom. 2008, 2008, 619832. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. Data, information, knowledge and principle: Back to metabolism in KEGG. Nucleic Acids Res. 2014, 42, D199–D205. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.; Halász, J.; Soltész, A.; Erös-Honti, Z.; Gutermuth, Á.; Szalay, L.; Höhn, M.; Vágújfalvi, A.; Galiba, G.; Hegedüs, A. Identification, Structural and Functional Characterization of Dormancy Regulator Genes in Apricot (Prunus armeniaca L.). Front. Plant Sci. 2019, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Miao, L.; Di, Q.; Sun, T.; Li, Y.; Duan, Y.; Wang, J.; Yan, Y.; He, C.; Wang, C.; Yu, X. Integrated Metabolome and Transcriptome Analysis Provide Insights into the Effects of Grafting on Fruit Flavor of Cucumber with Different Rootstocks. Int. J. Mol. Sci. 2019, 20, 3592. [Google Scholar] [CrossRef]
- Wang, X.; Liang, H.; Guo, D.; Guo, L.; Duan, X.; Jia, Q.; Hou, X. Integrated analysis of transcriptomic and proteomic data from tree peony (P. ostii) seeds reveals key developmental stages and candidate genes related to oil biosynthesis and fatty acid metabolism. Hortic. Res. 2019, 6, 111. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; Hu, F.; Qin, Y.; Wang, X.; Hu, G. Transcriptome changes between compatible and incompatible graft combination of Litchi chinensis by digital gene expression profile. Sci. Rep. 2017, 7, 3954. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Y.; Khan, A.; Ullah, N.; Li, Z.; Zaheer, S.; Zhou, R.; Zhang, Z. Quantitative Proteomics-Based Analysis Reveals Molecular Mechanisms of Chilling Tolerance in Grafted Cotton Seedlings. Agronomy 2022, 12, 1152. [Google Scholar] [CrossRef]
- Xiao, X.; Lv, J.; Xie, J.; Feng, Z.; Ma, N.; Li, J.; Yu, J.; Calderón-Urrea, A. Transcriptome Analysis Reveals the Different Response to Toxic Stress in Rootstock Grafted and Non-Grafted Cucumber Seedlings. Int. J. Mol. Sci. 2020, 21, 774. [Google Scholar] [CrossRef]
- Jiang, J.; Hou, R.; Yang, N.; Li, L.; Deng, J.; Qin, G.; Ding, D. Physiological and TMT-labeled proteomic analyses reveal important roles of sugar and secondary metabolism in Citrus junos under cold stress. J. Proteom. 2021, 237, 104145. [Google Scholar] [CrossRef]
- Li, J.F.; Lin, T.; Ren, D.D.; Wang, T.; Tang, Y.; Wang, Y.W.; Xu, L.; Zhu, P.K.; Ma, G.B. Transcriptomic and metabolomic stud-ies reveal mechanisms of effects of CPPU-mediated fruit-setting on attenuating volatile attributes of melon fruit. Agronomy 2021, 11, 1007. [Google Scholar] [CrossRef]
- Sakuradani, E.; Zhao, L.; Haslam, T.M.; Kunst, L. The CER22 gene required for the synthesis of cuticular wax alkanes in Arabidopsis thaliana is allelic to CER1. Planta 2013, 237, 731–738. [Google Scholar] [CrossRef]
- Chaudhary, K.; Geeta, R.; Panjabi, P. Origin and diversification of ECERIFERUM1 (CER1) and ECERIFERUM3 (CER3) genes in land plants and phylogenetic evidence that the ancestral CER1/3 gene resulted from the fusion of pre-existing domains. Mol. Phylogenet. Evol. 2021, 159, 107101. [Google Scholar] [CrossRef]
- Castorina, G.; Domergue, F.; Chiara, M.; Zilio, M.; Persico, M.; Ricciardi, V.; Horner, D.S.; Consonni, G. Drought-responsive ZmFDL1/MYB94 regulates cuticle biosynthesis and cuticle-dependent leaf permeability. Plant Physiol. 2020, 184, 266–282. [Google Scholar] [CrossRef]
- Lojkova, L.; Vranová, V.; Formánek, P.; Drápelová, I.; Brtnicky, M.; Datta, R. Enantiomers of Carbohydrates and Their Role in Ecosystem Interactions: A Review. Symmetry 2020, 12, 470. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant. 2021, 171, 739–755. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, A.; Song, T.; Jin, Y.; Xu, X.; Gao, Y.; Ye, X.; Qi, H. Transcriptome analysis reveals the effects of grafting on sugar and α-linolenic acid metabolisms in fruits of cucumber with two different rootstocks. Plant Physiol. Biochem. 2018, 130, 289–302. [Google Scholar] [CrossRef]





| Pathway ID | Gene | Description | Log2FC (PA) | Log2FC (AP) | ||
|---|---|---|---|---|---|---|
| Gene Level | Protein Level | Gene Level | Protein Level | |||
| map00062 | KCS | 3-ketoacyl-CoA synthase | −1.19 | 0.30 | ||
| map00062 | KCS | 3-ketoacyl-CoA synthase | −1.37 | 0.78 | ||
| map00073 | CER1 | aldehyde decarbonylase | −1.01 | 0.32 | ||
| map00073 | CYP77A | cytochrome P450 family 77 subfamily A | −1.46 | 0.73 | ||
| map00071 | ADH1_7 | alcohol dehydrogenase 1/7 | 1.52 | 0.65 | ||
| map00071 | ADH1_7 | alcohol dehydrogenase 1/7 | −1.35 | 1.72 | ||
| Pathway ID | Gene | Description | Log2FC (PA) | Log2FC (AP) | ||
|---|---|---|---|---|---|---|
| Gene Level | Protein Level | Gene Level | Protein Level | |||
| map00010 | PDC | pyruvate decarboxylase | −1.21 | 0.46 | ||
| map00010 | pckA | phosphoenolpyruvate carboxykinase (ATP) | −1.31 | 0.51 | ||
| map00010 | ENO | enolase | 1.10 | 1.07 | 1.41 | 1.06 |
| map00010 | ADH1_7 | alcohol dehydrogenase 1/7 | −2.17 | 1.38 | −1.35 | 1.72 |
| map00010 | ADH1_7 | alcohol dehydrogenase 1/7 | 1.52 | 0.65 | ||
| map00500 | EGLC | glucan endo-1,3-beta-D-glucosidase | 1.16 | 1.09 | ||
| map00500 | MGAM | maltase-glucoamylase | −1.03 | 0.28 | ||
| map00500 | SUS | sucrose synthase | −1.24 | 0.32 | ||
| map00500 | WAXY | granule-bound starch synthase | 1.24 | 0.55 | ||
| map00500 | INV | beta-fructofuranosidase | 2.58 | 0.58 | ||
| map00500 | INV | beta-fructofuranosidase | 2.58 | 1.04 | ||
| map00052 | INV | beta-fructofuranosidase | 2.58 | 1.04 | ||
| map00052 | MGAM | maltase-glucoamylase | −1.03 | 0.28 | ||
| map00052 | INV | beta-fructofuranosidase | 2.58 | 0.58 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Tian, L.; Xu, W.; Feng, L.; Jia, W.; Liu, Y.; Chen, Z.; Zhang, S.; Zhang, X.; Ru, G. Integrative Analysis of the Identified Transcriptome and Proteome Major Metabolism Pathways Involved in the Development of Grafted Apricot Hybrids. Forests 2023, 14, 417. https://doi.org/10.3390/f14020417
Sun X, Tian L, Xu W, Feng L, Jia W, Liu Y, Chen Z, Zhang S, Zhang X, Ru G. Integrative Analysis of the Identified Transcriptome and Proteome Major Metabolism Pathways Involved in the Development of Grafted Apricot Hybrids. Forests. 2023; 14(2):417. https://doi.org/10.3390/f14020417
Chicago/Turabian StyleSun, Xiying, Li Tian, Wanyu Xu, Luying Feng, Wenqing Jia, Yiteng Liu, Zhuo Chen, Shulin Zhang, Xianliang Zhang, and Guangxin Ru. 2023. "Integrative Analysis of the Identified Transcriptome and Proteome Major Metabolism Pathways Involved in the Development of Grafted Apricot Hybrids" Forests 14, no. 2: 417. https://doi.org/10.3390/f14020417
APA StyleSun, X., Tian, L., Xu, W., Feng, L., Jia, W., Liu, Y., Chen, Z., Zhang, S., Zhang, X., & Ru, G. (2023). Integrative Analysis of the Identified Transcriptome and Proteome Major Metabolism Pathways Involved in the Development of Grafted Apricot Hybrids. Forests, 14(2), 417. https://doi.org/10.3390/f14020417





