Abstract
Bretschneidera sinensis, the sole species of Bretschneidera, belonging to the family Akaniaceae, is a tertiary paleotropical flora. It is considered an endangered species by the International Union for Conservation of Nature (IUCN). It has an important protective and scientific value. The study of its seed dormancy and germination mechanisms contributes to better protection. In this study, the dormancy of fresh mature B. sinensis seeds released via low-temperature wet stratification was studied. In addition, the endogenous phytohormone levels, antioxidant enzyme activity, soluble sugar content, and the key metabolic enzyme activities of seeds at different stratification time nodes were determined. The goal was to analyze the mechanisms of seed dormancy release and germination comprehensively. Results show that low-temperature wet stratification under 5 °C can release seed dormancy effectively. During the seed dormancy release, the seed germination rate was positively correlated with soluble sugar, GA3, and IAA levels, as well as G-6-PDH, SOD, POD, CAT, and APX activity, but it was negatively correlated with MDH activity and ABA content. These imply that dormancy release might be attributed to the degradation of endogenous ABA and the oxidation of reactive oxygen species induced by low-temperature wet stratification. GA3, IAA, and the metabolism of energy substrates may be correlated with the induction and promotion of germination.
1. Introduction
Seed dormancy refers to the inability of viable seeds (or other germination units) to germinate under favorable environmental conditions (e.g., water, temperature, light) during a specific period [1,2]. Dormancy enables plant seeds to time germination until environmental conditions become favorable for seedling survival and growth. The dormancy characteristics of seeds are of important ecological adaptive significance and notable agricultural value [3,4].
Dormancy is a characteristic of many seed plants. Seeds exhibit a diverse range and degree of dormancy for environmental adaptation [5]. Further, seed dormancy release and the physiological mechanism of germination vary among different species. Until now, seed dormancy release and the physiological mechanisms of germination have been mainly attributed to endogenous phytohormone regulation, reactive oxygen substance production, and antioxidant generation, as well as energy metabolism.
The phytohormone regulation hypothesis states that GA3, cytokinin, ABA, and IAA collectively regulate seed dormancy and germination [6,7,8,9]. However, the regulation mechanisms are highly complex. Many studies have shown that abscisic acid (ABA) and gibberellin (GA) are the key factors for seed dormancy and germination. Mature seeds in a dormancy state contain high levels of ABA and low levels of GA. ABA induces and maintains seed dormancy, while GA antagonizes ABA and promotes seed germination [10,11,12,13]. Numerous studies have confirmed that the maintenance of seed dormancy depends on the ratio of ABA and GA3 in seeds, and the release of dormancy is closely related to the decrease in ABA and the increase in GA3 [14]. Studies also reported the role of other phytohormones in seed dormancy and germination directly or indirectly mediated via signaling of ABA and GA [15,16].
However, the role of IAA in seed dormancy remains controversial because IAA has shown opposite effects on seed germination. For example, IAA at low concentrations promoted the germination rate of unstratified and stratified Arabidopsis thaliana seeds, whereas IAA at high concentrations inhibited seed germination [9,17]. SA has been extensively reported to participate in the regulation of seed dormancy and germination. At low concentrations, SA can effectively inhibit oxidative damage to the cell membrane, protect membrane integrity, and promote seed dormancy release and germination under low-temperature stress [18,19]. Moreover, the interactions between phytohormones have a much stronger influence on seed dormancy and germination as compared with individual phytohormones [20].
Reactive oxygen species (ROS) production and antioxidant theory state that under normal conditions, plants maintain a balance between ROS production and elimination. However, plant cells under stress accumulate high levels of ROS, thereby disrupting the balance. The cellular antioxidant defense system rapidly eliminates surplus ROS at low levels. However, oxidative stress occurs when ROS production exceeds the short-period scavenging capacity, causing damage to the cytoplasmic membrane. The antioxidant defense system, which includes non-enzymatic antioxidants and antioxidant enzymes, scavenges excessive ROS through the water–water cycle, the ascorbate–glutathione cycle, the glutathione peroxidase cycle, and CAT [21,22]. Among these, SOD plays a pivotal role in the defense against oxidative damage caused by oxygen free radicals, effectively scavenging oxygen free radicals and protecting cells from oxidative damage [23]. Due to its high affinity for H2O2, APX is sensitive to minor changes in H2O2 content, thereby enabling the precise modulation of H2O2, which is of considerable importance in signal transduction [24]. CAT functions in scavenging high levels of ROS under oxidative stress [25,26].
According to the theory of energy metabolism, the release of plant seed dormancy is related to the internal respiratory metabolism of seeds and plant respiratory metabolism, including the Embden–Meyerhof Pathway (EMP), tricarboxylic acid cycle (TCA), and pentose phosphate pathway (PPP) [27]. A preliminary study reported that the process from seed dormancy release to germination depends on the transition from EMP to PPP and changes from NADH to NADPH [28]. PPP is an important pathway that is inhibited by TCA. Hence, seed dormancy release or germination is closely related to changes in the internal energy of seeds and the transformation of such internal energy into energy needed for seed germination via various metabolic pathways [29].
Bretschneidera sinensis Hemsl., the sole species in the genus Bretschneidera and the family Bretschneideraceae, is a perennial deciduous tree species endemic to China. As a paleotropical relict tree species that originated in the Tertiary Period, B. sinensis has considerable significance in the study of angiosperm phylogeny, flora, paleogeography, and paleoclimate [30]. However, B. sinensis is a critically endangered species due to habitat destruction and its difficulty in natural reproduction caused by a low growth rate, limited female tree flowering, and a low seed setting rate. Moreover, seed dormancy and a low natural germination rate seriously affect the regeneration of this species in vegetation communities and seedling production [31,32]. Therefore, it is important to clarify the physiological mechanisms underlying the release of their seed dormancy.
2. Materials and Methods
2.1. Materials
In October 2020 and 2021, seeds were collected from female trees aged 40 years in a natural B. sinensis population located in the Wufeng Houhe National Nature Reserve in Hubei Province, China (110°30′9.97″ E, 30°08′55.01″ N). Fruits with cracked skin were harvested and brought back to the laboratory. The red exocarp and endocarp were quickly removed. The seeds were rinsed with water and dried in the shade for 2 d at 25 °C.
2.2. Determination of Moisture Content
Test samples were crushed mechanically and mixed evenly. Four replicates (50 g each) were collected and recorded as G1. The samples were stored in a sample box with a diameter larger than 8 cm. The contents of the box were then dried in an oven, which was preheated to 110 °C for about (17 ± 1) h under (103 ± 2) °C. The box was covered before the samples were removed from the oven. The samples were transferred to a dryer, cooled, and weighed. This processed sample was recorded as G2. The mean of four replicate samples was used to calculate the water content (%) using the formula (G1 − G2)/G1 × 100%. Water content was calculated using fresh weight as the cardinal number.
2.3. Stratification Treatment
Fresh seeds were subjected to flotation, and the selected seeds were naturally dried. The seeds were mixed with moist perlite (Wperlite/Wseed = 3:1). Stratification was performed at 5 °C and 15 °C in the dark. Two portions of seeds were collected at 10, 20, 30, 40, 50, and 60 d. One portion was used for the germination experiment according to the method described in Section 2.4. Four replicates were conducted, and each replicate contained 30 seeds. The seeds that germinated during stratification were excluded from the calculation of the germination rate. The other portion was sealed in tin foil and stored in liquid nitrogen for 24 h. Then, the seeds were quickly ground in liquid nitrogen and stored in an ultra-low-temperature refrigerator at −80 °C. Measurements were performed according to the methods described in Section 2.5 Section 2.6 and Section 2.7.
2.4. Germination Experiment
Seed viability at different stages of stratification was determined using a seed germination experiment. Seeds were evenly placed in a glass box containing moist perlite. The seeds were covered with a 3 cm layer of moist perlite (approximately 85% moisture content) and cultured at 25 °C under light conditions. Seed germination was observed, and the seed germination rate was calculated at 30 d of cultivation. Germination was defined as the emergence of the radicle through the seed coat for a distance of 2 mm.
2.5. Determination of Soluble Sugar Content
Seeds obtained after different durations of stratification were stored in liquid nitrogen and pulverized. Later, 0.15 g samples were collected and treated with 1 mL distilled water to obtain a homogenate. The homogenate was transferred to a centrifuge tube with a cover, followed by heating in a water bath for 10 min at 95 °C. After cooling, the homogenate was centrifuged for 10 min at 25 °C and 8000× g. The supernatant (10 mL) was collected in a test tube and dissolved in distilled water to 10 mL, and vortexed for later use. The soluble sugar content was determined using visible spectrophotometry with an assay kit (Comin, Suzhou, China). Four biological replicates were performed for each sample.
2.6. Determination of Antioxidant Enzyme Activity
Seeds obtained after different periods of stratification were transferred to liquid nitrogen and pulverized. Later, 0.15 g samples were collected, and each sample was treated with 1 mL distilled water to prepare an ice-bath homogenate. The samples were centrifuged at the rate of 8000× g for 10 min at 4 °C. The supernatant was collected and placed on ice for the determination of antioxidant enzyme activity. The catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), ascorbate peroxidase (APX), malate dehydrogenase (MDH), and glucose-6-phosphate dehydrogenase (G-6-PDH) activities were measured using visible spectrophotometry according to the methods of Li et al. [33]. Four biological replicates were performed for each sample.
2.7. Determination of Endogenous Hormone Contents
Samples at different stratification levels treated with liquid nitrogen were collected from the ultra-low-temperature freezer and then pulverized. A total of 50 mg powder was collected and treated with the appropriate internal standard. The samples were extracted using 500 μL acetonitrile aqueous solution, and then centrifuged. The supernatant was used for secondary extraction. The supernatant was mixed. Later, 10 μL TEA and 10 μL BPTAB were added to the sample supernatant for 1 h at 90 °C and then dried under nitrogen. Samples were dissolved again with 100 μL acetonitrile aqueous solution, filtered through a 0.22 μm film, and then transferred to the sample bottle. According to the methods of Su et al. [34], the endogenous phytohormone contents were determined using high-performance liquid chromatography–mass spectrometry. Four biological replicates were performed for each sample.
2.8. Statistical Analysis
The calculated data were expressed as the mean ± standard error. To ensure the homogeneity of variance, seed germination data were subjected to arcsine transformation, followed by one-way analysis of variance and the Student–Newman–Keuls test for multiple comparisons (p = 0.05). Data processing was performed using WPS 13.0.503.101. Statistical analysis and plotting were conducted in R i386 3.5.2.
3. Results and Analysis
3.1. Effects of Cold Stratification on Seed Dormancy Release and Germination
As shown in Figure 1, cold stratification at 5 °C significantly promoted the seed germination rate (F = 700.46, p < 0.05). Under cold stratification (5 °C), the germination rate increased significantly with the extension of the treatment time. The seed coat gradually cracked and the maximum germination rate was 92.7 ± 0.3% at 50 d. Under warm stratification (15 °C), the maximum seed germination rate was 44 ± 1.1%, which was significantly lower than that under cold stratification (p < 0.05). These results suggest that stratification at 5 °C can effectively release the dormancy of B. sinensis seeds.
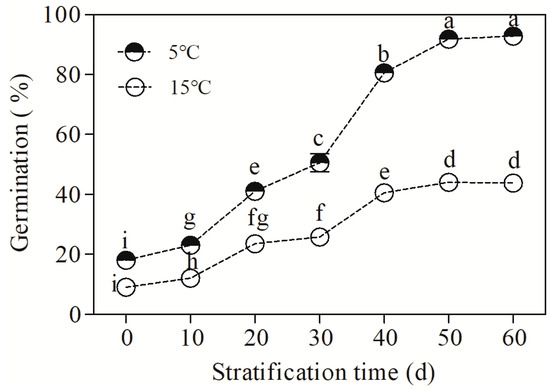
Figure 1.
Changes in the seed germination rate of Bretschneidera sinensis during cold stratification. The 5 °C and 15 °C represent cold and warm stratification, respectively. On the x axis, 0 is the control (CK, CK represents control unstratified seeds) and the numbers 10, 20, 30, 40, 50, and 60 represent the duration (days) of stratification. Each treatment had four replicates, and each replicate contained 30 seeds. Germination was performed at 25 °C under light conditions. The germination rate is expressed as the mean ± standard error. The same lowercase letters indicate insignificant differences (p = 0.05).
3.2. Changes in Endogenous Hormone Content during Cold Stratification
The GA3 content showed a trend of decreasing, increasing, and then decreasing during seed stratification (F = 1083.03, p < 0.05, Figure 2A). The GA3 content was 0.03 ng/g DW in unstratified seeds, which significantly decreased to a minimum value of 0.01 ng/g DW at 10 d of stratification. The GA3 content then increased sharply and reached a maximum value of 6.1 ng/g DW at 40 d of stratification. After that, the value decreased, but was always higher than that of the CK group. The GA3 content remained at 3.81 ng/g DW at the end of stratification (60 d).
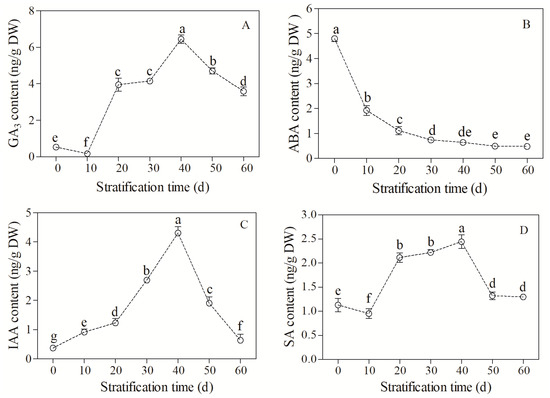
Figure 2.
Changes in endogenous hormone content in Bretschneidera sinensis seeds during cold stratification. (A–D) represent gibberellic acid (GA3), abscisic acid (ABA), indole-3-acetic acid (IAA), and salicylic acid (SA), respectively. Each treatment had four replicates. On the x axis, 0 is the control (CK) and the numbers 10, 20, 30, 40, 50, and 60 represent the duration (days) of stratification. The same lowercase letters indicate insignificant differences (p = 0.05).
The ABA content decreased continuously during seed stratification (F = 1374.40, p < 0.05, Figure 2B). The ABA content was the highest in unstratified seeds (4.83 ng/g DW). It decreased sharply during seed stratification, and reached the minimum value of 0.51 ng/g DW at 50 d.
The IAA content increased and then decreased during seed stratification (F = 9.92, p < 0.05, Figure 2C). The IAA content was the lowest in unstratified seeds (0.03 ng/g DW), increased sharply during stratification, and reached a maximum value of 4.21 ng/g DW at 40 d. Then, the value decreased dramatically, but was always higher than that of the CK group. The IAA content remained at 0.05 ng/g DW at the end of stratification (60 d).
The SA content showed a trend of decreasing, increasing, and then decreasing (F = 10.087, p < 0.05, Figure 2D). The SA content was 1.2 ng/g DW in unstratified seeds, which decreased to the lowest value of 0.08 ng/g DW at 10 d of stratification. The value then gradually increased, and reached the maximum of 2.43 ng/g DW at 40 d. After that, the value decreased, but always remained higher than that of the CK group. The SA content remained at 1.43 ng/g DW at the end of stratification (60 d).
These results suggested that the ABA content in the embryo decreased during cold stratification, while GA3, IAA, and SA increased before 40 d of stratification and then gradually decreased. These findings imply the synergism or antagonism of these hormones during the dormancy release and germination of B. sinensis seeds.
3.3. Changes in Antioxidant Enzyme Activities during Cold Stratification
The SOD activity showed a trend of decreasing, increasing, and then decreasing (F = 209.81, p < 0.05, Figure 3A). The SOD activity was 622 μg/g DW in unstratified seeds, which significantly decreased to a minimum value of 40.25 μg/g DW at 10 d of stratification. The value then increased sharply and reached a maximum of 123.3 μg/g DW at 40 d of stratification. After that, a notable decrease in SOD activity was observed.
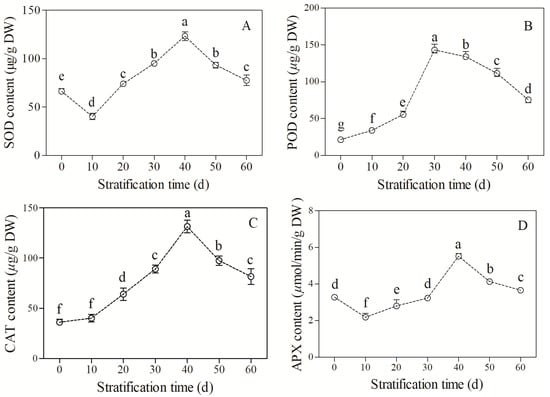
Figure 3.
Changes in antioxidant enzyme activity in Bretschneidera sinensis seeds during cold stratification. (A–D) represent superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX), respectively. Each treatment had four replicates. On the x axis, 0 is the control (CK) and the numbers 10, 20, 30, 40, 50, and 60 represent the duration (days) of stratification. The same lowercase letters indicate insignificant differences (p = 0.05).
The POD activity increased and then decreased (F = 724.52, p < 0.05, Figure 3B). The POD activity was the lowest in unstratified seeds (21.65 μg/g DW), then increased sharply with cold stratification and reached the maximum value of 144.2 μg/g DW at 30 d. The value then decreased, but was always higher than that of the CK group. The POD activity remained at 75.5 μg/g DW at the end of stratification (60 d).
The CAT activity increased and then decreased (F = 314.22, p < 0.05, Figure 3C). The CAT activity was the lowest in unstratified seeds (32 μg/g DW), then increased sharply with stratification and reached the maximum value of 131.47 μg/g DW at 40 d. The value then decreased, but always remained higher than that of the CK group. The CAT activity remained at 81.6 μg/g DW at the end of stratification (60 d).
The APX activity showed a trend of decreasing, increasing, and then decreasing (F = 115.58, p < 0.05, Figure 3D). The APX activity was 3.28 μmol/min/g DW in unstratified seeds, which decreased to the minimum value at 10 d of stratification, and then increased sharply and reached the maximum value of 5.51 μmol/min/g DW at 40 d of treatment. After that, the value declined, but was always higher than that of the CK group. The APX activity remained at 3.65 μg/g DW at the end of stratification (60 d).
These results suggest that the scavenging of excessive ROS mainly depends on SOD, POD, and CAT, while APX may play an important role in fine-tuning signal transduction during the dormancy release and germination of the fresh mature seeds of B. sinensis.
3.4. Changes in Soluble Sugar Content and Two Key Enzymes in Sugar Metabolism during Cold Stratification
With the extension of stratification time, the soluble sugar content increased and then decreased (F = 402.65, p < 0.05, Figure 4). The soluble sugar content was the lowest (20.17 mg/g dry weight (DW)) in unstratified seeds (CK), then increased rapidly with stratification, and peaked at 65.32 mg/g DW at 40 d of treatment. However, with the further extension of stratification, the soluble sugar content decreased but remained higher than that in the CK group. Notably, at the end of stratification (60 d), the soluble sugar content remained at a level of 42.23 ng/g DW. These observations indicate that B. sinensis seeds undergo a substantial consumption of soluble sugars during dormancy release and germination, and that soluble sugars facilitate seed germination. Nevertheless, the soluble sugar content gradually decreased with dormancy release at the late stage of stratification.
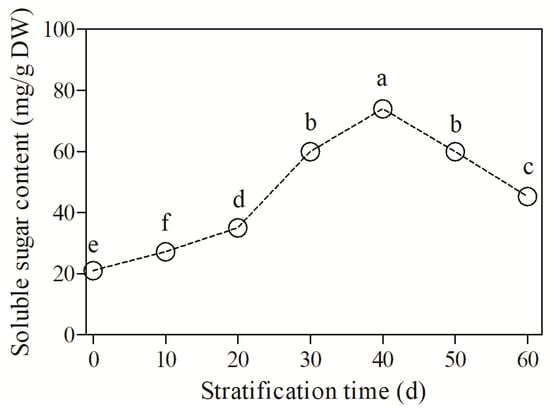
Figure 4.
Changes in soluble sugar content in Bretschneidera sinensis seeds during cold stratification. Each treatment had four replicates. On the x axis, 0 is the control (CK) and the numbers 10, 20, 30, 40, 50, and 60 represent the duration (days) of stratification. The same lowercase letters indicate insignificant differences (p = 0.05).
With the extension of seed stratification, the MDH activity followed the trend of decreasing, increasing, and then decreasing (F = 415.69, p < 0.05, Figure 5A). The activity of MDH in unstratified seeds was 6.72 μmol/min/g DW, which decreased after 10 d of stratification, then increased and reached the highest value of 8.86 μmol/min/g DW at 30 d of stratification. With the further extension of stratification, the MDH activity decreased sharply to the lowest value of 4.36 μmol/min/g DW at 60 d of stratification.
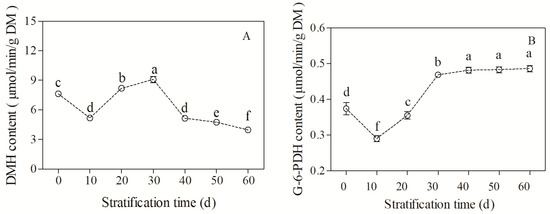
Figure 5.
Changes in key enzyme activity in sugar metabolism in Bretschneidera sinensis seeds during cold stratification. (A,B) represent malate dehydrogenase (MDH) and glucose-6-phosphate dehydrogenase (G-6-PDH), respectively. Each treatment had four replicates. On the x axis, 0 is the control (CK) and the numbers 10, 20, 30, 40, 50, and 60 represent the duration (days) of stratification. The same lowercase letters indicate insignificant differences (p = 0.05).
The G-6-PDH activity decreased and then increased during seed stratification (F = 440.62, p < 0.05, Figure 5B). The G-6-PDH activity was 0.37 μmol/min/g DW in unstratified seeds, which decreased at 10 d of stratification. The G-6-PDH activity then increased and reached the maximum value of 0.48 μmol/min/g DW at 40 d of stratification. The G-6-PDH activity remained stable with the further extension of cold stratification.
Therefore, the MDH and G-6-PDH activities fluctuated considerably during 30–40 d of stratification, suggesting significant changes in the respiratory pathways of sugar metabolism during dormancy release and germination.
3.5. Correlations of the Germination Rate with Physiological Indicators during Cold Stratification
As shown in Figure 6, the germination rate showed positive correlations with the soluble sugar content and G-6-PDH activity (p < 0.05), while demonstrating a negative correlation with MDH activity (p < 0.05). The germination rate showed positive correlations with GA3 and IAA contents (p < 0.05), but a negative correlation with ABA (p < 0.05). Moreover, SOD, POD, CAT, and APX activities were positively correlated with the seed germination rate (p < 0.05). These results highlight the interactions of these physiological indicators with seed dormancy release and germination.
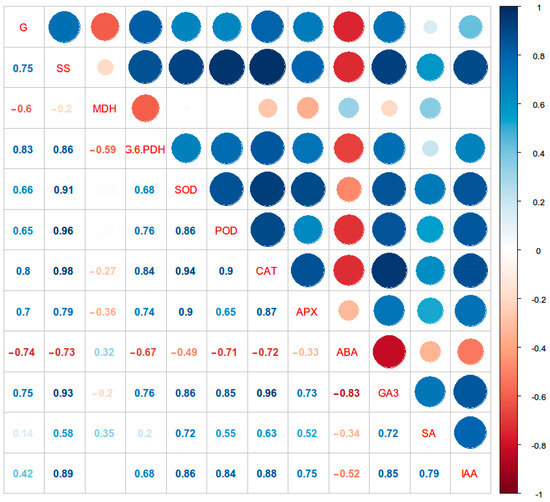
Figure 6.
Correlations between the average germination rate and soluble sugars, phytohormones, and antioxidant enzymes during cold stratification. Note: The right vertical axis represents the correlation between two datasets. The color of the circle indicates the direction of the correlation (red, positive correlation; blue, negative correlation). The size of the circle indicates the magnitude of the correlation coefficient, which is displayed by the corresponding value. G is the seed germination rate, SS is soluble sugars, and other abbreviations are physiological indicators as described in the text.
4. Discussion
4.1. Regulation of Seed Dormancy Release and Germination by Endogenous Hormones
Many studies have demonstrated that GA, ABA, and IAA are the most important endogenous factors regulating seed dormancy and germination [10]. B. sinensis seeds exhibit dormancy. The changes in hormone levels at different stages from the dry seed stage to imbibition and then to germination reflect the internal mechanisms of dormancy regulation. It is well known that ABA plays a pivotal role in inducing and maintaining seed dormancy. ABA reduces the osmotic potential and water absorption of the seed embryo, impeding radicle elongation and seed germination. Consequently, the release of seed dormancy is usually accompanied by a decline in ABA. GA3 is an important factor in releasing dormancy and promoting seed germination that mainly acts through increasing the vigor of the embryo, weakening the tissue around the embryo, and relieving the constraint imposed by the seed coat. The antagonism between ABA and GA, which are the major phytohormones, plays a key role in the regulation of seed dormancy. Hence, seed germination can be controlled by regulating the metabolism and signal transduction of ABA and GA [7,8]. The levels of ABA in B. sinensis seeds reached the maximum in the unstratified stage but decreased significantly with the increase in stratification time. As shown in Figure 2B, it decreased 9.47-fold at 50 d of seed stratification. The ABA content is negatively correlated with the germination rate of seeds (Figure 6). Conversely, the GA3 content increased significantly by 33.3-fold by the end of seed germination (Figure 2A). These findings indicate that the increase in the GA3/ABA ratio serves as a key determinant in seed dormancy release and germination, which is consistent with the classical theory highlighting the role of ABA and GA3 in regulating seed dormancy and germination.
IAA is another important endogenous hormone, which promotes seed germination by inducing elongation and differentiation of blastocytes [35]. For example, IAA content during dormancy release and germination of Rhizoma Paridis Yunnanensis, Paris polyphylla, and Medicago sativa seeds increased rapidly. In this study, the IAA content increased significantly in different stages of germination of B. sinensis seeds. The IAA content at the end of seed germination was 14-fold higher than the initial value (Figure 2C). This indicates that IAA plays a positive role in inducing seed germination. Nevertheless, seed germination does not depend on the increase or decrease in a single phytohormone, such as ABA, GA, or IAA. The spatiotemporal balance of ABA, GA, and IAA is the major factor determining seed germination [36,37]. Based on the analysis of hormone ratios at different stages, the ABA/GA3 and ABA/IAA ratios showed a significant decline. This is consistent with the regulation of germinating hormones in Taxus yunnanensis and Garcinia paucinervis seeds. This indicates that ABA might be the key inhibitor of germination of B. sinensis seeds. The antagonism with ABA was intensified by regulating the biosynthesis of the growth promoters GA3 and IAA to promote seed germination [38,39]. Additionally, it is widely accepted that SA regulates seed growth and development, including cell expansion and axial elongation of seed radicles. In this experiment, the SA level reached the maximum after 40 d of stratification (Figure 2D). This result can be explained by the role of B. sinensis seeds in regulating the growth of plumular axis and radicals from imbibition to germination stages by increasing SA synthesis, thus promoting the seed germination [40,41,42]. According to the endogenous hormone regulation theory of seed dormancy, seed germination requires interaction between hormones. The changes in hormone levels in different stages of germination suggest that the increase in GA3 and the decrease in ABA concentration promote seed germination in B. sinensis.
4.2. Antioxidative Stress during Seed Dormancy Release and Germination
Seed dormancy release and germination are related to changes in phytohormones. Further, different types of enzymes, such as antioxidant enzymes, may alter the response during seed dormancy release and germination [43]. The internal respiratory metabolism of seeds is enhanced from seed dormancy release to germination stages, which may generate substantial amounts of reactive oxygen species (ROS), leading to the accumulation of osmotic substances. ROS transform mutually via spontaneous or catalytic reactions. They are strong oxidants and induce lipid peroxidation, resulting in oxidative damage to cellular structures [44,45]. This has been established in physiological studies of seed germination [46,47]. SOD can cleave O2− into H2O2 and O2 rapidly with minimal toxicity [48]. CAT is an enzyme that uses H2O2 as the substrate and represents an important index of metabolic changes in plants. CAT can be used to characterize cell growth and development. The interaction between CAT and POD can further eliminate H2O2, thereby decreasing and eliminating the free radical-induced damage to cell membranes [49,50]. The study findings suggest an increase in the levels of SOD, POD, CAT, and APX during the release from seed dormancy stage in B. sinensis (Figure 3A–D). They are positively correlated with the seed germination rate (Figure 6). Thus, the seeds experience oxidative stress during stratification. The high levels of SOD, POD, CAT, and APX perform rapid free radical-scavenging activity. This indicates that the combination of SOD, POD, CAT, and APX activities not only contributes to seed dormancy release in B. sinensis, but also facilitates seed cell growth and structural integrity, ensuring smooth germination and prompt scavenging of internal free radicals.
4.3. The Role of Energy Metabolism in Seed Dormancy Release and Germination
The complex physiological transition from seed dormancy to germination requires a significant energy supply. Energy reserves are key to the activation of seed germination and metabolism and the changes in their content also indirectly reflect the mechanism of seed dormancy release [51]. Specifically, carbohydrate, the major reserve substrate in most types of seeds [52], represents an important source of energy for seed germination. Seed dormancy release originates in the glycolysis pathway, which generates the primary energy in seeds [53]; therefore, glucose metabolism plays an important role in seed germination [54]. Soluble sugars in seeds mainly include glucose, maltose, and saccharose. As the substrate of respiratory metabolism in the embryo, soluble sugar is the major source of energy during germination [55]. Soluble sugar exists in the embryo or endosperm. However, soluble sugar can be transformed from fat, starch, and soluble proteins [56]. This study found a dramatic increase in the levels of soluble sugar in B. sinensis seeds after stratification at an early stage, and the sugar was consumed significantly during seed dormancy release and reinforced the respiratory effect of the embryo (Figure 4). Further, the seed germination rate increased accordingly, suggesting that the soluble sugar level regulates the release of seed dormancy in B. sinensis.
Roberts et al. [57] hypothesized that the shift of sugar metabolism from glycolysis to the pentose phosphate pathway (PPP) is critical to seed dormancy release. MDH and 6-G-PDH are the key enzymes in the tricarboxylic acid cycle (TCA) and PPP, respectively, and their activities reflect the strength of seed respiration. In the present investigation, with the extension of cold stratification, there was a rapid decrease in the activity of MDH in TCA (Figure 5A), alongside a considerable increase in the activity of 6-G-PDH in PPP, showing a significant negative correlation (Figure 6). These results indicate a shift in the predominant respiratory metabolic pathway in B. sinensis seeds from TCA to PPP during dormancy release, which is similar to the findings of Li et al. [58]. The results of the present study provide new evidence for the hypothesis proposed by Roberts et al. [57]. In addition, B. sinensis seeds need to be released from physiological dormancy before germination. The natural cracking of the seed coat at 30 d of stratification indicates the development of the seed embryo and the increase in metabolic activity. This explains the significant changes in MDH and G-6-PDH activities during 30–40 d of stratification. During the process of seed stratification, therefore, 30–40 d is an important period for the shift from dormancy to germination preparation.
This study demonstrates that soluble sugars play an important role in protecting seeds against oxidative stress. It can be used to regulate osmotic balance and stabilize protein structure and biological membrane. Soluble sugars also protect phospholipids in the biological membrane by inducing vitrification in cytoplasm. The accumulation of soluble sugar increases significantly during seed dormancy release in B. sinensis (Figure 4), possibly due to the increased glycosylation of cell proteins [59,60]. According to Coue et al., the soluble sugar content is related to metabolic pathways associated with ROS generation. Conversely, the soluble sugar can induce metabolic pathways that generate NADPH, such as the oxidized pentose phosphate (OPP) pathway, to facilitate scavenging of ROS [61].
5. Conclusions
Low-temperature wet stratification under 5 °C can release seed dormancy effectively. During the seed dormancy release, the seed germination rate was positively correlated with soluble sugar, GA3, and IAA levels, as well as G-6-PDH, SOD, POD, CAT, and APX activity, but it was negatively correlated with MDH activity and ABA content. These imply that dormancy release might be attributed to the degradation of endogenous ABA and the oxidation of reactive oxygen species induced by low-temperature wet stratification. GA3, IAA, and the metabolism of energy substrates may be correlated with the induction and promotion of germination.
Author Contributions
S.D. and J.M. conceived and designed the study. L.X., L.Z. and H.D. collected the samples and performed the experiments. J.L., Z.D. and S.D. analyzed the data and drafted the manuscript. Z.D. provided financial support, supervised the study, and revised the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Sciences Foundation of China (31860073), the Science and Technology Research Project of Education Department of Hubei Province (B2021156) and Germplasm Engineering of Characteristic Plant Resources in Enshi Prefecture (2019–2021).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We are grateful to Hua Xue (Beijing Forestry University) for help during the experiments and for his continuous support throughout this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Elsevier Academic Press Inc.: San Diego, CA, USA, 2014. [Google Scholar]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.V.; Nicotra, A.B.; Godfree, R.C.; Guja, L.K. Polyploidy affects the seed, dormancy and seedling characteristics of a perennial grass, conferring an advantage in stressful climates. Plant Biol. 2020, 22, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Rhie, Y.H.; Kim, K.S. Dormancy breaking and germination requirements of seeds of Thalictrum uchiyamae (Ranuncula ceae) with underdeveloped embryos. Sci. Hortic. 2018, 231, 82–88. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef]
- Song, S.Q.; Liu, J.; Huang, H.; Wu, J.; Xu, H.; Zhang, Q.; Li, X.; Liang, J. Gibberellin metabolism and signaling and its molecular mechanism in regulating seed germina tion and dormancy. Sci. China Life Sci. 2020, 50, 599–615. [Google Scholar]
- Song, S.Q.; Liu, J.; Xu, H.H.; Liu, X.; Huang, H. ABA metabolism and signaling and their molecular mechanism regulating seed dormancy and germination. Sci. Agric. Sin. 2020, 50, 599–615. [Google Scholar]
- Song, S.Q.; Liu, J.; Tang, C.F.; Zhang, W.H.; Xu, H.H.; Zhang, Q.; Gao, J.D. Metabolism and signaling of auxins and their roles in regulating seed dormancy and germination. Chin. Sci. Bull. 2020, 65, 3924–3943. (In Chinese) [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Xiang, Y.; Tong, X.; Wojtyla, Ł.; Wang, Y. Editorial: Molecular basis of seed germination and dormancy. Front. Plant Sci. 2023, 14, 1242428. [Google Scholar] [CrossRef]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated role of ABA in seed maturation, dormancy, and germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef]
- Sano, N.; Marion-Poll, A. ABA Metabolism and Homeostasis in Seed Dormancy and Germination. Int. J. Mol. Sci. 2021, 22, 5069. [Google Scholar] [CrossRef]
- Longo, C.; Holness, S.; De Angelis, V.; Lepri, A.; Occhigrossi, S.; Ruta, V.; Vittorioso, P. From the Outside to the Inside: New Insights on the Main Factors That Guide Seed Dormancy and Germination. Genes 2020, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Bicalho, E.M.; Pintó-Marijuan, M.; Morales, M.; Müller, M.; Munné-Bosch, S.; Garcia, Q.S. Control of macaw palm seed germination by the gibberellin/abscisic acid balance. Plant Biol. 2015, 17, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Footitt, S.; Clewes, R.; Feeney, M.; Finch-Savage, W.E.; Frigerio, L. Aquaporins Influence seed dormancy and germination in response to stress. Plant Cell Environ. 2019, 42, 2325–2339. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Q.; Yang, L.; Li, X.; Wang, Z.; Zhang, Y. Changes in carbohydrate metabolism and endogenous hormone regulation during bulblet initiation and development in Lycoris radiata. BMC Plant Biol. 2020, 20, 180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, F.; Li, X.; Cao, H.; Ding, M.; Zhang, C.; Zuo, J.; Xu, C.; Xu, J.; Deng, X.; et al. Arabidopsis seed germination speed is controlled by SNL histone deacetylase-binding factor-mediated regulation of AUX1. Nat. Commun. 2016, 7, 13412. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Kumari, A.; Thakur, M.; Koul, A. Hydrogen peroxide signaling integrates with phytohormones during the germination of magnetoprimed tomato seeds. Sci. Rep. 2019, 9, 8814. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hao, W.; Liu, D.; Wang, L.; Qiu, S. Effects of SA on the germination of seeds and the stability of cell membrane under cold stress. J. Northwest AF Univ. (Nat. Sci. Ed.) 2010, 38, 183–188. [Google Scholar]
- Wang, Y.M.; Wang, L.J.; Yao, B.; Liu, Z.; Li, F. Changes in ABA, IAA, GA3, and ZR levels during seed dormancy release in Idesia polycarpa Maxim from Jiyuan. Pol. J. Environ. Stud. 2018, 27, 1833–1839. [Google Scholar]
- Katsuya-Gaviria, K.; Caro, E.; Carrillo-Barral, N.; Iglesias-Fernández, R. Reactive Oxygen Species (ROS) and Nucleic Acid Modifications during Seed Dormancy. Plants 2020, 9, 679. [Google Scholar] [CrossRef]
- Leymarie, J.; Vitkauskaité, G.; Hoang, H.H.; Gendreau, E.; Chazoule, V.; Meimoun, P.; Corbineau, F.; El-Maarouf-Bouteau, H.; Bailly, C. Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant Cell Physiol. 2012, 53, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Dai, Y.; Zheng, C.; Yang, Y.; Chen, W.; Wang, Q.; Chandrasekaran, U.; Du, J.; Liu, W.; Shu, K. The ABI4-RbohD/VTC2 regulatory module promotes reactive oxygen species (ROS) accumulation to decrease seed germination under salinity stress. New Phytol. 2021, 229, 950–962. [Google Scholar] [CrossRef]
- Wang, W.B.; Kim, Y.H.; Lee, H.S.; Kim, K.Y.; Deng, X.P.; Kwak, S.S. Analysis of antioxidant enzyme activity during Germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009, 47, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Oracz, K.; El-Maarouf-Bouteau, H.; Kranner, I.; Bogatek, R.; Corbineau, F.; Bailly, C. The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiol. 2009, 150, 494–505. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative signaling in seed germination and dormancy. Plant Signal. Behav. 2008, 31, 75–182. [Google Scholar] [CrossRef]
- Zaynab, M.; Pan, D.; Chen, W. Transcriptomic approach to address low germination rate in Cyclobalnopsis gilva seeds. S. Afr. J. Bot. 2018, 119, 286–294. [Google Scholar] [CrossRef]
- Roberts, E. Temperature and seed germination. Symp. Soc. Exp. Biol. 1988, 42, 109–132. [Google Scholar] [PubMed]
- Zhi, L.M.; Zhang, Y.H.; Yu, F.Y. Biochemical and physiological changes of Euscaphis japonica seeds during the period of stratifi. J. Cent. South Univ. For. Technol. 2016, 36, 36–40. [Google Scholar]
- Wu, Z.Y.; Lu, A.M.; Tang, Y.C. The Families and General of Angiosperm in China; Science Press: Beijing, China, 2003; p. 702. [Google Scholar]
- Zhang, J.R.; Cheng, G.F. National level to protect plants-bretschneidera sinensis Hemsl. Bull. Biol. 2009, 44, 7. [Google Scholar]
- Li, T.H.; Zhou, Y.X.; Duan, X.P. A preliminary study of physiology dormancy character bretschneidera sinensis Hemsl Seeds. J. Cent. South For. Univ. 1997, 17, 41–44. [Google Scholar]
- Li, X.Z.; Simpson, W.R.; Song, M.L.; Bao, G.S.; Niu, X.L.; Zhang, Z.H.; Xu, H.F.; Liu, X.; Li, Y.L.; Li, C.J. Effects of seed moisture content and Epichloe endophyte on germination and physiology of Achnatherum inebrians. S. Afr. J. Bot. 2022, 134, 407–414. [Google Scholar] [CrossRef]
- Ma, L.Y.; Cheng, N.L.; Han, G.J.; Li, L. Effects of exogenous salicylic acid on seed germination and physiological characteristics of Coronilla varia under drought stress. Chin. J. Appl. Ecol. 2017, 28, 3274–3280. [Google Scholar]
- Wu, M.J.; Wu, J.Y.; Gan, Y.B. The new insight of auxin functions: Transition from seed dormancy to germination and floral opening in plants. Plant Growth Regul. 2020, 91, 169–174. [Google Scholar] [CrossRef]
- Boter, M.; Calleja-Cabrera, J.; Carrera-Castaño, G.; Wagner, G.; Hatzig, S.V.; Snowdon, R.J.; Legoahec, L.; Bianchetti, G.; Bouchereau, A.; Nesi, N.; et al. An integrative approach to analyze seed germination in Brassica napus. Front. Plant Sci. 2019, 10, 1342. [Google Scholar] [CrossRef] [PubMed]
- Penfield, S. Seed dormancy and germination. Curr. Biol. 2017, 27, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Su, J.; Liu, W.; Li, S. Dormancy release and germination of Taxus yunnanensis seeds during wet sand storage. Sci. Rep. 2018, 8, 3205. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.G.; Park, C.M. Salicylic acid promotes seed germination under high salinity by modulating antioxidant activity in Arabidopsis. New Phytol. 2010, 188, 626–637. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wei, X.; Chai, S.F.; Wu, S.H.; Zou, R.; Qin, X.M.; Fu, R. Dormancy mechanism of the seeds of a rare and endangered plant, Garcinia paucinervis. Chin. J. Ecol. 2018, 37, 1371–1381. [Google Scholar]
- Su, H.L.; Zhou, X.Z.; Li, X. Physicochemical changes of Paris polyphylla var. Chinensis seed during different stages of germination. Chin. Tradit. Herb. Drugs 2017, 48, 4755–4763. [Google Scholar]
- Pluskota, W.E.; Pupel, P.; Głowacka, K.; Okorska, S.B.; Jerzmanowski, A.; Nonogaki, H.; Górecki, R.J. Jasmonic acid and ethylene are involved in the accumulation of osmotin in germinating tomato seeds. J. Plant Physiol. 2018, 232, 74–81. [Google Scholar] [CrossRef]
- Xu, L.; Wang, P.; Ali, B.; Yang, N.; Chen, Y.; Wu, F.; Xu, X. Changes of the phenolic compounds and antioxidant activities in germinated adlay seeds. J. Sci. Food Agric. 2017, 97, 4227–4234. [Google Scholar] [CrossRef]
- Oracz, K.; Bouteau, H.E.; Farrant, J.M.; Cooper, K.; Belghazi, M.; Job, C.; Job, D.; Corbineau, F.; Bailly, C. ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J. 2007, 50, 452–465. [Google Scholar] [CrossRef]
- Huang, W.; Mayton, H.S.; Amirkhani, M.; Wang, D.; Taylor, A.G. Seed dormancy,germination and fungal infestation of eastern gamagrass seed. Ind. Crops Prod. 2017, 99, 109–116. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Xu, Z.; Shi, Z.; Chen, S.; Huang, X.; Chen, J.; Wang, X. Involvement of reactive oxygen species in endosperm cap weakening and embryo elongation growth during lettuce seed germination. J. Exp. Bot. 2014, 65, 3189–3200. [Google Scholar] [CrossRef]
- Jeevan Kumar, S.P.; Rajendra Prasad, S.; Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Ann. Bot. 2015, 116, 663–668. [Google Scholar] [CrossRef]
- Amooaghaie, R. Triangular interplay between ROS, ABA and GA in dormancy alleviation of Bunium persicum seeds by cold stratification. Russ. J. Plant Physiol. 2017, 64, 588–599. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.X.; Xu, X. The activity of Principal antioxidant enzymes and the content of metabolites in dormancy breaking and germination of Davidia involucrate seeds. Plant Divers. Resour. 2015, 37, 779–787. [Google Scholar]
- Marta, B.; Szafrańska, K.; Posmyk, M. Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front. Plant Sci. 2016, 7, 575. [Google Scholar] [CrossRef]
- Kazmi, R.H.; Willems, L.A.J.; Joosen, R.V.L.; Khan, N.; Ligterink, W.; Hilhorst, H.W.M. Metabolomic analysis of tomato seed germination. Metabolomics 2017, 13, 145. [Google Scholar] [CrossRef]
- Alencar, N.L.; Innecco, R.; Gomes-Filho, E.; Gallão, M.I.; Alvarez-Pizarro, J.C.; Prisco, J.T.; De Oliveira, A.B. Seed reserve composition and mobilization during germination and early seedling establishment of Cereus jamacaru D.C. ssp. jamacaru (Cactaceae). An. Acad. Bras. Ciênc. 2012, 84, 823–832. [Google Scholar] [CrossRef]
- Han, C.; Zhen, S.; Zhu, G.; Bian, Y.; Yan, Y. Comparative metabolome analysis of wheat embryo and endosperm reveals the dynamic changes of metabolites during seed germination. Plant Physiol. Biochem. 2017, 115, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Sang, S.; Chen, Y.; Wei, Z.; Wang, P. The role of Arabidopsis inositol polyphosphate kinase AtIPK2β in glucose suppression of seed germination and seedling development. Plant Cell Physiol. 2017, 59, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, H.; Yan, H.; Qiu, L.; Baskin, C.C. Mobilization and role of starch,protein, and fat reserves during seed germination of six wild grassland species. Front. Plant Sci. 2018, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sakshi, G.; Munshi, S. Changes in lipid and carbohydrate composition of germinating soybean seeds under different storage conditions. Asian J. Plant Sci. 2007, 6, 596. [Google Scholar]
- Roberts, E.H.; Major, W. Dormancy in cereal seeds: II. The nature of gaseous exchange in imbibed barley and rice seeds. J. Exp. Bot. 1968, 19, 90–101. [Google Scholar]
- Li, Z.L.; Tong, K.; Yan, S.; Yang, H.; Wang, Q.; Tang, Y.B.; Deng, M.S.; Tian, M.L. Physiological and biochemical change of Paris seed in after-ripening during variable temperature stratifications. China J. Chin. Mater. Med. 2015, 40, 629–633. [Google Scholar]
- Smolikova, G.; Leonova, T.; Vashurina, N.; Frolov, A.; Medvedev, S. Desiccation Tolerance as the Basis of Long-Term Seed Viability. Int. J. Mol. Sci. 2021, 22, 101. [Google Scholar] [CrossRef]
- Livingston, D.P.; Hincha, D.K.; Heyer, A.G. Fructan and its relationship to abiotic stress tolerance in plants. Cell. Mol. Life Sci. 2009, 66, 2007–2023. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxide tive stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).