Abstract
The Sakhalin pine sawyer Monochamus saltuarius (Gebler) (Coleoptera: Cerambycidae) is a new vector of pine wood nematode in China, which has caused huge economic losses in the forestry industry. The mating process of M. saltuarius has been described in detail. However, mate choice and sexual selection in this species are not fully understood. In this study, we quantitatively evaluated the characteristics associated with contact between the sexes in mating and inferred the sex-specific characteristics under selection. We detected positive correlations between the morphological characteristics of females and males. Most female traits and all male traits differed significantly between mated and unmated individuals. The results of this study provide evidence for the selection of the mating preferences in M. saltuarius.
1. Introduction
Darwin [1] formulated the concept of sexual selection, which included two principal mechanisms: intrasexual competition (usually between males) for breeding access and mate choice (usually by the female) based on desirable traits in the opposite sex. Sexual selection theory predicts that females should be the more selective sex in choosing mates and show a preference for particular sexual ornaments and signals [2] due to their relatively greater investment in gametes [3]. Where females can choose a male among numerous males, they will commonly increase their fitness and that of their offspring by selecting genetically compatible mates, or mates with ‘good genes’, which the offspring may inherit [4].
Increasing evidence now shows that males can be choosy, too, even in apparently unexpected situations, such as in the absence of male parental care [5,6]. Males may exhibit mate choice for a variety of reasons, including seeking mates with greater fecundity [7]. For males, reproductive success tends to increase with the number of mates acquired [8], while males will inevitably reject available females if they lack the resources required to mate with them. Therefore, mate choice comprises not only decisions about whether to mate but also how many resources to allocate to each mate or mating [9]. In fact, regardless of whether the mate choice occurs in males or females, it is a central component of sexual selection theory [1,8,9], and understanding mate choice and the underlying preferences that lead to choice is, therefore, central to understanding sexual selection.
Sexual selection can lead to the rapid diversification of reproductive traits in both males and females. Such selection is often proposed as an explanation for the evolution of external morphological traits that confer a mating advantage upon their carriers of different genders. Sexual selection has diverse effects on individual traits, either by promoting success in competition for mates or by facilitating mate attraction and copulation. These traits can either be morphological or behavioral in nature and are subject to selection pressures [10].
The Sakhalin pine sawyer Monochamus saltuarius (Gebler) (Coleoptera: Cerambycidae) is widely distributed in Central and Eastern Europe, Siberia, the Russian Far East, and East Asia [11]. In China, M. saltuarius is mainly distributed in the Heilongjiang, Jilin, Liaoning, Hebei, Shaanxi, and Gansu provinces, Inner Mongolia Autonomous Region, and Xinjiang Uyghur Autonomous Region [12]. M. saltuarius has one generation per year in China and overwinters as larvae in the xylem canals of their host trees [13]. The second instar larvae of M. saltuarius feed on the sapwood, and the fourth instar larvae begin to drill the xylem [13]. The number of invasion holes of M. saltuarius increased first and then decreased with the increase in the trunk height of the host trees [14]. M. saltuarius began to emerge in early May; adults feed on the cortex of host tree branches to supplement nutrients and then spread to mate and lay eggs [15]. The oviposition groove of the female is a long prismatic. After eclosion, the M. saltuarius entered the stage of supplementary nutrition, gnawing bark and needles on host trees to cause wounds [16]. In the Japanese pine sawyer M. alternatus Hope, an insect vector of the pine wood nematode Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle has higher CO2 concentrations, resulting from the respiration cycles and bursts of CO2 that occur during maturation feeding, which drives the pine wood nematode off its insect vector [17]. Plausibly, a large number of pine wood nematodes carried by M. saltuarius were immediately introduced into healthy pine trees through the wound in the same way. Pine trees usually die 2–3 months after being infected by pine wood nematodes. The most important management action to control the pine wilt disease spread is based on the felling and elimination of declining trees during winter since the removal and destruction of these trees during winter months (before pine sawyer emergence) eliminates the pine wood nematode and the immature stages of pine sawyers that are still inside the pupal chambers [18]. M. salternatus is mainly spread by flight; the longest flight distance of M. saltuarius adults is about 5 km throughout their whole life cycle [19]. As a vector insect, M. salternatus has a great ability to transmit pine wood nematodes, which brings challenges to the prevention and control of pine wilt disease [12].
M. saltuarius mainly colonizes Korean white pine Pinus koraiensis Sieb. et Zucc., Chinese pine P. tabuliformis Carr. [20], and Mongolian Scots pine P. sylvestris var. mongolica Litv. [21]. In 2018, M. saltuarius was first identified as a new vector of pine wood nematode in China, which has resulted in huge economic losses to forestry production and development [16,22,23,24]. The mating process of M. saltuarius has been characterized in detail. Mating in the species could be divided into three stages: pair bonding, ejaculation, and post-copulatory guarding. The mating and spawning behaviors of M. saltuarius have circadian rhythms. The male remains motionless with an outstretched antenna or shows slight movement. The male mounts the approaching female upside down or turns the female so that they are facing the same direction. The male then grasps the female’s metathorax with the fore tarsal and holds the metasternum on either side. The male genitalia protrude during copulation. During mating, the male grasps the back of the female firmly using the forelegs and midlegs, with the hind legs on the bark of the tree [25,26].
We observed mating behavior in the species to determine the sequence and key body parts related to mate evaluation in both sexes. Next, we inferred sexually selected traits based on mate choice experiments, recorded successful matings, and measured traits that were in frequent contact between the sexes prior to insemination.
2. Materials and Methods
2.1. Sample Collection and Rearing Conditions
Wood segments (length: 57.6−74.3 cm) of P. koraiensis containing M. saltuarius larvae were collected from Dahuofang Forest Farm, Fushun City, Liaoning Province, China. (41°56′23.028″ N, 124°13′3.925″ E). The two ends of the wood segments were sealed with wax in order to prevent water loss. The temperature and relative humidity in the room were controlled at 22.3 ± 2.3 °C and 33.0% ± 7.0%, respectively. To ensure that M. saltuarius was unmated, newly emerged adults were obtained daily and placed in 800 mL plastic boxes. Individuals were fed cotton bales soaked in water with honey (10% concentration) [27] and young shoots of P. koraiensis separately, and foods were replaced every other day. Females of M. saltuarius are sexually mature at approximately 7-day-old post-emergence [28]; 14-day-old virgin females were used in the mating test. Males of M. saltuarius are sexually mature after emergence [29]; thus, 5-day-old virgin males were used in the mating test.
2.2. Mating Test
To determine female traits associated with mating success and to identify traits that might be affected by directional selection, mating was allowed at a female-biased sex ratio with a total of 45 replicates. To observe mating behavior, one 5-day-old virgin male and two 14-day-old virgin females were placed in an 800 mL plastic container for each replicate. To establish the male traits linked with successful mating and to identify characteristics that may undergo directional selection, mating experiments were carried out, releasing one 14-day-old virgin female and two 5-day-old virgin males in each replicate. Observations are usually conducted from 12:00 to 14:00, the time period coinciding with the peak period of mating activity of M. saltuarius [25]. A total of 45 replicates were conducted in this experiment. All individuals in the mating test were photographed, and body weights were measured before the mating test.
2.3. Morphological Measurements
ImageJ (NIH, Bethesda, MD, USA) was used to measure morphological parameters based on photos like body length, antennal length, elytra length, elytra width, head width, abdomen length, fore femur length, fore tibia length, fore tarsal length, hind femur length, hind tibia length. All length and width measurements reported here are the maximum values. For example, body length was defined as the length between the head and the abdomen.
2.4. Data Analyses
An index of body size for both sexes was generated by a principal component analysis based on five morphological characters of body parts: body length, elytra length, elytra width, head width, and abdomen length. A scatter plot of the relationship between body weight and body size was generated. We checked the normality of morphological data and conducted a multicollinearity analysis. To determine whether these morphological features are related to each other in both sexes, Spearman correlation coefficients were evaluated, and a Spearman correlation matrix was generated. To determine whether a morphological feature differs significantly between selected and unselected individuals, independent-sample t-test was performed when data satisfied the normal distribution. For the data that did not satisfy the normal distribution, we carried out ln(x) transformation before independent-sample t-test. Mann–Whitney U test was performed for the data that still did not meet the normal distribution after data transformation. Values of p < 0.05 indicated that a morphological feature was significantly correlated with mating success. Excel 2019 (Microsoft, New York, NY, USA) and IBM SPSS 23 Statistics (IBM, Armonk, NY, USA) were used for data processing and analysis, and Origin 2023b (Origin Lab, Northampton, MA, USA) was used to draw plots.
3. Results
3.1. Sexual Selection on Female Traits
Mating was successful in all 45 replicates. Based on multicollinearity analysis, only the variance inflation factor (VIF) value for body length (10.667) was more than 10 (Table 1), so we removed it [30]. Spearman correlation coefficients showed there were positive correlations among the 12 traits measured, and the correlation coefficient between antennal length and elytra length was highest at 0.817 (Table 2). The first principal component for females explained 68.984% of the total variance and was regarded as an index of the body size of females (Table 3). For females, body size PC1 = 0.517 × body length + 0.475 × elytra length + 0.424 × elytra width + 0.443 × head width + 0.362 × abdomen length. Female body weight and female body size were positively correlated (r = 0.338, p < 0.01, n = 90 females) (Figure 1a).

Table 1.
Multicollinearity analysis for morphological traits in females.

Table 2.
Spearman correlation matrix for morphological traits in females (* p < 0.05; ** p < 0.01).

Table 3.
Component score coefficient matrix of morphological characters on the first principal component and proportions of variance explained by these components.
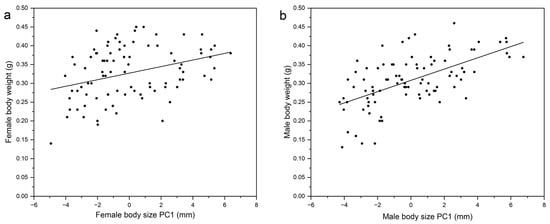
Figure 1.
Correlations between female body size PC1 and female body weight (a) and between male body size PC1 and male body weight (b).
Independent-sample t-tests revealed that significant differences existed in body weight (t = 2.010, p = 0.047), fore femur length (t = 2.335, p = 0.022) and fore tibia length (t = 2.576, p = 0.012), and extremely significant differences existed in body length (t = −3.696, p = 0.000), antennal length (t = 13.804, p = 0.000), abdomen length (t = 2.978, p = 0.004), fore tarsal length (t = 7.596, p = 0.000), hind femur length (t = 2.805, p = 0.006), and hind tibia length (t = 2.686, p = 0.009) between chosen and nonchosen females (Figure 2a,b). Mann–Whitney U test revealed that extremely significant differences existed in elytra length (z = −3.389, p = 0.001) and body size PC1 between chosen and nonchosen females (z = −3.623, p = 0.000) (Figure 2a).
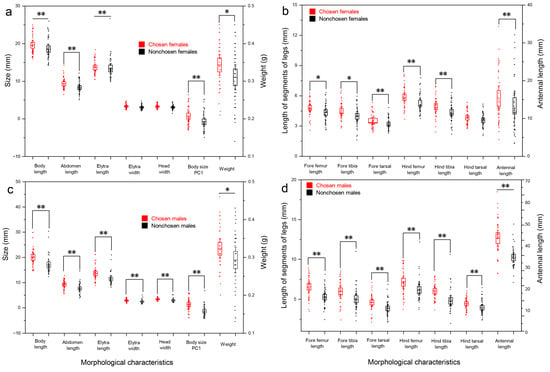
Figure 2.
Comparison of morphological characteristics between females and males that successfully mated (n = 45) and failed to mate (n = 45). (a) Female weight and morphological characteristics, (b) female leg length and antennal length, (c) male weight and morphological characteristics, and (d) male leg length and antennal length. * p < 0.05; ** p < 0.01.
3.2. Sexual Selection on Male Traits
Mating was successful in all 45 replicates. Based on multicollinearity analysis, the VIF value for the body length (48.259) and elytra length (40.091) was more than 10 (Table 4), so we removed it [30]. Spearman correlation coefficients revealed positive correlations between the 11 traits measured. The correlation coefficient was highest for the relationship between abdomen length and foretarsal length (i.e., 0.781) (Table 5). The first principal component for males explained 79.775% of the total variance and was regarded as an index of the body size of males (Table 3). For males, body size PC1 = 0.486 × body length + 0.485 × elytra length + 0.451 × elytra width + 0.462 × head width + 0.334 × abdomen length. Male body weight and male body size were positively correlated (r = 0.595, p < 0.01, n = 90 males) (Figure 1b).

Table 4.
Multicollinearity analysis for morphological traits in males.

Table 5.
Spearman correlation matrix for morphological traits in males (* p < 0.05; ** p < 0.01).
Independent-sample t-tests showed that there were extremely significant differences in body length (z = −4.305, p = 0.000), body weight (t = 2.127, p = 0.036), antennal length (t = 6.202, p = 0.000), elytra width (t = 3.120, p = 0.002), abdomen length (t = 3.960, p = 0.000), fore femur length (t = 4.738, p = 0.000), fore tibia length (t = 3.121, p = 0.002), fore tarsal length (t = 3.475, p = 0.001), hind femur length (t = 3.010, p = 0.003), hind tarsal length (t = 3.010, p = 0.003) and body size PC1 (t = 3.044, p = 0.004) between chosen and nonchosen males (Figure 2c,d). Mann–Whitney U test revealed that extremely significant differences existed in head width (z = −4.237, p = 0.000), elytra length (z = −3.240, p = 0.001), and hind tibia length (z = −2.825, p = 0.005) between chosen and nonchosen males (Figure 2c,d).
4. Discussion
In the realm of sexual competition, female preference for high-quality mates takes precedence over quantity. The result is that females can exhibit both choosiness and competitiveness in their pursuit of mates [31,32,33]. Morphological characteristics in females also play an important role in mate selection and have been shaped by sexual selection [34,35,36,37,38]. In competition for access to males, larger females may be at an advantage because they are better able to dominate other females and possess higher reproductive fitness [36].
In the hollyhock weevil Rhopalapion longirostre (Olivier), individuals with large elytra are favored by the opposite sex. Prior to mounting, the prospective male R. longirostre seeks a large female with a long elytra, and females with a larger elytra enable males to crawl better and promote mating success [39], which is consistent with our results. The elytra length showed a significant positive correlation with the elytra width and other morphological characteristics of females. In general, the larger the elytra, the larger the female traits. The larger body size of female insects appears to be attractive to males. This could be due to the male’s ability to easily perceive them or to the possibility that larger females emit more pheromones [40]. Regardless, body size is a key characteristic that can provide insight into ecological, behavioral, and evolutionary patterns. In many insect species, females are larger than males, a phenomenon known as sexual dimorphism. Scientists believe this may be due to the fact that the females are responsible for egg production [40]. One popular theory, the fecundity advantage hypothesis, posits that larger females have higher fecundity rates. These insights help advance our understanding of the diverse and complex behaviors and adaptations of insects in the natural world [9].
In some insect species, the thickness of a female’s abdomen is a dependable indicator of the quantity and growth stage of her eggs. Males can gain reproductive advantages by selecting females with thicker abdomens for mating and fertilizing their more developed eggs. This is a common occurrence in the animal kingdom, so the abdomen of the female may be an important indicator of fecundity, which makes her more attractive to males [37,38,41]. In the seed bug Nysius huttoni White [35], the cotton bollworm Helicoverpa armigera (Hübner) [41], the long-tailed dance fly Rhamphomyia longicauda Loew [37], and the Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) [42], a positive correlation exists between female abdominal size and lifetime reproductive output. Males in these species show a preference for larger abdomens in females as potential mates. These findings highlight the importance of female body size in reproductive success across different taxa. We found that the abdominal length of mated females differed significantly from that of unmated females (Figure 2a), and the abdominal length of females was significantly positively correlated with the elytra length and elytra width (Table 2). Therefore, mate selection based on female abdomen size in this species via male orientation can be attributed to the fact that this feature is a reliable indicator of the lifetime reproductive success of females.
Many morphological characteristics in males play an important role in mate selection and are sexually selected [10,34,35,43,44]. Morphological features can be used as weapons in male competition [43,44,45,46,47,48,49,50] or to attract females [51,52,53,54]. Insect precopulatory interactions involve frequent contact on the antenna and legs. In many species, males with larger body sizes [10,43,55], longer antennal [35,43] and legs [44], and longer wings [56] may have better opportunities to mate.
In addition, there is some evidence that larger males have better genes and a larger ejaculatory supply than those of smaller males [57,58,59,60]. In certain insect species, males exhibit a preference for mating with larger females due to their increased ability to dominate other females and higher reproductive fitness [61]. For instance, Micrarchus hystriculeus (Westwood), a sexually dimorphic stick insect species, displays a positive correlation between body size in female–male pairs collected in the field. In some species, larger male individuals have the opportunity to copulate with females of various sizes, while small males primarily mate with small females [62]. This can occur because large females do not choose to mate with small males, and large males may physically be unable to mate with small females. Alternatively, if mating occurs in a confined space, it is possible for the body size of both participants to be restricted [63]. Mating with larger males may result in the production of larger offspring for females, potentially leading to better reproductive success through their offspring. In the three species in the genus Drosophila, D. bipectinata (Duda), D. rajasekari (Joshi), and D. nasuta Lamb, females showed a preference for mating with large males, regardless of their own size. This highlights the advantage that large males have in intrasexual competition. Female selection criteria appeared to be based on relative size alone, favoring the larger males. These findings suggest that size plays an important role in mate selection among these species of fruit flies [64]. Further research indicates that male Acheta domesticus (L.) exhibit a preference for larger females, which may be due to the increased detection of pheromones emitted by these females [65]. The size of an insect’s mate plays a crucial role in determining its reproductive success. Certain species exhibit selective behavior in choosing their mates, with large females having longer lifespans and larger males having a higher likelihood of mating. In situations where larger females are in competition, larger males tend to emerge victorious and mate with them, leaving smaller males with the opportunity to mate with less contested, smaller females. These factors allow for variations in reproductive success within individual insect populations.
The secondary sexual characteristics of insects in the mating process are generally sexually dimorphic (i.e., they are generally larger in males than in females [42]. Many species of longicorn beetles (Coleoptera Cerambycidae) often display sexual dimorphism in their antennal length and in the ratio of antennal length/body size. Typically, males have longer antennal than females and a higher ratio of antennal length/body size [66,67,68]. Antennal is a major secondary sexual trait of M. saltuarius. The length or structural dimorphism of antennae is common in species that use air pheromones to find a mate [69]. Male antennal play an important role in female choice and competition between males. For males, the obvious selective advantage of longer antennal is the effect on mating opportunities [67]. For example, in Cnephasia jactatana Walker (Lepidoptera: Tortricidae), females significantly preferred males with longer antennal for mating [43]. Hanks et al. [67] found that larger males of Phoracantha semipunctata (Fabricius) (Cerambycidae) with longer antennal, were able to detect females more efficiently than smaller males due to their ability to sweep a larger area. Larger males were also more successful in aggressive contests for mating partners. Research regarding the antenna morphology of Pseudomantis albofimbriata (Stal) suggests that this may also affect the speed at which males are able to locate females [70]. In mating systems based on scramble competition, the ability to efficiently locate mates can greatly increase male mating success, with faster males potentially gaining an advantage over slower males in this regard [70]. Previous research has demonstrated the importance of male cricket antennal in initiating courtship behavior, facilitating copulation, and allowing for the ability to engage in mate guarding. These findings have implications for understanding the mating strategies of these insects [71,72]. According to Svensson [73], male moths with greater sensitivity to pheromones are more likely to find mates, implying that the length of their antennal is under significant sexual selection pressure. We detected a significant difference in the length of antennal between selected and unselected males (Figure 2c).
The male leg also contributes to insect mating. The harlequin beetle, Acrocinus longimanus (L.) have enlarged forelegs that flank females during mating and mate guarding. This is important to note as it contributes to the unique mating behavior of these beetles [74]. In some species, the hind leg plays an important role in mate selection; for example, in the ambush bug (Heteroptera: Reduviidae), sexual selection appears to act on the length of the male hind leg [75]. In Pachyrhamma waitomoensis Richards (Orthoptera: Rhaphidophoridae), males with the longest hind legs could accrue approximately triple the number Phymata wolffii Stal of copulations [76]. Female bean bugs Riptortus pedestris (Fabricius) prefer males with higher frequencies of courtship display and larger hind legs, which indicate better abilities to compete for mates. This information is important for understanding the reproductive behavior of R. pedestris [77]. During copulation, male M. saltuarius stroke their legs along the edges of the female elytra. This behavior is crucial for sperm precedence and paternity success, as previous research has shown that female perception of this leg-rubbing plays a key role. Overall, this information is part of a student assignment aimed at enhancing their understanding of reproductive behavior in M. saltuarius. For example, in Tribolium castaneum (Herbst), when females mate with two males, leg amputation in males reduces the number of offspring significantly [78]. Sexual dimorphism in R. longirostre is attributed to the interplay of two forces: natural selection, favoring larger females, and sexual selection, which stabilizes the size of male hind legs. This pattern has been observed and studied in this species [39]. In Nicrophrus orbicollis Say, the length of the male tarsus (especially the fore tarsus) is selected by females [79]. Kelly et al. [80] showed that females of Deinacrida rugosa Buller tend to choose males with smaller body sizes and longer legs, as this phenotype clearly has an advantage with respect to competition for mates. In many species of insects, legs have evolved to carry individuals through their surroundings, but in certain instances, leg length is also a subject of sexual selection [81]. Our observations show that to mate successfully, three pairs of legs behind the female are needed to ensure that the female is firmly grasped and that the male genitalia are inserted appropriately. This also prevents the male from falling off during the mating process, resulting in interrupted mating [26]. Therefore, male M. saltuarius with longer legs may be more capable of grasping females, which may explain our observation that leg length is significantly related to mating success.
Our results indicate that differences in the female abdomen size and male antenna length, as well as other traits, may reliably reflect variance in reproductive fitness in M. saltuarius. These traits differed significantly between selected and unselected individuals and may have evolved under sexual selection. The differences in key traits among species suggest that sexual selection acts on different traits in different species, probably due to diverse mating systems [82].
5. Conclusions
M. saltuarius is a pest that causes significant damage to pine forests in temperate regions of China, and the losses associated with this species are expected to increase in the near future. We studied mate selection behavior in M. saltuarius and the effects of various morphological characteristics on mating preferences. We detected positive correlations between female and male morphological characteristics. Furthermore, we observed significant differences in most morphological characteristics between selected and unselected females and males. The differences between selected and unselected males were significant in all the morphological characters measured, and we refer to previous research to demonstrate the role of body size, elytra length, antennal length, and leg length in insect mating. The differences between selected and unselected females were significant in the morphological characteristics of the abdomen and the leg, and we also refer to previous research to demonstrate the role of the abdomen and the leg in the mating of insects. We also refer to previous research to demonstrate the role of abdominal length and leg length in insect mating.
Author Contributions
Conceptualization, S.Z. and Z.H.; methodology, Z.H. and C.Z.; software, Z.H. and C.Z.; validation, S.Z., Z.H., C.Z. and H.W.; formal analysis, C.Z.; investigation, C.Z.; resources, H.W.; data curation, C.Z.; writing—original draft preparation, C.Z.; writing—review and editing, Z.H. and S.Z.; visualization, C.Z.; supervision, S.Z. and Z.H.; project administration, Z.H.; funding acquisition, Z.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 32301293) (for Z.H.).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to express our deep gratitude to Fengming Shi and Yiming Niu (Beijing Forestry University), and three anonymous workers at Dahuofang Forest Farm, Fushun City, Liaoning Province, China for their help with specimen collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Darwin, C.R. The Descent of Man, and Selection in Relation to Sex; John Murray: London, UK, 1871. [Google Scholar]
- Jennions, M.D.; Petrie, M. Variation in Mate Choice and Mating Preferences: A Review of Causes and Consequences. Biol. Rev. 1997, 72, 283–327. [Google Scholar] [CrossRef]
- Trivers, R.L. Parental Investment and Sexual Selection; Aldine Publishing: Chicago, IL, USA, 1972. [Google Scholar]
- Tregenza, T.; Wedell, N. Genetic Compatibility, Mate Choice and Patterns of Parentage: Invited Review. Mol. Ecol. 2000, 9, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Edward, D.A.; Chapman, T. The Evolution and Significance of Male Mate Choice. Trend. Ecol. Evol. 2011, 26, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Pollo, P.; Nakagawa, S.; Kasumovic, M.M. The Better, the Choosier: A Meta-Analysis on Interindividual Variation of Male Mate Choice. Ecol. Lett. 2022, 25, 1305–1322. [Google Scholar] [CrossRef] [PubMed]
- Bertram, S.M.; Loranger, M.J.; Thomson, I.R.; Harrison, S.J.; Ferguson, G.L.; Reifer, M.L.; Gowaty, P.A. Choosy Males in Jamaican Field Crickets. Anim. Behav. 2017, 133, 101–108. [Google Scholar] [CrossRef]
- Bateman, A.J. Intra-sexual Selection in Drosophila. Heredity 1948, 2, 349–368. [Google Scholar] [CrossRef]
- Andersson, M. Sexual Selection; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Danielsson, I. Antagonistic Pre- and Post-copulatory Sexual Selection on Male Body Size in a Water Strider (Gerris lacustris). Proc. R. Soc. Lond. B. Biol. Sci. 2001, 268, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Marescalchi, O.; Francardi, V.; Mantovani, B. Taxonomy and Phylogeny of European Monochamus species: First Molecular and Karyological Data. J. Zool. Syst. Evol. Res. 2005, 43, 1–7. [Google Scholar] [CrossRef]
- Hu, S.; Sun, M.; Luo, L.P. Research Progress on Bioecological Characteristics and Control Measures of Monochamus saltuarius. Forest Sci. Technol. 2023, 11, 27–31. [Google Scholar]
- Zhao, S.G. Advances in Studies on Biological and Ecological Characteristics of Monochamus saltuarius. Forest Pest and Disease 2021, 40, 37–43. [Google Scholar]
- Shi, Y.; Zhang, Y.L.; Wang, J.; Zheng, Y.A. Distribution Rule of Monochamus saltuarius Larvae in the Trunk of Pinus koraiensis. Forest Res. 2022, 58, 128–133. [Google Scholar]
- Pan, J.L.; Li, J.; Dong, Y.Q. Feeding Preference of Monochamus saltuarius Gebler (Coleoptera: Cerambycidae) for Pinus koraiensis, Pinus tabulaeformis and Larix kaempferi. Forest Pest and Disease 2020, 39, 19–22. [Google Scholar]
- Li, M.; Li, H.; Sheng, R.C.; Sun, H.; Sun, S.H.; Chen, F.M. The First Record of Monochamus saltuarius (Coleoptera; Cerambycidae) as Vector of Bursaphelenchus xylophilus and Its New Potential Hosts in China. Insects 2020, 11, 636. [Google Scholar] [CrossRef]
- Wu, Y.; Wickham, J.D.; Zhao, L.; Sun, J. CO2 Drives the Pine Wood Nematode off Its Insect Vector. Curr. Biol. 2019, 29, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.R. Epidemic Status of Pine Wilt Disease in China Prevention and Control Techniques and Counter Measures. Forest Res. 2019, 55, 1–10. [Google Scholar]
- Kwon, H.J.; Jung, J.K.; Jung, C.; Han, H.; Koh, S.H. Dispersal Capacity of Monochamus saltuarius on Flight Mills. Entomol Exp. Appl. 2018, 166, 420–427. [Google Scholar] [CrossRef]
- Hou, Z.; Shi, F.; Ge, S.; Tao, J.; Ren, L.; Wu, H.; Zong, S. Comparative Transcriptome Analysis of the Newly Discovered Insect Vector of the Pine Wood Nematode in China, Revealing Putative Genes Related to Host Plant Adaptation. BMC Genom. 2021, 22, 189. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Shi, F.M.; Pei, J.H.; Hou, Z.H.; Zong, S.X.; Ren, L.L. Gut Bacteria Associated with Monochamus saltuarius (Coleoptera: Cerambycidae) and Their Possible Roles in Host Plant Adaptations. Front. Microbiol. 2021, 12, 211–687. [Google Scholar] [CrossRef]
- Niu, Y.; Zhao, Y.; Shi, F.; Li, M.; Zhang, S.; Yang, J.; Zong, S.; Tao, J. An Efficient and Simple Method for Collecting Haemolymph of Cerambycidae (Insecta: Coleoptera) Adults. Insects 2023, 14, 29. [Google Scholar] [CrossRef]
- Pan, L.; Li, Y.X.; Cui, R.; Lui, Z.K.; Zhang, X.Y. Monochamus saltuarius Endangers Pinus tabuliformis Carr. and Carries Bursaphelenchus xylophilus (Steiner and Buhrer) in China. Forests 2020, 11, 1051. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhang, X.Y. Analysis on the Trend of Invasion and Expansion of Bursaphelenchus xylophilus. Forest Pest and Disease 2018, 37, 1–4. [Google Scholar]
- Wang, J.; Shi, Y.; Fan, L.C.; Zhang, Y.L.; Zheng, Y.N. Reproductive Behavior of Monochamus saltuarius (Coleoptera: Cerambycidae). Forest Res. 2023, 36, 22–30. [Google Scholar]
- Kim, M.; Kim, J.; Han, J.H.; Kim, Y.J.; Yoon, C.; Kim, G.H. Mating Behavior of Pine Sawyer, Monochamus saltuarius Gebler (Coleoptera: Cerambycidae). J. Asia. Pac. Entomol. 2006, 9, 275–280. [Google Scholar] [CrossRef]
- Yilmaz, C.; Genc, H. Egg Production and Adult Longevity of The Olive Leaf Moth, Palpita unionalis Hübner (Lepidoptera: Pyralidae) on Selected Adult Diets. J. Tekirdag Agric. Fac. 2012, 9, 1–5. [Google Scholar]
- Jung, J.K.; Kwon, H.; Kim, J.; Nam, Y.; Kim, D.; Jung, C. Changes in Catch Rate of Monochamus saltuarius (Coleoptera: Cerambycidae) Relation to Sexual Maturation. Korean J. Appl. Entomol. 2020, 59, 295–301. [Google Scholar]
- Jikumaru, S.; Togashi, K.; Taketsune, A.; Takahashi, F. Oviposition Biology of Monochamus saltuarius (Coleoptera: Cerambycidae) at a Constant Temperature. Appl. Entomol. Zool. 1994, 29, 555–561. [Google Scholar] [CrossRef]
- Liu, L.H.; Qiu, X.Y. On the Factors Affecting the Willingness to Choose the Inherent Level of Right of Landless Farmers’ ‘Empowerment’ Mechanism. Sci. Technol. Manag. Land Resour. 2023, 40, 50–65. [Google Scholar]
- Halliday, T. The Study of Mate Choice. In Mate Choice; Bateson, P., Ed.; C.U.P.: Cambridge, UK, 1983; pp. 3–32. [Google Scholar]
- Petrie, M. Female Moorhens Compete for Small Fat Males. Science 1983, 220, 413–415. [Google Scholar] [CrossRef]
- Owens, I.P.F.; Burke, T.; Thompson, D.B.A. Extraordinary Sex-Roles in the Eurasian dotterel—Female Mating Arenas, Female-Female Competition, and Female Mate Choice. Am. Nat. 1994, 144, 76–100. [Google Scholar]
- Wang, Q.; Zeng, W.Y. Sexual Selection and Male Aggression of Nadezhdiella cantori (Hope) (Coleoptera: Cerambycidae: Cerambycinae) in Relation to Body Size. Environ. Entomol. 2004, 33, 657–661. [Google Scholar] [CrossRef]
- Yang, L.H.; Wang, Q. Precopulation Sexual Selection in Nysius huttoni White (Heteroptera: Lygaeidae) in Relation to Morphometric Traits. J. Insect. Behav. 2004, 17, 695–707. [Google Scholar] [CrossRef]
- Stuart-Smith, J.; Swain, R.; Wapstra, E. The Role of Body Size in Competition and Mate Choice in an Agamid with Female-biased Size Dimorphism. Behaviour 2007, 144, 1087–1102. [Google Scholar]
- Bussiere, L.F.; Gwynne, D.T.; Brooks, R. Contrasting Sexual Selection on Males and Females in a Role-Reversed Swarming Dance Fly, Rhamphomyia longicauda Loew (Diptera: Empididae). J. Evol. Biol. 2008, 21, 1683–1691. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Q. Form and Nature of Premating Sexual Selection in Both Sexes of a Moth. Naturwissenschaften 2010, 97, 617–625. [Google Scholar] [CrossRef]
- Wilhelm, G.; Handschuh, S.; Plant, J.; Nemeschkal, H.L. Selection Becomes Visible: Enforced Sexual Dimorphism Caused by Sexual Selection in the Weevil Rhopalapion longirostre (Olivier 1807) (Coleoptera: Curculionoidea: Brentidae). Biol. J. Linn. Soc. 2015, 115, 38–47. [Google Scholar] [CrossRef]
- Sisodia, S.; Singh, B.N. Size Dependent Sexual Selection in Drosophila ananassae. Genetica 2004, 121, 207–217. [Google Scholar] [CrossRef]
- Li, Z.; Li, D.; Xie, B.; Ji, R.; Cui, J. Effect of Body Size and Larval Experience on Mate Preference in Helicoverpa armigera (Hübner) (Lep., Noctuidae). J. Appl. Entomol. 2005, 129, 574–579. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Q. Male Moths Undertake Both Pre- and In-copulation Mate Choice Based on Female Age and Weight. Behav. Ecol. Sociobiol. 2009, 63, 801–808. [Google Scholar] [CrossRef]
- Jiménez-Pérez, A.; Wang, Q. Sexual Selection in Cnephasia jactatana (Lepidoptera: Tortricidae) in Relation to Age, Virginity, and Body Size. Ann. Entomol. 2004, 97, 819–824. [Google Scholar] [CrossRef]
- Willemart, R.H.; Osses, F.; Chelini, M.C.; Macias-Ordonez, R.; Machado, G. Sexually Dimorphic Legs in a Neotropical Harvestman (Arachnida, Opiliones): Ornament or Weapon? Behav. Proc. 2009, 80, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, A.; Alcock, J. Female Mimicry and Resource Defense Polygyny by Males of a Tropical Rove Beetle, Leistotrophus versicolor (Coleoptera: Staphylinidae). Behav. Ecol. Sociobiol. 1990, 26, 325–330. [Google Scholar] [CrossRef]
- Eberhard, B.; Gutiérrez, E. Male Dimorphisms in Beetles and Earwigs and the Question of Developmental Constraints. Evolution 1991, 45, 18–28. [Google Scholar] [CrossRef]
- Simmons, W.L.; Tomkins, L.J. Sexual Selection and the Allometry of Earwig Forceps. Evol. Ecol. 1996, 10, 97–104. [Google Scholar] [CrossRef]
- Palestrini, C.; Rolando, A.; Laiolo, P. Allometric Relationships and Character Evolution in Onthophagus taurus (Coleoptera: Scarabaeidae). Can. J. Zool. 2000, 78, 1199–1206. [Google Scholar] [CrossRef]
- Hanley, R. Mandibular Allometry and Male Dimorphism in a Group of Obligately Mycophagous Beetles (Insecta: Coleoptera: Staphylinidae: Oxyporinae). Biol. J. Linn. Soc. 2001, 72, 451–459. [Google Scholar] [CrossRef][Green Version]
- Tatsuta, H.; Mizota, K.; Akimoto, S. Allometric Patterns of Heads and Genitalia in the Stag Beetle Lucanus maculifemoratus (Coleoptera: Lucanidae). Ann. Entomol. Soc. Am. 2001, 94, 462–466. [Google Scholar] [CrossRef]
- Kelly, C.D. Allometry and Sexual Selection of Male Weaponry in Wellington Tree Weta, Hemideina crassidens. Behav. Ecol. 2005, 16, 145–152. [Google Scholar] [CrossRef][Green Version]
- Cothran, R.D.; Jeyasingh, P.D. Condition Dependence of a Sexually Selected Trait in a Crustacean Species Complex: Importance of the Ecological Context. Evolution 2010, 64, 2535–2546. [Google Scholar] [CrossRef] [PubMed]
- Alatalo, R.V.; Höglund, J.; Lundberg, A. Patterns of Variation in Tail Ornament Size in Birds. Biol. J. Linn. Soc. 1988, 34, 363–374. [Google Scholar] [CrossRef]
- Baker, R.; Wilkinson, G. Phylogenetic Analysis of Sexual Dimorphism and Eye-span Allometry in Stalk-eyed Flies (Diopsidae). Evolution 2001, 55, 1373–1385. [Google Scholar]
- Partridge, L.; Hoffman, A.; Jones, J.S. Male Size and Mating Success in Drosophila melanogaster and D. pseudoobscura under Field Conditions. Anim. Behav. 1987, 35, 468–476. [Google Scholar]
- Nijhout, H.F. The Development and Evolution of Butterfly Wing Patterns; Smithsonian: Washington, DC, USA, 1991. [Google Scholar]
- Phelan, P.L.; Barker, J.C. Male Size Related to Courtship Success and Intersexual Selection on Tobacco Moth Ephesia cautella. Experientia 1986, 42, 1291–1293. [Google Scholar] [CrossRef]
- Kempenaers, B.; Verheyen, G.R.; Vandenbroeck, M.; Burke, T.; Vanbroeckhoven, C.; Dhondt, A. Extra-pair Paternity Results from Female Preference for High-quality Males in the Blue Tit. Nature 1992, 357, 494–496. [Google Scholar] [CrossRef]
- Keller, L.; Reeve, H.K. Why do Females Mate with Multiple Males? The Sexually Selected Sperm Hypothesis. Adv. Stud. Behav. 1995, 24, 291–315. [Google Scholar]
- Bissoondath, C.J.; Wiklund, C. Effect of Male Mating History and Body Size on Ejaculate Size and Quality in two Polyandrous Butterflies, Pieris napi and Pieris rapae (Lepidoptera: Pieridae). Funct. Ecol. 1996, 10, 457–464. [Google Scholar] [CrossRef]
- Kraak, S.B.M.; Bakker, T.C.M. Mutual Mate Choice in Sticklebacks: Attractive Males Choose Big Females, which Lay Big Eggs. Anim. Behav. 1998, 56, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.D. Sexual Selection, Phenotypic Variation, and Allometry in Genitalic and Non-genitalic Traits in the Sexually Size-dimorphic Stick Insect Micrarchus hystriculeus. Biol. J. Linn. Soc. 2014, 113, 471–484. [Google Scholar] [CrossRef]
- Brown, W.D. The Cause of Size-Assortative Mating in the Leaf Beetle Trirhabda canadensis (Coleoptera: Chrysomelidae). Behav. Ecol. Sociobiol. 1993, 33, 151–157. [Google Scholar] [CrossRef]
- Krishna, M.S.; Hegde, S.N. Influence of Body Size in Mating Success in three Sympatric Species of Drosophila. Ital. J. Zool. 2003, 70, 47–52. [Google Scholar] [CrossRef]
- Khadka, K.K.; Shek, J.; Hoffman, J.; Vulin, R.; Foellmer, M. Longer Antennal for Romeo: Assessing Effect of Antennal Length on Courtship and Mating Success in Male Crickets, Acheta domesticus (Orthoptera, Gryllidae). J. Insect Behav. 2012, 25, 96–103. [Google Scholar] [CrossRef]
- Sama, G. Coleoptera Cerambycidae. In Catalogo Topografico e Sinonimico; Calderini: Bologna, Italy, 1988. [Google Scholar]
- Hanks, L.M.; Millar, J.G.; Paine, T.D. Body Size Influences Mating Success of the Eucalyptus Longhorned Borer (Coleoptera: Cerambycidae). J. Insect Behav. 1996, 9, 369–382. [Google Scholar] [CrossRef]
- Saikia, K.; Thakur, N.S.A.; Ao, A.; Gautam, S. Sexual Dimorphism in Pseudonemorphus versteegi (Ritsema) (Coleoptera: Cerambycidae), Citrus Trunk Borer. Fla. Entomol. 2012, 95, 625–629. [Google Scholar] [CrossRef]
- Holwell, G.I.; Barry, K.L.; Herberstein, M.E. Mate Location, Antennal Morphology, and Ecology in Two Praying Mantids (Insecta: Mantodea). Biol. J. Linn. Soc. 2007, 91, 307–313. [Google Scholar] [CrossRef]
- Jayaweera, A.; Barry, K.L. Male Antenna Morphology and Its Effect on Scramble Competition in False Garden Mantids. Sci. Nat. 2017, 104, 75. [Google Scholar] [CrossRef] [PubMed]
- Dulling the Senses Ryan, K.M.; Sakaluk, S.K. The Role of Antennal in Materecognition, Copulation and Mate Guarding in Decorated Crickets. Anim. Behav. 2009, 77, 1345–1350. [Google Scholar] [CrossRef]
- Murakami, S.; Itoh, M.T. Removal of both Antennal Influences the Courtship and Aggressive Behaviors in Male Crickets. J. Neurobiol. 2003, 57, 110–118. [Google Scholar] [CrossRef]
- Svensson, M. Sexual Selection in Moths: The Role of Chemical Communication. Biol. Rev. Cambridge Phil. Soc. 1996, 71, 113–135. [Google Scholar] [CrossRef]
- Zeh, D.W.; Zeh, J.A. Sexual Selection and Sexual Dimorphism in the Harlequin Beetle Acrocinus longimanus. Biotropica 1992, 24, 86–96. [Google Scholar] [CrossRef]
- McLain, D.K.; Boromisa, R.D. Stabilizing Sexual Selection and Density-dependent Correlates of Copulatory Success in the Ambush Bug Phymata wolffii (Hemiptera: Reduviidae). Am. Midl. Nat. 1987, 118, 94–102. [Google Scholar] [CrossRef]
- Fea, M.; Holwell, G.I. Exaggerated Male Legs Increase Mating Success by Reducing Disturbance to Females in the Cave wētā Pachyrhamma waitomoensis. Proc. Royal Soc. B. 2018, 285, 20180401. [Google Scholar] [CrossRef]
- Suzaki, Y.; Katsuki, M.; Miyatake, T.; Okada, Y. Male Courtship Behavior and Weapon Trait as Indicators of Indirect Benefit in the Bean Bug, Riptortus pedestris. PLoS ONE 2013, 8, e83278. [Google Scholar] [CrossRef]
- Edvardsson, M.; Arnqvist, G. Copulatory Courtship and Cryptic Female Choice in Red Flour Beetles Tribolium castaneum. Proc. R. Soc. Lond. B. 2000, 267, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Trume, S.T.; Sike, D.S. Sexual Selection and Leg Morphology in Nicrophorus orbicollis and Ptomascopus morio. Entomol. Sci. 2000, 3, 585–589. [Google Scholar]
- Kelly, C.D.; Bussiere, L.F.; Gwynne, D. Sexual Selection for Male Mobility in a Giant Insect with Female-biased Size Dimorphism. Am. Nat. 2008, 172, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Bonduriansky, R. Sexual Selection and Allometry: A Critical Reappraisal of the Evidence and Ideas. Evolution 2007, 61, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wang, Q.; Xu, J.; Lv, J.; Qin, A.Z. Mating Behavior and Sexual Selection in a Polygamous Beetle. Curr. Zool. 2013, 59, 257–264. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).