Abstract
The aim of this study was to assess the antioxidant response towards urban air pollution of three widespread ornamental species—Tilia tomentosa, Fraxinus excelsior and Pinus nigra. Saplings were planted in four urban plots with different anthropogenic impacts, and periodic observations were performed on their development. Three types of biochemical markers, representing plant responses by three different mechanisms, were analyzed: photosynthetic pigments, free proline and guaiacol peroxidase activity. Our study confirmed that plant responses and adaptation to the environment are complex biological processes including physiological and biochemical changes. As a whole, these experiments revealed that the studied trees react by specific mechanisms towards urban air pollution, and antioxidant responses are significantly correlated with the enhancement of traffic (p < 0.05). Fraxinus excelsior was assessed as being very suitable for urban landscaping due to the significant tolerance to environmentally stressful conditions. Tilia tomentosa was also evaluated as a suitable ornamental species as it demonstrated good development in the urban environment. Pinus nigra was proven as more sensitive to the urban air pollution versus the other two studied trees. These findings could be very useful as a scientific basis for the landscaping practice in terms of the sustainable development and management of urban forestry.
1. Introduction
Rapid urbanization and globalization have been well known for several decades as two of the greatest threats to biodiversity at a local and global scale [1,2]. Urban development processes lead to significant landscape changes, destroying natural ecosystems and replacing them with anthropogenic ones. In this context, urban green infrastructures have a crucial role in sustaining various ecosystem services for human well-being [3,4] and urban quality of life [5]. There are various relationships between humanity, settlements and nature, among which greening and the formation of a green system of functionally different green spaces in cities play a major role, as this creates the environment required to carry out basic human activities. Urban green infrastructure has an important role to play as one of the most significant components that creates the conditions for sustainable urban development at all levels—national, regional and local. The green system, as a collection of different types of green spaces in the urban and suburban area of the settlement, is intended to maintain a biological balance between the elements of the socio-ecological system of man–society–nature. It plays an essential role in connecting the various elements and structural zones of the urban structure in a composite whole, and through the suburban green areas, it connects the urban structure with the environment [6].
According to the Millennium Ecosystem Assessment report [7], ecosystem services are defined as benefits that people obtain from ecosystems, and they are divided into four categories—supporting, regulating, provisioning and cultural services. Urban green infrastructures are related mainly to several regulating services in urban ecosystems [8] as follows: (i) regulation of air quality in urban areas [9,10]; (ii) mitigation of urban heat island effects [11]; (iii) regulation of runoff and flooding [12]. Regarding the supporting and provisioning services of green infrastructures, they have a range of different functions, such as biodiversity conservation [13,14,15,16], water management [14,15,16], sustainable land management [13,15] and urban regeneration [14,17]. However, all ecosystem services are crucial for the sustainable development of human settlements, but, to fulfill these functions, the green infrastructure needs an appropriate species composition, especially in terms of ornamental trees.
The process of urbanization has become more intensive recently, which results in the permanent worsening of environmental factors for urban biota. Exceeding values and the permanent presence of toxic gases, aerosols and dust in urban air, even at chronic concentrations, significantly damage more sensitive plant species [18,19,20]. Salt stress, water deficits and heat stress should also be considered as major factors affecting plant development in urban areas. To address these problems and to build a sustainable urban green infrastructure, the first step should be related to studying the reaction specificity and the tolerance to air pollution of the ornamental tree species in the actual cities’ conditions, and then to perform a revision and revaluation of the adaptation ability of the newly planted ornamental trees to the degraded urban environment.
It is well known that the adaptation of plants towards extreme values of any factor includes two basic reactions (to avoid stress and/or stress resistance) and has three main stages [21]. The first stage involves primary stress reactions, especially oxidative stress effects due to the increased production of reactive oxygen species (ROS) in plant tissues. This leads to an increment in the lipid peroxidation (LP) rate in cell membranes because some ROS, especially hydroxyl radicals (•OH), can initiate LP [22]. The second stage is connected with the adaptive responses towards stress due to the antioxidant defensive system of the plant organism. Antioxidants are a group of different substances (vitamins, minerals, enzymes, etc.) whose main function is to defend the plant organism from the synthesis of ROS or to block the action of generated ROS [23]. Generally, ROS have a short lifetime in plant tissues but their negative impact could be significant. They link with cell molecules and destroy cell membranes, so they cause faster aging and cell death. This is why each plant species has developed its own mechanism to protect itself against ROS’ influence. Two groups of substances are involved in this process—enzymatic and non-enzymatic. Non-enzymatic ones are presented by carotenoids, vitamins, flavonoids, free proline, etc. The enzymatic antioxidant system includes many enzymes, such as catalase, guaiacol peroxidase, glutathione peroxidase and reductase and superoxide dismutase [24]. If the plant has a lower ability to overcome the negative impact of the environmental factors, the third stage leads to weight loss and death.
The aim of the present study is to (1) assess the antioxidant response towards the urban environment (with a focus on air pollution) of Tilia tomentosa Moench, Fraxinus excelsior L. and Pinus nigra J. F. Arnold—three of the most widespread ornamental trees both in urban and rural areas of Europe [25], easy to identify and sample and frequently used as biomonitors; and (2) propose a reference scale for the early determination of the anthropogenic pressure extent based on the different levels of biochemical stress markers in urban trees.
2. Materials and Methods
2.1. Experimental Plots
The survey was conducted in the city of Plovdiv, Bulgaria (42°8′9.9492″ N; 24°44′31.8048″ E), one of the most polluted cities in Europe for some years [26] and which, hence, could be characterized as creating more severe conditions for plants. Domestic heating (during the cold period) and motor traffic are the major sources of air and soil pollution in the most Bulgarian cities, including Plovdiv, which have been caused by the rapid growth in the number of cars [27].
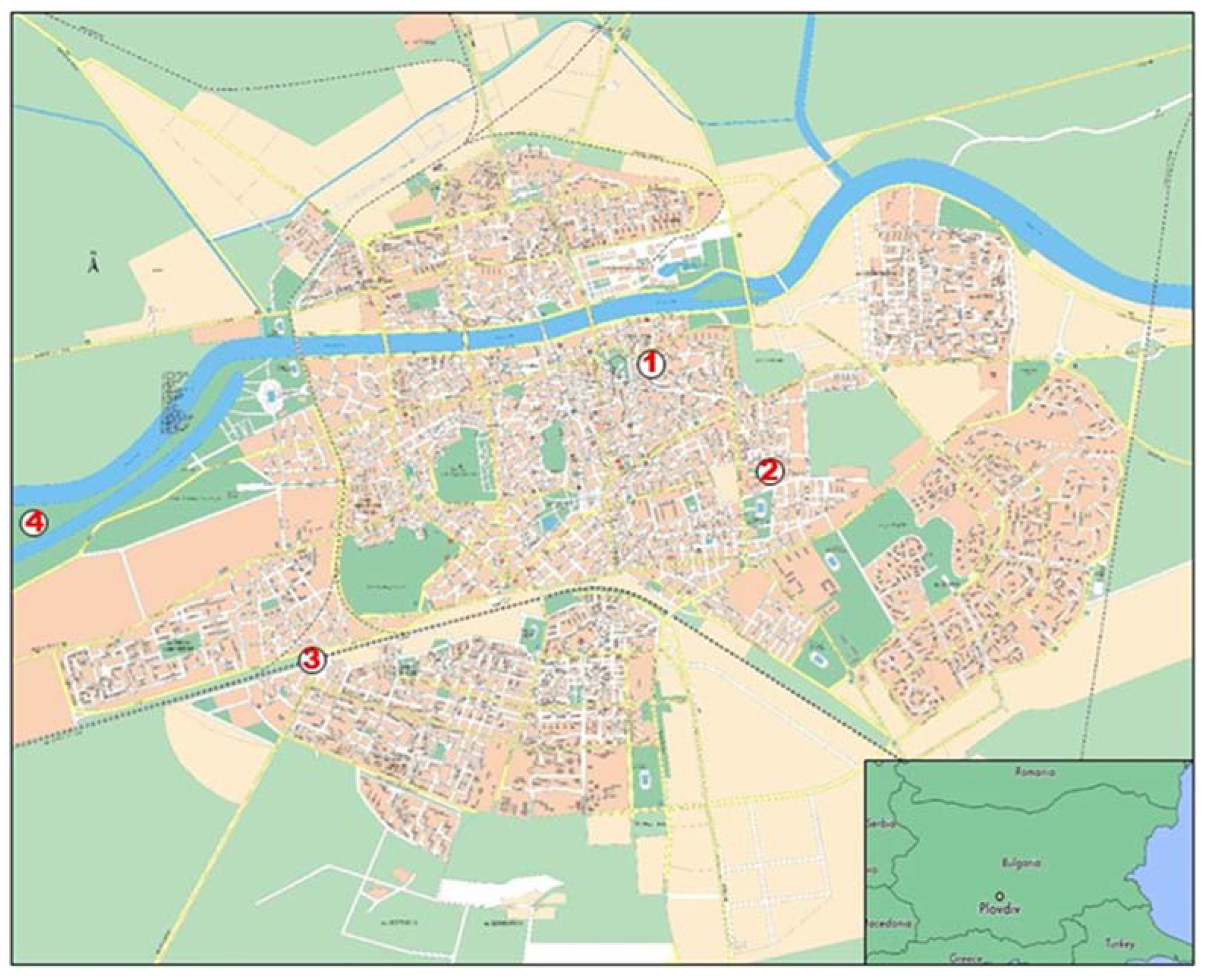
Four plots were selected on the basis of the typology of the urban environment that they represent and the type of anthropogenic impact (Figure 1). They were situated in the NE, SE, SW and NW directions from the city center, aiming to represent areas with different traffic intensity and load, as well as without any other sources of air pollution (such as plants, factories, etc.). The level of air pollution in each place was evaluated in the first week of August, according to the different intensities of road traffic (main cause of air pollution in this period). The intensity of road traffic was assessed on the basis of the number of cars (or motorcycles) in a period of ten minutes at 8 a.m. on a working day [28] (Table 1). The intensity of urbanization was determined according to the City General Layout Plan (Municipality of Plovdiv).
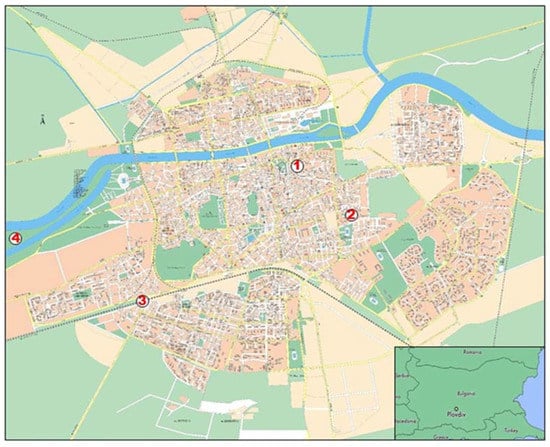
Figure 1.
Map of the city of Plovdiv (Bulgaria) and locations of the four selected plots (1—Plot 1; 2—Plot 2; 3—Plot 3; 4—Plot 4).

Table 1.
Level of air pollution evaluated on the basis of car traffic and intensity of urbanization.
Plot 1 (68 m2 surface area) was located in the central part of the city, NE direction (42°09′06.7″ N 24°45′03.1″ E), along a busy road junction with heavy traffic and a high level of air pollution. Saplings were planted in a green belt along one of the boulevards, located at a distance of around 5–10 m from the junction.
Plot 2 (52 m2 surface area) was situated in the central part of the city, SE direction (42°08′35.3″ N 24°45′56.9″ E), and was characterized by moderate motor traffic and a medium level of air pollution. Saplings were planted in a small green patch, located between two inner streets, 10–15 m away from tall buildings and 80–100 m away from the nearest boulevards.
Plot 3 (234 m2 surface area) was located in the west suburb, SW direction (42°07′54.0″ N 24°43′25.9″ E). It was characterized by very heavy railroad and vehicle traffic, resulting in a very high level of air pollution. Trees were planted in a large green area, situated between two railroad tracks and the Komatevo road junction, at a distance of around 5–10 m from the street and 20–25 m from the railroad.
Plot 4 (183 m2 surface area) was situated in the west suburb, NW direction (42°08′28.8″ N 24°41′59.9″ E), but in the largest city park, subjected to a low anthropogenic impact. It was characterized by very low traffic and a low level of air pollution. Saplings were planted in a large green area, close to the city outskirts; thus, we aimed to use them as a “conditional control” of the urban environment.
All plots were characterized by similar environmental conditions: soil type fluvisol with anthropogenic-influenced upper horizons; soil pH in the range 6.9–7.1; similar sun exposure; normal moistening regime. Differences were found in respect to the content of some chemical elements in the topsoil (0–30 cm layer) before planting (Table 2). The most abundant of the studied elements were Fe (12,391–21,503 mg/kg), Mg (4096–6135 mg/kg), Mn (387–613 mg/kg) and Zn (29–204 mg/kg kg), followed by Sr (33–69 mg/kg), Pb (7.6–56 mg/kg), Cu (7.6–58.4 mg/kg), Ni (13–41 mg/kg), V (28–39 mg/kg) and Cr (15–44 mg/kg). A general trend was established for six of the elements—Fe, Mg, Mn, As, U, Cd—with the first three being in the largest quantities, and the last three in the smallest, which is a consequence of the soil-forming process and their content in the matter rocks. The remaining seven elements—Zn, Sr, Cu, Pb, V, Cr and Ni—show a different tendency as a result of the anthropogenic impact.

Table 2.
Content of some chemical elements (mg/kg) in the studied urban soil samples and maximal permissible content (MPC) in urban soils according to the Bulgarian legislation.
2.2. Experimental Design
During the spring of 2015, 8-year-old seedling material was purchased from a certified nursery and planted by our team at the abovementioned experimental sites, representing 3 individuals per species per plot as group planting. Periodic observations were made on their development, physiology and health status throughout 2015–2020 and the average results from this 6-year period are presented below.
Leaf samples for analyses were taken in August of each year, when the leaves were fully developed. In order to obtain a homogeneous sample, a large number of leaves (one-year-old needles), comparable in size and shape, were sampled, taking care to minimize contact with the leaf surface. Usually, 80–100 fully expanded leaves (needles) per tree were collected and a composite sample was prepared for analyses. All the samples were stored in clean, labeled polyethylene bags, closed tightly to avoid contamination during transport.
2.3. Analyses of the Biochemical Markers of Stress
Three types of biochemical markers, representing the plant response by three different mechanisms, were analyzed, as follows:
- -
- Photosynthetic pigment content. Pigment analysis was performed as quickly as possible after sampling, following the standardized procedure. Spectrophotometric determination was performed at 440.5 nm (carotenoids), 644 nm (chlorophyll b) and 662 nm (chlorophyll a) wavelengths after extraction with 90% acetone (Acetone, 90% (v/v), Ricca Chemical). Concentrations of chlorophyll a, chlorophyll b, total chlorophyll and carotenoids were calculated for each sample and presented in mg g−1 fresh weight [29].
- -
- Proline content. Each leaf sample (0.2 g) was mashed with quartz sand and 10 mL buffer 100 mM KH2PO4/K2HPO4, pH = 7. Plant extract was than centrifuged at 10,000× g for 15 min at 4 °C. Reaction cocktail was prepared using 40:60 vol. % ethanol and distilled water. The concentration of free proline in the samples was determined spectrophotometrically at 520 nm against a standard curve prepared with proline (L-proline, TCI Europe N.V., Zwijndrecht, Belgium) [30] and presented in mg g−1 fresh weight.
- -
- Guaiacol peroxidase activity. The GPX activity was determined according to the method of Ridge and Osborne [31], with the modifications of Shevyakova et al. [32]. One gram of fresh leaves was homogenized in an ice-cold 0.066 mol/L K-Na phosphate buffer (pH 7.4), with the addition of polyvinylpyrrolidone (PVP 40, Sigma-Aldrich, Sofia, Bulgaria). The homogenate was centrifuged at 10,000× g at 4 °C for 20 min. The activity of GPX in the supernatant was determined spectrophotometrically by measuring the increase in absorbance at 470 nm. The reaction mixture contained 80 mmol/l guaiacol (W253200, Sigma-Aldrich) and 10 mmol/L H2O2 (Hydrogen peroxide solution, 30 % (w/w) in H2O, Merck, Sofia, Bulgaria) in 0.066 mol/L phosphate buffer, pH 7.4. The enzymatic activity is expressed in U mg−1 protein per 1 g leaves fresh weight.
2.4. Data Processing
All data presented are the averages of triplicate analysis of three separate subsamples. For the statistical evaluation of the data obtained, the raw values of the three subsamples per species per site were used and the differences were considered significant when p < 0.05. The Shapiro–Wilk test was used to test the normality of the data as a more appropriate method for small sample sizes (<50 samples). ANOVA and Student/Fisher test were used for testing the differences in studied parameters, both between the three plant species in one plot and also between the four studied sampling plots and between studied biochemical markers (p < 0.05). Regression analysis was applied to evaluate the dependence of the studied parameters on traffic intensity and urban air pollution. All statistical analyses were performed with STATISTICA version 7 (data analysis software system), Statsoft Inc. (Tulsa, OK, USA), 2004.
3. Results
3.1. Photosynthetic Pigments
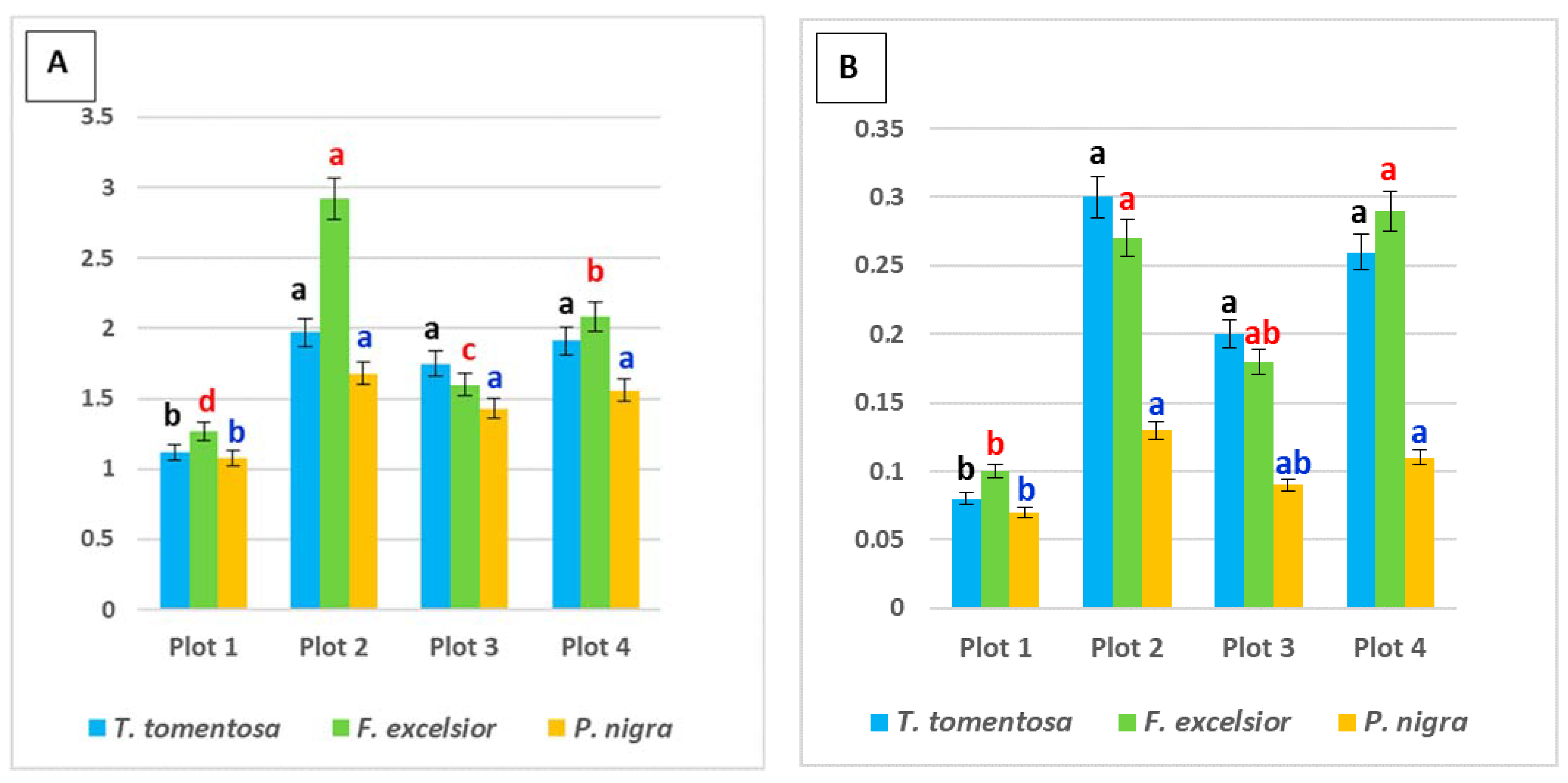
Data about the measured content of total chlorophyll, chlorophyll a, chlorophyll b and carotenoids in plant leaves, as well as calculated values of the ratios total chlorophyll/carotenoids and chlorophyll a/chlorophyll b, are presented in Figure 2 and Figure 3.
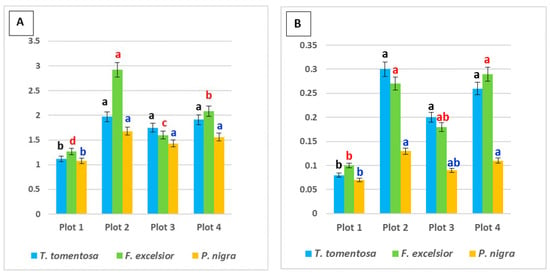
Figure 2.
Content (mg g−1 fresh weight) of photosynthetic pigments in the leaves of studied tree species, planted in four urban plots with different types and intensity of anthropogenic impact: (A) total chlorophyll content; (B) carotenoid content. For each tree species (Tilia tomentosa in black, Fraxinus excelsior in red; Pinus nigra in blue), means showing different letters are significantly different according to Student’s LSD comparison test (p < 0.05).
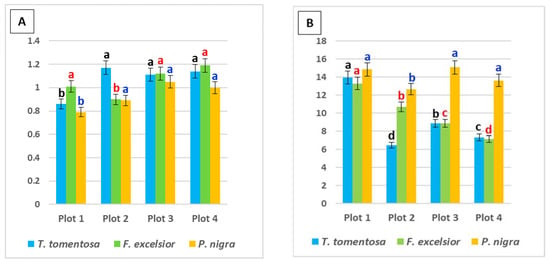
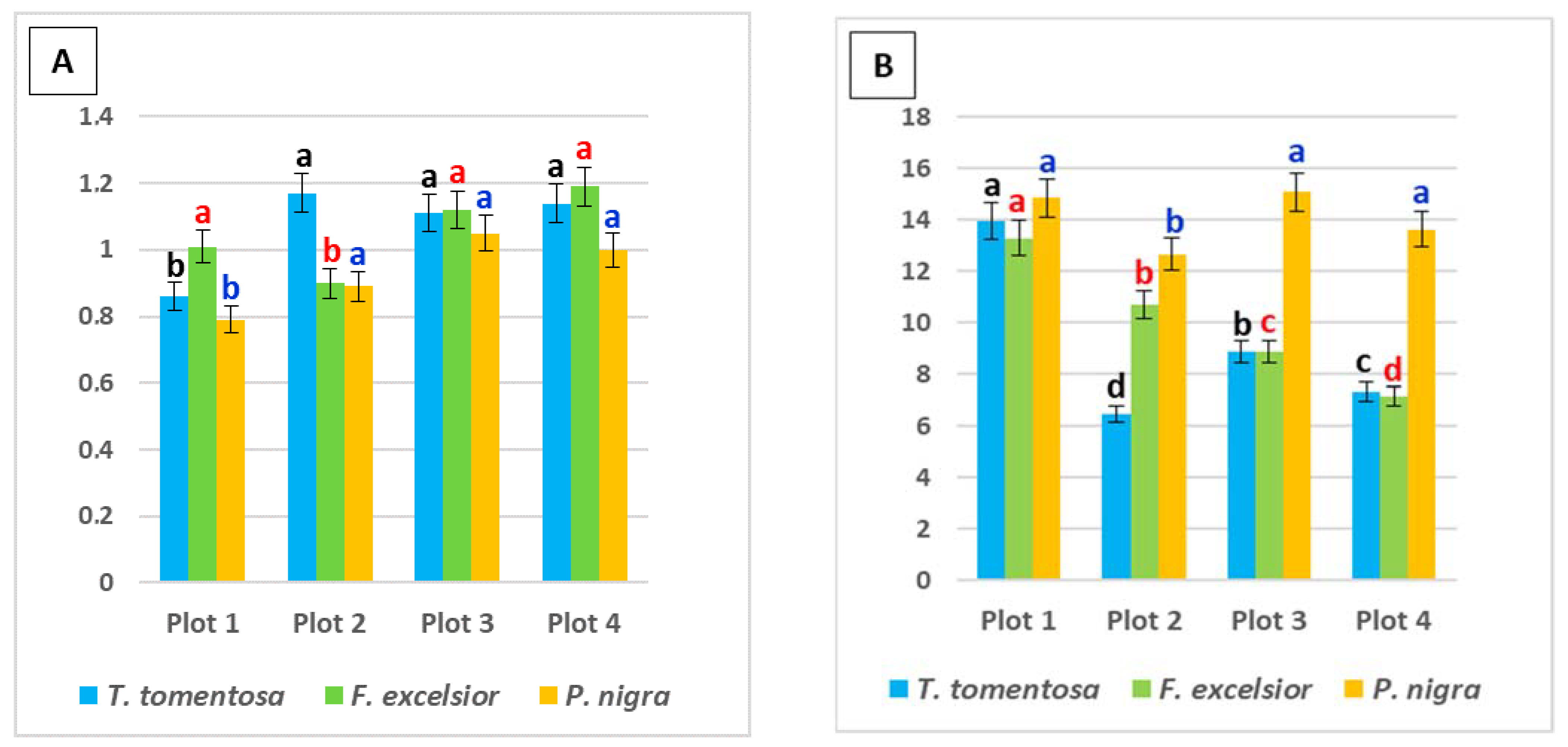
Figure 3.
Ratio between photosynthetic pigments in the leaves of studied tree species, planted in four urban plots with different types and intensity of anthropogenic impact: (A) chlorophyll a/chlorophyll b; (B) total chlorophyll/carotenoids. For each tree species (Tilia tomentosa in black, Fraxinus excelsior in red; Pinus nigra in blue), means showing different letters are significantly different according to Student’s LSD comparison test (p < 0.05).
Content of both total chlorophyll and carotenoids in all studied ornamental species was significantly lower in leaf samples from Plot 1, followed by Plot 3 (p < 0.05). Maximum values were found in Plot 2, also for the three studied species (p < 0.05). These sites could be classified as more affected by traffic pollution according to the selection criteria (Figure 1). Our results are in agreement with data from Merakchiyska-Nikolova et al. [33], who found a significant reduction in pigments in Tilia tomentosa under the influence of urban pollution in Sofia city (Bulgaria).
Regarding species specifics, it was obvious that the highest level of both types of photosynthetic pigments was in Fraxinus excelsior, with an average content of 2.01 mg g−1 fw total chlorophyll and 0.21 mg g−1 fw carotenoids (p < 0.05) (Figure 2). Despite this fact, we found that in Plot 3 (most influenced by traffic), the maximum pigment content was in T. tomentosa leaves. This fact can be related to the statements of some authors [34,35,36] whereby the changes in total chlorophyll content characterize the sustainability and adaptation extent of the plants to the permanently high level of air pollution.
Tilia tomentosa shows an average content of 1.76 mg g−1 fw total chlorophyll and 0.20 mg g−1 fw carotenoids, while Pinus nigra demonstrates significantly lower values of 1.48 mg g−1 fw total chlorophyll and 0.10 mg g−1 fw carotenoids (Figure 2). Our simultaneous observations on some other P. nigra individuals, growing nearby but planted for more than 20 years in the urban environment, showed quite elevated pigment values in the range 1.6–1.82 mg g−1 fw total chlorophyll and 0.19–0.24 mg g−1 fw carotenoids. Parallel observations on some other individuals of T. tomentosa, growing nearby but planted for more than 15 years in the urban environment, also revealed some pigment values in the range 2.75–3.81 mg g−1 fw total chlorophyll and 0.46–0.76 mg g−1 fw carotenoids. These results can be explained by the suppression of pigment synthesis under the stressful urban conditions, as reported in other studies [20,34,36]. The results obtained are consistent with the notion that photosynthesis is one of most sensitive processes to environmental stresses. As some authors have mentioned previously, low values of carotenoid content could characterize the resistance and the degree of adaptation of plants to constant and high levels of atmospheric pollution in the environment [34,36,37].
The chl a/chl b ratio in the urban environment is an effective indicator both for photosynthetic activity and the health status of plants [19,20,36,38,39]. In the present study, this ratio had higher values in leaf samples from Plot 3 and Plot 4 for F. excelsior (1.12 and 1.19, respectively) and P. nigra (1.05 and 1.0, respectively), but for T. tomentosa, the maximum is found in Plot 2 (1.17) and Plot 4 (1.14) (Figure 3A).
The total chl/carotenoid ratio had higher values in leaf samples from Plot 1 and Plot 3 for T. tomentosa (13.96 and 8.87, respectively) and P. nigra (14.85 and 15.08, respectively), but for F. excelsior, the maximum is found in Plot 1 (13.3) and Plot 2 (10.69) (Figure 3B). Higher ratio values can be a result of both the stimulation of chlorophyll synthesis and the suppression of carotenoid synthesis. According to some authors [18], carotenoid pigments exhibit several substantial functions in plant physiology (plant growth and development, adaptation to the environmental conditions, etc.), among which antioxidant defense is the most important. Many authors revealed that carotenoids were present in smaller concentrations in plants growing close to a source of contamination [40,41].
3.2. Free Proline
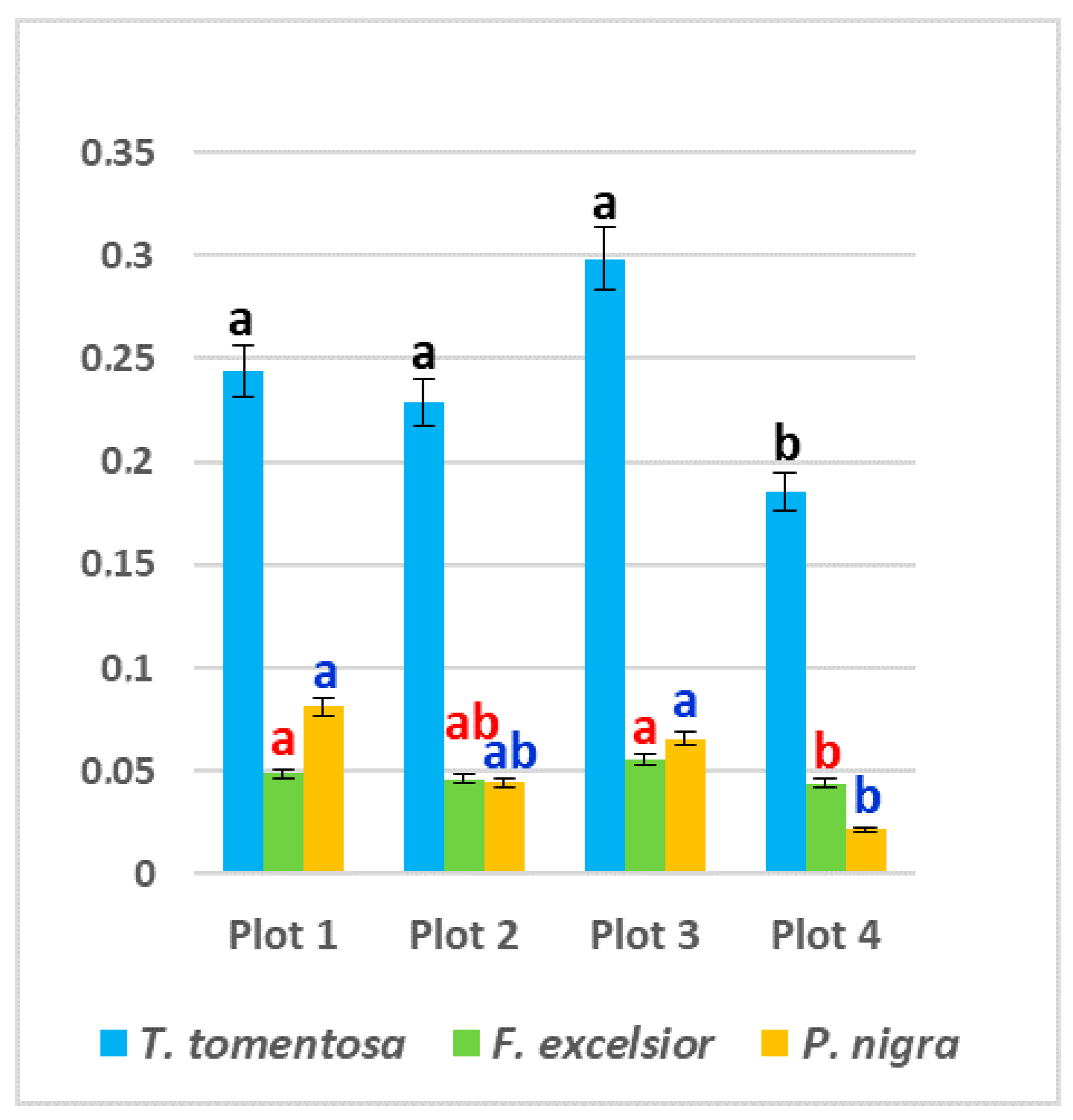
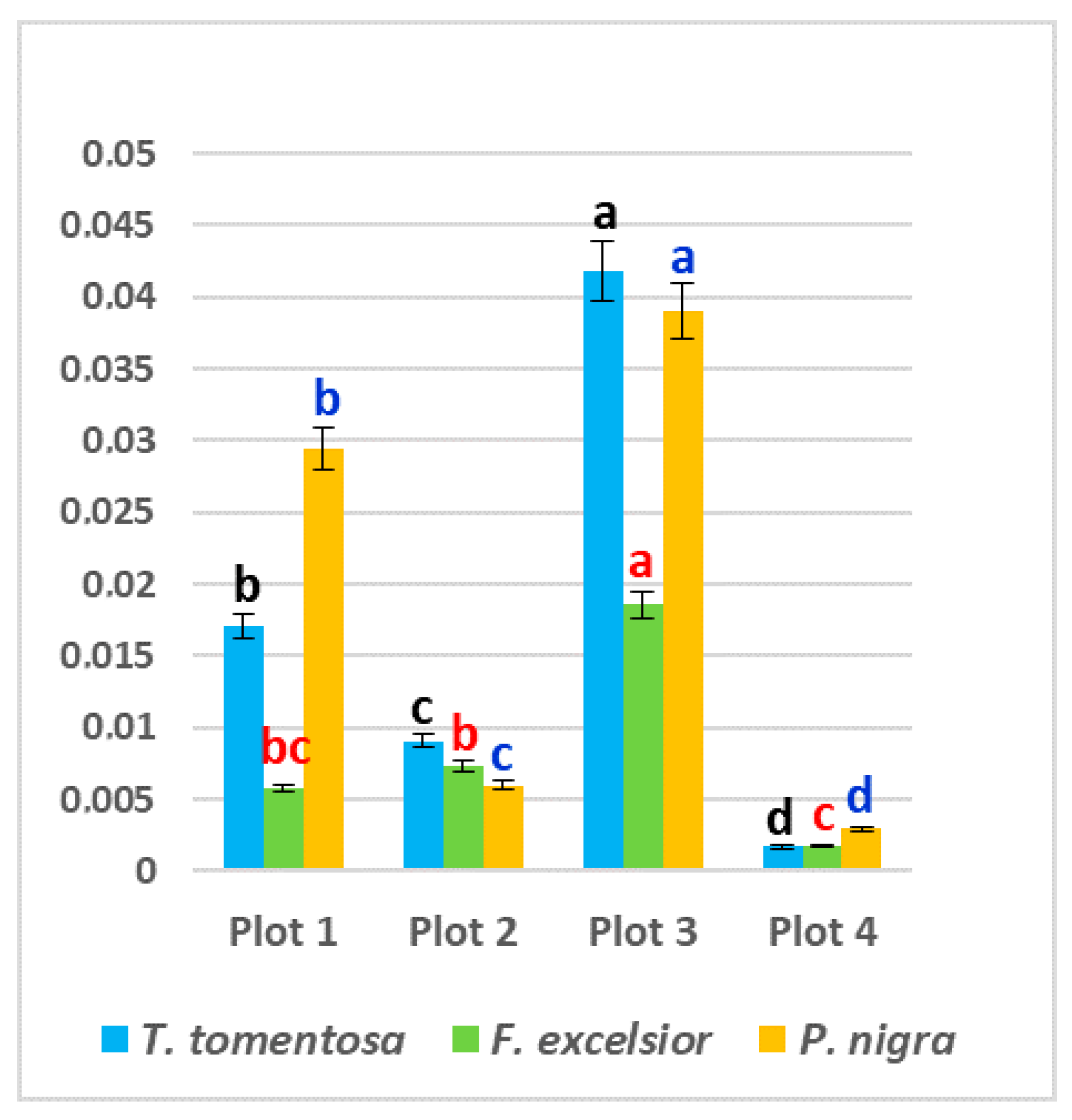
The content of free proline in all studied ornamental species was significantly higher in leaf samples from the three experimental plots subjected to higher anthropogenic impacts (Plot 1, Plot 2 and Plot 3) in comparison with the “control” Plot 4—up to 68% in P. nigra, up to 61% in T. tomentosa and up to 26% in F. exselsior (p < 0.05). Maximum values were found in Plot 3 for T. tomentosa (0.2985 mg g−1 fw) and F. excelsior (0.0554 mg g−1 fw), and in Plot 1 for P. nigra (0.0814 mg g−1 fw) (Figure 4). Regarding species specifics, it is obvious that the highest level of free proline was found in T. tomentosa, with up to 10-fold greater values in comparison with the other two studied trees (p < 0.05) (Figure 4).
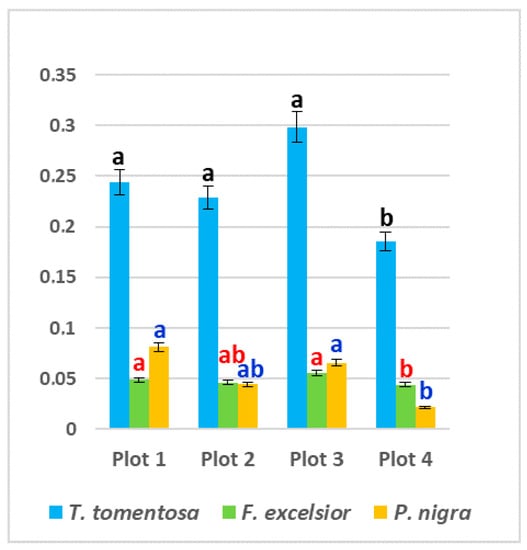
Figure 4.
Content of free proline (mg g−1 fw) in leaves of studied tree species, planted in four urban plots with different types and intensity of anthropogenic impact. For each tree species (Tilia tomentosa in black, Fraxinus excelsior in red; Pinus nigra in blue), means showing different letters are significantly different according to Student’s LSD comparison test (p < 0.05).
3.3. GPX Activity
The activity of GPX varied between 0.0017 U mg−1 protein (T. tomentosa, Plot 4) and 0.0418 U mg−1 protein (T. tomentosa, Plot 3) (Figure 5). The highest values of GPX activity in the leaves of all studied species were found in Plot 3 (intensive vehicle and railroad traffic), followed by Plot 1 (intensive motor traffic) (p < 0.05). These results show a clear tendency towards increased GPX activity with an increase in the traffic intensity and correlate with the findings of many authors [28,41,42,43,44].
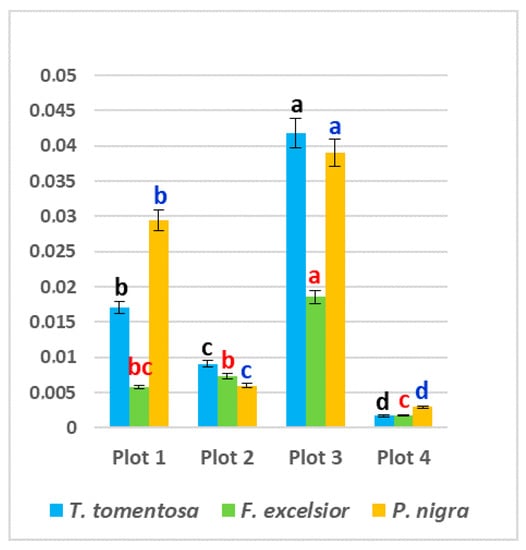
Figure 5.
Activity of guaiacol peroxidase (GPX) (U mg−1 protein) in leaves of studied tree species, planted in four urban plots with different types and intensity of anthropogenic impact. For each tree species (Tilia tomentosa in black, Fraxinus excelsior in red; Pinus nigra in blue), means showing different letters are significantly different according to Student’s LSD comparison test (p < 0.05).
Generally, peroxidase functions are associated with the neutralization of excess hydrogen peroxide, which is mainly accumulated in plant tissues due to stress effects [45]. Therefore, the results obtained indicate significant metabolic adjustments in tree leaves in the course of their adaptation towards the urban environment’s impacts. It is likely that such changes affect the accumulation of phenols and sugars, since Allison and Schultz [46] emphasized that peroxidases appear to play an important role in these metabolic pathways.
3.4. Assessment of the Antioxidant Response in Relation to the Intensity of Traffic and Urban Air Pollution
Considering the present achievements and some preliminary data, the values of all analyzed biochemical markers of stress were divided into the following four classes: low, medium, high and very high. These correspond well with the levels of air pollution, given in Table 1. The values of these classes were used as a reference scale (in relation to different tree species) for the evaluation of the different levels of air pollution in the experimental plots (Table 3).

Table 3.
Reference scale for early determination of the anthropogenic impact extent based on the different levels of biochemical stress markers in urban trees.
The GPX activity of T. tomentosa leaves directly correlates with the enhancement in traffic intensity and the level of air pollution (R = 0.91, p < 0.05) (Table 4). Positive relationships are found also between GPX activity and the level of free proline in leaves, as well as between the free proline level and ratio of total chlorophyll/carotenoids (p < 0.05). Negative relationships are found between photosynthetic pigment content and the level of free proline, on one hand, as well as the ratio of total chlorophyll/carotenoids on the another (p < 0.05). Thus, it can be assumed that the response of T. tomentosa towards urban air pollution is expressed both by an increment in enzyme activity and proline synthesis, and the inhibition of photosynthetic pigment synthesis (Table 4).

Table 4.
Pearson correlations between the level of air pollution and levels of studied biochemical markers in leaves of T. tomentosa from four experimental plots (n = 72, 3 individuals × 4 plots × 6 years).
The GPX activity in F. excelsior leaves directly correlates with the enhancement in traffic intensity and the level of air pollution also (R = 0.77, p < 0.05), but the content of free proline is found to be more significant (R = 0.94, p < 0.05) (Table 5). Negative relationships are found between photosynthetic pigment content and the level of free proline (p < 0.05). As a whole, this species shows weaker relationships between the studied parameters, both positive and negative, among all three plants included in the experiment. This finding can be interpreted as evidence of its higher tolerance to the urban environment and thus better appropriateness and efficacy for urban landscaping activities.

Table 5.
Pearson correlations between the level of air pollution and levels of studied biochemical markers in leaves of F. excelsior from four experimental plots (n = 72, 3 individuals × 4 plots × 6 years).
P. nigra is found to be the most sensitive among all studied tree species as stress responses with significant correlations with urban air pollution are identified for each type of biochemical marker used (Table 6). The most pronounced are in the groups “air pollution–GPX activity” (R = 0.95, p < 0.05), “air pollution–chl a/chl b ratio” (R = 0.94, p < 0.05) and “air pollution–total chl/carotenoid ratio” (R = 0.94, p < 0.05), followed by “air pollution–free proline” (R = 0.80, p < 0.05) and “air pollution–carotenoids” (R = −0.89, p < 0.05). GPX activity correlates positively with the free proline level and two ratios, and negatively with the content of total chlorophyll and carotenoids (p < 0.05). Similar tendencies are established for the free proline level in the needles. All these results clearly indicate that significant stress reactions have occurred and different mechanisms of antioxidant defense have been involved.

Table 6.
Pearson correlations between the level of air pollution and levels of studied biochemical markers in leaves of P. nigra from four experimental plots (n = 72, 3 individuals × 4 plots × 6 years).
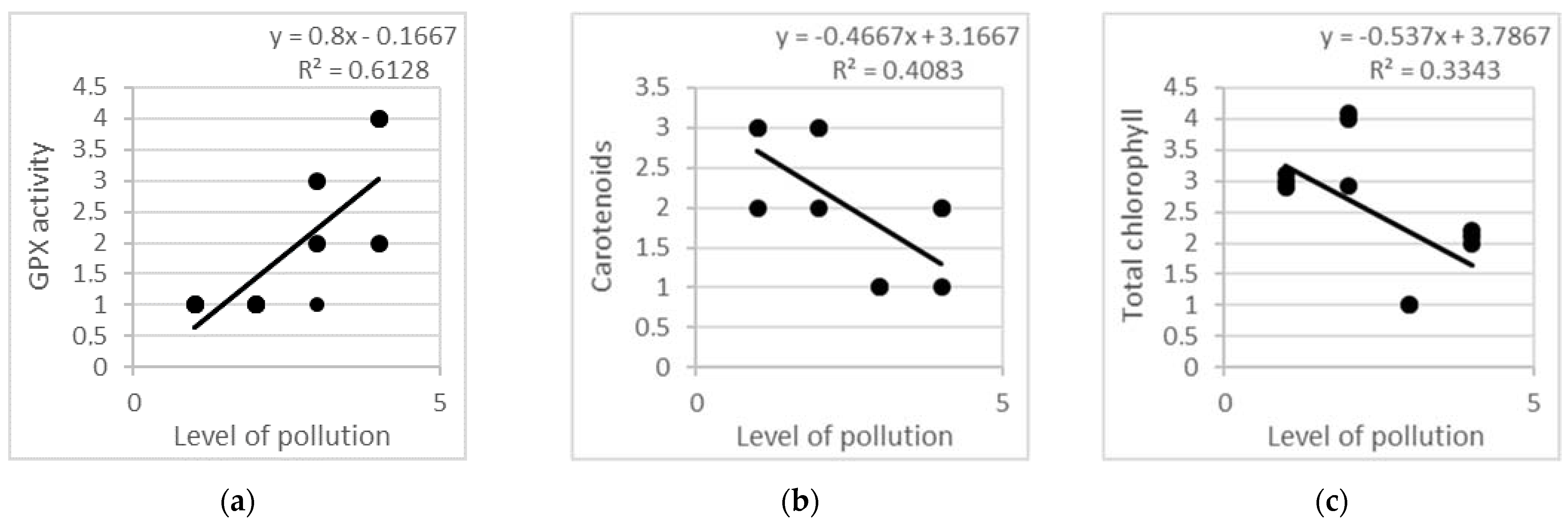
Linear regression models created on the basis of all studied leaf samples (n = 216) were evaluated in order to explain the relationships between the level of air pollution (due to traffic intensity, as explained in Table 1) and the antioxidant response of the tree species according to the proposed reference scale in Table 3 (Figure 6). The linear regression models, linear equations and determination coefficients confirm that a strong correlation exists between air pollution and the levels of some biochemical markers in all four urban plots (p < 0.05). Approximately 61% of the dispersion of GPX activity and 33%–40% of the dispersion of the carotenoid and total chlorophyll content can be explained by the dispersion of air pollution (Figure 6), while a further 39%–60% represent results of the impact of factors not included in the model (p < 0.05). ANOVA analysis revealed the coefficient values as follows: F = 15.824 at p = 0.003 for GPX activity, F = 5.947 at p = 0.035 for carotenoid content, F = 6.901 at p = 0.025 for total chlorophyll. Thus, there are strong, significant relationships between these biochemical markers and traffic pollution.
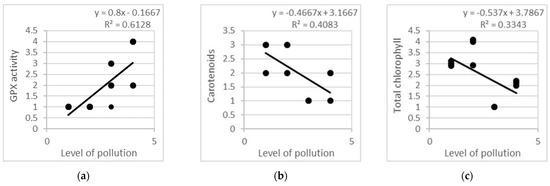
Figure 6.
Linear regression models on the basis of all studied leaf samples from three tree species, planted in four urban plots with different types and intensity of anthropogenic impact: (a) GPX activity; (b) carotenoid content; (c) total chlorophyll content.
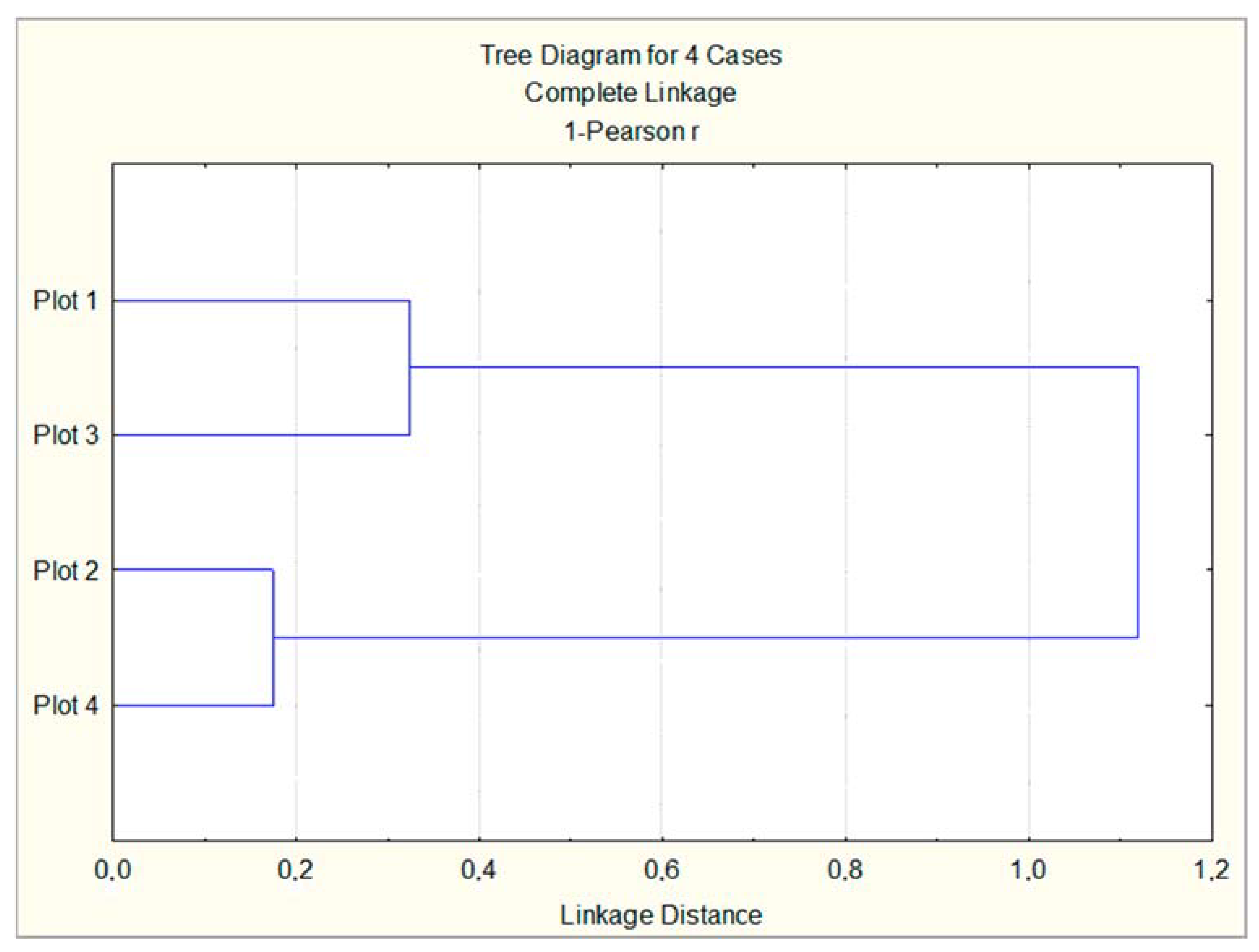
Cluster analysis performed on the basis of all studied leaf samples highlighted the existence of a significant, close relationship between Plot 2 and Plot 4, which were classified as low and moderately polluted according to Table 1 (Figure 7). Plot 3 and Plot 1 form another cluster in correspondence with the level of air pollution given in Table 1—high and very high. The descending order of the experimental urban plot according to the antioxidant response of the studied trees is found to be as follows: Plot 3 > Plot 1 > Plot 2 > Plot 4. This coincides with the descending order of plots according to the pollution level and traffic intensity.
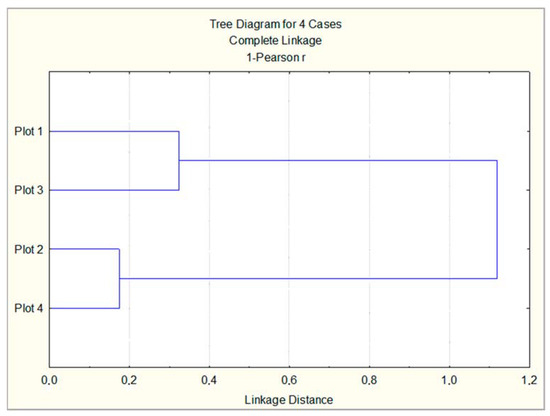
Figure 7.
Cluster analysis on the basis of all studied leaf samples from three tree species, planted in four urban plots with different types and intensity of anthropogenic impact.
4. Discussion
Since plants are constantly exposed to air, they are the primary receptors for both gaseous and particulate pollutants from the atmosphere. In terrestrial plant species, the enormous foliar surface area acts as a natural sink for pollutants, especially particulate ones.
The harmful impact of air pollutants on plants is firstly manifested by changes in their pigment content [20,28,36,39]. Toxic gases from the ambient air enter into the leaf lamella through the stomata, whereupon they interact with water from the intercellular spaces, so various anions are produced. Anions easily penetrate into the cytoplasm due to membrane transport and then accumulate in the chloroplasts. Such presence leads to irreversible changes in the structure and functions of chloroplasts and significantly damages the photosynthetic process [47]. Chlorophyll measurement is an important tool to evaluate the effect of air pollutants on plants, as it plays an important role in plant metabolism. The reduction in chlorophyll concentration in the polluted leaves could be due to chloroplast damage, inhibition of chlorophyll biosynthesis or enhanced chlorophyll degradation. The results obtained in the present study confirm the findings of Ianovici et al. [48] and other researchers [49,50] whereby, unlike chlorophyll b, the labile form prevails significantly over the tightly bound form in chlorophyll a, which implies its easier destruction under the influence of extreme environmental factors. The decline in the ratio of chl a/chl b found in the leaves of studied tree species was also considered as an efficient biomarker for the assessment of the adaptation ability of plants towards non-favorable environments and an indicator of their resistance to toxic gases [38,39].
Proline is an α-amino acid that exists in the plant cells in a free or bound state. It interacts directly or indirectly with macromolecules, stabilizing their spatial structures. According to the literature, proline acts as an osmoprotectant against turgor loss and dehydration in plants [43,51]. The main functions of osmoprotectants (proline, glycine-betaine, etc.) overlap with those of some stress-inducible proteins (dehydrins, chaperones, etc.) to some extent, but, of course, they are involved at different levels of cell organization. A high concentration of proline correlates with its role in osmolysis, where, due to its high hydrophilicity, it increases the osmotic pressure of the cells, improving their water balance and preventing the deleterious effects of an insufficient water supply on cytoplasmic colloids [52].
More recently, free proline’s functions in the cell have been associated with the disposal of reactive oxygen species, including singlet oxygen 1O2 [52,53]. Moreover, it is also involved in neutralizing and eliminating the formed reactive oxygen species in the thylakoid membranes during water stress. Despite the well-known antioxidant and osmoregulatory functions, proline’s role in plants is often related to the control of gene expression [54].
A number of authors have agreed that the content of free proline could characterize the physiological state of plant organisms, where its minimal content in plant cells means optimal growth conditions [50,54]. According to other authors [55], its accumulation in plants could be explained as a protective reaction in overcoming adverse growth conditions [56]. Fahmy et al. [57] revealed that free proline accumulation in plants is associated with premature leaf aging and delayed chlorophyll synthesis.
The high content of free proline in the T. tomentosa leaves in our study confirms the literature data regarding a dramatic increase in its level under various stress effects—up to 50 times the normal levels in a plant cell [54]. The increase in the endogenous proline concentration can be interpreted as a general, non-specific protective response of plants towards oxidative processes in their tissues due to adverse environmental conditions in the urban ecosystem. Our data confirm the negative correlation associated with changes in chlorophyll and proline content (p < 0.05), where higher proline content can be explained by a decrease in chlorophyll content, as previously proved by Gupta et al. [58].
Antioxidant systems in plants may be used as early indicators of environmental stress in target organisms, preceding morphological or ultrastructural damage, and as warning indicators for the ecosystem. Guaiacol peroxidases (GPXs) are a group of proteins that contain a heme cofactor in their active sites and catalyze reactions using oxidize aromatic electron donors such as guaiacol and pyragallol [59]. Their optimal substrate is hydrogen peroxide. The main role of guaiacol peroxidase in plants is the defense against abiotic and biotic stresses, so the GPXs are widely accepted as stress enzymes. A wide range of stressful environmental conditions (pollutants) are shown to induce the production of GPX in plants: heavy metals [42,43,44,45,46], herbicides [60], ozone [61], polycyclic aromatic hydrocarbons [62], etc. Many authors have revealed both increments and decrements in the GPX activity in plants upon exposure to different pollutants, including motor traffic pollution, depending on the species’ properties [36,63,64,65,66].
Estimation of plant ecophysiological responses in a field study may be useful in pollution biomonitoring, as well as in verifying the effect of metal contamination on plant physiology [66]. Future research directions may be focused on developing a similar fast and reliable method and reference scales based on other plant properties, e.g., anatomical, histological, or molecular. The functional traits and growth of tree selection for urban planting require further study, focusing on adaptation properties and environmental stress tolerance, in order to achieve a better overview of the relations between urban greenery and its environment.
5. Conclusions
Urban vegetation is crucial for maintaining a biological balance between the elements of the socio-ecological system (man–society–nature). Our study confirms that plant responses and adaptation to environmental conditions are complex biological processes including physiological and biochemical changes. As a whole, all studied trees reacted via specific mechanisms towards urban air pollution, and the antioxidant responses correlated significantly with the increase in traffic. Reference scales for the evaluation of the different levels of biochemical stress markers in relation to the urban air pollution have been proposed. These findings are very useful as a scientific basis for the landscaping practice in terms of the sustainable development and management of urban forestry.
Fraxinus excelsior is very suitable for urban landscaping due to the significant tolerance to environmentally stressful conditions. This species maintained a moderate level of biochemical markers, even in highly and very highly polluted experimental plots. Moreover, it seems to have a considerable reserve of adaptation capacity that not has yet been expressed.
Tilia tomentosa is also a suitable ornamental species as, in the course of the study, it demonstrated good development in the urban environment. The stress level in the plant was quite elevated and correlated significantly with the traffic intensity and urban air pollution. Antioxidant responses were observed for each pathway—physiological, non-enzymatic and enzymatic—but they were considerably below the critical level for plant well-being.
Pinus nigra is more sensitive to urban air pollution in comparison with the other two studied trees. As an evergreen species, it is subjected to the constant impact of the urban environment throughout the whole year, not only in the active vegetation period, as with the deciduous ones, which could explain our results. The antioxidant response is strongly expressed by an increment in the enzymatic and non-enzymatic activity with an increase in traffic intensity, both combined with the increment in the ratios of total chlorophyll/carotenoids and chl a/chl b. The high ratio values could be a consequence of low carotenoid levels and low chlorophyll b levels, respectively, which is a signal of alterations in the photosynthetic process.
Aiming to achieve optimal development, urban trees exhibit constant adaptation towards the dynamics of urban load. Although the adaptation mechanisms have not been clarified yet, it is known that different signaling pathways in plant cells are involved. More studies will be needed for a better understanding of the biochemical stress responses of plants and to enhance the plant resistance towards unfavorable factors in the environment.
Author Contributions
Conceptualization, S.P. and I.V.; methodology, T.V. and V.B.; software, S.P.; validation, S.P., B.N. and I.V.; formal analysis, S.P.; investigation, S.P. and I.V.; resources, S.P. and B.N.; data curation, all; writing—original draft preparation, S.P.; writing—review and editing, all; visualization, S.P.; supervision, I.V.; project administration, S.P.; funding acquisition, I.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department for Scientific Research, University of Plovdiv “Paisii Hilendarski”, Plovdiv, Bulgaria, grant numbers SP15-BF14 and MU17-BF22.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kondratyeva, A.; Knapp, S.; Durka, W.; Kühn, I.; Vallet, J.; Machon, N.; Martin, G.; Motard, E.; Grandcolas, P.; Pavoine, S. Urbanization Effects on Biodiversity Revealed by a Two-Scale Analysis of Species Functional Uniqueness vs. Redundancy. Front. Ecol. Evol. 2020, 8, 73. [Google Scholar] [CrossRef]
- McDonald, R.I.; Mansur, A.V.; Ascensão, F.; Colbert, M.; Crossman, K.; Elmqvist, T.; Gonzalez, A.; Güneralp, B.; Haase, D.; Hamann, M.; et al. Research gaps in knowledge of the impact of urban growth on biodiversity. Nat. Sustain. 2020, 3, 16–24. [Google Scholar] [CrossRef]
- Speak, A.; Escobedo, F.J.; Russo, A.; Zerbe, S. An ecosystem service-disservice ratio: Using composite indicators to assess the net benefits of urban trees. Ecol. Indic. 2018, 95, 544–553. [Google Scholar] [CrossRef]
- Wolf, K.L.; Lam, S.T.; McKeen, J.K.; Richardson, G.R.A.; van den Bosch, M.; Bardekjian, A.C. Urban Trees and Human Health: A Scoping Review. Int. J. Environ. Res. Public Health 2020, 17, 4371. [Google Scholar] [CrossRef] [PubMed]
- Mirici, M.E. The Ecosystem Services and Green Infrastructure: A Systematic Review and the Gap of Economic Valuation. Sustainability 2022, 14, 517. [Google Scholar] [CrossRef]
- Kovachev, A. The Green System of Sofia; Pensoft Publishers: Sofia, Bulgaria, 2005. (In Bulgarian) [Google Scholar]
- Millennium Ecosystem Assessment (MA). Ecosystems and Human Well-Being: Biodiversity Synthesis; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- Valente, D.; Pasimeni, M.R.; Petrosillo, I. The role of green infrastructures in Italian cities by linking natural and social capital. Ecol. Indic. 2020, 108, 105694. [Google Scholar] [CrossRef]
- Barwise, Y.; Kumar, P. Designing vegetation barriers for urban air pollution abatement: A practical review for appropriate plant species selection. Clim. Atmos. Sci. 2020, 3, 12. [Google Scholar] [CrossRef]
- Jayasooriya, V.M.; Muthukumaran, S.; Perera, B.J.C. Green infrastructure practices for improvement of urban air quality. Urban For. Urban Green. 2017, 21, 34–47. [Google Scholar] [CrossRef]
- Gunawardena, K.R.; Wells, M.J.; Kershaw, T. Utilising green and bluespace to mitigate urban heat island intensity. Sci. Total Environ. 2017, 584–585, 1040–1055. [Google Scholar] [CrossRef] [PubMed]
- Pearlmutter, D.; Calfapietra, C.; Samson, R.; O’Brien, L.; Ostoić, S.K.; Sanesi, G.; Alonso del Amo, R. The Urban Forest, Cultivating Green Infrastructure for People and the Environment; Springer: Berlin/Heidelberg, Germany, 2017; p. 351. [Google Scholar]
- Song, X.P.; Tan, P.Y.; Edwards, P.; Richards, D. The economic benefits and costs of trees in urban forest stewardship: A systematic review. Urban For. Urban Green. 2018, 29, 162–170. [Google Scholar] [CrossRef]
- Gotsch, S.G.; Draguljić, D.; Williams, C.J. Evaluating the effectiveness of urban trees to mitigate storm water runoff via transpiration and stemflow. Urban Ecosyst 2018, 21, 183–195. [Google Scholar] [CrossRef]
- Maragno, D.; Gaglio, M.; Robbi, M.; Appiotti, F.; Fano, E.A.; Gissi, E. Fine-scale analysis of urban flooding reduction from green infrastructure: An ecosystem services approach for the management of water flows. Ecol. Model. 2018, 386, 1–10. [Google Scholar] [CrossRef]
- European Commission (EC). Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: Green Infrastructure—Enhancing Europe’s Natural Capital; Publications Office of the European Union: Brussels, Belgium, 2013; Available online: http://eur-lex.europa.eu/resource.html?uri=cellar:d41348f2-01d5-4abe-b8174c73e6f1b2df.0014.03/DOC_1&format=PDF (accessed on 19 August 2021).
- Trentanovi, G.; Campagnaro, T.; Kowarik, I.; Munafò, M.; Semenzato, P.; Sitzia, T. Integrating spontaneous urban woodlands into the green infrastructure: Unexploited opportunities for urban regeneration. Land Use Policy 2021, 102, 105221. [Google Scholar] [CrossRef]
- Chaudhary, I.J.; Rathore, D. Dust pollution: Its removal and effect on foliage physiology of urban trees. Sustain. Cities Soc. 2019, 51, 101696. [Google Scholar] [CrossRef]
- Chaudhary, I.J.; Rathore, D. Suspended particulate matter deposition and its impact on urban trees. Atmos. Pollut. Res. 2018, 9, 1072–1082. [Google Scholar] [CrossRef]
- Kamble, P.; Bodhane, P.S.; Beig, G.; Awale, M.; Mukkannawar, U.; Mane, A.V.; Mujumdar, M.; Kuchekar, S.R.; Patil, V.N. Impact of transport sector emissions on biochemical characteristics of plants and mitigation strategy in Pune, India. Environ. Chall. 2021, 4, 100081. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic Stress Responses and Microbe-Mediated Mitigation in Plants: The Omics Strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic Stress Tolerance in Plants: Myriad Roles of Ascorbate Peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Chorological Maps for the Main European Woody Species. 2020. Available online: https://www.genres.eu/results/content/31f4-chorological-maps-for-the-main-european-woody-species/ (accessed on 30 July 2022).
- ISTAT. Available online: http://www.en.istat.it (accessed on 11 September 2019).
- European Environmental Agency. Report 2018. Available online: http://eea.government.bg/bg/soer/2017/air/emisii-na-vredni-veshtestva-vav-vazduha (accessed on 28 September 2021).
- Puccinelli, P.; Anselmi, N.; Bragaloni, M. Peroxidases: Usable markers of air pollution in trees from urban environment. Chemosphere 1998, 36, 889–894. [Google Scholar] [CrossRef]
- Morley, P.G.; Jump, A.S.; West, M.D.; Donoghue, D.N.M. Spectral response of chlorophyll content during leaf senescence in European beech trees. Environ. Res. Commun. 2020, 2, 071002. [Google Scholar] [CrossRef]
- Carillo, P.; Gibon, Y. Protocol: Extraction and determination of proline. PrometheusWiki 2011, 2011, 1–5. [Google Scholar]
- Ridge, I.; Osborne, D.J. Role peroxidase when hydroxyprolin-rich protein in plant cell wall is increased by ethylene. Nat. New Biol. 1971, 229, 205–208. [Google Scholar] [CrossRef]
- Shevyakova, N.I.; Stetsenko, L.A.; Meshcheryakova, A.B.; Kuznetsov, V.V. The activity of the peroxidase system in the course of stress-induced CAM development. Rus. J. Plant Physiol. 2002, 49, 598–604. [Google Scholar] [CrossRef]
- Merakchiyska-Nikolova, M.; Kurteva, M.; Delcheva, M.; Paunova, S.; Gecheva, V. Impact of industrial and urban pollution on pigments content in ornamental tree and shrub species. For. Sci. 2002, 1, 49–61. (In Bulgarian) [Google Scholar]
- Dezhban, A.; Shirvany, A.; Attarod, P.; Delshad, M.; Matinizadeh, M.; Khoshnevis, M. Cadmium and lead effects on chlorophyll fluorescence, chlorophyll pigments and proline of Robinia pseudoacacia. J. For. Res. 2015, 26, 323–329. [Google Scholar] [CrossRef]
- Pavlović, D.; Pavlović, M.; Marković, M.; Karadžić, B.; Kostić, O.; Jarić, S.; Mitrović, M.; Gržetić, I.; Pavlović, P. Possibilities of assessing trace metal pollution using Betula pendula Roth. leaf and bark—Experience in Serbia. J. Serb. Chem. Soc. 2017, 82, 1–15. [Google Scholar] [CrossRef]
- Khosropour, E.; Attarod, P.; Shirvany, A.; Pypker, T.G.; Bayramzadeh, V.; Hakimi, L.; Moeinaddini, M. Response of Platanus orientalis leaves to urban pollution by heavy metals. J. For. Res. 2019, 30, 1437–1445. [Google Scholar] [CrossRef]
- Sen, A.; Khan, I.; Kundu, D.; DAS, K.; Datta, J.K. Ecophysiological evaluation of tree species for biomonitoring of air quality and identification of air pollution-tolerant species. Env. Monit Assess 2017, 189, 262. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Shafiq, M.; Zaidi, S.; Athar, M. Effect of automobile pollution on chlorophyll content of roadside urban trees. Glob. J. Environ. Sci. Manag. 2015, 1, 283–296. [Google Scholar] [CrossRef]
- Molnár, V.É.; Tóthmérész, B.; Szabó, S.; Simon, E. Urban tree leaves’ chlorophyll-a content as a proxy of urbanization. Air Qual Atmos Health 2018, 11, 665–671. [Google Scholar] [CrossRef]
- Uka, U.N.; Belford, E.J.D.; Hogarh, J.N. Roadside air pollution in a tropical city: Physiological and biochemical response from trees. Bull. Natl. Res. Cent. 2019, 43, 90. [Google Scholar] [CrossRef]
- Elloumi, N.; Zouari, M.; Mezghani, I.; Ben Abdallah, F.; Woodward, S.; Kallel, M. Adaptive biochemical and physiological responses of Eriobotrya japonica to fluoride air pollution. Ecotoxicology 2017, 26, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Kandziora-Ciupa, M.; Ciepał, R.; Nadgórska-Socha, A.; Barczyk, G. Accumulation of heavy metals and antioxidant responses in Pinus sylvestris L. needles in polluted and non-polluted sites. Ecotoxicology 2016, 25, 970–981. [Google Scholar] [CrossRef]
- Zouari, M.; Elloumi, N.; Labrousse, P.; Ben Rouina, B.; Ben Abdallah, F.; Ben Ahmed, C. Olive trees response to lead stress: Exogenous proline provided better tolerance than glycine betaine. South Afr. J. Bot. 2018, 118, 158–165. [Google Scholar] [CrossRef]
- Skrynetska, I.; Karcz, J.; Barczyk, G.; Kandziora-Ciupa, M.; Ciepał, R.; Nadgórska-Socha, A. Using Plantago major and Plantago lanceolata in environmental pollution research in an urban area of Southern Poland. Environ. Sci. Pollut. Res. 2019, 26, 23359–23371. [Google Scholar] [CrossRef]
- Xie, X.; He, Z.; Chen, N.; Tang, Z.; Wang, Q.; Cai, Y. The Roles of Environmental Factors in Regulation of Oxidative Stress in Plant. BioMed Res. Int. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Allison, S.D.; Schultz, J.C. Differential activity of peroxidase isozymes in response to wounding, Gypsy moth, and plant hormones in Northern Red Oak (Quercus rubra L.). J. Chem. Ecol. 2004, 30, 1363–1379. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, E.; Kontunen-Soppela, S. Plants have different strategies to defend against air pollutants. Curr. Opin. Environ. Sci. Health 2021, 19, 100222. [Google Scholar] [CrossRef]
- Ianovici, N.; Batalu, A.; Hriscu, D.; Datcu, A.D. Phytomonitoring study on intra urban variations of leaves of some evergreen and deciduous trees. Ecol. Indic. 2020, 114, 106313. [Google Scholar] [CrossRef]
- Saxena, P.; Kulshrestha, U. Biochemical Effects of Air Pollutants on Plants. In Plant Responses to Air Pollution; Kulshrestha, U., Saxena, P., Eds.; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Dhir, B. Air Pollutants and Photosynthetic Efficiency of Plants. In Plant Responses to Air Pollution; Kulshrestha, U., Saxena, P., Eds.; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Sanaeirad, H.; Majd, A.; Abbaspour, H.; Peyvandi, M. The Effect of Air Pollution on Proline and Protein Content and Activity of Nitrate Reductase Enzyme in Laurus nobilis L. Plants. J. Mol. Biol. Res. 2017, 7, 99–105. [Google Scholar] [CrossRef]
- Gupta, G.P.; Kumar, B.; Kulshrestha, U.C. Impact and pollution indices of urban dust on selected plant species for green belt development: Mitigation of the air pollution in NCR Delhi, India. Arab. J. Geosci. 2016, 9, 136. [Google Scholar] [CrossRef]
- Dadkhah-Aghdash, H.; Rasouli, M.; Rasouli, K.; Salimi, A. Detection of urban trees sensitivity to air pollution using physiological and biochemical leaf traits in Tehran, Iran. Sci. Rep. 2022, 12, 15398. [Google Scholar] [CrossRef] [PubMed]
- Velinova, K.; Naydenova, T.; Dakov, A. Contents of pigments, total protein and free proline in the assimilating apparatus of 12-year-old European provenances of European beech (Fagus sylvatica L.). Silva Balc. 2008, 9, 59–66. [Google Scholar]
- Singh, H.; Sharma, R.; Sinha, S.; Kumar, M.; Kumar, P.; Verma, A.; Sharma, S.K. Physiological functioning of Lagerstroemia speciosa L. under heavy roadside traffic: An approach to screen potential species for abatement of urban air pollution. 3 Biotech 2017, 7, 61. [Google Scholar] [CrossRef]
- Rai, P.K. Impacts of particulate matter pollution on plants: Implications for environmental biomonitoring. Ecotoxicol. Environ. Saf. 2016, 129, 120–136. [Google Scholar] [CrossRef]
- Fahmy, G.; Hegazy, A.; Hassan, H. Phenology, pigment content and diurnal change of praline in green and senescing leaves of three Zygophyllum species. Flora 1990, 184, 423–436. [Google Scholar] [CrossRef]
- Gupta, D.; Nicoloso, F.; Schetinger, M.; Rossato, L.; Pereira, L.; Castro, G.; Srivastava, S.; Tripathi, R. Antioxidant defense mechanism in hydroponically grown Zea mays saplings under moderate lead stress. J. Hazard. Mater. 2009, 172, 479–484. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Hasanuzzaman, M.; Lao, M.T. Chapter 12: Oxidative Stress and Antioxidant Defense in Plants under Salinity. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; Available online: https://doi.org/10.1002/9781119468677.ch12 (accessed on 30 July 2022). [CrossRef]
- Ivanov, S.; Shopova, E.; Kerchev, P.; Sergiev, I.; Miteva, L.; Polizoev, D.; Alexieva, V. Long-term impact of sublethal atrazine perturbs the redox homeostasis in pea (Pisum sativum L.) plants. Protoplasma 2013, 250, 95–102. [Google Scholar] [CrossRef]
- Li, C.-H.; Wang, T.-Z.; Li, Y.; Zheng, Y.-H.; Jiang, G.-M. Flixweed is more competitive than winter wheat under ozone pollution: Evidences from membrane lipid peroxidation, antioxidant enzymes and biomass. PLoS ONE 2013, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wang, Y.-S.; Sun, C.-C.; Wang, Y.-T.; Peng, Y.-L.; Cheng, H. Effects of pyrene on antioxidant systems and lipid peroxidation level in mangrove plants, Bruguiera gymnorrhiza. Ecotoxicology 2012, 21, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Malik, Z.; Parveen, A.; Zong, Y.; Abbasi, G.H.; Rafiq, M.T.; Shaaban, M.; Mustafa, M.; Bashir, S.; Rafay, M.; et al. Biochar alleviates Cd phytotoxicity by minimizing bioavailability and oxidative stress in pak choi (Brassica chinensis L.) cultivated in Cd-polluted soil. J. Environ. Manag. 2019, 250, 109500. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Saifullah, M.; Zia-ur-Rehman, M.; Akhtar, T.; Zia, M.H.; Aslam, M. Silicon nutrition lowers cadmium content of wheat cultivars by regulating transpiration rate and activity of antioxidant enzymes. Environ. Pollut. 2018, 242 Pt A, 126–135. [Google Scholar] [CrossRef]
- Kandziora-Ciupa, M.; Nadgórska-Socha, A.; Barczyk, G.; Ciepał, R. Bioaccumulation of heavy metals and ecophysiological responses to heavy metal stress in selected populations of Vaccinium myrtillus L. and Vaccinium vitis-idaea L. Ecotoxicology 2017, 26, 966–980. [Google Scholar] [CrossRef] [PubMed]
- Nadgórska–Socha, A.; Kandziora-Ciupa, M.; Trzęsicki, M.; Barczyk, G. Air pollution tolerance index and heavy metal bioaccumulation in selected plant species from urban biotopes. Chemosphere 2017, 183, 471–482. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).