Abstract
The planted forest area in Vietnam increased from 3.0 to 4.4 million hectares in the period 2010–2020, but the loss of productivity from pests and diseases continues to be a problem. During this period, frequent and systematic plantation forest health surveys were conducted on 12 native and 4 exotic genera of trees as well as bamboo across eight forest geographic regions of Vietnam. Damage caused by insects and pathogens was quantified in the field and laboratory in Hanoi. The threats of greatest concern were from folivores (Antheraea frithi, Arthroschista hilaralis, Atteva fabriciella, Hieroglyphus tonkinensis, Lycaria westermanni, Krananda semihyalina, and Moduza procris), wood borers (Batocera lineolata, Euwallacea fornicatus, Tapinolachnus lacordairei, Xyleborus perforans, and Xystrocera festiva), sap-sucking insects (Aulacaspis tubercularis and Helopeltis theivora) and pathogens (Ceratocystis manginecans, Fusarium solani, and Phytophthora acaciivora). The number of new and emerging pests and pathogens increased over time from 2 in 2011 to 17 in 2020, as the damage became more widespread. To manage these pests and diseases, it is necessary to further invest in the selection and breeding of resistant genotypes, improve nursery hygiene and silvicultural operations, and adopt integrated pest management schemes. Consideration should be given to developing forest health monitoring protocols for forest reserves and other special-purpose forests.
1. Introduction
Vietnam has made great strides in regreening the country in recent decades [1,2]. Due to national policies and targets for afforestation and the rehabilitation of natural forests, the forest area expanded from 9.40 million ha in 1990 to 14.67 million ha in 2020 [3]. Vietnam’s 2006–2020 Forest Development Strategy expressed the goal of increasing the total forest cover from 37% in 2006 [4] to 42% by 2020 [3]. Forest development efforts of the past 10 years have greatly contributed to Vietnam’s sustainability strategy [5]. In particular, the plantation forestry sector has made a significant contribution to the growth in forest cover and now comprises 26.4% (ca. 4.4 million ha) of the total forest area in Vietnam [3]. About half of the forest plantations are managed by small stakeholders and the remainder by private companies [3,6]. Therefore, the plantation forest sector is vitally important to the livelihood of millions of rural households [7,8]. Exotic species, mainly acacias and eucalypts, have been widely planted in Vietnam [6]. It has been estimated that fast-growing Acacia hybrids can provide 33–56% of the total household income [9]. Furthermore, the export of wood and forest products earned US$9.4 billion in 2018 [6] and increased to US$12.3 billion in 2020 [10]. Even though the plantation area has continued to increase over time, it is insufficient to meet the demand of the local wood processing industry [11]. Reducing losses from damage caused by insect pests and pathogens can help to secure the future wood supply that Vietnam needs for its domestic and international markets.
Over time, the incidence of pest and disease problems in Acacia and Eucalyptus stands has increased globally [12,13,14,15], including in Vietnam. Old et al. described 13 fungal pathogens associated with Eucalyptus plantations in Vietnam [16,17]. The most common Eucalyptus diseases were caused by Cryptosporiopsis eucalypti, Cylindrocladium reteaudii, and Ralstonia solanacearum. Illustrations of the most common biotic problems (21 pests, 23 pathogens) in Acacia, Eucalyptus, and Pinus plantations were provided in a field guide for advisors and growers [18]. With the exception of native Pinus, most of the studies on forest health in Vietnam have focused on exotic species, mainly Acacia and Eucalyptus [19,20]. Currently, there are five major pest species (Ericeia sp., Helopeltis sp., Phalera grotei, Pteroma plagiophleps, and Xylosandrus crassiusculus) and four major pathogens (Ceratocystis sp., Corticium salmonicolor, Phytophthora cinnamomi, and Pythium vexans) threatening the productivity of Acacia plantations in Vietnam. Furthermore, six major pest species (Aristobia testudo, A. approximator, Biston suppressaria, Leptocybe invasa, Sarothrocera lowi, and Trabala vishnou) and five major pathogens (Cylindrocladium sp., Cryptosporiopsis eucalypti, Ralstonia solanacearum, Teratosphaeria destructans, and T. zuluensis) have been damaging Eucalyptus plantations. As the research effort on forest health in Vietnam has increased over the past two decades, many reports focusing on individual pests or pathogens of interest have been published [18,20,21,22,23,24,25,26,27].
However, new insect pests and pathogens continue to emerge and to cause damage in plantation forests in Vietnam [21,28,29,30,31]. This is the first comprehensive study conducted over a decade to quantify the diversity of new and emerging pests and pathogens and the damage they cause to exotic and native planted species in Vietnam. The national survey provides an opportunity to evaluate the extent of the new threats and to identify management options.
2. Materials and Methods
2.1. Field Surveys—General Procedures
Forest health surveys were undertaken annually from 2011 to 2020, in the eight forest geographic regions of Vietnam (Figure 1), with support from the Ministry of Agriculture and Rural Development. Field observations were carried out on forests planted with 16 tree species (Table 1). We relied on information gathered by local foresters as well as our own observations to select the surveyed plantations in each studied forest region. Three fixed plots (40 × 25 m) were randomly set up in plantations comprising the tree species present in each region and they were assessed over ten years. Selected plots were at least 20 m from plantation edges, roads, or forest gaps. About 25% of trees (at least 30 trees) in each plot were randomly selected and assessed for damage from insect pests and/or pathogens. Surveys usually involved three repeat visits a year undertaken in spring (February–April), summer (May–July), and autumn (August–October). Where tree injury was observed (e.g., defoliation, leaf senescence, shoot dieback, tree death), we quantified the damage.
Figure 1.
Map of Vietnam showing the eight forest geographic regions.

Table 1.
Details of forest plantation species that were surveyed for pests and pathogens.
The damage was classified at five levels using methods described by [27,32], where: 0 = healthy trees; 1 = low damage; 2 = medium damage; 3 = high damage; 4 = severe damage. The damage incidence (p%) in each plot was calculated as follows: p% = (n/N) × 100, where: n = the number of trees/culms attacked; N = total number of trees/culms assessed.
The average damage index (DI) was calculated as follows: , where: ni = the number of trees infected at damage index i; vi = the damage index at level i; and N = total number of plants assessed.
Values for p% and DI are reported in the results as ranges and are based on the plot data over the years of plot assessment.
2.2. Field Surveys—Insects
Insect survey methods included eye tracking, sweep netting, suction sampling, and lure traps. Adult folivores were captured with collecting nets on aluminum poles, and placed in killing jars. Plastic boxes (VietNhat Plastic Joint Stock Company, Hanoi, Vietnam) with nylon mesh covers were employed to transport the living larvae, pupae, and eggs to the laboratory. Fresh leaves were included for the larvae to feed.
For wood borers, samples were mostly obtained by the felling of affected trees, then chopping logs to obtain collections. When adults were not present, logs 1.0–1.5 m in length were transported to the laboratory in Hanoi. Some logs were dissected to capture the adults and/or larvae. The cuts of other logs were sealed with Parafilm® (Bemis Company Inc., Neenah, WI, USA) and were taken to the laboratory for rearing adults for identification.
In addition, more intensive sampling was undertaken through trapping in the field for adult ambrosia beetles. Black funnel Lindgren traps (BioQuip Products, Inc., Compton, CA, USA) and self-made plastic-bottle traps baited with 70% ethanol and para-menthenol (1S, 4R)-p-menth-2-en-1-ol) (Synergy Semiochemicals Corp. (Burnaby, BC, Canada) were used to attract adults. Propylene glycol (Merck, Darmstadt, Germany) and water (50:50) solution was used in the collection cups. Each trap was suspended at least 10 m apart in a plantation and 1.5 m above the ground to avoid damage by wild animals. The baits were replaced once a week. Baiting was undertaken from April to June. Trap collections were stored in 70% ethanol in Eppendorf® (Eppendorf Manufacturing Corp., Hamburg, Germany) tubes and then sorted in the laboratory.
2.3. Field Surveys—Pathogens
Samples (leaves, branches, and roots) were systematically taken from diseased trees. Diseased boles were cut into 0.5–1.0 m length sections. Where the felling of trees was not permitted, bark and wood samples were taken from the edge of lesions using a sharp knife. Samples were placed in paper bags and transported to the laboratory in Hanoi.
2.4. Rearing Insects in the Laboratory
Larvae of folivores were reared in mesh cages (1 × 1 × 1.5 m in length × width × height) at 25 ± 2 °C and 75 ± 5% relative humidity in the laboratory. Ten larvae were released in each cage and observed daily to capture the adults when they emerged.
Logs containing larva of wood borers were placed in insect cages as above, and the appearance of frass and adult emergence were recorded daily. For wood borers, an artificial diet was also used to feed the larvae following the method of [33,34] with modification. Larvae were reared in 15–50 mL Falcon® tubes (Corning Life Sciences Company, Corning, NY, USA) on artificial media. The media for longhorn beetles contained 30 g agar, 40 g sucrose, 25 g yeast extract, 60 g oat powder, 4 g sodium benzoate, 2 g sorbic acid, 150 g wood powder from the host tree, and 300 mL distilled water. The diet mixture for ambrosia beetles contained 30 g agar, 5 g sucrose, 1.25 g Wesson’s salt mixture, 10 g casein, 200 g sawdust from the host plant, and 500 mL distilled water. The rearing tubes were checked daily to observe the development of larvae and pupae, and to collect adults.
2.5. Isolation and Culturing Pathogens
Three main procedures were used as follows. For the direct isolation method, plant samples were prepared as above and either placed on selective PARPH-V8 (pimaricin + ampicillin + rifamycin + PCNB (C6Cl15NO2) + hymexazol + V8 juce + Agar) medium [35] and incubated at 20 °C (for Oomycetes such as Phytophthora sp.), or placed on PDA (Potato dextrose agar) medium supplemented with tetracycline and incubated at 26 °C (Fusarium sp.). The carrot baiting method [36] was used to isolate Ceratocystis. Briefly, wood samples (18–25 mm length, 1.0–1.5 mm thick) were sandwiched between two slices of fresh carrot and placed on sterile dry paper in plastic boxes at 6 °C. After 5–8 days, the hat-shaped spores of putative pathogens were placed on PDA medium and incubated at 26 °C.
When cultures had grown 1–2 cm in diameter, hyphal tips were sub-cultured onto new media (V8 and PDA). Isolates cultured at 20 °C (Phytophthora sp.) and 26 °C (other pathogens) were used for microscopy and DNA extraction.
2.6. Identification
Insect pests were mainly identified based on their morphology in taxonomic and other works as follows: Lepidoptera—keys [37,38,39,40]; Coleoptera—keys [41,42,43,44]; Hemiptera—keys [45,46]; Orthoptera—keys [47]. Phylogenetic analyses using the cytochrome c oxidase subunit I were undertaken to help confirm the identity of Batocera lineolata [48], Euwallacea fornicatus [31], and Tapinolachnus lacordairei [49]. Protocols were performed as described by [50,51,52,53].
For the plant pathogens, the ITS1/ITS4 [54] region was used to help identify Fusarium solani [55], the βT1a/βT1b gene region [56] was used for Ceratocystis manginecans [30], and in Phytophthora the primers DC6 [57] and ITS4 were used [58].
2.7. Specimens
The collected insect pests and plant pathogens associated with the investigated forest plantation have been deposited in the collection of the Forest Protection Research Centre, 46 Duc Thang Street, Hanoi 11910, Vietnam and Vietnam National Museum of Nature, 18 Hoang Quoc Viet Street, Hanoi 11350, Vietnam.
3. Results
3.1. Change over Time
Forest health surveys undertaken from 2011 to 2020 on the plantations of different tree species in Table 1 revealed 14 new or emerging insect pest species and major disease threats from two plant pathogens (Table 2). The total number of new or emerging insect pest species and pathogens increased from 2 in 2011 to 17 in 2020 (Figure 2). Hence, on average, 1–2 new pests per year were recorded damaging forest plantations in Vietnam. The temporal and geographical occurrence of the observed pests and pathogens is detailed in Table 3. Of particular note is the apparently rapid spread of Aulacapsis tubercularis in Cinnamomum cassia, Euwallacea fornicatus in Acacia spp. and C. cassia, Tapinolachrus lacordairea in Chukrasia tabularis, and Xyleborus perforans in Acacia and Eucalyptus. The two main types of damage from insect pest species are from foliar feeding by folivores (5 species of Lepidoptera), and bark and/or wood feeding (6 species of Coleoptera). The fungal pathogen Ceratocystis manginecans has extended its host range from exotic Acacia and Eucalyptus to the native Dalbergia tonkinensis and C. tabularis.

Table 2.
Pests and pathogens recorded in forest health surveys in Vietnam causing significant damage to host trees.
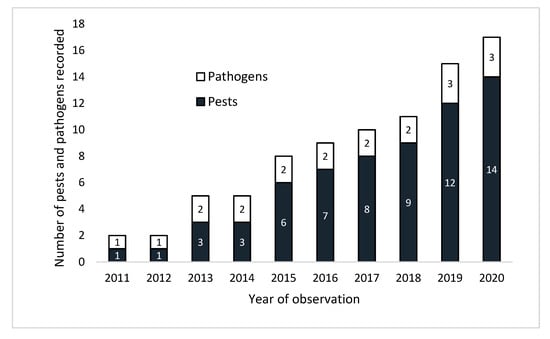
Figure 2.
Change in the number of pest and pathogen species recorded each year.

Table 3.
Temporal and geographical occurrence of pest and pathogen species in forest health surveys in Vietnam from 2011 to 2020.
3.2. Defoliation from Folivores
3.2.1. Antheraea frithi (Saturniidae) Wild Tasar Silkmoth (Vietnamese: Sau Tam)
This species was first recorded in southern Vietnam in 1970 [59], but it only recently has become a pest of D. alatus and H. odorata plantations. Affected plantations are from 5 to 15 years old, with p% of 82–97%, and DI of 2.9–3.6. Single tree deaths can occur with repeat defoliation (Figure 3a). Adults (Figure 3f) spread quickly and there is high risk of further damage [60]. Control using living Beauveria bassiana and Metarhizium anisopliae has been attempted by local authorities, but the effectiveness is low.
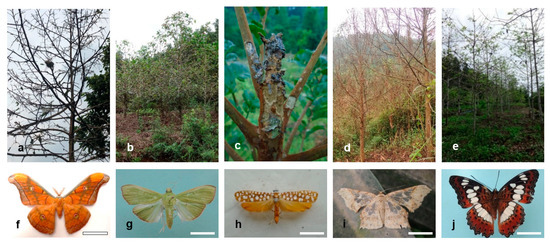
Figure 3.
Emerging Lepidoptera threats to forest plantations in Vietnam. (a–e) damaged to trees from folivores; (f–j) adults. (a,f) Antheraea frithi damage in Dipterocarpus alatus; (b,g) Arthroschista hilaralis damage in Neolamarckia cadamba; (c,h) Atteva fabriciella damage in Ailanthus triphysa; (d,j) Krananda semihyalina damages in Cinnamomum cassia; (e,l) Moduza procris damage in Nauclea orientalis. Scale bar: f = 3.0 cm; g = 1.5 cm; h = 0.5 cm; i = 1.8 cm; j = 2.0 cm.
3.2.2. Arthroschista hilaralis (Crambidae) Kadam Defoliator Moth (Vietnamese: Sau Cuon La Xanh Ngoc)
This pest (Figure 3g) was recorded in 2015 in the South West region (Figure 1) where it defoliated 15 ha of Neolamarckia cadamba and 20 ha of Nauclea orientalis plantations [61]. So far, more than 90 ha of plantations have been attacked. Young plantations (2 to 5 year-old) are most at risk of damage (Figure 3b) and defoliation occurs 2 to 3 times a year. p% is 75–99%, and DI is 2.6–3.8. Repeat damage of the new emerging foliage reduces tree growth and may cause tree mortality.
However, two Ne. cadamba provenances (Ca Mau and Dong Thap) and two Na. orientalis provenances (Dong Thap and Binh Phuoc) showed good recovery after defoliation [62]. The two host species are also attacked by Moduza procris (Figure 3j).
3.2.3. Atteva fabriciella (Attevidae) Ailanthus Webworm Moth (Vietnamese: Sau An La Vang Hoa Trang)
This pest was recorded in 2016 in 10 ha of Ailanthus triphysa in the North East region. Foliage and shoot tips (Figure 3c) are attacked 2–3 times a year. Young plantations (2–5 years old) are severely affected; p% is 100%, DI is 2.6–2.8, and some tree death occurs. Insecticides (abamectin and chlorpyrifos ethyl) have been applied by local authorities with 85–90% effectiveness. The pest (Figure 3h) spreads quickly and has the potential to cause wide damage [63].
3.2.4. Krananda semihyalina (Geometridae) Cinnamon Looper (Vietnamese: Sau Do Canh Nua Trong)
This pest (Figure 3i) was recorded in 2020 in the North East and North Plain regions damaging about 5 ha of Cinnamomum cassia plantations, aged 3–10 years. The foliage is devoured 4–5 times a year and this greatly reduces tree growth. p% is 26–90% (Figure 3d), DI is 0.7–3.5, and tree deaths have been reported.
3.2.5. Moduza procris (Crambidae) Commander Butterfly (Vietnamese: Sau Gai An La)
Moduza procris (Figure 3j) was recorded in the North Central region in 1970 [64], and its status as a forest pest was established in 2015 due to foliar damage to 1–5 year-old Neolamarckia cadamba and Nauclea orientalis plantations in Ca Mau province [62]. p% is 3–7% (Ne. cadamba) and 31–58% (Na. orientalis) (Figure 3e), and DI is 0.1–0.2 (Ne. cadamba) and 1.1–2.0 (Na. orientalis). Moduza procris and Arthroschista hilaralis are reported to cause concurrent damage in Ne. cadamba and Na. orientalis plantations.
3.3. Damage from Wood Borers
3.3.1. Batocera lineolata (Cerambycidae) Striped Longhorn Beetle (Vietnamese: Xen Toc Den Soc Trang)
This pest (Figure 4m) was recorded in 2019 in the North West damaging 30 ha of clonal Eucalyptus plantations. Larvae feed in the sapwood. Some severely damaged trees (Figure 4g) are so weakened that they are easily broken by strong winds, while others remain standing and die (Figure 4a). Tree decline and death are most prevalent in 1–3 year-old plantations. Resistance has been observed in some clones of E. urophylla × E. pellita and E. urophylla [48,65]. A number of insecticides are being evaluated for control in the field [48]. So far, about 350 ha of Eucalyptus hybrid plantations have been seriously damaged. p% is 24–52% and DI is 0.8–1.6.

Figure 4.
Wood borer and beetle damage to forest plantations in Vietnam. (a–f) damaged trees; (g–l) close-up of damage symtoms; (m–s) adults. (a,g,m) Batocera lineolata damage in Eucalyptus hybrid; (b,h,o) Euwallacea fornicatus damages in Acacia hybrid; (c,i,p) Lycaria westermanni damage in Fernandoa brilletii; (d,j,q) Tapinolachnus lacordairei damage in Chukrasia tabularis; (e,k,r) Xyleborus perforans damage in Eucalyptus urophylla; (f,l,s) Xystrocera festiva damage in Acacia mangium. Scare bar: m = 1.5 cm, o = 1.0 mm; p,q,s = 1.0 cm; r = 1.2 mm.
3.3.2. Euwallacea fornicatus (Curculionidae) Polyphagous Shot Hole Borer (Vietnamese: Mot Nuoi Nam Forni)
This species was recorded in Tonkin Island (Vietnam) in 1992 [66]. In 2013 it was recorded in Acacia hybrid plantations in the North West, North Central, South Central, and Highland Central regions [31]. By 2016, E. fornicatus (Figure 4o) had spread into Acacia auriculiformis, A. mangium and Acacia hybrid (Figure 4b) plantations across seven forest zones [21]. Three-year-old stands are especially vulnerable with p% of 29–33%, DI of 0.31–0.45, and 2–5% mortality. Trees from 1.5 to 15 years old have been attacked in some locations. The gallery system is created (Figure 4h) to lay eggs in broods leads to the degradation of wood quality. In addition, the beetle-transported fungus Fusarium euwallaceae [21,67] causes the wood to become discolored and decay.
3.3.3. Lycaria westermanni (Chrysomelidae) Leaf Beetle (Vietnamese: Bo Canh Cung An La)
This species was recorded in Vietnam in 1981 [68]. In 2018, L. westermanni (Figure 4p) was recorded damaging 18 ha of Fernandoa brilletii plantations in the North Central region. By 2020, over 50 ha of F. brilletii plantations had been damaged across three forest zones. The foliage of 1–10 year-old trees can be completely consumed (Figure 4c). p% is 45–68%, DI is 1.4–2.3, and some mortality has been reported [69].
3.3.4. Tapinolachnus lacordairei (Cerambycidae) Brown Longhorn Beetle (Vietnamese: Xen Toc Nau Den)
This species was recorded in the North West in 1933 [70]. In 2019 this pest (Figure 4q) was recorded damaging 34 ha of Chukrasia tabularis plantations (5–15 year-old) in North West [49]. The boles of attacked trees have numerous exit holes (Figure 4j). Damage from larvae feeding in the wood can lead to tree senescence and death (Figure 4d). p% is 13–23%, and DI is 0.3–0.6. By 2020, hundreds of hectares of C. tabularis plantations had been damaged as this pest can spread quickly. Cross-vane traps with ethanol have been used, with low effectiveness, to trap adults in C. tabularis plantations.
3.3.5. Xyleborus perforans (Curculionidae) Island Pinhole Borer (Vietnamese: Mot Nuoi Nam Perfor)
The species was recorded in Tonkin Island in Vietnam in 1992 [66]. In 2019 this pest was recorded damaging 155 ha of Eucalyptus urophylla plantations in the North East [71].
Compartments from 6-months-old to 3-year-old are vulnerable with p% of 26–32% and DI of 0.42–0.54. Xyleborus perforans (Figure 4r) create galleries (Figure 4k) that extend from the bark to the heartwood and this reduces tree growth and wood quality. More recently, X. perforans was reported in Acacia hybrid plantations in the South East (Dong Nai province) and South West (Ca Mau province). So far, the damage is low in Acacia plantations (p% is 7–10%, DI 0.1–0.3), but this pest is a threat to the future development of Acacia plantations in Vietnam.
3.3.6. Xystrocera festiva (Cerambycidae) Stem Borer (Vietnam: Xen Toc Canh Xanh)
The pest (Figure 4s) was reported in 2013 damaging 3 ha of Acacia mangium plantations in the Highland Central region [72]. It has now spread to the South Central region. The borer causes tree decline (Figure 4f) and death. Trees of all diameters of trees are attacked, and infestation increases with stand age. p% is 12–46%, and DI is 0.3–1.5.
3.4. Other Sap-Sucking Damage
3.4.1. Aulacaspis tubercularis (Diaspididae) White Mango Scale (Vietnamese: Rep Xoai Trang)
The pest was reported damaging Mangifera indica plantations in the North East region in 2008 [73]. In 2015 A. tubercularis (Figure 5g) was observed damaging 5 ha of Cinnamomum cassia plantations in the South West region.

Figure 5.
Sap-sucking insect damage to forest plantations in Vietnam. (a–c) damaged trees and bamboo; (d–f) larvae; (g–i) adults. (a,d,g) Aulacaspis tubercularis damage in Cinnamomum cassia; (b,e,h) Helopeltis theivora damage in C. cassia; (c,f,i) Hieroglyphus tonkinensis damage in Dendrocalamus barbatus. Scare bar: g = 0.2 cm; h = 0.5 cm; i = 1.0 cm.
It has now spread to C. cassia plantations in two further regions. The sap-sucking scale poses a severe threat to nursery stock and 1–5-year-old plantations (Figure 5a,d). p% and DI are 9–31% and 0.1–1.0 in nurseries, and 15–41% and 0.2–1.3 in plantations, respectively.
3.4.2. Helopeltis theivora (Miridae) Tea Mosquito Bug (Vietnamese: Bo Xit Muoi Mau Xanh)
This pest was reported in 2010 in the North Central region causing damage to Acacia hybrid (7 ha), Acacia mangium (15 ha), Cinnamomum cassia (8 ha), Melaleuca cajuputi (2 ha) and M. leucadendra (2 ha) [18]. It has now spread over much of Vietnam. Nursery stock (cuttings and hedge trees) is particularly vulnerable to attack. Nymphs (Figure 5e) and adults (Figure 5h) feed on new shoots and young leaves (Figure 5b). Damage appears initially as a lesion or area of necrosis around the feeding site and progresses to wilt, dieback and shoot death [74]. p% is 13–79%, and DI is 0.2–2.2.
3.4.3. Hieroglyphus tonkinensis (Acrididae) Grasshopper (Vietnamese: Chau Chau Mia Chay Xanh)
This species was recorded in 1912 in the North Plain [47]. In 1976 it was reported to occur in the North West, North East, and North Centre regions [75]. Later records include the North Central region in 2003 [76] and 2008 [77], and the North East region in 2020 [78]. Since 2011, the pest has damaged 80–150 ha of Dendrocalamus barbatus and D. latiflorus plantations in the North West and North East, with p% of 22–63% and DI of 0.3–1.8 (Figure 5c). A periodic survey was conducted by local officers to detect the occurrence of nymphs and adults (Figure 5i). When outbreaks occurred, the insecticides cypermethrin + chlorpyrifos, abamectin, and emamectin benzoate have been applied by local authorities to reduce the impact of the pest.
3.5. Damage from Pathogens
3.5.1. Ceratocystis manginecans (Ceratocystidaceae) Ceratocystis Wilt Disease (Vietnamese: Benh Chet Heo)
The pathogen was recorded in 2011 in Acacia hybrid and A. mangium plantations in the North East, North West, and North Central regions. Over time, it has spread to A. auriculiformis (Figure 6a), Eucalyptus camaldulensis, E. urophylla (Figure 6d), Chukrasia tabularis, and Dalbergia tonkinensis plantations in the eight forest geographic regions (Figure 1) in Vietnam. Damage is most severe in the North West and North Central zones. Diseased trees typically have wilted foliage and stem cankers with sap exudate. Wood beneath the lesioned areas become blue or gray and then turn grey-brown [29,30,79,80,81]. Ceratocystis manginecans causes severe wilt disease in 1–3 year-old Acacia hybrid (Figure 6b) and A. mangium (Figure 6c) plantations. p% and DI are 15–25% and 0.4–0.9, respectively. Trees can die 3–6 months after infection [79,81]. Some fungicides (carbendazim, mancozeb, and metalaxyl) and biological agents (Trichoderma spp., Bacillus spp.) have been used in experiments to try and control C. manginecans [82]. Pruning in the dry season and decreasing bark tearing during pruning can reduce the levels of infection and disease [28]. There is evidence of resistance to C. manginecans in some clonal Acacia [83,84] and in some families of C. tabularis [29].

Figure 6.
Pathogens damaging forest plantations and nurseries in Vietnam. (a–d) Ceratocystis manginecans wilt disease; (a) Acacia auriculiformis; (b) Acacia hybrid; (c) A. mangium; (d) Eucalyptus urophylla; (e) Shoot rot in Dendrocalamus latiflorus caused by Fusarium solani; (f) Decline in Acacia hybrid mother plants caused by Phytophthora acaciivora.
3.5.2. Fusarium solani (Nectriaceae) Fusarium Rot Disease (Vietnamese: Benh Thoi Mang)
Fusarium solani was recorded in 1999 on lychee trees in the North East and on coffee plants in the Highland Central [85]. Later, this species was mainly found on agricultural hosts such as pepper, citrus, and peanut. In 2020, this species was recorded damaging 700 ha of Dendrocalamus latiflorus plantations in the North East (Yen Bai province) [55]. The pathogen causes greyish brown lesions surrounded by dark brown margins on the outer protective sheath of the shoot (Figure 6e). The lesions spread quickly to the inner sheaths causing rot and a strong odor. This disease appears mainly during the humid season (July to October), when young shoots are harvested for food. p% of rot disease is 16–20% and DI is 0.2–0.3.
3.5.3. Phytophthora acaciivora (Peronosporaceae) Phytophthora Disease (Vietnamese: Benh Thoi Re)
This pathogen was first collected in 2013 in an Acacia mangium nursery in the North East [58]. In recent years P. acaciivora has been reported causing damage to Acacia hybrid and A. mangium nurseries in the same region. Infested seedlings, cuttings, and mother tree banks become wilted (Figure 6f) and then die from root rot. The fungicide potassium phosphonate is being used by local people to reduce P. acaciivora damage in forest nurseries. p% and DI in nurseries are 25–30% and 0.6–1.1, respectively. The mortality rate is 18–21%. There is concern that the pathogen poses a high risk to young Acacia plantations in some geographic regions.
4. Discussion
This is the first comprehensive study of new and emerging insect pest and pathogen threats to plantation forests in Vietnam. It comprised 10 years of field surveys monitoring the health of 12 native and 8 exotic plantation species. The surveillance was undertaken across all the eight geographic forest regions of Vietnam and was representative of the 493,000 ha of native and the 2,447,000 ha of exotic tree species and hybrids in plantations. Overall, 14 species of insects and 3 species of plant pathogens were recognized as either new or emerging threats to the forestry sector. It is worrying that the number of pests increased from 1 in 2011 to 14 in 2020, and the trajectory indicates that further outbreaks are likely to occur. In addition, the field surveys have shown that the range of some pests, notably Aulacaspis tubercularis and Euwallacea fornicatus, has rapidly expanded. Of the three plant pathogens that were classified as threats, Ceratocystis manginecans is of the greatest concern due to its increase in geographical range and spread from Acacia and Eucalyptus to the native Chukrasia tabularis and Dalbergia tonkinensis.
Apart from Ceratocystis manginecans, which has a wide distribution across South and Southeast Asia [86,87], and Batocera lineolata, which is a pest in forest plantations in southern China [52], probably all the species of concern are native in Indo-China. Some of the insect pests have broader natural geographical ranges in Asia [59,88], and some have become invasive alien species. Some wood borers and bark beetles have been distributed around the world, such as B. lineolata in India [89] and Europe [90], Euwallacea fornicatus in Indonesia [51] and the USA [31], and Tapinolachnus lacordairei in Indochina and Borneo [70]. Recent research has clarified the species boundaries for the E. fornicatus complex [91] and highlighted the roles of the Fusarium symbiont in determining host tree susceptibility [92].
Some of the new plantation forest pests in Vietnam are well-known damaging agricultural and horticultural crops. For example, the white mango scale Aulacaspis tubercularis causes global damage to fruit trees [93], and Helopeltis theivora damages cacao in Malaysia [94] and Camellia sinensis plantations in India [95]. In Vietnam, Hieroglyphus tonkinensis was recorded damaging field crops in Hoa Binh Province in 1976 [75], Thanh Hoa Province in 2003 [76], Yen Bai Province in 2020 [78], and Phu Tho Province in 2008 [77]. Fusarium solani was documented in roots of lychees in 1999 in Bac Giang Province and on coffee in Dak Lak Province [85]. Regarding the pathogens, Ceratocystis manginecans was first described in mango in Oman and Pakistan [96]. Since then, it has become very invasive in Acacia plantations across Southeast Asia [86,87] and its host range now includes Dalbergia tonkinensis [30] and Chukrasia tabularis [29]. High mortality in Acacia plantations in Sumatra led to their replacement with Eucalyptus pellita [97]. In the past, much attention had been placed on Phytophthora species causing damage to field and horticultural tree crops [98]. It is only recently that studies have been undertaken in conservation and production forests in Vietnam [99,100].
There is a high diversity of forest tree species in Vietnam and almost nothing is known about the diversity and severity of pests and pathogens and their impact in natural and secondary forests. Many tree species deserve consideration for domestication and plantation production [101]. Furthermore, there is an additional 1,458,000 ha of plantation forests that were not included in this study. The nearly 300,000 ha of pine plantations (Pinus massoniana, P. merkusii, P. kesya) have been well-studied in the past and damage from pests and pathogens is well known [18,19,22], so they were excluded from MARD’s priority lists of plantation species for forest health research. The remaining 1,158,000 ha includes mangroves, other bamboo species, small monocultures of other native species, and some mixed forests. At present, there is no national strategy to investigate the health of these forests even though tree mortality has been observed [102,103]. These planted forests in Vietnam have potential pests and diseases, some of which may become invasive and damage new production forests in the future. From 2021–2030, Vietnam has a target to plant an additional 4000–6000 ha of native trees each year [5]. Therefore, increased funding is needed for forest health monitoring for the whole forest estate in Vietnam.
To manage invasive pests and diseases, it is necessary to further invest in the selection and breeding of resistant hosts. So far, the effort to identify resistant genotypes has been limited. Some Acacia clones [83,84] and families of Chukrasia tabularis [29] have shown resistance to Ceratocystis maginecans; several provenances of Neolamarckia cadamba and Nauclea orientalis were resistant to Arthroschista hilaralis and Moduza procris [62]; Five Acacia hybrid clones were resistant to Euwallacea fornicatus [104]; and three Eucalyptus hybrid clones had high resistance to Batocera lineolata [48]. Hundreds of other new varieties have been selected only on their growth attributes [105]. As a priority, future tree breeding programs in Vietnam should embrace the main biotic threats in their objectives.
A number of studies have recommended improvements to nursery hygiene [27,106]. At present, many small forest nurseries are using unpasteurized soil or growing plants on the ground, and there is a high risk that nurseries may be sources of pathogens such as Phytophthora [58]. Silvicultural measures such as improved pruning techniques [28], limiting damage to roots, stems and branches [28,106,107], and diversification of the tree species composition of plantations [108] are opportunities to limit invasions of pests and diseases. Integrated pest management (IPM) has been successfully implemented for some pest species [109,110,111]. Although IPM has been applied to manage some pests in Vietnam such as Dasychira auxutha and Dendrolimus punctatus in Pinus spp. [112], Biston suppressaria and Krananda semihyalina in Cinnamomum cassia [113], and Ceratocystis manginecans in Acacia spp. [114], the field is in its infancy in forestry in Vietnam compared to IPM for horticultural tree crops [115].
The observed increase in plantation forest damage from new pests and pathogens in Vietnam parallels observations from around the world. These events are impacting livelihoods, economic development, and biodiversity [116]. The increase in invasive insect pests and pathogens in North America and Europe is concurrent with climate change and globalization [117,118]. The huge expansion in global trade and changes in trade routes have increased the risk of accidental introduction of pests and pathogens.
The policy of plant protection and quarantine in Vietnam includes import quarantine, resistant varieties, cultivation techniques, pest monitoring, and priority deployment of biological agents [119]. Recently, a technical support project was employed by FAO to set up a strategy framework and national plan for integrated pest management [120].
5. Conclusions
Extensive monitoring of forest plantations over a decade across Vietnam has revealed dynamic temporal changes in biotic threats to tree health. To guard against future commercial loss in the forest economy, steps need to be taken now to minimize future impacts of pests and disease. This requires improving nursery hygiene, adopting silvicultural practices that reduce physical damage to trees, selecting resistant genotypes, and strengthening national biosecurity and quarantine. However, the latter is difficult to achieve given the long land and sea borders in Vietnam. Early detection is critical [121], and for Vietnam, this necessitates developing forest health monitoring protocols for the extensive forest reserves in protection—special-purpose as well as urban forests. This will require the use of sentinel plantings [122] and remote sensing to detect trees under stress.
Author Contributions
These authors contributed equally to this work; Conceptualization, P.Q.T. and B.D.; investigation and sampling, P.Q.T., L.V.B., D.N.Q., N.M.C. and T.X.H.; rearing and isolation, D.N.Q., N.M.C., L.V.B. and T.X.H.; identification, P.Q.T. and B.D.; analysis, D.N.Q., T.X.H., and N.M.C.; methodology, D.N.Q., N.M.C., L.V.B. and T.X.H.; validation, P.Q.T. and B.D.; visualization, P.Q.T. and B.D.; writing—original draft, P.Q.T. and N.M.C.; writing—review and editing, P.Q.T. and B.D. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Ministry of Agriculture and Rural Development of Vietnam, projects “Forest health surveillance in Vietnam, phase I, code: 505/BVTV”, “Forest health surveillance in Vietnam, phase II, code: 148/QĐ-BNN-KH”, “Study on integrated management for insect pests in Cinnamomum cassia in Northern region and Quang Nam province, Vietnam, code: 03/HĐ-ĐTKHCN”, “Study on integrated pest management of major leave insects and stem borer beetles in Acacia hybrid, A. mangium and A. auriculiformis in Vietnam, code: 04/HĐ-ĐTKHCN”, and “Breeding and planting method development of Chukrasia tabularis for high productivity and tolerance of shoot-tip borer in Vietnam, code: 3710/QĐ-BNN-KHCN”.
Acknowledgments
We thank our colleagues for their support in collecting samples and data, and Gondess Pty Ltd. for travel support to B.D. and covering publication costs.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Meyfroidt, P.; Lambin, E.F. The causes of the reforestation in Vietnam. Land Use Policy 2008, 25, 182–197. [Google Scholar] [CrossRef]
- Cochard, R.; Nguyen, V.H.T.; Ngo, D.T.; Kull, C.A. Vietnam’s forest cover changes 2005–2016: Veering from transition to (yet more) transaction? World Dev. 2020, 135, 105051. [Google Scholar] [CrossRef]
- MARD. Decision No. 1439/QD-BNN-TCLN Dated 13/4/2021 of the Ministry of Agriculture and Rural Development of Vietnam on Announcing the State of Forests Nationwide in 2020; Ministry of Agriculture and Rural Development: Hanoi, Vietnam, 2021; p. 8.
- Vietnam Report on the Implementation Result of the Five Million Hectares New Afforestation Project and the Forest Protection and Development Program in the 2011–2020 Periods; The Socialist Republic of Vietnam: Hanoi, Vietnam, 2011; p. 34.
- Vietnam Decision No. 523/QD-TTg Dated 1/4/2021 of the Prime Minister of the Socialist Republic of Vietnam on Approving the Vietnam Forestry Development Strategy for the Period of 2021–2030, with a Vision to 2050; The Socialist Republic of Vietnam: Hanoi, Vietnam, 2021; p. 23.
- MARD. Wood Processing Industry, Wood and Forest Product Export in 2018-Successes. Lessons Learned. Breakthrough Solutions in 2019; Ministry of Agriculture and Rural Development: Hanoi, Vietnam, 2019; p. 15.
- Cuong, T.; Chinh, T.T.Q.; Zhang, Y.; Xie, Y. Economic performance of forest plantations in Vietnam: Eucalyptus, Acacia mangium, and Manglietia Conifera. Forests 2020, 11, 284. [Google Scholar] [CrossRef]
- Khuc, Q.V.; Le, T.A.T.; Nguyen, T.H.; Nong, D.; Tran, B.Q.; Meyfroidt, P.; Tran, T.; Duong, P.B.; Nguyen, T.T.; Tran, T.; et al. Forest cover change, households’ livelihoods, trade-offs, and constraints associated with plantation forests in poor upland-rural landscapes: Evidence from North Central Vietnam. Forests 2020, 11, 548. [Google Scholar] [CrossRef]
- Tham, L.T.; Darr, D.; Pretzsch, J. Contribution of small-scale Acacia hybrid timber production and commercialization for livelihood development in Central Vietnam. Forests 2020, 11, 1335. [Google Scholar] [CrossRef]
- MOIT. Vietnam Import and Export Report 2020; Industry and Trade Publisher: Hanoi, Vietnam, 2021; p. 252. [Google Scholar]
- Iwanaga, S.; Hoang, D.T.; Kuboyama, H.; Duong, D.T.; Tuan, H.H.; Minh, N.V. Changes in the Vietnamese timber processing industry: A case of Quang Tri province, North Central region. Forests 2021, 12, 984. [Google Scholar] [CrossRef]
- Andjic, V.; Dell, B.; Barber, P.; Hardy, G.; Wingfield, M.; Burgess, T. Plants for planting; indirect evidence for the movement of a serious forest pathogen, Teratosphaeria destructans, in Asia. Eur. J. Plant Pathol. 2011, 131, 49–58. [Google Scholar] [CrossRef]
- Burgess, T.I.; Wingfield, M.J. Pathogens on the move: A 100-year global experiment with planted eucalypts. Bioscience 2017, 67, 14–25. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Cheewangkoon, R.; Carnegie, A.J.; Burgess, T.I.; Summerell, B.A.; Edwards, J.; Taylor, P.W.J.; Groenewald, J.Z. Foliar pathogens of eucalypts. Stud. Mycol. 2019, 94, 125–298. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Roux, J.; Wingfield, B.D. Insect pests and pathogens of Australian acacias grown as non-natives—An experiment in biogeography with far-reaching consequences. Divers. Distrib. 2011, 17, 968–977. [Google Scholar] [CrossRef]
- Old, K.M.; Dudzinski, M.J.; Pongpanich, K.; Yuan, Z.Q.; Thu, P.Q.; Nguyen, T.N. Cryptosporiopsis leaf spot and shoot blight of eucalypts. Australas. Plant Pathol. 2002, 31, 337–344. [Google Scholar] [CrossRef]
- Old, K.M.; Wingfield, M.J.; Yuan, Z.Q. A Manual of Diseases of Eucalyptus in South-East Asia; CIFOR: Canberra, Australia, 2003; p. 106. [Google Scholar]
- Thu, P.Q.; Griffiths, M.W.; Pegg, G.S.; McDonald, J.; Wylie, F.R.; King, J.; Lawson, S.A. Healthy Plantations. A Field Guide to Pests and Pathogens of Acacia, Eucalyptus and Pinus in Vietnam; Department of Employment, Economic Development and Innovation: Queensland, Australia, 2010; p. 124. [Google Scholar]
- Dell, B.; Xu, D.; Thu, P.Q. Managing threats to the health of tree plantations in Asia. In New Perspectives in Plant Protection; Bandani, A.R., Ed.; InTech: Rijeka, Croatia, 2012; pp. 63–92. [Google Scholar]
- Thu, P.Q.; Dell, B.; Burgess, T.I. Susceptibility of 18 eucalypt species to the gall wasp Leptocybe invasa in the nursery and young plantations in Vietnam. ScienceAsia 2009, 35, 113–117. [Google Scholar]
- Thu, P.Q. Results of a survey of insect pests and diseases of the main forest plantation species in Vietnam. Vietnam J. For. Sci. 2016, 1, 4257–4264. [Google Scholar]
- Thu, P.Q. Status of a Pine wilt nematode in Vietnam. N. Z. J. For. Sci. 2003, 33, 336–342. [Google Scholar]
- Thu, P.Q. Die-back disease of Eucalyptus urophylla caused by bacteria Ralstonia solanacearum (Yabauchi et al. 1995) Smith. Sci. Tech. J. Agric. Rural Dev. 2006, 5, 90–91. [Google Scholar]
- Thu, P.Q.; Binh, L.V. Longhorned beetle (Aeolesthes holosericea (Fabricus)) damages stem of Melia azedarach Linnaeus. Sci. Tech. J. Agric. Rural Dev. 2010, 7, 84–88. [Google Scholar]
- Thu, P.Q.; Binh, L.V.; Sinh, L.V. Longhorn beetle damages Rhizophora apiculata plantation in mangrove forest in Can Gio, Ho Chi Minh city. Sci. Tech. J. Agric. Rural Dev. 2008, 8, 84–87. [Google Scholar]
- Thu, P.Q.; Quang, D.N.; Dell, B. Threat to cedar, Cedrela odorata, plantations in Vietnam by the weevil, Aclees sp. J. Insect Sci. 2010, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.T.; Dao, X.T. Pests and Diseases and Plant Protection Measures; Agricultural Publisher: Hanoi, Vietnam, 2004; p. 168. [Google Scholar]
- Chi, N.M.; Thu, P.Q.; Hinh, T.X.; Dell, B. Management of Ceratocystis manginecans in plantations of Acacia through optimal pruning and site selection. Australas. Plant Pathol. 2019, 48, 343–350. [Google Scholar] [CrossRef]
- Chi, N.M.; Trang, T.T.; Nhung, N.P.; Quang, D.N.; Son, V.M.; Tuan, T.A.; Mai, L.T.; Hung, T.X.; Nam, N.V.; Thu, P.Q.; et al. Ceratocystis wilt in Chukrasia tabularis in Vietnam: Identification, pathogenicity and host tolerance. Australas. Plant Pathol. 2021, 50, 17–27. [Google Scholar] [CrossRef]
- Chi, N.M.; Nhung, N.P.; Trang, T.T.; Thu, P.Q.; Hinh, T.X.; Nam, N.V.; Quang, D.N.; Dell, B. First report of wilt disease in Dalbergia tonkinensis caused by Ceratocystis Manginecans. Australas. Plant Pathol. 2019, 48, 439–445. [Google Scholar] [CrossRef]
- Stouthamer, R.; Rugman-Jones, P.; Thu, P.Q.; Eskalen, A.; Thibault, T.; Hulcr, J.; Wang, L.; Jordal, B.H.; Chen, C.; Cooperband, M. Tracing the origin of a cryptic invader: Phylogeography of the Euwallacea fornicatus (Coleoptera: Curculionidae: Scolytinae) species complex. Agric. For. Entomol. 2017, 19, 366–375. [Google Scholar] [CrossRef]
- McMaugh, T. Guidelines for Surveillance for Plant Pests in Asia and the Pacific; No. 435-2016-33725; Union Offset: Canberra, Australia, 2005; p. 192. [Google Scholar]
- Gichuhi, J.M.; Ndegwa, P.N.; Mugo, H.M.; Guandaru, E.K.; Babin, R. Rearing method and developmental biology of the African coffee white stem borer, Monochamus leuconotus (Coleoptera: Cerambycidae). J. Econ. Entomol. 2016, 110, 1120–1126. [Google Scholar] [CrossRef]
- Biedermann, P.H.W.; Klepzig, K.D.; Taborsky, M. Fungus cultivation by ambrosia beetles: Behavior and laboratory breeding success in three Xyleborine species. Environ. Entomol. 2009, 38, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.J.; Jeffers, S.N. Detecting multiple species of Phytophthora in container mixes from ornamental crop nurseries. Plant Dis. 1999, 83, 1129–1136. [Google Scholar] [CrossRef]
- Moller, W.J.; DeVay, J.E. Insect transmission of Ceratocystis fìmbriata in deciduous fruit orchards. Phytopathology 1968, 58, 1499–1508. [Google Scholar]
- Carter, D. Butterflies and Moths (Eyewitness Handbooks); Dorling Kindersley Inc.: New York, NY, USA, 1992. [Google Scholar]
- Gressitt, J.L. Pacific Insects Monographs; Bernice Pauahi Bishop Museum: Honolulu, HI, USA, 1961; p. 314. [Google Scholar]
- Scoble, M.J. The Lepidoptera. Form, Function and Diversity; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Wang, Y.; Li, C.-D.; Han, H.-L. A newly recorded species of the genus Krananda (Lepidoptera: Geometridae) from China. Entomotaxonomia 2010, 32, 74–76. [Google Scholar]
- Gomez, D.F.; Rabaglia, R.J.; Fairbanks, K.E.O.; Hulcr, J. North American Xyleborini north of Mexico: A review and key to genera and species (Coleoptera, Curculionidae, Scolytinae). ZooKeys 2018, 768, 19. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, S.; Geng, X.; Fang, L.; Zhang, W.; Shu, J.; Wang, H. Population differentiation of Batocera lineolata (Coleoptera: Cerambycidae) whose larvae fed on different host tree species. Sci. Silvae Sin. 2020, 56, 91–103. [Google Scholar]
- Thomson, J. Systema Cerambycidarum ou Expose de Tous les Genres Compris dans la Famille des Cerambycides et Familles Limitrophes; Société royale des sciences de Liège. Dessain: Luxembourg, 1864; p. 352. [Google Scholar]
- Vitali, F.; Gouverneur, X.; Chemin, G. Revision of the tribe Cerambycini: Redefinition of the genera Trirachys Hope, 1843, Aeolesthes Gahan, 1890 and Pseudaeolesthes Plavilstshikov (Coleoptera, Cerambycidae). Les Cah. Magellanes 2017, 26, 40–65. [Google Scholar]
- Newstead, R. On a collection of Coccidae and other insects affecting some cultivated and wild plants in Java and in Tropical Western Africa. J. Econ. Biol. 1908, 3, 33–42. [Google Scholar]
- Waterhouse, C.O., XVII. Some observations on the tea-bugs (Helopeltis) of India and Java. Trans. R. Entomol. Soc. Lond. 1886, 34, 457–460. [Google Scholar] [CrossRef]
- Bolívar, I. Estudios Entomológicos: El Género Hieroglyphus Krauss y Otros Próximos; Junta para Ampliación de Estudios é Investigaciones Científicas: Madrid, Spain, 1912; pp. 46–62. [Google Scholar]
- Quang, D.N.; Chi, N.M.; Thao, D.V.; Thanh, L.B.; Son, L.T.; Chung, D.H.; Minh, L.N.; Dell, B. Damage caused by Batocera lineolata Chevrolat (Coleoptera: Cerambycidae) in Eucalyptus and its management in Vietnam. Int. J. Trop. Insect Sci. 2021. accepted. [Google Scholar]
- Chi, N.M.; Thanh, N.V.; Quang, D.N.; Thanh, L.B.; Thao, D.V.; Son, L.T.; Hinh, T.X.; Thu, P.Q.; Dell, B. First report of Tapinolachnus lacordairei (Coleoptera: Cerambycidae) damage in Chukrasia Tabularis. Int. J. Trop. Insect Sci. 2020, 41, 909–914. [Google Scholar] [CrossRef]
- Liu, J.H.; Jia, P.F.; Luo, T.; Wang, Q.M. Complete mitochondrial genome of white-striped long-horned beetle, Batocera lineolata (Coleoptera: Cerambycidae) by next-generation sequencing and its phylogenetic relationship within superfamily Chrysomeloidea. Mitochondrial DNA Part B 2017, 2, 520–521. [Google Scholar] [CrossRef][Green Version]
- Lynn, K.M.T.; Wingfield, M.J.; Durán, A.; Marincowitz, S.; Oliveira, L.S.S.; de Beer, Z.W.; Barnes, I. Euwallacea perbrevis (Coleoptera: Curculionidae: Scolytinae), a confirmed pest on Acacia crassicarpa in Riau, Indonesia, and a new fungal symbiont; Fusarium rekanum sp. nov. Antonie Van Leeuwenhoek 2020, 113, 803–823. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, Y.; Chen, X. Complete sequence and gene organization of the mitochondrial genome of Batocera lineolata Chevrolat (Coleoptera: Cerambycidae). Chin. Sci. Bull. 2012, 57, 3578–3585. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Dai, X.Y.; Xu, X.D.; Zhang, Z.Y.; Yu, D.N.; Storey, K.B.; Zhang, J.Y. The complete mitochondrial genomes of five longicorn beetles (Coleoptera: Cerambycidae) and phylogenetic relationships within Cerambycidae. PeerJ 2019, 7, e7633. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Hung, T.X. An initial determination of the cause of emerging shoot rot disease associated with sweet bamboo (Dendrocalamus latiflorus) in Yen Bai province. Vietnam J. For. Sci. 2020, 6, 118–125. [Google Scholar]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Cooke, D.E.L.; Drenth, A.; Duncan, J.M.; Wagels, G.; Brasier, C.M. A molecular phylogeny of Phytophthora and related Oomycetes. Fungal Genet. Biol. 2000, 30, 17–32. [Google Scholar] [CrossRef]
- Burgess, T.I.; Dang, Q.N.; Le, B.V.; Pham, N.Q.; White, D.; Pham, T.Q. Phytophthora acaciivora sp. nov. associated with dying Acacia mangium in Vietnam. Fungal Syst. Evol. 2020, 6, 243–252. [Google Scholar]
- Arora, G.S.; Gupta, J.J. Non mulberry silkmoths. Mem. Zool. Surv. India 1979, 17, 25–28. [Google Scholar]
- Quang, D.N.; Dieu, N.K.; Dat, K.T.; Chi, N.M. First report of Antheraea frithi damaging Dipterocarpus alatus and Hopea odorata in Southest Vietnam. Vietnam J. For. Sci. 2021, 1, 68–74. [Google Scholar]
- Thu, P.Q.; Binh, L.V.; Thao, V.N.; Chi, N.M. Main insect pests damaging Neolamarckia cadamba and Nauclea orientalis plantations in Ca Mau province. Vietnam J. For. Sci. 2016, 4, 4731–4738. [Google Scholar]
- Chi, N.M.; Thao, V.N. Growth and insect pests damaging characterizations from Neolamarckia cadamba and Nauclea orientalis provenance trials in Ca Mau province. Sci. Tech. J. Agric. Rural Dev. 2017, 1, 96–101. [Google Scholar]
- Quang, D.N.; Thu, P.Q.; Nguyet, N.T.A.; Chi, N.M. First report on two catepillars damaging Ailanthus triphysa plantation in Phu Tho province. Vietnam J. For. Sci. 2020, 6, 135–141. [Google Scholar]
- Lien, V.V. Butterfly species list (Lepidoptera: Rhopolocera) of Natural forest on mountain of Pu Mat National park, Nghe An province. In Proceedings of the 6th National Scientific Conference on Ecology and Biological Resources, Hanoi, Vietnam, 21 October 2015; pp. 1493–1499. [Google Scholar]
- Thanh, L.B.; Toan, M.N.; Huong, N.T.T.; Chi, N.M.; Minh, L.N.; Bac, B.V. First report of Batocera lineolata (Coleoptera: Cerambycidae) damage in eucalypt in Hoa Binh province. J. For. Sci. Tech. 2021, 3, 106–112. [Google Scholar]
- Wood, S.L. A catalog of Scolytidae and Platypodidae (Coleoptera), Part 2: Taxonomic index. Great Basin Nat. Mem. 1992, 13, 1–1553. [Google Scholar]
- Freeman, S.; Sharon, M.; Maymon, M.; Mendel, Z.; Protasov, A.; Aoki, T.; Eskalen, A.; O’Donnell, K. Fusarium euwallaceae sp. nov.—A symbiotic fungus of Euwallacea sp., an invasive ambrosia beetle in Israel and California. Mycologia 2013, 105, 1595–1606. [Google Scholar]
- Shinsaku, K.; Gressitt, J.L. Chrysomelidae (Coleptera) of Thailand, Cambodia, Laos and Vietnam. Pac. Insects 1981, 3, 286–391. [Google Scholar]
- Quang, D.N.; Chi, N.M.; Son, V.M. Leaf beetle (Lycaria westermanni) damaging Fernandoa brilletii plantations in Vietnam. In Proceedings of the 10th National Entomology Conference, Hanoi, Vietnam, 22–23 October 2020; pp. 691–696. [Google Scholar]
- Miroshnikov, A.I. The longicorn beetle tribe Cerambycini Latreille, 1802 (Coleoptera: Cerambycidae: Cerambycinae) in the fauna of Asia. 4. New or little-known taxa, mainly from Indochina and Borneo, with reviews or annotated checklists of species of some genera. Cauc. Entomol. Bull. 2018, 14, 197–246. [Google Scholar]
- Hung, T.X.; Chi, N.M.; Quang, D.N.; Thu, P.Q. First report of polyphagous shot hole borer associated with Eucalyptus urophylla in Phu Tho and Bac Giang provinces, Vietnam. Sci. Tech. J. Agric. Rural Dev. 2019, 15, 109–114. [Google Scholar]
- Thu, P.Q.; Binh, L.V.; Long, P.D. Longhorn beetle, Xystrocera festiva Thomson, 1860 (Col.: Cerambycidae) a new stem borer of Acacia in Ngoc Hoi district, Kon Tum province. J. Plant Prot. 2013, 2, 5–9. [Google Scholar]
- Ung, N.H.; Nam, L.Q.; Dong, P.T.; Nuong, N.H.; Thuy, N.T.H. Morphological and biological characteristics of Aulacaspis tubercularis (Diaspididae, Homoptera) in mango in Cang Long district, Tra Vinh province. In Proceedings of the Plant Protection Conference, Ho Chi Minh City, Vietnam, 6 November 2015; pp. 429–435. [Google Scholar]
- Binh, L.V.; Thanh, N.V.; Thang, T.V.; Thu, N.H.; Thong, N.Q.; Tong, T.A. Biological characteristics and management of Helopeltis theivora damage in Melaleuca leucadendra and M. cajuputi in Mekong delta. Sci. Tech. J. Agric. Rural Dev. 2021, 12, 91–97. [Google Scholar]
- PPRI. Report of Insect Survellience 1967–1968; Rural Publisher: Hanoi, Vietnam, 1976; p. 66. [Google Scholar]
- Thanh Hoa Report on the Situation of Locust and Control No. 88/BVTV; Thanh Hoa Horticulture and Plant Protection Branch: Thanh Hoa, Vietnam, 2003; p. 56.
- Phu Tho Report of Project 242/BC-TT&BVTV Date 5/10/2018; Phu Tho Horticulture and Plant Protection Branch: Phu Tho, Vietnam, 2018; p. 53.
- Thanh, N.V.; Binh, L.V.; Quang, D.N.; Hung, T.X.; Tong, T.A.; Thang, T.V. Species composition and biological characteristics of some insect pests in Dendrocalamus latiflorus in Tran Yen district, Yen Bai Province. Vietnam J. For. Sci. 2020, 5, 103–111. [Google Scholar]
- Chi, N.M.; Thu, P.Q.; Huy, P.D.; Anh, N.T. Ceratocystis wilt disease of Acacia plantations in Vietnam Paper Corporation. Vietnam J. For. Sci. 2020, 2, 91–100. [Google Scholar]
- Chi, N.M.; Thu, P.Q. Ceratocystis wilt disease of Eucalyptus in Vietnam. Sci. Tech. J. Agric. Rural Dev. 2016, 6, 119–123. [Google Scholar]
- Thu, P.Q.; Chi, N.M.; Tam, T.T.T. Ceratocystis wilt disease of Acacia auriculiformis, Acacia mangium and Acacia hybrid in Vietnam. Sci. Tech. J. Agric. Rural. Dev. 2016, 8, 134–140. [Google Scholar]
- Tran, T.T.T.; Pham, T.Q.; Barber, P.A.; Nguyen, C.M. Control of Ceratocystis manginecans causing wilt disease on Acacia mangium seedlings. Australas. Plant Pathol. 2018, 47, 579–586. [Google Scholar] [CrossRef]
- Chi, N.M.; Thu, P.Q.; Mohammed, C. Screening disease resistance of Acacia auriculiformis clones against Ceratocystis manginecans by artificial and natural inoculation methods. Australas. Plant Pathol. 2019, 48, 617–624. [Google Scholar] [CrossRef]
- Trang, T.T.; Eyles, A.; Davies, N.; Glen, M.; Ratkowsky, D.; Mohammed, C. Screening for host responses in Acacia to a canker and wilt pathogen, Ceratocystis manginecans. For. Pathol. 2018, 48, e12390. [Google Scholar] [CrossRef]
- Burgess, L.W.; Burgess, J.S. Capacity building in plant pathology: Soilborne diseases in Vietnam, 1993–2009. Australas. Plant Pathol. 2009, 38, 325–333. [Google Scholar] [CrossRef]
- Fourie, A.; Wingfield, M.J.; Wingfield, B.D.; Barnes, I. Molecular markers delimit cryptic species in Ceratocystis sensu stricto. Mycol. Prog. 2015, 14, 1020. [Google Scholar] [CrossRef]
- Fourie, A.; Wingfield, M.J.; Wingfield, B.D.; Thu, P.Q.; Barnes, I. A possible centre of diversity in South East Asia for the tree pathogen, Ceratocystis manginecans. Infect. Genet. Evol. 2016, 41, 73–83. [Google Scholar] [CrossRef]
- Shantibala, T.; Devi, K.M.; Lokeshwari, R.K.; Anju, S.; Luikham, R. Complete mitochondrial genome of a latent wild oak tasar silkworm, Antheraea frithi (Lepidoptera: Saturniidae). Mitochondrial DNA Part B 2018, 3, 15–16. [Google Scholar] [CrossRef]
- Boyane, S.S.; Subba, B.; Rajan, P.D.; Ghate, H.V. First illustrated report of Batocera lineolata Chevrolat, 1852 (Cerambycidae, Lamiinae, Batocerini) from India. Check List 2020, 16, 1609. [Google Scholar] [CrossRef]
- Arias, A.; Torralba-Burrial, A. First detection of the exotic longhorn beetle Batocera parryi (Hope) (Coleoptera: Cerambycidae) in Europe. Coleopt. Bull. 2020, 74, 327–330. [Google Scholar] [CrossRef]
- Smith, S.M.; Beaver, R.A.; Cognato, A.I.; Hulcr, J.; Redford, A. Southeast Asian Ambrosia Beetle; USDA APHIS Identification Technology Program and Michigan State University: Fort Collins, CO, USA, 2019; Available online: idtools.org/id/wbb/sea-ambrosia (accessed on 28 March 2020).
- Mendel, Z.; Lynch, S.C.; Eskalen, A.; Protasov, A.; Maymon, M.; Freeman, S. What determines host range and reproductive performance of an invasive ambrosia beetle Euwallacea fornicatus; Lessons from Israel and California. Front. For. Glob. Chang. 2021, 4, 29. [Google Scholar] [CrossRef]
- Labuschagne, T.I.; Van Hamburg, H.; Froneman, I.J. Population dynamics of the mango scale, Aulacaspis tubercularis (Newstead) (Coccoidea: Diaspididae), in South Africa. Isr. J. Entomol. 1995, 29, 207–217. [Google Scholar]
- Tan, G.S. Helopeltis theivora theobromae on cocoa in Malaysia. I. Biology and population fluctuations. Malays. Agric. Res. 1974, 3, 127–132. [Google Scholar]
- Roy, S.; Muraleedharan, N.; Mukhapadhyay, A.; Handique, G. The tea mosquito bug, Helopeltis theivora Waterhouse (Heteroptera: Miridae): Its status, biology, ecology and management in tea plantations. Int. J. Pest Manag. 2015, 61, 179–197. [Google Scholar] [CrossRef]
- Van Wyk, M.; Al Adawi, A.O.; Khan, I.A.; Deadman, M.L.; Al Jahwari, A.A.; Wingfield, B.D.; Ploetz, R.; Wingfield, M.J. Ceratocystis manginecans sp. nov., causal agent of a destructive mango wilt disease in Oman and Pakistan. Fungal Divers. 2007, 27, 213–230. [Google Scholar]
- Inail, M.A.; Hardiyanto, E.B.; Mendham, D.S. Growth responses of Eucalyptus pellita F. Muell plantations in South Sumatra to macronutrient fertilisers following several rotations of Acacia mangium Willd. Forests 2019, 10, 1054. [Google Scholar]
- Drenth, A.; Guest, D.I. Diversity and Management of Phytophthora in Southeast Asia; ACIAR Monograph; Australian Centre for International Agricultural Research: Canberra, Australia, 2004; Volume 114, p. 238. [Google Scholar]
- Jung, T.; Scanu, B.; Brasier, C.M.; Webber, J.; Milenković, I.; Corcobado, T.; Tomšovský, M.; Pánek, M.; Bakonyi, J.; Maia, C.; et al. A survey in natural forest ecosystems of Vietnam reveals high diversity of both new and described Phytophthora Taxa including P. ramorum. Forests 2020, 11, 93. [Google Scholar] [CrossRef]
- Dang, Q.N.; Pham, T.Q.; Arentz, F.; Hardy, G.E.S.; Burgess, T.I. New Phytophthora species in clade 2a from the Asia-Pacific region including a re-examination of P. colocasiae and P. meadii. Mycol. Prog. 2021, 20, 111–129. [Google Scholar] [CrossRef]
- Crowther, J.; Zimmer, H.; Le Thi, H.; Quang, T.L.; Nichols, J.D. Forestry in Vietnam: The potential role for native timber species. For. Policy Econ. 2020, 116, 102182. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Hardy, G.E.S.; Le, T.V.; Nguyen, H.Q.; Nguyen, H.H.; Nguyen, T.V.; Dell, B. Mangrove forest landcover changes in coastal Vietnam: A case study from 1973 to 2020 in Thanh Hoa and Nghe An provinces. Forests 2021, 12, 637. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Hardy, G.E.S.; Le, T.V.; Nguyen, H.Q.; Hoang, D.L.; Nguyen, T.V.; Dell, B. Mangrove dieback and leaf disease in Sonneratia apetala and Sonneratia caseolaris in Vietnam. Forests 2021, 12, 1273. [Google Scholar] [CrossRef]
- Nhung, N.P.; Hung, L.D.; Kien, T.T.; Chi, N.M. Results on trial and evaluation the stems borer beetle of approved Acacia hybrid clones in the Centre and Northwest of Vietnam. Sci. Tech. J. Agric. Rural Dev. 2018, 16, 123–129. [Google Scholar]
- Anh, N.T. Preserve and Preserve Genetic Resources of Pulpwood Species; Vietnam Paper Corporation: Phu Tho, Vietnam, 2020; p. 68. [Google Scholar]
- Thu, P.Q. Investigation of Causes of Diseases and Proposing Measures to Quickly Handle Diseases of Acacia Hybrid and A. mangium Plantations; Vietnam Paper Corporation: Phu Tho, Vietnam, 2016; p. 59. [Google Scholar]
- Chi, N.M. Pathogenicity of Ceratocystis manginecans in inoculated Acacia roots. Indian Phytopathol. 2021, 74, 1–7. [Google Scholar]
- Macpherson, M.F.; Kleczkowski, A.; Healey, J.R.; Quine, C.P.; Hanley, N. The effects of invasive pests and pathogens on strategies for forest diversification. Ecol. Model. 2017, 350, 87–99. [Google Scholar] [CrossRef]
- Del Pino, M.; Bienvenido, C.; Boyero, J.R.; Vela, J.M. Biology, ecology and integrated pest management of the white mango scale, Aulacaspis tubercularis Newstead, a new pest in southern Spain—A review. Crop Protect. 2020, 133, 105160. [Google Scholar] [CrossRef]
- Hu, J.; Angeli, S.; Schuetz, S.; Luo, Y.; Hajek, A.E. Ecology and management of exotic and endemic Asian longhorned beetle Anoplophora glabripennis. Agric. For. Entomol. 2009, 11, 359–375. [Google Scholar] [CrossRef]
- Mohammed, C.L.; Rimbawanto, A.; Page, D.E. Management of basidiomycete root and stem—Rot diseases in oil palm, rubber and tropical hardwood plantation crops. For. Pathol. 2014, 44, 428–446. [Google Scholar] [CrossRef]
- Quang, D.N. Management of Needle Eating Caterpillars Associated with Pinus merkusii and Pinus massoniana in North and North Central Vietnam; Vietnamese Academy of Forest Sciences: Hanoi, Vietnam, 2019; p. 86. [Google Scholar]
- Binh, L.V. Study and Develop an Integrated Management Process for Insect Pests in Cinnamomum cassia Plantations in Vietnam; Vietnamese Academy of Forest Sciences: Hanoi, Vietnam, 2020; p. 167. [Google Scholar]
- Thu, P.Q.; Chi, N.M. Management of wilt disease causing by Ceratocystis manginecans in Acacia plantations in Vietnam. In Proceedings of the National Scientific Conference on Forestry, Hanoi, Vietnam, 15 August 2021; pp. 1–8. [Google Scholar]
- Tran, H.; Van, H.N.; Muniappan, R.; Amrine, J.; Naidu, R.; Gilbertson, R.; Sidhu, J. Integrated pest management of longan (Sapindales: Sapindaceae) in Vietnam. J. Integr. Pest Manag. 2019, 10, 18. [Google Scholar] [CrossRef]
- Graziosi, I.; Tembo, M.; Kuate, J.; Muchugi, A. Pests and diseases of trees in Africa: A growing continental emergency. Plants People Planet 2020, 2, 14–28. [Google Scholar] [CrossRef]
- Freer-Smith, P.H.; Webber, J.F. Tree pests and diseases: The threat to biodiversity and the delivery of ecosystem services. Biodivers. Conserv. 2017, 26, 3167–3181. [Google Scholar] [CrossRef]
- Spence, N.; Hill, L.; Morris, J. How the global threat of pests and diseases impacts plants, people, and the planet. Plants People Planet 2020, 2, 5–13. [Google Scholar] [CrossRef]
- Vietnam Decision No. 35/VBHN-VPQH Dated 10/12/2018 of the Vietnamese National Assembly on Approving the Law of Plant Protection and Quarantine; Vietnamese National Assembly: Hanoi, Vietnam, 2018; p. 18.
- MARD. Action Framework of Integrated Plant Health Management; Code TCP/VIE/3802; Ministry of Agriculture and Rural Development: Hanoi, Vietnam, 2021; p. 8. [Google Scholar]
- Silva, G.; Tomlinson, J.; Onkokesung, N.; Sommer, S.; Mrisho, L.; Legg, J.; Adams, I.P.; Gutierrez-Vazquez, Y.; Howard, T.P.; Laverick, A.; et al. Plant pest surveillance: From satellites to molecules. Emerg. Top. Life Sci. 2021, 5, 275–287. [Google Scholar] [PubMed]
- Britton, K.O.; White, P.; Kramer, A.; Hudler, G. A new approach to stopping the spread of invasive insects and pathogens: Early detection and rapid response via a global network of sentinel plantings. N. Z. J. For. Sci. 2010, 40, 109–114. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).