Abstract
Development of hybrid pines of Pinus radiata D. Don for commercial forestry presents an opportunity to diversify the current resource of plant material. Climate change and different land uses pose challenges, making alternative species necessary to guarantee wood and non-wood products in the future. Pinus radiata var. cedrosensis × Pinus attenuata hybrid possesses different attributes, such as tolerance to drought conditions, better growth and resistance to snow damage at higher altitudes, and more importantly, different wood quality characteristics. Embryogenic cell lines were successfully initiated reciprocal hybrids using as initial explants megagametophytes, excised zygotic embryos and excised zygotic embryos plus nurse culture. However, the questions raised were: does the initiation environment affect the conversion to somatic plantlets months later? Does the mother tree or the cross have an effect on the conversion to somatic plantlets? In the present work we analysed the maturation rate, number of somatic embryos, germination rate, and the ex-vitro growth in cell lines derived from different initiation treatments, mother tree species, and crosses. Differences were not observed for in vitro parameters such as maturation and germination. However, significant differences were observed due to the mother tree species in relation with the ex-vitro growth rates observed, being higher those in which P. radiata acted as a mother. Moreover, embryogenic cell lines from these hybrids were stored at −80 °C and regenerated after one and five years.
1. Introduction
Pinus spp. are frequently used in reforestation programs and, specifically, Pinus radiata D. Don is one of the most cultivated species in New Zealand and Spain because of its fast growth and wood production. However, several stresses derived from climate change can affect the productivity of plantations. This has contributed to a growing interest in the potential of pine hybrids. For instance, the commercial development of the P. patula × P. tecunumanii hybrid in South Africa to improve Fusarium circinatum (Pitch canker) resistance of susceptible P. patula has been a major success story in plantation forestry [1,2]. Hybrids of US southern pines with Mexican pines [2,3,4] may offer opportunities to improve wood properties and create ‘‘varieties’’ better adapted to specific exotic environments or to variable climatic conditions. According to the predictions, climatic change will increase the incidence and severity of droughts in ecosystems worldwide [5]. Different pine species, in comparison with radiata pine, possess different attributes, such as tolerance to subtropical climatic conditions, better growth at higher altitudes, susceptibility to disease, and, importantly, different wood quality characteristics [6]. P. attenuata, for instance, can potentially contribute to drought, cold and snow damage resistance acquisition in P. radiata, which does not have those attributes [6]. In this sense, hybrid pines have been recognised as an opportunity for the future [7,8]. Bearing in mind the information about P. attenuata pure species, some years ago we started studying the drought stress tolerance mechanism of the hybrid P. attenuata × P. radiata trying to understand the mechanisms involved in this process [9]. This hybrid showed higher drought tolerance than breeds from pure species from different geographical and climatological growth areas [10,11,12]. Moreover, some field studies have been carried out to evaluate the performance of the P. attenuata × P. radiata hybrid for afforestation in New Zealand [7].
Conifer propagation methodologies such us somatic embryogenesis (SE) facilitate multiplication and storage of embryogenic tissue while field testing takes place. These technologies are well established for P. radiata [13,14,15] but have not been developed for the Pinus radiata × Pinus attenuata hybrid. For this reason, SCION (New Zealand) and Neiker (Spain) focused on testing and modifying existing P.radiata protocols to produce hybrid plants for field trials [8].
Several studies carried out in our lab in Pinus spp. have showed the effect of environmental conditions during initiation of SE process in the subsequent stages of the process [16,17,18]. Taking into account our previous studies, we were interested in following the effect of the physical and chemical environment of SE process in P. radiata × P. attenuata hybrid and in answering to the following questions:
- Does the presence of initiation environment inhibit or enhance the success of the process?
- What is the effect of the mother (i.e., P. radiata or P. attenuata as the female parent) in embryonal mass conversion to somatic plants?
2. Materials and Methods
2.1. Plant Material
Proseed, a commercial seed orchard located at Amberley (North Canterbury, New Zealand) provided all the test material for this investigation. Green cones were collected from two hybrid crosses, Pinus attenuata × P. radiata var. cedrosensis and Pinus radiata var. cedrosensis × P. attenuata. Both hybrid crosses were control pollinated with a mixture of pollen parents (polycross) of the respective species. Details of crosses in respect to their relatedness were described in [8].
Green cones were stored at 4 °C for one-three days depending on when each cross was put into culture [19]. As described in [8] four collections were made from December to January corresponding to zygotic embryo stages 1 to 6 according to scoring system given by [13].
2.2. Culture Conditions and Initiation of Embryonal Masses
Immature seeds were aseptically removed from the seed coat and given one of the following three treatments (treatment and media details follow the treatment outlines):
- Treatment 1:
- Megagametophyte + Glitz medium
- Treatment 2:
- Excised zygotic embryo (ZE) + Glitz medium
- Treatment 3:
- Excised ZE + Glitz medium + Nurse
Glitz medium [13] is a modified Litvay medium [20] (Table 1) supplemented with 4.5 µM 2,4-dichlorophenoxyacetic acid, 2.7 µM benzyladenine, and 3 g L−1 of gellam gum (Gelrite®; Duchefa, Haarlem, The Netherlands). Amino acids were filter-sterilised but not pH-adjusted and were added to the autoclaved medium.

Table 1.
Basal media composition for the different media used along the somatic embryogenesis process in Pinus attenuata × Pinus radiata and the reciprocal hybrid: Glitz medium, embryo development medium (EDM) and half-strength modified Lepoivre medium (LP ½ Modified).
For treatment 3 each dissected ZE was placed on a sterile piece (3 cm × 3 cm) of Nybolt nylon screen (Scapa Filtration, 30-µm-diameter mesh) on top of a P. radiata nurse culture. The nurse tissue used consisted of a vigorously growing P. radiata cell line that had been maintained by routine subculture to Glitz 2 [8] at 14-day intervals for approximately ten years. This line has no regenerative capability using current Scion somatic embryo maturation protocols. There were three individual nurse cultures per Petri dish, each consisting of approximately 150–250 mg of fresh tissue with a square of Nybolt lightly pressed on to the top of the callus tissue; care was taken to ensure that the callus was vented, and the nylon screen was not forming a complete seal on the media surface. The nurse treatments were applied for seven days and then the screen was shifted to a fresh dish of Glitz for further culture.
Following the initial application of treatments, cultures were incubated under low light (5 μmol m−2 s−1) at 24 ± 1 °C. Four weeks after initiation, all responding explants were transferred to Glitz medium and sub-cultured at 14-day intervals (Figure 1a,b). Following 8–10 weeks of subculture, the embryogenic cell lines (ECLs) had to have in excess of 50 mg of tissue to be scored as established, the results were shown in previous studies [8].
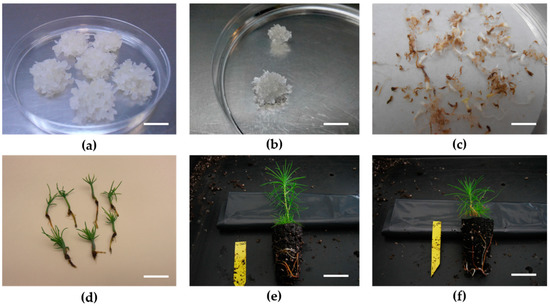
Figure 1.
(a) Pinus attenuata × Pinus radiata embryonal masses at proliferation stage, bar: 13 mm. (b) Pinus radiata × Pinus attenuata embryonal masses regenerated after five years of storage at −80 °C, bar: 10 mm. (c) Pinus attenuata × Pinus radiata somatic embryos, bar: 7 mm. (d) Pinus radiata × Pinus attenuata somatic plantlets after six weeks of in vitro germination, bar: 15 mm. (e) Pinus radiata × Pinus attenuata somatic plantlet after two months in the greenhouse, bar: 35 mm. (f) Pinus attenuata × Pinus radiata somatic plant after two months in the greenhouse, bar: 26 mm.
2.3. Maturation and Germination of Embryos
ECLs from each of the initiation treatments (1,2 and 3) were subjected to maturation; 23 ECLs from treatment 1 (12 with P. attenuata as the mother tree, 11 with P. radiata var. cedrosensis as the mother tree), 19 ECLs from treatment 2 (8 with P. attenuata as the mother tree, 11 with P. radiata var. cedrosensis as the mother tree), and 23 ECLs from treatment 3 (10 with P. attenuata as the mother tree, 13 with P. radiata var. cedrosensis as the mother tree). ECLs with P. attenuata as the mother tree belonged to three crosses (887.301, 887.302, 887.315) and ECLs with P. radiata var. cedrosensis as the mother tree belonged to four crosses (886.975, 886.977, 886.981, 886.987). Maturation and germination were performed following the methodology described by [14]. For maturation, samples of 100 mg of proliferating embryogenic tissue, 14 days after their last transfer to fresh medium, were placed in 5 mL of liquid embryo development medium (EDM) ([21], Table 1) devoid of growth regulators and gently suspended. The suspension was then poured onto a filter paper (Whatman no. 2, 70 mm) in a Büchner funnel; a vacuum pulse was applied for 10 s, and the filter paper with the attached tissue was transferred to maturation medium. Maturation medium was EDM medium supplemented with 60 µM abscisic acid (ABA) and 9 g L−1 Gelrite® (Duchefa, Haarlem, The Netherlands); medium was poured in 90 mm × 9 mm × 20 mm Petri dishes. Four Petri dishes per ECL were assayed, giving a total of 260 Petri dishes. Cultures were incubated at 22 °C in the dark. Mature somatic embryos (Se’s) were harvested 15 weeks later (Figure 1c) and placed on half strength macronutrients LP medium [22] modified by [23] (1/2 LP, Table 1) with 2 g L−1 of activated charcoal and 9 g L−1 of gellan gum (DifcoAgar®; Becton Dickinson; Franklin Lakes, NJ, USA). Petri dishes (90 mm × 9 mm × 15 mm) were used as containers, with the root caps of the Se’s pointing downwards at an angle of approximately 60° and placed under dim light (10 µmol m−2 s−1) for seven days. Twenty embryos Petri dish were cultured, the number of Petri dishes cultured depended on the number of Se’s obtained for each ECL. After six weeks, germinated Se’s (Figure 1d) were subcultured to glass jars with medium of the same composition (seven somatic plantlets per jar). Cultures were maintained 5 weeks at 23 °C under 16 h photoperiod at 100 µmol m−2 s−1 provided by cool white fluorescent tubes (TFL 58 W/33).
2.4. Acclimatization
After 12 weeks from the start of germination stage, plantlets showing roots and an aerial part of more than 15 mm were considered suitable to be transplanted to 43 cm3 pots containing a wet sterile peat: perlite mixture (3:1, v/v). After transplanting, the plantlets were acclimatized in a greenhouse under controlled conditions at 21 ± 2 °C and progressively decreasing humidity from 100% to 70% over four weeks. Then, after two months in the greenhouse the plants were transplanted to bigger pots and six months later in the greenhouse, the height (cm) was measured in 368 plants coming from different mother species and treatments (Figure 1e,f).
2.5. Long Term Conservation at −80 °C
43 ECLs were chosen, 17 EMs from P. radiata × P. attenuata (14 of them used in maturation experiments) and 26 EMs from P. attenuata × P. radiata were used for this study, (21 of them used in maturation experiments). Conservation of EMs at −80 °C was carried out following the procedure described by [24]. Briefly, 1.5 g of vigorously growing EMs were resuspended in 5.4 mL of EDM liquid medium supplemented with 180 g L−1 sucrose. After one hour of incubation in this medium, the same volume of EDM liquid medium containing 180 g L−1 sucrose plus 15% dimethyl sulfoxide was gradually added (3 times 1.8 mL in 15 min intervals) to reach a final concentration of 7.5%. The suspension was maintained in darkness in an ice bath on a shaker at 120 rpm during this process.
Finally, the suspension was pipetted into cryovials (six times 1.8 mL), and five cryovials, each containing 250 mg fresh mass of tissue, were arranged in a Mr. Frosty container (Nalgene® Labware; Thermo Fisher Scientific, Waltham, MA, USA) and then in an ultra-low temperature freezer (Sanyo V.I.P.™; Osaka, Japan) at −80 °C. After 90 min, the cryovials with cell suspensions were rapidly removed from the Mr. Frosty and plunged into liquid nitrogen for 5 min. Then the cryovials were stored again in the ultra-low temperature freezer (−80 °C) for one year and for five years. Thawing of samples was carried out after one and five years as follows: cryovials were removed from the −80 °C freezer and left at room temperature for 1 min. Then, they were immersed in a water bath at 40 °C for 3 min. Finally, the suspension was poured onto a filter paper disc (Whatman® no. 2.70 mm; Maidstone, UK) in a Büchner funnel. A vacuum pulse was applied for 10 s, and the filter paper with the attached tissue was transferred to EDM basal medium supplemented with a combination of 4.5 μM 2, 4-dichlorophenoxyacetic acid, 2.7 μM 6-benzylaminopurine, 30 g L−1 sucrose and 3.5 g L−1 gellan gum (Gelrite®; Duchefa, Haarlem, The Netherlands). After 24 h, the filter papers were transferred to fresh EDM medium and the cultures were kept in darkness at 23 ± 1 °C.
Control samples (one cryovial per ECL) treated as frozen ones but not cooled in the Mr. Frosty or immersed in liquid nitrogen, were subjected to the same procedure in order to assess whether they had lost regeneration potential prior to storage at −80 °C.
2.6. Data Collection and Analysis
After 15 weeks on maturation medium, the ECLs producing Se’s were recorded and the percentage of maturation was calculated by dividing the number of ECLs that produced mature Se’s by the total number of ECLs subjected to maturation.
After 15 weeks on maturation medium, the number of Se’s in ECLs producing Se’s was counted and the number of Se’s per gram of fresh tissue was calculated. Regarding the number of Se’s, two analyses were conducted. Firstly, a linear mixed effects model including the cross as a fixed effect and the ECL as a random effect to cope with variability was fitted. Additionally, the number of Se’s was transformed using the square root. Secondly, a similar linear mixed effects model including the treatment, the mother, and their interaction as fixed effects, and the ECL as a random effect was considered to evaluate the effect of the treatment on the number of Se’s. The same square root transformation was applied to the number of Se’s to reduce variability.
To assess the effect of the cross on the germination percentage, a logistic mixed model including the ECLs a random effect was considered. The ECL was included to improve the fit and to cope with variability. The effects of the treatment, the mother and their interaction were assessed using a logistic mixed model including the ECLs a random effect. In this model the treatment, the mother, and their interaction were fixed effects.
The height of somatic plantlets after six months in the greenhouse was evaluated by two-way analysis of variance (ANOVA). Data were transformed by LN (height). Significant differences between means were determined by Tukey’s post hoc test at a significance level of 5%.
For data on ECL storage at −80 °C after one year and five years, the number of ECLs that showed growth two months after thawing was recorded (Figure 1b) and the regeneration percentages were calculated.
3. Results
The analyses on the effect of treatment, the mother species and the cross were reported previously [25]; as described in this study, there was a significant effect of treatment, and cross and an interaction among these factors.
To evaluate the effect of the cross on the maturation percentage, an initial exploratory data analysis was conducted revealing that this percentage was above 80% for all levels of cross but one. Moreover, the maturation percentage was 100% in two levels of cross. With these data, it was not possible to fit any model. Regarding the effect of the treatment and the mother, the exploratory analysis revealed that in four out of the six levels defined by the interaction of both factors, the maturation percentage was above 80% with one level reaching 100%. Therefore, results were based on descriptive statistics. Maturation percentages for the different mother trees and crosses are shown in Figure 2a. Maturation percentages by treatment and mother are shown in Figure 2b (three crosses when P. attenuata acted as the mother tree and four crosses when P. radiata var. cedrosensis acted as the mother tree). Different ECLs analysed for each cross showed a different maturation rate ranging from 67 % (cross 887.302, P. attenuata as mother tree) to 100% (cross 887.301 with P. attenuata as mother tree and cross 886.975 with P. radiata as the mother tree, ECLs from the rest of crosses showed maturation rates between 83 and 89% (Figure 2a). On the other hand, different initiation treatments provoked a variation in the success of the process depending on the species that acted as mother; Treatment 1 was the most successful when P. attenuata was the mother and the worst when this species was the father. As it can be observed in Figure 2b, there was an opposite effect of the treatments: maturation percentage decreased from 1 to 3 when P. attenuata acted as mother and increased when P. radiata was the mother. So, there was a strong effect of the species that acted as mother in the maturation rates obtained.
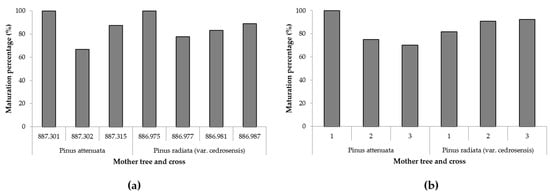
Figure 2.
(a) Maturation percentages in embryogenic cell lines in which P. attenuata acted as the mother tree (crosses 887.301, 887.302, 887.315) and in which P. radiata var. cedrosensis acted as the mother (crosses 886.975, 886.977, 886.981, 886.987). (b) Maturation percentages in embryogenic cell lines from different initiation treatments (1, 2 and 3) in the two mother species.
When the effect of mother, cross and treatment on the number of Se’s was analysed, significant differences were not found (p > 0.05), even when the interaction was analysed (p > 0.05). Table 2 displays the sample mean (and its standard error) of the number of Se’s for the levels of cross and the two mothers. Table 3 displays the same information for the treatment levels and the two mothers. Though at first glance, some differences seem to be important, the statistical models did not detect such apparent discrepancies. The reason is that the variability within the levels of cross and the combination of treatment and mother were rather large, and the statistical model could hardly cope with it. Much of this variability persisted even with a square root transformation of the data (which typically reduces variance) and the introduction of the ECL as a random effect. However, it is noticeable that within the ECLs from each mother tree (P. attenuata or P. radiata) the best results were obtained in ECLs from treatment 2 and the lowest number os Se’s were recorded in ECLs from treatment 1.

Table 2.
Number of somatic embryos g−1 fresh weight in embryogenic cell lines in which P. attenuata acted as the mother tree (crosses 887.301, 887.302, 887.315) and in which P. radiata var. cedrosensis acted as the mother (crosses 886.975, 886.977, 886.981, 886.987). Sample mean and its standard error.

Table 3.
Number of somatic embryos g−1 fresh weight for the different levels of mother and initiation treatments (1, 2, and 3). Sample mean and its standard error.
The germination percentage did not show significant differences when the cross was analysed for each mother (Table 4 and Figure 3a). In the same way, when the mother, treatment and interaction were evaluated not significant differences were found (Table 3 and Figure 3b). In both cases, the germination percentages were always above 75%.

Table 4.
Analysis of deviance of the mixed logistic regression for germination (%) according to cross, mother tree, treatment and their interaction.
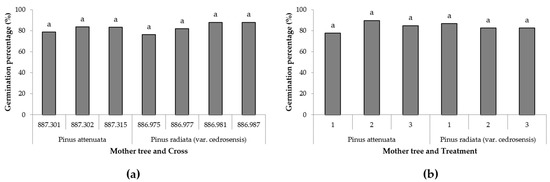
Figure 3.
(a) Germination percentages in embryogenic cell lines in which P. attenuata acted as the mother tree (crosses 887.301, 887.302, 887.315) and in which P. radiata var. cedrosensis acted as the mother (crosses 886.975, 886.977, 886.981, 886.987). (b) Maturation percentages in embryogenic cell lines from different initiation treatments (1, 2 and 3) in the two mother species. Different letters indicate significant differences by Tukey’s post hoc test (p < 0.05).
Table 5 and Table 6 show the height of plants coming from different treatments and from different crosses when each species acted as mother, respectively. No significant differences were found between initiation treatments. However, when P. radiata acted as mother, plants showed a significantly higher height (13.1 cm on average) than when P. attenuata was the mother (9.2 cm on average). When P. radiata acted as a mother somatic plants from ECLs from cross 886.977 displayed where significantly higher than somatic plants from the other crosses tested.

Table 5.
Height of somatic plants (cm) from embryogenic cell lines in which P. attenuata or P. radiata var. cedrosensis acted as the mother and from different initiation treatments (1, 2 and 3) after six months in the greenhouse.

Table 6.
Height of somatic plants (cm) from embryogenic cell lines in which P. attenuata or P. radiata var. cedrosensis acted as the mother and from different initiation treatments (1, 2 and 3) after six months in the greenhouse.
The regeneration percentage in samples before freezing (controls) was 100%. After one year of storage at −80°C, regeneration percentages were 82% and 81% in ECLs from P. radiata and P. attenuata mother trees, respectively. After five years of storage the regeneration percentages decreased to 12% and 8% in embryogenic cell lines from P. radiata and P. attenuata mother trees, respectively.
4. Discussion
There is very little information on the effect of the mother on the development of embryogenic processes in Pinus spp. hybrids, these together with the fact that there is also little information on the effect that a certain treatment applied at initial steps of SE might have on the subsequent steps of the process, led us to carry out this study.
We found that the initiation treatment did not affect the in vitro conversion of EMs to somatic plants, this is in agreement with results reported in Pinus halepensis [26] and opposite the effect of environmental conditions at initial stages of SE process on plant conversion reported by different authors [26,27]. Although a great variability was observed among ECLs, this is common in SE with conifer species and the strong effect of genotype has been reported by several authors [28,29]; this is why a high number of genotypes (65 ECLs) were screened in this study.
Regarding maturation percentages, the lowest was obtained when P. attenuata acted as mother (63%) in one of the crosses tested, however the rest of crosses assessed presented maturation percentage above 80% regardless of the species that acted as a mother. These results are high when compared with those reported in other Pinus species such as P. pinea [30] or P. nigra [31] but in accordance with those reported previously in this species [14,17]. The number of Se’s produced by different ECLs showed a high variability, this fact has been widely reported in the literature [32]. However, it was observed that independently of the species used as mother, initiation treatment 2 (excised zygotic embryo) was the one that gave the best results. Our results are in line with those reported by [33] in the hybrid Pinus elliottii var. elliottii × P. caribaea var. hondurensis where they reported a high variability in somatic embryo production among the genotypes tested; these authors also observed that there was a significant effect of proliferation medium on the obtaining of Se’s. On the other hand, while the nurse culture seems to be beneficial in certain occasions at initial stages of the process [8] the ECLs developed from this treatment presented the lowest number of Se’s.
Germination was carried out without any post-maturation treatment such as desiccation and the percentages obtained in this study were high (always above 75%) considering that this phase has been reported as a bottleneck for the SE process in this genus [34]. Germination percentages did not depend on the initiation treatment, the mother species or de cross. As reported for white spruce [34], variation among cell lines will be apparent in all stages of the SE process, affecting the overall effective yield of somatic plants, however this variance due to ECLs seems to be higher for SE initiation, lower for maturation and still lower for germination. Although an effect of the environment at proliferation stage on germination percentage was described in P. halepensis [26] or in P. pinaster [27], our results are in line with those reported for P. radiata in previous studies in our laboratory [19].
Size of somatic plantlets after six months of hardening was significantly higher in crosses in which P. radiata was the mother, showing the same behaviour as pure species; in this sense, P. attenuata grows much more slowly on most New Zealand sites where commercial forests are grown, when compared with P. radiata [35]. However, even when growth is slower than in P. radiata, P. attenuata is drought, snow and frost tolerant, and also capable of growing on poor soils [35]. Although in gymnosperms it has been believed that the mitochondrial DNA is inherited from the female parent and the chloroplast DNA is only inherited from the male [36], recent studies in Pinus mugo × Pinus sylvestris hybrid [37] have shown a biparental inheritance of chloroplast DNA. It could be interesting for future research to study the plastid inheritance in our reciprocal hybrids to try to elucidate the origin of the better ex vitro growth of the P. radiata × P. attenuata hybrids.
Finally, in this work we have also explored the possibility of storing ECLs from the hybrid at −80 °C. After one year, regeneration percentages were similar to those found by [24] in P. radiata using the same methodology and storage time. This storage time, although insufficient for carrying out ex vitro testing of clones, enables a preliminary assessment of the in vitro performance of different ECLs. Results are encouraging as with this storage period and with the development of genomic techniques even an early selection could be performed [38]. Although there are several studies were conifer ECLs has been cryopreserved in liquid nitrogen [39], this is the first report where regeneration of embryogenic tissues has been reported after a long storage period at −80 °C (five years). Regeneration percentages were very low after this storage period, further research will be carried out to try to improve regeneration by means of evaluating different protectant solutions for storage and testing other post-thawing treatments. Additionally, the use of nurse will be evaluated for thawing ECLs stored at −80 °C as it has been successful used for the regeneration of cryopreserved cell lines of P. radiata [24].
5. Conclusions
The physical and chemical environment derived from the presence of megagametophytes and/or nurse did not provoke any effect on the maturation percentages, the number of Se’s developed or the germination percentages. However, P. radiata × P. attenuata somatic plantlets growing in the greenhouse showed significantly higher heights than P. attenuata × P. radiata plantlets.
It is possible to preserve ECLs at −80 °C for one year without loss of regeneration capacity. Regeneration has also been obtained from some ECLs stored at −80 °C up to five years. The application of this technique could represent a great economic saving because it does not require cryotanks with liquid nitrogen.
Author Contributions
Conceptualization, I.A.M., C.L.H. and P.M.; data curation, I.A.M., A.C.-O., T.G. and M.D.U.; formal analysis, I.A.M. and P.M.; funding acquisition, P.M.; investigation, I.A.M., C.L.H., K.G., C.B.R., S.v.B. and P.M.; methodology, I.A.M., A.C.-O., K.G., C.B.R. and S.v.B.; writing—review and editing, I.A.M., A.C.-O. and P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MINECO (Spanish Government) project (AGL2016-76143-C4-3R), CYTED (P117RT0522), DECO (Basque government, “Ayudas de formación a jóvenes investigadores y tecnólogos”). OECD Co-operative Research Programme Fellowship (Biological Resource Management for Sustainable Agricultural Systems, 2013) for supporting the visit of Paloma Moncaleán to Scion and Scion Core Funding for supporting the hybrid pine initiative.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Acknowledgments
Shaf van Ballekom of Proseed Ltd. New Zealand is thanked for the provision of seed and warm support of this research. We also thank the MULTIFOREVER (Forest Value, ERANET program, EU). Project MULTIFOREVER is supported under the umbrella of ERA-NET Cofund ForestValue by ANR (FR), FNR (DE), MINCyT (AR), MINECO-AEI (ES), MMM (FI) and VINNOVA (SE). ForestValue has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 773324.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Mitchell, R.G. Reducing the Risks of Pitch Canker Disease (Caused by Fusarium circinatum) to Pinus patula in South Africa. Ph.D. Thesis, Faculty of Natural and Agricultural Science, University of Pretoria, Pretoria, South Africa, 2012. [Google Scholar]
- Mitchell, R.G.; Wingfield, M.J.; Hodge, G.R.; Dvorak, W.S.; Continho, T.A. The tolerance of Pinus patula × Pinus tecunumanii, and other pine hybrids, to Fusarium circinatum in greenhouse trials. New For. 2013, 44, 443–456. [Google Scholar] [CrossRef]
- Camcore. Progress in pine hybrid testing. In 2012 Camcore Annual Report; Department of Forestry and Environmental Resources, NC State University: Raleigh, NC, USA, 2012; p. 18. [Google Scholar]
- Camcore. Status of pine hybrid project. In 2013 Camcore Annual Report; Department of Forestry and Environmental Resources, NC State University: Raleigh, NC, USA, 2013; p. 20. [Google Scholar]
- Sheffield, J.; Wood, E.F. Global trends and variability in soil moisture and drought characteristics, 1950–2000, from observation-driven simulations of the terrestrial hydrologic cycle. J. Clim. 2008, 21, 432–458. [Google Scholar] [CrossRef]
- Dungey, H.S.; Carson, M.J.; Low, C.B.; King, N.G. Potential and niches for inter-specific hybrids with Pinus radiata in New Zealand. N. Z. J. For. Sci. 2003, 33, 295–318. [Google Scholar]
- Hargreaves, C.; Sigley, M.; Menzies, M.; Dungey, H. Hybrid pines: Opportunities for life on the edge. Proc. Int. Plant Propagators Soc. 2008, 58, 62–167. [Google Scholar]
- Hargreaves, C.; Reeves, C.; Gough, K.; Montalbán, I.A.; Low, C.; van Ballekom, S.; Dungey, H.S.; Moncaleán, P. Nurse tissue for embryo rescue: Testing new conifer somatic embryogenesis methods in a F1 hybrid pine. Trees 2017, 31, 273–283. [Google Scholar] [CrossRef]
- De Diego, N.; Pérez-Alfocea, F.; Cantero, E.; Lacuesta, M.; Moncaleán, P. Physiological response to drough in radiata pine: Phytohormone implication at leaf level. Tree Physiol. 2012, 32, 435–449. [Google Scholar] [CrossRef] [PubMed]
- De Diego, N.; Sampedro, M.C.; Barrio, R.J.; Saiz-Fernández, I.; Moncaleán, P.; Lacuesta, M. Solute accumulation and elastic modulus changes in six radiata pine breeds exposed to drought. Tree Physiol. 2013, 33, 69–80. [Google Scholar] [CrossRef]
- De Diego, N.; Rodríguez, J.L.; Dodd, I.C.; Pérez-Alfocea, F.; Moncaleán, P.; Lacuesta, M. Immunolocalization of IAA and ABA in roots and needles of radiata pine (Pinus radiata) during drought and rewatering. Tree Physiol. 2013, 33, 537–549. [Google Scholar] [CrossRef]
- De Diego, N.; Saiz-Fernández, I.; Rodríguez, J.L.; Pérez-Alfocea, P.; Sampedro, M.C.; Barrio, R.J.; Lacuesta, M.; Moncaleán, P. Metabolites and hormones are involved in the intraspecific variability of drought hardening in radiata pine. J. Plant Physiol. 2015, 188, 64–71. [Google Scholar] [CrossRef]
- Hargreaves, C.L.; Reeves, C.B.; Find, J.I.; Gough, K.; Josekutty, P.; Skudder, D.B.; van der Maas, S.A.; Sigley, M.R.; Menzies, M.I.; Low, C.B.; et al. Improving initiation, genotype capture, and family representation in somatic embryogenesis of Pinus radiata by a combination of zygotic embryo maturity, media, and explant preparation. Can. J. For. Res. 2009, 39, 1566–1574. [Google Scholar] [CrossRef]
- Montalbán, I.A.; De Diego, N.; Moncaleán, P. Bottlenecks in Pinus radiata somatic embryogenesis: Improving maturation and germination. Trees 2010, 24, 1061–1071. [Google Scholar] [CrossRef]
- Montalbán, I.A.; De Diego, N.; Moncaleán, P. Enhancing initiation and proliferation in radiata pine (Pinus radiata D.Don) somatic embryogenesis through seed family screening, zygotic embryo staging and media adjustments. Acta Physiol. Plant. 2012, 34, 451–460. [Google Scholar] [CrossRef]
- Castander-Olarieta, A.; Montalbán, I.A.; De Medeiros Oliveira, E.; Dell’Aversana, E.; D’Amelia, L.; Carillo, P.; Steiner, S.; De Freitas Fraga, H.P.; Guerra, M.P.; Goicoa, T.; et al. Effect of thermal stress on tissue ultrastructure and metabolite profiles during initiation of radiata pine somatic embryogenesis. Front. Plant Sci. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- García-Mendiguren, O.; Montalbán, I.A.; Goicoa, T.; Ugarte, M.D.; Moncaleán, P. Environmental conditions at the initial stages of Pinus radiata somatic embryogenesis affect the production of somatic embryos. Trees Struct. Funct. 2016, 30, 949–958. [Google Scholar] [CrossRef]
- Pereira, C.; Montalbán, I.A.; García-Mendiguren, O.; Goicoa, T.; Ugarte, M.D.; Correia, S.; Canhoto, J.M.; Moncaleán, P. Pinus halepensis somatic embryogenesis is affected by the physical and chemical conditions at the initial stages of the process. J. For. Res. 2016, 21, 143–150. [Google Scholar] [CrossRef]
- Montalbán, I.A.; García-Mendiguren, O.; Goicoa, T.; Ugarte, M.D.; Moncaleán, P. Cold storage of initial plant material affects positively somatic embryogenesis in Pinus radiata. New For. 2015, 46, 309–317. [Google Scholar] [CrossRef]
- Litvay, J.D.; Verma, D.C.; John, M.A. Influence of loblolly pine (Pinus taeda L.) culture medium and its components on growth and somatic embryogenesis of the wild carrot (Daucus carota L.). Plant Cell Rep. 1985, 4, 325–328. [Google Scholar] [CrossRef]
- Walter, C.; Find, J.I.; Grace, L.J. Somatic embryogenesis and genetic transformation in Pinus radiata. In Protocol for Somatic Embryogenesis in Woody Plants; Jain, S.M., Gupta, P.K., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 11–24. [Google Scholar]
- Quoirin, M.; Lepoivre, P. Études des milieux adaptés aux cultures in vitro de Prunus. Acta Hort. 1977, 78, 437–442. [Google Scholar] [CrossRef]
- Aitken-Christie, J.; Singh, A.P.; Davies, H. Multiplication of meristematic tissue: A new tissue culture system for radiata pine. In Genetic Manipulation of Woody Plants; Hanover, J.W., Keathley, D.E., Eds.; Plenum: New York, NY, USA, 1988; pp. 413–432. [Google Scholar]
- Montalbán, I.A.; Moncaleán, P. Long term conservation at −80 °C of Pinus radiata embryogenic cell lines: Recovery, maturation and germination. CryoLetters 2017, 38, 202–209. [Google Scholar]
- Hargreaves, C.L.; Grace, L.J.; Holden, D.G. Nurse culture for efficient recovery of cryopreserved Pinus radiata D. Don embryogenic cell lines. Plant Cell Rep. 2002, 21, 40–45. [Google Scholar]
- Pereira, C.; Castander-Olarieta, A.; Montalbán, I.A.; Pěnčík, A.; Petřík, I.; Pavlović, I.; Medeiros Oliveira, E.D.; de Freitas Fraga, H.P.; Guerra, M.P.; Novák, O.; et al. Embryonal masses induced at high temperatures in Aleppo pine: Cytokinin profile and cytological characterization. Forests 2020, 11, 807. [Google Scholar] [CrossRef]
- Arrillaga, I.; Morcillo, M.; Zanón, I.; Lario, F.; Segura, J.; Sales, E. New approaches to optimize somatic embryogenesis in maritime pine. Front. Plant Sci. 2019, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Miguel, C.; Gonçalves, S.; Tereso, S.; Marum, L.; Maroco, J.; Oliveira, M.M. Somatic embryogenesis from 20 open-pollinated families of Portuguese plus trees of maritime pine. Plant Cell Tissue Org. Cult. 2004, 76, 121–130. [Google Scholar] [CrossRef]
- Niskanen, A.-M.; Lu, J.; Seitz, S.; Keinonen, K.; Von Weissenberg, K.; Pappinen, A. Effect of parent genotype on somatic embryogenesis in Scots pine (Pinus sylvestris). Tree Physiol. 2004, 24, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Carneros, E.; Celestino, C.; Klimaszewska, K.; Park, Y.-S.; Toribio, M.; Bonga, J.M. Plant regeneration in Stone pine (Pinus pinea L.) by somatic embryogenesis. Plant Cell Tissue Org. Cult. 2009, 98, 165–178. [Google Scholar] [CrossRef]
- Salaj, T.; Moravcikova, J.; Salaj, J. Somatic embryogenesis in Pinus nigra arn.: Some physiological, structural and molecular aspects. Plant Cell Mongr. 2005, 2, 141–156. [Google Scholar]
- Salaj, T.; Matusova, R.; Salaj, J. Conifer somatic embryogenesis—An efficient plant regeneration system for theoretical studies and mass propagation. Dendrobiology 2015, 74, 69–76. [Google Scholar] [CrossRef]
- Nunes, S.; Marum, L.; Farinha, N.; Pereira, V.T.; Almeida, T.; Sousa, D.; Mano, N.; Figueiredo, J.; Dias, M.C.; Santos, C. Somatic embryogenesis of hybrid Pinus elliottii var. elliottii × P. caribaea var. hondurensis and ploidy assessment of somatic plants. Plant Cell Tissue Org. Cult. 2018, 132, 71–84. [Google Scholar] [CrossRef]
- Egertsdotter, U. Plant physiological and genetical aspects of the somatic embryogenesis process in conifers. Scan. J. For. Res. 2019, 34, 360–369. [Google Scholar] [CrossRef]
- Miller, J.T.; Knowles, F.B. Introduced Forest Trees in New Zealand: Recognition, Role and Seed Source Pinus attenuata Lemmon—Knobcone Pine; New Zealand Ministry of Forestry, Forest Research Institute: Rotorua, New Zealand, 1988. [Google Scholar]
- Lüttge, U. Father hands it down: Patrilineal inheritance of chloroplast DNA in conifers may help to uncover relations of reproduction, population biology and longevity of trees. Trees Struct. Func. 2016, 30, 73–74. [Google Scholar] [CrossRef][Green Version]
- Kormutak, A.; Galgoci, M.; Sukenikova, D.; Bolecek, P.; Libantova, J.; Gőmőry, D. Maternal inheritance of chloroplast DNA in Pinus mugo Turra: A case study of Pinus mugo × Pinus sylvestris crossing. Plant Syst. Evol. 2018, 304, 71–76. [Google Scholar] [CrossRef]
- Chamberland, V.; Robichaud, F.; Perron, M.; Gélinas, N.; Bousquet, J.; Beaulieu, J. Conventional versus genomic selection for white spruce improvement: A comparison of costs and benefits of plantations on Quebec public lands. Tree Genet. Genomes 2020, 16, 17. [Google Scholar] [CrossRef]
- Krajňáková, J.; Bertolini, A.; Gömöry, D.; Vianello, A.; Häggman, H. Initiation, long-term cryopreservation, and recovery of Abies alba Mill. embryogenic cell lines. Cel. Dev. Biol. Plant 2013, 49, 560–571. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).