Abstract
Liriodendron hybrid (L. chinense × L. tulipifera), an essential medium-sized tree generally famous for its timber, is also used as an ornamental and greenery tool in many places around the world. The Liriodendron hybrid (L. hybrid) tree goes through many hurdles to achieve its maximum strength and vigor, such as loss of habitat, vast genetic variation, and low seed setting rate. The establishment of an effective and well-organized somatic embryogenesis (S.E.) system could be used to overcome these obstacles, rather than the old-fashioned seed culture and organogenesis. This study is based on the impact of chitosan oligosaccharide (COS) and its role in the induction of S.E. on the callus of four genotypes of the L. hybrid. The optimal concentration of COS could enhance the momentum and effectiveness in S.E.’s mechanism, which further improves the growth rate of the L. hybrid tree’s plantlets. This study shows that COS has a prominent role in endogenous hormones like indole acetic acid (IAA), zeatin (Z.T.), and gibberellic acid (GA3). Furthermore, COS improves the growth development, growth speed, as well as the development situation of plant germination ability. COS can also regulate branch development and root growth, which could be linked to the antagonistic effect on growth factors to some extent or by affecting auxin synthesis and polar transport.
1. Introduction
The L. hybrid tree, also known as the Liriodendron hybrid, is a large growing tree species in the East Asia region. L. hybrid, produced from the cross between Liriodendron chinense (Hemsley) Sargent and Liriodendron tulipifera Linn, is considered a vicarious species [1]. However, little has been known about its genetic divergence and evolutionary trajectories [2]. The hybrid is primarily grown for its timber quality and landscaping purposes [3] and is one of the most abundant tree species in China’s temperate regions. The hybrid is a separate species and has distinct morphological and biological characteristics that have been widely cultivated and used in China [4]. Typically, the genus of Liriodendron belongs to Magnoliaceae, which occupy a critical evolutionary position [5,6,7,8,9,10,11]. These two relict species of Liriodendron have been suggested to be divaricated in the early ages during the middle to late Miocene [2,12]. It is an excellent garden ornamental and garden cultivation tree species and grows fast with light and soft wood and is cultivated in many temperate mountains of America and China for wood production [13,14,15,16,17]. The tree is tall, the trunk is perfect and straight, the wood structure is fine and uniform, the color is light, easy to dry, and easy to process, and the fiber is longer. In the sexual reproduction of L. chinense, seed setting percentage is very low, and it is considered its prominent trait. In a natural environment, the seed setting ratio of Liriodendron is very low and is not more than 10% [9]. It is an excellent tree species for pulp, paper, artificial board, and furniture. It is suitable for afforestation in mountains and hills and is also a remarkable tree species for building industrial raw material forests. Furthermore, Liriodendron is valued as a source material for honey production, chemical extract [18,19,20], and biofuels [21,22]. Liriodendron chinense (Hemsl.) Sarg. (L. chinense) has been classified as a very rare and endangered species because of its limited occurrence [23]; therefore, back in 1992, L. chinense was listed in the Red List of Endangered Plants in China [24]. Later on, in 1998, the International Union for Conservation of Nature and Natural Resources has enlisted L. chinense as a near-threatened species in the IUCN Red List of Threatened Species.
S.E. is a synthetic developmental technique of acquiring a plant or embryo from a single somatic cell. Under appropriate conditions, this newly formed embryo can further develop into a whole plant [25]. S.E. is a stable and more reliable tool, and this process, controlled by the relevant genes, has a vital role in the entire development of somatic embryos [26]. The primary step in acquiring the embryo cell involves the transition of cell fate from a somatic cell. S.E. has widely been practiced in many woody plants and others by directly introducing somatic cells, such as in Bacopa monnieri (L.) Wettst [27], or indirect induction through the callus stage, as in Gentiana decumbens L.f., Theobroma cacao L. [28], and Ledebouria revolute [29]. S.E. has a vital role in clonal propagation in woody plants and is an essential source for synthetic seed production, germplasm conservation, and cryopreservation [25]. Moreover, S.E. has a significant role in mass propagation in vitro, germplasm conservation, and woody plants’ genetic improvement [30,31,32]. In vitro propagation not only plays a significant role in the clonal propagation of desirable genotypes, but it could also provide suitable target material for genetic transformation [33,34].
Many artificial growth regulators negatively affect the eco-friendly environment; therefore, natural polysaccharides could stimulate plant growth and development [35,36]. Chitin, a fibrous substance comprising polysaccharides, is an abundant biopolymer [37]. Chitin is one of the main components of invertebrate exoskeletons and fungi cell walls [38]. In the past, chitin and its deacetylated product chitosan have been widely used to improve agricultural crop plants in various ways [39,40,41]. The structure of chitosan contains 2-acetamido-2-deoxy-β-d-glucose (NAG) monomers linked together by β (1→4) linkages [37]. When the degree of acetylation (DA) (expressed as a molar percentage), used to differentiate chitin from chitosan, is lower than 50 mol%, the product is named chitosan [42]. Oligosaccharides have been reported earlier, in 1983 [43], through enzymatic hydrolysis of the plant cell wall and different crop plants. Besides, it has positive results when applied exogenously [44,45]. In plants, oligosaccharides can be used as a growth regulator for development and survival in the ecosystem [46]. Chitosan oligosaccharides’ (COS) hydrolysis of chitosan or chitin has been paid extensive attention in recent years. This is mainly due to their physicochemical properties like lower molecular weight, lower viscosity, higher water solubility, biocompatibility, and biodegradability [47,48].
Coating the bulbs of Ornithogalum saundersiae with COS enhances the growth vigor, early flowering, and improves the content of pigments, polyphenols, L-ascorbic acid, potassium, phosphorus, zinc, and manganese [49]. COS, carrageenan, alginate, and their depolymerized derivatives could positively enhance plant growth and development [49,50,51]. Exogenous treatment of oligosaccharides can stimulate fruit ripening in Lycopersicon esculentum (Mill.) and improve natural fruit softening [52]. Spraying COS at the tillering stage increases the yield in wheat [53]. COS induces fruits’ antioxidant activity and improves the fruit texture, quality, and taste in strawberries [54]. Due to its non-toxic and high solubility properties, COS has been the subject of increased attention in terms of its pharmaceutical and medicinal applications [55]. Although much research has shown the significance of COS involved in plant growth and development, the concise mechanism of COS on plant S.E. has not been studied in detail.
Our primary studies showed the improvement in the efficiency of S.E. of L. hybrid. More specifically, we have demonstrated the analyzed data indicating the significant amount of development in the germinated seedlings grown from somatic embryos. Furthermore, we have shown the effect of COS and obtained promising results that show the increased efficiency of S.E. of L. hybrid. Lastly, we examined endogenous hormones’ influence during the formation of somatic embryos treated with COS.
2. Materials and Methods
2.1. Plant Material and Culture Initiation
In this study, we used the embryogenic callus of L. hybrid, and the reagent used was of COS in the basic 3/4 MS solid medium. The genotypes used were callus 154102, 155202, 166302, and BxB. The four callus lines all belong to the same species, i.e., Liriodendron hybrid, but they differ based on time, and their parental lines are different. Genotypes 154102 and 155202 originated in 2015, while genotypes 166203 and BxB were created in 2016 and 2019, respectively. All the calli were yellowish, dense, and fine, with a moderate growth rate and stable state.
Callus of L. hybrid is a good combination of L. hybrid bred by the Nanjing Forestry University research group from 2012 to 2014, induced by the immature embryo. The medium used before treatment was M13, 3/4 MS + 2,4-d 1.0 mg L−1 + 6-ba 0.2 mg L−1 + VC 5.0 mg L−1 + Sucrose 30 g L−1 + CH 0.5 g L−1, pH: 5.7–5.8. MS denotes Murashige & Skoog, BA is 6-benzylaminopurine, VC indicates vitamin C, and CH is casein hydrolysate.
2.2. Configuration of COS Stock Solution
COS is easily soluble in water, and its autoclaved activity remains unchanged. The product of chitosan oligosaccharide was bought from Sinopharm Chemical Reagent Co., Ltd. (Catalogue No. TCI-C2849). The solution of chitosan oligosaccharide was prepared in the Nanjing Forestry University laboratory by diluting 1 g of the product in 20 mL of double-distilled water in a beaker. The volume is fixed to 1 L, which is the stock solution of COS with a concentration of 1 mg mL−1. Finally, it is transferred to a brown bottle and stored and kept away from light at 4 °C for later use. Solution and culture media were autoclaved at 120 °C for 20 min, and then transferred to a brown bottle and stored and kept away from light at 4 °C for later use.
2.3. Concentration of COS
The somatic embryo induction medium of L. hybrid was prepared using 3/4 MS as the basic medium and adding different COS concentrations. COS-free 3/4 MS basic medium with ABA (abscisic acid) was used as a control. Four genotypes with three concentration gradients set were used in the experiment. Each concentration was inoculated with at least three dishes, and each dish was connected with five calli, where each callus was about 0.5 cm in diameter and 1.6 g/dish in weight (Table 1). The initial culturing environment was 24 °C dark culture.

Table 1.
Culture medium with different concentrations of COS.
2.4. In Vitro Growth of Somatic Embryo Seedlings
The somatic embryo seedlings were transferred into the 3/4 MS medium without plant growth regulators to enhance the growth of regenerated seedlings. The regenerated plantlets were cultured under a photoperiod of the 16 h light/8 h dark at 25 °C.
2.5. Ex Vitro Adaptation of Somatic Embryo Seedlings
All the regenerated plantlets were transferred to the pots (plastic pots) after eight weeks on a growth medium filled with vermiculite:perlite:soil (1:1:3, v/v). The plantlets were carried to the greenhouse to acclimatize under a 16 h photoperiod at 120 µmol m−2 133 s−1 provided by cool white fluorescent tubes at 21 ± 2 °C and relatively high humidity (70–90%).
2.6. Somatic Embryo Observation
For induced somatic embryos of L. hybrid, stereoscopic and optical microscopy were used to observe the somatic embryos. Photographs were taken to study the morphological formation and induction during the S.E. of each genotype different media. To avoid errors caused by callus block size, the somatic embryo induction amount was determined using the mass method. The number of somatic embryos induced by callus per unit mass was taken as the statistical object to analyze the optimal concentration of COS for inducing somatic embryos on a solid medium.
2.7. Acquisition and Growth Potential Measurement of Regenerated Plants
After the embryoid body grew from the callus’ surface and developed into the cotyledon embryo stage, the culture conditions were adjusted. The material was transferred to the tissue culture bottles in the light culture room, and the regenerated plants were further cultured in the 3/4 MS basic medium. One month later, using the 3/4 MS basic medium as a control, the differences in regeneration rate, branching rate, and plant height of the regenerated plants were compared. For the plants that produced branches, they were divided into three modes according to the number of branches, the total number of leaves (TNL), total stem length (TSL), the average number of leaves per branch (LN/B), and average stem diameter per branch (BD/B) of branches 1, 2, and 3, respectively. When the regenerated seedlings grew up, they were domesticated and transplanted into small pots with a 5–8 cm diameter. To understand the plant growth more clearly, the statistical standard was formulated as the following formula:
Seedling rate = (No. of regenerated somatic embryos)/(No. of somatic embryos) × 100%
Branching rate = (No. of branches)/(No. of total plants) × 100%
2.8. Seedling Effects of COS
To verify whether COS has a strong effect on seedling of L. hybrid, about 60 seedlings with the same basal stem and growth from the same genotype were selected, and 30 days of the seedlings were divided into four groups and cultured in 3/4 MS basic medium supplemented with 0.01, 0.05, and 0.1 mg L−1 COS. After two months, plant growth of the two groups was compared, and plant height, stem length, stem diameter, leaf size, root length, and root diameter were statistically determined.
2.9. Analysis of Endogenous Hormone Levels
The embryogenic callus of COS- and ABA-induced somatic embryos for all the four genotypes was randomly quantified to check endogenous hormones such as control (C.K.), ABA (2 mg L−1), and COS. The samples were randomly taken at days 0, 15, 30, and 60 after different treatments to detect endogenous hormones. Besides, the samples were immediately frozen in liquid nitrogen and stored at −80 °C until analysis. The samples treated without any plant growth regulators were selected as the controls.
3. Data Analysis
SPSS 16.0 statistical software was used for one-way ANOVA, and Duncan’s multiple tests were used to compare the mean values. The significance level was set as alpha = 0.05 indicated as * and alpha = 0.01 denoted as **. GraphPad Prism was used to plot the analysis results of SPSS software.
4. Results
COS improves the efficacy of S.E.
The embryogenic callus of four genotypes of L. hybrid was cultured on the medium containing 0.01, 0.05, and 0.1 mg L−1 COS to study its impact and observe whether COS regulates somatic embryos’ induction. The number of somatic embryos induced in the callus of all four genotypes was significantly different in different concentration media (Figure 1). In the embryogenic callus of all four genotypes, the total number of somatic embryos produced by the medium having 0.05 mg L−1 COS was more than twice as compared to the control group (Figure 1). The number of mature somatic embryos induced by COS (0.05 mg L−1) for all the genotypes was significantly higher than the control (Figure 2). The number of total somatic embryos and mature somatic embryos induced by the medium with 0.05 mg L−1 COS was significantly higher than that induced by the two other media containing 0.01 and 0.1 mg L−1, as well as with the control group. A low concentration of 0.05 mg L−1 of COS could promote S.E. of the callus of L. hybrid, while a high concentration of COS could inhibit the induction of S.E. In the genotype 166302, when the COS concentration was 0.05 mg L−1, the average number of somatic embryos produced per gram of callus was the highest, up to 2456 on average, more than two times that of the control group (Figure 1d). When the concentration of COS was >0.05 mg L−1, the somatic embryo induction ability was significantly decreased. Besides, mature somatic embryos’ occurrence was consistent with the trend of overall embryos, and the number reached the highest at 0.05 mg L−1 for all the genotypes. Therefore, the optimal concentration of COS for S.E. of all the four genotypes is 0.05 mg L−1. Compared with the control, the embryogenic callus of the L. hybrid treated with COS is relatively good, with a pale-yellow color, moderate water content, and a more significant number of S.E.; however, the callus of the control group showed uneven status (Figure 1a–d). Some calli grew well and could produce somatic embryos, while the other calli showed browning and water-stained shape and could not produce somatic embryos or rarely produced deformed embryos. The number of mature somatic embryos of genotype 166302 is significantly higher than that of other genotypes, which could have three reasons (Figure 1g). First, genotype 166302 itself has a more robust S.E. and development ability than the other genotypes. Secondly, it is more sensitive to COS than the other genotypes; therefore, the somatic embryo induction effect of COS can be fully utilized. Third, the callus particles were smaller than the other genotypes; thus, more callus particles per unit mass and the number of somatic embryos and mature somatic embryos are more extensive (Figure 1a–g).
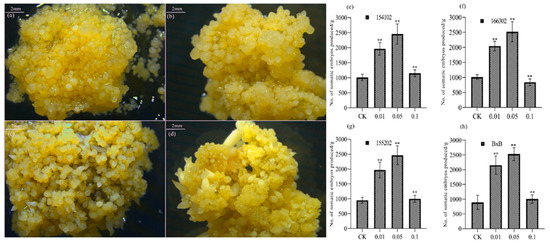
Figure 1.
Several somatic embryos with different concentrations of COS. The (a–d) represent (e–h) at the interval of 0, 15, 30, and 60 days respectively, of genotype 166302. The significance level was set as alpha = 0.05 indicated as * and alpha = 0.01 denoted as **. GraphPad Prism was used to plot the analysis results of SPSS software. The scale bar level was set as 2 mm, and all the genotypes show almost similar results; therefore, we represent only genotype 166302 here.
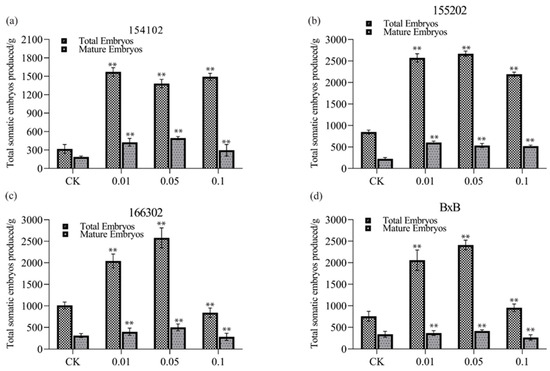
Figure 2.
The total embryos and mature embryos at different COS concentrations for all the four genotypes of L. hybrid, (a–d) represent 154102, 155202, 166302, and BxB, respectively. The significance level was set as alpha = 0.05 indicated as * and alpha = 0.01 denoted as **. GraphPad Prism was used to plot the analysis results of SPSS software. All four genotypes show approximately parallel results.
The number of mature somatic embryos of genotype 166302 was higher at 0.05 mg L−1, and the number of mature somatic embryos is higher than that of genotypes 154102, 155202, and BxB. Its peak appears at a concentration of 0.05 mg L−1 (Figure 2c). This study found that the number of mature somatic embryos was also the highest when the COS concentration was 0.01 or 0.05 mg L−1. The difference between the two concentrations was highly significant (Figure 2). The total number of embryos induced by 0.01 or 0.05 mg L−1 was relatively high because this concentration was more suitable for the growth and development of somatic embryos of L. hybrid. There was no significant difference in the number of mature somatic embryos between the control and 0.1 mg L−1. Still, the number of somatic embryos was significantly different, indicating that high concentrations of COS may inhibit the growth and development of L. hybrid (Figure 2). Unlike the total somatic embryos’ statistics, the number of mature somatic embryos of genotype 154102 was higher at 0.01 mg L−1. The number of mature somatic embryos of all four genotypes varies largely with different COS concentrations. The number of mature somatic embryos was higher when the concentration reached 0.01 and 0.05 mg L−1 for all the four genotypes, and its peak appears at a concentration of 0.05 mg L−1 (Figure 2).
4.1. COS Regulating the Level of Endogenous Hormones
When the embryogenic callus of the genotype 154102 was cultured on a medium containing 0.05 mg L−1 COS, the IAA content was significantly increased at days 15, 30, and 60 (Figure 3a). After 30 days of culture, a large number of spherical embryos began to appear, and torpedo embryos developed around 30 days, and a large number of globular embryos were continuously produced (Figure 3b). After 45 days of culture, most of the somatic embryos developed from the callus’s surface matured early and could be transferred to the light culture room for cultivation to regenerate plants. On the other hand, many embryoid bodies were brewed inside and on the original callus’ surface, which continuously emerges and grows rapidly. Most cotyledon embryos were transferred to the light culture when they were mature at 60 days (Figure 4a–d).
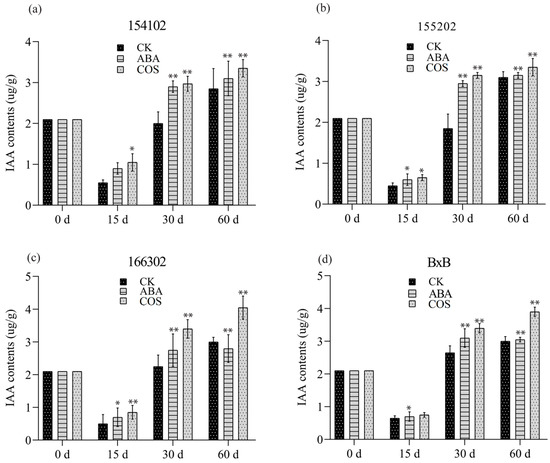
Figure 3.
IAA content of all four (a–d) genotypes induced by COS at the concentration of 0.05 mg L−1, and ABA at the concentration of 2 mg L−1, recorded after 0, 15, 30, and 60 days, respectively. CK represents control, while the significance level was set as alpha = 0.05 indicated as * and alpha = 0.01 denoted as **. GraphPad Prism was used to plot the analysis results of SPSS software.

Figure 4.
Column-wise representation of somatic embryogenesis of all the four genotypes of L. hybrid in response to COS 0.05 mg L−1 after 0, 30, 45, and 60 days. Genotypes 154102, 155202, 166302, and BxB at different time intervals of 0 (a), 30 (b), 45 (c), and 60 days (d) were observed after treating with COS 0.05 mg L−1. The scale bar level was set as 2 mm, and all the pictures were captured on the same given day and time.
Furthermore, for the genotype of 154102, the content of endogenous Z.T. significantly increased in the COS-treatment group before the stage of the globular embryo and then increased slightly in the subsequent development. Endogenous ZT’s content in ABA treatment was almost the same and was not different from that in COS, which had a slight drop trend at the early developmental stage (Figure 5a). The contents of GA3 in the ABA treatment were initially significantly increased, while they decreased for the COS treatment after 15 days. After 30 days, an abrupt decrease was observed for GA3 for both ABA and COS treatments and the control group. At 60 days, it was increased significantly for both ABA and COS, with COS slightly higher than the rest of the treatments (Figure 6a).
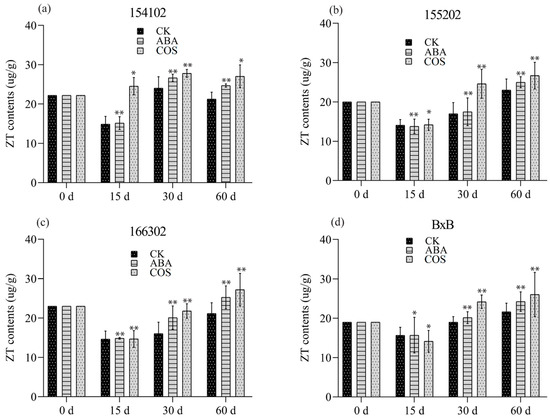
Figure 5.
The ZT content of all the four (a–d) genotypes induced by COS at the concentration of 0.05 mg L−1 and ABA at the concentration of 2 mg L−1, recorded after 0, 15, 30, and 60 days, respectively. CK represents control, while the significance level was set as alpha = 0.05 indicated as * and alpha = 0.01 denoted as **. GraphPad Prism was used to plot the analysis results of SPSS software.
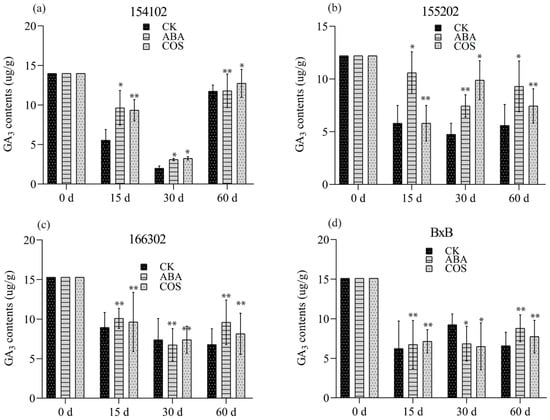
Figure 6.
The GA3 content of all the four (a–d) genotypes induced by COS at the concentration of 0.05 mg L−1, and ABA at the concentration of 2 mg L−1, recorded after 0, 15, 30, and 60 days, respectively. CK represents control, while the significance level was set as alpha = 0.05 indicated as * and alpha = 0.01 denoted as **. GraphPad Prism was used to plot the analysis results of SPSS software.
For the genotype 155202, when it was cultured on a medium supplemented with 0.05 mg L−1 COS, the IAA content was significantly increased at 30 and 60 days (Figure 4b). At 30 days, spherical and torpedo embryos appeared, and the number of globular embryos increased after 30 days. In contrast, a large number of embryoid bodies were developed, which grow very fast. Most cotyledon embryos were shifted to the light culture at 60 days (Figure 3a–d). The contents of Z.T. and GA3 initially decreased, then enhanced significantly at 30 and 60 days for COS treatment (Figure 5b and Figure 6b).
For the genotype of 166302, the content of endogenous IAA and Z.T. increased before the globular embryo stage for both ABA and COS, at 30 and 60 days (Figure 3a–d, Figure 4c, and Figure 5c). In the genotype 166302, the contents of GA3 in the ABA treatment initially increased and then decreased for the COS treatment after 15 days. After 30 days, an unexpected decrease was observed for GA3 and ABA; however, the control and COS treatments increased in almost the same manner. After 60 days, the content of GA3 for ABA increased and decreased for both control and COS treatments (Figure 6c).
The genotype BxB was slightly different when it comes to the contents of IAA. Initially, the IAA content was high, but after 15 days, it dropped significantly lower; however, a significant increase in the IAA content was observed at 30 and 60 days for both ABA and COS (Figure 4d). The contents of Z.T. for COS and ABA increased significantly at 30 and 60 days in the given period (Figure 5d). The GA3 content in the ABA and COS treatments was lower than the control group after 30 days and was higher after 60 days (Figure 3a–d and Figure 6d).
4.2. Germination Ability
Among all the four genotypes, the somatic embryo development rate of genotype 166302 was higher. The cycle of genotype 166302 was earlier, and the rate of S.E. among other genotypes in the treatment group was consistent with that of the control group. Still, the occurrence of S.E. of genotypes 166302 and 155202 was very much earlier than that in the control group (Figure 7). Genotype 154102 was 1–2 weeks later than that in the control group. Combined with the growth of callus, it is not difficult to find that COS could improve the callus state of L. hybrid, when used at the concentration of 0.05 mg L−1, and induce S.E. and ensure the normal occurrence of somatic embryos (Figure 7).
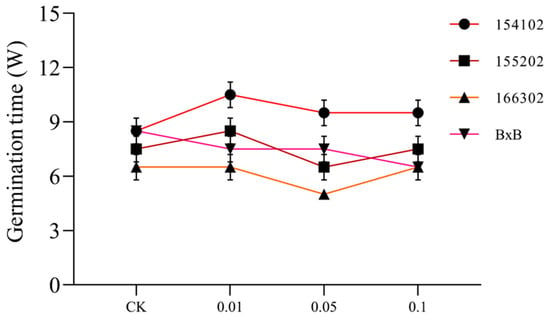
Figure 7.
The somatic embryo germination rate of each genotype under COS action at different concentrations, with control as C.K., and W denotes week(s).
The callus of L. hybrid was cultured for a long time on a basic medium without adding any hormones, which caused slow growth, increased water content, browning, and other phenomena, and caused inevitable stress on the cells. Thus, certain stresses are formed on the cells, and some studies have shown that these stresses can stimulate the production of somatic embryos in plants. Therefore, it is speculated that the early appearance of somatic embryos of genotypes 166302 and 155202 has an important relationship with callus status deterioration. To sum up, the optimal concentration of COS for S.E. and development of L. hybrid is 0.01~0.5 mg L−1. Based on the S.E. ability, S.E. rate, and somatic embryo development of each genotype, it was found that the regeneration ability of genotype 166302 is far better than all of the other genotypes (Figure 8).
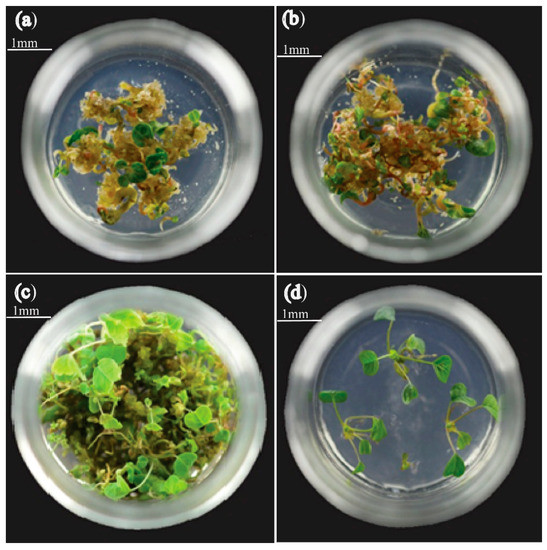
Figure 8.
Plantlets from somatic embryo induced by COS, (a,b) somatic embryos’ germination plantlets of the control group under 15 and 30 days of illumination, bar = 1 mm, (c,d) somatic embryos’ regeneration plantlets of COS under 15 and 30 days of lighting.
4.3. Acquisition and Growth Potential Measurement of Somatic Embryo Regenerated Plants
The mature somatic embryo of the L. hybrid and its base callus was transferred into a culture flask and gently flattened with forceps to fully contact the culture medium. We took five pieces per bottle with a diameter of about 0.8 cm. A basic medium was used to culture the germinated plantlets under 24 °C light culture. After five days, the cotyledon embryo became green and developed into seedling after 15–20 days, and some immature embryos continued to grow and developed into somatic embryo germinated plants (Figure 8).
The somatic embryos induced by COS can develop into somatic embryo germinated plants better than that of the control group (Figure 8). COS induces the early stage of the embryo plant regeneration and enhances the growth development, growth speed, as well as development situation of plant germination, which was far better than the control group. We further compared plantlet germination, the growth rate of 2-month-old plant growth, and growth potential, and found that the number of germinated plants, growth rate, and development is better than the control group.
The somatic embryo seedling rate induced by COS was significantly higher than that of the control group (80.12%), and significant differences were found between the treatment group with COS 0.01~0.1 mg L−1 (Figure 9a). In terms of plant growth morphology, a significant difference was found in the number of leaf blades in all three concentrations (Figure 9b), and highly significant differences were found for both the number of roots (Figure 9c) and stem length at the concentration of 0.05 mg L−1 (Figure 9d). Furthermore, highly significant differences were recorded for plant height at 0.05 and 0.1 mg L−1 (Figure 9e) and root length and branching rate at the concentrations of 0.01 and 0.05 mg L−1, respectively (Figure 9f,g). It can be seen that COS can not only inhibit the elongation growth of the hypocotyl of pea induced by auxins, such as 2, 4-d, and IAA, but also regulates the branch development and root growth of L. hybrid, which could be related to the antagonistic effect on growth factors to some extent, or by affecting auxin synthesis and polar transport.
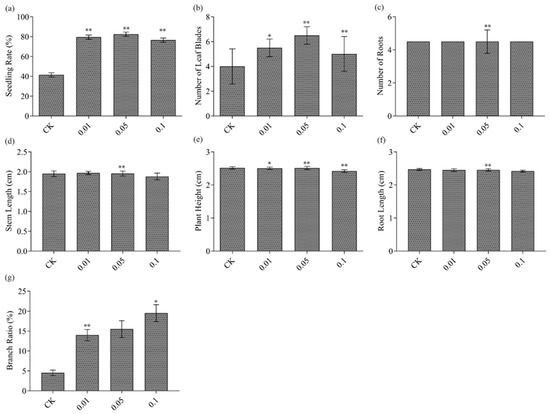
Figure 9.
Growth vigor of L. hybrid induced by COS, the seedling rate (a), number of leaf blades (b), number of roots (c), stem length (d), plant height (e), root length (f), and branching rate (g), respectively. The control group was 3/4 MS without adding COS. The significance level was set as alpha = 0.05 indicated as * and alpha = 0.01 denoted as **. GraphPad Prism was used to plot the analysis results of SPSS software.
Measurements of the average number of leaves per branch, LN/B (Figure 10d), average stem length per branch, SL/B (Figure 10e), and average stem or branch diameter, BD/B (Figure 10f), of L. hybrid somatic embryo regeneration plants showed that LN/B and SL/B of plants were higher than those of four branched plants, and BD/B was significantly higher than that of plants with four branches (Figure 10f). The total leaf number (L.N.) difference between the four branching modes was highly significant (Figure 10a). Those with four branches had the most leaves, but the average number of leaves per branch (LN/B) was the least (Figure 10a,d). It is speculated that the increase in the total number of leaves of the plant may increase the competition between branches for the light environment, which reduces the photosynthetic capacity of the leaves and eventually limits the accumulation of material. The SL/B of the L. hybrid decreased with the number of branches, which may result from the plant’s nutrition distribution.
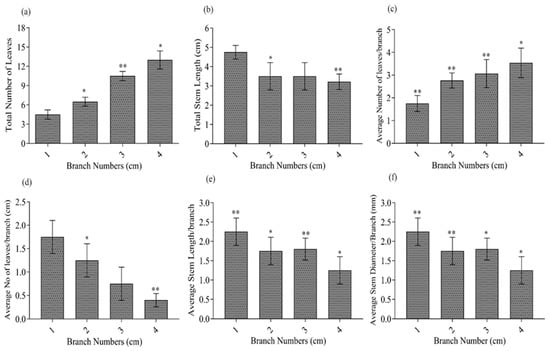
Figure 10.
Effects of COS on branching development of L. hybrid, the total number of leaves (a), stem length (b), the average number of leaves per branch (c), average no of average leaves per branch (d), average stem length per branch (e), and average stem diameter per branch (f). The significance level was set as alpha = 0.05 indicated as * and alpha = 0.01 denoted as **. GraphPad Prism was used to plot the analysis results of SPSS software.
5. Discussion
It is well-known that oligosaccharides can stimulate or inhibit growth and development in different plants [56]. In the wheat crop, the number of spikes, grains per spike, and total grain yield were increased by spraying COS at the tillering stage [53]. Moreover, COS has low-cost production and has a number of promising biological properties, namely biodegradability, biocompatibility, and non-allergenicity [57]. In plants, the mechanism of chitosan is yet to be fully understood. Chitosan, with its oligomers, has been widely used in plants to confer resistance against abiotic stresses, such as water deficit, salinity, and heat stress [58], and enhances photosynthetic activities as well as increases the chitinase activity in seedlings by 30–50% [59]. These characteristics make COS valuable for many industries, such as cosmetology, food, biotechnology, pharmacology, and medicine [60,61]. Our hypothesis that COS could positively impact the growth and development of L. hybrid leads to promising results. The optimal concentration of somatic embryos induced by COS in L. hybrid is 0.05 mg L−1. The addition of 0.05 mg L−1 of exogenous COS can increase the rate and number of somatic embryos, ultimately promoting somatic embryos’ growth and development. The number of somatic embryos in callus of L. hybrid was higher in solid medium supplemented with COS, and the average number of somatic embryos per gram of callus was up to a maximum of 2500 in two genotypes of L. hybrid.
The present work shows that after COS treatment, 2000~2500 individual embryos were produced on average per gram of callus, significantly different from the basic medium (3/4 MS) (Figure 1a–d). Statistical analysis of the number of mature somatic embryos showed that the trend was consistent with the number of total embryos, and the somatic embryos developed well (Figure 2a–d and Figure 3). In this study, the S.E. number was significantly enhanced 2–3-fold by COS induction compared with control or without any plant growth regulators. In tobacco, the oligo-chitosan can be precisely bound to the walls and the membranes of tobacco cells, which increases the accumulation of IAA and decreases the concentration of IAA peroxidase in the cells in suspension to promote plant growth and development [62,63]. Alginate oligosaccharides regulate auxin-related genes’ expression to accelerate auxin biosynthesis and transport and reduce IAA oxidase activity and induce calcium signaling in the roots of rice [64]. In this study, different genotypes have different S.E. abilities and different sensitivity to COS. In general, COS induces the efficiency of somatic embryos of L. hybrid. Among all the four genotypes, 166302 produced better results in response to COS 0.05 mg L−1 when added to the culture medium. Statistics of somatic embryo regeneration plants induced by COS showed that somatic embryos developed well under COS induction. Most of them were able to grow into typical regenerated plants. The somatic embryo seedling rate was as high as 82.14%, which was significantly higher than that of the control group. The root length of somatic embryo germinated plants induced by COS was significantly longer than that of the control group. The number of leaves, plant height, stem length, and rooting number were also significantly different from those of the control group, indicating that COS is beneficial to the radical development of a somatic embryo and root growth elongation somatic embryo germinated plants. In plants, the rapid metabolism, light isomerization in vitro, complicated synthetic routes, and too expensive cost hinder ABA use [65]. Compared to ABA, COS is economical, diverse, and can be utilized in many advanced ways [66]. The enzymatic process to obtain COS was considered the perfect and environment-friendly process with low cost, which controls the DA and degree of polymerization (D.P.) of the products. COS is easily soluble in neutral water compared to ABA, which is insoluble in water [67].
Most importantly, COS significantly induces S.E., and improved tolerance of low-temperature stress, U.V., and salt stress in soybean [68], and in our study, in L. sino-americanum. In our study, the statistical data of somatic embryo regeneration plants induced by COS showed that somatic embryos developed well under COS induction. Most of them were able to grow into normal germinated plants. Signaling induced by the chitosan molecule comprises specific cellular receptors that are transduced by secondary messenger(s) such as reactive oxygen species (ROS), H2O2, nitric oxide (NO), and phytohormones inside the cell to induce physiological responses. Its inhibitory effects of radicals by chitosan treatment has been stated [69].
The branching rate of somatic embryo germinated plants induced by COS was significantly higher. When the COS concentration was 0.1 mg L−1, the two-month-old somatic embryo germinated plant had the highest branching rate, as high as 18.32%, which was significantly different from the 4.91% of the control group (p < 0.01). The branching rate of somatic embryo germinated plants with a COS concentration of 0.05 mg L−1 was 14.92%, which was higher than the control group. The average number of leaves per branch, stem diameter, and the average stem length of each branch decreased with an increase in the number of branches.
6. Conclusions
COS increased the efficacy of somatic embryogenesis when used at a particular concentration, such as 0.05 mg L−1. Our results showed that adding a certain concentration of COS to the growth medium could promote the growth of stem and root of regenerated plantlets in somatic embryos of L. hybrid. The plant height was 19.21% higher than that of the control group, and the stem increased by 17.36%, the stem diameter increased by 18.03%, and the root increased by 36.12%. Highly significant differences were observed for leaf growth and root thickening when the concentration was 0.05 mg L−1.
Author Contributions
Data curation, A.A. and M.Z.; L.S.; statistical analysis, Formal analysis, S.u.R.; Funding acquisition, J.S. and J.C.; Investigation, S.H. and J.Z.; Writing—original draft, A.A and T.C.; Writing—review and editing, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Nature Science Foundation of China (32071784), the Distinguished Professor Project of Jiangsu province, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data for the manuscript can be provided at the request of readers.
Acknowledgments
The authors are grateful for the support from the Nature Science Foundation of China (32071784), Distinguished Professor Project of Jiangsu province, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Chen, J.; Hao, Z.; Guang, X.; Zhao, C.; Wang, P.; Xue, L.; Zhu, Q.; Yang, L.; Sheng, Y.; Zhou, Y.; et al. Liriodendron genome sheds light on angiosperm phylogeny and species–pair differentiation. Nat. Plants 2019, 5, 18–25. [Google Scholar] [CrossRef]
- Parks, C.R.; Wendel, J.F. Molecular divergence between Asian and North American species of Liriodendron (Magnoliaceae) with implications for interpretation of fossil floras. Am. J. Bot. 1990, 77, 1243–1256. [Google Scholar] [CrossRef]
- Zhen, Y.; Li, C.; Chen, J.; Chen, Q.; Shi, J. Proteomics of embryogenic and non-embryogenic calli of a liriodendron hybrid. Acta Physiol. Plant. 2015, 37, 211. [Google Scholar] [CrossRef]
- Shang, C.; Wang, Z. A new scientific name of hybrid Liriodendron-L. Sino-americanum. J. Nanjing For. Univ. 2012, 36, 1–2. [Google Scholar]
- Cheng, Y.; Li, H. Interspecies evolutionary divergence in Liriodendron, evidence from the nucleotide variations of the lcdhn-like gene. BMC Evol. Biol. 2018, 18, 195. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, M.; Luo, Q.; Wang, J.; Li, H. De novo transcriptome analysis of Liriodendron chinense petals and leaves by Illumina sequencing. Gene 2014, 534, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Rudall, P. Comparative floral anatomy and ontogeny in Magnoliaceae. Plant Syst. Evol. 2006, 258, 1–15. [Google Scholar] [CrossRef]
- Endress, P.K. The evolution of floral biology in basal angiosperms. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 411–421. [Google Scholar] [CrossRef]
- Li, M.; Wang, K.; Wang, X.; Yang, P. Morphological and proteomic analyses reveal the role of pistil under pollination in Liriodendron chinense (hemsl.) Sarg. PLoS ONE 2014, 9, e99970. [Google Scholar]
- Li, B.; Li, Y.; Cai, Q.; Lin, F.; Meng, Q.; Zheng, Y. The complete chloroplast genome of a Tertiary relict species Liriodendron chinense (Magnoliaceae). Conserv. Genet. Resour. 2016, 8, 279–281. [Google Scholar] [CrossRef]
- Soltis, P.S.; Soltis, D.E. The origin and diversification of angiosperms. Am. J. Bot. 2004, 91, 1614–1626. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.-L.; Wen, J.; Azuma, H.; Qiu, Y.-L.; Sun, H.; Meng, Y.; Sun, W.-B.; Zimmer, E.A. Phylogenetic and biogeographic complexity of Magnoliaceae in the Northern Hemisphere inferred from three nuclear data sets. Mol. Phylogenetics Evol. 2008, 48, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D. Magnolias and Their Allies; Published for the International Dendrology Society and the Magnolia Society: London, UK, 1998. [Google Scholar]
- Moody, R.C. Yellow Popular Glulam Timber Beam Performance; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 1993; Volume 520.
- Hernandez, R. Strength and Stiffness of Reinforced Yellow-Poplar Glued-Laminated Beams; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 1997; Volume 554.
- Williams, R.S.; Feist, W.C. Durability of yellow-poplar and Sweetgum and service life of finishes after long-term exposure. For. Prod. J. 2004, 54, 96–101. [Google Scholar]
- Kim, K.D.; Lee, E.J. Potential Tree Species for Use in the Restoration of Unsanitary Landfills. Environ. Manag. 2005, 36, 1–14. [Google Scholar] [CrossRef]
- Moon, M.K.; Oh, H.M.; Kwon, B.-M.; Baek, N.-L.; Kim, S.-H.; Kim, J.S.; Kim, D.K. Farnesyl protein transferase and tumor cell growth inhibitory activities of lipiferolide isolated from Liriodendron tulipifera. Arch. Pharmacal Res. 2007, 30, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Kudi, A.; Umoh, J.; Eduvie, L.; Gefu, J. Screening of some Nigerian medicinal plants for antibacterial activity. J. Ethnopharmacol. 1999, 67, 225–228. [Google Scholar] [CrossRef]
- Celen, I.; Harper, D.; Labbe, N. A multivariate approach to the acetylated poplar wood samples by near-infrared spectroscopy. Holzforschung 2008, 62, 189–196. [Google Scholar] [CrossRef]
- Xiang, Q.; Lee, Y.Y.; Torget, R.W. Kinetics of glucose decomposition during dilute-acid hydrolysis of lignocellulosic biomass. In Proceedings of the Twenty-Fifth Symposium on Biotechnology for Fuels and Chemicals Held, Breckenridge, CO, USA, 4–7 May 2003; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1127–1138. [Google Scholar]
- Berlin, A.; Maximenko, V.; Bura, R.; Kang, K.-Y.; Gilkes, N.; Saddler, J. A rapid microassay to evaluate enzymatic hydrolysis of lignocellulosic substrates. Biotechnol. Bioeng. 2006, 93, 880–886. [Google Scholar] [CrossRef]
- Ri-Ming, H.; Shan-An, H.; Shi-Jie, T.; Shou-Peng, W. Geographical distribution of Liriodendron chinense in china and its significance. J. Plant Resour. Environ. 1995, 1, 1–6. [Google Scholar]
- Fu, L.; Jin, J. Red List of Endangered Plants in China; Science Press: Beijing, China, 1992. [Google Scholar]
- Guan, Y.; Li, S.-G.; Fan, X.-F.; Su, Z.-H. Application of Somatic Embryogenesis in Woody Plants. Front. Plant Sci. 2016, 7, 938. [Google Scholar] [CrossRef]
- Lu, D.; Wei, W.; Zhou, W.; McGuigan, L.D.; Ji, F.-Y.; Li, X.; Xing, Y.; Zhang, Q.; Fang, K.-F.; Cao, Q.-Q.; et al. Establishment of a somatic embryo regeneration system and expression analysis of somatic embryogenesis-related genes in Chinese chestnut (Castanea mollissima Blume). Plant Cell Tissue Organ Cult. 2017, 130, 601–616. [Google Scholar] [CrossRef]
- Khilwani, B.; Kaur, A.; Ranjan, R.; Kumar, A. Direct somatic embryogenesis and encapsulation of somatic embryos for in vitro conservation of Bacopa monnieri (L.) Wettst. Plant Cell Tissue Organ Cult. 2016, 127, 433–442. [Google Scholar] [CrossRef]
- Guillou, C.; Fillodeau, A.; Brulard, E.; Breton, D.; Maraschin, S.D.F.; Verdier, D.; Simon, M.; Ducos, J.-P. Indirect somatic embryogenesis of Theobroma cacao L. in liquid medium and improvement of embryo-to-plantlet conversion rate. Vitr. Cell. Dev. Biol. Anim. 2018, 54, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.M.; Ghosh, B. High-frequency somatic embryogenesis and artificial seeds for mass production of true-to-type plants in Ledebouria revoluta: An important cardioprotective plant. Plant Cell Tissue Organ Cult. 2016, 127, 71–83. [Google Scholar] [CrossRef]
- Merkle, S.A.; Dean, J.F. Forest tree biotechnology. Curr. Opin. Biotechnol. 2000, 11, 298–302. [Google Scholar] [CrossRef]
- Chiancone, B.; Germanà, M.A. Micropropagation of Citrus spp. by Organogenesis and Somatic Embryogenesis. In Protocols for Micropropagation of Selected Economically-Important Horticultural Plants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 99–118. [Google Scholar]
- Ozudogru, E.A.; Lambardi, M. Cryotechniques for the long-term conservation of embryogenic cultures from woody plants. In Vitro Embryogenesis in Higher Plants; Springer: Berlin/Heidelberg, Germany, 2016; pp. 537–550. [Google Scholar]
- Rugh, C.L.; Senecoff, J.F.; Meagher, R.B.; Merkle, S.A. Development of transgenic yellow poplar for mercury phytoremediation. Nat. Biotechnol. 1998, 16, 925–928. [Google Scholar] [CrossRef]
- Vidal, N.; Mallón, R.; Valladares, S.; Meijomín, A.; Vieitez, A. Regeneration of transgenic plants by agrobacterium-mediated transformation of somatic embryos of juvenile and mature Quercus robur. Plant Cell Rep. 2010, 29, 1411–1422. [Google Scholar] [CrossRef]
- Cabrera, J.C.; Wégria, G.; Onderwater, R.C.A.; González, G.; Nápoles, M.C.; Falcón-Rodríguez, A.B.; Costales, D.; Rogers, H.J.; Diosdado, E.; González, S.; et al. Practical use of oligosaccharins in agriculture. Acta Hortic. 2013, 1009, 195–212. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle-based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Cadano, J.R.; Jose, M.; Lubi, A.G.; Maling, J.N.; Moraga, J.S.; Shi, Q.Y.; Vegafria, H.M.; VinceCruz-Abeledo, C.C. A comparative study on the raw chitin and chitosan yields of common bio-waste from philippine seafood. Environ. Sci. Pollut. Res. 2021, 28, 11954–11961. [Google Scholar] [CrossRef]
- Fadlaoui, S.; Asri, O.E.; Mohammed, L.; Sihame, A.; Melhaoui, M. Isolation and characterization of chitin from shells of the freshwater crab Potamon algeriense. Prog. Chem. Appl. Chitin Its Deriv. 2019, 14, 23–35. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef]
- Hadwiger, L.A. Multiple effects of chitosan on plant systems: Solid science or hype. Plant Sci. 2013, 208, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, S.; Héloir, M.-C.; Poinssot, B.; Gauthier, A.; Paris, F. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Albersheim, P.; Darvill, A.G.; McNeil, M.; Valent, B.S.; Sharp, J.K.; Nothnagel, E.A.; Davis, K.R.; Yamazaki, N.; Gollin, D.J.; York, W.S.; et al. Oligosaccharins: Naturally occurring carbohydrates with biological regulatory functions. In Structure and Function of Plant Genomes; Springer: Berlin/Heidelberg, Germany, 1983; pp. 293–312. [Google Scholar]
- Enríquez-Guevara, E.A.; Aispuro-Hernández, E.; Vargas-Arispuro, I.; Martínez-Téllez, M.Á. Cell wall oligosaccharine derivatives: Biological activity and participation in the response of plant defense. Rev. Mex. Fitopatol. 2010, 28, 144–155. [Google Scholar]
- López-Guerrero, A.G.; Rodríguez-Hernández, A.M.; Mounzer, O.; Zenteno-Savín, T.; Rivera-Cabrera, F.; Izquierdo-Oviedo, H.; Soriano-Melgar, L.d.A.A. Effect of oligosaccharins on the vase life of lisianthus (Eustoma grandiflorum raf.) cv. ‘Mariachi blue’. J. Hortic. Sci. Biotechnol. 2019, 95, 316–324. [Google Scholar] [CrossRef]
- Cté, F.; Hahn, M.G. Oligosaccharins: Structures and signal transduction. Plant Mol. Biol. 1994, 26, 1379–1411. [Google Scholar]
- Meng, Q.-Y.; Wang, H.; Cui, Z.-B.; Yu, W.-G.; Lu, X.-Z. Chitosan oligosaccharides attenuate amyloid formation of hiapp and protect pancreatic β-cells from cytotoxicity. Molecules 2020, 25, 1314. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, J.; Jiao, S.; Cheng, G.; Feng, C.; Du, Y.; Liu, H. A review on the preparation of chitosan oligosaccharides and application to human health, animal husbandry and agricultural production. Carbohydr. Polym. 2019, 220, 60–70. [Google Scholar] [CrossRef]
- Salachna, P.; Grzeszczuk, M.; Soból, M. Effects of chitooligosaccharide coating combined with selected ionic polymers on the stimulation of Ornithogalum saundersiae growth. Molecules 2017, 22, 1903. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Azad, M.; Kalam, A.; Lin, Y.; Kim, S.W.; Tian, Y.; Liu, G.; Wang, H. Biological Effects and Applications of Chitosan and Chito-Oligosaccharides. Front. Physiol. 2019, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Necha, L.L.B.; Bautista-Baños, S. Chapter 8–prospects for the use of chitosan and other alternatives in ornamental conservation. Chitosan Preserv. Agric. Commod. 2016, 221–249. [Google Scholar]
- Dumville, J.C.; Fry, S.C. Solubilisation of tomato fruit pectins by ascorbate: A possible non-enzymic mechanism of fruit softening. Planta 2003, 217, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Y.; Zhang, R.; Wang, W.; Zhao, X.; Du, Y.; Yin, H. Effects of chitosan oligosaccharides on the yield components and production quality of different wheat cultivars (Triticum aestivum L.) in northwest China. Field Crop. Res. 2015, 172, 11–20. [Google Scholar] [CrossRef]
- He, Y.; Santosh, B.; Wang, W.; Jia, X.; Lu, H.; Yin, H. Pre-harvest treatment of chitosan oligosaccharides improved strawberry fruit quality. Int. J. Mol. Sci. 2018, 19, 2194. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.S.; Hwang, J.W.; Kim, S.K.; Jeon, Y.J.; Je, J.Y.; Ahn, C.B.; Moon, S.H.; Jeon, B.T.; Park, P.J. Chitooligosaccharide and its derivatives: Preparation and biological applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef]
- González, A.; Castro, J.; Vera, J.; Moenne, A. Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation, basal metabolism, and cell division. J. Plant Growth Regul. 2013, 32, 443–448. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitosan Effects on Plant Systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Reactive Oxygen and Nitrogen Species in Defense/Stress Responses Activated by Chitosan in Sycamore Cultured Cells. Int. J. Mol. Sci. 2015, 16, 3019–3034. [Google Scholar] [CrossRef]
- Hirano, S.; Yamamoto, T.; Hayashi, M.; Nishida, T.; Inui, H. Chitinase activity in [several plants] seeds coated with chitosan derivatives. Agric. Biol. Chem. 2014, 54, 2719–2720. [Google Scholar]
- Choi, C.; Nam, J.-P.; Nah, J.-W. Application of chitosan and chitosan derivatives as biomaterials. J. Ind. Eng. Chem. 2016, 33, 1–10. [Google Scholar] [CrossRef]
- Guo, W.; Ye, Z.; Wang, G.; Zhao, X.; Yuan, J.; Du, Y. Measurement of oligochitosan–tobacco cell interaction by fluorometric method using europium complexes as fluorescence probes. Talanta 2009, 78, 977–982. [Google Scholar] [CrossRef]
- Guo, W.; Yin, H.; Ye, Z.; Zhao, X.; Yuan, J.; Du, Y. A comparison study on the interactions of two oligosaccharides with tobacco cells by time-resolved fluorometric method. Carbohydr. Polym. 2012, 90, 491–495. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, H.; Zhao, X.; Wang, W.; Du, Y.; He, A.; Sun, K. The promoting effects of alginate oligosaccharides on root development in Oryza sativa L. mediated by auxin signaling. Carbohydr. Polym. 2014, 113, 446–454. [Google Scholar] [CrossRef]
- Wan, C.; Wang, M.; Yang, D.; Han, X.; Che, C.; Ding, S.; Xiao, Y.; Qin, Z. Synthesis and biological activity of 2′, 3′-iso-aryl-abscisic acid analogs. Molecules 2017, 22, 2229. [Google Scholar] [CrossRef]
- Yin, H.; Du, Y.; Dong, Z. Chitin oligosaccharide and chitosan oligosaccharide: Two similar but different plant elicitors. Front. Plant Sci. 2016, 7, 522. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.-J.; Park, R.-D. Bioproduction of Chitooligosaccharides: Present and Perspectives. Mar. Drugs 2014, 12, 5328–5356. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Yamaguchi, H.; Yuasa, T.; Iwaya-Inoue, M.; Arima, S.; Zheng, S.-H. Hydrogen peroxide spraying alleviates drought stress in soybean plants. J. Plant Physiol. 2011, 168, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, X.; Chen, X.S. Inhibitory effects of chitosan on superoxide anion radicals and lipid free radicals. Chin. Sci. Bull. 2002, 47, 887–889. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).