Abstract
The successful establishment of many plants in tropical forests often depends on species-specific adaptations related to light availability and forest successional stage. Species that are present in early successional stages generally do not occur in later successional stages. In this study, we documented the diversity, distribution, and abundance of terrestrial invasive plants across the (sub)tropical island of Hainan, China, and tested the germination of specific invasive plants in forested environments. In 97 transects positioned randomly across the island, we found nine invasive herb and shrub species were present in all human-modified habitats but not in intact forest interiors. In separate forest-specific transects, we documented a sharp drop in the abundance of invasive plants >5 m into the forest. High numbers of invasive plant seeds germinated from the soil seed bank sampled at the forest edge, but very few seeds germinated from soil sampled any distance into the forest. Finally, in experiments with four focal invasive plant species, overall germination rates were low; and much lower in shaded sites compared to full gap sites. In conclusion, our findings demonstrated that invasive herbs and shrubs do not yet form a serious threat to native species in the closed-canopy forests of Hainan.
1. Introduction
Biological invasions can have serious environmental and economic impacts (Dennis 2004), for example through reduced forest productivity [1] and disrupted ecosystem services [2]. Nevertheless, biological invasions may also have positive impacts, including economic benefits from the introduction of new food sources for humans [3], improved soil microbial biomass and soil mineralization [4], and positive bottom-up effects on higher trophic levels [5]. Because of the many ways introduced species can impact ecosystem function, understanding the tradeoffs and establishment conditions of invasive species is vital for determining the resilience of vulnerable ecosystems against invasives.
In plant communities, invasive species can have detrimental effects on the native flora, either directly via competition, or indirectly by altering soil chemistry [6], nutrient cycling [7], or community assembly [8]. The mechanisms of plant invasion largely involve two major hypotheses: one based on the invasive ability of the exotic species [9,10,11,12], and another based on the invasibility of the habitat [13,14]. Based on succession theory, most invasive species of forests are pioneers that can only survive in specific early successional stages (likewise for native pioneer species). Pioneer species are heliophilous plants that are generally characterized by rapid growth, shade-intolerance, fast reproductive cycles, and short lifespans (REFS). However, the development of the post-invasion community, especially during subsequent succession in tropical forests, remains largely unknown because of a lack of long-term monitoring of the communities affected by invasive plants [13,14,15].
Globally, there are approximately 289 invasive pioneer species documented to occur in the tropics and subtropics [16,17,18,19]. In general, tropical forests tend to be far less affected by invasive plant species than other forest types [20,21]. Complex forest communities and high connectivity, such as tropical forests, can resist invasion by exotic species through niche occupation and local adaptation [22,23,24,25]. Invasive species are often able to encroach on secondary habitats (e.g., abandoned fields and roadsides), secondary forests and forest gaps [26,27], and they could be well integrated in the local spatial-phylogenetic landscape [28], but few are able to establish within mature forest stands, suggesting that environmental barriers prevent their establishment there [29]. The seeds of exotic plants can reach the forest interior and remain in the soil seed bank, but subsequent germination and seedling growth are limited by environmental factors such as light availability [30,31].
The species richness and abundance of invasive species, both in the vegetation and the soil seed bank, generally decreases rapidly with increased distance from the forest edge [32]. However, the influence of edge effects on plant invasions may be confounded by other factors, such as disturbance, propagule source, physical barriers, and light intensity [29,33]. For example, invasive species can potentially colonize forest interiors following disturbance events [19], suggesting that the presence of invasive species in forest interiors could be limited by the process of forest succession [26]. Therefore, studies focusing on the distribution and abundance of invasive species along the forest succession gradient are necessary to ascertain the ecological limitations of plant invasions.
In 2012, the Chinese Ministry of Agriculture identified 20 invasive plant species as ‘malignant’ (That is, species that have caused huge losses and serious impacts on the economy or the ecological environment at the national level, and the invasion range exceeds more than one natural geographic area), including Chromolaena odorata, Praxelis clematidea, Mimosa pudica, Parthenium hysterophorus, Mikania micrantha, Eichhornia crassipes, Pseudosorghum fasciculare, Sphagneticola trilobata, Conyza sumatrensis, Alternanthera philoxeroides, Lantana camara, Flaveria bidentis, Solanum rostratum, Lactuca serriola, Solidago canadensis, Sicyos angulatus, Ageratina adenophora, Ambrosia artemisiifolia, Cenchrus pauciflorus, Euphorbia dentate [34]. Among these species, Mikania micrantha is an herbaceous vine that can reach the forest canopy and Eichhornia crassipes is an aquatic species found in freshwater wetlands. The remaining species are terrestrial invasive herbs and shrubs (TIHS) found in secondary habitats, but generally not in primary tropical rain forests [34].
In this study, we investigated the presence of TIHS in habitats of various successional stages in order to determine the ecological barriers that prevent the establishment of TIHS in tropical rain forests. Our study consisted of three components: (1) a survey of the diversity and distribution of TIHS across 97 plots distributed randomly across Hainan Island, (2) a study on the distribution of TIHS along forest edge gradients, and (3) an experiment to examine the germination and seedling vigor of TIHS under different canopy openness conditions in the forest interior of the Baiwang-lin Nature Reserve in Hainan Island. Specifically, we aimed to answer the following research questions: (1) Are TIHS present in the interior of tropical rain forests? (2) Does distance from the forest edge limit the occurrence of TIHS? (3) Does canopy shading negatively affect the germination rate and seedling vigor of TIHS?
2. Methods
2.1. Diversity and Distribution of Terrestrial Invasive Herbs and Shrubs in Hainan Island
We surveyed the species diversity and distribution of TIHS in Hainan Island, China, from 2011 to 2014. Hainan Island (33,000 km2) is located at 18°10′–20°10′ N, 108°37′–111°03′ E. A total of 300 locations were randomly plotted on a map of the island. Of these, 203 turned out to be located inside towns, rural houses, or water bodies, and were discarded. The remaining 97 locations used for further study were located in abandoned fields, grasslands, road sides, plantations, rural vegetation, secondary forests, and old-growth forests. At each location, four 30-m transects were established that ran in North, East, South and West directions from a central point. Along each transect, five 2 × 2 m plots were established at 5-m intervals, resulting in a total of 20 survey plots per location. In each 2 × 2 m plot, the presence, number, and coverage of the following nine TIHS were recorded: C. odorata, P. clematidea, M. pudica, P. hysterophorus, P. fasciculare, S. trilobata, C. sumatrensis, L. camara and A. philoxeroides (Table 1).

Table 1.
Study taxa and ecological traits.
2.2. Forest Edge Effects on Distribution and Abundance of Terrestrial Invasive Herbs and Shrubs
During 2014–2015, we surveyed the distribution of TIHS along transects running along the forest edge of tropical rain forest in Baiwang-lin Nature Reserve in Hainan Island (18°52′–19°12′ N, 108°53′–109°20′ E). The Nature Reserve has a circumference road (3–6 m wide) of 26 km and covers an altitudinal range of 250 m to 1265 m. Baiwang-lin Nature Reserve consists of four forest types: rubber plantation, lowland tropical rain forest (low elevation, no clear dominant tree species), hillside tropical rain forest (intermediate to high elevation, dominant species Dacrydium pierrei), and tropical coniferous forest (intermediate to high elevation, dominated by Pinus latteri). We established 13 transects of 250 m length, spaced a minimum of 1 km apart, along forest edges, covering all three natural forest types (i.e., excluding the rubber plantations) (Figure 1). Along each transect we established three belt transects (2 m × 20 m) starting at and perpendicular to the forest edge. Each belt was subdivided into four quadrats (2 m × 5 m) at a distance of 0–5, 5–10, 10–15, 15–20 m from the forest edge. In each quadrat, the presence and abundance of TIHS were recorded, as well as environmental data such as elevation (measured with GPS), distance from forest edge, forest type, and canopy cover (arcsine-transformed). The canopy cover of the quadrat was estimated using a concave spherical crown densiometer (Lemmon, Bartesville, OR, USA). Readings were taken at approximately 1.5 m above ground as the percent cover in the center of the quadrat. While this method of canopy cover estimation can suffer from bias [35], we were mainly interested in an index of relative canopy cover that could be compared between quadrats within this study. A two-sided Pearson correlation test was performed between the species richness and density of TIHS, and each environmental factor.
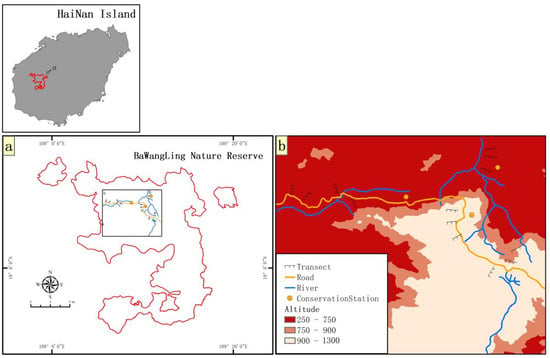
Figure 1.
Location of soil sampling transects in Baiwang-lin Nature Reserve, Hainan Island, China. (a): Baiwang-lin Nature Reserve; (b): soil sample transects (dotted lines). The range in altitude of transects is 250–900 m. 13 transects of 250 m length were established, and along each transect there are three belt transects (2 m × 20 m) starting at and perpendicular to the forest edge.
2.3. Forest Edge Effects on Soil Seed Bank of Terrestrial Invasive Herbs and Shrubs
In each belt transect, five soil samples (volume = 20 × 20 × 10 cm) were taken at 0 m, 5 m, 10 m, 15 m, and 20 m from the forest edge, resulting in a total of 195 soil samples for all transects combined. Each soil sample was then placed in a 30 × 40 × 10 cm plastic frame for germination under full sun light in the greenhouse at Hainan University. Moisture of the soil samples was maintained and seed contamination was prevented by covering the trays with a fine sieve mesh. The number of species emerging was monitored daily and seedlings were removed from the tray once identified. The germination experiment lasted from 11 April to 11 June 2014 (60 days), after which date no new seedlings were recorded. The accumulated frequency of TIHS in the soil seed bank was calculated for each distance class as the number of soil samples with germinated seedlings/total number of soil samples. Seed density was calculated as the number of germinated plants per m2.
2.4. Seed Germination of Terrestrial Invasive Herbs and Shrubs in Forest Gaps
During 2014–2015, we tested the seed germination rate in three forest gaps (I, II, and III, Table 2) in the hillside tropical rain forest at the Baiwang-lin Nature Reserve in Hainan Island. The seeds of C. odorata, P. clematidea, Mimosa diplotricha and L. camara were collected in Bawang-ling, Hainan. In each gap, a total of five seedling plots (1 m × 1 m) were set up: one in the center of the gap, two at the edge of the gap, and two in the understory next to the forest gap. Each seedling plot contained four plastic circular frames (radius = 10 cm, depth = 3 cm). Each frame was filled with local forest soil and filled with 50 seeds of each of the four TIHS species. Frames were watered regularly to avoid drying up of the soil. We recorded seed germination in the frames daily after sowing and continued monitoring the frames for 60 days.

Table 2.
The three study forest gaps in Baiwang-lin of Hainan, China.
2.5. Statistical Analyses
To test if TIHS are present in the interior of tropical rain forests (Q1), we calculated the incidence of nine species of terrestrial invasive herbs or shrubs in different vegetation types on Hainan Island.
To test if distance from the forest edge limits the occurrence of TIHS (Q2), a two-sided Pearson correlation test was performed between the species richness and density of TIHS, and each environmental factor.
To test if canopy shading negatively affected the germination rate and seedling vigor of TIHS (Q3), we ran bivariate correlation analyses and distance correlation analyses using SPSS 19.0 (REF).
The Poisson generalized linear models (GLM) and negative binomial models (NBM) were used to analyze the influence of canopy rate and altitude on exotic plants (Table 3).

Table 3.
The response variables included in the GLM models *.
3. Results
3.1. Species Diversity and Distribution of Terrestrial Invasive Herbs and Shrubs in Hainan Island
Across all 97 sample sites, the nine study species were detected in all habitat types except for the tropical rain forest interior (Table 4). Several TIHS were found in all non-rain forest habitats (e.g., Co, Cs and Lc) and others were found in fewer habitats (e.g., Ap, Pf, and Ph).

Table 4.
Incidence of nine species of terrestrial invasive herbs or shrubs in different vegetation types in Hainan Island, China. For species codes, see Table 1 (+ = present, 0 = absent).
Of all invaded vegetation types, shrub land and forest edges had the lowest frequency of TIHS occurrence, while (abandoned) fields had the highest frequency (Table 5).

Table 5.
Comparison of terrestrial invasive herbs and shrubs frequencies (TIHS/site) among invaded vegetation types (mean ± SE) *.
3.2. Forest Edge Effects on Terrestrial Invasive Herbs or Shrubs
Elevation and canopy cover had a significant negative correlation with TIHS species numbers (Figure 2A,C). In addition, distance to forest edge and canopy closure had a significant negative correlation with TIHS abundance (Figure 2B,D).
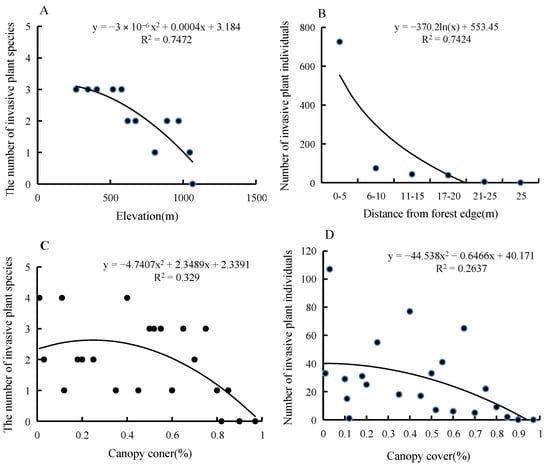
Figure 2.
The relationship between the distribution characters and environmental factors. (A). The relationship between the number of invasive plant species and elevation; (B). The relationship between the number of invasive plant individuals and distance from forest edge; (C). The relationship between the number of invasive plant species and canopy coner; (D). The relationship between the number of invasive plant individuals and canopy cover.
Most TIHS individuals were found in sites 0–5 m from the forest edge, some at 5–10 m, and only a few at 10–15 m, 15–20 m, or 20–25 m (Figure 3A–C).
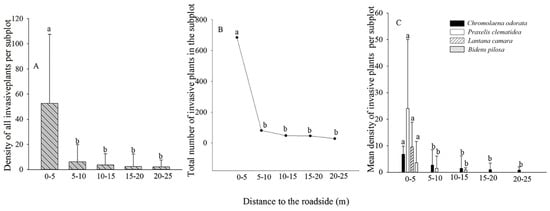
Figure 3.
Abundance of terrestrial invasive herbs and shrubs in relation to distance from forest edge. (A). Density of invasive plants at different distances from forest edge; (B). The number of invasive plants at different distances from forest edge; (C). The density of four invasive plants at different distances from forest edge. Different lowercase letters indicate significant differences (p < 0.05).
A total of 57,138 individual seedlings from 72 plant species were germinated from the 195 soil samples collected from the Baiwang-lin Nature Reserve, among which 1545 seedlings of 12 TIHS were observed (2.7% of all seedlings). The 12 TIHS were P. clematidea, C. odorata, L. camara, M. pudica, M. repens, M. diplotricha, Crassocephalum crepidioides, Tridax procumbens, Bidens pilosa, Spermacoce alata, Ageratum conyzoides, and Stachytarpheta jamaicensis. Among these species, P. clematidea, C. odorata, L. camara, and M. pudica are classified as malignant TIHS in China (REF). The seed germination density of these TIHS ranged from 25 to 7000 seeds m−2 (Table 6). Seed density of the TIHS in the soil seed bank decreased rapidly with distance from the forest edge into the forest interior (Figure 4).

Table 6.
Germination results of the terrestrial invasive herbs and shrubs seeds in the soil seed banks of tropical forest edges.
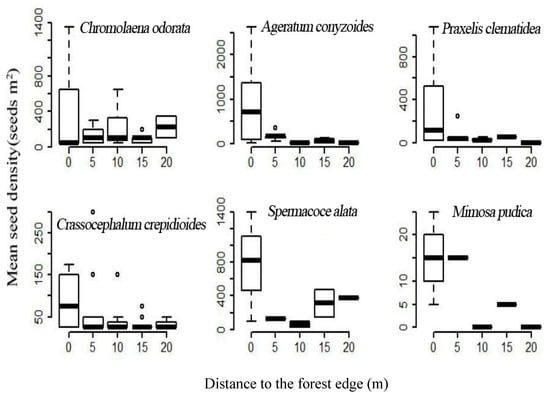
Figure 4.
The relationship between distance from the forest edge (m) and the number of seeds germinated per m2 in our seed germination trails of soil samples collected at different distances from the forest edge.
The best-supported model explaining the canopy cover included a significant effect of altitude (LRT: χ2 = 27.234, df = 1, p < 0.001) and the distance from the forest edges (χ2 = 6.1831, df = 1, p = 0.129) (Table 7). The canopy cover increased significantly as the distance from edge and altitude increased. The second best-supported model had similar AIC to the first model and also included the forest type, though the significance was marginal (χ2 = 5.87, df = 3, p = 0.08). Low-stature forests generally had lower canopy cover than other forest types. Transects in higher areas in the study site usually had a more dense canopy cover (estimate of difference in higher altitude effect for the surveyed transects = 0.551; 95% confidence interval = 0.329, 0.774).

Table 7.
The best-supported models explaining (A) the pooled species richness of exotic species and (B) the pooled abundance of exotic species (C) the presence of exotic plants in the soil seed bank. Parameter estimates and their standard errors are shown, as are standard deviations of model random effects.
For the pooled abundance of alien plants in the soil seed bank, the best-fitting model according to Akaike weights retained the significant effects of the distance from the edge (χ2 = 46.81, df = 1, p < 0.001), and the seed mass of the alien plants (χ2 = 8.682, df = 1, p = 0.003; Table 4), as well as a weak effect of canopy cover (χ2 = 1.802, df = 1, p = 0.179), while the second best model had similar AIC values to the first model and included the influence of altitude besides canopy cover and distance from edge (Table 7). For all the surveyed forests, the pooled abundance of alien species declined markedly with increasing distance from the edge. The seed traits of the exotic plants significantly affected their abundance in the soil seed bank. Light-seeded species were more abundant compared with the large-seeded species. We also found that species with lower seed mass usually have a higher chance of occurrence in the soil seed banks. This may suggest a biological barrier for the large-seeded exotic species to penetrate into the forest interior.
3.3. Seed Germination and Seedling Survival of Terrestrial Invasive Herbs and Shrubs in Forest Gaps
The average germination rate of TIHS was very low, being only 12.1%, 5.9%, and 2.1% in the gap core area (CA), boundary of forest gap (EA), and understory near the forest gap (UA), respectively. The germination rates for all TIHS were higher in the center of the gaps, lower at the edge of the gap, and even lower at the forest interior (Table 4, Figure 5 and Figure 6), except for M. diplotricha. The seed germination of M. diplotricha occurred within 2 to 10 days after sowing, with 31, 21, and 27 seedlings in CA, EA, and UA, respectively. The seed germination of P. clematidea occurred within 5 to 15 days after sowing, with 209, 133, and 37 seedlings in CA, EA, and UA, respectively. The seed germination of C. odorata occurred within 6 to 25 days after sowing, with 120, 23, and no seedlings in CA, EA, and UA, respectively. The seed germination of L. camara occurred within 20 to 30 days after sowing, with two, one, and no seedling(s) in CA, EA, and UA, respectively. All seedlings died within a year.
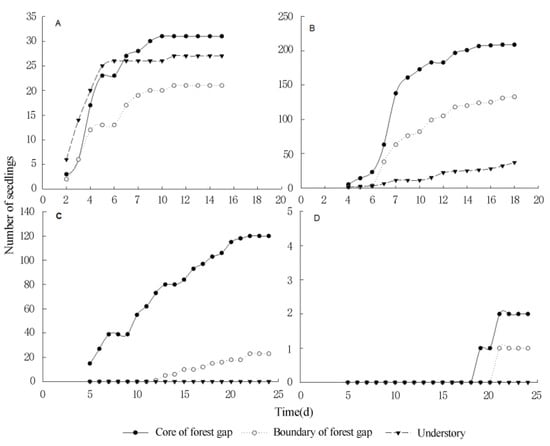
Figure 5.
The germination rates of four species of terrestrial invasive herbs and shrubs in different locations of the forest gap ((A): M. diplotricha, (B): P. clematidea, (C): C. odorata, (D): L. camara).
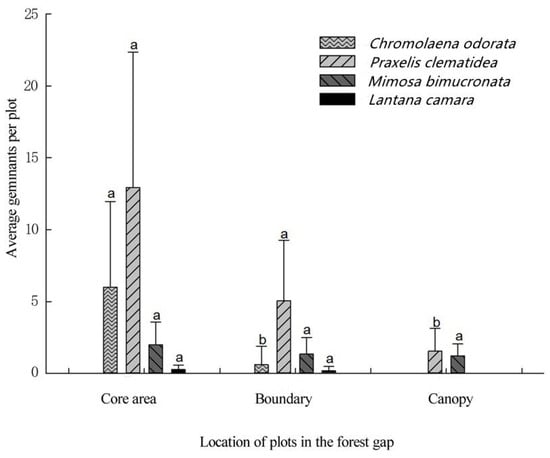
Figure 6.
Comparison of mean germinants per plot of four species of terrestrial invasive herbs and shrubs in three different positions in the forest gap. Different lowercase letters indicate significant differences (p < 0.05).
4. Discussion
Most of the 289 globally recognized invasive species are heliophilous plants with a preference for early successional vegetation [16]. In China, approximately 100 invasive species are known to be heliophilous plants [17]. From a classical forest succession perspective, plants are usually adapted to specific successional stages, i.e., heliophilous pioneer species will generally be replaced by mid- or late-successional species as succession progresses [36,37]. Pioneer species, both invasive and native, occur at early forest successional stages and may occupy a broad range of novel habitats [38]. The presence of these species is limited in mature forests, as they are usually replaced by other species during succession. However, some shade-tolerant invasive species are found in both disturbed and undisturbed habitats. For example, Microstegium vimineum, an exotic annual grass, is highly shade tolerant and found in both open and shady forest [16]. Nevertheless, our results from the randomly assigned survey sites across Hainan Island found no evidence of TIHS in forest interiors, indicating that none of the invasive species presently occurring in Hainan has been able to adapt to late-successional forest. Similar patterns were found for other studies conducted in tropical lowland forests [32].
The absence of TIHS from mature forest has been attributed to the presence of functionally diverse plant communities that are locally adapted and have a strong resistance towards biological invasion [24,39,40]. However, our results indicate that light conditions may be the main factor responsible for the absence of invasives in intact forest understories. Almost all our study species were found near forest edges where light conditions were favorable for them. Additionally, most invasives only managed to establish and maintain themselves under high-light conditions. Even species that did germinate in low-light conditions, did not survive for long unless there was enough light available (such as forest edges and in forest gaps). Therefore, the present set of exotic species in Hainan are unlikely to penetrate into tropical forest interiors. The depth that exotics were able to penetrate into tropical forest edges was associated with forest identity on Hainan, with exotic species concentrated within 5 m of the forest edge in mountain rain forest edges, but up to 15–25 m depth in rubber plantations.
The distribution survey and the germination study both further showed that TIHS grew rampantly in secondary habitats such as abandoned fields and roadsides, less so at forest edges, but were almost completely absent in forests interiors (>20 m from edges into forest). It was evident from our findings that TIHS were excluded from forest interior through low seed germination resulting from high canopy cover. The seed germination rate and seedling vigor of invasive species could be limited by weak light intensity [27,30,31]. Even though moderate germination was observed in our study for some invasive species, all the seedlings eventually died. Therefore, it is essential to consider the relationship between invasive species and forest succession stages when evaluating the environmental destruction of invasive plants.
5. Conclusions
In this study, we demonstrated that in Hainan Island, China, terrestrial invasive herbs and shrubs are widespread pioneer plants but are restricted to gaps in forest habitats. The TIHS mostly colonized and thrived in disturbed environments and their expansion was restricted by forest canopy cover. During forest succession, TIHS are likely replaced by native mid- to late-successional species, further limiting their growth in the understory of tropical rain forests. Thus, TIHS are likely not an immediate threat to the native species of the intact interiors of most tropical rain forests.
Author Contributions
Conceptualization, X.Y. and W.L. (Wenqi Luo); methodology, X.Y.; software, X.L.; validation, C.L., A.K.S.W., W.L. (Wenxing Long) and L.L.; formal analysis, X.L.; investigation, W.L. (Wenqi Luo); resources, X.Y. and W.L.; data curation, L.L.; writing—original draft preparation, W.L. (Wenqi Luo); writing—review and editing, L.L.; visualization, X.Y.; supervision, X.Y.; project administration, D.L.; funding acquisition, D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31460120, 31760170).
Acknowledgments
We thank the National Natural Science Foundation of China for its support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peerbhay, K.; Mutanga, O.; Ismail, R. The identification and remote detection of alien invasive plants in commercial forests: An Overview. S. Afr. J. Geomat. 2016, 5, 49–67. [Google Scholar]
- Walsh, J.R.; Carpenter, S.R.; Zanden, V.M.J. Invasive species triggers a massive loss of ecosystem services through a trophic cascade. Proc. Natl. Acad. Sci. USA 2016, 113, 4081–4085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozlan, R.E.; Newton, A.C. Biological invasions: Benefits versus risks. Science 2009, 324, 1015–1016. [Google Scholar] [CrossRef] [PubMed]
- Meisner, A.; Boer, W.E.; Verhoeven, K.J.F.; Boschker, H.T.S.; Putten, W.H.V.D. Comparison of nutrient acquisition in exotic plant species and congeneric natives. J. Ecol. 2011, 99, 1308–1315. [Google Scholar] [CrossRef]
- David, P.; Thébault, E.; Anneville, O.; Duyck, P.-F.; Chapuis, E.; Loeuille, N. Chapter One—Impacts of Invasive Species on Food Webs: A Review of Empirical Data. Adv. Ecol. Res. 2017, 56, 1–60. [Google Scholar]
- Weidenhamer, J.D.; Callaway, R.M. Direct and indirect effects of invasive plants on soil chemistry and ecosystem function. J. Chem. Ecol. 2010, 36, 59–69. [Google Scholar] [CrossRef]
- Prescott, C.E.; Zukswert, J.M. Invasive plant species and litter decomposition: Time to challenge assumptions. New Phytol. 2016, 209, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Mollot, G.; Pantel, J.H.; Romanuk, T.N. Chapter Two—The Effects of Invasive Species on the Decline in Species Richness: A Global Meta-Analysis. Adv. Ecol. Res. 2017, 56, 61–83. [Google Scholar]
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Webb, C.O.; Salamin, N. Exotic taxa less related to native species are more invasive. Proc. Natl. Acad. Sci. USA 2006, 103, 5841–5845. [Google Scholar] [CrossRef] [Green Version]
- Broennimann, O.; Treier, U.A.; Müller-Schärer, H.; Thuiller, W.; Peterson, A.T.; Guisan, A. Evidence of climatic niche shift during biological invasion. Ecol. Lett. 2007, 10, 701–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catford, J.A.; Baumgartner, J.B.; Vesk, P.A.; White, M.; Buckley, Y.M.; McCarthy, M.A. Disentangling the four demographic dimensions of species invasiveness. J. Ecol. 2016, 104, 1745–1758. [Google Scholar] [CrossRef] [Green Version]
- Davies, K.W. Plant community diversity and native plant abundance decline with increasing abundance of an exotic annual grass. Oecologia 2011, 167, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Ström, L.; Jansson, R.; Nilsson, C. Invasibility of boreal wetland plant communities. J. Veg. Sci. 2014, 25, 1078–1089. [Google Scholar] [CrossRef]
- Koerner, S.E.; Avolio, M.L.; Chang, C.C.; Gray, J.; Hoover, D.L.; Smith, M.D. Invasibility of a mesic grassland depends on the time-scale of fluctuating resources. J. Ecol. 2015, 103, 1538–1546. [Google Scholar] [CrossRef] [Green Version]
- Martin, P.H.; Canham, C.D.; Marks, P.L. Why forests appear resistant to exotic plant invasions: Intentional introductions, stand dynamics, and the role of shade tolerance. Front. Ecol Env. 2009, 7, 142–149. [Google Scholar] [CrossRef]
- Yan, X.L.; Liu, Q.R.; Shou, H.Y.; Zeng, X.F.; Zhang, Y.; Chen, L.; Liu, Y.; Ma, H.Y.; Qi, S.Y.; Ma, J.S. The categorization and analysis on the geographic distribution patterns of Chinese alien invasive plants. Biodivers. Sci. 2014, 22, 667–676. [Google Scholar]
- Sanderson, L.A.; Antunes, P.M. The exotic invasive plant Vincetoxicum rossicum is a strong competitor even outside its current realized climatic temperature range. Neobiota 2016, 16, 1–15. [Google Scholar]
- Syamsuardi, N.Y.; Yulianti, W.; Usman, S. Floristic analysis of alien invasive plant species at some conservation areas in tropical forest of West Sumatra. Der. Pharm. Lett. 2016, 8, 237–245. [Google Scholar]
- Fine, P.V.A. The invasibility of tropical forests by exotic plants. J. Trop. Ecol. 2002, 18, 687–705. [Google Scholar] [CrossRef] [Green Version]
- Denslow, J.S.; Dewalt, S.J. Exotic plant invasions in tropical forests: Patterns and hypotheses. In Tropical Forest Community Ecology; Carson, W.P., Schnitzer, S.A., Eds.; Wiley-Blackwell: Oxford, UK, 2008. [Google Scholar]
- Miguel, J.M.D.; Martín-Forés, I.; Acosta-Gallo, B.; Pozo, A.D.; Ovalle, C.; Sánchez-Jardón, L.; Castro, L.; Casado, M.A. Non-random co-occurrence of native and exotic plant species in Mediterranean grasslands. Acta Oecologica 2016, 77, 18–26. [Google Scholar] [CrossRef]
- Ashbacher, A.C.; Cleland, E.E. Native and exotic plant species show differential growth but similar functional trait responses to experimental rainfall. Ecosphere 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Chen, B.M.; Li, S.; Liao, H.X.; Peng, S.L. Do forest soil microbes have the potential to resist plant invasion? A case study in Dinghushan Biosphere Reserve (South China). Acta Oecologica 2017, 81, 1–9. [Google Scholar] [CrossRef]
- Mavimbela, L.Z.; Sieben, E.J.J.; Procheş, Ş. Invasive alien plant species, fragmentation and scale effects on urban forest community composition in Durban, South Africa. N. Z. J. For. Sci. 2018, 48, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ghulam, A.; Porton, I.; Freeman, K. Detecting subcanopy invasive plant species in tropical rainforest by integrating optical and microwave (InSAR/PolInSAR) remote sensing data, and a decision tree algorithm. ISPRS J. Photogramm. Remote. Sens. 2014, 88, 174–192. [Google Scholar] [CrossRef]
- Klinczar, A.G. The Effect of Treefall Gaps and Propagule Rain on the Spatial Distribution of Four Invasive Plants in a Mature Upland Forest in Maryland; Miami University: Oxford, OH, USA, 2014. [Google Scholar]
- Procheş, Ş.; Forest, F.; Jose, S.; Dominicis, M.D.; Ramdhani, S.; Wiggill, T. How do alien plants fit in the space-phylogeny matrix? PLoS ONE 2015, 10, e0123238. [Google Scholar] [CrossRef] [PubMed]
- Parendes, L.A. Spatial Patterns of Invasion by Exotic Plants in a Forested Landscape; Oregon State University: Corvallis, OR, USA, 1997. [Google Scholar]
- Wijayabandara, S.; Jayasuriya, K.; Jayasinghe, J. Seed Dormancy, Storage Behavior and Germination of an Exotic Invasive Species, Lantana camara L. (Verbenaceae). Int. Res. J. Biol. Sci. 2013, 2, 7–14. [Google Scholar]
- Marques, A.R.; Costa, C.F.; Atman, A.P.F.; Garcia, Q.S. Germination characteristics and seedbank of the alien species Leucaena leucocephala (Fabaceae) in Brazilian forest: Ecological implications. Weed Res. 2015, 54, 576–583. [Google Scholar] [CrossRef]
- Dawson, W.; Burslem, D.F.R.P.; Hulme, P.E. Consistent effects of disturbance and forest edges on the invasion of a continental rain forest by alien plants. Biotropica 2015, 47, 27–37. [Google Scholar] [CrossRef]
- González-Moreno, P.; Pino, J.; Gassó, N.; Vilà, M. Landscape context modulates alien plant invasion in mediterranean forest edges. Biol. Invasions 2013, 15, 547–557. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.Q.; Fu, S.H.; Yang, X.B.; Chen, Y.K.; Zhou, W.; Yang, Q.; Tao, C.; Zhou, W.S. Distribution patterns of alien invasive plants and their influences on native plants of Hainan Island. Chin. J. Plant Ecol. 2015, 39, 486–500. [Google Scholar]
- Cook, J.G.; Stutzman, T.W.; Bowers, C.W.; Brenner, K.A.; Irwin, L.L. Spherical densiometers produce biased estimates of forest canopy cover. Wildl. Soc. Bulletin. 1995, 23, 711–717. [Google Scholar]
- Winkler, E.; Marcante, S.; Erschbamer, B. Demography of the alpine pioneer species Saxifraga aizoides in different successional stages at the glacier foreland of the Rotmoosferner (Obergurgl, Ötztal, Austria). Tuexenia 2015, 35, 267–283. [Google Scholar]
- Liénard, J.F.; Gravel, D.; Strigul, N.S. Data-intensive modeling of forest dynamics. Environ. Model. Softw. 2015, 67, 138–148. [Google Scholar] [CrossRef]
- Oduor, A.M.O.; Leimu, R.; Kleunen, M.V. Invasive plant species are locally adapted just as frequently and at least as strongly as native plant species. J. Ecol. 2016, 104, 957–968. [Google Scholar] [CrossRef] [Green Version]
- Tilman, D. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc. Natl. Acad. Sci. USA 2004, 101, 10854–10861. [Google Scholar] [CrossRef] [Green Version]
- Hooper, D.U.; Dukes, J.S. Dukes J.S. Functional composition controls invasion success in a California serpentine grassland. J. Ecol. 2010, 98, 764–777. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).