Abstract
The present research focused on the analysis of European beech (Fagus sylvatica L.) wood polyphenols in respect to red heartwood formation, which is a significant color and technological defect of the species. For the first time, high-performance liquid chromatography/tandem mass spectrometry and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) were applied for the investigation of the structure and the radial distribution of polyphenols. Altogether 125 compounds were characterized by their MSn spectra, of which 71 were tentatively identified, including procyanidins (dimers to pentamers), flavonoids (taxifolin, naringenin, isorhamnetin, (epi)afzelechin), (+)-catechin, (−)-epicatechin) and their glycoside conjugates, phenolic acids (gallic acid, vanillic acid, syringic acid) and their glycosides as well as gallic acid derivatives, many of which were identified for the first time in beech wood. It was found that the concentration of many compounds increased at the color boundary. In situ polyphenol synthesis and metabolism were clearly evidenced at the color boundary. Red heartwood contained only free aglycones (syringic acid, taxifolin, naringenin, isorhamnetin, naringenin, syringic acid). Contrary to earlier assumptions, the MALDI-TOF analysis did not indicate the presence of oxidized high-molecular-weight polymeric polyphenols in the red heartwood tissues. The role of individual compounds in the formation of the red heartwood chromophores are discussed.
1. Introduction
Climate change results in the decline of forests due to the increased occurrence of diseases, which leads to the forest dying [1]. European beech (Fagus sylvatica L.) is one of the most common broad-leaved tree species in Europe with significant ecological and economic importance [2]. The most significant color and technological defect of beech is the “red heartwood”, which results in a decrease in timber value [3]. In the past, red heartwood timber was considered as a lower value timber due to its color defects and this has limited its use for a long time [4,5,6]. However, about 20 years ago, intensive marketing efforts were undertaken to make red heartwood timber more accepted by the market [7]; this succeeded in enhancing the appreciation of red heartwood timber in many fields. Nevertheless, the recent literature still describes red heartwood in beech as a defect, resulting in lower quality timber [8,9,10,11].
The causes and the physiology of red heartwood formation are still not clearly understood, although extensive research has been carried out on the phenomenon in regard to its forestry, physiological and biochemical aspects for over 100 years, as reviewed by Seeling et al. [12]. In spite of the results, none of the theories could unequivocally explain the causes, triggering factors, and exact chemical processes behind beech red heartwood formation. According to the literature, the formation of red heartwood could be initiated and influenced by individual stem properties (diameter, age, canopy structure) [3,13,14] as well as by environmental (soil quality, drought, weather extremities, pollution) [15,16] and silvicultural (stand thinning, stand composition) [15,16,17] factors. To the best of our current knowledge, the process is initiated by biotic (fungi, bacteria, etc.) and abiotic (drought, temperature extremities, etc.) environmental factors causing oxidative stress, which leads to the formation of red heartwood chromophores. According to Dietrichs [18] and Hofmann et al. [19,20], it is the phenolic compounds that undergo oxidation and transformation at the color boundary as an effect of changes in the biochemical environment in the transition zone, which leads to the formation of chromophores. This is due to the increase in the pH to above 6.0 as well as the effect of peroxidase (POD), and polyphenol oxidase (PPO) enzymes, catechin-type polyphenols are oxidized. In contrast, other types of polyphenols remain unaffected by the enzymes [19,20], and characteristic brown/red colored substances are formed. These findings have been confirmed by the in vitro experiments of Hofmann et al. [21], and by the recent study of Sorz and Hietz [10] on the role of molecular oxygen in the process. Presumably, sugars are also involved in fueling the biochemical transformation reactions at the sapwood/heartwood boundary [22,23,24].
Due to a lack of detailed results, red heartwood formation processes were often compared to the reactions of obligatory colored heartwood forming species, such as Robinia pseudoacacia [25,26,27], Juglans spp. [28,29] or Pinus spp. [30]. In these cases, evidence was given for the in situ synthesis and subsequent transformation of polyphenols at the color boundary, and the contribution of several enzymes. However, colored heartwood formation is a normal, obligatory, and physiologically determined process in certain tree species [31]. Red heartwood formation in beech is facultative, that is, its occurrence frequency (number of trees affected in a stand), extent (size in diameter), and type (cloudy, star-shaped, etc.) are not predictable.
Up to now, there has been no high-performance separation and detailed mass spectrometric analysis done that focuses on the identification and radial distribution of beech wood polyphenols and the coloring substances. Additionally, the possible transformation pathways that result in the chromophores are yet to be unveiled It has been suggested [20,21], but never proven that the chromophores are high molecular mass polymers composed of the oxidized and polymerized compounds of the sapwood.
For the first time, high-performance liquid chromatography coupled with photodiode array and tandem electrospray mass spectrometry detection (HPLC-PDA-ESI-MS/MS) as well as matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry has been applied for the detailed profiling of the polyphenol pool of beech wood and for tracking of the radial variation in the concentrations of individual compounds from the outer sapwood to the inner red heartwood, to assess the role of compounds in the red heartwood formation process. The results not only contribute to the clarification of the role of individual polyphenolic compounds in the chemical processes of red heartwood formation, but also contribute to the understanding of the composition and the formation of the red color, which is essential for wood technology in terms of color stability and surface treatment issues.
2. Materials and Methods
2.1. Sample Collection and Extraction
Two beech trees were investigated in the present study. The trees originated from the forests of the TAEG (Tanulmányi Erdőgazdaság) Forestry Company, Sopron (Hungary), and were felled in December 2017. The ages of the trees were between 100–110 years. After felling, sample disks were cut from a height of 1.3 m. Both sample disks had a diameter of 36 cm and contained cloudy-shaped red heartwood with a diameter of 17 and 18 cm.
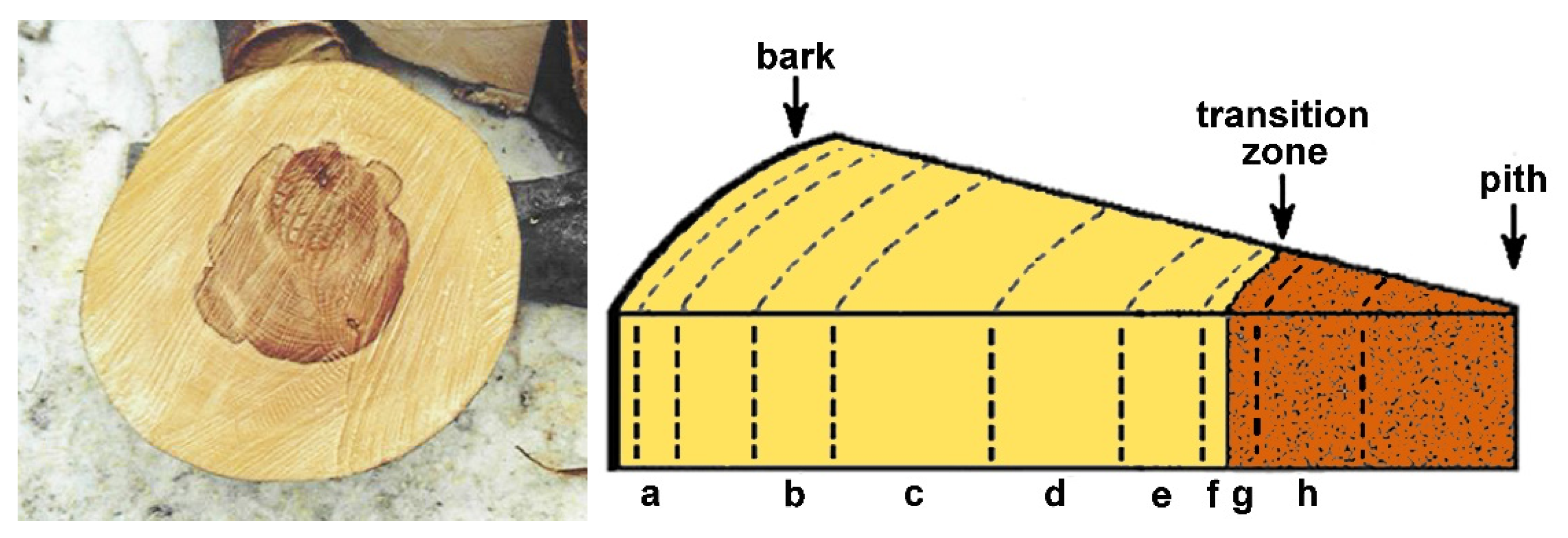
In order to investigate the radial distribution of polyphenols, half of the quarter was cut out of the disks and 8 sections (a–h) were assigned to the wood between the bark to the pith as depicted in Figure 1. The letters f and g represent the sapwood (f) and heartwood (g) on each side of the transition zone (color boundary). The bark and the tissues next to the bark and the pith were excluded from the investigation. After the different sections were separated, they were rasped and homogenized.
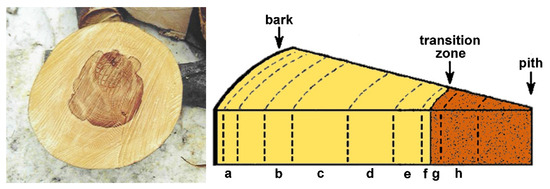
Figure 1.
The graphic description of the assignment of wood sections from sample disks; a–e: outer and inner sapwood tissues; f/g: sapwood/red heartwood transition zone; h: inner red heartwood.
For the liquid chromatographic investigations, wood samples were extracted as follows: 0.4 g wood was extracted with 20 mL methanol:water 80:20 (v/v) solution using ultrasonication (Elma Transsonic T570 ultrasonic bath, Elma Schmidbauer GmbH, Singen, Germany) for 2 × 15 min at room temperature. Extracts were filtered using 0.45 μm cellulose-acetate syringe filters, and 5 mL aliquots were transferred into glass tubes and evaporated to dryness under a gentle stream of N2 gas at 40 °C using an MD200-2 type sample concentrator (Hangzhou Allsheng Instruments, Hangzhou, China). The dry extracts were reconstituted into a final volume of 0.5 mL methanol:water 80:20 (v/v) solution and used for HPLC analysis.
For the MALDI analyses, 3 g of heartwood tissue (h) and sapwood tissue (c) were extracted with 100 mL acetone:water 50:50 (v/v) solution using a Soxhlet extractor for 24 h. The extract solutions were evaporated to dryness under reduced pressure at 40 °C using a Büchi Rotavapor device (Büchi, Flawil, Switzerland) and the dried material was collected.
2.2. The HPLC-PDA-ESI-MS/MS Separation and Relative Quantitative Determination of Beech Wood Polyphenols
The separation, identification, and quantitative assessment of leaf polyphenols was completed using the HPLC-PDA-ESI-MS/MS technique. For chromatographic separation, a Shimadzu LC-20 type high-performance liquid chromatograph was used coupled to a Shimadzu SPD-M20A type diode array detector (PDA) (Shimadzu Corporation, Kyoto, Japan) and an AB Sciex 3200 QTrap triple quadrupole/linear ion trap LC/MS/MS detector (AB Sciex, Framingham, MA, USA). A Phenomenex Kinetex C18, 150 mm × 4.6 mm, 2.6 µm core-shell column (Phenomenex, Torrance, CA, USA) was applied for the separation at 40 °C. The mobile phase (1.2 mL min−1) gradient of A (H2O + 0.1% HCOOH) and B (CH3CN + 0.1% HCOOH) was run as follows: 5% B (0–1 min), 15.5% B (18 min), 44% B (27 min), 100% B (33 min), 100% B (36 min), 5% B (37 min), 5% B (40 min). 6 µL of the samples were injected. Flow-splitting was applied in front of the mass spectrometer using a split valve, which allowed 0.6 mL min−1 flow to enter the ion source. Negative electrospray ionization mode was used with the following settings: ion spray voltage: −4500 V, curtain gas (N2) pressure: 35 psi, spray gas (N2) pressure: 40 psi, drying gas (N2) pressure: 30 psi, ion source temperature: 500 °C. The Q1 and Q3 scans were performed between m/z 160–1200.
Quantitative assessment of the compounds was done by relative quantification using PDA detector signals in the 250–300 nm range. Chromatographic data were acquired and processed using the Analyst software (Version: 1.6.1; AB Sciex, Framingham, MA, USA).
2.3. MALDI-TOF Analysis of the Extracts
The MALDI-TOF experiments were performed on a Bruker UltrafleXtreme instrument (Bruker Daltonik GmbH, Bremen, Germany). As a matrix, 2,5-dihydroxybenzoic acid (DHB) was used, prepared in 50% acetonitrile and 2.5% trifluoroacetic acid. Measurements were conducted in positive mode in the 0–2600 Da range. The final spectra were averaged from 3500 laser shots/spectra for each sample spot. The laser power was set 5–10% above the threshold. The mass spectrometer was calibrated using a peptide calibration mixture from Bruker (Bruker Daltonik GmbH, Bremen, Germany). FlexControl software (Version 3.4; Bruker Daltonik GmbH, Bremen, Germany) was used for operating the mass spectrometer, and a flexAnalysis software (Version 3.4; Bruker Daltonik GmbH, Bremen, Germany) was used for mass spectra annotation.
Before the MALDI-TOF analysis, the dried extracts were reconstituted in a solution of acetonitrile:water 50:50 (v/v) to achieve a concentration of 1 mg mL−1. One µL of each sample was deposited onto a spot of MALDI plate, air-dried, and then 1 µL of a DHB solution was applied and again air-dried. This was repeated three times. All samples were deposited in triplicates.
2.4. Chemicals
Conventional distillation equipment was used to produce double distilled water for extraction and chromatography. Acetonitrile (LC-MS grade), methanol, acetone, formic acid (98%), and trifluoroacetic acid were obtained from VWR-International (Budapest, Hungary) and 2,5-dihydroxybenzoic acid was purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.5. Statistical Evaluation
For the comparison of the relative concentrations of individual compounds in the tissues (a–h), one-way analysis of variance (ANOVA) was run using Statistica software (Version: 12; StatSoft Inc., Tulsa, OK, USA) software and the Tukey HSD calculation method was applied as a post-hoc test for n = 2, at p < 0.05 level. In order to fulfil the requirements of the ANOVA analysis, values of the measurements were first checked for normal distribution, and then the variables were checked for the homogeneity of variances using Bartlett’s Chi-square test.
3. Results and Discussion
3.1. Separation and Identification of Polyphenols Using HPLC-PDA-ESI-MS/MS
In order to obtain a complete list of the polyphenolic compounds of beech wood, one discolored (h) and one non-discolored (f) sample was chosen from one of the sample disks, and compound identification was done from these two samples. As a result, we accomplished the separation and tentative identification of 125 compounds from the investigated beech wood tissues. Of these compounds, altogether 71 were identified by name or structural traits. The majority of the compounds were identified from beech wood for the first time. The list of identified compounds is included in Table 1.

Table 1.
Identified polyphenols from European beech wood extracts—chromatographic and mass spectrometric data. * Detected as [M − H + HCOOH]−.
The most abundant types of compounds were the flavan-3-ols, including (+)-catechin (26), (−)-epicatechin (49) and their derivatives (procyanidins, gallic acid and hexose conjugates) as well as (epi)afzelechin-O-hexoside (107), which amounted to 35 compounds. The presence of (+)-catechin and of (−)-epicatechin has been evidenced earlier in various research works [18,19,32,33,34], yet the presence of oligomeric procyanidins have only been indicated indirectly using layer chromatographic techniques [19,20]. Oligomeric procyanidins (dimers: 21, 23, 25, 38, 46, 52, 67; trimers: 3, 4, 7, 27, 35, 40, 58, 60, 62, 73; tetramers: 31, 37, 63, 70; pentamers: 41, 48, 51, 68) were identified by the presence of (epi)catechin fragment ions (m/z 289, 245, 125) in their mass spectra and by the 288 amu mass differences, corresponding to catechin units; pentameric procyanidins were identified by their double-charged parent ion [35,36]. Gallic acid conjugates of procyanidin dimer (59, 64, 69) and (epi)catechin (85), as well as three isomers of the hexose conjugate of (epi)catechin, were also detected (14, 17, 19). The presence of gallic acid was confirmed by the neutral loss of a galloyl moiety (152 amu: m/z 729→577) and by the simultaneous presence of the [M–gallic acid–H]-fragment ion (m/z 559) in the mass spectra, or by the presence of gallic acid fragments (m/z 169, 125, 109). In the present work, the structure of hexose conjugates was verified by the neutral losses of [M–H–120]– and [M–H–162]– (loss of hexosyl unit) and [M–H–180]– (loss of hexose sugar). These neutral losses corresponded to m/z 331, 289, and 271 ions, respectively, in the mass spectra of the compounds 14, 17, and 19.
The second-largest group of polyphenols in beech wood extracts were flavonol (quercetin, taxifolin, isorhamnetin) and flavonon (naringenin) compounds and their conjugates (hexosides, pentosides), including 19 compounds. The presence of taxifolin and quercetin and their glycosides in beech wood [18,21,32,33,34], bark [35], and leaves [37] has already been reported earlier, whereas naringenin compounds have only been evidenced in the leaf tissues of beech [37]. To the best of our knowledge, this is the first research to report on the presence of isorhamnetin (91) and its pentose (101) and hexose conjugates (74, 86, 108) in beech wood. The identification of the aglycone unit was confirmed using fragmentation data from earlier works of the authors [35,36,37]. The presence of the hexose moiety was verified as described above; the presence of a pentose sugar was evidenced by the [M–H–132]– and [M–H–150]– ions in the mass spectra. The number of taxifolin-O-hexoside (57, 66, 78), taxifolin-O-pentoside (71, 75, 87, 93), quercetin-O-hexoside (94) and naringenin-C-hexoside (72, 76, 80) isomers was the same as had been found earlier for beech bark [35] and leaf [37] extracts.
Although no free gallic acid was found in beech wood, monogalloyl-glucose (1, 2, 6, 9) and its derivative (34), digalloyl glucose (30, 45, 55) and gallic acid + taxifolin derivative (39) were evidenced for the first time in beech wood by the fragments of gallic acid (m/z 169, 125), taxifolin (m/z 285) as well as by the neutral loss of the hexosyl moiety (162 amu).
Beech wood was found to contain simple phenols, phenolic acids, and aromatic aldehydes as well as their derivatives, including the O-hexoside conjugates of vanillic acid (11), dihydro-coumaric acid (22), syringic acid (16, 20), coniferyl alcohol (28, 65), syringic acid (44) and sinapaldehyde (95). Vek et al. [32] reported on the occurrence of free vanillic and syringic acid in the methanol extract of beech sapwood earlier, yet the other compounds were detected for the first time.
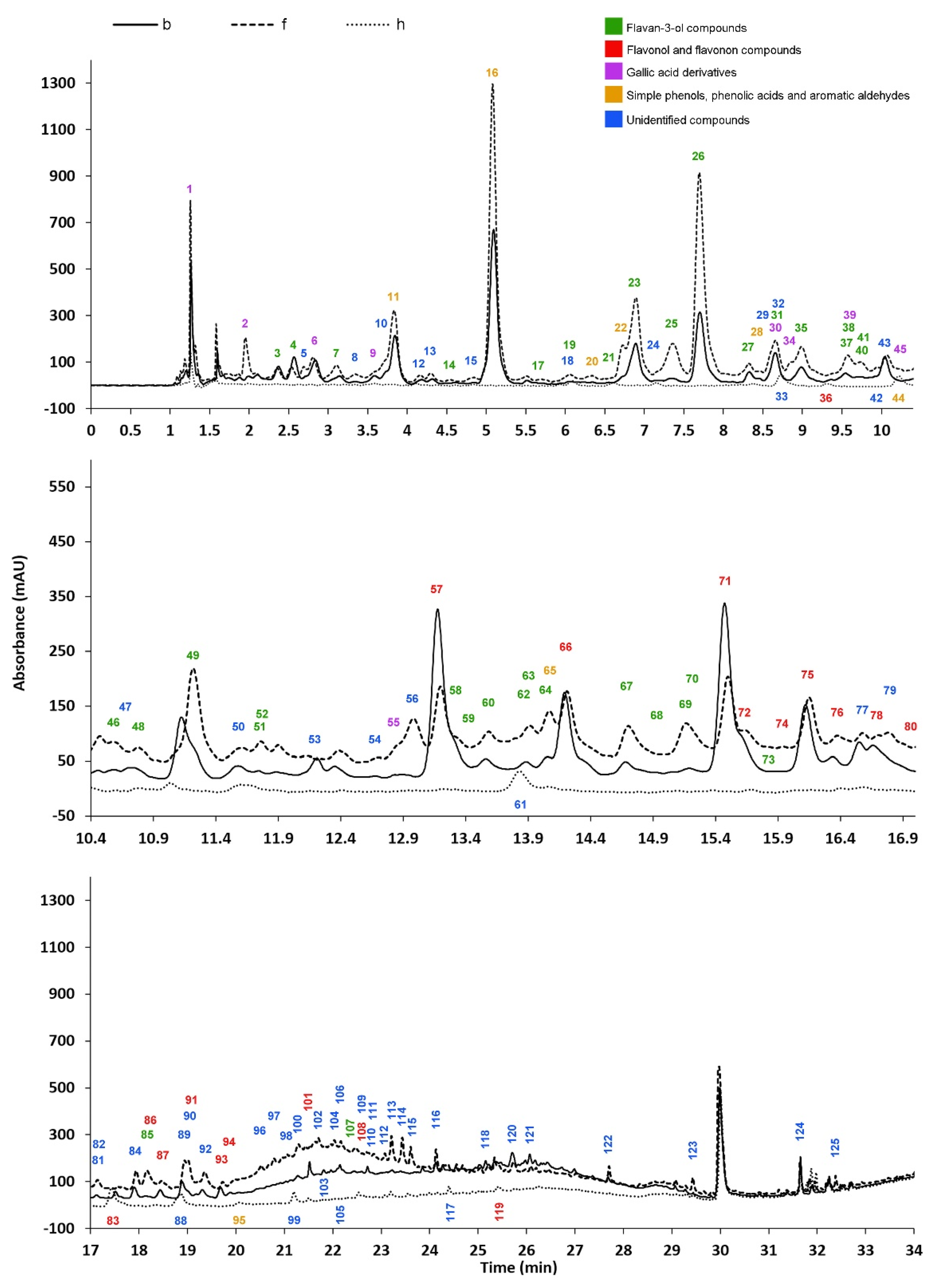
Over 50 compounds were documented and described with MSn spectra, yet their structures have been left unidentified. As they appeared as significant peaks in the chromatogram (Figure 2), they were included in the evaluation in the present article; their fragmentation spectra provide data for future structure elucidation.

Figure 2.
The typical HPLC PDA (250–300 nm) chromatograms of the b, f, and h beech wood tissue extracts.
According to Figure 2, there are differences not only in the quality but also in the concentrations of the compounds between the tissues, suggesting that some of the compounds are characteristic of red heartwood or non-discolored tissues. In this regard, the transitional zone between sapwood/heartwood boundary is of utmost importance as this is the “reaction zone” of heartwood formation and the discoloration process. This transitional zone is where the local synthesis of polyphenolic compounds takes place, as determined in the obligatory colored heartwood forming Juglans and Robinia species [25,28], with increased enzymatic activity [26,27] and clear transformation of phenolic compounds [29]. In the case of beech wood, increased POD and PPO enzyme activities have already been documented at the heartwood boundary [20], as well as the increase in (+)-catechin and (−)-epicatechin levels [19,20]; however, detailed concentration profiles and transformation pathways have not been described yet. The molecular processes involved in the transformation and roles of individual polyphenolic compounds can be tracked and assessed from the radial variation in the identified compounds.
3.2. Relative Quantitative Assessment of Polyphenols
As no standards were available for most of the compounds, the comparison of concentrations was achieved by using relative quantification, which involved the manual integration of the peaks of the identified compounds between the wavelengths ranging from 250 to 300 nm. This wavelength interval was chosen because it showed the highest absorbance intensity and best signal-to-noise ratio in general. The results of the integration and the statistical evaluation of the results are summarized in Supplementary Materials, Table S1. Peak areas were indicated as mean ± standard deviation, and the statistical comparison of radial variation was assessed using ANOVA analysis.
Regarding flavan-3-ols, the compound with the highest peak area was (+)-catechin (26), while its epimer, (−)-epicatechin (49) showed lower levels. Both compounds had a significant concentration increase in f tissue, while red heartwood (g,h) samples contained only traces. These results confirmed earlier findings [18,19,20] that indicated a radial increase in concentration from the bark to the color boundary, with a “jump” at the non-discolored side of the red heartwood, and a sharp decrease and an almost complete absence in inner red heartwood tissues [18].
Basically, the same radial tendency was established for all of the procyanidin B dimers (21, 23, 25, 38, 46, 52, 67) and for some of the procyanidin C trimers (7, 35, 40, 60, 62). For the rest of the procyanidin trimers (3, 4, 27, 58, 73), and for all the tetramers (31, 37, 63, 70) and pentamers (41, 48, 51, 68), no radial variation was established and there was no increase at the color boundary. The hexose conjugates of (epi)afzelechin (107) and (epi)catechin (14, 17) showed low levels in non-discolored wood, with no distinct radial variation or increase at the color boundary, with the only exception being (epi)catechin-O-hexoside (19), which showed a significant increase at the color boundary. Beritognolo et al. [28] indicated the same increase in the concentration of flavanols as well as an increase in the activities of phenylalanine-ammonia-lyase (PAL) and chalcone synthase (CHS) enzymes in the sapwood cells undergoing transformation to heartwood in Juglans spp. According to Hofmann et al. [21], increased pH and the presence of POD and PPO enzymes are necessary for the production of coloring substances of red heartwood from catechins. Increased syntheses of the most flavan-3-ols with highest levels (monomers, dimers and trimers) also indicate that oligomerized compounds transform and take part in the formation of red heartwood chromophoric substances.
The radial variation of flavonol and flavonon derivatives in trees is dependent on the species and type of the compound, which indicates the specificity of the heartwood formation processes of individual species. In Robinia pseudoacacia, the most prominent polyphenolic compounds, robinetin and dihydrorobinetin, have low concentrations in sapwood, show elevated amounts at the sapwood/heartwood boundary and reach maximum levels within the heartwood [25]. In Juglans nigra and Juglans regia, the main flavonol compound involved in the coloring and heartwood formation processes are glycosides of quercetin, which gradually increase in concentration from the sapwood to the sapwood/heartwood transition zone, and are detected only in traces in the heartwood itself. Heartwood chromophores are produced through the hydrolysis of glycosides and heartwood formation is marked by the accumulation of new soluble compounds [29].
In beech wood, a wide variety of flavonols (taxifolin, quercetin, isorhamnetin) and flavonons (naringenin) were detected, all of which were present as sugar conjugates in the sapwood, while free aglycones were only detected in the inner heartwood for all of the four compounds. The results confirm the earlier findings of Hofmann [20], who evidenced free taxifolin and quercetin from the inner red heartwood tissues using HPTLC technique. Similar to Juglans spp., these glycosides have a more or less increasing gradient from sapwood to the heartwood boundary (or if no gradient then a significant “jump” between e and f tissues), where they are hydrolyzed and appear as free aglycones (quercetin: 36, taxifolin: 83, isorhamnein: 91, naringenin: 119) in the heartwood tissues. There is no evidence, however, from the present data that shows if further transformations of these compounds take place inside the red heartwood tissues. Nevertheless, Hofmann et al. [21] showed that free taxifolin and quercetin are not oxidized in vitro in the presence of beech wood enzyme extracts at the pH of the red heartwood and postulated that these compounds are left unaffected by the environment of the red heartwood and are only accumulated and stored in these tissues.
Isorhamnetin (91) and its compounds were evidenced for the first time in beech wood tissues and their radial variation was similar to that of quercetin and taxifolin and its conjugates, with a mild or statistically non-significant radial increase from sapwood to the red heartwood and the occurrence of the free aglycone in the red heartwood tissues. The same radial tendencies were found for naringenin (119) and its derivatives.
The increase in the levels of some of the flavonol and flavonon glycosides at the sapwood/red heartwood boundary proves the in situ biosynthesis of these compounds, which is presumably fueled by the hydrolysis of starch [24] and by the elevated levels of simple sugars (glucose, fructose) [22].
The present study is the first to report on the presence of gallotannins and gallic acid derivatives in the wood of beech. Although present in minor amounts, monogalloyl glucose (1, 2, 6, 9) and digalloyl glucose (30, 45, 55) isomers mainly show a significantly elevated level in the f tissue compared to other sapwood (a-e) tissues. Behind the color boundary, peak areas decrease sharply, and none of these compounds were detected in the inner red heartwood tissues. A similar tendency was also established for the galloylglucose derivative (34) and for the taxifolin derivative of gallic acid (39). The in situ synthesis of gallic acid derivatives in the transition zone tissues proves that despite their low amount, these compounds are also synthesized and take part in the reactions of red heartwood formation. In Rhus succedanea L., gallotannins are the only type of polyphenols present in the sapwood [38]; however, the heartwood contains a variety of flavonoids (fisetin, fustin, garbanzol, etc.) and phenolic acids (ellagic acid, gallic acid), which suggest the in situ synthesis and transformation of polyphenols at the sapwood/heartwood boundary. In Eucalyptus nitens, Barry et al. [39] identified over 30 gallotannins, ellagitannins and phenols in the sapwood and transition zone and found that ellagitannins and many of the gallic acid derivatives are more abundant in the reaction zone compared to healthy sapwood. In Toxicodendron vernicifluum (syn. Rhus verniciflua), the concentration of gallotannins was found to increase gradually from sapwood to the heartwood boundary, and also showed high values in the heartwood tissues, and for some compounds (pentagalloyl-glucose), highly elevated levels were found at the sapwood/heartwood boundary compared to other tissues [40]. The diverse behavior of tannins was also confirmed in Juglans spp., for example, Burtin et al. [29] demonstrated that the content of ellagic acid derivative E1 increased gradually in the sapwood, peaked in the sapwood/heartwood transition zone, and decreased drastically in the heartwood, while the content of the other derivative, E2 was low in the sapwood and increased in the heartwood. The synthesis and transformation of tannins at the heartwood boundary is associated with the heartwood’s color formation and antimicrobial effects [29,39].
The contents of the derivatives of vanillic acid (11), syringic acid (16, 20), coumaric acid (22), and of the coniferin isomers (28, 65) also showed significantly elevated concentrations in the heartwood boundary tissue (f) compared to preceding sapwood (a–e), while colored wood only contained these compounds in low amounts or in traces. Free syringic acid (44) was not detected in the sapwood, but was found in high amounts behind the color boundary tissues, proving that its derivatives (16, 20) are hydrolyzed in the color boundary tissues. In Robinia pseudoacacia, Magel et al. [25] found that the hydroxycinnamic acid (HCA) concentration increased from sapwood towards the heartwood and decreased again in the inner heartwood parts. They concluded that starch is hydrolyzed at the sapwood/heartwood boundary and provides the primary source of hydroxycinnamic acid and flavonoid biosynthesis.
Altogether 54 compounds were left unidentified and were only characterized by MSn spectra and radial variation tendencies. The mass spectra will provide data for future elucidation of the structure of these compounds. Although they seem to be of minor interest in regard to their role in red heartwood formation in beech, many of them show elevated concentrations at the sapwood/red heartwood boundary tissues, thus they seem to have a yet unknown role in the heartwood formation processes, which needs to be clarified in the future.
One of the major questions that has remained unresolved for beech wood is how the individual compounds are transformed, that is, what is the composition of red heartwood chromophores. This question is not only interesting from the plant physiology perspective, but also for wood technology in regard to color stability and wood durability issues. To the best of our knowledge, phenolic compounds are accumulated at the color boundary due to radial transport and/or in situ synthesis and are supposed to transform into condensed chromophores with high molecular weight. The reaction requires elevated pH levels (pH > 6.0) and the presence of POD and PPO enzymes [20]. The products were characterized as high molecular weight and high polarity substances based on their behavior in the separation in a HPTLC phase system; however, the structure of these compounds has not been studied yet in detail [21].
In order to study the chemical composition and structure of heartwood chromophores, other analytical techniques are also required. MALDI-TOF mass spectrometry is ideal for the study of the structure of high molecular weight compounds; it is sensitive, and facilitates a soft ionization, thus enabling the study of intact molecule ions, polymerization sequences, and composition in the >1,000,000 m/z range.
3.3. MALDI-TOF Analysis of Sapwood and Red Heartwood Extracts
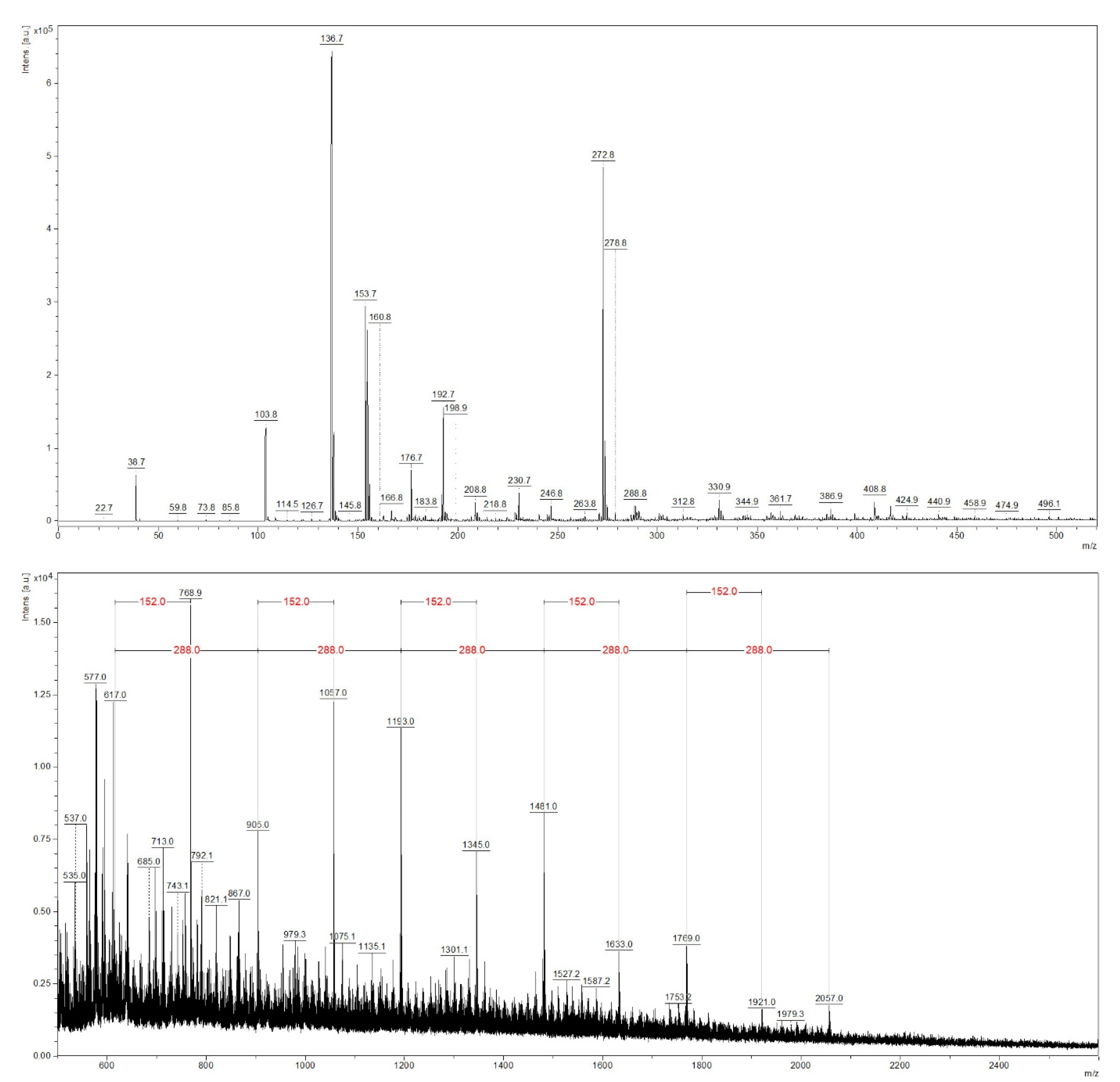
Figure 3 and Figure 4 depict the typical MALDI-TOF spectra of a sapwood and of a red heartwood extract. In the spectrum of the sapwood sample, many of those compounds can be recognized as they were shown in Table 1, especially high molecular mass compounds. However, in the MALDI-TOF spectrum, [M + K]+ ion adduct formation takes place instead of a [M − H]− parent ion formation, which causes a difference in the corresponding peaks of a given compound by 40 amu. Thus, procyanidin trimers, tetramers, pentamers, hexamers, and heptamers appear at m/z 905, 1193, 1481, 1769, and 2057 respectively. Above m/z 2057, no peaks could be identified; thus, heptamers were the highest degree of condensation of catechin units in beech sapwood. The peak at m/z 769 corresponded to the K+ adduct of the procyanidin dimer monogallate (59, 64, 69). Additionally, the gallic acid conjugates of the other procyanidins were also found; their structure was confirmed by the 152 amu mass difference (galloyl moiety) [41,42] compared to the respective procyanidin oligomers (m/z 1057: [procyanidin trimer monogallate + K]+, m/z: 1345: [procyanidin tetramer monogallate + K]+, m/z 1633: [procyanidin pentamer monogallate + K]+, m/z 1921: [procyanidin hexamer monogallate + K]+).

Figure 3.
The MALDI-TOF mass spectrum of the beech sapwood extract.
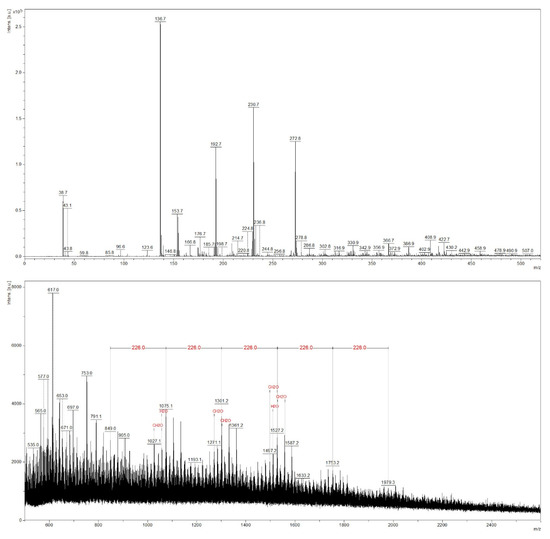
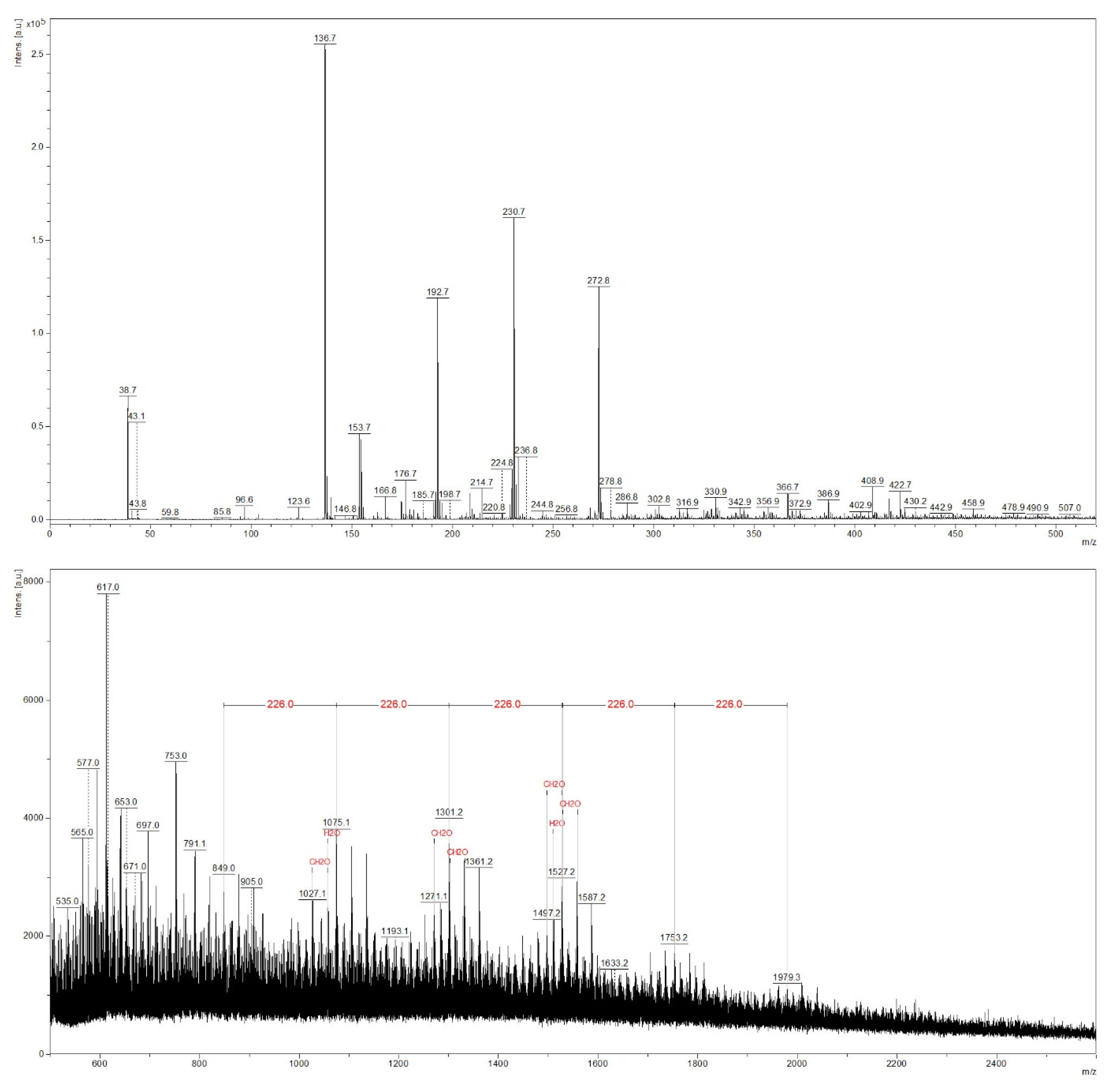
Figure 4.
The MALDI-TOF mass spectrum of the beech red heartwood extract.
In the case of the red heartwood sample (Figure 4), peaks were generally less intense, and high molecular weight compounds (above m/z 1300) were barely detectable, although the prepared red heartwood extract was brown in color; thus, red heartwood chromophores were apparently extracted and investigated. In the MALDI-TOF spectrum, repeating units of m/z 226 were found at m/z 849, 1075, 1301, 1527, 1753, and 1979. This was probably caused by contamination with polyamide 66 originating from the sample preparation [43]. The possibility of contamination was also confirmed by the fact that these peaks were also detected in the spectrum of the sapwood sample with similar intensities (Figure 3). Thus, these compounds cannot be extractives characteristic of the red heartwood. The peaks differing by m/z 30 were interpreted as a common neutral loss of CH2O units as recognized by a flexAnalysis software.
According to these results, two possible explanations can be given on the role and transformation of polyphenolic compounds during the red heartwood formation processes in beech. One of the explanations is that the chromophores of the red heartwood are mostly not high molecular weight compounds as supposed earlier but are composed of the mixture of small molecules (quercetin, taxifolin, isorhamnetin, naringenin, syringic acid) formed at the red heartwood boundary from the hydrolysis of glycosides as well as of the compounds that are detected mostly or exclusively in red heartwood (e.g., compounds 33, 61, 88, 99, 113, 114, 117). According to Vek et al. [32], red heartwood contains significantly larger amounts of saturated fatty acids, fatty alcohols, and triterpenoids than sapwood, however, these compounds do not contribute to wood color. The structural identification of newly formed compounds requires further research.
The other explanation is that the chromophoric substances are indeed molecules of high molecular weight; however, they become chemically bound to the structural polymers of the cell wall as non-extractable polyphenols [44]. Thus, they contribute to the color of red heartwood, yet they are not extractable by neutral solvents or only to a minor extent. This theory is supported by the observation that sapwood contains a large variety of flavonoid-, flavan-3-ol- and phenolic acid derivatives, which after an increase in concentration and subsequent transformation (hydrolysis) in the transition zone (tissue f) seem to “disappear” behind the color boundary or are detected only in relatively small amounts compared to their respective glycosides. Supposedly, they react and are incorporated into the cell wall structure of red heartwood tissues. However, this hypothesis has not been justified by the present research data, and it requires further investigation.
In our opinion, red heartwood chromophores are formed both ways, that is, small molecular weight compounds accumulate while other compounds react and become chemically bound to structural polymers of the cell wall.
4. Conclusions
In the present work, the distribution and the role of the polyphenolic compounds in the processes involved in the formation of the chromophoric compounds of red heartwood have been discussed. Using HPLC-PDA-ESI-MS/MS technique, a total of 125 compounds have been tentatively identified and described, many for the first time. We identified carbohydrate conjugates of the quercetin, taxifolin, isorhamnetin, naringenin, vanillic acid, syringic acid, dihydrocoumaric acid and coniferyl alcohol. (+)-Catechin and (−)-epicatechin were found as free aglycones, as hexose conjugates, and as polymeric (dimeric to pentameric) flavan-3-ols. Gallic acid derivatives were evidenced as monogalloyl- and digalloyl glucose and as their conjugates with taxifolin. Altogether 54 compounds were left unidentified. The MALDI-TOF analysis of sapwood and red heartwood extracts revealed that sapwood contains polymeric flavan-3-ols in the heptameric range as well as their galloyl conjugates. Red heartwood was not found to contain polymeric compounds, which contradicts earlier assumptions that red heartwood chromophores are a highly condensed type.
Radial variation in the polyphenols levels indicated that the concentration of most of the compounds increased in the transition zone in front of the color boundary, and decreased sharply behind it. In the red heartwood, only free aglycones could be evidenced in low amounts. According to the results, beech sapwood polyphenols behave differently during the red heartwood formation process. Based on their structure, function, and reactivity, some polyphenols undergo hydrolysis and accumulate as free aglycones or as their metabolites in the red heartwood tissues, while other compounds are bound to the cell wall structure as non-extractable polyphenols and contribute to the color and resistance of red heartwood tissues. The results contribute to the understanding of the physiological processes of the red heartwood formation of beech and also to the understanding of the color stability of red heartwood beech wood. Based on the present study, further analysis of beech red heartwood is needed using other instrumental analytical techniques to acquire more and detailed information on the chromophoric substances.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/f13010010/s1, Table S1: Relative concentrations via average peak areas of the identified polyphenols from beech wood tissue extracts. Results are indicated as mean ± std. deviation (n = 2). Different superscript letters for a given compound indicate significant differences between tissues at p < 0.05 level. -: not detected, tr: traces.
Author Contributions
Conceptualization, T.H., E.V.-R. and L.A.; methodology, E.V.-R., T.H. and R.G.; software, T.H., R.G. and O.Z.; formal analysis, L.A.; investigation, T.H., L.A. and O.Z.; resources, writing—original draft preparation, T.H., E.V.-R., L.A., R.G. and O.Z.; writing—review and editing, T.H. and L.A.; visualization, T.H. and R.G.; project administration, L.A.; funding acquisition, L.A. All authors have read and agreed to the published version of the manuscript.
Funding
The research was conducted within the framework of the “EFOP-3.6.1-16-2016-00018, Improving the role of research, development, and innovation in higher education through institutional developments assisting intelligent specialization in Sopron and Szombathely”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to their complexity.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Molnár, S. Faanyagismeret; Mezőgazdasági Szaktudás Kiadó: Budapest, Hungary, 2004. [Google Scholar]
- Knoke, T. Predicting red heartwood formation in beech trees (Fagus sylvatica L.). Ecol. Model. 2003, 169, 295–312. [Google Scholar] [CrossRef]
- Nečesany, V. Forstliche Aspekte bei der Entstehung des Falschkerns bei der Rotbuche. Holz Zent. Bl 1969, 95, 563–564. [Google Scholar]
- Seeling, U. Kerntypen im Holz—Konsequenzen für die Verwertung am Beispiel der Buche (Fagus sylvatica L.). Schweiz Z Forstwes 1998, 149, 991–1004. [Google Scholar]
- Tarp, P.; Helles, F.; Holten-Andersen, P.; Bo Larsen, J.; Strange, N. Modelling near-natural silvicultural regimes for beech—An economic sensitivity analysis. For. Ecol. Manag. 2000, 130, 187–198. [Google Scholar] [CrossRef]
- Zell, J.; Hanewinkel, M.; Seeling, U. Financial optimisation of target diameter harvest of European beech (Fagus sylvatica) considering the risk of decrease of timber quality due to red heartwood. For. Policy Econ. 2004, 6, 579–593. [Google Scholar] [CrossRef]
- Hörnfeldt, R.; Drouin, M.; Woxblom, L. False heartwood in beech Fagus sylvatica, birch Betula pendula, B. papyrifera and ash Fraxinus excelsior—An overview. Ecol. Bull. 2010, 53, 61–75. [Google Scholar]
- Klement, I.; Vilkovska, T. Color characteristics of red false heartwood and mature wood of beech (Fagus sylvatica L.) determining by different chromacity coordinates. Sustainability 2019, 11, 690. [Google Scholar] [CrossRef]
- Sorz, J.; Hietz, P. Is oxygen involved in beech (Fagus sylvatica) red heartwood formation? Trees 2008, 22, 175–185. [Google Scholar] [CrossRef]
- Bonifazi, G.; Calienno, L.; Capobianco, G.; Lo Monaco, A.; Pelosi, C.; Picchio, R.; Serranti, S. Modeling color and chemical changes on normal and red heart beech wood by reflectance spectrophotometry, Fourier transform infrared spectroscopy and hyperspectral imaging. Polym. Degrad. Stab. 2015, 113, 10–21. [Google Scholar] [CrossRef]
- Seeling, U.; Becker, G.; Schwarz, C. Stand der Buchenrotkernforschung und Zerstörungsfreie Erfassung des Rotkerns bei Buche (Fagus sylvatica L.); Interner Abschlußbericht; Institut für Forstbenutzung und Forstliche Arbeitswissenschaft Universität Freiburg, University of Freiburg: Freiburg, Germany, 1999. [Google Scholar]
- Herrmann, E. Über die Kernbildung der Rotbuche. Z. F Forst-u Jagdwes. 1902, 34, 596–617. [Google Scholar]
- Mayer-Wegelin, H. Die Verkernung des Buchenholzes. In Silvae Orbis; CIS: Berlin, Germany, 1944; Volume 15, pp. 227–236. [Google Scholar]
- Sachsse, H. Kerntypen der Rotbuche. Forstarchiv 1991, 62, 238–242. [Google Scholar]
- Beimgraben, T. Stand der Rotkernforschung aus Holzanatomischer und Holztechnologischer Sicht, Materialien RVNA 1/03—Regionales Vermarktungsprojekt Rotkernige Buche; Regionalverband Neckar-Alb: Mössingen, Germany, 2003. [Google Scholar]
- Büren, S.V. Der Farbkern der Buche in der Schweiz Nördlich der Alpen—Untersuchung über die Verbreitung, die Erkennung am Stehendem Baum und die Ökonomischen Auswirkungen. Ph.D. Thesis, ETH Zürich, Zürich, Switzerland, 1997. [Google Scholar]
- Dietrichs, H.H. Studies of the chemistry and physiology of the transformation of sapwood into heartwood in Fagus sylvatica L. A contribution to the problem of heartwood formation. Mitt BundesforschAnst Forst Holzw 1964, 58, 141. [Google Scholar]
- Hofmann, T.; Albert, L.; Rétfalvi, T. Quantitative TLC analysis of (+)-catechin and (-)-epicatechin from Fagus sylvatica L. with and without red heartwood. J. Planar Chromatogr.-Mod. 2004, 17, 350–354. [Google Scholar] [CrossRef]
- Hofmann, T. A Kémiai Paraméterek Szerepe a Bükk (Fagus sylvatica L.) álgesztesedésében. Ph.D. Thesis, University of West Hungary, Sopron, Hungary, 2006. [Google Scholar]
- Hofmann, T.; Albert, L.; Rétfalvi, T.; Visi-Rajczi, E.; Brolly, G. TLC analysis of the in-vitro reaction of beech (Fagus sylvatica L.) wood enzyme extract with catechins. J. Planar Chromatogr.-Mod. 2008, 21, 83–88. [Google Scholar] [CrossRef]
- Visi-Rajczi, E.; Albert, L.; Hofmann, T.; Sárdi, É.; Koloszár, J.; Varga Sz Csepregi, I. Storage and accumulation of nonstructural carbohydrates in trunks of Fagus sylvatica L. in relation to discoloured Wood. In Proceedings of the International Conference Chemical Technology of Wood, Pulp and Paper, Bratislava, Slovak Republic, 17–18 September 2003; pp. 330–334. [Google Scholar]
- Dietrichs, H.H. The behaviour of carbohydrates during heartwood formation. Holzforschung 1964, 18, 14–24. [Google Scholar] [CrossRef]
- Magel, E.A.; Höll, W. Storage carbohydrates and adenine nucleotides in trunks of Fagus sylvatica in relation to discoloured wood. Holzforschung 1993, 47, 19–25. [Google Scholar] [CrossRef]
- Magel, E.; Jay-Allemand, C.; Ziegler, H. Formation of heartwood substances in the stemwood of Robinia pseudoacaia L. II: Distribution of nonstructural carbohydrates and wood extractives across the trunk. Trees 1994, 8, 165–171. [Google Scholar] [CrossRef]
- Magel, E.; Hübner, B. Distribution of phenylalanine ammonia lyase and chalcone synthase within trunks of Robinia pseudoacacia L. Bot. Acta 1997, 110, 314–322. [Google Scholar] [CrossRef]
- Yang, J.; Kamdem, D.P.; Keathley, D.E.; Han, K.-H. Seasonal changes in gene expression at the sapwood–heartwood transition zone of black locust (Robinia pseudoacacia) revealed by cDNA microarray analysis. Tree Physiol. 2004, 24, 461–474. [Google Scholar] [CrossRef]
- Beritognolo, I.; Magel, E.; Abdel-Latif, A.; Charpentier, J.P.; Jayallemand, C.; Breton, C. Are flavonoids de novo synthesised in Juglans nigra L. sapwood tissues being transformed into heartwood? Tree Physiol. 2002, 22, 291–300. [Google Scholar] [CrossRef]
- Burtin, P.; Jay-Allemand, C.; Charpentier, J.P.; Janin, G. Natural wood colouring process in Juglans sp. (J. nigra, J. regia and hybrid J. nigra x J. regia) depends on native phenolic compounds accumulated in the transition zone between sapwood and heartwood. Trees 1998, 12, 258–264. [Google Scholar] [CrossRef]
- Lim, K.-J.; Paasela, T.; Harju, A.; Venäläinen, M.; Paulin, L.; Auvinen, P.; Kärkkäinen, K.; Teeri, T.H. Developmental changes in Scots pine transcriptome during heartwood formation. Plant Physiol. 2016, 172, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Hillis, W.E. Chemical aspects of heartwood formation. Wood Sci. Technol. 1968, 2, 241–259. [Google Scholar] [CrossRef]
- Vek, V.; Oven, P.; Poljanšek, I.; Ters, T. Contribution to understanding the occurrence of extractives in red heart of beech. BioResources 2015, 10, 970–985. [Google Scholar] [CrossRef][Green Version]
- Vek, V.; Oven, P.; Poljanšek, I. Review on lipophilic and hydrophilic extractives in tissues of common beech. Drv. Ind. 2016, 67, 85–96. [Google Scholar] [CrossRef]
- Mämmelä, P. Phenolics in selected European hardwood species by liquid chromatography–electrospray ionisation mass spectrometry. Analyst 2001, 126, 1535–1538. [Google Scholar] [CrossRef]
- Hofmann, T.; Nehebaj, E.; Albert, L. The high-performance liquid chromatography/multistage electrospray mass spectrometric investigation and extraction optimization of beech (Fagus sylvatica L.) bark polyphenols. J. Chromatogr. A 2015, 1393, 96–105. [Google Scholar] [CrossRef]
- Hofmann, T.; Nehebaj, E.; Albert, L. Antioxidant properties and detailed polyphenol profiling of European hornbeam (Carpinus betulus L.) leaves by multiple antioxidant capacity assays and high-performance liquid chromatography/multistage electrospray mass spectrometry. Ind. Crop. Prod. 2016, 87, 340–349. [Google Scholar] [CrossRef]
- Visi-Rajczi, E.; Hofmann, T.; Albert, L.; Mátyás, C. Tracing the acclimation of European beech (Fagus sylvatica L.) populations to climatic stress by analyzing the antioxidant system. iForest 2021, 14, 95–103. [Google Scholar] [CrossRef]
- Hillis, W.E.; Inoue, T. The formation of polyphenols in trees—III.: The effect of enzyme inhibitors. Phytochemistry 1966, 5, 491–499. [Google Scholar] [CrossRef]
- Barry, K.M.; Davies, N.W.; Mohamed, C.L. Identification of hydrolysable tannins in the reaction zone of Eucalyptus nitens wood by high performance liquid chromatography–electrospray ionisation mass spectrometry. Phytochem. Anal. 2001, 12, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Hashida, K.; Tabata, M.; Kuroda, K.; Otsuka, Y.; Kubo, S.; Makino, R.; Kubojima, Y.; Tonosaki, M.; Ohara, S. Phenolic extractives in the trunk of Toxicodendron vernicifluum: Chemical characteristics, contents and radial distribution. J. Wood Sci. 2014, 60, 160–168. [Google Scholar] [CrossRef]
- Ricci, A.; Parpinello, G.P.; Palma, A.S.; Teslić, N.; Brilli, C.; Pizzi, A.; Versari, A. Analytical profiling of food-grade extracts from grape (Vitis vinifera sp.) seeds and skins, green tea (Camellia sinensis) leaves and Limousin oak (Quercus robur) heartwood using MALDI-TOF-MS, ICP-MS and spectrophotometric methods. J. Food Compost. Anal. 2017, 59, 95–104. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Jungfer, E.; Ritter, C.; Santiago-Schübel, B.; Thiele, B.; Fett, R.; Galensa, R. Characterization of flavan-3-ols in seeds of grape pomace by CE, HPLC-DAD-MSn and LC-ESI-FTICR-MS. Food Res. Int. 2012, 48, 848–855. [Google Scholar] [CrossRef]
- Tran, J.C.; Doucette, A.A. Cyclic polyamide oligomers extracted from nylon 66 membrane filter disks as a source of contamination in liquid chromatography/mass spectrometry. Am. Soc. Mass. Spectrom. 2006, 17, 652–656. [Google Scholar] [CrossRef]
- Pérez-Jimnez, J.; Díaz-Rubio, M.; Saura-Calixto, F. Non-extractable polyphenols in plant foods: Nature, isolation and analysis. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: London, UK, 2014; pp. 203–218. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).