Abstract
Global climate change creates new environmental scenarios and selective pressures; thus, a better understanding of the plasticity of plant functional traits is needed to predict how plant species will respond to shifts in climate. Among the important functional traits for plants are their hydraulic properties which ultimately determine their photosynthetic capacity, growth rate, and survival in a changing environment. In this study, the light sensitivity of leaf (KL) and branch hydraulic conductance (KB) to fast changes in irradiance, and hydraulic plasticity (PIh) was studied in two broadleaved tree species differing in water-use strategy—silver birch (Betula pendula) and hybrid aspen (Populus × wettsteinii). The KL increased by a factor of 3.5 and 1.5 from minimal values recorded in darkness to maximal values in high light conditions for birch and aspen, respectively, indicating a significantly higher PIh for birch (0.72) than for aspen leaves (0.35). KB increased 1.5-fold from dark to light conditions for both species. The high light sensitivity of KL and KB provides a regulatory mechanism to maintain a balance between transpirational demand and hydraulic supply. The plasticity of these traits increases the ability of plants to cope with a rapidly changing environment and to adapt to global climate change.
1. Introduction
Today, plant scientists solidly acknowledge the integral role of water transfer in soil–plant–atmosphere systems (i.e., plant hydraulics) in understanding plant and ecosystem functioning [1,2]. Water is an essential resource that all living organisms need for existence. On a global scale, water availability is an important limiting factor for plants, determining the productivity of crops and forests that humans rely on [3,4]. The structure of the water transport system places a physical limit on plant functioning, as the efficiency of water transport through their bodies determines their leaf water status, photosynthetic capacity, and growth rate [5,6,7]. Thus, plant hydraulic properties are fundamental determinants of plant fitness and their competitiveness and survival in plant communities. Moreover, hydraulic traits mediate the ways in which plants interact with their abiotic and biotic environments [1].
Leaf hydraulic conductance (KL) is one of the most important physiological parameters in plant–water relations. It is the measure of water flow efficiency through the leaf (from stem to the sites of evaporation) and is defined as the water flow rate through the leaf per water potential difference across the leaf and generally expressed on a leaf area basis [8]. Leaves comprise more than 1/3 of the total hydraulic resistance of a plant, thus representing a major bottleneck of the water transport pathway in plants [8,9,10]. Any changes in leaf hydraulic efficiency will have a significant impact on the water balance of the whole plant.
Plants, as sessile organisms inhabiting temporally and spatially variable environments, present a sizeable capacity to acclimate to their growth environment to survive, grow, compete, and reproduce successfully [11]. Phenotypic plasticity is considered one of the major means by which plants can cope with heterogeneous and variable conditions [12]. By influencing the organisms’ fitness and competitive ability, phenotypic plasticity plays a role in the survival of individuals and persistence of populations under changing climate conditions [13,14,15]. Phenotypic plasticity is defined as the capacity of a genotype to produce different phenotypes in response to environmental conditions [13,16]. A vast number of studies show that plants are plastic in numerous functionally important traits, including morphology, physiology, and anatomy [16,17], and plastic adjustments occur in response to changes in various environmental factors, such as water availability, light gradients, temperature, atmospheric CO2 concentration, pollution, or combinations of global change drivers [18]. In terms of future perspectives, it is important to identify plant functional traits, the plasticity of which may play a determinant role in plant acclimation to global climate change [12].
Global climate change creates considerable geographical variability in climate, in which trends are different on a regional scale. For instance, although an increasing incidence of climate extremes is predicted for the whole northern hemisphere, future climate scenarios predict wetter conditions at high latitudes, including increasing precipitation in northern Europe, while southern Europe will experience substantial warming and drying [19]. Climate change scenarios for the year 2100 predict an increase in air temperature (by 2.3–4.5 °C) and precipitation (by 5–30%) in the Baltic region [20]. The rising amount and frequency of rainfall will increase soil water content, as well as air relative humidity (RH), on a local scale [21,22,23], especially within forest canopies [24]. Changes in precipitation and weather patterns will have significant consequences on the growth of trees and the functioning of forest ecosystems [25,26,27]. Results from the Free Air Humidity Manipulation (FAHM) experiment show that under increasing atmospheric humidity, trees experience less water loss and there is weaker environmental pressure to invest resources into developing effective water conducting tissues, resulting in reduced hydraulic conductance in leaves [28,29]. In the case of climate extremes (heat waves, severe droughts), which have been predicted to become more frequent during the 21st century across Europe, reduced hydraulic efficiency might represent a potential threat in hemi-boreal forest ecosystems [30]. Understanding which hydraulic traits are phenotypically plastic and to what extent is essential for understanding how tree species will respond to shifts in climate [31].
KL is a highly plastic trait, showing great variability and dynamics both between and within species, on different timescales, and in response to various environmental factors [8]. Light is one of the most important environmental factors besides water availability and temperature that influences leaf hydraulic conductance. KL has been shown to exhibit sensitivity to irradiance, e.g., [32,33,34,35], but the magnitude of the light response (i.e., plasticity) varies between species [36,37]. To our knowledge, only a few studies have specifically quantified the hydraulic plasticity of different species. Scoffoni et al. [38] studied the plasticity of KL in differential growth light environments in endemic Hawaiian lobeliads and found species native to higher irradiance to show greater hydraulic plasticity. In one of our previous studies [39], we quantified the intracanopy plasticity of KL to incident light in common hazel (Corylus avellana L.) and found sun-exposed leaves to be considerably more plastic than leaves growing in deep shade. In the context of global climate change, a better understanding of the plasticity of plant functional traits is necessary to predict the adaptations of forest ecosystems to future conditions and to elaborate appropriate management practices. As plant hydraulics represents a central hub integrating plant and ecosystem function, greater hydraulic plasticity (i.e., a fast and responsive adjustment of hydraulic conductance depending on environmental factors) is likely to allow trees to adapt more easily to climate change. Thus, it is important to investigate the variation of the plasticity of plant hydraulic traits responsible for adequate leaf water supply in more detail.
In this study, light sensitivity and plasticity of KL was measured in silver birch (Betula pendula Roth) and hybrid aspen (Populus × wettsteinii Hämet-Ahti). Both species are considered typical light-demanding fast-growing species that are able to quickly colonise disturbed sites [40]. Silver birch is commercially the most important fast-growing early-successional tree species widely distributed in northern Europe [41]. Hybrid aspen is the fastest-growing deciduous tree species in the boreal zone and is commercially a valuable and promising species for short-rotation plantation forestry in northern Europe [42]. These two species were chosen for this study primarily because they grow at the study site. From an ecological perspective, these species were suitable for this study because they are similar in their geographical distribution and commercial importance, but at the same time they differ in their water-use strategy and thus might react to and cope with climate change differently. Silver birch represents an isohydric species with a conservative water-use strategy [43,44,45], whereas aspen inclines to anisohydric behaviour with a prodigal water-use strategy [40,45,46,47]. The objective of this study was to test whether and to what extent the plasticity of KL to incident light differs between tree species with contrasting water-use strategies. Evidence is also presented on the light-mediated short-term modulation of branch hydraulic conductance. The results of this research will improve forest management in the future by broadening the understanding of the ability of trees to acclimate in changing environmental conditions and by providing additional information to maintain ecologically stable, diverse, and productive ecosystems. Hydraulically plastic tree species are more likely to adapt to a rapidly fluctuating environment. Hence, through forest selection and the identification of plastic genotypes, it is possible to shape the development of forest ecosystems that would be more stable against climate change.
2. Materials and Methods
2.1. Study Site and Sample Trees
This study was performed in the summer of 2019 at the Free Air Humidity Manipulation (FAHM) experimental site situated at Rõka village (58°14′ N, 27°17′ E, 40–48 m ASL) in eastern Estonia. The site belongs to the hemi-boreal forest zone and to the maritime to continental transitional climate zone. The mean annual precipitation in this area is 650 mm, with a mean air temperature of 17.0 °C in July and −6.7 °C in January. The growing period lasts 175–180 days, from mid-April to October. The total annual global short-wave radiation in the region averages 3518 MJ m−2, and the annual radiation budget is 2552 MJ m−2 [48]. The soil type at the site is fertile Endogleyic Planosol (WRB).
Based on climate change scenarios that predict increasing precipitation and concomitant rise in air relative humidity for northern Europe [19,49,50], the FAHM facility was established to investigate the effects of increasing air humidity on tree performance and the functioning of a deciduous forest ecosystem [51]. The experimental design and technical setup are described in detail by Kupper et al. [51], Tullus et al. [52], and Sellin et al. [53].
This study was performed on excised shoots of seven-year-old trees of silver birch (Betula pendula Roth clonal plants micropropagated from meristem cultures that originated from southern Finland, Vehmersalmi (62°45′ N, 28°10′ E); [54]) and six-year-old trees of hybrid aspen (Populus × wettsteinii Hämet-Ahti; root and stump regenerated offspring of clone C05-99–34 micropropagated from meristem culture) growing at the FAHM site. For hydraulic measurements, 252 shoots (14 shoots × 9 repetitions × 2 species) in total were sampled.
In the evening, prior to the measurement day, 14 birch (average length 28.0 cm) or aspen (20.5 cm) shoots were cut, recut under water to avoid airseeding of the shoot xylem, and inserted into test tubes with their basal ends submerged in water. Shoots were then transported to the laboratory and put into plastic flasks filled with deionised, filtered (Direct-Q3 UV water purification system; Millipore SAS, Molsheim, France), and freshly degassed water (T-04-125 ultrasonic-vacuum degasser; Terriss Consolidated Industries, Asbury Park, NJ, USA). Shoots were then left to rehydrate overnight in a dark room until the hydraulic measurements were performed the next day.
2.2. Measuring Hydraulic Conductance
Hydraulic measurements were carried out by applying the water perfusion method using a high-pressure flow meter (HPFM; Dynamax, Houston, TX, USA) applied in quasi-steady state mode [32]. In the morning, the first two shoots were brought sequentially from the dark room and immediately measured for hydraulic conductance under dark conditions (light treatment dark-dark, DD). The shoots were recut under water and immediately attached to the HPFM apparatus. Water was perfused into the shoot until hydraulic conductance values became constant. Then, the leaves were removed with a razor blade, and the perfusion continued until again values became constant. Next, another two shoots were brought sequentially from the dark room and sampled for hydraulic conductance under dark conditions. Subsequently, the light was switched on, and the same shoots were sampled under light (PPFD 900–1000 μmol m−2 s−1) conditions (light treatment dark-light, DL). Then, the light was switched off again, until the readings in the darkness became constant again (light treatment light-dark, LD). The leaf blades were then removed, and new constant values were again achieved. The remaining 10 shoots to be sampled that day were brought into the light at the same time (light treatment light-light, LL). Two shoots were exposed to light for 1, 2, 3, 4, or 5 h (1 hL, 2 hL, 3 hL, 4 hL, 5 hL). Each shoot was sampled first under light conditions; then, the light was turned off, and the shoot was sampled under dark conditions. Finally, the leaves were cut off and the leafless branch was sampled. The irradiance level chosen for our experiment was in accordance with Xiong et al. [37], who showed that the light intensity level required to saturate leaf hydraulic conductance (KL; kg m−2 s−1 MPa−1) is around 1000 μmol m−2 s−1 for most species. LED Fyto-Panels (Photon Systems Instruments, Drásov, Czech Republic) consisting of neutral white light sources supplemented with far-red LEDs (735 nm) were used for illumination prior to and in the course of the measurements. To minimise small-scale temperature gradients, the air in the vicinity of the shoots was stirred with a ventilator. During the measurements, leaf and branch temperature were recorded with an MT2 fast response temperature sensor (Delta-T Devices, Burwell, UK).
After hydraulic measurements, the shoot length was measured with a ruler. Additionally, shoot diameter was measured under bark from two perpendicular directions with a digital calliper for calculations of xylem cross-sectional area. The total leaf blade area of the shoot (AL; m2) was determined with a LI-3100C optical area meter (LI-COR Biosciences, Lincoln, NE, USA). Leaves were then dried in an oven at 80 °C for 48 h, and the total leaf dry weight of the shoot (DW; g) was recorded. Leaf mass per area (LMA; g m−2) was calculated for each shoot. The Huber value (HV) of each shoot was calculated by dividing the xylem cross-sectional area by AL. The hydraulic conductance values were scaled by AL and corrected for the dynamic viscosity of water at 23 °C. KL was calculated as follows:
where KS is the total shoot hydraulic conductance, and KB (kg m−2 s−1 MPa−1) is the hydraulic conductance of the leafless branch. KL in the LL treatment represents the maximum leaf hydraulic conductance (KLmax), and KL in the DD treatment represents the minimum leaf hydraulic conductance (KLmin).
The up-regulation time (tup; s) and down-regulation time (tdown; s) of KL were determined as the time elapsed between turning on or off the light until hydraulic conductance values became constant, respectively. The up-regulation rate of KL (dKLup/dt; kg m−2 s−2 MPa−1) was calculated as follows:
The down-regulation rate of KL (dKLdown/dt; kg m−2 s−2 MPa−1) was calculated as follows:
where KL_LD is leaf hydraulic conductance in the LD treatment. The initial up-regulation rate of KL (bup; kg m−2 s−2 MPa−1) was calculated as the slope of the linear part of KL vs. time regression. Relative hydraulic resistance residing in leaves (RL; %) was calculated as follows:
2.3. Plasticity Index
The plasticity index [55] was calculated for both species and used to characterise hydraulic plasticity (PIh):
where Kmax and Kmin represent the species mean maximum and minimum hydraulic conductance, respectively, of leaves (KL) or leafless branches (KB).
2.4. Data Analysis
Statistical data analysis was performed using Statistica, Version 7.1 (StatSoft Inc., Tulsa, OK, USA). The normality of the data and the homogeneity of variance were checked using the Kolmogorov–Smirnov D-statistic and Levene’s test, respectively. Light exposition time (1–5 h) did not influence KL; thus, KL data from light treatments 1 hL to 5 hL were pooled together and treated as a unitary light treatment (LL). To analyse the effects of categorical factors (species, light treatment) on leaf and branch hydraulic traits, an analysis of variance (ANOVA) was performed using the ‘general linear model’ module. Type IV sums of squares were used in the calculations. To fulfil the assumptions for ANOVA, logarithmic or complex transformations were applied to the data when necessary. Post hoc mean comparisons were conducted using Tukey’s HSD test. Bivariate relationships between the studied characteristics were assessed by Pearson’s correlations and simple linear or nonlinear least-squares regressions.
3. Results
Morphological traits of the sampled shoots are given in Table 1. AL (p < 0.001), LMA (p < 0.001), and HV (p = 0.024) were significantly higher in aspen than in birch.

Table 1.
Mean values (±SE) of shoot morphological traits in silver birch (Betula pendula) and hybrid aspen (Populus × wettsteinii). LS—shoot length, AL—total leaf blade area of the shoot, LMA—leaf mass per area, HV—Huber value.
Light treatment had a significant effect (p ≤ 0.001) on both leaf and branch hydraulic conductance (Table 2). For both species, KL was lowest in the initial dark conditions (treatment DD, KLmin), intermediate a few minutes after the light was turned on (treatment DL), highest in light lasting over an hour (treatment LL, KLmax), and it declined again in the second dark period (treatment LD) (Figure 1). The mean KL for birch was 0.80 ± 0.05 × 10−4, 1.94 ± 0.14 × 10−4, 2.82 ± 0.07 × 10−4, and 2.26 ± 0.04 × 10−4 kg m−2 s−1 MPa−1 for DD, DL, LL, and LD treatments, respectively. For aspen, KL averaged 1.90 ± 0.07 × 10−4, 2.23 ± 0.08 × 10−4, 2.92 ± 0.05 × 10−4, and 2.84 ± 0.06 × 10−4 kg m−2 s−1 MPa−1 in DD, DL, LL, and LD treatments, respectively.

Table 2.
Results of ANOVA for the effects of species and light treatment on leaf and branch hydraulic properties.
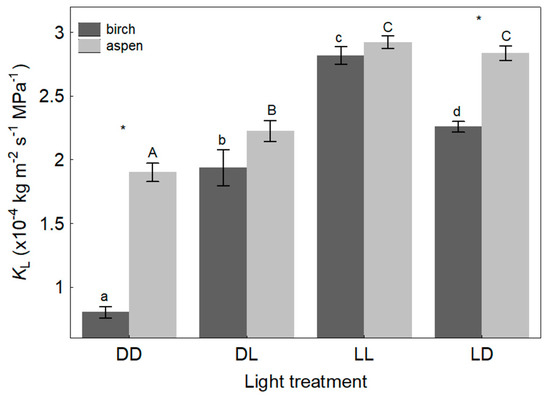
Figure 1.
Mean values (±SE) of leaf hydraulic conductance (KL) depending on species and light treatment. DD—initial dark conditions; DL—initial light conditions (exposure to light for less than 1 h); LL—light conditions (exposure to PPFD 900–1000 μmol m−2 s−1 for 1–5 h); LD—final dark conditions (light turned off after exposure to light for 1–5 h). Different letters express statistically significant (p < 0.001) differences between light treatments within a species; asterisks denote statistically significant (p < 0.01) differences between species within a light treatment.
KL varied significantly (p < 0.001) between the species (Table 2); the mean KLmin in aspen was 2.4 times higher (p < 0.001) than that in birch (Figure 1). KL in the DL treatment tended to be higher in aspen, but the difference was statistically not significant (p = 0.08). The means of KLmax did not differ (p = 0.23) between species. In the LD treatment, KL for aspen was on average 1.26 times higher (p < 0.01; Figure 1) compared to birch leaves.
The interaction between species and light treatment was significant (p < 0.001); the species differed in the amplitude of KL (i.e., the difference between KLmax and KLmin). KL increased 3.5 (p < 0.01) and 1.5 times (p < 0.01) from dark to light conditions for birch and aspen, respectively (Figure 1). Thus, birch leaves exhibited a greater hydraulic plasticity than aspen; the PIh for birch leaves (PIh_L) was 0.72 compared to 0.35 for aspen. The species also differed significantly in the decrease of leaf hydraulic conductance when transferring shoots from light to dark. Birch exhibited a 25% decrease (p < 0.01) and aspen a 3% decrease (p = 0.263) in KL after the light was switched off (Figure 1). KLmax was significantly and positively correlated to HV for both birch (r = 0.27, p = 0.017) and aspen (r = 0.26, p = 0.014).
Branch hydraulic conductance also responded to the application of light and differed between the species (Table 2). KB was lowest in the dark (DD and DL treatments) and reached a maximum after 3 h of light exposure (3 hL) in birch (Figure 2). In aspen, KB was lowest under DD treatment and reached a maximum in light lasting for one hour (1 hL; Figure 2). For birch, the minimal and maximal KB averaged 4.03 ± 0.29 × 10−4 and 6.06 ± 0.54 × 10−4 kg m−2 s−1 MPa−1, respectively. For aspen, the minimal and maximal values of KB were 7.54 ± 0.60 × 10−4 and 11.47 ± 0.83 × 10−4 kg m−2 s−1 MPa−1, respectively. KB increased 1.5 times (p < 0.01) from dark to light conditions for both species. Both minimal and maximal KB were 1.9-fold (p < 0.01) higher for aspen compared to birch. The species did not differ in the hydraulic plasticity of branches; the PIh for birch branches (PIh_B) was 0.33 and for aspen branches 0.34. KB in light conditions was significantly and positively correlated with HV for both birch (r = 0.54, p < 0.001) and aspen (r = 0.33, p = 0.001). In light, KB and KL were positively correlated in birch (r = 0.36, p = 0.001) and aspen (r = 0.40, p < 0.001), but they were not correlated in the dark.
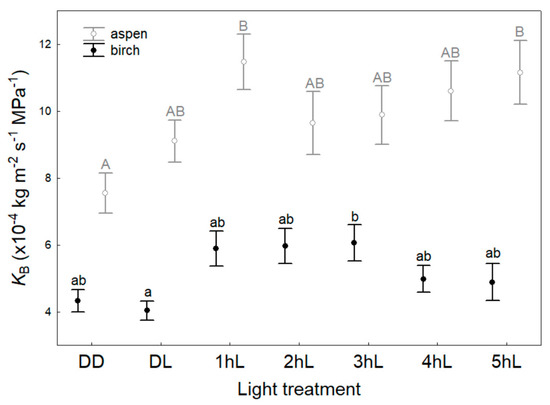
Figure 2.
Mean values (±SE) of branch hydraulic conductance (KB) depending on species and light treatment. DD—initial dark conditions; DL—initial light conditions (exposure to light for less than 1 h); 1 hL–5 hL—light conditions (exposure to PPFD 900–1000 μmol m−2 s−1 for 1–5 h). Different letters express statistically significant (p < 0.001) differences between the light treatments within a species.
The times of up- and down-regulation of KL (tup and tdown) in response to turning the light on and off differed significantly between the species (Table 2). The mean tup was 483 ± 32 s for birch and 150 ± 12 s for aspen (p < 0.001), and the mean tdown was 210 ± 5 s for birch and 251 ± 4 s for aspen (p < 0.001; Figure 3). tup and tdown differed significantly from each other. In birch, tup was 2.3 times longer (p < 0.01) than tdown, while the opposite trend was observed in aspen, in which tdown was 1.7 times longer (p < 0.01) than tup (Figure 3). Across species, tup was strongly and inversely related to LMA (r2 = 0.60, p < 0.001; Figure 4).
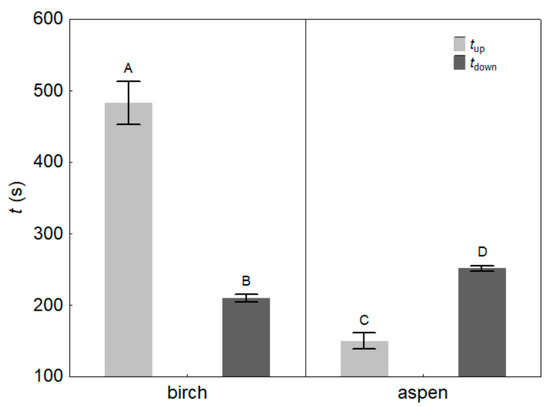
Figure 3.
Mean values (±SE) of the up- (tup) and down-regulation (tdown) times of leaf hydraulic conductance of silver birch (Betula pendula) and hybrid aspen (Populus × wettsteinii). Different letters express statistically significant (p < 0.01) differences between the means.
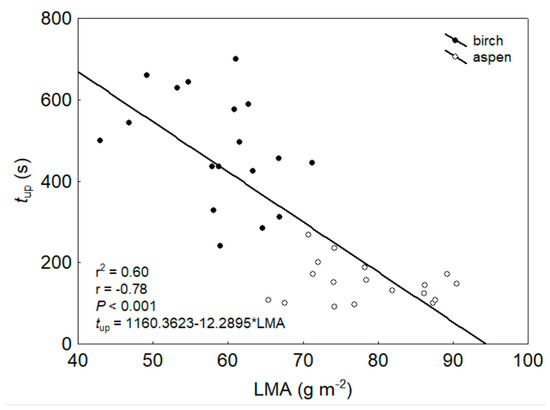
Figure 4.
Relationship between leaf mass per area (LMA) and time of KL up-regulation (tup) across species. All data points correspond to one shoot.
The up-regulation rate of KL (dKLup/dt) did not differ between the species; the corresponding mean values were 2.94 ± 0.44 × 10−7 and 2.72 ± 0.22 × 10−7 kg m−2 s−2 MPa−1 for birch and aspen, respectively (p = 0.667; Figure 5). Additionally, the initial up-regulation rate of KL (bup) did not differ significantly between the species (p = 0.193). However, the down-regulation rate of KL (dKLdown/dt) differed substantially between species, and the corresponding means were 3.50 ± 0.47 × 10−7 and 0.83 ± 0.08 × 10−7 kg m−2 s−2 MPa−1 for birch and aspen, respectively (p < 0.01), exhibiting 4.2 times faster down-regulation in birch leaves (Figure 5). For birch, there was no significant difference between the up- and down-regulation rates of KL, whereas for aspen, dKLup/dt was 3.3-fold faster than dKLdown/dt (p < 0.01).
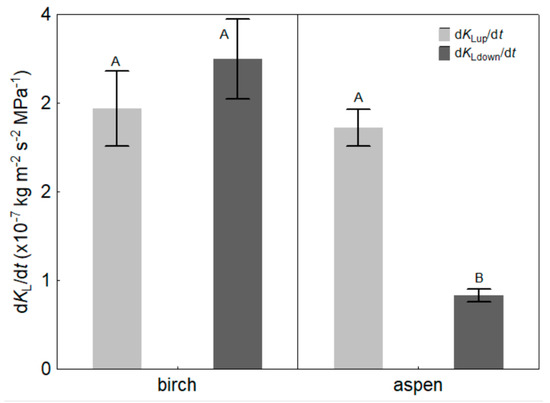
Figure 5.
Mean values (±SE) of the rates of up- (dKLup/dt) and down-regulation of KL (dKLdown/dt) of silver birch (Betula pendula) and hybrid aspen (Populus × wettsteinii). Different letters express statistically significant (p < 0.01) differences between the means.
The changes in KL induced by exposure to light brought about a considerable redistribution of the hydraulic resistance within the sample shoots. The relative contribution of leaves to the whole-shoot hydraulic resistance (RL) under dark conditions was similar for the two study species, averaging 83 and 80% for birch and aspen, respectively (Figure 6). After at least 1 h of illumination, RL decreased to 65 and 77% for birch and aspen, respectively, indicating a significantly greater decline in birch.
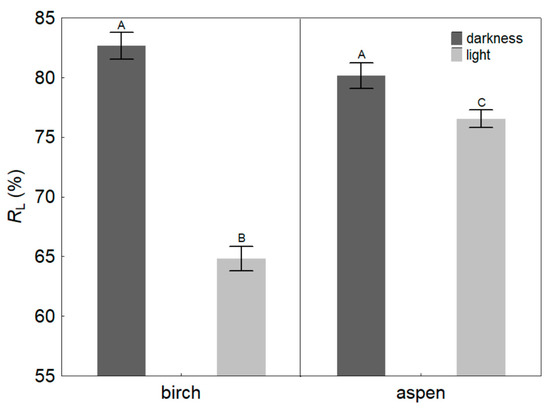
Figure 6.
Relative hydraulic resistance (mean ±SE) residing in leaves (RL) of silver birch (Betula pendula) and hybrid aspen (Populus × wettsteinii) under dark or light conditions (exposure to PPFD 900–1000 μmol m−2 s−1 for at least 1 h). Different letters express statistically significant (p < 0.01) differences between the means.
4. Discussion
4.1. Light Effects on Leaf Hydraulic Properties
The results of this study confirm the high light sensitivity of leaf hydraulic conductance in silver birch and hybrid aspen; a rapid and substantial modulation of KL took place in response to switching light on and off in both species (Figure 1 and Figure 3). Nearly 20 years have passed since the earliest studies on the light sensitivity of KL [32,56,57], and the enhancement of KL by light has been generally acknowledged and amply confirmed in various species [35,36,37,39,58]. The light-induced rapid enhancement of KL has been shown to originate from the outside-xylem compartment of the leaf [34,57] being specifically related to the up-regulation of aquaporins (AQPs) through enhanced gene expression and/or post-translational protein activation in response to irradiance. In B. pendula, a genome analysis has identified 33 putative genes encoding full-length AQP sequences [59]. The light-dependent increase of KL shows a tight correlation with AQP gene transcript abundance [60,61,62,63,64]. The activation of AQPs by phosphorylation is also associated with enhanced leaf water transport capacity [65,66]. At the present time, it is not yet clear which mechanism is primarily responsible for light-induced rapid KL enhancement, whether it is AQP expression or activation.
In the current study, KL was lowest in the initial dark conditions, intermediate after the light was turned on, and highest in light lasting for an hour or more (Figure 1). Variation of the exposure time from 1 to 5 h did not influence leaf hydraulic capacity. In our earlier study [33], also carried out in silver birch, KL increased up to 9 h of illumination. This discrepancy is attributable to different experimental conditions; in that study, substantially lower irradiance was applied, which is why more time was needed for AQP up-regulation. We propose that the rapid changes in KL that occurred directly after turning the light on/off probably took place through the post-translational activation of AQPs and/or their trafficking to the plasma membrane, whereas the maximal values of KL were achieved through de novo AQP synthesis. In such a context, the PIP (plasma membrane intrinsic protein) and TIP (tonoplast intrinsic proteins) subfamilies would be appropriate candidates, as their functions in water and CO2 homeostasis are very specialised [67]. Our present results concur with studies indicating that maximal KL values and AQP transcript amounts occur at least 1 h after switching on the light [60,61,62]. Xiong et al. [37] indicated that for most species the sufficient light intensity required to saturate KL is around 1000 μmol m−2 s−1. Our results suggest that, for correct estimations of light-saturated KL, it is also important to take into account the time of light exposure and that 1 h of illumination is enough to reach maximum KL when really saturating light is applied. The light-induced rapid enhancement of KL is a means of maintaining stable gas exchange in the event of rapid increases in VPD and evaporative water loss, while avoiding water potential drop and xylem embolism [35,60,68].
The magnitude of light sensitivity of KL is highly species-specific, ranging from zero to a several-fold increase in KL in response to incident light [32,34,37,60]. Xiong et al. [37] reported the lack of light sensitivity of KL of ferns, gymnosperms, and one angiosperm, whereas the other angiosperms under study responded to light. In the current study, the species differed substantially in the magnitude of their response; KL increased 3.5 and 1.5 times from dark to saturating light conditions for birch and aspen, respectively, reflecting higher hydraulic plasticity in birch compared to aspen. The differences in sensitivity of KL to light between the species sampled in the current study probably ensued from the extravascular compartment of the leaf, specifically from species specificity in AQP gene expression and/or activation.
Additionally, the differing distribution of resistance between the vascular and extravascular compartments within leaves of different species and changes in preferential water transport pathways with varying light conditions probably contribute to the differences in KL light sensitivity between the species. Cochard et al. [60] argued that, in the dark, when AQP accumulation/activation is low, hydraulic resistance of the extravascular (Rext) compared to the vascular (Rvasc) compartment in the leaf is high and water flows preferentially through the vascular and apoplasmic compartment. At the onset of light, AQPs start to accumulate, thus reducing Rext, and the preferential water pathway changes to the cell-to-cell route. The current consensus is that within the leaves the relative contribution of Rvasc and Rext to total leaf hydraulic resistance is relatively equal [8], but depending on species specificity and environmental factors, either one can prevail. Trifilo et al. [69] reported that Rext for Populus nigra contributes about 80% to the total leaf hydraulic resistance under low light, while at a high irradiance Rext and Rvasc are equal. Considering this information, we can assume that in the current study, the higher hydraulic plasticity resulted, in part, from the higher proportion of Rext under dark conditions in birch leaves compared to aspen.
This study revealed a strong inverse relationship between LMA and tup across the species (Figure 4). No doubt, this is attributable to species specificity, but leaf general morphology probably also plays a role in this relationship: a lower LMA is accompanied by lower leaf vein density (vein length per area, VLA) in birch leaves compared to aspen [29,70]. The lower the VLA, the greater the contribution of the extravascular pathway and the smaller the role of vascular pathway in water movement in the leaf. Therefore, for birch leaves with lower LMA (and VLA) and thus with a higher proportion of Rext, the time for KL up-regulation tended to be longer. To unravel the basis of KL light sensitivity more deeply, future studies on KL light responses should involve the estimations of Rext and Rvasc contributions to total leaf hydraulic resistance with concomitant AQP expression analyses.
High hydraulic plasticity is important from many aspects of tree biology, contributing to whole-tree performance through effects on energy, carbon, and water balance of individual leaves in changing or spatially heterogeneous environments. A high PIh of B. pendula is associated with its isohydric behaviour; in addition to the strict stomatal control over water losses [71,72], an efficient and highly dynamic water conducting system is also necessary to maintain the leaf water status within definite limits. Silver birches coordinate their hydraulic capacity with changes in stomatal conductance depending on environmental conditions to prevent leaf water potential from reaching critical values, supporting the isohydric water-use strategy. Moreover, this coordination can also be seen from the leaf anatomical structure. Leaf vascular (size of vascular bundles and vessels, vessel number and density, vein density, etc.) and stomatal traits (stomatal size, density, pore area index) exhibit coordinated adjustment, reflecting the balance between transpirational demand and hydraulic supply in silver birch [29]. On the one hand, exposure of birch foliage to high irradiance (changing patterns of clouds and sun flecks within forest canopies) affords, through a fast increase in KL and reduction of leaf resistance to water movement (Figure 1 and Figure 6), an adequate hydraulic supply to keep stomata open, and supports evaporative cooling. On the other hand, the capability for efficient down-regulation of KL helps to prevent excessive water loss in birches in the dark (i.e., arrival of the night) or in the case of shade (Figure 1 and Figure 3).
In hybrid aspen, low PIh and dKLdown/dt and high KL in the dark (Figure 3 and Figure 5) afford lavish water use at night, as evidenced by high nocturnal stomatal conductance and low predawn leaf water potential [45,73]. However, high KL cannot compensate for high water loss due to weak stomatal control day and night, which causes substantial native embolism (percentage loss of hydraulic conductivity 16–41%) in aspen coppice during drought periods [74]. At the leaf level, no solid evidence of coordination was found between vascular and stomatal traits and hydraulic functions, which were most likely associated with the anisohydric behaviour of this species [70].
The up-regulation time of KL was significantly longer for birch compared to aspen; however, with a much bigger amplitude of KL between dark and light values, the resulting up-regulation rate of KL did not differ between the species (Figure 5). We measured similar values of dKLup/dt also in Corylus avellana, a shade-tolerant species [39]. The similar up-regulation rates of KL among species may imply that the mechanism behind the fast light-induced enhancement of KL is universal for vascular plants. However, birch exhibited a down-regulation rate over four times faster than aspen, and dKLup/dt and dKLdown/dt did not differ for birch, whereas for aspen, dKLup/dt was 3.3 times faster than dKLdown/dt (Figure 5). At present, an unequivocal explanation cannot be provided for why the down-regulation rate for aspen was so much slower than for birch. It may be related to aquaporin metabolism, principally with phosphorylation and dephosphorylation of AQP monomers that form the water channels in cell membranes [75,76]. The exact process of AQP activation and deactivation is subject to research even today [77].
4.2. Light Effects on Branch Hydraulic Properties
The results of this study confirm the light sensitivity and light-induced rapid (time scale in hours) enhancement of branch hydraulic conductance. KB increased by approximately 50% from dark to light conditions for both species (Figure 2). Water movement in xylem was previously considered a passive process [78,79], determined only by the driving forces and anatomical features of the conducting system (i.e., density, length, diameter of the xylem conduits, structure of interconduit pits) and modified on a developmental time scale. The changes in xylem hydraulic efficiency on a short time scale were attributable to embolism. Presently, there is consensus that xylem hydraulic efficiency can be modulated by the plant on a short time scale also through the modification of xylem sap ionic concentration [80,81,82]. This ionic effect has been considered to be responsible for the rapid enhancement of KB in response to increases in incident light [43,83]. The ion-mediated modulation of xylem hydraulic efficiency is related to the interaction of cations (mainly K+) with the pectin hydrogels of pit membranes that separate adjacent xylem vessels, and which alters the hydraulic resistance of the bordered pits through changes in the thickness of the pit membrane [84]. The ecophysiological implication of the phenomenon is probably to provide a regulatory mechanism of branch hydraulics to facilitate water flow towards fully illuminated foliage by reducing hydraulic resistance and water potential drop, maximising stomatal aperture and photosynthesis [43,83]. This is a crucial point for fast-growing light-demanding species, such as silver birch and hybrid aspen, contributing to their competitive advantage. A coordinated adjustment of different segments of the water transport pathway is also an essential point in this context. In both species, KB and KL were positively correlated in the light but not in the dark, i.e., at night when stomata are closed and atmospheric evaporative demand is low.
5. Conclusions
B. pendula and P. × wettsteinii, both fast-growing and light-demanding pioneer species, exhibit high light sensitivity in leaf and branch hydraulic conductance that provide a regulatory mechanism to maintain a balance between transpirational demand and hydraulic supply. However, the tree species differ in their hydraulic plasticity associated with their different water-use strategies; B. pendula with its isohydric behaviour exhibits a significantly higher hydraulic plasticity, allowing for a strict control over the water balance of the plant in changing or spatially heterogeneous environments, compared to P. × wettsteinii inclined to anisohydric behaviour. In light of climate change one can suppose that hybrid aspen as a species with weak stomatal control and low hydraulic plasticity will be potentially more susceptible to hydraulic impairment compared to silver birch, which will more likely suffer from carbon starvation in case of weather extremes. From a future perspective, it is necessary not merely to determine the plasticity in regard to light conditions but also to investigate possible shifts in the plasticity of plant functional traits (incl. leaf hydraulic conductance) in response to changes in other environmental factors, because most likely they play a determinant role in plant acclimation to climate change.
Author Contributions
Conceptualization, E.Õ.-P. and A.S.; Methodology, E.Õ.-P. and A.S.; Validation, A.S.; Formal Analysis, E.Õ.-P.; Investigation, E.Õ.-P. and A.S.; Resources, A.S.; Writing—Original Draft Preparation, E.Õ.-P.; Writing—Review and Editing, E.Õ.-P., J.-S.V., P.L. and A.S.; Supervision, A.S.; Project Administration, A.S.; Funding Acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from the Estonian Research Council (personal research grant PRG1434).
Data Availability Statement
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sack, L.; Ball, M.C.; Brodersen, C.; Davis, S.D.; Des Marais, D.L.; Donovan, L.A.; Givnish, T.J.; Hacke, U.G.; Huxman, T.; Jansen, S.; et al. Plant hydraulics as a central hub integrating plant and ecosystem function: Meeting report for ‘Emerging Frontiers in Plant Hydraulics’ (Washington, DC, May 2015). Plant Cell Environ. 2016, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.L.; Kumar, M.; Katul, G.G.; Feng, X.; Konings, A.G. Plant hydraulics accentuates the effect of atmospheric moisture stress on transpiration. Nat. Clim. Chang. 2020, 10, 691–695. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology, 2nd ed.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2010. [Google Scholar]
- Brodribb, T.J. Xylem hydraulic physiology: The functional backbone of terrestrial plant productivity. Plant Sci. 2009, 177, 245–251. [Google Scholar] [CrossRef]
- Scoffoni, C.; Chatelet, D.S.; Pasquet-kok, J.; Rawls, M.; Donoghue, M.J.; Edwards, E.J.; Sack, L. Hydraulic basis for the evolution of photosynthetic productivity. Nat. Plants 2016, 2, 16072. [Google Scholar] [CrossRef]
- Xiong, D.; Nadal, M. Linking water relations and hydraulics with photosynthesis. Plant J. 2020, 101, 800–815. [Google Scholar] [CrossRef]
- Sack, L.; Holbrook, N.M. Leaf hydraulics. Annu. Rev. Plant Biol. 2006, 57, 361–381. [Google Scholar] [CrossRef] [Green Version]
- Sack, L.; Cowan, P.D.; Jaikumar, N.; Holbrook, N.M. The ‘hydrology’ of leaves: Co-ordination of structure and function in temperate woody species. Plant Cell Environ. 2003, 26, 1343–1356. [Google Scholar] [CrossRef] [Green Version]
- Õunapuu, E.; Sellin, A. Daily dynamics of leaf and soil-to-branch hydraulic conductance in silver birch (Betula pendula) measured in situ. Plant Physiol. Biochem. 2013, 68, 104–110. [Google Scholar] [CrossRef]
- Newman, J.A.; Anand, M.; Henry, H.A.L.; Hunt, S.; Gedalof, Z. Climate Change Biology; CABI Publishing: Wallingford, UK, 2011. [Google Scholar]
- Gratani, L. Plant Phenotypic Plasticity in Response to Environmental Factors. Adv. Bot. 2014, 208747. [Google Scholar] [CrossRef] [Green Version]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Henn, J.J.; Buzzard, V.; Enquist, B.J.; Halbritter, A.H.; Klanderuds, K.; Maitner, B.S.; Michaletz, S.T.; Pötsch, C.; Seltzer, L.; Telford, R.J.; et al. Intraspecific trait variation and phenotypic plasticity mediate alpine plant species response to climate change. Front. Plant Sci. 2018, 9, 1548. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla, S.; Martin, I.; Babiano, J.; Escudero, A. Foliar plasticity related to gradients of heat and drought stress across crown orientations in three Mediterranean Quercus species. PLoS ONE 2019, 14, e0224462. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F.; Gianoli, E.; Gomez, J.M. Ecological limits to plant phenotypic plasticity. New Phytol. 2007, 176, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.E. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci. 2000, 5, 537–542. [Google Scholar] [CrossRef]
- Matesanz, S.; Gianoli, E.; Valladares, F. Global change and the evolution of phenotypic plasticity in plants. Ann. N. Y. Acad. Sci. 2010, 1206, 35–55. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Kont, A.; Jaagus, J.; Aunap, R. Climate change scenarios and the effect of sea-level rise for Estonia. Glob. Planet. Chang. 2003, 36, 1–15. [Google Scholar] [CrossRef]
- Betts, A.K.; Desjardins, R.; Worth, D.; Beckage, B. Climate coupling between temperature, humidity, precipitation, and cloud cover over the Canadian Prairies. J. Geophys. Res. -Atmos. 2014, 119, 13305–13326. [Google Scholar] [CrossRef]
- Westra, S.; Fowler, H.J.; Evans, J.P.; Alexander, L.V.; Berg, P.; Johnson, F.; Kendon, E.J.; Lenderink, G.; Roberts, N.M. Future changes to the intensity and frequency of short-duration extreme rainfall. Rev. Geophys. 2014, 52, 522–555. [Google Scholar] [CrossRef]
- Busuioc, A.; Birsan, M.V.; Carbunaru, D.; Baciu, M.; Orzan, A. Changes in the large-scale thermodynamic instability and connection with rain shower frequency over Romania: Verification of the Clausius-Clapeyron scaling. Int. J. Climatol. 2016, 36, 2015–2034. [Google Scholar] [CrossRef]
- Fanourakis, D.; Aliniaeifard, S.; Sellin, A.; Giday, H.; Körner, O.; Nejad, A.R.; Delis, C.; Bouranis, D.; Koubouris, G.; Kambourakis, E.; et al. Stomatal behavior following mid or long-term exposure to high relative air humidity: A review. Plant Physiol. Biochem. 2020, 153, 92–105. [Google Scholar] [CrossRef]
- Niu, S.L.; Luo, Y.Q.; Li, D.J.; Cao, S.H.; Xia, J.Y.; Li, J.W.; Smith, M.D. Plant growth and mortality under climatic extremes: An overview. Environ. Exp. Bot. 2014, 98, 13–19. [Google Scholar] [CrossRef]
- Zang, C.; Hartl-Meier, C.; Dittmar, C.; Rothe, A.; Menzel, A. Patterns of drought tolerance in major European temperate forest trees: Climatic drivers and levels of variability. Glob. Chang. Biol. 2014, 20, 3767–3779. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, E.; Lihavainen, J.; Keinänen, M.; Keski-Saari, S.; Kontunen-Soppela, S.; Sellin, A.; Sõber, A. Northern forest trees under increasing atmospheric humidity. In Progress in Botany; Canovas, F.M., Lüttge, U., Matyssek, R., Pretzsch, H., Eds.; Springer: Cham, Switzerland, 2018; Volume 80. [Google Scholar]
- Sellin, A.; Tullus, A.; Niglas, A.; Õunapuu, E.; Karusion, A.; Lõhmus, K. Humidity-driven changes in growth rate, photosynthetic capacity, hydraulic properties and other functional traits in silver birch (Betula pendula). Ecol. Res. 2013, 28, 523–535. [Google Scholar] [CrossRef]
- Sellin, A.; Taneda, H.; Alber, M. Leaf structural and hydraulic adjustment with respect to air humidity and canopy position in silver birch (Betula pendula). J. Plant Res. 2019, 132, 369–381. [Google Scholar] [CrossRef]
- Sellin, A.; Niglas, A.; Õunapuu-Pikas, E.; Kupper, P. Rapid and long-term effects of water deficit on gas exchange and hydraulic conductance of silver birch trees grown under varying atmospheric humidity. BMC Plant Biol. 2014, 14, 72. [Google Scholar] [CrossRef] [Green Version]
- Pritzkow, C.; Williamson, V.; Szota, C.; Trouve, R.; Arndt, S.K. Phenotypic plasticity and genetic adaptation of functional traits influences intra-specific variation in hydraulic efficiency and safety. Tree Physiol. 2020, 40, 215–229. [Google Scholar] [CrossRef]
- Tyree, M.T.; Nardini, A.; Salleo, S.; Sack, L.; El Omari, B. The dependence of leaf hydraulic conductance on irradiance during HPFM measurements: Any role for stomatal response? J. Exp. Bot. 2005, 56, 737–744. [Google Scholar] [CrossRef]
- Sellin, A.; Õunapuu, E.; Kupper, P. Effects of light intensity and duration on leaf hydraulic conductance and distribution of resistance in shoots of silver birch (Betula pendula). Physiol. Plant. 2008, 134, 412–420. [Google Scholar] [CrossRef]
- Voicu, M.C.; Zwiazek, J.J.; Tyree, M.T. Light response of hydraulic conductance in bur oak (Quercus macrocarpa) leaves. Tree Physiol. 2008, 28, 1007–1015. [Google Scholar] [CrossRef] [Green Version]
- Guyot, G.; Scoffoni, C.; Sack, L. Combined impacts of irradiance and dehydration on leaf hydraulic conductance: Insights into vulnerability and stomatal control. Plant Cell Environ. 2012, 35, 857–871. [Google Scholar] [CrossRef]
- Ohtsuka, A.; Sack, L.; Taneda, H. Bundle sheath lignification mediates the linkage of leaf hydraulics and venation. Plant Cell Environ. 2018, 41, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.L.; Douthe, C.; Flexas, J. Differential coordination of stomatal conductance, mesophyll conductance, and leaf hydraulic conductance in response to changing light across species. Plant Cell Environ. 2018, 41, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Scoffoni, C.; Kunkle, J.; Pasquet-Kok, J.; Vuong, C.; Patel, A.J.; Montgomery, R.A.; Givnish, T.J.; Sack, L. Light-inducec plasticity in leaf hydraulics, venation, anatomy, and gas exchange in ecologically diverse Hawaiian lobeliads. New Phytol. 2015, 207, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Õunapuu-Pikas, E.; Sellin, A. Plasticity and light sensitivity of leaf hydraulic conductance to fast changes in irradiance in common hazel (Corylus avellana L.). Plant Sci. 2020, 290, 110299. [Google Scholar] [CrossRef] [PubMed]
- Possen, B.J.H.M.; Oksanen, E.; Rousi, M.; Ruhanen, H.; Ahonen, V.; Tervahauta, A.; Heinonen, J.; Heiskanen, J.; Kärenlampi, S.; Vapaavuori, E. Adaptability of birch (Betula pendula Roth) and aspen (Populus tremula L.) genotypes to different soil moisture conditions. For. Ecol. Manag. 2011, 262, 1387–1399. [Google Scholar] [CrossRef]
- Hynynen, J.; Niemisto, P.; Vihera-Aarnio, A.; Brunner, A.; Hein, S.; Velling, P. Silviculture of birch (Betula pendula Roth and Betula pubescens Ehrh.) in northern Europe. Forestry 2010, 83, 103–119. [Google Scholar] [CrossRef]
- Tullus, A.; Rytter, L.; Tullus, T.; Weih, M.; Tullus, H. Short-rotation forestry with hybrid aspen (Populus tremula L. × P. tremuloides Michx.) in Northern Europe. Scand. J. For. Res. 2012, 27, 10–29. [Google Scholar] [CrossRef]
- Sellin, A.; Õunapuu, E.; Karusion, A. Experimental evidence supporting the concept of light-mediated modulation of stem hydraulic conductance. Tree Physiol. 2010, 30, 1528–1535. [Google Scholar] [CrossRef] [Green Version]
- Zapater, M.; Breda, N.; Bonal, D.; Pardonnet, S.; Granier, A. Differential response to soil drought among co-occurring broad-leaved tree species growing in a 15-to 25-year-old mixed stand. Ann. For. Sci. 2013, 70, 31–39. [Google Scholar] [CrossRef]
- Kupper, P.; Ivanova, H.; Sõber, A.; Rohula-Okunev, G.; Sellin, A. Night and daytime water relations in five fast-growing tree species: Effects of environmental and endogenous variables. Ecohydrology 2018, 11, e1927. [Google Scholar] [CrossRef]
- Aasamaa, K.; Sõber, A.; Hartung, W.; Niinemets, Ü. Drought acclimation of two deciduous tree species of different layers in a temperate forest canopy. Trees-Struct. Funct. 2004, 18, 93–101. [Google Scholar]
- Aasamaa, K.; Kõivik, K.; Kupper, P.; Sõber, A. Growth environment determines light sensitivity of shoot hydraulic conductance. Ecol. Res. 2014, 29, 143–151. [Google Scholar] [CrossRef]
- Russak, V. Päikesekiirgus. Tartu Kliima Ja Selle Muutumine Viimastel Kümnenditel; Ross, J., Ed.; Eesti Teaduste Akadeemia: Tartu, Estonia, 1990. [Google Scholar]
- Jaagus, J.; Mändla, K. Climate change scenarios for Estonia based on climate models from the IPCC Fourth Assessment Report. Est. J. Earth Sci. 2014, 63, 166–180. [Google Scholar] [CrossRef]
- Jacob, D.; Petersen, J.; Eggert, B.; Alias, A.; Christensen, O.B.; Bouwer, L.M.; Braun, A.; Colette, A.; Déqué, M.; Georgievski, G.; et al. EURO-CORDEX: New high-resolution climate change projections for European impact research. Reg. Environ. Chang. 2014, 14, 563–578. [Google Scholar] [CrossRef]
- Kupper, P.; Sõber, J.; Sellin, A.; Lõhmus, K.; Tullus, A.; Räim, O.; Lubenets, K.; Tulva, I.; Uri, V.; Zobel, M.; et al. An experimental facility for free air humidity manipulation (FAHM) can alter water flux through deciduous tree canopy. Environ. Exp. Bot. 2011, 72, 432–438. [Google Scholar] [CrossRef]
- Tullus, A.; Kupper, P.; Sellin, A.; Parts, L.; Sõber, J.; Tullus, T.; Tullus, H. Climate change at Northern latitudes: Rising atmospheric humidity decreases transpiration, N-uptake and growth rate of hybrid aspen. PLoS ONE 2012, 7, e42648. [Google Scholar] [CrossRef] [PubMed]
- Sellin, A.; Alber, M.; Keinanen, M.; Kupper, P.; Lihavainen, J.; Lõhmus, K.; Tullus, A. Growth of northern deciduous trees under increasing atmospheric humidity: Possible mechanisms behind the growth retardation. Reg. Environ. Chang. 2017, 17, 2135–2148. [Google Scholar] [CrossRef]
- Heimonen, K.; Valtonen, A.; Kontunen-Soppela, S.; Keski-Saari, S.; Rousi, M.; Oksanen, E.; Roininen, H. Insect herbivore damage on latitudinally translocated silver birch (Betula pendula)—Predicting the effects of climate change. Clim. Chang. 2015, 131, 245–257. [Google Scholar] [CrossRef]
- Valladares, F.; Martinez-Ferri, E.; Balaguer, L.; Perez-Corona, E.; Manrique, E. Low leaf-level response to light and nutrients in Mediterranean evergreen oaks: A conservative resource-use strategy? New Phytol. 2000, 148, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Sack, L.; Melcher, P.J.; Zwieniecki, M.A.; Holbrook, N.M. The hydraulic conductance of the angiosperm leaf lamina: A comparison of three measurement methods. J. Exp. Bot. 2002, 53, 2177–2184. [Google Scholar] [CrossRef] [Green Version]
- Nardini, A.; Salleo, S.; Andri, S. Circadian regulation of leaf hydraulic conductance in sunflower (Helianthus annuus L. cv Margot). Plant Cell Environ. 2005, 28, 750–759. [Google Scholar] [CrossRef]
- Scoffoni, C.; Pou, A.; Aasamaa, K.; Sack, L. The rapid light response of leaf hydraulic conductance: New evidence from two experimental methods. Plant Cell Environ. 2008, 31, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Venisse, J.-S.; Õunapuu-Pikas, E.; Dupont, M.; Gousset-Dupont, A.; Saadaoui, M.; Faize, M.; Chen, S.; Chen, S.; Petel, G.; Fumanal, B.; et al. Genome-wide identification, structure characterization, and expression pattern profiling of the aquaporin gene family in Betula pendula. Int. J. Mol. Sci. 2021, 22, 7269. [Google Scholar] [CrossRef] [PubMed]
- Cochard, H.; Venisse, J.-S.; Barigah, T.S.; Brunel, N.; Herbette, S.; Guilliot, A.; Tyree, M.T.; Sakr, S. Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiol. 2007, 143, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Ben Baaziz, K.; Lopez, D.; Bouzid, S.; Cochard, H.; Venisse, J.S.; Sakr, S. Early gene expression in the walnut tree occurring during stimulation of leaf hydraulic conductance by irradiance. Biol. Plant. 2012, 56, 657–666. [Google Scholar] [CrossRef]
- Ben Baaziz, K.; Lopez, D.; Rabot, A.; Combes, D.; Gousset, A.; Bouzid, S.; Venisse, J.S. Light-mediated K-leaf induction and contribution of both the PIP1s and PIP2s aquaporins in five tree species: Walnut (Juglans regia) case study. Tree Physiol. 2012, 32, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Lopez, D.; Venisse, J.S.; Fumanal, B.; Chaumont, F.; Guillot, E.; Daniels, M.J.; Gousset-Dupont, A. Aquaporins and leaf hydraulics: Poplar sheds new light. Plant Cell Physiol. 2013, 54, 1963–1975. [Google Scholar] [CrossRef] [Green Version]
- Miniussi, M.; Del Terra, L.; Savi, T.; Pallavicini, A.; Nardini, A. Aquaporins in Coffea arabica L.: Identification, expression, and impacts on plant water relations and hydraulics. Plant Physiol. Biochem. 2015, 95, 92–102. [Google Scholar] [CrossRef]
- Prado, K.; Boursiac, Y.; Tournaire-Roux, C.; Monneuse, J.M.; Postaire, O.; Da Ines, O.; Maurel, C. Regulation of Arabidopsis leaf hydraulics involves light-dependent phosphorylation of aquaporins in veins. Plant Cell 2013, 25, 1029–1039. [Google Scholar] [CrossRef] [Green Version]
- Prado, K.; Cotelle, V.; Li, G.W.; Bellati, J.; Tang, N.; Tournaire-Roux, C.; Martinière, A.; Santoni, V.; Maurel, C. Oscillating aquaporin phosphorylation and 14-3-3 proteins mediate the circadian regulation of leaf hydraulics. Plant Cell 2019, 31, 417–429. [Google Scholar] [CrossRef]
- Groszmann, M.; Osborn, H.L.; Evans, J.R. Carbon dioxide and water transport through plant aquaporins. Plant Cell Environ. 2017, 40, 938–961. [Google Scholar] [CrossRef] [PubMed]
- Scoffoni, C.; Albuquerque, C.; Cochard, H.; Buckley, T.N.; Fletcher, L.R.; Caringella, M.A.; Sack, L. The causes of leaf hydraulic vulnerability and its influence on gas exchange in Arabidopsis thaliana. Plant Physiol. 2018, 178, 1584–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trifilo, P.; Petruzzellis, F.; Abate, E.; Nardini, A. The extra-vascular water pathway regulates dynamic leaf hydraulic decline and recovery in Populus nigra. Physiol. Plant. 2021, 172, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Sellin, A.; Alber, M.; Jasińska, A.K.; Rosenvald, K. Adjustment of leaf anatomical and hydraulic traits across vertical canopy profiles of young broadleaved forest stands. Trees-Struct. Funct. 2022, 36, 67–80. [Google Scholar] [CrossRef]
- Sellin, A.; Kupper, P. Variation in leaf conductance of silver birch: Effects of irradiance, vapour pressure deficit, leaf water status and position within a crown. For. Ecol. Manag. 2005, 206, 153–166. [Google Scholar] [CrossRef]
- Sellin, A.; Eensalu, E.; Niglas, A. Is distribution of hydraulic constraints within tree crowns reflected in photosynthetic water-use efficiency? An example of Betula pendula. Ecol. Res. 2010, 25, 173–183. [Google Scholar] [CrossRef]
- Kangur, O.; Kupper, P.; Sellin, A. Predawn disequilibrium between soil and plant water potentials in light of climate trends predicted for northern Europe. Reg. Environ. Chang. 2017, 17, 2159–2168. [Google Scholar] [CrossRef]
- Sellin, A.; Alber, M.; Kupper, P. Increasing air humidity influences hydraulic efficiency but not functional vulnerability of xylem in hybrid aspen. J. Plant Physiol. 2017, 219, 28–36. [Google Scholar] [CrossRef]
- Azad, A.K.; Sawa, Y.; Ishikawa, T.; Shibata, H. Phosphorylation of plasma membrane aquaporin regulates temperature-dependent opening of tulip petals. Plant Cell Physiol. 2004, 45, 608–617. [Google Scholar] [CrossRef] [Green Version]
- Hedfalk, K.; Tornroth-Horsefield, S.; Nyblom, M.; Johanson, U.; Kjellbom, P.; Neutze, R. Aquaporin gating. Curr. Opin. Struct. Biol. 2006, 16, 447–456. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- Tyree, M.T.; Zimmermann, M.H. Xylem Structure and the Ascent of Sap, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Zwieniecki, M.A.; Melcher, P.J.; Holbrook, N.M. Hydrogel control of xylem hydraulic resistance in plants. Science 2001, 291, 1059–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardini, A.; Salleo, S.; Jansen, S. More than just a vulnerable pipeline: Xylem physiology in the light of ion-mediated regulation of plant water transport. J. Exp. Bot. 2011, 62, 4701–4718. [Google Scholar] [CrossRef]
- Zwieniecki, M.A.; Secchi, F. Getting variable xylem hydraulic resistance under control: Interplay of structure and function. Tree Physiol. 2012, 32, 1431–1433. [Google Scholar] [CrossRef] [Green Version]
- Nardini, A.; Grego, F.; Trifilo, P.; Salleo, S. Changes of xylem sap ionic content and stem hydraulics in response to irradiance in Laurus nobilis. Tree Physiol. 2010, 30, 628–635. [Google Scholar] [CrossRef]
- Lee, J.; Holbrook, N.M.; Zwieniecki, M.A. Ion induced changes in the structure of bordered pit membranes. Front. Plant Sci. 2012, 3, 55. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).