Abstract
Heritage trees have important ecological, historical, and landscape values in cities. Rapid urbanization may cause dramatic change of ecosystem functions of cities, thereby inevitably affecting the growth performance of ancient trees. However, few studies have explored their species diversity and spatial differentiation on the medium scale in the scenario of urbanization in China. Here, we took Jiangsu Province in China, with developed economy in recent decades, as a typical case. Based on the provincial forest inventory data, we addressed the abundance, species richness, tree density, and species diversity of ancient trees in 13 cities, and their tree habitat, growth status, and tree age, as well. Then, we compared the spatial differentiation of tree attributes by 13 districts and nine tree habitats. We also applied detrended correspondence analysis (DCA) and redundancy analysis (RDA) to determine the leading factor influencing their distribution pattern. The 7678 heritage trees in Jiangsu belonged to 215 species. More than half of the trees were native with domination by Ginkgo biloba. Villages and farmlands accommodated the most heritage trees while parks and gardens harbored the most species. This indicates that sparsely-populated rural community and scenic areas with open space are conducive to accommodating more urban heritage trees. The tier 3 heritage trees (100–299 years) accounted for about 80% of the total. Overall, most ancient trees in Jiangsu grew well. The species diversity index (H) of 13 cities was between 1.98 and 3.39. The H value among the 13 cities was largely affected by elevation range shift, while the tree density by GDP per capita. DCA showed that the ratio of unique species was >40%, and that dominant species presented little habitat preference. Therefore, species diversity among different cities are affected by climate and topography, as well as human factors. With the accelerating urbanization process, tree habitat, cultural tradition, and urban history should be taken into consideration for management and conservation of heritage trees in the future.
1. Introduction
Heritage trees are essential components of urban ecosystems because they can offer direct and indirect ecosystem services [1,2,3]. As large trees, they, per se, are part of floristic composition in a city, playing an important role in urban forests. Simultaneously, they can provide food, shelter, and living space or create distinct habitats for other plants and animals, especially for some birds and insects [4,5,6].
Heritage trees, at least 100 years old, tend to be large in size: tree height, crown spread, and/or trunk girth [7]. Therefore, they can form the urban landscape, and create an amenity urban environment for human beings [8,9]. For example, ancient trees can provide shade for pedestrians through spreading branches and thick foliage and help them keep from overheating by transpiration during the summer. This is of high importance in densely populated areas of a city. Most ancient trees also have a variety of ornamental value, such as thick trunks, fragrant flowers and fruit, or colorful leaves in different seasons [10]. Take Cinnamomum camphora as an example; it contains a special aroma and volatile oil when in blossom. Another example is Ginkgo biloba, whose green leaves in summer turn into golden yellow in autumn. In addition, its seeds are edible. Therefore, ancient trees in tandem with urban architecture can become a unique landscape unit or a beautiful landmark of the city.
Besides, some ancient urban trees can be seen as a witness of city’s vicissitudes or regarded as mascots on which people place their hope and feeling [11,12].
Currently, the studies concerning urban ancient trees cover a variety of themes, such as field investigation [13,14,15], spatial distribution [7,16], rejuvenation and pest control [17,18,19], and protection legislation [20,21,22], as well as the aesthetic and spiritual aspects of ancient trees [23,24]. Among them, most studies have focused on species diversity and spatial differentiation [7,25]. According to different dimensions, the current studies can be roughly divided into three major categories. On a large scale (i.e., national or international level), it seems reasonable to determine the factors contributing to the distribution pattern because of a wide variation of climate [26]. However, due to required data being hard to obtain, such studies are likely conducted using indirect data collected from diverse sources. Accordingly, it is difficult to ensure the accuracy of such research. On a small scale (i.e., county or city level), there have been a number of studies of heritage trees by field investigation [1,8,12]. Nevertheless, most of them only provide a simple description of heritage trees therein [27]. More importantly, these studies applied different methods, thereby making it difficult to compare distribution pattern of ancient trees in different areas. In contrast, there is little research on ancient trees on the medium scale (i.e., provincial or regional level) [10,28,29]. However, studies at this scale can better reveal the spatial differentiation of ancient urban trees in distinct civic space.
A growing body of work has been carried out on reporting the species diversity and spatial distribution of urban heritage trees in a certain region recently. However, little is concerned with the underlying factors [30,31,32]. Generally, the factors influencing spatial distribution can be divided into two categories, including natural and human factors. The former includes longitude, latitude, topography (e.g., average elevation and elevation range shift) [31], and climate (e.g., mean annual temperature, mean annual precipitation, mean temperature of the warmest month, and mean temperature of the coldest month) [33]. The latter (i.e., anthropogenic influence) can be characterized by GDP per capita and population density [7,34]. For example, a recent study demonstrated that the tree density of old ginkgos in China increased significantly with growth of population density and GDP [16]. Therefore, as urban ancient trees exist for a long time in cities, their diversity and distribution pattern may be comprehensively affected by natural geographical conditions and social human factors, especially in areas with rapid population and urban growth.
Eastern China has witnessed rapid economic development in recent decades. With the increase of urban population, rapid and sustained urbanization may cause dramatic change of ecosystem functions of cities [35,36], thereby inevitably affecting the survival, distribution, and growth of ancient trees. As an eastern–central coastal province of China, Jiangsu has experienced rapid social and economic development over the past four decades. Jiangsu is the third smallest in land area, but the fourth most populous and the most densely populated of the 23 provinces of China, according to the seventh national census. In the past few decades, Jiangsu has witnessed a high level of urbanization. The urban population of Jiangsu rose from 15.2% in 1980 to 73.4% in 2020. Jiangsu had the highest GDP per capita among 31 Chinese provinces and second-highest GDP of those provinces, only behind Guangdong in 2020 [37]. Among China’s economic hundred counties in 2020, Jiangsu had 25 of them, accounting for 25%. In addition, each of the 13 cities in Jiangsu has had top counties (i.e., top 100 economic counties of China) in recent years.
The rapid economic development and urban population explosion have caused pressure on local environment and urban heritage trees [12]. Besides, different city histories also affect the preservation and growth of ancient trees. Accordingly, the diversity and distribution of urban ancient trees may be affected by multiple aspects such as climate, geography, population, economic, social, and city history in the process of urbanization.
Here, we took Jiangsu Province in eastern China, which has experienced rapid and sustainable economic development in recent decades, as a typical case. According to provincial forest inventory data, we assessed the abundance, species richness, distribution pattern, and driving factors of heritage trees in this area. Specifically, the object of our study is to: (1) characterize overall species richness and diversity; (2) explore the spatial distribution patterns by cities and tree habitats; (3) determine the leading factor (i.e., climate, geography, and anthropogenic interferences) influencing the distribution pattern; (4) inform scientific baseline and recommendations for management and conservation of heritage trees in Jiangsu and other provinces in eastern China.
2. Methods
2.1. Study Area
Jiangsu Province (30°45′–35°08′ N, 116°21′–121°56′ E) covers 107,200 km2 of land area. It is located in the central part of the eastern coast region of China. Jiangsu borders Shandong in the north, Anhui to the west, and Zhejiang to the south, and is on the west side of the Yellow Sea. Its landform is mainly plain with multitudinous lakes, and the highest elevation is 624.4 m. It has a warm, temperate humid monsoon climate in the north and subtropical humid monsoon climate in the south. The annual average temperature is 13–16 °C, and the annual average precipitation is 998.5 mm [38]. The main soil types of Jiangsu from north to south are brown soil, leached cinnamon soil, yellow–brown soil, and red–yellow soil. The province covers a forest area of 15,600 km2, with 24.0% forest coverage rate. Geographically, it can be divided into three subregions: southern Jiangsu (S. Jiangsu), central Jiangsu (C. Jiangsu), and northern Jiangsu (N. Jiangsu), with 13 prefecture-level cities in total (Figure 1). These cities’ key information is summarized in Table 1 [37].
Figure 1.
Sketch map of study area of heritage trees in Jiangsu Province, eastern China.

Table 1.
Land area, population density, and GDP per capita of 13 cities in Jiangsu Province, eastern China.
2.2. Study Method
Our analysis was based on provincial forest inventory data of field surveys from 2016 to 2020 in Jiangsu Province, which was downloaded from Cloud Platform for Forest Genetic Resources Information of Jiangsu Province (with restricted access). The investigations of ancient trees were made following “Technical Guidelines for Document Establishment of General Survey of National Ancient-Famous Trees”. It was issued by the State Forestry Bureau in 2001 and has been widely used in China recently [7,12]. Based on tree ages, three protection categories were classified as tier 3 (100–299 years of age), tier 2 (300–499 years of age), and tier 1 (≥500 years of age).
We collated the data of these heritage trees, including their coordinates, diameter at breast height (DBH), tree age, growth status, geographical origin, photos, etc. Firstly, botanical names and taxonomic classifications of all species were identified following the Flora of China [39] and the Catalogue of Life China: 2021 Annual Checklist (accessed on 30 September 2021). We also checked their species name. For example, the ancient tree was identified as Zelkova schneideriana in Nantong, but we corrected its name to Celtis sinensis according to its photos and morphological description. According to DBH and tree growth characteristics, we first checked the tree age of each individual, and then removed the records of such trees less than 100 years old. The remained trees were divided into four categories as “good”, “fair”, “poor”, and “dying” by the description of growth status (Table 2) [7,8]. Moreover, nine tree habitat types were classified based on the detailed records of ancient trees’ growing sites (Table 3) [1,12]. For geographical origin of species, natives refer to the species naturally occurring in Jiangsu Province while exotics refer to the species cultivated in Jiangsu (Table A1). In addition, a total of 12 ancient trees were deleted because they were recorded as dead, and no further statistical analysis was performed.

Table 2.
Gradients division of growth status of heritage trees in Jiangsu Province, eastern China.

Table 3.
Nine tree habitat types which accommodate heritage trees were identified in Jiangsu Province, eastern China.
2.3. Data Analysis
The species diversity of heritage trees was assessed by Shannon–Wiener index (H), which was calculated as follows:
In the formula, pi is the proportion of individuals of species i in the sum of individuals [40].
Tree density (D) was selected as indicator reflecting the distribution pattern of 13 cities in Jiangsu Province:
For each city, N is the number of heritage trees and A is the city land area. The formula could avoid the influence of area effects on tree density [41,42].
Because of the sample size (n = 13) being small and not normally distributed, we conducted Spearman rank correlation analysis between the density and number of ancient trees in 13 cities, and then used Mann–Whitney U-test to compare the difference between them.
The distribution pattern and influencing factors of heritage trees were investigated by ordination analysis. We built a habitats × species matrix, analyzing the species composition of ancient trees in different habitats through detrended correspondence analysis (DCA) [12,43]. To understand the relationship between species diversity/tree density and environmental variables, ten factors were selected as explanatory variables to build up the matrix. Those factors can be categorized into three types representing four geographic (i.e., longitude, Long, °; latitude, Lat, °; average elevation, ELE, m; and elevation range shift, ELR, m), four climatic (i.e., mean annual temperature, MAT, °C; mean annual precipitation, MAP, mm; mean temperature of the warmest month, MWMT, °C; and mean temperature of the coldest month, MCMT, °C), and two anthropogenic variables (i.e., GDP per capita, GDPpc, $; population density, PD, person/km2). These data were obtained from the Bureau of Statistics of Jiangsu Province (http://tj.jiangsu.gov.cn/col/col80733/index.html, accessed on 30 August 2021). In the study, the applicability of different ordination models was determined by the first ordination axis of DCA. Response data had a gradient <3 SD units long, so redundancy analysis (RDA) was recommended. Explanatory variables were converted by lg(x + 1) to eliminate the difference between dimensions. Collinear variables were eliminated based on forward selection, while the significance of each factor was calculated by using a total of 999 Monte Carlo permutation tests [44].
Data collation and analysis was carried out with Excel 2019, DCA and RDA were performed using the Canoco 5.0 software [45], and bar charts were drawn using Origin 2021.
3. Results
3.1. Species Composition and Growth Status
We obtained 7678 records of heritage tree individuals and identified 215 species belonging to 129 genera and 64 families across 13 cities in Jiangsu Province, China (Table A1). The species were grouped in frequency classes as dominant (over 100 trees per species), common (10 ≤ trees ≤ 100), rare (2 ≤ trees ≤ 9), and solitary (only one tree). Only 12 dominant species were among the four categories, accounting for 5.58% of all species. Ginkgo biloba achieved supremacy with 2566 individuals and highest proportion at 33.29%, which was the most typical ancient tree species with an absolute numerical advantage in Jiangsu Province. Except the dominant species, the other three categories had similar proportion of species for about 30% per type.
As shown in Table 4, Rosaceae, Cupressaceae, Fagaceae, and Sapindaceae were the top four families, including 55 species, which accounted for 25.58% of the total species. In contrast, the dominant genera were not pronounced, and the largest genera Acer only had six species, representing 2.79% of the total species. In Jiangsu, the majority of the heritage trees were native species (i.e., 119), which accounted for 55.35%, whereas the 96 exotics accounted for 44.56% of total species.

Table 4.
The top-ranking ten families and genera with number of heritage tree species in Jiangsu Province, eastern China.
In terms of tree age, there were 6110 (79.58%) heritage trees of tier 3 (i.e., the youngest category) corresponding to 208 species, accounting for 96.74% in Jiangsu Province. Compared with tier 3, there were fewer trees classified as tier 1 and 2 with aggregate species of 82 and individuals of 1568, contributing 38.14% of species and 24.42% of heritage trees (Figure 2).
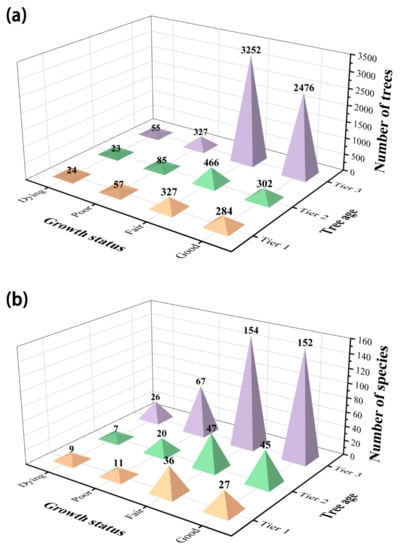
Figure 2.
The counts and species of heritage trees in Jiangsu Province, eastern China: (a) number of trees; (b) number of species across four growth status and three tree ages.
The growth status of ancient trees in Jiangsu Province can be divided into four types: “good” (3062 trees), “fair” (4045 trees), “poor” (469 trees), and “dying” performance (102 trees), accounting for 39.88%, 52.68%, 6.11%, and 1.33% of the whole heritage trees, respectively. In terms of species, the number of “fair” performance was the largest, amounting to 160 species (74.42%). Collectively, the ancient trees in Jiangsu grew well (Figure 2).
3.2. Spatial Distribution and Differentiation by Cities
There was a considerable difference in the distribution of heritage trees among 13 cities of Jiangsu Province. Of them Suzhou ranked the first with 1734 ancient trees (22.58% in total), and far more than the other cities (Figure 3). In terms of species, there were 94 species (43.72%) of ancient trees in Wuxi, followed by Suzhou with 92 species (42.79%) (Figure 3). Conversely, Yancheng had the smallest number of individuals (144 trees, 1.88%) and species (27 species, 12.56%) of heritage trees.
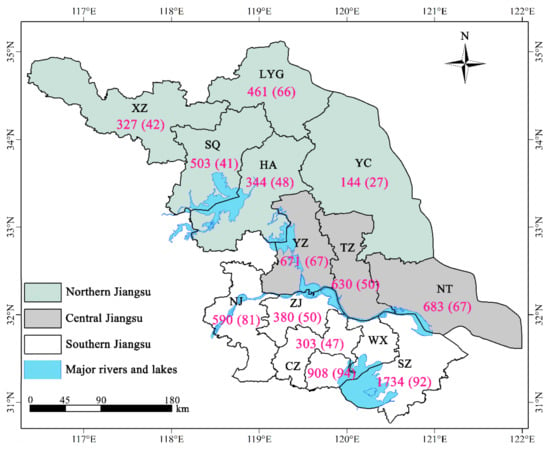
Figure 3.
The distribution map showing number and species of heritage trees by 13 cities in Jiangsu Province, eastern China. For each city, the first number on the map indicates individuals, while the second indicates species of heritage trees. Refer to Table 1 for the meaning of the abbreviated cities.
The species diversity index (H) of 13 cities was between 1.98 and 3.39, with the average of 2.77. In light of H, the first three cities were Lianyungang (H = 3.39), Nanjing (H = 3.30), and Huai’an (H = 3.18) (Figure 4a). There was a significantly positive correlation between the tree density of 13 cities and number distribution (r = 0.989, p = 0.000 < 0.01; Z = -3.821, p = 0.000 < 0.01). Suzhou had the largest tree density (D = 440.40), while Yancheng had the lowest (D = 34.05) (Figure 4b).
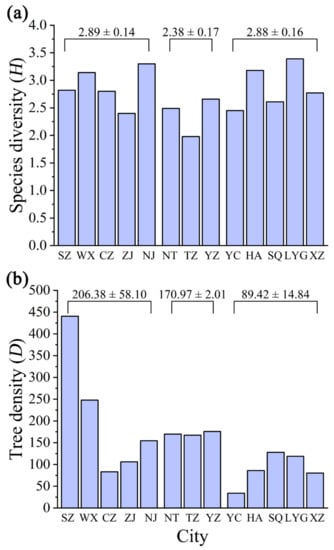
Figure 4.
Comparison of spatial distribution by 13 cities in Jiangsu Province, eastern China: (a) species diversity and (b) tree density. The different data above the bars in (a,b) indicate the mean ± standard error of southern Jiangsu (including SZ, WX, CZ, ZJ, and NJ), central Jiangsu (NT, TZ, and YZ) and northern Jiangsu (YC, HA, SQ, LYG, and XZ), respectively. Refer to Table 1 for the meaning of the abbreviated cities.
As shown in Figure 5a, the number of four growth states of heritage trees varied greatly in each city. Suzhou had the most heritage trees with “good” growth (i.e., 1101 trees) while Nantong had the most trees with “fair” growth (i.e., 594 trees). For each city, the sum of “good” and “fair” was greater than 90% of the total number of trees, whereas there were very few “poor” and “dying” trees therein (Figure 6a).
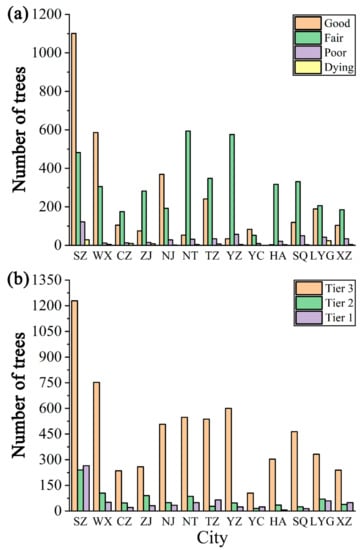
Figure 5.
Heritage trees’ abundance by growth status (a) and tree age (b) in 13 cities of Jiangsu Province, eastern China. Refer to Table 1 for the meaning of the abbreviated cities.
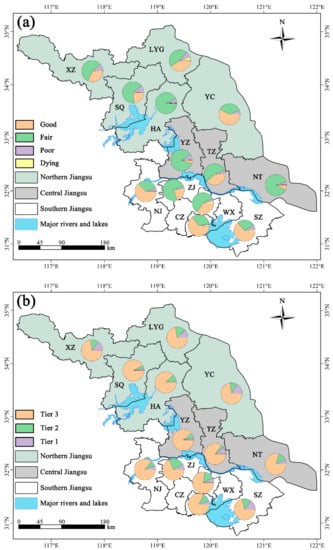
Figure 6.
Proportion of growth status (a) and tree age (b) of heritage trees by 13 cities in Jiangsu Province, eastern China. Refer to Table 1 for the meaning of the abbreviated cities.
Similarly, the age distribution also varied a lot in each city, and the number of tier 3 heritage trees was the largest for each city. Suzhou had the largest number of tier 3 trees (i.e., 1229 trees), followed by Wuxi (i.e., 752 trees), and Yangzhou (i.e., 600 trees), respectively (Figure 5b). For each city, the proportion of tier 3 heritage trees was more than 60%. Suqian had the highest proportion of tier 3 trees, while Zhenjiang had the lowest (68%) (Figure 6b).
3.3. Spatial Distribution and Growth Performance by Tree Habitats
In terms of their number and species, heritage trees were unevenly distributed in different tree habitats. In Jiangsu Province, VF (30.58%) and PG (24.26%) had the first two highest tree counts, followed by RC (14.67%) and GC (14.34%). The number of species distributed in the PG habitat was 149 (69.30%), and 106 species were found in VF habitat ranking the second. GC ranked third with 104 species, while RC ranked fourth with 92 species. RS had the least 108 trees (1.41%) and 26 species (Figure 7a). As expected, PG had the highest biodiversity in light of Shannon–Wiener index, followed by WP and GC (Figure 7b).
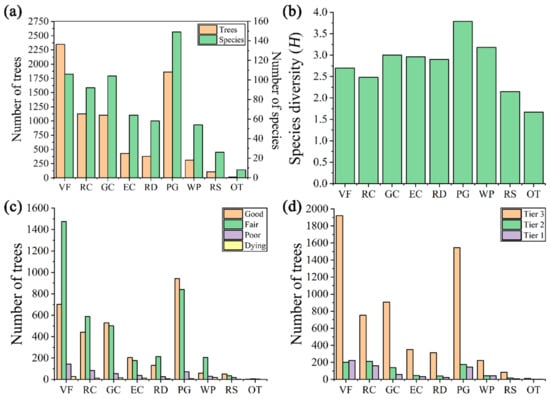
Figure 7.
Comparison of spatial distribution by nine tree habitats in Jiangsu Province, eastern China: (a) number of trees and species, (b) species diversity index, (c) abundance by growth status, and (d) abundance by tree age. Refer to Table 3 for the meaning of the abbreviated tree habitats.
On the whole, the growth states of ancient trees were mostly “good” and “fair” in different habitats. The distribution of “fair” ancient trees was the largest in the VF habitat, while the PG had the largest number of “good” trees (Figure 7c). Of the nine habitat types, only PG habitat had more than 50% of “good” performance heritage trees (i.e., 50.51%). In each habitat type, “good” and “fair” performances were dominant. Except for OT habitat (71.43%), the sum of “good” and “fair” was greater than 80% in the other eight habitat types.
The age distribution of ancient trees varied largely in terms of habitats. The number of tier 3 heritage trees was the largest in each habitat type. VF had the most tier 1 ancient trees, while RC harbored the most tier 2 ancient trees (Figure 7d). In contrast, ancient trees over 300 years old had a higher proportion in RC and WP.
3.4. Influencing Factors
Species spatial distribution by tree habitats was illustrated in Figure 8. Unique species were found in seven habitats except roadsides and others. Firstly, PG had the most unique species with 41 species. For example, all 17 of Carya illinoinensis (No. 94) were restricted to PG, and the species was a “common” species in this study. Secondly, there were 18 unique species in GC, and then 17 unique species, including the nine counts of Juniperus procumbens (No. 19), four of Pyrus calleryan (No. 115), and three of Buxus bodinieri (No. 47), were restricted to VF. Ginkgo biloba (No. 2), Cinnamomum camphora (No. 31), Chaenomeles sinensis (No. 105), and Celtis sinensis (Mo. 136) were found in all nine habitats. These species were “dominant” or “common” species in Jiangsu Province. The other nine dominant species (e.g., Juniperus chinensis, No. 16; Zelkova schneideriana, No. 132; Osmanthus fragrans, No. 195, etc.) were found in most habitats.
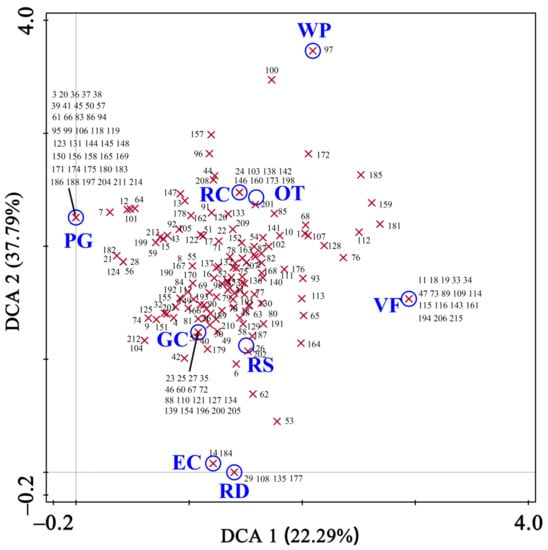
Figure 8.
The first two axes of the DCA ordination of nine tree habitats and heritage tree species composition in Jiangsu Province, eastern China. Tree habitat types are presented as circles, and species as crosses. Refer to Table 3 for the meaning of the abbreviated tree habitats, and Table A1 for the numerical order of species names.
Secondly, there were 18 unique species in GC, and then 17 unique species, including nine of Juniperus procumbens (No. 19), four of Pyrus calleryana (No. 115), and three of Buxus bodinieri (No. 47) were restricted to GC.
Ginkgo biloba (No. 2), Cinnamomum camphora (No. 31), Chaenomeles sinensis (No. 105), and Celtis sinensis (Mo. 136) were found in all nine habitats. These species were “dominant” or “common” species in Jiangsu Province. The other nine dominant species (e.g., Juniperus chinensis, No. 16; Zelkova schneideriana, No. 132; Osmanthus fragrans, No. 195, etc.) were found in most habitats.
Nine factors were selected to build an RDA model, which explained 84.1% of the total variation. In this model, axes 1 and 2 explained 84.06% and 15.94% of the total variance, respectively. By screening, ELR was the strongest (F = 8.4) and only significant (p = 0.01) explanatory variable for the species diversity variation, which explained 43.2% of the total variation, and accordingly was the most important factor affecting the species diversity of heritage trees in Jiangsu. The strength (F-value) of all other drivers included in the RDA was obviously lower, which, ranked by strength, were ELE (F = 3.9) > PD (F = 1.0) > MAP (F = 0.5) > Lat (F = 0.3) > MWMT (F = 0.2) > MCMT (F = 0.2) > GDPpc (F = 0.2), while MAT explained very little (F < 0.1) of the additional variation (Figure 9a).
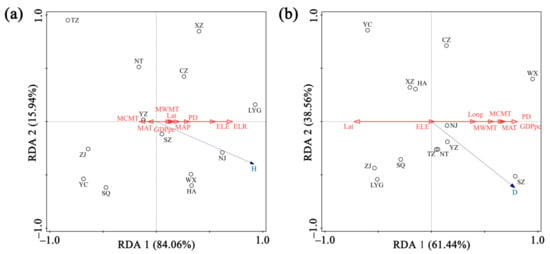
Figure 9.
Redundancy analysis between species diversity (a), tree density (b), and environmental variables, respectively, in Jiangsu Province, eastern China. Blue dashed arrows represent different types of response variables. Red solid arrows represent different environmental factors mentioned in the text. Hollow circles represent the 13 studied cities in Jiangsu. Long: longitude, Lat: latitude, ELE: average elevation, ELR: elevation range shift, MAT: mean annual temperature, MAP: mean annual precipitation, MWMT: mean temperature of the warmest month, MCMT: mean temperature of the coldest month, GDPpc: GDP per capita, PD: population density. Refer to Table 1 for the meaning of the abbreviated cities.
First, ELR explained 51.4% of the model, and then MWMT explained 14.4% of all. The two factors accounted for a total of 57.6% of the variation (68.5% of the model). For MCMT and MAT, RDA identified negative associations with species diversity along the constrained axes.
Eight of the ten factors were selected to generate a better model, which together explained 61.4% of the total variation. The first and second axes accounted for 61.44% and 38.56%, respectively. GDPpc (F = 7.4), PD (F = 7.4), and Lat (F = 5.3) were the three principal explanatory variables of the tree density variation (P < 0.05), respectively, explaining 40.1%, 3.6%, and 1.3% of all. The other factors included in the RDA were ranked by F-value from greatest to least: MAT (F = 4.7) > MCMT (F = 4.3) > MWMT (F = 2.9) > Long (F = 1.3), meanwhile ELE explained very little (F < 0.1) (Figure 9b).
In this model, GDPpc first explained 65.3% of the model, and MCMT explained 8.6% of all. However, no significant correlation existed between MCMT and tree density. There was negative association between Lat and the tree density along the constrained ordination axes.
4. Discussion
4.1. The Heritage Tree Diversity Assessment
As a representative province of rapid urbanization in the eastern coastal areas of China, Jiangsu had 7678 heritage trees belonging to 215 species. The number of heritage trees in Jiangsu is higher than that in Shandong Province (7179 trees) to the north of Jiangsu [15], but much lower than that in Zhejiang Province (65,067 trees, 338 species, 2001; 213, 700 trees, 459 species, 2005) to the south of Jiangsu [13,14].
Jiangsu is mainly characterized by a wide plain and low mountain in topography. Meanwhile, there are many lakes and rivers therein. At the same time, Jiangsu is located in a transition zone between the temperate and subtropical climate [46]. The topography and climate provide distinct microhabitats and hydrothermal conditions for the growth of urban trees. In addition, Jiangsu has the second highest GDP of 31 provinces in mainland China, which provides an economic foundation for the protection of urban heritage trees.
The review of current studies indicates that various indexes have been applied to evaluate the heritage tree diversity in different regions. Generally, they include single index (i.e., number, species, density, etc.) [28,32,47] and composite index (i.e., importance value, Shannon–Wiener index, evenness index, etc.) [1,12]. There is no uniform approach for investigating ancient trees in practice, and, furthermore, such surveys are conducted by different scholars at different time. Accordingly, it is difficult to ensure data accuracy when comparing ancient trees of different areas. For example, Yu (2001) reported that Zhejiang had 65,067 heritage trees of 338 species [13], whereas Du et al. (2005) documented that it had 213,700 trees of 459 species [14]. For reasons given above, it seems difficult to compare the ancient trees among different provinces or large cities.
In the current study, all the data and materials were obtained from provincial forest inventory data, which ensures that the 13 cities have consistent standards and methods for ancient tree survey. Based on the raw data, we then checked each item of heritage trees (e.g., species name, tree habitat, locality, and tree age) so that the reliability of analysis data can be guaranteed in this study.
To our knowledge, this is the first time that species diversity and distribution patterns of heritage trees have been evaluated by a scientific and unified method on the provincial scale. This study can provide a baseline for the protection of ancient trees in Jiangsu Province and offer a scientific reference for heritage tree investigations in other provinces.
4.2. Spatial Pattern of Heritage Trees in Jiangsu Province
There is a significant difference in distribution of heritage trees in Jiangsu Province by cities or by habitats. The results indicate that trees, species, and density of ancient trees declined from south to north (Figure 3 and Figure 4b), mainly resulting from climatic conditions. Compared to C. or N. Jiangsu, S. Jiangsu has the more subtropical humid monsoon climate, with higher mean annual temperature and more precipitation, which may be more suitable for the survival of heritage trees. Many studies have confirmed that ancient trees can better tolerate heat than drought [31,48,49,50], especially in dry or hot seasons. The Shannon–Wiener diversity index (H) in S. Jiangsu is similar to that in N. Jiangsu, but each of them is higher than that in C. Jiangsu. This may be largely due to different topography. Either S. or N. Jiangsu has relatively higher mountain areas than C. Jiangsu, which mainly comprises plains. Therefore, both S. and N. Jiangsu may harbor much richer tree species than C. Jiangsu, thereby providing the abundant species pool for heritage trees.
Collectively, the distribution pattern of heritage trees of the 13 cities presents a consistent declining trend of three regions from south to north in Jiangsu. However, there is a considerable variation in urban tree number among the 13 cities. Take Nanjing (in S. Jiangsu) as an example: it has more counts of heritage trees than in Yancheng (in N. Jiangsu), but less than in Nantong (in C. Jiangsu). In fact, the differences in urban history, economy level, and cultural tradition play a significant role in urban planning and landscape greening, and they may further affect the abundance, species, and diversity of urban heritage trees [7,51]. Jiangsu has a variety of regional cultures. For example, Lianyungang, Yancheng, and Nantong, bordering the Yellow Sea, boasted navigation and salt-making technology in history, thus developing Marine Culture. In contrast, Changzhou, Wuxi, and Suzhou, surrounding Tai Lake, have been well developed in agricultural and industrial production, thus developing Wu Culture [52]. Wu Culture is characterized by garden construction, which will certainly benefit the bequeath of local heritage trees. Our results demonstrate that species diversity and density of heritage trees in 13 cities of Jiangsu Province may be shaped by different types of influencing factors.
Redundancy analysis (RDA) indicated that diversity of heritage trees was mainly influenced by elevation range shift (ELR) among 13 cities of Jiangsu (Figure 9a). Elevation range shift could be used to reflect habitat heterogeneity [53,54]. Different trees species have distinctive characteristics, thereby affording them extensive adaptability for temperature, water, and elevation ranges. In a certain area, habitat heterogeneity increases with ELR, which can contribute to a greater variety of microhabitats for accommodating more trees [55,56]. Furthermore, there is no remarkable difference in climatic conditions at small and medium geographic scales. Accordingly, topographic heterogeneity is considered as a leading factor influencing distribution pattern of species diversity [57]. Thus, ELR becomes a key factor shaping the spatial differentiation of ancient tree diversity at the medium scale of distribution area.
Unlike species diversity, the heritage tree density of Jiangsu was largely shaped by anthropogenic factors (i.e., GDP per capita, GDPpc; population density, PD) and latitude (Lat) (Figure 9b). This is similar to the results of other studies, in which the density of heritage trees increased with population density [16,49]. GDPpc is the most significant of the three factors. Generally, the higher the GDPpc a city has, the higher its tree density is. In terms of GDPpc, the first two cities are Suzhou and Wuxi, and their corresponding tree densities are also ranked as the top two of the 13 cities (Figure 9b). We notice that in S. Jiangsu, with higher GDPpc where urban residents usually have higher conservation awareness of ancient trees, they may not destroy the urban trees. On the contrary, they are willing to protect trees from insect attack, plant disease, and lightning strikes. Studies have confirmed that higher earners are likely to pay more than lower ones for urban ancient tree conservation [58]. Meanwhile, the local governments in economically developed cities will invest more in the protection of ancient trees. In addition, with the development of economy, citizens expect to have a more comfortable and better environment. Indeed, heritage trees are conducive to improving environmental quality, particularly in densely populated urban areas. Suzhou boasts a developed economy and has high population density. Accordingly, it has the largest density of ancient trees among the 13 cities. Indeed, Suzhou’s abundant heritage trees are also linked to classical gardens and city history. Suzhou was founded in 514 BC, with a history of more than 2500 years. There are nine classical Chinese gardens (i.e., Humble Administrator Garden, Lingering Garden, Pavilion of Surging Waves, etc.), which have been listed in the World Cultural Heritage by the United Nations [37]. The ancient trees within these gardens complement city buildings and satisfy people’s pursuit for beauty.
Among the nine tree habitats in Jiangsu Province, PG, VF, GC, and RC had the highest numbers and species of heritage trees (Figure 7a and Figure 10b,d,e). DCA showed that over 40% of the 215 species were unique species, which indicates that the relationships between species and habitats is selective. Meanwhile, the four high-diversity habitats predominate with 84 unique species (Figure 8). For example, PG provides a tree habitat with more open, large, and natural conditions, which can allow heritage trees to flourish with enough room and less stressful environment. Furthermore, this habitat is often used to introduce and cultivate unusual species for ornamental, economic, scientific, and other purposes [59]. For twelve dominant species of heritage trees in Jiangsu, they can be found in most of the nine habitats, indicating that they have no obvious preference for tree habitats. Nine of these dominant species are native, while the other three are exotic. All the three species (i.e., Ginkgo biloba) have a long history of cultivation in Jiangsu Province (Figure 10f) [7]. This indicates that all of the 12 species may have adapted to different tree habitats in Jiangsu Province for a long time. In addition, the other reason may be persistent cultivated preference of tree managers [10]. Ginkgo biloba is extensively cultivated as ornamental plants or edible nuts in Taizhou, which is known as the Ginkgo Village in China [7]. Zelkova schneideriana and Cinnamomum camphora are common ornamental tree species in the 13 cities of Jiangsu (Figure 10a,g). Castanea mollissima has sweet and edible fruit which can be eaten directly or used as an ingredient in Chinese dishes. Nevertheless, Styphnolobium japonicum is often endowed with religious and cultural values in traditional Chinese stories.

Figure 10.
Photographs of main heritage trees and their tree habitats in Jiangsu Province, eastern China. (a) Zelkova schneideriana in the school (GC); (b) Celtis sinensis in the government (GC); (c) Gleditsia sinensis in the roadside (RS); (d) Podocarpus macrophyllus in the temple (RC); (e) Ulmus parvifolia in the park (PG); (f) Ginkgo biloba in the village (VF); (g) Cinnamomum camphora in the village (VF). The photographs were provided by Zhang G.F.
4.3. Implication for Heritage Tree Conservation and Management
Being economically developed and densely populated, Jiangsu is a rapidly urbanizing province in the eastern coast region of China. Its urbanized population has increased by 58.2% over the past four decades [37]. Nonetheless, there are a large number of well-preserved heritage trees which have been bequeathed in urbanized areas. Most of them grow well in the 13 cities or nine tree habitats because Jiangsu, located in the warm and humid monsoon climate zone, is an economically developed region in eastern China (Table 1). The majority age of 7678 trees is tier 3 (Figure 2), suggesting that they have a great potential of utilization in the near future. These urban heritage trees are becoming assets which may play a significant role in urban landscape construction.
Among the total 215 species of heritage trees in Jiangsu, twelve are dominant species, which contain 5221 trees, accounting for 68.00% of the total. These dominant species demonstrate their adaptability and tenacity despite city development stresses, and they may obtain high recovery capability after experiencing different extent artificial and nature damage. These dominant trees are integral parts of urban greening of Jiangsu since they generally have graceful tree form and dense canopy and can develop a pleasant scent in blossom or bear a large number of fruits/seeds for ornament or food. As a result, they can offer a livable environment for city dwellers. Due to many advantages, they can be regarded as candidate tree species for urban greening and future planning.
Unlike dominant species widely distributed in most of the nine tree habitats, the other heritage tree species have strong preferences or high fidelities to habitats. This probably indicates that land-use change may result in landscape differentiation in quality, quantity, and style during the process of rapid urbanization, thereby shaping the spatial pattern of heritage trees. In fact, there is a considerable variation in number, species, diversity, and health status of these trees (Figure 7). This also reflects the effect of distinctive habitats on urban tree distribution. In such habitats as VF and PG, more urban trees occur with higher species richness because they are protected much better than those in other habitats due to the little influence of urbanization. This implies the need to protect not only the heritage trees themselves from damages, but also their microhabitats. At present, localized management in Jiangsu might have weaknesses for urban heritage trees because it overlooked their habitats and settings. Indeed, distinctive habitat types have obvious effects on distribution of tree species (Figure 8). Therefore, at province scale, protection and management systems of heritage trees should embrace tree habitats and develop individually targeted conservation plans based on current and future land uses.
Heritage trees witness the vicissitudes of a region. Accordingly, the distribution pattern of heritage trees is the result of nature interacting with humans in a province. The distribution of ancient trees in Jiangsu is jointly affected by geographical, climatic, and anthropogenic factors. Based on their analysis of geography and climate, we can identify the species diversity differentiation in different cities, thereby enabling administrative management to create appropriate protection policies. As an example, S. Jiangsu supports most of the individuals and species of heritage trees, most likely because of abundant rainfall, appropriate temperature, and heterogeneous habitats. As such, conservation planning of heritage trees should consider potential threats of environmental changes and extreme climate events (i.e., sustained extreme low temperature in winter). Urban residents’ influence for heritage trees has its pros and cons. On the one hand, rapid urbanization and high population density limit the original living space and worsen environmental stresses for heritage trees, thereby making it difficult to survive or grow. On the other hand, our results indicate that anthropogenic factors (i.e., GDPpc and PD) have a positive and significant impact on tree density (Figure 9b). More specifically, residents and the government have taken considerable measures to protect them, such as hanging tags, designing tree grates, and bracing the trunk. Accordingly, these efforts contribute to making most of them grow well in Jiangsu (Figure 2). In addition, for a city, long history and cultural tradition are also conducive to heritage tree conservation. Therefore, economic level, traditional culture, and urban planning should be integrated into management and conservation plans for heritage trees at the provincial level alongside natural factors.
5. Conclusions
In this study, we took the rapidly urbanizing Jiangsu as a representative province in the eastern coastal areas of China, analyzed its abundance, species richness, tree density, and species diversity of ancient trees in 13 cities, and further explored the spatial differentiation of tree attributes by districts and tree habitats. Our results indicate that species diversity, tree health, and age are associated with tree habitats, and that species diversity among these cities is largely affected by elevation range shift, while the tree density is mainly affected by GDP per capita, population density, and latitude. It was also found that anthropogenic factors (i.e., GDPpc) have a significant effect on heritage tree density. Our findings highlight that management or stakeholders should not only take steps to protect ancient urban trees, per se, but also their habitats and settings (i.e., open space, sufficient light, good soil, etc.) at the provincial level in the future. More importantly, ancient urban trees should be treated as green infrastructure and protected in combination with urban construction and landscape planning. In addition, such protection needs to consider their natural influencing factors, such as topography and climate, and human factors, including social economy, cultural tradition, and urban history, as well.
Author Contributions
K.L.: data analysis, writing original draft. G.Z.: conceiving the study and leading the writing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank L.Y. Shao, G. Yan, and X. Lu for their valuable advice on an earlier draft of the manuscript. Thanks are extended to Y.F. Qu for his assistance in statistical analysis. We thank the anonymous reviewers for their constructive comments on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Checklist of heritage trees in Jiangsu Province, eastern China. “Native” in the column of “Geographical origin of species” refers to the species naturally occurring in Jiangsu Province while “Exotic” refers to the species cultivated in Jiangsu.
Table A1.
Checklist of heritage trees in Jiangsu Province, eastern China. “Native” in the column of “Geographical origin of species” refers to the species naturally occurring in Jiangsu Province while “Exotic” refers to the species cultivated in Jiangsu.
| No. | Species Name | Genus Name | Family Name | Geographical Origin of Species |
|---|---|---|---|---|
| 1 | Cycas revoluta | Cycas | Cycadaceae | Exotic |
| 2 | Ginkgo biloba | Ginkgo | Ginkgoaceae | Exotic |
| 3 | Abies firma | Abies | Pinaceae | Exotic |
| 4 | Cedrus deodara | Cedrus | Pinaceae | Exotic |
| 5 | Pinus bungeana | Pinus | Pinaceae | Exotic |
| 6 | Pinus densiflora | Pinus | Pinaceae | Native |
| 7 | Pinus massoniana | Pinus | Pinaceae | Native |
| 8 | Pinus parviflora | Pinus | Pinaceae | Exotic |
| 9 | Pinus thunbergii | Pinus | Pinaceae | Exotic |
| 10 | Pseudolarix amabilis | Pseudolarix | Pinaceae | Native |
| 11 | Chamaecyparis lawsoniana | Chamaecyparis | Cupressaceae | Exotic |
| 12 | Cryptomeria japonica | Cryptomeria | Cupressaceae | Exotic |
| 13 | Cryptomeria japonica var. sinensis | Cryptomeria | Cupressaceae | Exotic |
| 14 | Cupressus duclouxiana | Cupressus | Cupressaceae | Exotic |
| 15 | Cupressus funebris | Cupressus | Cupressaceae | Exotic |
| 16 | Juniperus chinensis | Juniperus | Cupressaceae | Native |
| 17 | Juniperus formosana | Juniperus | Cupressaceae | Native |
| 18 | Juniperus pingii var. wilsonii | Juniperus | Cupressaceae | Exotic |
| 19 | Juniperus procumbens | Juniperus | Cupressaceae | Exotic |
| 20 | Juniperus virginiana | Juniperus | Cupressaceae | Exotic |
| 21 | Metasequoia glyptostroboides | Metasequoia | Cupressaceae | Exotic |
| 22 | Platycladus orientalis | Platycladus | Cupressaceae | Native |
| 23 | Taxodium distichum | Taxodium | Cupressaceae | Exotic |
| 24 | Taxodium distichum var. imbricatum | Taxodium | Cupressaceae | Exotic |
| 25 | Taxodium mucronatum | Taxodium | Cupressaceae | Exotic |
| 26 | Podocarpus macrophyllus | Podocarpus | Podocarpaceae | Exotic |
| 27 | Taxus wallichiana var. mairei | Taxus | Taxaceae | Exotic |
| 28 | Torreya grandis | Torreya | Taxaceae | Exotic |
| 29 | Illicium lanceolatum | Illicium | Schisandraceae | Native |
| 30 | Chimonanthus praecox | Chimonanthus | Calycanthaceae | Exotic |
| 31 | Cinnamomum camphora | Cinnamomum | Lauraceae | Native |
| 32 | Cinnamomum longepaniculatum | Cinnamomum | Lauraceae | Exotic |
| 33 | Laurus nobilis | Laurus | Lauraceae | Exotic |
| 34 | Machilus thunbergii | Machilus | Lauraceae | Native |
| 35 | Phoebe chekiangensis | Phoebe | Lauraceae | Exotic |
| 36 | Phoebe sheareri | Phoebe | Lauraceae | Native |
| 37 | Sassafras tzumu | Sassafras | Lauraceae | Native |
| 38 | Liriodendron chinense | Liriodendron | Magnoliaceae | Native |
| 39 | Liriodendron tulipifera | Liriodendron | Magnoliaceae | Exotic |
| 40 | Magnolia grandiflora | Magnolia | Magnoliaceae | Exotic |
| 41 | Michelia figo | Michelia | Magnoliaceae | Exotic |
| 42 | Yulania biondii | Yulania | Magnoliaceae | Exotic |
| 43 | Yulania denudata | Yulania | Magnoliaceae | Exotic |
| 44 | Yulania liliiflora | Yulania | Magnoliaceae | Exotic |
| 45 | Yulania zenii | Yulania | Magnoliaceae | Native |
| 46 | Trachycarpus fortunei | Trachycarpus | Arecaceae | Exotic |
| 47 | Buxus bodinieri | Buxus | Buxaceae | Exotic |
| 48 | Buxus sinica | Buxus | Buxaceae | Native |
| 49 | Buxus sinica var. parvifolia | Buxus | Buxaceae | Exotic |
| 50 | Meliosma myriantha | Meliosma | Sabiaceae | Native |
| 51 | Platanus acerifolia | Platanus | Platanaceae | Exotic |
| 52 | Platanus orientalis | Platanus | Platanaceae | Exotic |
| 53 | Nandina domestica | Nandina | Berberidaceae | Exotic |
| 54 | Paeonia suffruticosa | Paeonia | Paeoniaceae | Exotic |
| 55 | Liquidambar formosana | Liquidambar | Altingiaceae | Native |
| 56 | Distylium racemosum | Distylium | Hamamelidaceae | Exotic |
| 57 | Fortunearia sinensis | Fortunearia | Hamamelidaceae | Native |
| 58 | Loropetalum chinense var. rubrum | Loropetalum | Hamamelidaceae | Exotic |
| 59 | Parrotia subaequalis | Parrotia | Hamamelidaceae | Native |
| 60 | Euonymus alatus | Euonymus | Celastraceae | Native |
| 61 | Euonymus fortunei | Euonymus | Celastraceae | Native |
| 62 | Euonymus japonicus | Euonymus | Celastraceae | Exotic |
| 63 | Euonymus maackii | Euonymus | Celastraceae | Native |
| 64 | Populus adenopoda | Populus | Salicaceae | Native |
| 65 | Populus tomentosa | Populus | Salicaceae | Exotic |
| 66 | Salix × aureo-pendula | Salix | Salicaceae | Exotic |
| 67 | Salix babylonica | Salix | Salicaceae | Exotic |
| 68 | Salix matsudana | Salix | Salicaceae | Native |
| 69 | Xylosma congesta | Xylosma | Salicaceae | Native |
| 70 | Triadica sebifera | Triadica | Euphorbiaceae | Native |
| 71 | Bischofia polycarpa | Bischofia | Phyllanthaceae | Native |
| 72 | Flueggea suffruticosa | Flueggea | Phyllanthaceae | Native |
| 73 | Albizia julibrissin | Albizia | Fabaceae | Native |
| 74 | Albizia kalkora | Albizia | Fabaceae | Native |
| 75 | Dalbergia hupeana | Dalbergia | Fabaceae | Native |
| 76 | Gleditsia japonica | Gleditsia | Fabaceae | Native |
| 77 | Gleditsia sinensis | Gleditsia | Fabaceae | Native |
| 78 | Ormosia hosiei | Ormosia | Fabaceae | Exotic |
| 79 | Robinia pseudoacacia | Robinia | Fabaceae | Exotic |
| 80 | Styphnolobium japonicum | Styphnolobium | Fabaceae | Native |
| 81 | Wisteria sinensis | Wisteria | Fabaceae | Native |
| 82 | Castanea mollissima | Castanea | Fagaceae | Native |
| 83 | Castanea seguinii | Castanea | Fagaceae | Native |
| 84 | Castanopsis sclerophylla | Castanopsis | Fagaceae | Native |
| 85 | Cyclobalanopsis glauca | Cyclobalanopsis | Fagaceae | Native |
| 86 | Lithocarpus glaber | Lithocarpus | Fagaceae | Native |
| 87 | Quercus acutissima | Quercus | Fagaceae | Native |
| 88 | Quercus aliena | Quercus | Fagaceae | Native |
| 89 | Quercus chenii | Quercus | Fagaceae | Native |
| 90 | Quercus fabri | Quercus | Fagaceae | Native |
| 91 | Quercus variabilis | Quercus | Fagaceae | Native |
| 92 | Myrica rubra | Myrica | Myricaceae | Native |
| 93 | Carya cathayensis | Carya | Juglandaceae | Exotic |
| 94 | Carya illinoinensis | Carya | Juglandaceae | Exotic |
| 95 | Juglans mandshurica | Juglans | Juglandaceae | Native |
| 96 | Juglans regia | Juglans | Juglandaceae | Exotic |
| 97 | Platycarya strobilacea | Platycarya | Juglandaceae | Native |
| 98 | Pterocarya stenoptera | Pterocarya | Juglandaceae | Native |
| 99 | Alnus cremastogyne | Alnus | Betulaceae | Exotic |
| 100 | Carpinus turczaninowii | Carpinus | Betulaceae | Native |
| 101 | Armeniaca mume | Armeniaca | Rosaceae | Exotic |
| 102 | Armeniaca vulgaris | Armeniaca | Rosaceae | Native |
| 103 | Cerasus × yedoensis | Cerasus | Rosaceae | Exotic |
| 104 | Cerasus serrulata | Cerasus | Rosaceae | Native |
| 105 | Chaenomeles sinensis | Chaenomeles | Rosaceae | Native |
| 106 | Crataegus pinnatifida | Crataegus | Rosaceae | Native |
| 107 | Eriobotrya japonica | Eriobotrya | Rosaceae | Exotic |
| 108 | Malus × micromalus | Malus | Rosaceae | Exotic |
| 109 | Malus halliana | Malus | Rosaceae | Exotic |
| 110 | Photinia bodinieri | Photinia | Rosaceae | Exotic |
| 111 | Photinia serratifolia | Photinia | Rosaceae | Native |
| 112 | Pyrus × michauxii | Pyrus | Rosaceae | Exotic |
| 113 | Pyrus betulifolia | Pyrus | Rosaceae | Native |
| 114 | Pyrus bretschneideri | Pyrus | Rosaceae | Exotic |
| 115 | Pyrus calleryana | Pyrus | Rosaceae | Native |
| 116 | Pyrus pyrifolia | Pyrus | Rosaceae | Exotic |
| 117 | Rosa banksiae | Rosa | Rosaceae | Exotic |
| 118 | Rosa banksiae f. lutea | Rosa | Rosaceae | Exotic |
| 119 | Rosa banksiae var. banksiae | Rosa | Rosaceae | Exotic |
| 120 | Rosa banksiae var. normalis | Rosa | Rosaceae | Exotic |
| 121 | Elaeagnus argyi | Elaeagnus | Elaeagnaceae | Native |
| 122 | Elaeagnus pungens | Elaeagnus | Elaeagnaceae | Native |
| 123 | Elaeagnus umbellata | Elaeagnus | Elaeagnaceae | Native |
| 124 | Hovenia acerba | Hovenia | Rhamnaceae | Native |
| 125 | Sageretia thea | Sageretia | Rhamnaceae | Native |
| 126 | Ziziphus jujuba | Ziziphus | Rhamnaceae | Native |
| 127 | Ulmus chenmoui | Ulmus | Ulmaceae | Native |
| 128 | Ulmus laevis | Ulmus | Ulmaceae | Exotic |
| 129 | Ulmus parvifolia | Ulmus | Ulmaceae | Native |
| 130 | Ulmus pumila | Ulmus | Ulmaceae | Native |
| 131 | Ulmus szechuanica | Ulmus | Ulmaceae | Native |
| 132 | Zelkova schneideriana | Zelkova | Ulmaceae | Native |
| 133 | Aphananthe aspera | Aphananthe | Cannabaceae | Native |
| 134 | Celtis biondii | Celtis | Cannabaceae | Native |
| 135 | Celtis bungeana | Celtis | Cannabaceae | Native |
| 136 | Celtis sinensis | Celtis | Cannabaceae | Native |
| 137 | Pteroceltis tatarinowii | Pteroceltis | Cannabaceae | Native |
| 138 | Broussonetia papyrifera | Broussonetia | Moraceae | Native |
| 139 | Ficus pumila | Ficus | Moraceae | Native |
| 140 | Maclura tricuspidata | Maclura | Moraceae | Native |
| 141 | Morus alba | Morus | Moraceae | Native |
| 142 | Morus alba var. multicaulis | Morus | Moraceae | Exotic |
| 143 | Euscaphis japonica | Euscaphis | Staphyleaceae | Native |
| 144 | Firmiana simplex | Firmiana | Malvaceae | Native |
| 145 | Tilia henryana var. subglabra | Tilia | Malvaceae | Native |
| 146 | Tilia mandshurica | Tilia | Malvaceae | Native |
| 147 | Tilia miqueliana | Tilia | Malvaceae | Native |
| 148 | Edgeworthia chrysantha | Edgeworthia | Thymelaeaceae | Exotic |
| 149 | Lagerstroemia indica | Lagerstroemia | Lythraceae | Native |
| 150 | Lagerstroemia subcostata | Lagerstroemia | Lythraceae | Exotic |
| 151 | Punica granatum | Punica | Lythraceae | Exotic |
| 152 | Pistacia chinensis | Pistacia | Anacardiaceae | Native |
| 153 | Acer buergerianum | Acer | Sapindaceae | Native |
| 154 | Acer buergerianum var. yentangense | Acer | Sapindaceae | Exotic |
| 155 | Acer palmatum | Acer | Sapindaceae | Exotic |
| 156 | Acer palmatum var. thunbergii | Acer | Sapindaceae | Exotic |
| 157 | Acer pictum subsp. mono | Acer | Sapindaceae | Native |
| 158 | Acer truncatum | Acer | Sapindaceae | Native |
| 159 | Aesculus chinensis | Aesculus | Sapindaceae | Exotic |
| 160 | Koelreuteria bipinnata | Koelreuteria | Sapindaceae | Exotic |
| 161 | Koelreuteria paniculata | Koelreuteria | Sapindaceae | Exotic |
| 162 | Sapindus saponaria | Sapindus | Sapindaceae | Native |
| 163 | Citrus medica | Citrus | Rutaceae | Exotic |
| 164 | Citrus wilsonii | Citrus | Rutaceae | Exotic |
| 165 | Orixa japonica | Orixa | Rutaceae | Native |
| 166 | Ailanthus altissima | Ailanthus | Simaroubaceae | Native |
| 167 | Melia azedarach | Melia | Meliaceae | Native |
| 168 | Toona sinensis | Toona | Meliaceae | Native |
| 169 | Tamarix chinensis | Tamarix | Tamaricaceae | Native |
| 170 | Camptotheca acuminata | Camptotheca | Nyssaceae | Native |
| 171 | Cornus officinalis | Cornus | Cornaceae | Exotic |
| 172 | Cornus walteri | Cornus | Cornaceae | Native |
| 173 | Cornus wilsoniana | Cornus | Cornaceae | Exotic |
| 174 | Ternstroemia gymnanthera | Ternstroemia | Pentaphylacaceae | Exotic |
| 175 | Diospyros armata | Diospyros | Ebenaceae | Exotic |
| 176 | Diospyros kaki | Diospyros | Ebenaceae | Exotic |
| 177 | Diospyros kaki var. silvestris | Diospyros | Ebenaceae | Native |
| 178 | Diospyros lotus | Diospyros | Ebenaceae | Native |
| 179 | Camellia japonica | Camellia | Theaceae | Exotic |
| 180 | Camellia sasanqua | Camellia | Theaceae | Exotic |
| 181 | Camellia sinensis | Camellia | Theaceae | Native |
| 182 | Symplocos paniculata | Symplocos | Symplocaceae | Native |
| 183 | Sinojackia xylocarpa | Sinojackia | Styracaceae | Native |
| 184 | Rhododendron simsii | Rhododendron | Ericaceae | Native |
| 185 | Eucommia ulmoides | Eucommia | Eucommiaceae | Exotic |
| 186 | Trachelospermum jasminoides | Trachelospermum | Apocynaceae | Native |
| 187 | Ehretia acuminata | Ehretia | Boraginaceae | Native |
| 188 | Ehretia dicksonii | Ehretia | Boraginaceae | Exotic |
| 189 | Lycium chinense | Lycium | Solanaceae | Native |
| 190 | Chionanthus retusus | Chionanthus | Oleaceae | Native |
| 191 | Fontanesia phillyreoides subsp. fortunei | Fontanesia | Oleaceae | Native |
| 192 | Fraxinus chinensis | Fraxinus | Oleaceae | Native |
| 193 | Ligustrum lucidum | Ligustrum | Oleaceae | Native |
| 194 | Ligustrum quihoui | Ligustrum | Oleaceae | Native |
| 195 | Osmanthus fragrans | Osmanthus | Oleaceae | Exotic |
| 196 | Osmanthus fragrans var. aurantiacus | Osmanthus | Oleaceae | Exotic |
| 197 | Osmanthus fragrans var. semperflorens | Osmanthus | Oleaceae | Exotic |
| 198 | Osmanthus fragrans var. thunbergii | Osmanthus | Oleaceae | Exotic |
| 199 | Campsis grandiflora | Campsis | Bignoniaceae | Exotic |
| 200 | Campsis radicans | Campsis | Bignoniaceae | Exotic |
| 201 | Catalpa bungei | Catalpa | Bignoniaceae | Native |
| 202 | Catalpa ovata | Catalpa | Bignoniaceae | Native |
| 203 | Catalpa speciosa | Catalpa | Bignoniaceae | Exotic |
| 204 | Vitex negundo | Vitex | Lamiaceae | Native |
| 205 | Vitex negundo var. cannabifolia | Vitex | Lamiaceae | Native |
| 206 | Paulownia tomentosa | Paulownia | Paulowniaceae | Native |
| 207 | Pittosporum tobira | Pittosporum | Pittosporaceae | Native |
| 208 | Kalopanax septemlobus | Kalopanax | Araliaceae | Native |
| 209 | Ilex chinensis | Ilex | Aquifoliaceae | Native |
| 210 | Ilex cornuta | Ilex | Aquifoliaceae | Native |
| 211 | Ilex macrocarpa | Ilex | Aquifoliaceae | Exotic |
| 212 | Viburnum macrocephalum | Viburnum | Adoxaceae | Exotic |
| 213 | Viburnum macrocephalum f. keteleeri | Viburnum | Adoxaceae | Native |
| 214 | Viburnum odoratissimum var. awabuki | Viburnum | Adoxaceae | Exotic |
| 215 | Lonicera maackii | Lonicera | Caprifoliaceae | Native |
References
- Jim, C.; Zhang, H. Species diversity and spatial differentiation of old-valuable trees in urban Hong Kong. Urban For. Urban Green. 2013, 12, 171–182. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Laurance, W.F.; Franklin, J.F.; Likens, G.E.; Banks, S.C.; Blanchard, W.; Gibbons, P.; Ikin, K.; Blair, D.; McBurney, L.; et al. New Policies for Old Trees: Averting a Global Crisis in a Keystone Ecological Structure. Conserv. Lett. 2013, 7, 61–69. [Google Scholar] [CrossRef]
- Mahmoud, T.; Gairola, S.; El-Keblawy, A. Large old trees need more conservation attention: A case of Tamarix aphylla in the arid deserts of the United Arab Emirates. J. Asia-Pac. Biodivers. 2015, 8, 183–185. [Google Scholar] [CrossRef] [Green Version]
- Lindenmayer, D.B. Conserving large old trees as small natural features. Biol. Conserv. 2017, 211, 51–59. [Google Scholar] [CrossRef]
- Liu, J.J.; Lindenmayer, D.B.; Yang, W.J.; Ren, Y.; Campbell, M.J.; Wu, C.P.; Luo, Y.Q.; Zhong, L.; Yu, M.J. Diversity and density patterns of large old trees in China. Sci. Total Environ. 2019, 655, 255–262. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, B.; Yuan, W.; Shen, A.; Yang, S.; Yao, S.; Liu, J. On the Management of Large-Diameter Trees in China’s Forests. Forests 2020, 11, 111. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Jiang, R.-Y.; Zhang, G.-F. Number and distribution of large old ginkgos in east China: Implications for regional conservation. Nat. Conserv. 2020, 42, 71–87. [Google Scholar] [CrossRef]
- Zhang, H.; Lai, P.Y.; Jim, C. Species diversity and spatial pattern of old and precious trees in Macau. Landsc. Urban Plan. 2017, 162, 56–67. [Google Scholar] [CrossRef]
- Townsend, J.B.; Barton, S. The impact of ancient tree form on modern landscape preferences. Urban For. Urban Green. 2018, 34, 205–216. [Google Scholar] [CrossRef]
- Huang, L.; Tian, L.; Zhou, L.; Jin, C.; Qian, S.; Jim, C.; Lin, D.; Zhao, L.; Minor, J.; Coggins, C.; et al. Local cultural beliefs and practices promote conservation of large old trees in an ethnic minority region in southwestern China. Urban For. Urban Green. 2020, 49, 126584. [Google Scholar] [CrossRef]
- Aird, P.L. Heritage, natural heritage, cultural heritage and heritage tree defined. For. Chron. 2005, 81, 593. [Google Scholar] [CrossRef]
- Lai, P.Y.; Jim, C.; Da Tang, G.; Hong, W.J.; Zhang, H. Spatial differentiation of heritage trees in the rapidly-urbanizing city of Shenzhen, China. Landsc. Urban Plan. 2018, 181, 148–156. [Google Scholar] [CrossRef]
- Yu, X.J. The current status and measures on management and conservation of ancient and famous trees in Zhejiang Province. Land Green. 2001, 17, 14. [Google Scholar]
- Du, Q.; Chen, Z.H.; Liu, A.X.; Zhu, G.G. Study on species diversity of ancient trees in Zhejiang Province. J. Zhejiang Univ. Agric. Life Sci. 2005, 31, 215–219. [Google Scholar] [CrossRef]
- Fan, X.L.; Liang, Y.; Fang, Y.; Wang, Q.; Gao, L.; Bai, S.W. The current status and protective measures on ancient and famous trees in Shandong Province. J. Jiangsu For. Sci. Technol. 2018, 45, 55–57. [Google Scholar] [CrossRef]
- Chi, X.L.; Yang, G.; Sun, K.; Li, X.L.; Wang, T.L.; Zhang, A.; Li, Y.; Cheng, M.; Wang, Q.G. Old ginkgo trees in China: Distribution, determinants and implications for conservation. Glob. Ecol. Conserv. 2020, 24, e01304. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, C.Y. Research progress in ancient trees health assessment. World For. Res. 2013, 26, 37–42. [Google Scholar]
- Yang, L.; Kang, Y.X.; Li, X.J.; Wang, F.; Wang, D.Y.; Guo, L. Health of ancient Platycladus orientalis in the Mausoleum of the Yellow Emperor. J. Zhejiang Agric. For. Univ. 2014, 31, 779–784. [Google Scholar] [CrossRef]
- Takács, M.; Szénási, Á.; Malatinszky, Á. Polypores, Agrobacterium and ivy damage on Hungarian ancient trees. Nat. Conserv. 2020, 40, 1–38. [Google Scholar] [CrossRef]
- Zhou, H.H.; Wang, S.L. Analysis of legal protection of our country’s old and famous tree resources. Ecol. Environ. 2007, 15, 153–155. [Google Scholar] [CrossRef]
- Hakkenberg, C.R. Biodiversity and sacred sites: Vernacular conservation practices in northwest Yunnan, China. Worldviews Environ. Cult. Relig. 2008, 12, 74–90. [Google Scholar] [CrossRef]
- Wang, F.; Qin, Z.; Chen, X.L. Analysis and proposals of local legislation for the protection of ancient and famous trees. Resour. Dev. Mark. 2021, 37, 51–55. [Google Scholar] [CrossRef]
- Dong, D.; Zhou, Z.X.; He, Y.H.; Li, G. Landscape aesthetic assessment of old-tree communities in Jiuhua Mountain Scenic Area of Anhui Province. Chin. J. Ecol. 2011, 30, 1786–1792. [Google Scholar] [CrossRef]
- Lan, Y.; Yu, W.; Du, H.Y.; Bao, Z.Y. Landscape evaluation of old trees in the West Lake Scenic Area. Acta Agric. Zhejiangensis 2015, 27, 1192–1197. [Google Scholar] [CrossRef]
- Salick, J.; Amend, A.; Anderson, D.; Hoffmeister, K.; Gunn, B.; Zhendong, F. Tibetan sacred sites conserve old growth trees and cover in the eastern Himalayas. Biodivers. Conserv. 2007, 16, 693–706. [Google Scholar] [CrossRef]
- Zapponi, L.; Mazza, G.; Farina, A.; Fedrigoli, L.; Mazzocchi, F.; Roversi, P.F.; Peverieri, G.S.; Mason, F. The role of monumental trees for the preservation of saproxylic biodiversity: Re-thinking their management in cultural landscapes. Nat. Conserv. 2017, 19, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Wu, Y.J.; Han, M.L.; Zhao, F.; Zhang, J.Q.; Zhang, J.N. Composition and distribution characteristics of individual old and notable trees in Lanzhou City. J. Arid. Land Resour. Environ. 2020, 34, 182–188. [Google Scholar] [CrossRef]
- Jin, C.; Zheng, M.; Huang, L.; Qian, S.; Jim, C.; Lin, D.; Zhao, L.; Minor, J.; Coggins, C.; Chen, B.; et al. Co-existence between humans and nature: Heritage trees in China’s yangtze River region. Urban For. Urban Green. 2020, 54, 126748. [Google Scholar] [CrossRef]
- Asanok, L.; Kamyo, T.; Norsaengsri, M.; Yotapakdee, T.; Navakam, S. Assessment of the diversity of large tree species in rapidly urbanizing areas along the Chao Phraya River Rim, central Thailand. Sustainability 2021, 13, 10342. [Google Scholar] [CrossRef]
- Thaiutsa, B.; Puangchit, L.; Kjelgren, R.; Arunpraparut, W. Urban green space, street tree and heritage large tree assessment in Bangkok, Thailand. Urban For. Urban Green. 2008, 7, 219–229. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Blanchard, W.; Blair, D.; McBurney, L.; Banks, S.C. Environmental and human drivers influencing large old tree abundance in Australian wet forests. For. Ecol. Manag. 2016, 372, 226–235. [Google Scholar] [CrossRef]
- Huang, L.; Jin, C.; Zhen, M.; Zhou, L.; Qian, S.; Jim, C.; Lin, D.; Zhao, L.; Minor, J.; Coggins, C.; et al. Biogeographic and anthropogenic factors shaping the distribution and species assemblage of heritage trees in China. Urban For. Urban Green. 2020, 50, 126652. [Google Scholar] [CrossRef]
- Wan, J.-Z.; Li, Q.-F.; Wei, G.-L.; Yin, G.-J.; Wei, D.-X.; Song, Z.-M.; Wang, C.-J. The effects of the human footprint and soil properties on the habitat suitability of large old trees in alpine urban and periurban areas. Urban For. Urban Green. 2019, 47, 126520. [Google Scholar] [CrossRef]
- Hartel, T.; Hanspach, J.; Moga, C.I.; Holban, L.; Szapanyos, Á.; Tamás, R.; Hováth, C.; Réti, K.-O. Abundance of large old trees in wood-pastures of Transylvania (Romania). Sci. Total Environ. 2018, 613–614, 263–270. [Google Scholar] [CrossRef]
- Chen, S.; Li, G.; Zhuo, Y.F.; Xu, Z.G.; Ye, Y.M.; Thorn, J.P.R.; Marchant, R. Trade-offs and synergies of ecosystem services in the Yangtze River Delta, China: Response to urbanizing variation. Urban Ecosyst. 2021, 24, 1–16. [Google Scholar] [CrossRef]
- Xu, W.; Jin, X.; Liu, J.; Zhou, Y. Analysis of influencing factors of cultivated land fragmentation based on hierarchical linear model: A case study of Jiangsu Province, China. Land Use Policy 2021, 101, 105119. [Google Scholar] [CrossRef]
- Bureau of Statistics of Jiangsu Province. Jiangsu Statistical Yearbook 2020; Bureau of Statistics of Jiangsu Province of the People’s Republic of China: Nanjing, China, 2020. [Google Scholar]
- Li, H.; Liu, Z.; James, N.; Li, X.; Hu, Z.; Shi, H.; Sun, L.; Lu, Y.; Jia, X. Agricultural transformations and their influential factors revealed by archaeobotanical evidence in Holocene Jiangsu Province, eastern China. Front. Earth Sci. 2021, 9, 661684. [Google Scholar] [CrossRef]
- Flora of China Editorial Committee. Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2013. [Google Scholar]
- Shannon, E.C.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1963. [Google Scholar]
- Rejmánek, M.; Randall, J.M. Invasive alien plants in California: 1993 summary and comparison with other areas in North America. Madroño 1994, 41, 161–177. Available online: https://www.jstor.org/stable/41425013.
- Jiang, R.; Zhang, G. Distribution patterns and influencing factors of different parasitic angiosperm types in China. Glob. Ecol. Conserv. 2021, 27, e01533. [Google Scholar] [CrossRef]
- Hill, T.C.; Walsh, K.A.; Harris, J.A.; Moffett, B.F. Using ecological diversity measures with bacterial communities. FEMS Microbiol. Ecol. 2003, 43, 1–11. [Google Scholar] [CrossRef]
- Hasanaliyeva, G.; Chatzidimitrou, E.; Wang, J.; Baranski, M.; Volakakis, N.; Pakos, P.; Seal, C.; Rosa, E.; Markellou, E.; Iversen, P.; et al. Effect of organic and conventional production methods on fruit yield and nutritional quality parameters in three Traditional Cretan grape varieties: Results from a farm survey. Foods 2021, 10, 476. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination (Version 5.0); Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Liu, Q.X. Flora of Jiangsu; Jiangsu Phoenix Science and Technology Press: Nanjing, China, 2015; Volume 1. [Google Scholar]
- Orłowski, G.; Nowak, L. The importance of marginal habitats for the conservation of old trees in agricultural landscapes. Landsc. Urban Plan. 2007, 79, 77–83. [Google Scholar] [CrossRef]
- Bennett, A.C.; McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.J. Larger trees suffer most during drought in forests worldwide. Nat. Plants 2015, 1, 15139. [Google Scholar] [CrossRef]
- Liu, J.; Yang, B.; Lindenmayer, D.B. The oldest trees in China and where to find them. Front. Ecol. Environ. 2019, 17, 319–322. [Google Scholar] [CrossRef]
- Nolan, V.; Reader, T.; Gilbert, F.; Atkinson, N. The ancient tree inventory: A summary of the results of a 15 year citizen science project recording ancient, veteran and notable trees across the UK. Biodivers. Conserv. 2020, 29, 3103–3129. [Google Scholar] [CrossRef]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- He, Y.A. Features and highlights of Jiangsu regional culture—General summary to Jiangsu local cultural history. Jiangsu Local Chron. 2020, 34, 4–12. [Google Scholar]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Shen, Z.; Ying, L.; Zang, R.; Jiang, Y. Effects of current climate, paleo-climate, and habitat heterogeneity in determining biogeographical patterns of evergreen broad-leaved woody plants in China. J. Geogr. Sci. 2019, 29, 1142–1158. [Google Scholar] [CrossRef] [Green Version]
- López-Pujol, J.; Zhang, F.-M.; Sun, H.-Q.; Ying, T.-S.; Ge, S. Centres of plant endemism in China: Places for survival or for speciation? J. Biogeogr. 2011, 38, 1267–1280. [Google Scholar] [CrossRef]
- Zou, D.-T.; Wang, Q.-G.; Luo, A.; Wang, Z.-H. Species richness patterns and resource plant conservation assessments of Rosaceae in China. Chin. J. Plant Ecol. 2019, 43, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.Y.; Gu, Y.Y.; Liu, C.; Xing, S.H. Effects of spatial scales on topographic heterogeneity—Species richness relationships: Grain and extent. Chin. J. Ecol. 2021, 40, 577–585. [Google Scholar] [CrossRef]
- Chen, W.Y. Public willingness-to-pay for conserving urban heritage trees in Guangzhou, south China. Urban For. Urban Green. 2015, 14, 796–805. [Google Scholar] [CrossRef]
- Jim, C. Spatial differentiation and landscape-ecological assessment of heritage trees in urban Guangzhou (China). Landsc. Urban Plan. 2004, 69, 51–68. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).