Abstract
Successfully restoring degraded forest areas depends on seedlings adapting their growth to suit harsh environments. Hence, the requirements for seedlings’ growth need to be addressed before replanting degraded sites. The present study determines the effect of abiotic factors viz. light irradiance (8%, 30%, and 100%), nutrient addition (no fertiliser (NF), NPK, and vermicompost) on the growth performance and photosynthetic capacity of two dipterocarp species seedlings, Shorea leprosula Miq. and Shorea acuminata Dyer. The morphological characteristics assessed for growth performance comprised plant height, stem diameter, leaf count, leaf area, relative chlorophyll concentration, biomass, and root-to-shoot ratio. Li-Cor 6400 and 6800 were used to measure the leaf gas exchange traits, including photosynthetic rate (A), transpiration rate (E), intercellular CO2 concentration (Ci), stomatal conductance (gsw), and water-use efficiency (WUE). Our results demonstrated that different levels of light intensity and nutrient amendment significantly impacted plant-growth performance. Plants grown in 30% irradiance showed better growth performance in terms of relative height growth rate (RHGR), mean number of leaves, and leaf areas 41%, 24%, and 32% higher than the control. The A value was also higher in 30% irradiance, but no significant differences were observed between each level of light irradiance. The addition of vermicompost gave better growth for RHGR, relative diameter growth rate (RDGR), mean number of leaves, biomass, and relative chlorophyll concentrations 47%, 40%, 131%, 19%, and 27% higher than the control, respectively. However, the results obtained for photosynthetic parameters were contrary to growth performance. The photosynthesis rate (A) was higher (14.8%) in NPK compared to the control, and the other photosynthetic parameters did not differ significantly despite different nutrient amendments. In terms of species, S. leprosula has better growth performance and photosynthetic characteristics than S. acuminata in different light irradiance and nutrient amendments, thereby rendering S. leprosula the preferred rehabilitation species. Generally, nutrient addition of either NPK or vermicompost and 30% light irradiance gave better morphological and physiological growth for both species. The outcome of this study could provide a better understanding on the forest rehabilitation strategy to reduce the seedling-mortality rate, particularly for climax tree species.
1. Introduction
Most of the lowland forests of Southeast Asia are dipterocarp forests [1,2,3] with Dipterocarpaceae the most dominant family over vast areas in Southeast Asia’s forests [4]. It is an essential plant in Malaysia, not only for its valuable timber tree species but also for the presence of the genus Shorea [5]. Shorea Roxb. is the largest genus of Dipterocarpaceae and the most dominant emergent tree genus in tropical Asia [6]. There have been minimal conservation efforts of dipterocarps in recent decades as the family was regarded as a common species free of threats. However, due to the changes in land-use patterns to meet the increasing demands of resources, the sustainability of dipterocarp species needs to be addressed.
Deforestation is defined as converting forests for other land uses [7]. Farming, agriculture, mining, urbanisation, and plantation are leading causes of deforestation. Between 2001 and 2018, Malaysia estimated that around 34.0% of total tree cover was lost due to deforestation [8]. Uncontrolled deforestation will lead to forest degradation, which lowers productivity and hampers the essential forest services. Forest rehabilitation was identified as a reliable effort to restore the forest area. The reduction of forest area as the primary terrestrial carbon sink disrupts the global carbon cycle and increases atmospheric carbon dioxide concentration. This reduction impacts global warming in two ways: the acceleration of greenhouse gas emissions, such as carbon dioxide, methane, and nitrogen oxides; and reduction in carbon sequestration by tropical trees, such as Shorea species, during photosynthesis [9]. Forest destruction has led to carbon emissions with 4.3–5.5 Gt CO2 eq/year [10]. Thus, protecting and restoring this vast carbon sink is essential for mitigating climate change.
There is an urge to combat climate change and its impacts due to the increment of the 2020 global average temperature. The temperature was reported at 1.2 °C above the pre-industrial baseline which is woefully off track to stay at or below 1.5 °C, as stated in the Paris Agreement [11]. The Bonn Challenge and New York Declaration on Forest (NYDF) aim to restore 150 million hectares of degraded land by 2020 and 350 million hectares by 2030 [10]. In addition, these efforts were aligned with the existing international commitment, such as the Aichi target from the Convention on Biological Biodiversity [12], the UNFCC REDD+ goal [13], and the sustainable development goals (SDGs) by the United Nations [11]. In Malaysia, the rehabilitation efforts are actively executed through a tree-planting program by the government which is known as Greening Malaysia. To date, 46,298,820 trees from 1349 species were planted to achieved the goal of 100 million trees planted by 2025 [14].
Forest rehabilitation is arguably the most promising way to restore forest capacity. However, the selection of tree species should be prioritised to achieve the ultimate goals of rehabilitation. The use of native tree species rather than exotic tree species should be emphasised to preserve the well-known tropical rainforest dominant tree species. The use of native tree species could influence the structure and function of the tropical forest [5]. Shorea is a fast-growing hardwood and the most promising plant for dipterocarp plantation in Peninsular Malaysia [5,15,16,17,18]. The success of each rehabilitation effort differs based on site conditions. Factors that determine the success of rehabilitation include the type of disturbance, forest remnant, climate [19], seed dispersal [20], wood density [21], species selection, seedling quality [22], and site maintenance in the early stage of the establishment [7]. Nevertheless, the real determinant for restoration success is still a mystery as the success of each restoration differs based on the condition of the local environment [19].
Generally, various environmental conditions affect plant growth, such as light, soil water content, temperature, and soil nutrients [23]. Among these, light is the most important environmental factor affecting plant establishment, growth, and survival [24,25]. Taiz and Zeiger [26] also supported the importance of light in plant growth as light energy was used to produce ATP and NADPH in the light reactions of photosynthesis. The optimum light irradiance to support plant growth, especially in an altered environment, is scarce and varies depending on plant species. Additionally, the need for nutrient addition to the available nutrient content in soil needs to be addressed, especially in dipterocarp species, to achieve optimum growth. The present study aims to investigate the growth performance and photosynthetic responses of two Shorea species in different light irradiance and nutrient amendments.
2. Materials and Methods
2.1. Study Area
The study site is located at Tembat Forest Reserve (FR), consisting of Tembat and Puah Dams, north of the existing Kenyir Dam in Kuala Berang, Hulu Terengganu, Peninsular Malaysia (Figure 1). It is around 50 km from Gua Musang-Hulu Terengganu highway and around 65 km west of Kuala Terengganu. The estimated terrain elevation of Tembat FR is 284 m above sea level. Its latitude is 5°05′03″ N, and the longitude is 102°44′13″ E. Tembat FR is a part of the Central Forest Spine (CFS), running parallel to the Main Range. Its existence is crucial for biodiversity and environmental protection. The study site can be categorised as a lowland dipterocarp forest to a hill dipterocarp forest in which the altitudes range between 150 m and 420 m.
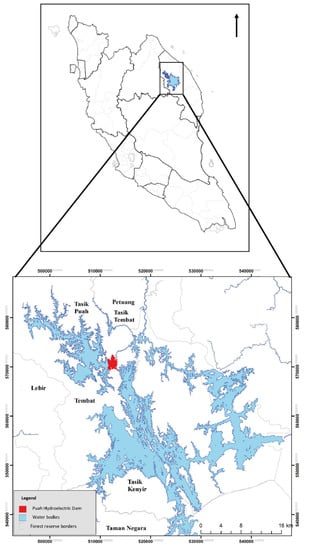
Figure 1.
Study area, Tembat Forest Reserve, Terengganu, Peninsular Malaysia.
2.2. Plant Materials and Experimental Design
The experiment was conducted for seven months from October 2018 until May 2019 at Hulu Terengganu, Peninsular Malaysia, and the factorial RCBD design was implemented. In this study, both Shorea leprosula Miq. and S. acuminata Dyer were used because these species assemble good traits and have the survival potential that matches the rehabilitation sites. Nineteen-month-old seedlings of S. leprosula and S. acuminata were obtained from Ajil’s Nursery owned by Forestry Department Terengganu. Three seedlings from both species were planted in a 914.4 cm2 customised planter box (n = 3). Two treatments were applied: light irradiance and nutrient amendments with three replicates for each level of treatment. The levels of light irradiance were 100% (control), 30%, and 8%, while the nutrient applications were divided into the control (without fertiliser), NPK, and vermicompost (VC) (Figure 2).

Figure 2.
Shorea leprosula Miq. and Shorea acuminata Dyer tree seedlings in three levels of light irradiance; (a) 100% (control); (b) 30%; and (c) 8%.
2.3. Data Collection
2.3.1. Growth Parameters
The plant height, stem diameter, number of leaves, and relative chlorophyll concentration were measured monthly for seven months. Plant height was measured from the soil surface until the tips of the upper shoot using a ruler. Using a digital Vernier calliper, the stem diameter was measured 2 cm from the soil surface. A chlorophyll content meter (Hansatech Instruments Ltd., King’s Lynn, Norfolk, UK) was used to measure relative chlorophyll concentration. At the end of the experiment, the shoots and roots of each plant were sampled and immediately dried at 80 °C in an oven for 72 h to measure dry weight and later the biomass accumulation. The leaf area was measured using Image-J software (Version 1.52a, Bethesda, MD, USA). Each young, matured leaf was photographed using a digital camera on a whiteboard with a customised 3 cm scale. Then, the images were processed using the software to obtain the leaf area.
2.3.2. Photosynthetic Parameters
Photosynthesis rate (A, µmol m−2 s−1), transpiration rate (E, mmol−2 s−1), stomatal conductance (gsw, mol m−2 s−1), water-use efficiency (WUE, µmol CO2 mmol−1 H2O), and intercellular carbon concentration (Ci, µmol mol−1) were measured for the leaf gas exchange traits. All gaseous-exchange measurements were conducted with portable photosynthesis-measurement equipment (Li-6400XT and Li-6800; LI-COR, Inc., Lincoln, NE, USA) referring to Yong et al. [27] with some modification. The measurements were taken at an ambient CO2 concentration of 400 μmol mol−1, air temperature of 29–30 °C, relative humidity of 55–65%, and photosynthetic photon flux density (PPFD) of 1300 μmol mol−1 in the leaf chamber. One fully expanded leaf per plant was selected for each of the measurements.
2.4. Data Analysis
The collected data were subjected to analysis of variance (ANOVA) using R Studio (Version 1.2.5033, Boston, MA, USA) to find out if there were any significant differences among the treatment levels of light intensity and nutrient application with a significant level of p < 0.05. When significant differences were found, the means were separated using Tukey’s HSD test. Principal component analysis (PCA) was performed using morphological data and leaf gas exchange measurements to reduce data dimensionality.
3. Results
The growth performances for both species were the highest in 30% irradiance (Table 1) except for the biomass, relative chlorophyll concentration, and root-to-shoot ratio. The leaf gas exchange parameters showed the highest photosynthesis rate (A) and stomatal conductance (gsw) in 30% irradiance (Table 2). In terms of the nutrient amendment, all growth traits showed higher responses when treated with vermicompost than the control except for leaf area and root-to-shoot ratio. The leaf area was higher with the amendment of NPK instead of vermicompost. The growth traits also gave significant differences among nutrient levels and species. Transpiration rate (E), water-use efficiency (WUE), and intercellular carbon concentration (Ci) have higher values with the application of vermicompost compared to the control, while photosynthesis rate (A) and stomatal conductance (gsw) favoured the application of NPK (Table 2). Three out of five photosynthetic characteristics were also higher with vermicompost amendments compared to the control (no fertiliser).

Table 1.
The effects of light irradiance and nutrient amendments on morphological growth characteristics in two Shorea species.

Table 2.
The effects of light irradiance and nutrient amendments on physiological characteristics in two Shorea species.
3.1. Growth and Photosynthetic Responses of S. leprosula and S. acuminata Grown under Different Light Irradiance
Comparing three different levels of light irradiance, the control showed the highest photosynthetically active radiation (PAR) compared to 30% (single layer of shading) and 8% (double layer of shading) irradiance (Figure 3). The peak of light irradiance was at noon for all light-intensity levels. The control recorded the highest PAR which reached up to 1200 μmol m−2 s−1 followed by 30% (400 μmol m−2 s−1) and 8% (150 μmol m−2 s−1) irradiance.
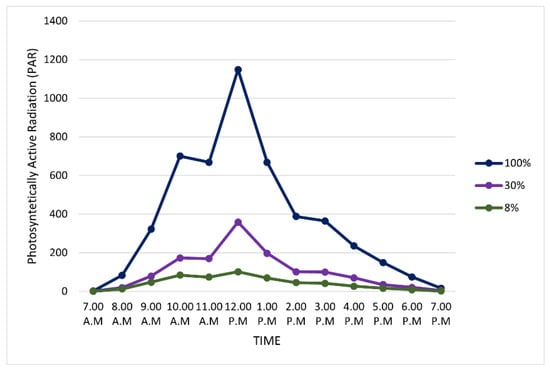
Figure 3.
Photosynthetically active radiation (PAR) differences between three levels of light irradiance.
Generally, S. leprosula showed better growth performance in all growth parameters compared to S. acuminata except for the root-to-shoot ratio (Figure 4). Shorea leprosula showed the highest RHGR, RDGR, and the mean number of leaves in 30% light irradiance (Figure 4a–c). The RHGR and mean number of leaves for S. leprosula grown in 30% irradiance were doubled compared to the control. RDGR was 18.2% higher in 30% irradiance (0.30 mm mm−1 month−1) than the control (0.25 mm−1 month−1). In contrast, the highest biomass and relative chlorophyll concentration (RCC) were recorded in 8% irradiance with both 24.4% and 38.3% higher than the control, respectively. The root-to-shoot ratio was twofold lower in 8% irradiance than the control (Figure 4f).
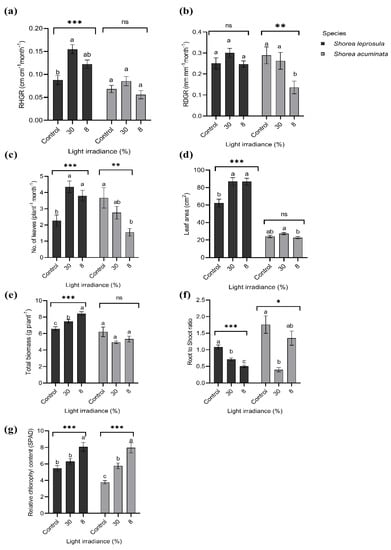
Figure 4.
The growth performance of Shorea leprosula Miq. and Shorea acuminata Dyer to different light irradiances; (a) relative height growth rate (RHGR) (cm cm−1 month−1); (b) relative diameter growth rate (RDGR) (mm mm−1 month−1); (c) mean number of leaves (plant−1 month−1); (d) leaf area (cm2); (e) biomass (g plant−1); (f) root-to-shoot ratio; and (g) relative chlorophyll concentration (SPAD) (mean ± SE). Alphabets denote significant differences between light irradiance treatments with *, **, and *** indicating significant at p < 0.05, p < 0.01, and p < 0.001, respectively; ns = non-significant.
Shorea acuminata showed different growth responses compared to S. leprosula. Four out of seven parameters for S. acuminata showed lower values in both 8% and 30% compared to the control viz. RDGR, means number of leaves, biomass, and root-to-shoot ratio (Figure 4). The RDGR and means number of leaves were two times and three times lower, respectively, in 8% irradiance than the control. Biomass did not show a significant difference in 30% irradiance (4.95 g plant−1) compared to the control (6.21 g plant−1), while the root-to-shoot ratio was almost four times lower in 30% irradiance than the control. RCC doubled in 8% irradiance compared to the control (Figure 4g).
For all the leaf gas exchange characteristics, S. leprosula has greater values compared to S. acuminata (Figure 5), with a significant difference between these two species (Table 2). Shorea leprosula recorded the highest photosynthesis rate (A) in 30% light irradiance compared to the control (Figure 5a), but the differences were insignificant. The transpiration rate (E), stomatal conductance (gsw), and water-use efficiency (WUE) showed the lowest values in 8% irradiance compared to the control (Figure 5b,d,e).
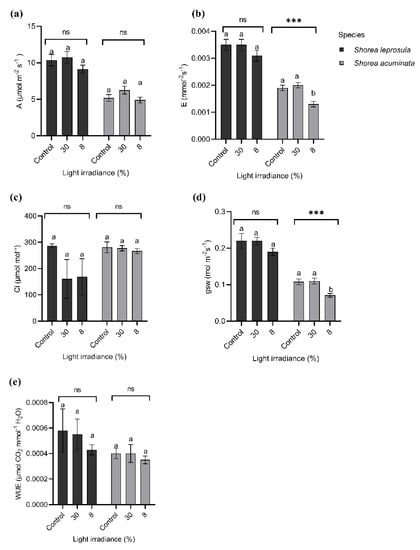
Figure 5.
Shorea leprosula Miq. and Shorea acuminata Dyer gas exchange characteristics in different light irradiances (mean ± SE); (a) photosynthesis rate (A, µmol m−2 s−1); (b) transpiration rate (E, mmol−2 s−1); (c) intercellular carbon concentration (Ci, µmol mol−1); (d) stomatal conductance (gsw, mol m−2 s−1); (e) and water-use efficiency (WUE, µmol CO2 mmol−1 H2O). Alphabets denote significant differences between light irradiance treatments with *** indicating significant at p < 0.001, respectively; ns = non-significant.
All the leaf gas exchange parameters for S. acuminata were higher in 30% irradiance than the control except for WUE and intercellular CO2 concentration (Ci), which showed the opposite trend (Figure 5a–e). Surprisingly, S. acuminata has a significantly higher Ci value than S. leprosula despite different light irradiance levels.
3.2. Growth and Photosynthetic Responses of S. leprosula and S. acuminata Grown in Different Nutrient Amendments
Similarly, in response to different light irradiances, S. leprosula has better growth performance than S. acuminata except for the root-to-shoot ratio (Figure 6). The RHGR, RDGR, mean number of leaves, biomass, and RCC for S. leprosula showed the highest values with vermicompost amendment compared to the control (no fertiliser). The RHGR, RDGR, and biomass were 36%, 50%, and 6% higher in vermicompost, respectively, when compared to the control. Additionally, the mean number of leaves was twofold higher with the addition of VC compared to the control. The leaf area and RCC showed the opposite trend, with the highest value observed in NPK. The leaf area and RCC were 40% and 20% higher than the control, respectively. The root-to-shoot ratio was approximately twofold lower in VC compared to the control.
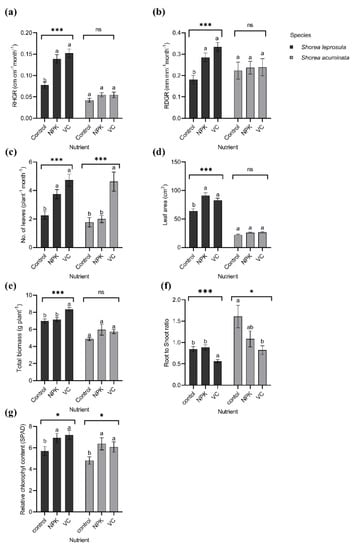
Figure 6.
The growth performance of Shorea leprosula Miq. and Shorea acuminata Dyer to different nutrient amendments; (a) relative height growth rate (RHGR) (cm cm−1 month−1); (b) relative diameter growth rate (RDGR) (mm mm−1 month−1); (c) mean number of leaves (plant−1 month−1); (d) leaf area (cm2); (e) biomass (g plant−1); (f) root-to-shoot ratio; and (g) relative chlorophyll concentration (SPAD) (Mean ± SE). Alphabets denote significant differences between light irradiance treatments with *, and *** indicating significant at p < 0.05, and p < 0.001, respectively; ns = non-significant.
The growth responses of S. acuminata only showed mean number of leaves as the highest value (approximately twofold) in VC compared to the control (Figure 6c). The RHGR, RDGR, and leaf area for both NPK and VC shared the same value and were 27%, 9%, and 17% higher than the control, respectively (Figure 6a,b,d). The RCC and total biomass were higher in NPK with 35% and 20%, respectively, compared to the control (Figure 6e,g). The root-to-shoot ratio was twice as low in VC than the control (Figure 6f).
Shorea leprosula has higher leaf gas exchange characteristics compared to S. acuminata. The photosynthesis rate (A), transpiration rate (E), and stomatal conductance (gsw) were higher in the application of NPK with approximately 55%, 6%, and 15% higher compared to the control, respectively (Figure 7a,b,d). In contrast, WUE was 9% higher with the addition of vermicompost compared to the control (Figure 7e).
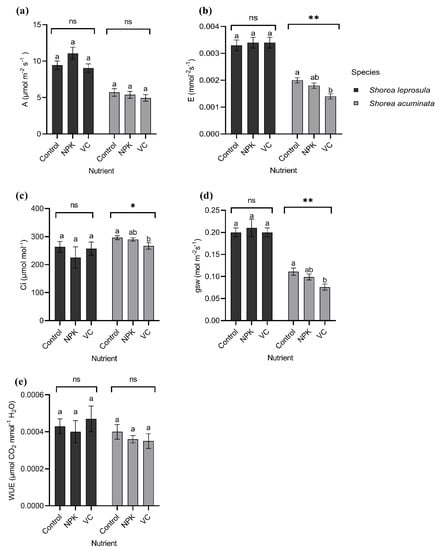
Figure 7.
Shorea leprosula Miq. and Shorea acuminata Dyer gas exchange characteristics in different light irradiances (Mean ± SE); (a) photosynthesis rate (A, µmol m−2 s−1); (b) transpiration rate (E, mmol−2 s−1); (c) intercellular carbon concentration (Ci, µmol mol−1); (d) stomatal conductance (gsw, mol m−2 s−1); (e) and water-use efficiency (WUE, µmol CO2 mmol−1 H2O). Alphabets denote significant differences between light irradiance treatments with *, and ** indicating significant at p < 0.05, and p < 0.01, respectively; ns = non-significant.
The trend observed in S. acuminata was contrary to S. leprosula. All the leaf gas exchange characteristics showed a decreased value in VC compared to the control (Figure 7a–e).
The interaction between light and nutrients showed 8% irradiance × VC (8%*VC) obtained the highest total biomass for S. leprosula (Figure 8a), while the control treatment C × NF (100% irradiance × no fertiliser) had the lowest total biomass. As for S. acuminata, the highest total biomass was obtained using C × NPK, while the lowest was the 30% × NPK (Figure 8b). However, the differences were not significant in S. acuminata. The root-to-shoot ratio showed the highest value in C × NPK and C × NF (control), while the lowest root-to-shoot ratio for S. leprosula was observed in 8% × VC (Figure 9a). Shorea acuminata showed the highest root-to-shoot ratio in the control treatment (C × NF) and the lowest in 8% × NPK (Figure 9b).
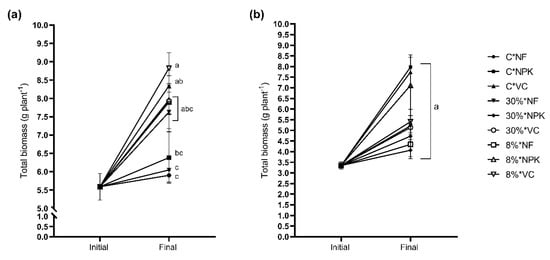
Figure 8.
Total biomass of Shorea leprosula Miq. (a) and Shorea acuminata Dyer (b) in response to the interaction factor, light × nutrient. Alphabets denote significant differences between light × nutrient interaction.
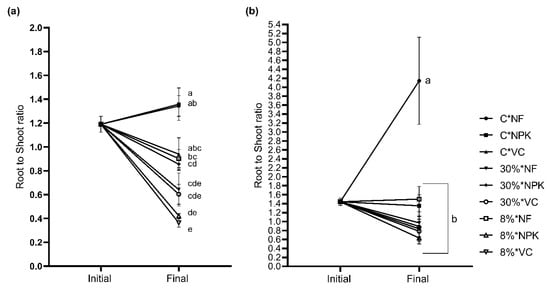
Figure 9.
Root-to-shoot ratio of Shorea leprosula Miq. (a) and Shorea acuminata Dyer (b) in response to the interaction factor, light × nutrient. Alphabets denote significant differences between light × nutrient interactions.
3.3. Establishing the Optimum Level of Light Irradiance and Nutrient Amendments
Principal components analysis based on 12 plant traits explained 79.6% of the variance in the first three principal components (Table 3 and Figure 10). The first component (PC1) represented 53.0% of the variability and accounted primarily for RHGR, RDGR, leaf area, biomass, and WUE. The second component (PC2) represented 15.1% of the variance and primarily comprised the mean number of leaves, root-to-shoot ratio, E, A, Ci, and gsw (Table 3). Biplots from PCA analysis showed S. leprosula with 30% light irradiance were scattered on the right-hand side of the biplot (Figure 10). The results of PCA indicated that RHGR and leaf area were two key factors (with major positive effects) in PC1 (Table 3). SLVC30 had higher PC1 scores, indicating better performance for the traits measured than other treatments (Figure 10).

Table 3.
Variable loading scores of 12 parameters for two Shorea species (S. leprosula Miq. and S. acuminata Dyer) in different levels of light irradiance and nutrient amendment and the proportion of variation of each principal component.
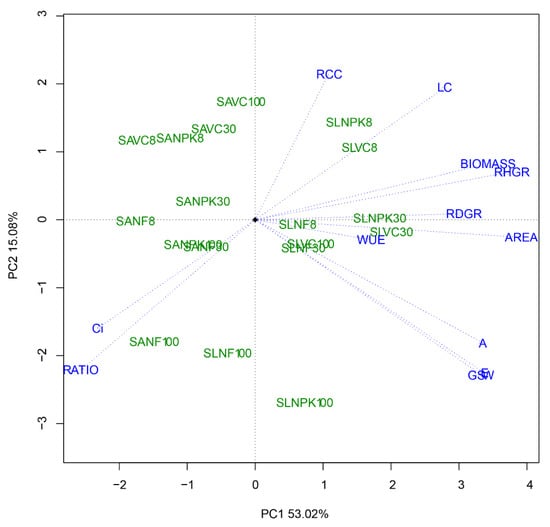
Figure 10.
Principal component analysis (PCA) biplot for two Shorea species, S. leprosula (SL) and S. acuminata (SA), growing under different levels of light irradiance (8%, 30%, 100%) and nutrient amendments (NF, NPK, VC). Growth responses: relative height growth rate (RHGR) (cm cm−1 month−1), relative diameter growth rate (RDGR) (mm mm−1 month−1), mean number of leaves (LC) (plant−1 month−1), leaf area (AREA) (cm2), biomass (g plant−1), Root-to-shoot ratio (RATIO), and relative chlorophyll concentration (RCC) (SPAD). Gas exchange measurements: photosynthesis rate (A, µmol m−2 s−1), transpiration rate (E, mmol−2 s−1), stomatal conductance (gsw, mol m−2 s−1), water-use efficiency (WUE, µmol CO2 mmol−1 H2O), and intercellular carbon concentration (Ci µmol mol−1).
4. Discussion
4.1. Growth and Photosynthetic Responses of S. leprosula and S. acuminata Grown in Different Light Irradiances
The present study showed that plants grown in 30% irradiance had the highest RHGR and photosynthetic rate (A) compared to plants grown in 8% irradiance and exposed to direct sunlight (control). The growth results were in line with the rate of photosynthesis (A), with the highest value in 30% irradiance. Plants grown in 8% irradiance recorded the lowest rate of photosynthesis as they received the lowest light irradiance. Lack of light irradiance will cause the limitation of energy sources to undergo photosynthesis [28] as plant growth and the leaf eco-physiological traits are closely related to the light environment [17,29,30,31], although various environmental factors exist in the forest. Contrary to the current results, a high-growth rate of Dyera costulata and Dipterocarpus baudii were observed under high-light conditions that correspond to the high photosynthetic rate [32]. This is because each plant has its own optimal light irradiance range to support growth [33]. Light can become a stress factor if supplied too much or too little. The increase in illumination may also cause photoinhibition and decreased photosynthetic efficiency if the input of photons exceeds the plant’s photosynthetic capacity [34,35,36,37]. Excessive light irradiance will cause over energisation of the photosynthetic apparatus, leading to photoinhibition or even photo-destruction [28]. The plants grown under control showed photoinhibition symptoms with lower growth performance and yellowish leaf appearance, but they had developed a specific mechanism to adapt to changes to survive. Roeber et al. [38] stated that plants exhibit two different systems to perceive environmental light information viz. photoreceptors and chloroplast to cope with oxidative damage under high-light stress as an adaptive mechanism. In addition, we believe the rate of photosynthesis fluctuates within the growth time frame. The initial, mid, and final photosynthetic measurements might have different results as the microclimatic changes also affect the whole process. Usuda [39] also supported that the rate of photosynthesis changes very much during development.
Direct sunlight caused S. leprosula and S. acuminata to gain the highest root-to-shoot ratio and intercellular carbon concentration (Ci). A high level of intercellular CO2 induces closure of the stomata in the same way as ABA-induced closure [40] which also resulted in lower A under 100% light irradiance in this study. Carbon dioxide diffuses through stomatal pores on the leaf surfaces, altering the intercellular carbon concentration (Ci) [41]. The intercellular CO2 was essential to indicate the CO2 substrate available for photosynthetic assimilation. Additionally, Ci also controls the opening and closing of the stomata. A high concentration of CO2 in intercellular spaces causes partial closure of the stomata during daytime [42]. Once the stomata are closed, external CO2 concentration does not affect stomatal movement.
4.2. Growth and Photosynthetic Responses of S. leprosula and S. acuminata Grown in Different Nutrient Amendments
The nutrient application showed positive plant growth performance for both species. Vermicompost (VC) recorded better growth performance than the well-known chemical fertiliser, NPK, but the results were more prominent in S. leprosula. The present study showed that the application of vermicompost had a significantly lower root-to-shoot ratio than the control. Despite better morphological features observed with the application of VC, the rate of photosynthesis (A) showed better responses towards NPK.
According to Lynch et al. [43], the reduction in the root-to-shoot ratio is associated with the increment of soil fertility, which displays that the shoot growth was higher than root growth. Blouin et al. [44] mentioned that the addition of vermicompost significantly increased shoot biomass by 78% and root biomass by 57%. The present study showed that organic fertilisers can provide better growth than chemical fertilisers. This result also supports the previous study which proved the use of biostimulants (with low NPK values) can produce the same plant growth promotion effect comparable to chemical fertiliser (high NPK values) application [45]. Vermicompost has been widely used in agriculture to increase crop production [46,47,48,49]. Using vermicompost to rehabilitate degraded soil would be the most promising way to enhance soil physicochemical recovery. Lal [50] and Dignac et al. [51] mentioned that the large-scale use of composts is a good way to increase the soil content in organic matter, which is critical for their long-term fertility. Altogether, the growth responses tend to be greater under conditions where plants have access to adequate nutrients [52].
Surprisingly, S. leprosula grown in vermicompost application had the lowest A. According to Longstreth and Nobel [53], plant mineral status markedly affects the rate of photosynthesis. Two types of mineral nutrients often limit plant growth. These nutrients are nitrogen (N) and phosphorus (P) which are required in large amounts to their availability in soil [54]. Plants require a certain amount of nutrients to support their growth. The amount could be minimum, optimum, or beyond optimum, which can cause toxicity and later reduce growth and photosynthetic rates. Worse come to worst, plants will eventually die [55,56]. The nutrient content in the control soil might have sufficient amounts of nutrients to support the photosynthesis processes. Thereby, the plants do not show any response toward the additional nutrient. The results were opposed to the results obtained by Wright et al. [57] in tree seedlings, saplings, and poles of Alseis blackiana, a lowland tropical forest tree species that showed increased growth by enhancing photosynthesis in response to fertilisation.
4.3. Interaction between Species or Factors
Shorea leprosula and S. acuminata showed different light preferences, with S. leprosula exhibiting better growth. Both species were known as high light-demanding and for rapid early growth [58,59,60], but the present results were less pronounced in S. acuminata. Competition between both species for resources might be why both were growing together. Shorea leprosula were investing more on height increment, while S. acuminata were observed to have higher leaf proliferation. Schmitt and Wuff [61] stated that the presence of taller neighbours will decrease the red or far-red light ratio above the crowns of shorter trees, triggering shoot elongation of shorter trees. This would be a morphological adaptation for S. acuminata to anticipate and avoid increasing competitive intensity for light. According to Appanah and Weiland [4], S. leprosula has been known as a dipterocarp species tolerant to water stress and a light demander in the early stage of growth. In addition, this species can adapt to a wide range of site distribution [62]. Shorea leprosula was also a fast-growing, light hardwood species and high light demander [63,64]. Suzuki et al. [64] also stated that this species requires relatively higher light levels for survival or growth. Lack of research addressing the effect of light on S. acuminata as this species was reported to have adaptations similar to S. leprosula.
The difference between these two species was easily spotted as S. leprosula has a bigger leaf, while S. acuminata has a smaller pinnately compound leaf. This feature explains the significant difference between both species leaf areas. Size and shape of leaves are largely genetically controlled, but the developmental flexibility exists even within an individual plant depending on environmental circumstances prevailing during leaf formation [65,66]. Leaves expand to intercept light for photosynthesis, take up carbon dioxide, and transpire water for cooling and circulation [65,67]. The sophisticated sensing mechanisms in plants help determine the available nutrients in soil, atmosphere, and light, thus conveying a response by regulating biochemical processes.
The addition of any resources should increase plant growth because plants adjust the allocation of resources until growth is equally limited by all resources [68]. Both species showed different nutrient acquisition strategies. The nutrient-uptake efficiency influenced both species’ growth performances and photosynthetic responses towards nutrients. Shorea leprosula showed that the root efficiency might cause better responses in absorbing the available nutrients, and S. acuminata does not show any responses with any nutrient amendments. The justification for this scenario might be the types of nutrient storage applied by plants. As mentioned by Chapin et al. [69], there were three types of nutrient storage: accumulation, reserve formation, and recycling. Accumulation of nutrient compounds does not directly promote growth, while reserve formation involves the synthesis of storage compounds from resources that might otherwise promote growth. Recycling storage involves the compounds breaking down and possibly mobilising for later growth.
Competition among plants for resources has long been measured to generate stress for plants and is important for determining the distribution of species [70]. In this study, the competition for resources between species had caused S. acuminata to be outcompeted by S. leprosula as the growth performance was more pronounced in S. leprosula. As Craine and Dybzinski [70] mentioned, the presence of multiple plants in a given volume of soil can induce nutrient stress in a given plant as neighbors acquire limitations. Each plant individual differentially captures a potentially common limiting resource supply. Additionally, the responses of individuals in a mixture also reflects their interactions with their biotic and abiotic environments [71,72].
5. Conclusions
The alarming state of the current climate has forced the urge to initiate the rehabilitation of degraded tropical forests. Rehabilitation or restoration should emphasise bringing back the forest service instead of forest cover. The species selection should be prioritised to mitigate climate change by absorbing more carbon and optimising the conditions under which increased photosynthesis can lead to maximal growth increases. In addition, the tree selection should not restrict their tolerance to available light but also the ability to adapt to their microclimate. Light irradiance and nutrient amendment were proven to affect the growth performances of S. leprosula and S. acuminata, and both species showed different preferences. Plants grown in 30% irradiance and vermicompost applications were considered an ideal condition for better growth performance. Generally, both treatments contributed to an approximately 50% increase in height, diameter, and leaf proliferations compared with the control plants. Vermicompost was widely used and known to bring success in agriculture by providing a better yield, but the application of vermicompost towards dipterocarps tree species was limited. Based on the results, vermicompost application was highly recommended in large-scale dipterocarp plantations to rehabilitate degraded tropical forests as it provides both short-term and long-term benefits. This will conserve dipterocarp species from extinction and improve the productivity of the tropical rainforest.
Author Contributions
A.R.S.N.; writing—original draft preparation, W.A.W.J.; writing—review and editing, K.S. and M.N.S.; supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Tenaga Nasional Berhad Research (TNBR) research grant (ST-2017-010) and Research University Grants (GUP) (GUP-2018-022) and in collaboration with Terengganu Forestry Department (JPNT).
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the anonymous reviewers for their valuable suggestions and comments on the manuscript. Thanks to Jean Wan Hong Yong from Swedish University of Agriculture Sciences for all the valuable insights, especially on site, and Mohd Ikmal Asmuni for the helpful guidance in data analysis.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Slik, J.; Poulsen, A.; Ashton, P.; Cannon, C.; Eichhorn, K.; Kartawinata, K.; Lanniari, I.; Nagamasu, H.; Nakagawa, M.; van Nieuwstadt, M.; et al. A Floristic Analysis of the Lowland Dipterocarp Forests of Borneo. J. Biogeogr. 2003, 30, 1517–1531. [Google Scholar] [CrossRef]
- Brearley, F.Q.; Banin, L.F.; Saner, P. The Ecology of the Asian Dipterocarps. Plant Ecol. Divers. 2016, 9, 429–436. [Google Scholar] [CrossRef]
- Ghazoul, J. A Short and Sweet (but Not Too Sweet), Introduction to Our Forests Forests: A Very Short Introduction. Biotopica 2016, 48, 927. [Google Scholar]
- Appanah, S.; Ashton, M.; Bawa, K.; Curtet, L.; Elouard, C.; Jantan, I.; Krishnapillay, B.; Lee, S.; Maury-Lechon, G.; Shiva, M.; et al. A Review of Dipetrocarps: Taxonomy, Ecology and Silviculture; Center for International Forestry Research: Bogor, Indonesia, 1998. [Google Scholar]
- Fatma, N.; Wan Juliana, W.A.; Shaharuddin, M.I.; Wickneswari, R. Stand Structure of Shorea and Spatial Distribution of Shorea Acuminata in a Rehabilitated Area of Kenaboi Forest Reserve. J. Trop. For. Sci. 2020, 32, 70–80. [Google Scholar] [CrossRef]
- Ashton, P. Dipterocarpaceae. In Flowering Plants. Dicotyledons. The Families and Genera of Vascular Plants; Kubitzki, K., Bayer, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 5, pp. 182–197. [Google Scholar] [CrossRef]
- FAO. Assessing Forest Degradation: Towards the Development of Globally Applicable Guidelines; FAO: Rome, Italy, 2011; p. 177. [Google Scholar]
- Mongabay. Deforestation Statistics for Malaysia. Available online: Rainforest.mongabay.com (accessed on 12 December 2020).
- Kira, T. Forest Ecosystems of East and Southeast Asia in a Global Perspective. Ecol. Res. 1991, 6, 185–200. [Google Scholar] [CrossRef]
- International Union for Conservation Nature. Bonn Challenge. Available online: https://www.bonnchallenge.org/ (accessed on 21 August 2021).
- United Nations. The Sustainable Development Goals Report 2021; UN: New York, NY, USA, 2021. [Google Scholar]
- United Nations. Quick guide to the aichi biodiversity targets. In Convention on Biological Divresity; UN: New York, NY, USA, 2013; pp. 1–42. [Google Scholar]
- UNFCCC. Key Decisions Relevant for Reducing Emissions from Deforestation and Forest Degradation in Developing Countries (REDD+); UNFCCC: Bonn, Germany, 2014. [Google Scholar]
- Kementerian Tenaga dan Sumber Asli. Penghijauan Malaysia: Kempen Penanaman 100 Juta Pokok. Available online: https://100jutapokok.gov.my/ (accessed on 11 September 2021).
- Kobayashi, S. Landscape Rehabilitation of Degraded Tropical Forest Ecosystems: Case Study of CIFOR/Japan Project in Indonesia and Peru. For. Ecol. Manag. 2004, 201, 13–22. [Google Scholar] [CrossRef]
- Kettel, J. Ecological Considerations for Using Dipterocarps for Restoration of Lowland Rainforest in Southeast Asia. Biodivers. Conserv. 2010, 19, 1137–1151. [Google Scholar] [CrossRef]
- Daisuke, H.; Tanaka, K.; Joseph Jawa, K.; Ikuo, N.; Katsutoshi, S. Rehabilitation of Degraded Tropical Rainforest Using Dipterocarp Trees in Sarawak, Malaysia. Int. J. For. Res. 2013, 2013, 683017. [Google Scholar] [CrossRef]
- Widiyatno, W.; Soekotjo, S.; Naiem, M.; Purnomo, S.; Setiyanto, P.E. Early Performance of 23 Dipterocarp Species Planted in Logged-over Rainforest. J. Trop. For. Sci. 2014, 26, 259–266. [Google Scholar]
- Crouzeilles, R.; Ferreira, M.S.; Chazdon, R.L.; Lindenmayer, D.B.; Sansevero, J.B.B.; Monteiro, L.; Iribarrem, A.; Latawiec, A.E.; Strassburg, B.B.N. Ecological Restoration Success Is Higher for Natural Regeneration than for Active Restoration in Tropical Forests. Sci. Adv. 2017, 3, e1701345. [Google Scholar] [CrossRef]
- Gallegos, S.C.; Hensen, I.; Schleuning, M. Secondary Dispersal by Ants Promotes Forest Regeneration after Deforestation. J. Ecol. 2014, 102, 659–666. [Google Scholar] [CrossRef]
- Charles, L.S.; Dwyer, J.M.; Smith, T.J.; Connors, S.; Marschner, P.; Mayfield, M.M. Species Wood Density and the Location of Planted Seedlings Drive Early-Stage Seedling Survival during Tropical Forest Restoration. J. Appl. Ecol. 2018, 55, 1009–1018. [Google Scholar] [CrossRef]
- Blakesley, D.; Anusarnsunthorn, V.; Kerby, J.; Navakitbumrung, P.; Kuarak, C.; Zangkum, S.; Hadwick, K.; Elliott, S. Nursery technology and tree species selection for restoring forest biodiversity in Northern Thailand. In Forest Restoration for Wildlife Conservation; Elliott, S., Kerby, J., Blakesley, D., Hardwick, K., Woods, K., Anusarnsunthorn, V., Eds.; International Tropical Timber Organization dan Forest: Chiang Mai, Thailand, 2000; pp. 207–222. [Google Scholar]
- Rhie, Y.H.; Lee, S.Y.; Jung, H.H.; Kim, K.S. Light Intensity Influences Photosynthesis and Crop Characteristics of Jeffersonia Dubia. Korean J. Hortic. Sci. Technol. 2014, 32, 584–589. [Google Scholar] [CrossRef][Green Version]
- Niinemets, Ü.; Sun, Z.; Talts, E. Controls of the Quantum Yield and Saturation Light of Isoprene Emission in Different-Aged Aspen Leaves. Plant Cell Environ. 2015, 38, 2707–2720. [Google Scholar] [CrossRef]
- Gitelson, A.; Chiykunova, O.; Zhigalova, T.; Solovchenko, A. In Situ Optical Properties of Foliar Flavonoids: Implication for Non-Destructive Estimation of Flavonoid Content. J. Plant Physiol. 2017, 218, 258–264. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Photosynthesis: Physiological and ecological considerations. In Plant Physiology; Sinauer Associates: Sunderland, UK, 2006; pp. 249–264. [Google Scholar]
- Yong, J.W.H.; Ng, Y.F.; Tan, S.N.; Chew, A.Y.L. Effect of Fertilizer Application on Photosynthesis and Oil Yield of Jatropha curcas L. Photosynthetica 2010, 48, 208–218. [Google Scholar] [CrossRef]
- Lüttge, U. Plant physiology. In Encyclopedia of Ecology; Fath, B., Ed.; Elsevier Inc.: Darmstadt, Germany, 2019; pp. 549–557. [Google Scholar] [CrossRef]
- Sasaki, S. Ecology and physiology of Dipterocarpaceae. In Plantation Technology in Tropical Forest Science; Springer: Berlin/Heidelberg, Germany, 2006; pp. 3–22. [Google Scholar] [CrossRef]
- Turner, I.M. The Ecology of Trees in the Tropical Rain Forest, 1st ed.; Ashton, P., Hubbell, S., Janzen, D., Raven, P., Tomlinson, P., Eds.; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Kenzo, T.; Yoneda, R.; Azani, M.A. Artificial Shade Shelters Mitigate Harsh Microclimate Conditions and Enhance Growth in Tropical Tree Seedlings Planted in Degraded Land. Tropics 2020, 29, 121–132. [Google Scholar] [CrossRef]
- Kenzo, T.; Yoneda, R.; Matsumoto, Y.; Mohamad Azani, A.; Nik Majid, M. Growth and Photosynthetic Response of Four Malaysian Indigenous Tree Species under Different Light Conditions. J. Trop. For. Sci. 2011, 23, 271–281. [Google Scholar]
- Pan, J.; Guo, B. Effects of Light Intensity on the Growth, Photosynthetic Characteristics, and Flavonoid Content of Epimedium Pseudowushanense B.L.Guo. Molecules 2016, 21, 1475. [Google Scholar] [CrossRef]
- Dai, Y.; Shen, Z.; Liu, Y.; Wang, L.; Hannaway, D.; Lu, H. Effects of Shade Treatments on the Photosynthetic Capacity, Chlorophyll Fluorescence, and Chlorophyll Content of Tetrastigma Hemsleyanum Diels et Gilg. Environ. Exp. Bot. 2009, 65, 177–182. [Google Scholar] [CrossRef]
- Idris, A.; Tun, U.; Onn, H.; Linatoc, A.; Tun, U.; Onn, H.; Fadzelly, M.; Bakar, A. Effect of Light Intensity on the Photosynthesis and Stomatal Density of Selected Plant Species of Gunung Ledang, Johor. Malays. Appl. Biol. 2019, 48, 133–140. [Google Scholar]
- Liu, M.; Gong, J.; Yang, B.; Ding, Y.; Zhang, Z.; Wang, B.; Zhu, C.; Hou, X. Differences in the Photosynthetic and Physiological Responses of Leymus Chinensis to Different Levels of Grazing Intensity. BMC Plant Biol. 2019, 19, 558. [Google Scholar] [CrossRef]
- Formisano, L.; Ciriello, M.; Cirillo, V.; Pannico, A.; El-nakhel, C.; Cristofano, F.; Duri, L.G.; Giordano, M.; Rouphael, Y.; De Pascale, S. Divergent Leaf Morpho-Physiological and Anatomical Adaptations of Four Lettuce Cultivars in Response to Different Greenhouse Irradiance Levels in Early Summer Season. Plants 2021, 10, 1179. [Google Scholar] [CrossRef]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmülling, T.; Cortleven, A. Light Acts as a Stressor and Influences Abiotic and Biotic Stress Responses in Plants. Plant Cell Environ. 2021, 44, 645–664. [Google Scholar] [CrossRef]
- Usuda, H. Evaluation of the Effect of Photosynthesis on Biomass Production with Simultaneous Analysis of Growth and Continuous Monitoring of CO2 Exchange in the Whole Plants of Radish, Cv Kosena under Ambient and Elevated CO2. Plant Prod. Sci. 2004, 7, 386–396. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Plant Physiology, Development and Metabolism; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Farquhar, G.; von Caemmerer, S.; Berry, J. A Biochemical Model of Photosynthetic CO2 Assimilation in Leaves of C3 Species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Driesen, E.; Van den Ende, W.; De Proft, M.; Saeys, W. Influence of Environmental Factors Light, Co2, Temperature, and Relative Humidity on Stomatal Opening and Development: A Review. Agronomy 2020, 10, 1975. [Google Scholar] [CrossRef]
- Lynch, J.; Marschner, P.; Rengel, Z. Effect of internal and external factors on root growth and development. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 331–346. [Google Scholar] [CrossRef]
- Blouin, M.; Barrere, J.; Meyer, N.; Lartigue, S.; Barot, S.; Mathieu, J. Vermicompost Significantly Affects Plant Growth. A Meta-Analysis. Agron. Sustain. Dev. 2019, 39, 34–50. [Google Scholar] [CrossRef]
- Wong, W.S.; Zhong, H.T.; Cross, A.T.; Yong, J.W.H. Plant biostimulants in vermicomposts: Characteristics and plausible mechanisms. In The Chemical Biology of Plant Biostimulants; Gelen, D., Xu, L., Eds.; John Wiley & Sons Ltd.: Singapore, 2020; pp. 156–180. [Google Scholar]
- Devi, C.; Khwairakpam, M. Vermicompost for sustainable agriculture and bioconversion of terrestrial weed biomass into vermicompost. In New Generation of Organic Fertilizers; Turan, M., Yildiri, E., Eds.; IntechOpen: London, UK, 2022. [Google Scholar]
- Lim, S.L.; Wu, T.Y.; Lim, P.N.; Shak, K.P.Y. The Use of Vermicompost in Organic Farming: Overview, Effects on Soil and Economics. J. Sci. Food Agric. 2015, 95, 1143–1156. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Solarte, Z.; Arancon, N.Q.; Solarte, Z. Vermiculture in Greenhouse Plants, Field Crop Production, and Hydroponics; Oxford University Press: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Vermicompost Affects Soil Properties and Spinach Growth, Physiology, and Nutritional Value. HortScience 2016, 51, 847–855. [Google Scholar] [CrossRef]
- Lal, R. Soil Carbon Sequestration Impacts on Global Climate Change and Food Security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Dignac, M.F.; Derrien, D.; Barré, P.; Barot, S.; Cécillon, L.; Chenu, C.; Chevallier, T.; Freschet, G.T.; Garnier, P.; Guenet, B.; et al. Increasing Soil Carbon Storage: Mechanisms, Effects of Agricultural Practices and Proxies. A Review. Agron. Sustain. Dev. 2017, 37, 14. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F.F. Does Enhanced Photosynthesis Enhance Growth? Lessons Learned from CO2 Enrichment Studies. Plant Physiol. 2011, 155, 117–124. [Google Scholar] [CrossRef]
- Longstreth, D.J.; Nobel, P.S. Nutrient Influences on Leaf Photosynthesis. Plant Physiol. 1980, 65, 541–543. [Google Scholar] [CrossRef]
- Harpole, W.S.; Ngai, J.T.; Cleland, E.E.; Seabloom, E.W.; Borer, E.T.; Bracken, M.E.S.; Elser, J.J.; Gruner, D.S.; Hillebrand, H.; Shurin, J.B.; et al. Nutrient Co-Limitation of Primary Producer Communities. Ecol. Lett. 2011, 14, 852–862. [Google Scholar] [CrossRef]
- Ingestad, T.; Lund, A. Nitrogen Stress in Birch Seedlings: II. N, K, P, Ca, and Mg Nutrition. Physiol. Plant. 1979, 45, 149–157. [Google Scholar] [CrossRef]
- Knecht, M.F.; Göransson, A. Terrestrial Plants Require Nutrients in Similar Proportions. Tree Physiol. 2004, 24, 447–460. [Google Scholar] [CrossRef]
- Wright, S.J.; Yavitt, J.B.; Wurzburger, N.; Turner, B.I.; Tanner, E.V.J.; Sayer, E.J.; Santiago, L.S.; Kaspari, M.; Hedin, L.O.; Harms, K.E.; et al. Potassium, Phosphorus, or Nitrogen Limit Root Allocation, Tree Growth, or Litter Production in a Lowland Tropical Forest. Ecology 2011, 92, 1616–1625. [Google Scholar] [CrossRef]
- Pamoengkas, P.; Zamzam, A.; Dwisutono, A. Vegetation Recovery of Logged-over Dipterocarp Forests In Central Kalimantan, Indonesia. Floresta Ambient. 2019, 26, e20171239. [Google Scholar] [CrossRef]
- Widiyatno; Hidayati, F.; Hardiwinoto, S.; Indrioko, S.; Purnomo, S.; Jatmoko; Tani, N.; Naiem, M. Selection of Dipterocarp Species for Enrichment Planting in a Secondary Tropical Rainforest. For. Sci. Technol. 2020, 16, 206–215. [Google Scholar] [CrossRef]
- Ghazoul, J. Dipterocarp Biology, Ecology, and Conservation, 1st ed.; Oxford University Press: New York, NY, USA, 2016. [Google Scholar]
- Schmitt, J.; Wulff, R.D. Light Spectral Quality, Phytochrome and Plant Competition. Trends Ecol. Evol. 1993, 8, 47–51. [Google Scholar] [CrossRef]
- Newman, M.; Burgess, P.; Whitmore, T. Manuals of Dipterocarps for Foresters, Borneo Island Light Hardwoods: Anisoptera, Parashorea, Shorea (Red, White and Yellow Meranti); Royal Botanic Garden: Edinburgh, UK, 1996. [Google Scholar]
- Hirasawa, Y. Pasoh Ecology of a Lowland Rain Forest in Southeast Asia, 1st ed.; Okuda, T., Manokaran, N., Matsumoto, Y., Niiyama, K., Thomas, S., Ashton, P., Eds.; Springer: Tokyo, Japan, 2003. [Google Scholar]
- Suzuki, R.O.; Numata, S.; Okuda, T.; Nur Supardi, M.N.; Kachi, N. Growth Strategies Differentiate the Spatial Patterns of 11 Dipterocarp Species Coexisting in a Malaysian Tropical Rain Forest. J. Plant Res. 2009, 122, 81–93. [Google Scholar] [CrossRef]
- Van Volkenburgh, E. Leaf Expansion—An Integrating Plant Behaviour. Plant Cell Environ. 1999, 22, 1463–1473. [Google Scholar] [CrossRef]
- Mathur, S.; Jain, L.; Jajoo, A. Photosynthetic Efficiency in Sun and Shade Plants. Photosynthetica 2018, 56, 354–365. [Google Scholar] [CrossRef]
- Terashima, I.; Hanba, Y.T.; Tholen, D.; Niinemets, Ü. Leaf Functional Anatomy in Relation to Photosynthesis. Plant Physiol. 2011, 155, 108–116. [Google Scholar] [CrossRef]
- Pasquini, S.C.; Santiago, L.S. Nutrients Limit Photosynthesis in Seedlings of a Lowland Tropical Forest Tree Species. Oecologia 2012, 168, 311–319. [Google Scholar] [CrossRef]
- Chapin, F.S.; Schulze, E.D.; Mooney, H.A. The Ecology and Economics of Storage in Plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Craine, J.M.; Dybzinski, R. Mechanisms of Plant Competition for Nutrients, Water and Light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- Connolly, J. On the Use of Response Models in Mixture Experiments. Oecologia 1987, 72, 95–103. [Google Scholar] [CrossRef]
- Connolly, J.; Wayne, P.; Bazzaz, F.A. Interspecific Competition in Plants: How Well Do Current Methods Answer Fundamental Questions? Am. Nat. 2001, 157, 107–125. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).