Abstract
The temporal progress of candeia rust, caused by the fungus Puccinia velata, was monitored in an experimental field at Lavras municipality, Southern Minas Gerais state, Brazil. A plantation with 17 Eremanthus erythropappus clones was set at the site, and the temporal disease progress was analyzed based on visual assessments of disease severity on leaves. The disease was monitored monthly between September 2016 and August 2017. Progress curves based on disease severity were constructed and empirical models were fitted. The area under the disease progress curve (AUDPC) was calculated, and the means test was applied to select clones resistant to the disease. The Pearson coefficient was used to assess correlations between disease severity and environmental variables. The model that best described disease progress over the assessment period was the Gompertz model. The mean AUDPC values were grouped into four groups of resistance levels according to the Scott–Knott test. There was a negative correlation between air temperature and disease severity. Considering that the disease occurred in all clones and that the climatic conditions of Southern Minas Gerais are favorable to the candeia rust, it is important to adopt measures for the selection of clones resistant to this disease.
1. Introduction
Candeia (Eremanthus erythropappus (DC.) McLeish), is an important native species for wood production and the potential for essential oil extraction [1]. Candeia occurs naturally in South America and can be found in northeastern Argentina, Northern and Eastern Paraguay [2], and in several regions of Brazil, mainly in the Cerrado Biome [2,3]. The oil extracted from this tree contains the compound alpha-bisabolol, which has been used in the pharmaceutical and cosmetic industries worldwide [4]. Approximately 200 products contain alpha-bisabolol [5] due to its anti-inflammatory, anti-irritant, and antimicrobial properties [4]. Several plants produce this compound, but the essential oil of candeia has higher quality and concentration of this substance (70%), giving it greater economic viability [4]. In addition, after extraction of the oil, candeia wood residue is an excellent option for use in the production of particle board, which has higher added value than lumber [6].
Because of that and other characteristics, such as its ability to grow in difficult locations where it is not possible to grow other forest species or agricultural crops, candeia is an attractive option for forest cultivation [7]. The exploitation of this forest species has intensified in recent years. In many areas, candeia is grown exclusively, providing environments that favor the emergence of several diseases. Among them, rust caused by Puccinia velata [8] may become a limiting factor for the production of candeia due to the expansion of its planted area. This is because rusts in forest species are known to cause severe and premature defoliation, and the physiological damage reduces wood yield [9]. P. velata is described as occurring in trees over three years old and in adult leaves in the field [10], which may decrease plant growth due to nutrient removal and leaf area reduction resulting from the formation of signs of the pathogen’s fungal structures and early leaf drop [11]. Consequently, the photosynthetic area is reduced, delaying development of the plant resulting in reduced wood quality and yield.
In this context, despite the knowledge about the negative effect of rusts on hosts, there are no studies on the etiology or epidemiology of P. velata on candeia trees. The characteristics that hinder the study of rusts include their biotrophic parasitism strategy because they grow only on their specific living hosts [12]. After the first report of P. velata in E. erythropappus in 1897 by Dietel in Ouro Preto, Minas Gerais, few studies have been conducted on this pathogen. There is a report of its incidence in naturally occurring candeia forests [13]. However, due to the increase in planted area, which favors the incidence of the disease in the field, the pathogen may become a problem for producers, necessitating studies on its behavior and control.
Thus, to identify management strategies for the disease, it is important to study the pathogen, the host and the environment [14]. The environment is a determinant of the increase in disease severity, as it influences other factors. Environmental variables affect infection, colonization, sporulation, pathogen survival, and host physiological processes [15]. The factors typically correlated with disease progress are temperature, humidity, and light intensity [16], though each pathogen is differently affected by these variables. Thus, studying how these variables influence the severity and temporal dynamics of candeia rust can assist in the development of alternative measures for adequate management, as well as in the screening of more resistant genetic materials.
In addition, to understand the disease epidemiology, it is necessary to analyze the damage caused by the disease and its relationship with pathogen, host, and environmental variables [15,17]. In such studies, it is common to use statistical models to describe these phenomena [14]. Vanderplank [18] quantified disease using the progress rate (r), maximum disease intensity, and initial inoculum (y0), demonstrating how statistically calculated variable values could help compare management tactics. Currently, the disease progress is studied fitting the best empiric model to the observed disease incidence and severity data. The choice of the model most representative of the pathosystem is based on the analysis of the coefficient of determination (R2), the value of the mean square deviation and the plot of the standardized residuals as a function of the dependent variable [14].
The objectives of this study were to analyze temporal progress of candeia rust in E. erythropappus clones in the field; correlate disease severity with meteorological variables; select rust-resistant clones; and fit empirical models to describe the candeia rust progress.
2. Materials and Methods
2.1. Experimental Site Description
The experiment was conducted between September 2016 and August 2017 in the experimental field located in Lavras municipality, southern region of Minas Gerais state, Brazil, 21°14’ S latitude, 45°00’ W longitude Gr. and 918 m altitude. The climate is temperate rainy (mesothermal), or Cwa in the Köppen classification, with rainy periods in the summer and dry winters, a mean annual temperature of 20.4 °C, and annual rainfall of 1460 mm [19]. The soil was classified as dystroferric Red Latosol according to the Brazilian soil classification system.
The soil was plowed, harrowed, and corrected with the incorporation of lime into the total area, in according to the soil analysis. The planting furrow was opened with a subsoiler at 80 cm depth, incorporating 300 kg of natural phosphate per hectare.
The disease was assessed in 17 five-year-old clones of E. erythropappus, C4, C6, C7, C12, C19, C20, C24, C25, C26, C27, C30, C33, C35, C36, C37, C40, and C49. The disease occurred naturally in the experimental area of candeia, and the severity of the disease was assessed based on the symptoms of the pustules containing signs (urediniospores) under the abaxial face of the leaves (Figure 1E). In addition, we observed yellowed edges to the lesions on the adaxial face (Figure 1D), as well dead leaves on the plant (Figure 1F). The experimental plot consisted of 350 trees with 11 rows and 3 × 3 m spacing. To remove the border effect, eight trees per treatment in the central rows were evaluated. The experimental design was completely randomized because the local area, soil type, and climatic conditions were homogeneous.

Figure 1.
Naturally occurring candeia trees (A), 5-year-old candeia trees from the experimental area (B), purple inflorescences at the ends of the branches (C), necrotic lesions surrounded by a bright yellow halo at the leaf margin or interior of the leaf (D), pustules containing signs (urediniospores) under abaxial face of the leaves (E), leaf tissue necrosis and dead leaves (F), urediniospores by light microscopy (G), SEM micrographs of urediniospores (H). Scale bars: 20 µm (G) and 10 µm (H).
2.2. Disease Assessment and Area under the Disease Progress Curve (AUDPC)
Disease severity was assessment monthly. A nondestructive method was used, sampling 30 leaves per plant randomly, 10 from the apical third, 10 from the middle third, and 10 from the basal third of each tree. The disease severity percentage was determined using the Pereira et al. [20] diagrammatic scale. The values obtained in the evaluation of candeia rust severity were transformed into area under the disease progress curve (AUDPC), according to Campbell and Madden [14], using the equation below.
where:
- AUDPC = area under the disease progress curve
- yi = proportion of disease at the ith observation
- ti = time in days at the ith observation
- n = total number of observations
2.3. Disease Progress Curve and Its Relationship with Climatic Variables
Climatological data were obtained from the Main Climatological Station of Lavras municipality, belonging to the 5th Meteorology District, in partnership with the National Meteorological Institute and Federal University of Lavras. Insolation (hrs), rainfall (mm), maximum, mean, and minimum temperatures (°C), relative humidity (%), actual evapotranspiration (mm), and wind speed (m/s) were analyzed.
2.4. Fitting of Empirical Models to Disease Progression
The candeia rust progress curves were constructed using the mean severity values obtained in the evaluations of four clones, selected according to the clustering test performed with the AUDPC values. The data were fitted to four empirical models, monomolecular, logistic, gompertz, and exponential [14]. The variables for the model were yi (disease severity at the ith evaluation), y0 (initial severity), and r (progress rate). The selection of the best model was based on the analysis of the coefficient of determination (R2), the mean square deviation and the analysis of the plots of residuals versus the predicted values.
2.5. Statistical Analysis
The assumptions for the analysis of variance of disease severity and of AUDPC were tested with the normality (Shapiro–Wilk) and homogeneity (Bartlett) tests. The data were submitted to the analysis of variance. The means were compared by the F test (p < 0.05). The AUDPC data were compared between clones by the Scott–Knott test (p < 0.05). All analyses were performed in R statistical software [21].
The mean disease severity index for clones C34, C25, C27, and C35 was plotted in a graph of the disease progress curve together with the means of the environmental variables. These clones were selected for plotting because they were representative of each significance group according to the means test.
To evaluate the relationship between disease severity and the climatological data collected in this study, Pearson’s correlation analysis (p < 0.05) was used for monthly mean insolation (h/month), maximum, mean, and minimum temperatures (°C), relative humidity (%), actual evapotranspiration (mm), wind speed, cumulative monthly rainfall (mm), and disease severity (%).
3. Results
3.1. Area under the Disease Progress Curve
There was a significant difference in the AUDPC between the clones (p < 0.05), forming four groups according to the AUDPC variation (Figure 2). The clone with the highest AUDPC was C27 (280.25), followed by clone C25 (182.96), which were significantly different from each other. Clones C26, C6, C36, C19, C35, C49, C4, and C7 formed an intermediate group, with AUDPC values ranging between 116.4 and 55.2. Clones C40, C37, C33, C12, C24, C20, and C34 showed the lowest AUDPC values, with means ranging between 33.4 and 2.0.
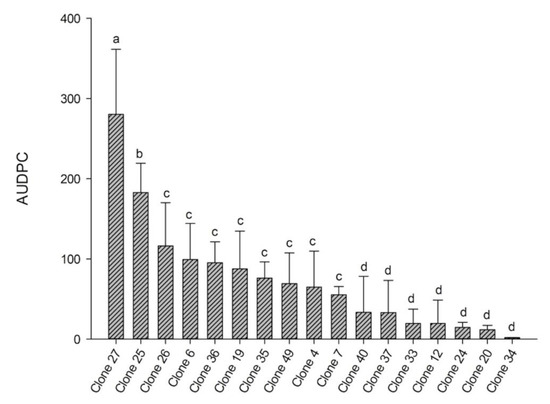
Figure 2.
Area under the disease progress curve (AUDPC) of candeia rust for 17 candeia clones between September 2016 and August 2017. Values are means (n = 5) and error bars indicate ± SD of the mean.
3.2. Disease Progress Curve and Its Relationship with Climatic Variables
Candeia rust occurred throughout the experimental evaluation period. The disease was seen in all clones, but there was variation in severity among the genetic materials (Figure 3A).
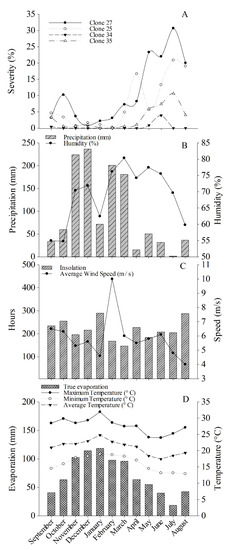
Figure 3.
(A) Rust severity progress curve of candeia clones representative of each significance group. (B) Monthly means of climatic variables: relative humidity (%) and cumulative monthly rainfall. (C) Mean wind speed (m/s) and insolation (h/month). (D) Mean, maximum, and minimum temperatures (°C) and actual evapotranspiration (mm) in the period from September 2016 to August 2017.
The onset of disease progress was observed in March 2017 (Figure 3), under a mean temperature of 22.7 °C, relative humidity of 71.9%, 146 h/month of insolation, and cumulative monthly rainfall of 181 mm (Figure 3B–D, respectively). Mean severity indices increased during the following months until July, when the incidence in the field peaked and then started to go down. The highest severities were observed in clones C27 and C25, reaching 32% and 20% disease, respectively.
In the evaluated period, the highest mean relative humidity, rainfall, actual evapotranspiration, and temperature were observed from November to March. However, there was no disease in most clones during this period, except for clones C27 and C25. Following the highest mean relative humidity recorded in March (80.37%), there were consecutive decreases in the means of all these variables in the following months until July (Figure 3), a period during which disease severity increased in most of the clones studied.
The lowest mean rust incidence occurred in December and January, months in which the highest mean temperatures in the evaluated period were recorded (23 and 24 °C, respectively). In addition, December was the month with the highest cumulative monthly rainfall over the entire assessment period. In contrast, in July, the lowest mean actual evapotranspiration and cumulative monthly rainfall were observed, a month coinciding with the highest mean disease severity for most clones. In this sense, the beginning of the increase in disease severity occurred in April, reaching the highest means in July, the period in which the lowest mean temperature (19 °C) was recorded (Figure 3D). After this period of elevated disease severity, the beginning of the severity reduction was recorded in August. In this same month, increases in mean temperature, actual evapotranspiration, insolation, and rainfall were also observed. August was the month with the lowest mean wind speed (4 m/s).
In January, the highest mean insolation (289 h/month) and highest mean actual evapotranspiration (118 mm) over the evaluation period were observed. In March, there was a lower mean insolation (146 h/month) along with the increase in disease severity that started in this month (Figure 3C).
Climatic variables were correlated with the mean disease severity of the clones (Table 1). actual evapotranspiration and rainfall showed negative correlations with the severity data for clones C25, C27, and C35, but the same was not observed for clone C34 (Table 1). The mean, maximum, and minimum temperature were negatively correlated with disease severity for all clones (Table 1).

Table 1.
Correlation coefficients between the climatic variables and candeia rust severity, caused by Puccinia velata in September 2016 to August 2017. Lavras, Minas Gerais.
3.3. Fitting of Empirical Models to Candeia Rust Progress
To fit the rust progress curve in the nonlinear models, the most representative clones of each means group (C24, C25, C27, and C35) were chosen to explain the disease behavior in the period between March and July, when the disease progressed at positive rates (Figure 4). The model with the best fit to the temporal disease progress in all clones was the Gompertz model (Table 2). The best-fit parameters were based on lower residuals and higher coefficients of determination (R2). Furthermore, the progress rate (r) was a significant parameter for all clones, while initial inoculum was not significant.
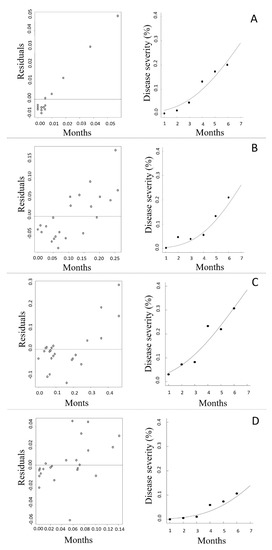
Figure 4.
Dispersion plot of residuals and disease severity fitted by the Gompertz model in the months from February to August 2017 for clones (A) 34, (B) 25, (C) 27, and (D) 35. Dispersion plots are on the left and disease progress on the right-hand side.

Table 2.
Parameters used in the regression analysis of the nonlinear model to compare the fit of the linear and nonlinear models for candeia rust progress curve.
4. Discussion
This study presents the first temporal analysis under field conditions of candeia rust in E. erythropappus, which is the main disease in plantations to date. The climatic variables influenced the incidence and severity of rust throughout the evaluation period. Candeia rust occurred throughout the year, but an increase in severity was observed only after March. In addition, the evaluated clones presented different levels of resistance to rust.
All clones showed incidence of the disease, but seven clones were considered resistant to rust (C40, C37, C33, C12, C24, C20, and C35), having lower AUDPC values, eight clones were considered moderately resistant (C7, C4, C49, C35, C19, C36, C6, and C26), one was moderately susceptible (C25), and one was susceptible (C27). Clones C27 and C25 presented the highest disease intensity, showing even under conditions not favorable to its occurrence, indicating that the disease progress depends not only on the climatic conditions but also on the physiology of the host.
The resistance and susceptibility of clones to pathogens have been frequently studied using the AUDPC in other forest species. However, due to the relatively recent recognition of candeia as a promising forest species for exploitation [7], data on the productivity of clones and damage caused by diseases to this pathosystem are lacking. Consequently, studies correlating the incidence and severity of the disease with the development of resistant candeia clones are necessary. Considering that the regions where candeia plantations are established can offer climatic conditions favorable to the disease and that its incidence can cause damage to the physiological development of the plant, the best control option is the use of resistant varieties. Thus, the results of this study suggest that knowledge about genetic resistance can assist in screening clones for disease management.
This initial step in the selection of candeia rust-resistant genetic materials is important, because the development of clonal tests for the selection of desirable characteristics are still scarce for candeia. The implementation of new commercial plantations is recommended in areas where the species occurs naturally. Thus, since the area of natural occurrence extends to different regions with different climatic conditions, the use of resistant clones selected in one region may be susceptible in others. Thus, the selection of candeia rust resistant clones must be carried out in the respective planting regions. Our study was based on the evaluation of disease that occurred naturally in a candeia population. However, the selection of resistant clones can be accessed in the early stages of plant development through artificial inoculation of the pathogen, thus facilitating the identification and selection of resistant progenies in the future. A protocol for this has not yet been developed for phenotyping, as there is a need for studies involving controlled conditions of humidity and temperature, which is in phase of development.
During the experimental period, the incidence and severity of candeia rust were correlated with environmental variables. The evapotranspiration, rainfall, and temperature were inversely correlated with disease progress. This relationship is negative because the evaluations were based on the presence of symptoms of the pustules containing signs (urediniospores) under the abaxial face of the leaves. However, lesions with yellow edges were commonly observed on the adaxial face as well as dead leaves on the plant. Rust sporulation is favored by adverse environmental conditions, such as low temperature, without rain, and low air humidity. Water and leaf wetness are important for germination and infection, and these occur before April in Brazil. Once environmental conditions become unfavorable for sporulation of the pathogen, it is still possible to visualize disease symptoms.
Thus, the negative correlation observed between disease severity and monthly mean temperature shows that mild temperatures favor the incidence of fungal sporulation. Sales et al. [9] stated that low temperatures can favor the disease because they increase the stress experienced by the plant, thus increasing its susceptibility to the pathogen, since these conditions favor the sporulation of the pathogen. In addition, Ruiz et al. [22] observed correlations between the severity of P. psidii in Eucalyptus grandis and temperatures between 18 and 25 °C and high humidity.
In the present study, relative humidity did not influence the disease severity, but the microclimatic conditions of the leaf surface were not considered. These conditions directly affect disease severity. The influence of microclimatic factors on the release of urediniospores from the pustules of P. psidii for new infections was well explained in a study conducted by Zauza et al. [23], where it was observed that variations in irradiance, temperature, relative humidity, and leaf wetness influenced the release of P. psidii spores in eucalyptus. Along these lines, our results suggest that the high mean insolation and temperature in January, a month in which one of the lowest mean disease severities was observed, contributed to the inhibition of P. velata spore germination. This behavior was explained by Ruiz et al. [24], who observed that exposure to light and to high temperatures inhibits the germination of P. psidii spores.
In addition, a negative correlation between rainfall and disease severity was observed. However, in the months before the beginning of disease progression, such as February and March, there was high rainfall. In this case, the high rainfall combined with the high relative humidity observed before the increase in disease severity in the field favored the establishment of the pathogen in the area, since these conditions contribute to the germination of urediniospores to initiate infection [9]. In turn, the highest mean rainfall, recorded in December, coincided with the lowest mean disease severity. This is due to the reduction in the number of spores in the air thanks to the increased rainfall, which washes away the pustules and deposits the urediniospores in the soil, preventing the release of these spores into the air [25]. This information is important because it contributes to the selection of adverse climatic regions to rust infection for the establishment of new plantations of candeia, based on the principle of controlling the evasion or escape of the pathogen.
Regarding disease progression over time, the Gompertz model showed the best fit to the data for candeia rust. This model was described by Vanderplank [18] for modeling diseases, who showed that the infection rate increases during the epidemic because it is not limited by factors such as the amount of host tissue. Sales et al. [9] studied the temporal progression of teak rust and observed that the Gompertz model had the best fit to the data, indicating that control measures to reduce the disease progress rate are the most effective. In addition, Oliveira et al. [26] obtained the best fit to bacterial leaf blight (Xanthomonas axonopodis) progression data with the Gompertz model for most of their studied clones. This indicates the better efficiency of the Gompertz model than others to describe disease progression in several pathosystems [27], especially polycyclic diseases, as observed by Bergamin Filho et al. [11].
The incidence of candeia rust reached 90% of the evaluated trees in May, and there were no changes in the number of infected trees after this period. My-de Mio et al. [28] observed the same behavior in relation to the incidence of poplar rust, since the incidence of the disease did not serve as a discriminatory resistance variable, as quickly 100% of the evaluated leaves showed symptoms, regardless of the resistance level of the clone. Zauza et al. [23] also observed a small variation in the incidence of P. psidii throughout the experimental period in eucalyptus, but the annual seasonal effect within a region and climatic variations in distinct regions should be noted. These variations were referred to as “ecological zoning” of eucalyptus rust by Masson et al. [29]. Thus, there may be differences in disease intensity between years due to natural climatic variations [23], so the results obtained in the present study are intrinsic to the region and the experimental period in which they were evaluated.
In contrast to the incidence of candeia rust, the severity of the disease continued to increase until July due to environmental conditions. The high incidence of rust in the experimental field is a worrisome factor for growing because the disease has rapid dissemination and increased severity under favorable conditions. In the field, the constant production of inoculum and its dispersion by wind and rainwater, added to a favorable environment, can accelerate disease progress if the host is susceptible [23]. Thus, it is important to select genotypes resistant to P. velata for planting in new areas and for renewal of established plantations, such as the clones classified as resistant in this study, in order to contain the spread of the disease in regions of commercial or naturally occurring candeia forests.
5. Conclusions
The candeia rust occurred in all clones, but the intensity of disease was different among clones. Clones C4, C37, and C34 were resistant to rust and therefore the most suitable for candeia plantations in southern Minas Gerais.
The temporal progress of the disease occurred between March and July, occurring during the coldest seasons, such as autumn and winter. The sporulation of P. velata increases in these months, that present milder temperatures (19–22 °C), cumulative rainfall of 31 mm, and relative humidity of approximately 75% at the beginning of progression, which favored the establishment of the disease. Further, studies are needed to evaluate the damage caused by the pathogen to tree productivity.
Author Contributions
For Conceptualization, M.A.F.; Methodology, M.A.F. and E.A.P.; Software, R.C.M.P.; Validation, R.C.M.P.; Formal Analysis, R.C.M.P.; Investigation, R.C.M.P., M.F.C.M.A., M.L.M.A. and C.A.L.F.; Resources, M.A.F.; Data Curation, R.C.M.P.; Writing Original Draft, R.C.M.P., T.P.F.S.; Preparation, R.C.M.P., T.P.F.S.; Writing-Review & Editing, M.A.F., E.A.P., and L.A.M.; Visualization, M.A.F.; Supervision, M.A.F.; Project Administration, M.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível—Superior Coordination for the Improvement of Higher Education Personnel (CAPES) and Citroleo.
Conflicts of Interest
The authors declare no conflict of interest.
References
- de Araújo, E.J.G.; Pelissari, A.L.; David, H.C.; Scolforo, J.R.S.; Netto, S.P.; Morais, V.A. Relação hipsométrica para candeia (Eremanthus erythropappus) com diferentes espaçamentos de plantio em Minas Gerais, Brasil. Pesqui. Florest. Bras. 2012, 32, 257–268. [Google Scholar] [CrossRef]
- Carvalho, P.E.R. Espécies Florestais Brasileiras: Recomendações Silviculturais, Potencialidade e Uso Da Madeira; EMBRAPA-CNPF: Brasília, Brazil, 1994; p. 640. [Google Scholar]
- Teixeira, M.C.B.; Nunes, Y.R.F.; Maia, K.M.P.; Ribeiro, R.N. Influência da Luz na Germinação de Sementes de Candeia (Vanillosmopsis erythropappa Shuh. Bip.); Encontro regional de botânica, 28., 1996, Belo Horizonte; Anais. Belo Horizonte: SBB; Pontifícia Universidade Católica de Minas Gerais: Belo horizonte, Brazil, 1996; pp. 35–41. [Google Scholar]
- Scolforo, J.R.S.; Oliveira, A.D.; Davide, A.C. Manejo Sustentável da Candeia Eremanthus erythropappus e Eremanthus incanus; Relatório técnico científico; UFLA-FAEPE: Lavras, Brazil, 2002; p. 350. [Google Scholar]
- Prance, L. Cosmetics Design. Available online: https://www.cosmeticsdesign.com/Article/2007/06/07/Worldwide-manufacturers-harness-powers-of-bisabolol (accessed on 16 March 2020).
- Santos, R.C.; Mendes, L.M.; Mori, F.A.; Mendes, R.F. Aproveitamento de resíduos da madeira de candeia (Eremanthus erythropappus) para produção de painéis cimentomadeira. Cerne 2008, 14, 241–250. [Google Scholar]
- Silva, C.S.J.E.; de Oliveira, A.D.; Junior, L.M.C.; Scolforo, J.R.S.; de Souza, Á.N. Viabilidade econômica e rotação florestal de plantios de candeia (Eremanthus erythropappus), em condições de risco. Cerene 2014, 20, 113–122. [Google Scholar] [CrossRef]
- Dietel, P. Uredineae brasilienses a cl. E. Ulelectae. Hedwigia 1897, 26–37. [Google Scholar]
- Sales, N.I.S.; Correia, L.C.M.D.A.; Siqueira, C.D.A.; dos Santos, G.R.; Leão, E.U. Temporal progress of teak rust in a tropical area of Tocantins State, Brazil. Acta Amaz. 2017, 47, 277–280. [Google Scholar] [CrossRef]
- Galdino, A.P.P.; Brito, J.O.; Garcia, R.F.; Scolforo, J.R. Estudo sobre o rendimento e qualidade do óleo de candeia (Eremanthuss sp.) e an influência das diferentes origens comerciais da sua madeira. Rev. Brasil. Plantas Med. 2006, 8, 44–46. [Google Scholar]
- Bergamin, A.F.; Amorim, L.; Hau, B. Análise temporal e espacial de epidemias. In O essencial da fitopatologia: Epidemiologia de doenças de Planta; UFV: Viçosa, Brazil, 2014; pp. 101–165. [Google Scholar]
- Figueiredo, M.B.; Passador, M.M. Morfologia, Funções dos Soros e Variações dos Ciclos Vitais das Ferrugens; Arquivos do Instituto Biológico: São Paulo, Brazil, 2008; pp. 117–134. [Google Scholar]
- Carvalho, A.; Hennen, J.F. The species of Puccinia on Piptocarpha and Vanillosmopsis in the Neotropics. Mycologia 2012, 104, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; John Wiley: New, York, NY, USA, 1990; p. 560. [Google Scholar]
- Vale, F.X.R.; Fernandes Filho, E.I.; Liberato, J.R. QUANT. A software plant disease severity assessment. In Proceedings of the 8th International Congress of Plant Pathology, Christchurch, New Zealand, 2–7 February 2003; p. 105. [Google Scholar]
- Colhoun, J. Effects of Environmental Factors on Plant Disease. Annu. Rev. Phytopathol. 1973, 11, 343–364. [Google Scholar] [CrossRef]
- Junior, W.C.D.J.; Bassanezi, R.B. Análise da dinâmica e estrutura de focos da morte súbita dos citros. Fitopatol. Bras. 2004, 29, 399–405. [Google Scholar] [CrossRef][Green Version]
- Vanderplank, J.E. Plant Diseases: Epidemics and Control; Academic Press: New York, NY, USA, 1963. [Google Scholar]
- Dantas, A.A.A.; de Carvalho, L.G.; Ferreira, E. Classificação e tendências climáticas em Lavras, MG. Ciência Agrotecnol. 2007, 31, 1862–1866. [Google Scholar] [CrossRef]
- Pereira, R.C.M.; Filho, C.A.L.; Melo, L.A.; Alozen, P.C.; Mafia, R.G.; Barros, A.F.; Ferreira, M.A. Diagrammatic scale for severity evaluation of the Eremanthus erythropappus—Puccinia velata pathosystem. J. Phtyopathol. 2019, 168, 135–143. [Google Scholar] [CrossRef]
- Anonymous. The R Project for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 13 February 2012).
- Ruiz, R.A.R.; Alfenas, A.C.; Maffia, L.A.; Barbosa, M.D.M. Progresso da ferrugem do eucalipto, causada por Puccinia psidii, em condições de campo. Fitopatol. Brasil. 1989, 14, 73–81. [Google Scholar]
- Zauza, E.A.V.; Lana, V.M.; Maffia, L.A.; Araujo, M.M.F.C.; Alfenas, R.; Silva, F.F.; Alfenas, A.C. Wind dispersal of Puccinia psidii urediniospores and progress of eucalypt rust. For. Pathol. 2014, 45, 102–110. [Google Scholar] [CrossRef]
- Ruiz, R.A.R.; Alfenas, A.C.; Ferreira, F.A.; Vale, F.X.R. Influência da temperatura, do tempo de molhamento foliar, fotoperíodo e da intensidade de luz sobre an infeccão de Puccinia psidii em eucalipto. Fitopatol. Brasil. 1989, 14, 55–61. [Google Scholar]
- Fulton, J.D. Microorganisms of the upper atmosphere. V. Relationship between frontal activity and the micropopulation at altitude. J. Appl. Microbiol. 1966, 14, 245–250. [Google Scholar] [CrossRef]
- de Oliveira, M.E.S.; Fernandes, F.S.; Junior, M.A.G.; de Oliveira, A.S.; Mafia, R.G.; Ferreira, M.A. Temporal Analysis of Bacterial Leaf Blight in Clonal Eucalyptus Plantations in Brazil. Forests 2019, 10, 839. [Google Scholar] [CrossRef]
- Berger, R.D. Comparison of the Gompertz and Logistic Equations to Describe Plant Disease Progress. Phytopathology 1981, 71, 716. [Google Scholar] [CrossRef]
- de Mio, L.L.M.; Amorim, L.; Moreira, L.M. Progresso de epidemias e avaliação de danos da ferrugem em clones de álamo. Fitopatol. Bras. 2006, 31, 133–139. [Google Scholar] [CrossRef][Green Version]
- Masson, V.M.; Ohto, C.T.; Furtado, E.L.; Silva, A.S. Zoneamento climático do eucalipto no estado de São Paulo visando o controle da ferrugem. Summa Phytopathol. 2007, 33, 67. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).