Abstract
Given the ongoing climate change, estimating the amount of less degradable plant compounds that can be stored in the soil, such as lignin, is a topic of primary importance. There are few methods applicable to soils for the determination of lignin, such as the copper oxide (CuO) oxidation method (CuOL). Acetyl bromide spectrophotometric lignin (ABSL) could be a valid alternative providing information that is less detailed compared to CuOL, but it offers data on the bulk amount of lignin and may offer a valid, fast, and cheap alternative to the CuO method. The aim of this work was to compare ABSL with the CuO method on several soils receiving plant residues from different trees. Mineral soil samples from 0 to 10 cm depth were obtained from a former agricultural site in northern Italy (Brusciana, Tuscany), where different tree plantations were established 22 years ago. The plantations were white poplar and common walnut, which were also intercropped with other species such as hazelnut, Italian alder, and autumn olive. Soil samples under these plantations were also compared to soil under an adjacent agricultural field. In general, the amount of lignin in the afforested stands was approximately double than in the agricultural field as determined by either method. The two methods returned a largely different scale of values due to their different mechanisms of action. The acid-to-aldehyde ratio of syringyl structural units highlights that forest plantation provides a plant input material that is more slowly oxidatively degraded compared to arable soil. A linear mixed model proved that ABSL performed well in relation to CuOL, especially when considering the random variation in the model given by the plantation field design. In conclusion, ABSL can be considered a valid proxy of soil C pool derived from structural plant component, although further analyses are needed.
1. Introduction
There is a growing consensus that human activities in the last two centuries have induced dramatic and unprecedented changes in the global chemical and physical environment. Such climatic and environmental changes are predicted to exert strong effects on the ecosystem functioning, which in turn, are reflected at an infrastructural [1] and economic level [2]. This situation could have particularly strong consequences for dryland regions, as well as for Mediterranean areas [3], implying a significant loss of ecosystem services. The global drylands contain 1.1 billion hectares of forest, which is 27% of the global forest area, estimated at approximately 4 billion hectares and, given the ongoing climate change, forests in a more arid climate could decrease by about 12.5% [4]. All tropical, temperate, and boreal forests are predominantly carbon (C) sinks [5]. Through their C-sink role, forests contribute to regulating the atmospheric CO2 concentration level [6] and, by storing C, can mitigate global warming [7]. Thus, all actions such as afforestation, reforestation, as well as restoration of cultivated, abandoned, and marginal agricultural lands, can potentially reverse or reduce the process of C loss and increase ecosystem C storage [8], representing a valid tool to mitigate climate change. In such a context, it is a priority not only to apply management to induce the increase in C sink in the soil, but also to quickly and reliably quantify the C pool that is less prone to biological degradation and can be stored in the soil.
Lignin, cellulose, and hemicelluloses represent the main types of complex carbon compounds entering the soil environment, and their degradation represents a key step in the terrestrial carbon cycle [9]. The chemical analysis of lignin is difficult due to the intricate structure of the ligno-cellulose complex consisting of the polysaccharide and the phenol-based network of the lignin macromolecules. However, alkaline oxidation with CuO and the analysis of the products with gas chromatography-flame ionization detection (GC-FID) or gas chromatography-mass spectrometry (GC-MS) has proven to be a valuable method for the analyses of lignin and cutin in plants, soils, and sediments [10,11].
CuO oxidation yields single-ring phenols, with vanillyl (V), syringyl (S), and cinnamyl (C) structures and aldehyde, ketone, and acid sidechains. The sum of the concentrations of the specific lignin biomarkers of the V-, S-, and C-type (VSC) is used as an indicator of the quantitative lignin contribution to soils even if CuO oxidation does not completely depolymerize lignin, and is therefore, not quantitative, but the method cleaves aryl ether bonds and releases phenolic monomers and dimers from the outer part of the lignin polymer [12]. The lignin-derived phenolic acid to their corresponding aldehydes ratios, the (Ac/Al) ratios, instead, have been applied in numerous studies to determine the degradation stage of soil and riverine organic matter [13].
The CuO oxidation method, in addition to not providing a quantitative indication, requires long time and high costs, as well as the use of equipment that might be not readily available in all laboratories. In addition, the CuO oxidation method, being dependent on the different chemical structure of lignin, is strongly dependent on the type of plant from which the lignin originates [14]. The identification of a quick and inexpensive method for the determination of lignin into soils that require minimal laboratory instruments represents a challenge and could be a significant support in the study of the fate of organic matter in soil. Simple gravimetric methods such as Klason lignin or detergent fiber analysis can be applied to plant material [15,16,17] but, given their use of strongly oxidizing chemicals and their principle of loss-on-weight, their use on soil matrices cannot be applied, as the loss of inorganic substances (such as carbonates) would be computed as lignin.
In litter decomposition studies (i.e., when dealing with fully organic matrixes), lignin contents are often measured both by fast and relatively inexpensive spectrophotometric methods, typical procedures, which also proved to outmatch gravimetric methods in their resolution, reliability, and speed [16,18]. Spectrophotometric methods for the determination of lignin have already been applied to forest soils and proved to be significantly correlated with the concentration of humic and fulvic acid carbon [19], obtained using the Springer–Klee wet digestion that involves a back-titration system based on the oxidation by K2Cr2O7 [20,21]. However, despite their potential, there is a need to evaluate the results of the spectrophotometric method for lignin in soil to provide evidence as to whether these measurements are consistent not only with humic and fulvic acids carbon [19], but also with CuO oxidation results, which are usually used as a proxy for lignin-derived organic substances in soil.
The goals of this work were to (i) evaluate the impact of the different stands on the lignin amount, and to (ii) to test the spectrophotometric method on soil by comparing them with the results of the CuO oxidation method. Soils were obtained from a forest site planted on former agricultural land, characterized by different tree associations (stands) consisting of broad-leaves mixed plantations that include the presence of valuable species and nurse species. We approached this by linear mixed modelling, which allowed us also to take into account the spatial variability given by the forest plantation that had four different implants (stands) nested within three field replicates.
2. Material and Methods
2.1. Study Site
The experimental area is located in Tuscany, Central Italy, nearby Brusciana (Florence) (43°40′29′′ N, 10°55′21′′ E), at 35 m a.s.l. The location has a mean annual precipitation of 850 mm and a mean annual temperature of 15 °C. In 1996, an experimental tree farming plantation was established for the production of wood on agricultural land. In this type of plantation, namely polycyclic plantation, different crop trees with different fast-growing and rotation periods are planted together on the same surface. In this trial, two valuable broadleaved tree species, widely used in Italy for timber production, poplar (Populus alba L., Salicaceae) and walnut (Juglans regia L., Juglandaceae), were planted together, and intercropped with different nurse trees and shrubs (Danise et al., 2020 in press). In particular, the following planting plots were compared using a randomized blocks design with three replications:
- Poplar and walnut (stands PJ) were planted using an intimate mixture;
- Poplar, walnut intercropped with hazel (Corylus avellana L., Betulaceae) (stands PJC);
- Poplar, walnut intercropped with Autumn olive (Elaeagnus umbellata Thunb., Elaeagnaceae) (stands PJE), a N-fixing species;
- Poplar, walnut intercropped with Italian alder (Alnus cordata (Loisel.) Duby, Betulaceae) (stands PJA), a N-fixing species.
2.2. Sampling
The field experiment followed a randomized block design with three field replicates. In May 2018, for each plot, five microsites were identified. The center of each plot was identified and sampled along with four microsites 6 m away from the center, in the direction of the cardinal points, for a total of five microsites (i.e., 3 field replicates × 5 plots × 5 soil cores, n = 75). In each point, the first 10 cm of mineral soil was taken by means of a core sampler. Soil samples were also collected from an adjacent agricultural field (AL) using the same sampling technique. The three field replicates were virtually arranged along three axes (Plots), which will only be taken into account in the statistical analysis as it does not affect the graphical representation. The agricultural field does not provide the same experimental design, so it will not be included in the statistical analysis but will be considered as a nonforested reference soil. Once in the laboratory, the disturbed soil samples were left to air-dry and were then sieved to remove rock fragments, plant fragments, and roots (>2 mm).
2.3. Determination of CuO Oxidation Lignin (CuOL)
Soil samples were oxidized with CuO to release lignin-derived phenols (modified after Hedges and Ertel, 1982) [10]. Teflon-lined bombs were loaded with dry soil (from 100 to 800 mg according to C content), 500 mg CuO, 100 mg (NH4)2Fe(SO4)2·6 H2O, 50 mg glucose, 100 µL of ethyl vanillin (EV) stock solution (10 µg EV/100 µL NaOH 2 M) used as internal standard, and 15 mL of 2 M NaOH. The internal standard was used to normalize the yields of the CuO decomposition products, and its recovery must be at least 70%.
The Teflon beakers were purged with nitrogen gas, sealed, and heated for 3 h at 170 °C. We let them cool all night.
After the precipitation of humic acids induced by the addition of 6 M HCl, we proceeded with the solid-phase extraction of the samples using SPE columns. This process took place through the use of a filtration apparatus connected to a vacuum pump. Once the columns were fixed, they were prepared by filling them, in turn, with ethyl acetate, methanol (MeOH), and water, taking care that they never got dry. When the columns had been prepared, the sample was filtered. Subsequently, the columns were dried using N gas for one hour. The elution phase followed using 5 mL of ethyl acetate and, also in this phase, the support of the filtration apparatus connected to the vacuum pump was used. After adding first 100 μL pyridine and then 200 μL BSTFA (N,O-Bis(trimethylsilyl)trifluoroacetamide), vials were placed in the derivisation block for 20 min at 60 °C. The samples thus obtained were read on the GC-MS (5977 Series GC/MSD system with Agilent 7890B GC, City Stevens Creek Blvd., City of San Jose, CA, USA). The results were obtained on the basis of a calibration curve consisting of 9 points, i.e., 9 standards containing increasing concentrations of a standard mix solution and 1 mL of phenyl acetic acid (25 µg/1 mL MeOH). The standard mix solution contained (50 mg/100 mL MeOH) vanillin, ethyl vanillin, acetovanillin, syringaldehyde, vanillic acid, acetosyringone, syringic acid, p-coumaric acid, and ferulic acid. Excluding ethyl vanillin, each of these compounds represents the aldehyde, acid, or ketone residue afferent respectively to the vanillyl (V), syringyl (S), and cinnamyl (C) unit, and was identified and quantified by the GC-MS. In addition, a soil sample with a known VSC concentration was added to each batch of samples (no more than 10). Otto and Simpson (2006) [14] made the comparative CuO oxidation of three subsamples (2 g each) of the Orthic Black Chernozem, yielding 4.8, 5.0, and 5.5 mg of products, representing 94%–108% of the average and demonstrating the reproducibility of the method.
Besides the yield of vanillyl, syringyl and cinnamyl units (VSC), the ratios of lignin-derived phenolic acids and their corresponding aldehydes (Ac/Al) for vanillyl (Ac/Al)v and syringyl (Ac/Al)s units were calculated [22].
2.4. Determination of Acetyl-Bromide Spectrophotometric Lignin (ABSL)
We followed the acetyl-bromide spectrophotometric lignin determination method proposed by [23] with minor modifications. Dry soil samples (50–120 mg according to SOM content) were put into 15 mL polypropylene tubes and, in order to remove tannins, flavonoids, and proteins that might interfere with the analytical determination, 5 mL of 70% acetone solution in distilled water (v/v) were added. Samples and acetone were thoroughly mixed and then put into a sonic bath (40 kHz) for 40 min. Subsequently, samples were centrifuged at 200× g for 2 min and the supernatant carefully discarded. The procedure was repeated once more and then samples were oven-dried at 75 °C for 24 h. In a fume cupboard, 5 mL of 25% acetyl-bromide solution in glacial acetic acid (v/v) was added. Tubes were loosely capped and put into a thermostatic water bath at 50 °C for 2 h. Subsequently, 250 μL of the previous solution was transferred into new tubes, followed by 3.25 mL of glacial acetic acid and 1.00 mL of NaOH 0.3 M. Tubes were closed and thoroughly mixed, and then 500 μL of 0.5 m hydroxylamine hydrochloride solution was added. Absorbance of the solution (mixed immediately before the reading) was read in a UV-spectrophotometer at 280 nm against blank samples using quartz cuvettes. We used a mass attenuation coefficient (ε) of 20.619 g−1 L cm−1 that was obtained by creating a calibration curve using a lignin standard (Merck, CAS number 8068-05-1). In detail, from a stock solution containing 1 g lignin per 1 L of 25% acetyl-bromide in glacial acetic acid, we made the dilution in order to cover a range of lignin concentrations between 0.010 and 0.100 g L−1. Our mass attenuation coefficient was comparable to 23.08 g−1 L cm−1 that was reported by Fukushima and Kerley (2011) [24] after computation from the regression analysis of standard curves derived from 17 isolated lignin from different plant species. Furthermore, Fukushima and Kerley (2011) [24] also included three commercial laboratory lignin (Aldrich Chemical Co., Inc., Milwaukee, WI, USA): Hydrolytic, organosolv (propionate), and organosolv (2-acetoxyethyl ether) lignin that all rendered a mass attenuation coefficient comparable to the alkali lignin that we used as internal reference. The mean recovery rate for kraft lignin under our laboratory conditions was 99.8 ± 1.1% (N = 14) with a 4.1% coefficient of variation. Results were expressed as mg g−1 dry weight. All analyses were performed in triplicate.
2.5. Statistics
All data were checked for normality and homogeneity of variance before statistical analyses. One-way analysis of variance (ANOVA) was used to test the effects of land use on the response variables, followed by Tukey’s post-hoc test (α = 0.05)
The general relationship between independent (ABSL) and dependent variable (CuOL) was displayed by scatterplots with regression lines according to the stand factor after scaling the variables. We fitted linear mixed models (LMM) between predictors and the dependent variable using factors plot and stand as random factors, the latter nested in the former. All variables were scaled before analysis. Denominator degrees of freedom were computed with the Kenward-Roger method. The intraclass correlation coefficient (ICC) was reported as a proxy of the magnitude of the random variation in the models. The mixed-models goodness of fit was expressed as conditional and marginal determination coefficients (R2c and R2m) in order to quantify unbiased measurements of variance expressed by fixed and fixed + random factors, respectively [25]. All statistical analyses were done in R 4.0.3 [26].
3. Results
3.1. Lignin
The spectrophotometric lignin ABSL (Figure 1a) showed differences not only between the afforested stands and the arable soil, but also between the afforested stands themselves.
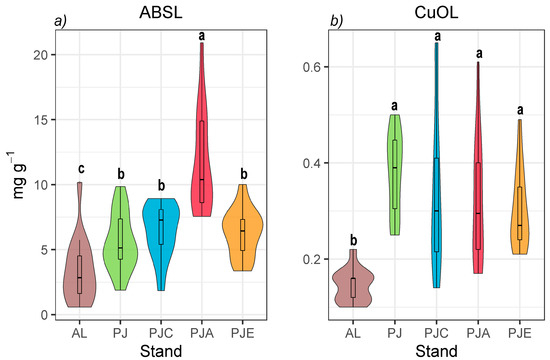
Figure 1.
(a) Acetyl-bromide spectrophotometric lignin (ABSL) and (b) CuO oxidation lignin (CuOL) (as the sum of the V + S + C components of the CuO oxidation method) contents in the afforested stands and agricultural land. Data are represented as violin plots with a nested boxplot. Violin plots that do not share at least one letter are significantly different (p ≤ 0.05, Tukey’s post-hoc test follows one-way ANOVA).
In the presence of alder (i.e., PJA), ABSL showed values (11.7 ± 1.05 mg g−1) that were twice as high as the other forested stands, while AL had the lowest values. Overall, in all the forested stands, CuOL presented a three-times larger content than the arable soil, while there were no differences between the values recorded in the forested stands (Figure 1b). The absolute values ranged from 0.14 ± 0.01 in AL to 0.38 ± 0.02 mg g−1 in PJ.
3.2. The Acid-To-Aldehyde Ratios
The lignin-derived phenolic acid to their corresponding aldehydes ratios of (Ac/Al) for syringyl units (Ac/Al)s and for vanillyl units (Ac/Al)v are shown in Figure 2.
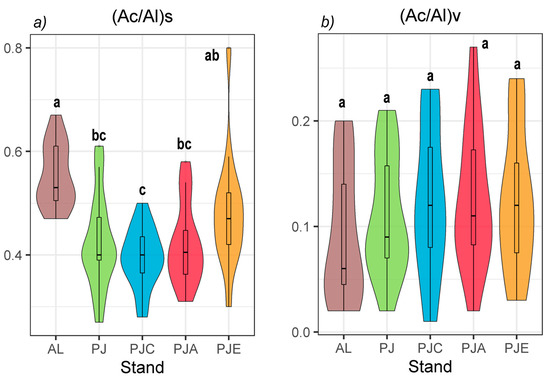
Figure 2.
The lignin-derived phenolic acid to their corresponding aldehydes ratios of (Ac/Al) for (a) syringyl units (Ac/Al)s and (b) for vanillyl units (Ac/Al)v in the afforested stands and agricultural land stands. Data are represented as violin plots with a nested boxplot. Violin plots that do not share at least one letter are significantly different (p ≤ 0.05, Tukey’s post-hoc test follows one-way ANOVA).
The acid-to-aldehydes ratio for syringyl units (Ac/Al)s varied from 0.55 ± 0.01 (AL) to 0.39 ± 0.01 (PJC), while in the soils under the other stands, it showed intermediate values (Figure 2a). On the contrary, the (Ac/Al)v ratio did not present any differences between the considered stands, showing largely overlapping values between 0.094 ± 0.01 in AL and 0.13 ± 0.01 in PJA (Figure 2b).
3.3. Linear Mixed Models (LMM)
The LMM summary with CuOL as a dependent variable and ABSL as an independent variable is shown in Table 1.

Table 1.
Results from the linear mixed models (LMM) between ABSL and CuOL. All continuous variables were scaled before analysis (i.e., the mean was subtracted from each observation and the result divided by the standard deviation). The fixed part of the model reports the estimates, the confidence interval (CI), and the p-value for both intercept and slope, while the random effects are reported as explained variance (σ2), random intercept variance (τ00), and intraclass correlation coefficient (ICC). Goodness-of-fit is reported as marginal and conditional R2 to account for the effect of fixed and fixed + random components of the model, respectively.
Although the marginal R2 was comparatively low (i.e., 0.103, the amount of variance explained only by the fixed terms in the model), ABSL proved to be a significant term in the model (p-value = 0.035). The explained variance largely improved when also considering the random variation in the model, explained by the nested structure of stands within plots (conditional R2 = 0.587). Noticeably, when considering the random intercept variance (τ00), which explains the between-subject variance, the effect of stands (as nested in plots) was larger than plots alone (τ00 0.34 vs. 0.29, respectively). Thus, the stand random effect had a large effect on the model, as can also be seen in Figure 3, where the scatterplot between scaled variables with simple regression lines (that were fitted only to aid visualization) showed a comparatively different behavior, as seen between PJ and PJA vs. PJC and PJE.
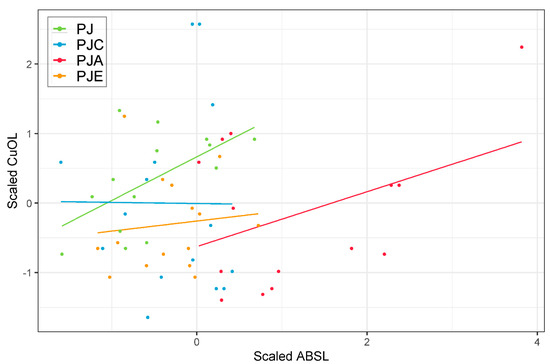
Figure 3.
Scatterplot between scaled data of ABSL and CuOL. Observations are colored according to the different plots, and simple regression lines have been added to aid a general visualization of the relationship between data.
4. Discussion
The key role in the formation of SOM with long residence times exerted from the soil microbial biomass, microbial products, and their accessibility to organic substrates has become increasingly more evident [27]. However, according to Sokol et al. (2019) [28], plant-derived substances can also be stabilized in soil via direct sorption processes to minerals. Hence, the use of, e.g., lignin-derived phenols, as biomarkers can improve the understanding of the transformation of lignin-derived plant residues into SOM in forest environments. In particular, the biodegradation of water-soluble leaf fractions is characterized by a preferential metabolization of carbohydrates [29], leaving the residual material relatively enriched in lignin-derived compounds that, in turn, contributes to the formation of a continuum of organic fragments that are continuously processed by the decomposer community toward smaller molecular size in forested soils [30]. Danise et al. (2018) [19] analyzed the lignin quantity in several Fagus sylvatica L. forest topsoil layers by applying the ABSL spectrophotometric method. Our results in afforested stands for ABSL (Table S1) are higher as compared to those published by Danise et al. (2018) [19], where lower contents of lignin (32.55 ± 2.26 mg g−1 and 106.32 ± 5.93 mg g−1 OC (organic carbon), 0–5 cm soil depth; 20.18 ± 1.08 mg g−1 and 61.87 ± 4.67 mg g−1 OC, 5–15 cm) were found. Similarly, our findings were higher than those reported in Innangi et al. (2017) [31] who reported, for a Alnus cordata forest topsoil (0–5 cm soil depth), 6.5 ± 0.6 mg g−1 lignin and 78.95 ± 2.82 mg g−1 OC. Yet, both aforementioned studies dealt with natural forests with little-to-no management, while in our case, we had mixed afforested plots that started from an agricultural soil with a low SOM content.
The concentrations of CuOL lignin (Table S1) were lower than those found in Traversa et al. (2011) [32] where a pure Quercus ilex L. forest was studied, showing a VSC content of 32.64 mg g−1 OC (0–2 cm soil depth), 23.43 mg g−1 OC (2–5 cm). Moreover, Traversa et al. (2011) [32] analyzed soil in a mixed Quercus trojana Webb. and Q. ilex forest showing higher VSC (i.e., CuOL) values (34.75 mg g−1 OC (0–2 cm), 31.33 mg g−1 OC (2–5 cm)) than the pure Q. ilex plantation, but the two broad-leaved trees, although belonging to different species, fall within the same genus. From the comparison between the stands (where CuOL shows the highest values in PJ and ABSL in PJA, Table S1) and in relation to the literature, a very varied pattern emerges. It has been demonstrated that different tree species mixtures can influence the microbial community and, consequently, soil C through litter quality and quantity and root dynamics [33,34]. In particular, a positive correlation has been found between the number of intercropped species and microbial residues able to lead an increase in stable SOC (soil organic carbon) content [35,36]. As each stand contains the same number of species, but different ancillary species, the differences between the various stands suggest that—more than the number of species present in the afforested plot—it was the species within the stands and their litter quality that had an effect. There have been many studies on the relationship between litter properties and their decomposition rates in soil, and the effects of litter composition on soil microbial structure have also been reported [17,37]. Berg and McClaugherty (2014) [17] showed that plant litter provides the substrate for soil microorganisms, and therefore, significantly influences the microbial community structure through the availability of nutrients and the unique soil microenvironment created by its different chemical components. Santonia et al. (2018) [38] showed that litter diversity effects on soil were mediated by litter species composition rather than litter species richness, highlighting the importance of litter species identity—and associated litter traits—as drivers of microbial communities, which in turn, affects the SOM [35]. Accordingly, the presence of Italian alder in PJA appeared to increase the amount of ABSL (Table S1). Previous studies have demonstrated the positive influence of alder on the chemical [39] and biological [40] characteristics of the soil, and our evidence suggests that it could also play a key role in territorial management from a climate change perspective. However, PJA soil also had a higher clay content (200 ± 20 g/kg) than the other stands soils (Danise et al. (2020), under review), which could imply that side-chain oxidation of lignin is retarded due to the protection by clay minerals and corresponds to the higher amounts of VSC-lignin in the clayey soils [41]. Probably for the same reason, the CuO oxidation method did not detect part of the lignin present in PJA, unlike the spectrophotometric method. Moreover, it has been clearly shown that mineral-bound lignin is underestimated by up to 44% with the CuO oxidation method [42] and takes into account only the outermost part of the lignin molecule. On the contrary, the spectrophotometric method, given the use of an acetyl-bromide and acetic acid solution, uses the ability of acetyl-bromide to acetylate phenols of the whole molecule [43], giving a more accurate estimate. Additionally, it is important to highlight that the ABSL method allows one to process up to 50 samples in one day, whereas the CuO oxidation method requires 3 days for 10 samples and does not use a large amount of organic solvents. On the other hand, the CuO oxidation method provides important qualitative information given by the acid-to-aldehyde ratios of vanillyl (Ac/Al)V and syringyl (Ac/Al)S structural units, as can be clearly seen above all in AL compared to the afforested stands. When the VSC values are expressed on an OC base (Table S1, 17.4 ± 0.80 mg g−1 OC), the values at AL are lower than those reported by Heim and Schmidt (2007) [44] for an arable soil (31 mg g−1 OC). While there were no significant differences of VSC between the arable soil and the afforested soils, the (Ac/Al)s ratio at AL is higher than in the soils under trees (Figure 2a). As this ratio reflects the degree of microbial alteration of residual lignin-derived phenols [22], it implies that the lignin is more decomposed in AL, suggesting that forest plantation provides a plant input material that is more slowly oxidatively degraded. By contrast, PJC presented the opposite situation (Figure 2a), showing the incidence of a nurse species in a mixed plantation, whereas in PJ, where there are no nurse species, the (Ac/Al)S values were intermediate despite having the greatest values of VSC (Table S1). This evidence pointed out that hazel could influence the oxidative decomposition of lignin in a mixed plantation. In all stands, the (Ac/Al)S ratios (Figure 2a) were significantly greater than the (Ac/Al)V ratios (Figure 2b), which confirms the greater availability of S units to microbial alteration [32].
The stabilization of lignin in the soil is a much-debated issue [45], as is the contribution of the polysaccharide component to the soil stable C pool [46]. The refractory C pool has a similar proportion of polysaccharides as the labile C pool, but refractory polysaccharides are mainly associated with fine separates and show a dominant contribution of microbial sugars [47]. Compound-specific analyses of carbohydrates suggested a relatively slow turnover, considering their high degradability [48]. Gleixner et al. (2002) [49] indicated that polysaccharides and N-containing compounds had longer residence times than lignin-derived compounds, suggesting that microbial recycling is an important process responsible for C stabilization in soils [50]. Heim and Schmidt (2007) [44] showed that it is difficult to discern if lignin that is no longer found in the soil has been mineralized to CO2, or has been transformed into more recalcitrant degradation products that are no longer detectable. Moreover, they found that residence times refer to unaltered lignin molecules and thus provide a minimum turnover time of lignin in soils. Thus, the residence time of modified lignin-derived C in soils could be substantially longer. Therefore, it is not easy to determine the lignin in the soil. To date, probably, the real amount of lignin in soils cannot be analyzed directly by any independent analysis [51], although spectroscopic methods remain a viable resource for analyzing soil organic matter in general [50].
In agreement with Danise et al. (2018) [19], LMM (Table 1) proved that ABSL performed well in relation to CuOL (Conditional R2 0.587), especially when considering the random variation in the model given by the nested structure of the field experiment spatial variations (i.e., plots), as well as afforestation design (i.e., stand) (Table 1). According to previous studies [52,53,54], there is a spatial variability due to the plot (Table 1), but the stand factor has the greatest impact (Table 1). As shown by the scatterplot (Figure 3), the presence of bushes (E. umbellata and C. avellana) negatively affected the performance of ABSL within the model, and further studies are needed to clarify this aspect. As previously shown, the plant species influence not only the amount of lignin in the soil, but also its quality. Previous studies [14,55], in fact, have demonstrated that the composition of lignin-derived phenols in soil closely matches the composition observed in their respective source plants, reflecting the preservation of characteristic lignin patterns in soils. Therefore, the different chemical nature of the lignin attributable to the different cover trees could influence the relationship identified between the methods. In fact, the apparent relationship between aromaticity and bulk OC sorption [42] could lead not only to a quantitative underestimation by the CuO oxidation method, but also to a selection of the lignin on the basis of its peculiar chemical characteristics, as the CuO oxidation method yield is strongly influenced by the absorbed lignin fraction. Further studies are needed to clarify the relationship between ABSL and absorbed lignin.
5. Conclusions
The impact of climate change on the carbon cycle is a major issue. The implementation of mixed deciduous tree plantations on former arable land provides a good option for the recovery of exploited soils. Besides an increase in the quantity of soil organic carbon, it also has a significant impact on the organic matter quality, as is clearly shown by the dichotomy between the agricultural field and PJC with respect to lignin. The two methods return a largely different scale of values due to their different mechanisms of action as the CuO oxidation method is influenced by the presence of clay and acts only on the outermost parts of the lignin molecule. The soil collected in PJA shows the highest ABSL values, highlighting that the alder can be a valid species in relation of climate change. The spectrophotometric method ABSL showed a good performance in relation to the CuO oxidation method, taking into account random variation (such as plots and, above all, origin of the plant material). We can therefore conclude that ABSL can be considered a valid proxy of soil C pool derived from a structural plant component.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/11/12/1262/s1, Table S1: Total organic carbon (TOC) contents of the investigated afforested and the arable soils along with the OC-based contents of vanillyl (V), syringly (S) and cinnamyl (C) units of lignin as determined by the CuO oxidation method and OC-based lignin contents as determined by spectrophotometric methods (ABSL). Stand labels: PJ = white poplar and common walnut; PJC = PJ intercropped with common hazel; PJA = PJ intercropped with Italian alder; PJE = PJ intercropped with autumn olive; AL = agricultural land. Values are mean and standard deviation of three samples. Superscript letters indicate significant differences within means in the column according to One-way ANOVA at p ≤ 0.05 and Tukey test.
Author Contributions
T.D. conceptualized the research design, was involved in the soil sampling and analyses, and led the writing of the manuscript with revision from all authors. A.F. and E.C. were involved in the soil sampling and analyses. M.I. was involved in the soil sampling and analyses, and performed the statistical analyses. G.G. conceptualized the research design and was involved in the soil analyses. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We are enormously grateful to Franca Testa, Flora Chiariello and Viola Rünzi for their invaluable support in laboratory analysis.
Conflicts of Interest
No author is involved in competing interests.
Abbreviations
| ABSL | acetyl-bromide spectrophotometric lignin |
| CuOL | CuO oxidation lignin |
| (Ac/Al)v | The lignin-derived phenolic acid to their corresponding aldehydes ratios of (Ac/Al) for vanillyl units. |
| (Ac/Al)s | The lignin-derived phenolic acid to their corresponding aldehydes ratios of (Ac/Al) for syringyl units. |
| PJ | Populs alba; Juglans regia (white poplar; common walnut) |
| PJA | Populs alba; Juglans regia; Alnus cordata (white poplar; common walnut; Italian alder) |
| PJC | Populs alba; Juglans regia; Corylus avellana (white poplar; common walnut; hazelnut) |
| PJE | Populs alba; Juglans regia; Elaeagnus umbellata (white poplar; common walnut; autumn olive) |
References
- Giudicianni, C.; Herrera, M.; di Nardo, A.; Carravetta, A.; Ramos, H.M.; Adeyeye, K. Zero-net energy management for the monitoring and control of dynamically-partitioned smart water systems. J. Clean. Prod. 2020, 252, 119745. [Google Scholar] [CrossRef]
- Tol, R.S.J. The Economic Effects of Climate Change. J. Econ. Perspect. 2009, 23, 29–51. [Google Scholar] [CrossRef]
- Schlaepfer, D.R.; Bradford, J.B.; Lauenroth, W.K.; Munson, S.M.; Tietjen, B.; Hall, S.A.; Wilson, S.D.; Duniway, M.C.; Jia, G.; Pyke, D.A.; et al. Climate change reduces extent of temperate drylands and intensifies drought in deep soils. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- FAO. Trees, forests and land use in drylands: The first global assessment. FAO For. 2019, 184, 31. [Google Scholar]
- Besnard, S.; Carvalhais, N.; Arain, M.A.; Black, A.; De Bruin, S.; Buchmann, N.; Cescatti, A.; Chen, J.; Clevers, J.G.P.W.; Desai, A.R. Quantifying the effect of forest age in annual net forest carbon balance. Environ. Res. Lett. 2018, 13, 124018. [Google Scholar] [CrossRef]
- Tang, X.; Li, H.; Ma, M.; Yao, L.; Peichl, M.; Arain, A.; Xu, X.; Goulden, M. How do disturbances and climate effects on carbon and water fluxes differ between multi-aged and even-aged coniferous forests? Sci. Total Environ. 2017, 599, 1583–1597. [Google Scholar] [CrossRef] [PubMed]
- Rebane, S.; Jõgiste, K.; Kiviste, A.; Stanturf, J.A.; Kangur, A.; Metslaid, M. C-exchange and balance following clear-cutting in hemiboreal forest ecosystem under summer drought. For. Ecol. Manag. 2020, 472, 118249. [Google Scholar] [CrossRef]
- Arevalo, C.B.M.; Bhatti, J.S.; Chang, S.X.; Sidders, D. Land use change effects on ecosystem carbon balance: From agricultural to hybrid poplar plantation. Agric. Ecosyst. Environ. 2011, 141, 342–349. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Zühlke, D.; Becher, D.; Riedel, K.; Baldrian, P. Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci. Rep. 2016, 6, 25279. [Google Scholar] [CrossRef]
- Hedges, J.I.; Ertel, J.R. Characterization of lignin by gas capillary chromatography of cupric oxide oxidation products. Anal. Chem. 1982, 54, 174–178. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. Analytical approaches for characterizing soil organic matter. Org. Geochem. 2000, 31, 609–625. [Google Scholar] [CrossRef]
- Johansson, M.-B.; Kögel, I.; Zech, W. Changes in the lignin fraction of spruce and pine needle litter during decomposition as studied by some chemical methods. Soil Biol. Biochem. 1986, 18, 611–619. [Google Scholar] [CrossRef]
- Ertel, J.R.; Hedges, J.I. The lignin component of humic substances: Distribution among soil and sedimentary humic, fulvic, and base-insoluble fractions. Geochim. Cosmochim. Acta 1984, 48, 2065–2074. [Google Scholar] [CrossRef]
- Otto, A.; Simpson, M.J. Evaluation of CuO oxidation parameters for determining the source and stage of lignin degradation in soil. Biogeochemistry 2006, 80, 121–142. [Google Scholar] [CrossRef]
- Sjöberg, G.; Nilsson, S.I.; Persson, T.; Karlsson, P. Degradation of hemicellulose, cellulose and lignin in decomposing spruce needle litter in relation to N. Soil Biol. Biochem. 2004, 36, 1761–1768. [Google Scholar] [CrossRef]
- Fukushima, R.S.; Hatfield, R.D. Comparison of the acetyl bromide spectrophotometric method with other analytical lignin methods for determining lignin concentration in forage samples. J. Agric. Food Chem. 2004, 52, 3713–3720. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9783642388200. [Google Scholar]
- Bauer, S.; Ibáñez, A.B. Rapid determination of cellulose. Biotechnol. Bioeng. 2014, 111, 2355–2357. [Google Scholar] [CrossRef]
- Danise, T.; Fioretto, A.; Innangi, M. Spectrophotometric methods for lignin and cellulose in forest soils as predictors for humic substances. Eur. J. Soil Sci. 2018, 69, 856–867. [Google Scholar] [CrossRef]
- Curcio, E.; Danise, T.; Innangi, M.; Coppola, E.; Alvarez-romero, M.; Fioretto, A.; Papa, S. Soil characterization and comparison of organic matter quality and quantity of two stands under different vegetation cover on Monte Faito (Campania, S-Italy). Fresenius Environ. Bull. 2017, 26, 8–18. [Google Scholar]
- Vitti, C.; Stellacci, A.M.; Leogrande, R.; Mastrangelo, M.; Cazzato, E.; Ventrella, D. Assessment of organic carbon in soils: A comparison between the Springer-Klee wet digestion and the dry combustion methods in Mediterranean soils (Southern Italy). Catena 2016, 137, 113–119. [Google Scholar] [CrossRef]
- Ertel, J.R.; Hedges, J.I. Sources of sedimentary humic substances: Vascular plant debris. Geochim. Cosmochim. Acta 1985, 49, 2097–2107. [Google Scholar] [CrossRef]
- Fukushima, R.S.; Kerley, M.S.; Ramos, M.H.; Porter, J.H.; Kallenbach, R.L. Comparison of acetyl bromide lignin with acid detergent lignin and Klason lignin and correlation with in vitro forage degradability. Anim. Feed Sci. Technol. 2015, 201, 25–37. [Google Scholar] [CrossRef]
- Fukushima, R.S.; Kerley, M.S. Use of lignin extracted from different plant sources as standards in the spectrophotometric acetyl bromide lignin method. J. Agric. Food Chem. 2011, 59, 3505–3509. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: 2019. Available online: https://www.r-project.org/ (accessed on 1 September 2020).
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Sokol, N.W.; Sanderman, J.; Bradford, M.A. Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry. Glob. Chang. Biol. 2019, 25, 12–24. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schwesig, D.; Schmerwitz, J.; Kaiser, K.; Haumaier, L.; Glaser, B.; Ellerbrock, R.; Leinweber, P. Changes in properties of soil-derived dissolved organic matter induced by biodegradation. Soil Biol. Biochem. 2003, 35, 1129–1142. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Innangi, M.; Danise, T.; Alessandro, F.; Curcio, E.; Fioretto, A. Dynamics of Organic Matter in Leaf Litter and Topsoil within an Italian Alder (Alnus cordata (Loisel.) Desf.) Ecosystem. Forests 2017, 8, 240. [Google Scholar] [CrossRef]
- Traversa, A.; Said-Pullicino, D.; D’Orazio, V.; Gigliotti, G.; Senesi, N. Properties of humic acids in Mediterranean forest soils (Southern Italy): Influence of different plant covering. Eur. J. For. Res. 2011, 130, 1045–1054. [Google Scholar] [CrossRef]
- Deveau, A.; Bonito, G.; Uehling, J.; Paoletti, M.; Becker, M.; Bindschedler, S.; Hacquard, S.; Hervé, V.; Labbé, J.; Lastovetsky, O.A.; et al. Bacterial-fungal interactions: Ecology, mechanisms and challenges. FEMS Microbiol. Rev. 2018, 42, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Santonja, M.; Rancon, A.; Fromin, N.; Baldy, V.; Hättenschwiler, S.; Fernandez, C.; Montès, N.; Mirleau, P. Plant litter diversity increases microbial abundance, fungal diversity, and carbon and nitrogen cycling in a Mediterranean shrubland. Soil Biol. Biochem. 2017, 11, 124–134. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Faucon, M.P.; Houben, D.; Lambers, H. Plant Functional Traits: Soil and Ecosystem Services. Trends Plant Sci. 2017, 22, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, R.; Lindo, Z. Short-term leaching dynamics of three peatland plant species reveals how shifts in plant communities may affect decomposition processes. Geoderma 2017, 285, 110–116. [Google Scholar] [CrossRef]
- Santonja, M.; Foucault, Q.; Rancon, A.; Gauquelin, T.; Fernandez, C.; Baldy, V.; Mirleau, P. Contrasting responses of bacterial and fungal communities to plant litter diversity in a Mediterranean oak forest. Soil Biol. Biochem. 2018, 125, 27–36. [Google Scholar] [CrossRef]
- Sroka, K.; Chodak, M.; Klimek, B.; Pietrzykowski, M. Effect of black alder (Alnus glutinosa) admixture to Scots pine (Pinus sylvestris) plantations on chemical and microbial properties of sandy mine soils. Appl. Soil Ecol. 2018, 124, 62–68. [Google Scholar] [CrossRef]
- Chodak, M.; Niklińska, M. The effect of different tree species on the chemical and microbial properties of reclaimed mine soils. Biol. Fertil. Soils 2010, 46, 555–566. [Google Scholar] [CrossRef]
- Neufeldt, H.; Resck, D.V.S.; Ayarza, M.A. Texture and land-use effects on soil organic matter in Cerrado Oxisols, Central Brazil. Geoderma 2002, 107, 151–164. [Google Scholar] [CrossRef]
- Hernes, P.J.; Kaiser, K.; Dyda, R.Y.; Cerli, C. Molecular trickery in soil organic matter: Hidden lignin. Environ. Sci. Technol. 2013, 47, 9077–9085. [Google Scholar] [CrossRef]
- Iiyama, K.; Wallis, A.F.A. Effect of acetyl bromide on the ultraviolet spectra of lignin model compounds. Holzforschung 1989, 43, 309–316. [Google Scholar] [CrossRef]
- Heim, A.; Schmidt, M.W.I. Lignin turnover in arable soil and grassland analysed with two different labelling approaches. Eur. J. Soil Sci. 2007, 58, 599–608. [Google Scholar] [CrossRef]
- Thevenot, M.; Dignac, M.F.; Rumpel, C. Fate of lignins in soils: A review. Soil Biol. Biochem. 2010, 42, 1200–1211. [Google Scholar] [CrossRef]
- Gunina, A.; Kuzyakov, Y. Sugars in soil and sweets for microorganisms: Review of origin, content, composition and fate. Soil Biol. Biochem. 2015, 90, 87–100. [Google Scholar] [CrossRef]
- Kiem, R.; Kögel-Knabner, I. Contribution of lignin and polysaccharides to the refractory carbon pool in C-depleted arable soils. Soil Biol. Biochem. 2003, 35, 101–118. [Google Scholar] [CrossRef]
- Derrien, D.; Marol, C.; Balabane, M.; Balesdent, J. The turnover of carbohydrate carbon in a cultivated soil estimated by 13C natural abundances. Eur. J. Soil Sci. 2006, 57, 547–557. [Google Scholar] [CrossRef]
- Gleixner, G.; Poirier, N.; Bol, R.; Balesdent, J. Molecular dynamics of organic matter in a cultivated soil. Org. Geochem. 2002, 33, 357–366. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Rumpel, C. Advances in Molecular Approaches for Understanding Soil Organic Matter Composition, Origin, and Turnover: A Historical Overview, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 149. [Google Scholar]
- Guggenberger, G.; Christensen, B.T.; Zech, W. Land-use effects on the composition of organic matter in particle-size separates of soil: I. Lignin and carbohydrate signature. Eur. J. Soil Sci. 1994, 45, 449–458. [Google Scholar] [CrossRef]
- Tan, Z.X.; Lal, R.; Smeck, N.E.; Calhoun, F.G. Relationships between surface soil organic carbon pool and site variables. Geoderma 2004, 121, 187–195. [Google Scholar] [CrossRef]
- Schöning, I.; Totsche, K.U.; Kögel-Knabner, I. Small scale spatial variability of organic carbon stocks in litter and solum of a forested Luvisol. Geoderma 2006, 136, 631–642. [Google Scholar] [CrossRef]
- Ide, J.; Ohashi, M.; Takahashi, K.; Sugiyama, Y.; Piirainen, S.; Kortelainen, P.; Fujitake, N.; Yamase, K.; Ohte, N.; Moritani, M.; et al. Spatial variations in the molecular diversity of dissolved organic matter in water moving through a boreal forest in eastern Finland. Sci. Rep. 2017, 7, 42102. [Google Scholar] [CrossRef] [PubMed]
- Traversa, A.; D’Orazio, V.; Senesi, N. Properties of dissolved organic matter in forest soils: Influence of different plant covering. For. Ecol. Manag. 2008, 256, 2018–2028. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).