Moisture, Not Temperature, in the Pre-Monsoon Influences Pinus wallichiana Growth along the Altitudinal and Aspect Gradients in the Lower Himalayas of Central Nepal
Abstract
1. Introduction
2. Materials and Methods
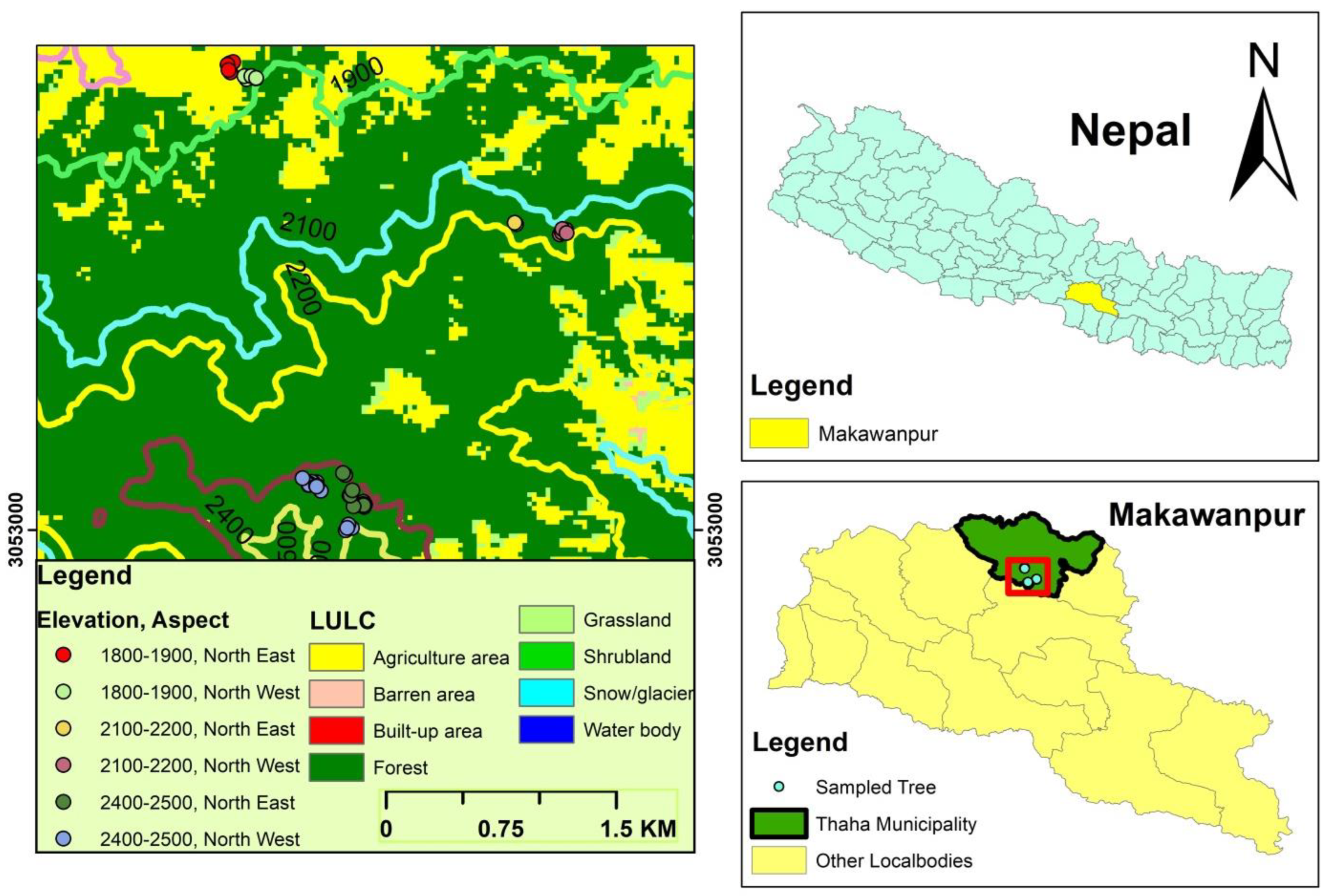
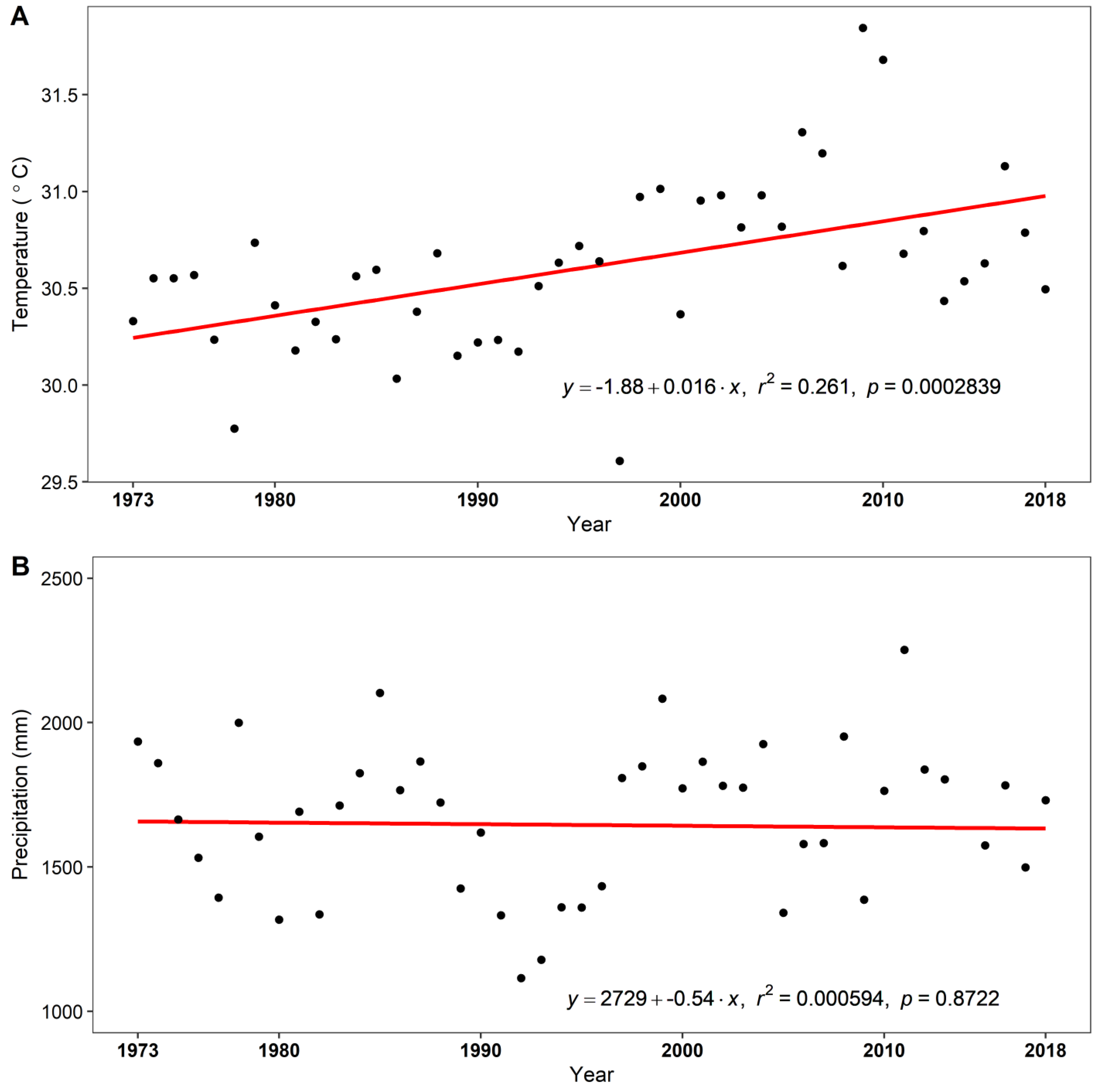
2.1. Study Area
2.2. Sample Collection and Chronology Development
2.3. Growth–Climate Relationship
2.4. Basal Area Increment (BAI) Analysis
3. Results
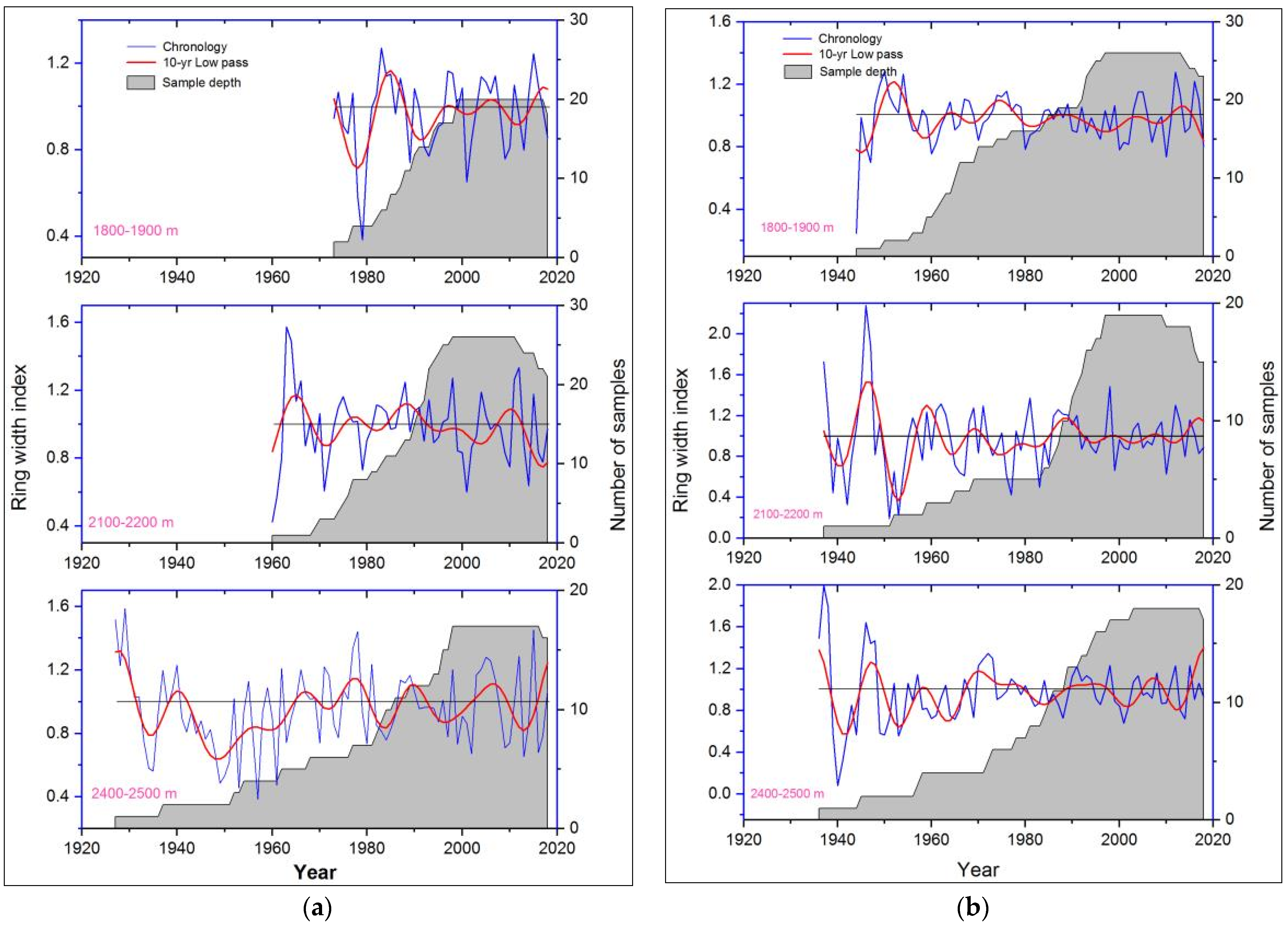
3.1. Tree-Ring Width Chronologies of P. wallichiana along the Elevation and Aspect Gradients
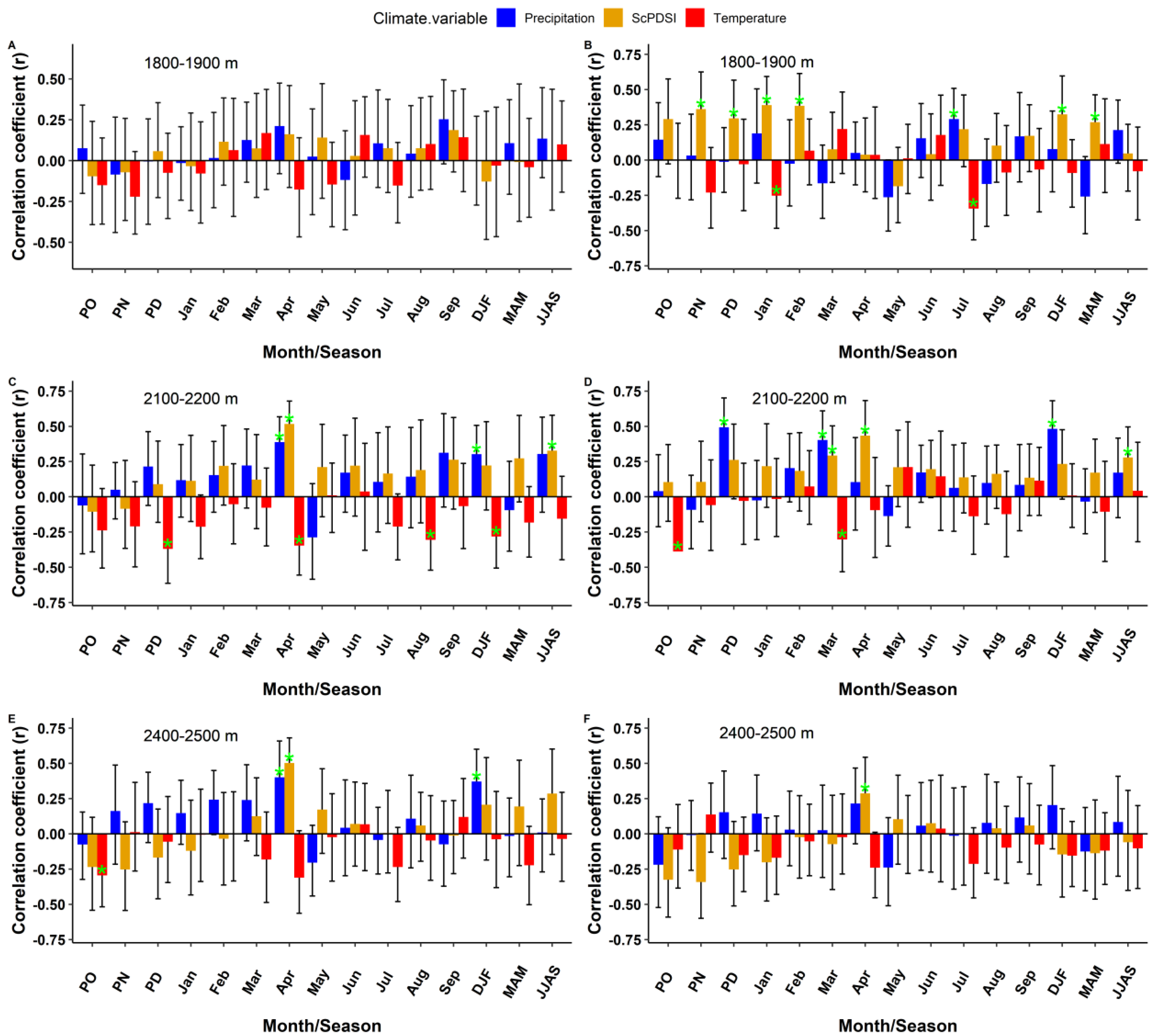
3.2. Climate–Growth Response
3.3. Basal Area Increment (BAI) Growth Trend
4. Discussion
4.1. Tree-Ring Growth Pattern
4.2. Climate–Growth Response
4.3. Basal Area Increment (BAI) Growth Trend
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Subedi, M.R. Climate change and its potential effects on tree line position: An introduction and analysis. Greenery—J. Environ. Biodiver. 2009, 7, 17–21. [Google Scholar]
- Ameztegui, A.; Coll, L.; Brotons, L.; Ninot, J.M. Land-use legacies rather than climate change are driving the recent upward shift of the mountain tree line in the Pyrenees. Glob. Ecol. Biogeogr. 2016, 25, 263–273. [Google Scholar] [CrossRef]
- García-Valdés, R.; Bugmann, H.; Morin, X. Climate change-driven extinctions of tree species affect forest functioning more than random extinctions. Divers. Distrib. 2018, 24, 906–918. [Google Scholar] [CrossRef]
- McKenney, D.W.; Pedlar, J.H.; Lawrence, K.; Campbell, K.; Hutchinson, M.F. Potential impacts of climate change on the distribution of North American trees. BioScience 2007, 57, 939–948. [Google Scholar] [CrossRef]
- IPCC (Ed.) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. Available online: https://www.ipcc.ch/report/ar5/wg1/ (accessed on 20 August 2022).
- Gaire, N.P.; Koirala, M.; Bhuju, D.R.; Borgaonkar, H.P. Treeline dynamics with climate change at the central Nepal Himalaya. Clim. Past 2014, 10, 1277–1290. [Google Scholar] [CrossRef]
- Chhetri, P.K.; Cairns, D.M. Contemporary and historic population structure of Abies spectabilis at treeline in Barun valley, eastern Nepal Himalaya. J. Mt. Sci. 2015, 12, 558–570. [Google Scholar] [CrossRef]
- Shrestha, K.B.; Hofgaard, A.; Vandvik, V. Tree-growth response to climatic variability in two climatically contrasting treeline ecotone areas, central Himalaya, Nepal. Can. J. For. Res. 2015, 45, 1643–1653. [Google Scholar] [CrossRef]
- Aryal, P.C.; Dhamala, M.K.; Gaire, N.P.; Bhatta, S.; Suwal, M.K.; Bhuju, D.R.; Chhetri, P.K. Tree-ring climate response of two Larix species from the Central Nepal Himalaya. Trop. Ecol. 2020, 61, 215–225. [Google Scholar] [CrossRef]
- Gaire, N.P.; Fan, Z.X.; Bräuning, A.; Panthi, S.; Rana, P.; Shrestha, A.; Bhuju, D.R. Abies spectabilis shows stable growth relations to temperature, but changing response to moisture conditions along an elevation gradient in the central Himalaya. Dendrochronologia 2020, 60, 125675. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: New York, NY, USA, 1976; p. 567. [Google Scholar]
- Enquist, B.J.; Enquist, C.A. Long-term change within a Neotropical forest: Assessing differential functional and floristic responses to disturbance and drought. Glob. Change Biol. 2011, 17, 1408–1424. [Google Scholar] [CrossRef]
- Feng, X.; Uriarte, M.; González, G.; Reed, S.; Thompson, J.; Zimmerman, J.K.; Murphy, L. Improving predictions of tropical forest response to climate change through integration of field studies and ecosystem modeling. Glob. Change Biol. 2018, 24, e213–e232. [Google Scholar] [CrossRef]
- Zhang, T.; Niinemets, Ü.; Sheffield, J.; Lichstein, J.W. Shifts in tree functional composition amplify the response of forest biomass to climate. Nature 2018, 556, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Dorado-Liñán, I.; Piovesan, G.; Martínez-Sancho, E.; Gea-Izquierdo, G.; Zang, C.; Cañellas, I.; Castagneri, D.; Di Filippo, A.; Gutierrez, E.; Ewald, J.; et al. Geographical adaptation prevails over species-specific determinism in trees’ vulnerability to climate change at Mediterranean rear-edge forests. Glob. Change Biol. 2019, 25, 1296–1314. [Google Scholar] [CrossRef] [PubMed]
- Muller-Landau, H.C.; Cushman, K.C.; Arroyo, E.E.; Martinez Cano, I.; Anderson-Teixeira, K.J.; Backiel, B. Patterns and mechanisms of spatial variation in tropical forest productivity, woody residence time, and biomass. New Phytol. 2021, 229, 3065–3087. [Google Scholar] [CrossRef]
- Fritts, H.C.; Smith, D.G.; Cardis, J.W.; Budelsky, C.A. Tree-ring characteristics along a vegetation gradient in northern Arizona. Ecology 1965, 46, 393–401. [Google Scholar] [CrossRef]
- Zhang, H.W.; Ma, J.Y.; Sun, W.; Chen, F.H. Altitudinal variation in functional traits of Picea schrenkiana var. tianschanica and their relationship to soil factors in Tianshan Mountains, Northwest China. Acta Ecol. Sin. 2010, 30, 5747–5758. [Google Scholar]
- Yang, B.; He, M.; Melvin, T.M.; Zhao, Y.; Briffa, K.R. Climate control on tree growth at the upper and lower treelines: A case study in the Qilian Mountains, Tibetan Plateau. PLoS ONE 2013, 8, e69065. [Google Scholar] [CrossRef]
- Leal, S.; Melvin, T.M.; Grabner, M.; Wimmer, R.; Briffa, K.R. Tree-ring growth variability in the Austrian Alps: The influence of site, altitude, tree species and climate. Boreas 2007, 36, 426–440. [Google Scholar] [CrossRef]
- Fritts, H.C.; Blasing, T.J.; Hayden, B.P.; Kutzbach, J.E. Multivariate techniques for specifying tree-growth and climate relationships and for reconstructing anomalies in paleoclimate. J. Appl. Meteorol. (1962–1982) 1971, 10, 845–864. [Google Scholar] [CrossRef]
- Liu, L.S.; Shao, X.M.; Liang, E.Y. Climate signals from tree ring chronologies of the upper and lower treelines in the Dulan region of the northeastern Qinghai-Tibetan Plateau. J. Integr. Plant Biol. 2006, 48, 278–285. [Google Scholar] [CrossRef]
- Liang, E.; Wang, Y.; Xu, Y.; Liu, B.; Shao, X. Growth variation in Abies georgei var. smithii along altitudinal gradients in the Sygera Mountains, southeastern Tibetan Plateau. Trees 2010, 24, 363–373. [Google Scholar] [CrossRef]
- Jackson, J.K. Manual of Afforestation in Nepal; Forest Research and Survey Centre: Kathmandu, Nepal, 1994.
- Gaire, N.P.; Dhakal, Y.R.; Shah, S.K.; Fan, Z.X.; Bräuning, A.; Thapa, U.K.; Bhandari, S.; Aryal, S.; Bhuju, D.R. Drought (scPDSI) reconstruction of trans-Himalayan region of central Himalaya using Pinus wallichiana tree-rings. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 514, 251–264. [Google Scholar] [CrossRef]
- Gautam, D.; Shrestha, N.M.; Gaire, N.P.; Roth, B.E.; Jandug, C.M.B.; Tong, X.J.; Liu, Q.J. A 99-year chronology of blue pine (Pinus wallichiana) tree-rings concerning interannual climate variability in the central Himalayas of Nepal. Appl. Ecol. Environ. Res. 2021, 19, 3519–3532. [Google Scholar] [CrossRef]
- Gautam, D.; Karki, J.; Gaire, N.P.; Roth, B.E.; Bhattarai, S.; Thapa, S.; Sharma, R.P.; Li, J.; Tong, X.; Liu, Q.J. Intra-and interannual climate variability drives the radial growth of Pinus wallichiana in the Nepalese Himalayas. Plant Ecol. Divers. 2020, 13, 391–400. [Google Scholar] [CrossRef]
- Thapa, U.K.; St. George, S.; Kharal, D.K.; Gaire, N.P. Tree growth across the Nepal Himalaya during the last four centuries. Prog. Phys. Geogr. 2017, 41, 478–495. [Google Scholar] [CrossRef]
- Yao, T.; Thompson, L.; Yang, W.; Yu, W.; Gao, Y.; Guo, X.; Yang, X.X.; Duan, K.Q.; Zhao, H.B.; Xu, B.Q.; et al. Different glacier status with atmospheric circulations in Tibetan Plateau and surroundings. Nat. Clim. change 2012, 2, 663–667. [Google Scholar] [CrossRef]
- Tech, R. TSAP-Win: Time Series Analysis and Presentation for Dendrochronology and Related Applications; Version 0.53; Rinntech: Heidelberg, Germany, 2010. [Google Scholar]
- Rinn, F. TSAP–Win: Time Series Analysis and Presentation for Dendrochronology and Related Applications; Version 0.55; Rinntech: Heidelberg, Germany, 2003; Available online: http://www.rimatech.comSalzer (accessed on 15 August 2022).
- Grissino–Mayer, H.D. Evaluating cross-dating accuracy: A manual and tutorial for the computer program COFECHA. Tree-Ring Res. 2001, 57, 205–221. [Google Scholar]
- Holmes, R.L. Computer assisted quality control. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Speer, J.H. Fundamentals of Tree Ring Research; The University of Arizona Press: Tucson, AZ, USA, 2010. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Briffa, K.R. Interpreting high-resolution proxy climate data—The example of dendroclimatology. In Analysis of Climate Variability; Springer: Berlin/Heidelberg, Germany, 1999; pp. 77–94. [Google Scholar] [CrossRef]
- Wigley, T.M.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Appl. Meteorol. Climatol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 2020, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Van der Schrier, G.; Barichivich, J.; Briffa, K.R.; Jones, P.D. A scPDSI-based global data set of dry and wet spells for 1901–2009. J. Geophys. Res. Atmos. 2013, 118, 4025–4048. [Google Scholar] [CrossRef]
- Qi, Z.; Liu, H.; Wu, X.; Hao, Q. Climate-driven speedup of alpine treeline forest growth in the Tianshan Mountains, Northwestern China. Glob. Change Biol. 2015, 21, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Thapa, U.K.; Shah, S.K.; Gaire, N.P.; Bhuju, D.R.; Bhattacharyya, A.; Thagunna, G.S. Influence of climate on radial growth of Abies pindrow in Western Nepal Himalaya. Banko Janakari 2013, 23, 14–19. [Google Scholar] [CrossRef][Green Version]
- Bhandari, S.; Gaire, N.P.; Shah, S.K.; Speer, J.H.; Bhuju, D.R.; Thapa, U.K. A 307-year tree-ring SPEI reconstruction indicates modern drought in western Nepal Himalayas. Tree-Ring Res. 2019, 75, 73–85. [Google Scholar] [CrossRef]
- Aryal, S.; Bhuju, D.R.; Kharal, D.K.; Gaire, N.P.; Dyola, N. Climatic upshot using growth pattern of Pinus roxburghii from western Nepal. Pak. J. Bot. 2018, 50, 579–588. [Google Scholar]
- Cook, E.R.; Krusic, P.J.; Jones, P.D. Dendroclimatic signals in long tree-ring chronologies from the Himalayas of Nepal. Int. J. Clim. 2003, 23, 707–732. [Google Scholar] [CrossRef]
- Kharal, D.K.; Thapa, U.K.; George, S.S.; Meilby, H.; Rayamajhi, S.; Bhuju, D.R. Tree-climate relations along an elevational transect in Manang Valley, central Nepal. Dendrochronologia 2017, 41, 57–64. [Google Scholar] [CrossRef]
- Rai, S.; Dawadi, B.; Wang, Y.; Lu, X.; Ru, H.; Sigdel, S.R. Growth response of Abies spectabilis to climate along an elevation gradient of the Manang valley in the central Himalayas. J. For. Res. 2020, 31, 2245–2254. [Google Scholar] [CrossRef]
- Panthi, S.; Fan, Z.X.; van der Sleen, P.; Zuidema, P.A. Long-term physiological and growth responses of Himalayan fir to environmental change are mediated by mean climate. Glob. Change Biol. 2020, 26, 1778–1794. [Google Scholar] [CrossRef]
- Lyu, L.; Suvanto, S.; Nöjd, P.; Henttonen, H.M.; Mäkinen, H.; Zhang, Q.B. Tree growth and its climate signal along latitudinal and altitudinal gradients: Comparison of tree rings between Finland and the Tibetan Plateau. Biogeosciences 2017, 14, 3083–3095. [Google Scholar] [CrossRef]
- Liang, E.; Dawadi, B.; Pederson, N.; Eckstein, D. Is the growth of birch at the upper timberline in the Himalayas limited by moisture or by temperature? Ecology 2014, 95, 2453–2465. [Google Scholar] [CrossRef]
- Fan, Z.X.; Bräuning, A.; Cao, K.F. Tree-ring based drought reconstruction in the central Hengduan Mountains region (China) since AD 1655. Int. J. Clim. 2008, 28, 1879–1887. [Google Scholar] [CrossRef]
- Lyu, L.; Zhang, Q.B.; Pellatt, M.G.; Büntgen, U.; Li, M.H.; Cherubini, P. Drought limitation on tree growth at the Northern Hemisphere’s highest tree line. Dendrochronologia 2019, 53, 40–47. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Deslauriers, A.; Bräuning, A. Intra-annual stem radial increment response of Qilian juniper to temperature and precipitation along an altitudinal gradient in northwestern China. Trees 2015, 29, 25–34. [Google Scholar] [CrossRef]
- Shi, L.; Liu, H.; Xu, C.; Liang, B.; Cao, J.; Cressey, E.L.; Quine, T.A.; Zhou, M.; Zhao, P. Decoupled heatwave-tree growth in large forest patches of Larix sibirica in northern Mongolian Plateau. Agric. For. Meteorol. 2021, 311, 108667. [Google Scholar] [CrossRef]
- Ding, J.; Yang, T.; Zhao, Y.; Liu, D.; Wang, X.; Yao, Y.; Peng, S.; Wang, T.; Piao, S. Increasingly important role of atmospheric aridity on Tibetan alpine grasslands. Geophys. Res. Lett. 2018, 45, 2852–2859. [Google Scholar] [CrossRef]
- Fang, O.; Alfaro, R.I.; Zhang, Q.B. Tree rings reveal a major episode of forest mortality in the late 18th century on the Tibetan Plateau. Glob. Planet. Change 2018, 163, 44–50. [Google Scholar] [CrossRef]
- Panthi, S.; Bräuning, A.; Zhou, Z.K.; Fan, Z.X. Tree rings reveal recent intensified spring drought in the central Himalaya, Nepal. Glob. Planet. Change 2017, 157, 26–34. [Google Scholar] [CrossRef]
- Tiwari, A.; Fan, Z.X.; Jump, A.S.; Li, S.F.; Zhou, Z.K. Gradual expansion of moisture sensitive Abies spectabilis forest in the Trans-Himalayan zone of central Nepal associated with climate change. Dendrochronologia 2017, 41, 34–43. [Google Scholar] [CrossRef]
- Sigdel, S.R.; Wang, Y.; Camarero, J.J.; Zhu, H.; Liang, E.; Peñuelas, J. Moisture-mediated responsiveness of treeline shifts to global warming in the Himalayas. Glob. Change Biol. 2018, 24, 5549–5559. [Google Scholar] [CrossRef] [PubMed]
- Baral, S.; Gaire, N.P.; Aryal, S.; Pandey, M.; Rayamajhi, S.; Vacik, H. Growth ring measurements of Shorea robusta reveal responses to climatic variation. Forests 2019, 10, 466. [Google Scholar] [CrossRef]
- Chhetri, P.K.; Bista, R.; Shrestha, K.B. How does the stand structure of treeline-forming species shape the treeline ecotone in different regions of the Nepal Himalayas? J. Mt. Sci. 2020, 17, 2354–2368. [Google Scholar] [CrossRef]


| Northeast Aspect (NEA) | |||
|---|---|---|---|
| Statistics | Upper | Middle | Lower |
| Elevation (masl) | 2400–2500 | 2100–2200 | 1800–1900 |
| Slope (°) | ≤40 | ≤40 | ≤40 |
| Chronology span | 1927–2018 (92) | 1960–2018 (59) | 1973–2018 (46) |
| Number of cores | 17 | 26 | 20 |
| Missing rings (%) | 0.013 | 0.010 | 0 |
| First order autocorrelation (AC1) | 0.125 | 0.210 | 0.199 |
| Standard deviation (SD) | 0.414 | 0.345 | 0.323 |
| Mean sensitivity (MS) | 0.435 | 0.305 | 0.323 |
| Mean series intercorrelation (Rbar) | 0.376 | 0.466 | 0.404 |
| Expressed population signal (EPS) | 0.855 | 0.881 | 0.852 |
| Northwest Aspect (NWA) | |||
| Statistics | Upper | Middle | Lower |
| Elevation (masl) | 2400–2500 | 2100–2200 | 1800–1900 |
| Slope (°) | ≤40 | ≤40 | ≤40 |
| Chronology span | 1936–2018 (83) | 1937–2018 (82) | 1944–2018 (75) |
| Number of cores | 18 | 19 | 26 |
| Missing rings (%) | 0.012 | 0.021 | 0.005 |
| First order autocorrelation (AC1) | 0.219 | 0.137 | 0.229 |
| Standard deviation (SD) | 0.381 | 0.382 | 0.294 |
| Mean sensitivity (MS) | 0.389 | 0.428 | 0.270 |
| Mean series intercorrelation (Rbar) | 0.223 | 0.324 | 0.356 |
| Expressed population signal (EPS) | 0.852 | 0.852 | 0.848 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam, D.; Gaire, N.P.; Subedi, M.; Sharma, R.P.; Tripathi, S.; Sigdel, R.; Basnet, S.; Miya, M.S.; Chhetri, P.K.; Tong, X. Moisture, Not Temperature, in the Pre-Monsoon Influences Pinus wallichiana Growth along the Altitudinal and Aspect Gradients in the Lower Himalayas of Central Nepal. Forests 2022, 13, 1771. https://doi.org/10.3390/f13111771
Gautam D, Gaire NP, Subedi M, Sharma RP, Tripathi S, Sigdel R, Basnet S, Miya MS, Chhetri PK, Tong X. Moisture, Not Temperature, in the Pre-Monsoon Influences Pinus wallichiana Growth along the Altitudinal and Aspect Gradients in the Lower Himalayas of Central Nepal. Forests. 2022; 13(11):1771. https://doi.org/10.3390/f13111771
Chicago/Turabian StyleGautam, Deepak, Narayan Prasad Gaire, Mukti Subedi, Ram P. Sharma, Shankar Tripathi, Rajesh Sigdel, Saroj Basnet, Mahamad Sayab Miya, Parveen K. Chhetri, and Xiaojuan Tong. 2022. "Moisture, Not Temperature, in the Pre-Monsoon Influences Pinus wallichiana Growth along the Altitudinal and Aspect Gradients in the Lower Himalayas of Central Nepal" Forests 13, no. 11: 1771. https://doi.org/10.3390/f13111771
APA StyleGautam, D., Gaire, N. P., Subedi, M., Sharma, R. P., Tripathi, S., Sigdel, R., Basnet, S., Miya, M. S., Chhetri, P. K., & Tong, X. (2022). Moisture, Not Temperature, in the Pre-Monsoon Influences Pinus wallichiana Growth along the Altitudinal and Aspect Gradients in the Lower Himalayas of Central Nepal. Forests, 13(11), 1771. https://doi.org/10.3390/f13111771









