Abstract
Natural regeneration of sessile oak forests is a complex process whose final outcome is influenced by numerous factors. The initial phase of development of sessile oak seedlings stands out as the most critical period in the process of natural regeneration of sessile oak forests. As the available light and competition from the accompanying woody species and ground vegetation are some of the main predictors of the success of sessile oak forest regeneration, this paper presents the results of studying the influence of these factors on the regeneration dynamics and development of sessile oak seedlings. The research was conducted in sessile oak forests in northeastern Serbia in the period from 2015 to 2020. At the end of the growing season each year, the following data were collected on 180 smaller sampling plots of 1 m2 in different conditions of canopy openness: the number, height, and root collar diameter of sessile oak seedlings. Also, the percent cover of competing woody species and ground vegetation was estimated on these sample plots. The obtained results indicated that the success of sessile oak forest regeneration largely depends on the initial number of sessile oak seedlings and silvicultural treatments during the rejuvenation period, which determine the microclimatic conditions in the stand and control the competing vegetation. They also indicate that with an increase in the available light, the impact of the competing vegetation on the dynamics of regeneration and development of sessile oak seedlings is less pronounced. Accordingly, as young sessile oak seedlings need a large amount of light for survival and development, it is necessary to increase the available amount of light intensively in a short period of time (six–eight years) by reducing canopy openness and thus providing optimal conditions in which sessile oak seedlings can gain an advantage over the competing vegetation.
1. Introduction
Sessile oak (Quercus petraea (Matt.) Liebl.) is one of the most valuable tree species in Europe considering its ecological and economic significance [1,2,3,4]. Climate change projections indicate that, due to its high tolerance to warm and dry climates, sessile oak will adapt well to future climate scenarios [5,6,7]. Its forests will thus play a significant role in European forestry in the future, especially within its natural range [8,9].
Although sessile oak often occurs together with pedunculate oak in habitats with a similar supply of water and nutrients [9,10,11,12], these two species build independent stands in Serbia, Southeast Europe. While pedunculate oak occurs in lowland conditions near rivers, sessile oak grows in the hilly-mountainous region at altitudes ranging from 300 to 1300 m. Sessile oak is the third most common species in the forest growing stock of Serbia, after beech (Fagus sylvatica L.) and Turkey oak (Quercus cerris L.). It occupies an area of 173,200 ha and accounts for 5.9% of volume and 6.1% of volume increment [13].
Natural regeneration of sessile oak forests is one of the crucial issues in European forestry, considering their current state, growing demand for products from these forests, and the impact of global climate change on their stability and existence. It is assumed that the failure of natural regeneration in oak forests in the absence of human intervention would lead to the extinction of most types of these important forest communities [14]. Natural regeneration of oak forests without human assistance succeeds only in extreme localities, with sufficient light and low ground vegetation [15]. Accordingly, the increased demand for oak products and the depletion of natural oak forests have significantly influenced the development of oak silviculture [3]. In this context, it is necessary to emphasize the importance of the concept of sustainability, which implies the application of close-to-nature silviculture in order to support sustainable, natural, and diverse forests [16].
At the beginning of the 20th century, anomalies were observed in the natural regeneration of oak forests in Europe. A large number of authors highlighted the problem of the survival of oak seedlings in the initial phase of development [2,17,18,19,20,21,22]. In the process of natural regeneration of sessile oak forests, several factors significantly affect the survival and development of sessile oak seedlings in the initial phase of their development. The amount of light needed by sessile oak seedlings changes with age and is one of the factors that play a dominant role in the process of natural regeneration of these forests. In the first three to four years, sessile oak can grow in the shade, while in the later phase of development, it needs a significantly larger amount of light [10,23,24,25,26]. About 15% of available light is needed for the survival of sessile oak seedlings, at least 20% for sustainable growth [24], while 30–60% of available light is needed to ensure a satisfactory increment [10,23,27,28]. Extreme temperatures can be one of the limiting factors in the natural regeneration of sessile oak forests. They can significantly affect the appearance, development, and survival of seedlings and reduce the success of regeneration. As sessile oak comes into leaf relatively late in the year (late April to early May), it is particularly sensitive to late spring frosts, and temperatures below −3 °C destroy young leaves [3,18,26,29,30]. In the initial phase of development, sessile oak is very sensitive to extremely high temperatures when it can easily be damaged if there is no protection from the canopy of the parent stand [23,25,31]. In addition to microclimatic conditions, the competition from woody species and ground vegetation significantly affects the initial development of seedlings and the dynamics of regeneration of sessile oak forests. The degree of competition in the medium and long term can hinder the process of natural regeneration of sessile oak forests and is often identified as one of the main reasons for the death of sessile oak seedlings [2,4,32,33]. In the area of Serbia in Southeast Europe, hornbeam (Carpinus betulus L.), white linden (Tilia tomentosa L.), and common ash (Fraxinus excelsior L.) are the strongest competitors among woody species in the process of natural regeneration of sessile oak forests in moist habitats, while it is manna ash (Fraxinus ornus L.) in drier habitats. In Central Europe, beech (Fagus sylvatica L.) and hornbeam (Carpinus betulus L.) are the most common competing woody species in the natural regeneration of sessile oak forests [2,4,33,34,35,36]. Besides these woody species, blackberry (Rubus spp.) is a very serious competitor in moist and rich soils. Blackberry can quickly outgrow and completely suppress sessile oak seedlings [26,36,37,38]. Successful control of competing vegetation depends on a combination of different measures: preventive, mechanical and chemical [26]. Other reasons for the failure in the natural regeneration of sessile oak forests often include the damage from wildlife, damage from insects and predators (birds and rodents), and plant diseases [15,25,39,40,41,42].
Natural regeneration of sessile oak forests is always given priority over artificial regeneration by seed sowing or planting seedlings that is used only when natural regeneration is not possible for any reason. Natural regeneration, with proper regulation, provides the best biological results which do not require high costs [43]. It also follows the principles of close-to-nature silviculture, preserves indigenous genetic diversity [44], and creates preconditions for future positive selection from a large number of trees of the desired species [40]. Modern silviculture promotes the methods of regeneration of sessile oak forests on small surfaces that support the principles of uniform shelterwood cutting [23,25,26,45], and continuous cover forestry system (gap-cutting) [10,32,46]. Despite numerous positive effects achieved by applying these methods, some authors believe that they have certain disadvantages. The concept of shelterwood cutting in the regeneration of oak forests implies the removal of all trees in a relatively short period of time (˂ten years), which can have adverse effects on habitat conditions and biodiversity protection [40,46]. On the other hand, the application of the continuous cover forestry system (gap-cutting) causes numerous dilemmas concerning the size of the openings in the stands and the required amount of light for the survival of oaks, biological strength of competing species, etc. [40,46]. Nevertheless, these methods have given the best results in practice so far, and with certain modifications and improvements in the future, they can find wide application in the natural regeneration of sessile oak forests.
Considering the above, and the fact that the available amount of light and the competition from woody species and ground vegetation are some of the main predictors of the success of sessile oak regeneration, this paper aims to study the joint effects of these factors on the regeneration dynamics and development of sessile oak seedlings. The starting hypotheses in the paper are the following:
- (a)
- The available amount of light and the presence of competing ground and woody vegetation significantly affect the success of natural regeneration of sessile oak forests;
- (b)
- As the amount of available light increases, the influence of competing ground and woody vegetation on the number of sessile oak seedlings decreases;
- (c)
- As the amount of available light increases, the influence of competing ground and woody vegetation on the growth characteristics of sessile oak seedlings decreases.
2. Materials and Methods
2.1. Study Site
The research was conducted in sessile oak forests (Quercetum montanum s.l.) in the area of northeastern Serbia in the period 2015–2020, at two sites: the MU Crna Reka within the Teaching Base “Majdanpečka Domena“of the Faculty of Forestry, University of Belgrade (44°21′ N; 21°55′ E) and the MU Ujevac within the State Enterprise “Srbijašume“, Belgrade (44°25′ N; 21°52′ E). The average annual temperature at the study sites was 9.3–10.3 °C (15.9–17.0 °C in the growing season), and the average annual precipitation was 679–705 mm (388–403 mm in the growing season). According to Thornthwaite climate classification [47], the studied localities have a subhumid moist climate (C2), while it is semi-humid (a climate of low-altitude forests) according to Lang′s climate classification [48].
The research in this paper was based on two repeated experiments at different localities.
There were three experimental units within the study site of the MU Crna Reka. The first experimental unit on which the final cut of shelterwood cutting (FC) was performed was 0.25 ha in size. The second experimental unit on which the preparatory-seed cut of shelterwood cutting was performed (P-SC) was 0.32 ha in size and the third experimental unit without any silvicultural treatments (Control) was 0.16 ha in size.
The experimental units were located at an altitude of 450–500 m, with a slope of 15–30° and a southwestern aspect. In terms of phytosociology, the stand was defined as an association of sessile oak with forest fescue (Festuco drymeiae-Quercetum petraeae Janković 1974) on a dystric cambisol over gneiss. Before performing silvicultural treatments, the total number of trees in the stand (d1.3 > 5.0 cm) was 1674 per ha, while the basal area amounted to 32.0 m2/ha, and the total volume to 287.5 m3/ha. The number of sessile oak trees was 178 per ha, the basal area 17.8 m2/ha, and the volume 190.7 m3/ha. The most common accompanying woody species that occured mainly in the understorey included hornbeam (Carpinus betulus L.) and silver linden (Tilia tomentosa L.), while common ash (Fraxinus excelsior L.), beech (Fagus sylvatica L.), field maple (Acer campestre L.) and Norway maple (Acer platanoides L.) occured individually.
There were also three experimental units within the study site of the MU Ujevac. They were all 0.25 ha in size: the first on which the final cut of shelterwood cutting (FC) was performed, the second on which the preparatory-seed cut of shelterwood cutting was performed (P-SC), and the third without any silvicultural treatments (Control).
The experimental units were located at an altitude of 300–350 m, with a slope of 20–25° and a western aspect. In terms of phytosociology, the stand was defined as an association of sessile oak with hairy sedge (Carici pilosae-Quercetum petraeae B. Jov. 1989) on a dystric and eutric cambisol over gneiss and neutral and basic eruptive rocks. Before performing silvicultural treatments, the total number of trees in the stand (d1.3 > 5.0 cm) was 562 per ha, while the basal area amounted to 28.2 m2/ha, and the total volume to 283.9 m3/ha. The number of sessile oak trees was 148 per ha, the basal area 17.6 m2/ha, and the volume 188.9 m3/ha. The most common accompanying woody species that also occured mainly in the understorey were silver linden (Tilia tomentosa L.) and hornbeam (Carpinus betulus L.), while common ash (Fraxinus excelsior L.) and wild service tree (Sorbus torminalis L.) occured individually.
2.2. Sampling Design
Within the experimental units, 180 sample plots of 1 m2 were established using a systematic sampling (Figure 1): 20 sample plots on the experimental unit of 0.16 ha, 120 sample plots on four experimental units of 0.25 ha, and 40 sample plots on the experimental unit of 0.32 ha. At the end of the growing season each year in the period between 2015 and 2020, data relating to the following elements were collected on these sample plots: the number, height, and root collar diameter of sessile oak seedlings at different ages. The height was measured with a measuring tape with an accuracy of 1.0 cm, and root collar diameter with a digital caliper with an accuracy of 1.0 mm. The percent cover of other competing woody species and ground vegetation (mainly blackberry (Rubus spp.)) was also estimated on these sample plots. It was done visually from above and recorded to the nearest 10% from 10% to 100%, and to the nearest 1% from 1% to 10%.
Figure 1.
The arrangement of sample plots in the experimental field of 0.25 ha.
Depending on the percent cover of competing woody species and ground vegetation, the degree of competition in the sample plots was defined as follows: low—the percent cover of competing species <30%; medium—the percent cover of competing species 30–60%; high—the percent cover of competing species >60%.
The age of sessile oak seedlings was determined by monitoring acorn production. To determine the age of seedlings more precisely, we cut fifteen naturally regenerated seedlings in each experimental unit (five tallest, five smallest, and five medium-sized seedlings). We counted the growth rings of these seedlings to determine their age accurately. As the previous abundant sessile oak acorn production was recorded in 2012, data about the occurrence, condition, and development of three- to eight-year-old sessile oak seedlings were collected in the experimental units at the end of growing seasons 2015–2020.
For the purpose of defining the canopy openness and determining the amount of available light, three hemispherical photographs were taken on each sample plot at a height of 2 m.
In order to define the influence of microclimatic conditions on the presence of sessile oak and the main competing species, air temperature and relative humidity were measured in all experimental units every 2 h in the period from 6 AM to 6 PM during three days in the sunniest month of July—successively from 1 to 3 July, 2020. For this purpose, we used a WS-GP1 portable automatic weather station, which allowed the measurement of basic meteorological parameters in the desired time intervals at a height of 2 m.
2.3. Data Analysis
The analysis of the canopy openness and the available amount of light was performed by processing hemispherical photographs in the specialized software Gap Light Analyzer 2.0.
The regeneration dynamics in different conditions of canopy openness and available amount of light was analyzed on the basis of the average number of sessile oak seedlings (per m2) of different ages. On the other hand, the maximum values of the height and root collar diameter of the seedlings, also of different ages, were analyzed as the best representatives of the influence of the mentioned factors on the development of sessile oak seedlings.
To evaluate the individual and joint influence of the canopy openness and competing woody species and ground vegetation on the number and basic growth parameters of eight-year-old sessile oak seedlings, linear mixed models (LMM) were used. The number (N), maximum height (hmax), and maximum root collar diameter (dmax) of sessile oak seedlings were dependent variables, the canopy openness after silvicultural treatment (final cut of shelterwood cutting (FC), preparatory-seed cut of shelterwood cutting (P-SC), control surface without silvicultural treatment (Control) and the percent cover of competing woody species and ground vegetation, i.e., the degree of competition (high, medium, low) were fixed factors, while the locality (Crna Reka vs. Ujevac) was the random factor.
The normality of all dependent variables was checked using Kolmogorov–Smirnoff and Shapiro–Wilk tests.
Redundancy analysis (RDA) was used to study the presence of sessile oak and the most common competing species in relation to the basic environmental factors (available light, air temperature, and relative humidity) and the canopy openness.
All statistical analyses were performed using the R 4.0.3 package [49]. Linear mixed models (LMM) were created using the lme4 package [50], redundancy analysis (RDA) was performed using vegan package [51], while the ggplot2 package [52] and the ggvegan package [53] were used to create the graphs.
3. Results
3.1. Canopy Openness and Light Availability
The canopy openness within both study sites was the lowest in the control experimental units without silvicultural intervention (Control) and ranged from 26.67% to 29.69%. In the experimental units where the preparatory-seed cut (P-SC) was performed, it ranged from 55.98% to 56.24%, while in the experimental units where the final cut (FC) was performed, the canopy of the stand was completely open (Table 1).

Table 1.
Canopy openness and light availability in the experimental units. FC, final cut; P-SC, preparatory-seed cut.
The average total radiation intensity (direct + diffuse radiation) was also the lowest in the control experimental units (Control) and ranged from 7.67 to 10.35 MJ/m2/d (24.8–33.5% compared to the radiation intensity in the open space). In the experimental units where the preparatory-seed cut (P-SC) was performed, the average total radiation intensity ranged from 20.90 to 22.66 MJ/m2/d (67.5–73.1% in relation to the intensity of the radiation in the open space), while in the experimental units where the final cut (FC) was performed, the average total radiation intensity ranged from 30.93 to 30.98 MJ/m2/d, which also represents the intensity of radiation in the open space (Table 1).
3.2. The Number and Growth Characteristics of Sessile Oak Seedlings and the Degree of Competition
Comparative analysis of the number and growth characteristics of sessile oak seedlings indicates significant differences due to the application of different silvicultural treatments (Figure 2).
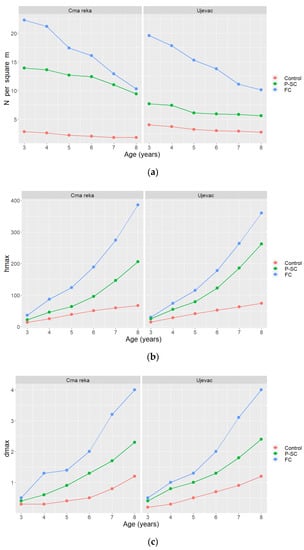
Figure 2.
The number and growth characteristics of 3–8-year-old sessile oak seedlings in experimental units with different silvicultural treatments. (a) The average number of sessile oak seedlings—N (per m2). (b) The maximum heights of sessile oak seedlings—hmax (cm). (c) The maximum root collar diameters of sessile oak seedlings—dmax (cm).
In the experimental units where the final cut (FC) was performed, the average number of seedlings constantly decreased with age and ranged from 19.6–22.3 per m2 in the 3rd year of age to 10.1–10.3 per m2 in the 8th year of age (Figure 2a). In contrast, as seedlings developed in full light conditions, their growth in these experimental units was the most intense, which was reflected in the achieved maximum heights ranged from 30–36 cm in the 3rd year of age to 360–386 cm in the 8th year of age (Figure 2b), as well as maximum root collar diameters ranged from 0.5 cm in the 3rd year of age to 4.0 cm in the 8th year of age (Figure 2c).
The high degree of competition within these experimental units was recorded on 13.3–20.0% of the area, the medium degree of competition on 20.0–26.7% of the area, and the low degree of competition on 53.3–66.7% of the area.
In the experimental units where the preparatory-seed cut (P-SC) was performed, the average number of seedlings also constantly decreased with age. It ranged from 7.7–13.9 per m2 in the 3rd year of age to 5.6–9.4 per m2 in the 8th year of age (Figure 2a). The sessile oak seedlings in these experimental units had significantly slower growth than the seedlings developed in full light conditions. The maximum heights ranged from 22–25 cm in the 3rd year of age to 206–262 cm in the 8th year of age (Figure 2b), while the maximum root collar diameters ranged from 0.4 cm in the 3rd year of age to 2.3–2.4 cm in the 8th year of age (Figure 2c).
The high degree of competition within these experimental units was recorded on 25.0–33.3% of the area, the medium degree of competition on 17.5–33.3% of the area, and the low degree of competition on 33.3–57.5% of the area.
In the control experimental units (Control), the average number of seedlings was the smallest, slightly decreased with age and ranged from 2.8–4.0 per m2 in the 3rd year of age to 1.8–2.7 per m2 in the 8th year of age (Figure 2a). The sessile oak seedlings in these experimental units had significantly slower growth than the seedlings in the experimental units where the mentioned silvicultural treatments were performed. The maximum heights of seedlings ranged from 14–15 cm in the 3rd year of age to 67–74 cm in the 8th year of age (Figure 2b), while the maximum root collar diameters ranged from 0.2–0.3 cm in the 3rd year of age to 1.2 cm in the 8th year of age (Figure 2c).
The high degree of competition within these experimental fields was recorded on 50.0–56.7% of the area, the medium degree of competition on 10.0–13.3% of the area, and the low degree of competition on 30.0–40.0% of the area.
In all situations, the most common competing woody species were silver linden (Tilia tomentosa), hornbeam (Carpinus betulus), and common ash (Fraxinus excelsior), while field maple (Acer campestre), wild cherry (Prunus avium), Cornelian cherry (Cornus mas), and common hawthorn (Crataegus monogyna) occurred individually. The most common ground vegetation species were blackberry (Rubus spp.), hairy sedge (Carex pilosa), and forest fescue (Festuca drymeia) on a smaller part of the surface.
Since the most common competing species in the study stands were silver linden (Tilia tomentosa), hornbeam (Carpinus betulus), common ash (Fraxinus excelsior), and blackberry (Rubus spp.), redundancy analysis (RDA) was used to analyze the presence of these species in relation to the basic environmental factors (available light, air temperature, relative humidity) and the canopy openness.
The obtained model was statistically significant at the level of p < 0.05 (p = 0.03889; R2adjusted = 0.981), where the first two axes represent 98.02 and 1.43% of the total explained variance.
The redundancy analysis indicated a positive correlation of sessile oak (Quercus petraea), common ash (Fraxinus excelsior), and blackberry (Rubus spp.) with air temperature, available light, and canopy openness and a negative correlation with relative humidity. In contrast, silver linden (Tilia tomentosa) and hornbeam (Carpinus betulus) were positively correlated with relative humidity, and negatively correlated with air temperature, available light, and canopy openness (Figure 3).
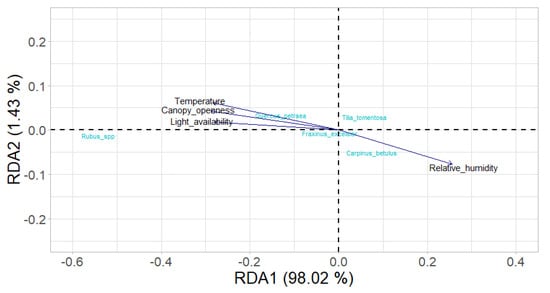
Figure 3.
Redundancy analysis (RDA) of the presence of eight-year-old sessile oak seedlings and major competing species in relation to basic environmental factors (available light, air temperature, and relative humidity) and canopy openness.
3.3. Influence of Canopy Openness and the Degree of Competition on the Regeneration Dynamics and Development of Sessile Oak Seedlings
The results of linear mixed models indicate that the canopy of the stand, i.e., the available amount of light and the presence of competing vegetation significantly affected the number and growth characteristics of sessile oak seedlings (Table 2), which confirmed the first starting hypothesis. Also, the influence of the analyzed fixed factors was statistically significant when it comes to the individual or joint influence of both factors (p < 0.05), where the obtained values of the coefficients R2fix. and R2tot. indicated that a significant share of variance was explained by fixed and random factors (Table 2).

Table 2.
Results of the linear mixed model for estimating the number (N), maximum height (hmax), and maximum root collar diameter (dmax) of eight-year-old sessile oak seedlings in relation to the canopy openness (silvicultural treatment) and the degree of competition (fixed factors) at different study sites (random factor).
The number of eight-year-old sessile oak seedlings increased with increasing available light and decreasing presence of competing vegetation. Furthermore, with increasing available light, the influence of competing woody and ground vegetation on the regeneration dynamics of sessile oak decreased, which confirms the second starting hypothesis (Figure 4a).
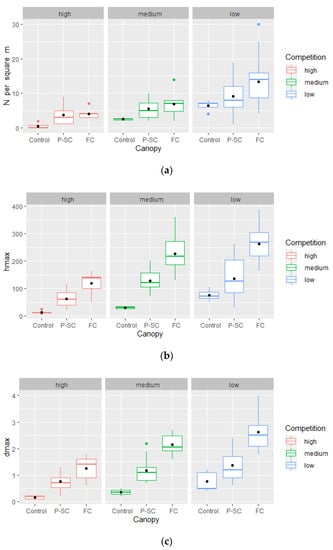
Figure 4.
The number and growth characteristics of eight-year-old sessile oak seedlings depending on the canopy openness and the degree of competition. (a) The average number of sessile oak seedlings—N (per m2). (b) The average maximum heights of sessile oak seedlings—hmax (cm). (c) The average maximum root collar diameters of sessile oak seedlings—dmax (cm).
In conditions of high degree of competition, the average number of sessile oak seedlings in the control experimental units (Control) was 0.5 per m2, in the experimental units with PS-C treatment 3.8 per m2, and in the experimental units with FC treatment 4.0 per m2 (Figure 4a). The average number of sessile oak seedlings in the control experimental units (Control) in the conditions of medium degree of competition was 2.5 per m2, in the experimental units with PS-C treatment 6.3 per m2, and in the experimental units with FC treatment 6.2 per m2 (Figure 4a). In conditions of low degree of competition, the average number of sessile oak seedlings in the control experimental units (Control) was 6.4 per m2, in the experimental units with PS-C treatment 9.2 per m2, and in the experimental units with FC treatment 13.4 per m2 (Figure 4a).
As in the case of the number of seedlings, the development of eight-year-old sessile oak seedlings was significantly more intense with an increase in the available light and a decrease in the degree of competition. It can also be stated that the influence of the degree of competition on the development of sessile oak seedlings decreased with an increase in the available light (Figure 4b,c), which confirms the third starting hypothesis.
In conditions of the high degree of competition, in the control experimental units (Control), the average hmax was 14.6 cm and the average dmax 0.2 cm, in the experimental units with the PS-C treatment, the average hmax was 63.5 cm and the average dmax 0.8 cm, and in the experimental units with the FC treatment, the average hmax was 119.6 cm and the average dmax was 1.3 cm (Figure 4b,c). In the conditions of the medium degree of competition, in the control experimental units (Control), the average hmax was 30.5 cm and the average dmax 0.3 cm, in the experimental units with the PS-C treatment, the average hmax was 129.3 cm and the average dmax 1.2 cm, and in the experimental units with the FC treatment, the average hmax was 226.1 cm and the average dmax was 2.1 cm (Figure 4b,c). In conditions of the low degree of competition, in the control experimental units (Control), the average hmax was 76.9 cm and the average dmax 0.8 cm, in the experimental units with the PS-C treatment, the average hmax was 136.1 cm and the average dmax 1.4 cm, and in the experimental units with the FC treatment, the average hmax was 262.4 cm and the average dmax was 2.6 cm (Figure 4b,c).
4. Discussion
Despite numerous and extensive research conducted in the past to define the most favorable way of natural regeneration of sessile oak forests, there are still numerous open questions and doubts that are mostly related to defining optimal conditions for survival and development of sessile oak seedlings in the initial phase of regeneration [2,4,26,33,40,46].
The final outcome of natural regeneration of sessile oak forests depends on a number of factors, among which the most important are the canopy openness, the amount of available light, as well as the presence of competing vegetation with pronounced biological strength and ability to take over sessile oak habitats [10,11,23,24,26,40,54,55].
4.1. Canopy Openness and Light Availability
The issue of optimal canopy openness that provides the appropriate amount of light and optimal microclimatic conditions for sustainable development of sessile oak seedlings is the key issue in the regeneration of sessile oak forests. Since the appropriate canopy openness directly affects the available amount of light and other microclimatic elements, it is necessary to take these factors into account together when defining the optimal conditions for survival and development of sessile oak seedlings.
Although sessile oak can develop in low light conditions in the initial stage of development, at a later stage, when the energy reserves of cotyledons are depleted, it needs large amounts of light for survival and development [2,56]. In the initial stage of development, sessile oak needs only 15% of the available light [24], but optimal conditions for the development of one-year-old seedlings are 20–40% of the available light, for two-year-old seedlings 25–50% of the available light [15], while optimal conditions for the development of older seedlings require 30–60% of the available light [10,23,27,28]. An insufficient amount of light leads to poor development of the root system, weakening of metabolism, reduced assimilation capacity, and generally less favorable growth characteristics [57].
Our results indicate a very pronounced dependence of the regeneration dynamics and the growth characteristics of sessile oak seedlings on the canopy openness and the available amount of light. The largest number of sessile oak seedlings was recorded in full light conditions (FC), and the maximum values of the height and root collar diameter in the 8th year of age were 124–154 cm, i.e., 1.6–1.7 cm higher than the seedlings that developed in conditions with 50–60% of the available amount of light (PS-C), and 293–312 cm, i.e., 2.8 cm higher than the seedlings that develop in conditions with 20–30% of the available amount of light (Control).
By creating the appropriate canopy openness, the available amount of light and microclimatic conditions, in general, are regulated. It indirectly affects the survival and development of sessile oak seedlings, but it also affects the competing vegetation as one of the key factors in the natural regeneration of sessile oak forests [10,26,32,36,40,46,55,57].
Based on the analysis of the influence of the canopy openness and basic microclimatic elements on the presence of sessile oak and main competing species (silver linden, hornbeam, common ash, and blackberry) in the study stands, it was found that increase of the canopy openness, available light, and air temperature and decrease of the relative humidity positively affect the presence of sessile oak, common ash, and blackberry. On the other hand, an increase in the relative humidity and a decrease in the canopy openness, available light, and air temperature had a positive effect on the presence of silver linden and hornbeam.
This confirmed the findings of many authors that the natural regeneration of sessile oak forests with continuous cover forestry system (gap-cutting) can be very problematic because unfavorable microclimatic conditions, especially the available amount of light, in this way significantly limit the potential of sessile oak to conquer the competing woody and ground vegetation [40,46,58].
Although the greater canopy openness has a positive effect on the survival and development of sessile oak seedlings, it is necessary to open it gradually, bearing in mind that in the initial stage of development sessile oak is very sensitive to extremely low and high temperatures [3,18,23,25,26,27,28,29,30,31]. Accordingly, in the initial phase of regeneration (first three to four years), it is necessary to maintain the canopy openness below 50% in order to protect sessile oak seedlings from the adverse effects of extreme temperatures, while later the canopy openness can be increased to 60–70%, given that sessile oak seedlings become more resistant with age [26,31].
4.2. Influence of Competing Vegetation on the Natural Regeneration of Sessile Oak
Competitive relationships between sessile oak and competing species of woody and ground vegetation represent one of the most delicate issues in the natural regeneration of sessile oak forests. Considering the bioecological characteristics of sessile oak, the seedlings of this species are usually in a subordinate position to the competing vegetation in the regeneration process, which is the reason why its survival largely depends on the implemented silvicultural treatments.
Consequently, it is of great importance to reduce interspecific competition, which can be achieved by increasing the presence of sessile oak in the mature stand before regeneration and by gradually improving the light conditions for the seedlings during stand regeneration [2,59]. In addition, the number of sessile oak seedlings in the initial phase of regeneration is of great importance, since the higher number of sessile oak seedlings negatively affects the development of competing vegetation [4].
The results obtained in the paper indicate that the presence of competing woody and ground vegetation significantly affects the number and growth characteristics of sessile oak seedlings (Figure 4).
Competing vegetation can inhibit the growth of sessile oak seedlings through allelopathy or reducing the available resources necessary for their growth and survival [60,61,62]. In addition, competing vegetation provides an environment conducive to the development of fungi, insects, and voles that can significantly damage sessile oak seedlings [60].
The influence of competing vegetation on the number and growth characteristics of sessile oak seedlings is significantly determined by the conditions of canopy openness, i.e., microclimatic conditions in the stand. The results obtained in the paper indicated that with an increase in the canopy openness, i.e., the available amount of light, there was a decrease in the influence of competing vegetation on the regeneration dynamics and the development of sessile oak seedlings. Also, our results indicated that the presence of competing vegetation decreased with the increase in the number of sessile oak seedlings. This is in accordance with the well-known fact that the most common competing woody species in sessile oak forests are significantly more tolerant to shade conditions and can overcome sessile oak seedlings if light conditions are unfavorable over a long period of time [1,4,26,36].
In contrast to competing woody species, blackberry, as the most important competing herbaceous species, mostly occur in places with greater canopy openness, i.e., available amount of light, whereby it prefers deep and moist soils, rich in nutrients. The marked ability of blackberry to expand and conquer space at high speed is one of the biggest problems in the natural regeneration of sessile oak forests [26,37,63]. In conditions when blackberry outgrows sessile oak seedlings, the chances for their survival are reduced to a minimum. Therefore, it is of utmost importance to control this factor in order to create favorable conditions for the growth and development of sessile oak seedlings.
Despite the fact that greater canopy openness and a greater amount of light affect blackberry, as well as sessile oak, in such conditions it is possible to intensify the growth of sessile oak, which with a satisfactory number of seedlings can be permanently more dominant than blackberry [4,36].
Following the above, in order to control the competing woody and ground vegetation, it is necessary to intensively increase the available amount of light by regulating the canopy openness and thus provide optimal conditions for survival and satisfactory development of sessile oak seedlings. Furthermore, it is necessary to stress that when for any reason competing vegetation can potentially overcome sessile oak seedlings, it is necessary to apply auxiliary measures to natural regeneration, which include mechanical or chemical removal of competing vegetation. The effects of these treatments were not considered in this paper, since this was not the aim of the research.
5. Conclusions and Silvicultural Considerations
Natural regeneration of sessile oak forests is a complex process that requires continuous adaptation and modernization in accordance with the dynamics of the conditions of these forests. In the process of natural regeneration of sessile oak forests, it is necessary to consider all factors that burden the final outcome of their regeneration.
The results obtained in this paper indicate that the success of sessile oak forest regeneration largely depends on the initial number of sessile oak seedlings and silvicultural treatments during the rejuvenation period which determine the microclimatic conditions in the stand and control the competing vegetation. As sessile oak seedlings need large amounts of light for survival and development, it is necessary to intensively increase the available amount of light by regulating the canopy openness in a short period of six to eight years, thus providing optimal conditions in which sessile oak can gain an advantage over competing vegetation, which is also confirmed by the results obtained in the paper. At the same time, the gradual opening of the canopy in this period provides adequate protection of sessile oak seedlings from the adverse effects of extreme temperatures [26,31], while the remaining parent trees have a role in the subsequent acorn production.
Following the above, the natural regeneration of sessile oak forests can be done on smaller surfaces, up to 1 ha, with respect to the principle of shelterwood cutting, which provides optimal conditions for the development of sessile oak seedlings, and also allows strict control of the regeneration process. In addition, other methods that involve the regeneration of sessile oak forests on smaller surfaces with an intensive increase of the available amount of light, such as gap-cutting, can give good results in the regeneration of these forests. At the same time, a common feature of these methods is the encouragement of natural regeneration and maintenance of the permanent structure of sessile oak forests, which is in accordance with the concept of close-to-nature silviculture (CNS).
Author Contributions
B.K. contributed with original data; B.K., M.K., V.B., and Z.G. conducted data analyses and manuscript writing. All authors designed basic idea, content and method of work, while B.K. performed technical preparation of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ligot, G.; Balandier, P.; Fayolle, A.; Lejeune, P.; Claessens, H. Height competition between Quercus petraea and Fagus sylvatica natural regeneration in mixed and uneven-aged stands. For. Ecol. Manag. 2013, 304, 391–398. [Google Scholar] [CrossRef]
- Annighöfer, P.; Beckschäfer, P.; Vor, T.; Ammer, C. Regeneration Patterns of European Oak Species (Quercus petraea (Matt.) Liebl., Quercus robur L.) in Dependence of Environment and Neighborhood. PLoS ONE 2015, 10, e0134935. [Google Scholar] [CrossRef]
- Eaton, E.; Caudullo, G.; Oliveira, S.; de Rigo, D. Quercus robur and Quercus petraea in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species, 1st ed.; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 160–163. [Google Scholar]
- Kuehne, C.; Pyttel, P.; Modrow, T.; Kohnle, U.; Bauhus, J. Seedling development and regeneration success after 10 years following group selection harvesting in a sessile oak (Quercus petraea [Mattuschka] Liebl.) stand. Ann. For. Sci. 2020, 77, 1–13. [Google Scholar] [CrossRef]
- Bolte, A.; Ammer, C.; Löf, M.; Madsen, P.; Nabuurs, G.-J.; Schall, P.; Spathelf, P.; Rock, J. Adaptive forest management in central Europe: Climate change impacts, strategies and integrative concept. Scand. J. For. Res. 2009, 24, 473–482. [Google Scholar] [CrossRef]
- Sáenz-Romero, C.; Lamy, J.-B.; Ducousso, A.; Musch, B.; Ehrenmann, F.; Delzon, S.; Cavers, S.; Chałupka, W.; Dağdaş, S.; Hansen, J.K.; et al. Adaptive and plastic responses of Quercus petraea populations to climate across Europe. Glob. Chang. Biol. 2017, 23, 2831–2847. [Google Scholar] [CrossRef] [PubMed]
- Nölte, A.; Yousefpour, R.; Hanewinkel, M. Changes in sessile oak (Quercus petraea) productivity under climate change by improved leaf phenology in the 3-PG model. Ecol. Model. 2020, 438, 109285. [Google Scholar] [CrossRef]
- Petritan, A.M.; Biris, I.A.; Merce, O.; Turcu, D.O.; Petritan, I.C. Structure and diversity of a natural temperate sessile oak (Quercus petraea L.)–European Beech (Fagus sylvatica L.) forest. For. Ecol. Manag. 2012, 280, 140–149. [Google Scholar] [CrossRef]
- Perkins, D.; Uhl, E.; Biber, P.; Du Toit, B.; Carraro, V.; Rötzer, T.; Pretzsch, H. Impact of Climate Trends and Drought Events on the Growth of Oaks (Quercus robur L. and Quercus petraea (Matt.) Liebl.) within and beyond Their Natural Range. Forests 2018, 9, 108. [Google Scholar] [CrossRef]
- Von Lüpke, B. Silvicultural methods of oak regeneration with special respect to shade-tolerant mixed species. For. Ecol. Manag. 1998, 106, 19–26. [Google Scholar] [CrossRef]
- Collet, C.; Manso, R.; Barbeito, I. Coexistence, association and competitive ability of Quercus petraea and Quercus robur seed-lings in naturally regenerated mixed stands. For. Ecol. Manag. 2017, 390, 36–46. [Google Scholar] [CrossRef]
- Jurkšienė, G.; Baliuckas, V. Pedunculate and Sessile Mixed Oak Forest Regeneration Process in Lithuania. Forests 2018, 9, 459. [Google Scholar] [CrossRef]
- Banković, S.; Medarević, M.; Pantić, D.; Petrović, N. The National Forest Inventory of the Republic of Serbia-The growing stock of the Republic of Serbia, 1st ed.; Ministry of Agriculture, Forestry and Water Management of the Republic of Serbia-Forest Directorate: Belgrade, Serbia, 2009; pp. 1–238.
- Shaw, M.W. Factors Affecting the Natural Regeneration of Sessile Oak (Quercus petraea) in North Wales: I. A Preliminary Study of Acorn Production, Viability and Losses. J. Ecol. 1968, 56, 565. [Google Scholar] [CrossRef]
- Reif, A.; Gärtner, S. Die natürliche Verjüngung der laubabwerfenden Eichenarten Stieleiche (Quercus robur L.) und Traub-eneiche (Quercus petraea Liebl.)-eine Literaturstudie mit besonderer Berücksichtigung der Waldweide. Wald. Online 2007, 5, 79–116. [Google Scholar]
- Govedar, Z.; Krstic, M.; Keren, S.; Babic, V.; Zlokapa, B.; Kanjevac, B. Actual and Balanced Stand Structure: Examples from Beech-Fir-Spruce Old-Growth Forests in the Area of the Dinarides in Bosnia and Herzegovina. Sustain. J. Rec. 2018, 10, 540. [Google Scholar] [CrossRef]
- Watt, A.S. On the Causes of Failure of Natural Regeneration in British Oakwoods. J. Ecol. 1919, 7, 173. [Google Scholar] [CrossRef]
- Worrell, R.; Nixon, C.J. Factors Affecting the Natural Regeneration of Oak in Upland Britain: A Literature Review; Forestry Commission Occasional Paper 31; Forestry Commission: Edinburgh, UK, 1991; pp. 1–28.
- Ovington, J.D.; Macrae, C. The Growth of Seedlings of Quercus Petraea. J. Ecol. 1960, 48, 549. [Google Scholar] [CrossRef]
- Löf, M.; Gemmel, P.; Nilsson, U.; Welander, N. The influence of site preparation on growth in Quercus robur L. seedlings in a southern Sweden clear-cut and shelterwood. For. Ecol. Manag. 1998, 109, 241–249. [Google Scholar] [CrossRef]
- Widdicombe, R.C. A comparative study of the regeneration of beech (Fagus sylvatica L.) and sessile oak (Quercus petraea (Matt.) Liebl.) in Kent and Cornwall. Arboric. J. Int. J. Urban For. 1999, 23, 125–137. [Google Scholar] [CrossRef]
- Harmer, R.; Boswell, R.; Robertson, M. Survival and growth of tree seedlings in relation to changes in the ground flora during natural regeneration of an oak shelterwood. Forestry 2005, 78, 21–32. [Google Scholar] [CrossRef]
- Krstić, M. Research on Ecological and Production Characteristics of Sessile Oak Forests and Selection of the Best Regeneration Methods in the Area of Northeastern Serbia. Ph.D. Thesis, University of Belgrade-Faculty of Forestry, Belgrade, Serbia, 1989. [Google Scholar]
- Röhrig, E.; Bartsch, N.; von Lüpke, B. Waldbau auf ökologischer Grundlage, 7th ed.; Verlag Eugen Ulmer: Stuttgart, Germany, 2006. [Google Scholar]
- Brezina, I.; Dobrovolný, L. Natural regeneration of sessile oak under different light conditions. J. For. Sci. 2011, 57, 359–368. [Google Scholar] [CrossRef]
- Kanjevac, B. Regeneration of Sessile Oak Forests with the Undergrowth of Accompanying Tree Species in Northeastern Serbia. Ph.D. Thesis, University of Belgrade-Faculty of Forestry, Belgrade, Serbia, 2020. [Google Scholar]
- Babić, V. Impact of Environmental Conditions and Stand Characteristics on the Natural Regeneration of Forests of Sessile Oak (Quercus petraea agg. Ehr.) on Fruška Gora. Ph.D. Thesis, University of Belgrade-Faculty of Forestry, Belgrade, Serbia, 2014. [Google Scholar]
- Babić, V.; Krstić, M.; Govedar, Z.; Todorić, J.; Vuković, N.; Milošević, Z. Temperature and other microclimate conditions in the oak forests on Fruška Gora (Serbia). Therm. Sci. 2015, 19, 415–425. [Google Scholar] [CrossRef]
- Liepe, K. Growth-chamber trial on frost hardiness and field trial on flushing of sessile oak (Quercus petraea Liebl). Ann. Sci. For. 1993, 50, 208s–214s. [Google Scholar] [CrossRef]
- Chaar, H.; Colin, F. Impact of late frost on height growth in young sessile oak regenerations. Ann. Sci. For. 1999, 56, 417–429. [Google Scholar] [CrossRef]
- Krstić, M.; Kanjevac, B.; Babić, V. Effects of extremely high temperatures on some growth parameters of sessile oak (Quercus petraea /Matt./Liebl.) seedlings in northeastern Serbia. Arch. Biol. Sci. 2018, 70, 521–529. [Google Scholar] [CrossRef]
- Tobisch, T. Gap-phase Regeneration of a Central-European Sessile Oak-Hornbeam Forest. South-East Eur. For. 2010, 1, 28–40. [Google Scholar] [CrossRef]
- Mölder, A.; Sennhenn-Reulen, H.; Fischer, C.; Rumpf, H.; Schönfelder, E.; Stockmann, J.; Nagel, R.-V. Success factors for high-quality oak forest (Quercus robur, Q. petraea) regeneration. For. Ecosyst. 2019, 6, 1–17. [Google Scholar] [CrossRef]
- Sikkema, R.; Caudullo, G.; de Rigo, D. Carpinus betulus in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species, 1st ed.; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 74–75. [Google Scholar]
- Bobiec, A.; Reif, A.; Öllerer, K. Seeing the oakscape beyond the forest: A landscape approach to the oak regeneration in Europe. Landsc. Ecol. 2018, 33, 513–528. [Google Scholar] [CrossRef]
- Modrow, T.; Kuehne, C.; Saha, S.; Bauhus, J.; Pyttel, P.L. Photosynthetic performance, height growth, and dominance of naturally regenerated sessile oak (Quercus petraea [Mattuschka] Liebl.) seedlings in small-scale canopy openings of varying sizes. Eur. J. For. Res. 2020, 139, 41–52. [Google Scholar] [CrossRef]
- Tobisch, T. Effects of Artificial Regeneration Methods on Mortality, Growth and Shape of Oak Seedlings in a Central European Oak-Hornbeam Stand. Acta Silv. Lignaria Hung. 2008, 3, 29–38. [Google Scholar]
- Krstić, M.; Kanjevac, B.; Babić, V.; Vasiljević, Ž. Uticaj uslova staništa i sastojinskih karakteristika na preživljavanje i razvoj podmlatka hrasta kitnjaka. [The impact of site conditions and stand characterstics on the survival and growth of young sessile oak trees.]. Šumarstvo 2017, 1–2, 25–42. [Google Scholar]
- Chaar, H.; Colin, F.; Leborgne, G. Artificial defoliation, decapitation of the terminal bud, and removal of the apical tip of the shoot in sessile oak seedlings and consequences on subsequent growth. Can. J. For. Res. 1997, 27, 1614–1621. [Google Scholar] [CrossRef]
- Kohler, M.; Pyttel, P.; Kuehne, C.; Modrow, T.; Bauhus, J. On the knowns and unknowns of natural regeneration of silvi-culturally managed sessile oak (Quercus petraea (Matt.) Liebl.) forests—a literature review. Ann. For. Sci. 2020, 77, 1–19. [Google Scholar] [CrossRef]
- Dobrosavljević, J.; Marković, Č.; Marjanović, M.; Milanović, S. Pedunculate Oak Leaf Miners’ Community: Urban vs. Rural Habitat. Forests 2020, 11, 1300. [Google Scholar] [CrossRef]
- Dobrosavljević, J.; Kanjevac, B.; Marković, Č. Uticaj gala Andricus kollari (Hartig, 1843) (Hymenoptera, Cynipidae) na rast podmlatka hrasta kitnjaka (Quercus petraea (Matt.) Liebl.). [Effects of Andricus kollari (Hartig, 1843) (Hymenoptera, Cynipidae) galls on the growth of sessile oak (Quercus petraea (Matt.) Liebl.) seedlings.]. Šumarstvo 2018, 3–4, 137–152. [Google Scholar]
- Vyskot, M. Pěstění Dubu. [Cultivation of Oak.], 1st ed.; SzN: Prague, Czech Republic, 1958. [Google Scholar]
- Burczyk, J.; Adams, W.T.; Birkes, D.S.; Chybicki, I.J. Using Genetic Markers to Directly Estimate Gene Flow and Reproductive Success Parameters in Plants on the Basis of Naturally Regenerated Seedlings. Genetics 2006, 173, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.-H. Die Natürliche und Künstliche Verjüngung der Eichenarten Quercus Robur und Quercus Petraea; Shaker Verlag: Aachen, Germany, 2001. [Google Scholar]
- Tinya, F.; Kovács, B.; Aszalós, R.; Tóth, B.; Csépányi, P.; Németh, C.; Ódor, P. Initial regeneration success of tree species after different forestry treatments in a sessile oak-hornbeam forest. For. Ecol. Manag. 2020, 459, 117810. [Google Scholar] [CrossRef]
- Thornthwaite, C.W. An Approach toward a Rational Classification of Climate. Geogr. Rev. 1948, 1, 55–94. [Google Scholar] [CrossRef]
- Lang, R. Versuch einer Exakten Klassifikation der Böden in Klimatischer und Geologischer Hinsicht. Internationale Mitteilungen für Bodenkunde 1915, 5, 312–346. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 15 November 2020).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Wagner, H. vegan: Community Ecology Package. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 November 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; p. 213. [Google Scholar]
- Simpson, G.L. ggvegan: ’ggplot2’ Plots for the ’vegan’ Package, R Package Version 0.1-0; 2019. Available online: https://www.rdocumentation.org/packages/ggvegan/versions/0.1-0 (accessed on 15 November 2020).
- Timal, G.; Balleux, P.; Ponette, Q. La régénération naturelle des chênes indigènes en Wallonie: État des lieux et experiences réussies. Forêt Wallonne 2014, 129, 8–18. [Google Scholar]
- Kanjevac, B.; Krstić, M.; Babić, V.; Govedar, Z.; Stajić, S.; Milenković, M.; Milošević, J. The ability of vegetative reproduction of hornbeam in the process of natural regeneration of the sessile oak forests in northeastern Serbia. In Proceedings of the XI International Scientific Agricultural Symposium “AgroSym 2020”, Jahorina, Bosnia and Herzegovina, 8–9 October 2020; pp. 1012–1017. [Google Scholar]
- Petersson, L.K.; Dey, D.C.; Felton, A.M.; Gardiner, E.S.; Löf, M. Influence of canopy openness, ungulate exclosure, and low-intensity fire for improved oak regeneration in temperate Europe. Ecol. Evol. 2020, 10, 2626–2637. [Google Scholar] [CrossRef] [PubMed]
- Kamler, J.; Dobrovolný, L.; Drimaj, J.; Kadavý, J.; Kneifl, M.; Adamec, Z.; Knott, R.; Martiník, A.; Plhal, R.; Zeman, J.; et al. The impact of seed predation and browsing on natural sessile oak regeneration under different light conditions in an over-aged coppice stand. iForest Biogeosci. For. 2016, 9, 569–576. [Google Scholar] [CrossRef]
- Von Lüpke, B. Einfluss unterschiedlicher Hiebsformen auf die Naturverjüngung eines Traub-eneichen-Buchen-Mischbestandes. Influence of various cutting types on natural regeneration of a sessile oak-beech mixed stand. Forstarchiv 2008, 79, 4–15. [Google Scholar]
- Evans, J. Natural Regeneration of Broadleaves; Forestry Commission Bulletin 78 HMSO; Her Majesty’s Stationery Office: London, UK, 1988. [Google Scholar]
- Löf, M. Establishment and growth in seedlings of Fagus sylvatica and Quercus robur: Influence of interference from herba-ceous vegetation. Can. J. For. Res. 2000, 30, 855–864. [Google Scholar] [CrossRef]
- Jarvis, P.G. The Adaptability to Light Intensity of Seedlings of Quercus petraea (Matt.) Liebl. J. Ecol. 1964, 52, 545. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Kramer, P.J.; Pallardy, S.G. The Physiological Ecology of Woody Plants; Academic Press Inc.: San Diego, CA, USA, 1991. [Google Scholar]
- Balandier, P.; Marquier, A.; Casella, E.; Kiewitt, A.; Coll, L.; Wehrlen, L.; Harmer, R. Architecture, cover and light interception by bramble (Rubus fruticosus): A common understorey weed in temperate forests. Forestry 2012, 86, 39–46. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).