White-Rot Fungi Control on Populus spp. Wood by Pressure Treatments with Silver Nanoparticles, Chitosan Oligomers and Propolis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Biological Material
2.2. Synthesis of the Antifungal Solutions
2.3. Vacuum-Pressure Treatments and Antifungal Tests
2.4. Wood Degradation Monitoring
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karimi, A.; Taghiyari, H.R.; Fattahi, A.; Karimi, S.; Ebrahimi, G.; Tarmian, A. Effects of wollastonite nanofibers on biological durability of poplar wood (Populus nigra) against Trametes versicolor. BioResources 2013, 8, 4134. [Google Scholar] [CrossRef]
- Casado, M.; Acuña, L.; Basterra, L.-A.; Ramón-Cueto, G.; Vecilla, D. Grading of structural timber of Populus × euramericana clone I-214. Holzforschung 2012, 66, 633. [Google Scholar] [CrossRef]
- Todaro, L.; Russo, D.; Cetera, P.; Milella, L. Effects of thermo-vacuum treatment on secondary metabolite content and antioxidant activity of poplar (Populus nigra L.) wood extracts. Ind. Crop. Prod. 2017, 109, 384–390. [Google Scholar] [CrossRef]
- Kollert, W.; Borodowski, E.D. Situación de las Salicáceas en el Mundo. In Proceedings of the Jornadas de Saliáceas 2014—IV Congreso Internacional de Salicáceas en Argentina, Buenos Aires, Argentina, 18–21 March 2014; p. 10. [Google Scholar]
- Diaz, B.; Murace, M.; Peri, P.; Keil, G.; Luna, L.; Otaño, M.Y. Natural and preservative-treated durability of Populus nigra cv Italica timber grown in Santa Cruz Province, Argentina. Int. Biodeterior. Biodegrad. 2003, 52, 43–47. [Google Scholar] [CrossRef]
- Xing, J.-Q.; Ikuo, M.; Wakako, O. Natural resistance of two plantation woods Populus × canadensis cv. and Cunninghamia lanceolata to decay fungi and termites. For. Stud. China 2005, 7, 36–39. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pitman, A.J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Singh, T.; Singh, A.P. A review on natural products as wood protectant. Wood Sci. Technol. 2011, 46, 851–870. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent advances in graft copolymerization and applications of chitosan: A review. ACS Sustainable Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Quiroga, E.N.; Sampietro, D.A.; Soberon, J.R.; Sgariglia, M.A.; Vattuone, M.A. Propolis from the northwest of Argentina as a source of antifungal principles. J. Appl. Microbiol. 2006, 101, 103–110. [Google Scholar] [CrossRef]
- Torlak, E.; Sert, D. Antibacterial effectiveness of chitosan–propolis coated polypropylene films against foodborne pathogens. Int. J. Biol. Macromol. 2013, 60, 52–55. [Google Scholar] [CrossRef]
- Kartal, S.N.; Green, F.; Clausen, C.A. Do the unique properties of nanometals affect leachability or efficacy against fungi and termites? Int. Biodeterior. Biodegrad. 2009, 63, 490–495. [Google Scholar] [CrossRef]
- Clausen, C.A.; Yang, V.W.; Arango, R.A.; Green, F., III. Feasibility of nanozinc oxide as a wood preservative. Proc. Am. Wood Prot. Assoc. 2009, 105, 255–260. [Google Scholar]
- Matsunaga, H.; Kiguchi, M.; Evans, P.D. Microdistribution of copper-carbonate and iron oxide nanoparticles in treated wood. J. Nanopart. Res. 2008, 11, 1087–1098. [Google Scholar] [CrossRef]
- Marzbani, P.; Mohammadnia-afrouzi, Y. Investigation on leaching and decay resistance of wood treated with nano-titanium dioxide. Adv. Environ. Biol. 2014, 8, 974–979. [Google Scholar]
- Nair, S.; Pandey, K.K.; Giridhar, B.N.; Vijayalakshmi, G. Decay resistance of rubberwood (Hevea brasiliensis) impregnated with ZnO and CuO nanoparticles dispersed in propylene glycol. Int. Biodeterior. Biodegrad. 2017, 122, 100–106. [Google Scholar] [CrossRef]
- Dorau, B.; Arango, R.; Green, F. An Investigation into the Potential of Ionic Silver as a Wood Preservative, Proceedings from the Woodframe Housing Durability and Disaster Issues Conference, Las Vegas, NV, USA, 4–6 October 2004; Forest Products Society: Las Vegas, NV, USA, 2004; pp. 133–145.
- Velmurugan, N.; Kumar, G.G.; Han, S.S.; Nahm, K.S.; Lee, Y.S. Synthesis and characterization of potential fungicidal silver nano-sized particles and chitosan membrane containing silver particles. Iran. Polym. J. 2009, 18, 383–392. [Google Scholar]
- Kaur, P.; Thakur, R.; Choudhary, A. An in vitro study of the antifungal activity of silver/chitosan nanoformulations against important seed borne pathogens. Int. J. Sci. Technol. Res. 2012, 1, 83–86. [Google Scholar]
- Narayanan, K.B.; Park, H.H. Antifungal activity of silver nanoparticles synthesized using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens. Eur. J. Plant Pathol. 2014, 140, 185–192. [Google Scholar] [CrossRef]
- Silva-Castro, I.; Martín-García, J.; Diez, J.J.; Flores-Pacheco, J.A.; Martín-Gil, J.; Martín-Ramos, P. Potential control of forest diseases by solutions of chitosan oligomers, propolis and nanosilver. Eur. J. Plant Pathol. 2017, 150, 401–411. [Google Scholar] [CrossRef]
- Kim, S.W.; Jung, J.H.; Lamsal, K.; Kim, Y.S.; Min, J.S.; Lee, Y.S. Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi. Mycobiology 2018, 40, 53–58. [Google Scholar] [CrossRef]
- Ivask, A.; ElBadawy, A.; Kaweeteerawat, C.; Boren, D.; Fischer, H.; Ji, Z.; Chang, C.H.; Liu, R.; Tolaymat, T.; Telesca, D.; et al. Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver. ACS Nano 2013, 8, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Bin Ahmad, M.; Lim, J.J.; Shameli, K.; Ibrahim, N.A.; Tay, M.Y. Synthesis of silver nanoparticles in chitosan, gelatin and chitosan/gelatin bionanocomposites by a chemical reducing agent and their characterization. Molecules 2011, 16, 7237–7248. [Google Scholar] [CrossRef] [PubMed]
- Goycoolea, F. Monografía XXVIII: Nanotecnología Farmacéutica. Available online: https://www.analesranf.com/index.php/mono/article/view/990/1024 (accessed on 7 October 2019).
- Alfredsen, G.; Eikenes, M.; Militz, H.; Solheim, H. Screening of chitosan against wood-deteriorating fungi. Scand. J. For. Res. 2011, 19, 4–13. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Rabea, E.I. Potential of the biopolymer chitosan with different molecular weights to control postharvest gray mold of tomato fruit. Postharvest Biol. Technol. 2009, 51, 110–117. [Google Scholar] [CrossRef]
- Silva-Castro, I.; Casados-Sanz, M.; Alonso-Cortés, A.; Martín-Ramos, P.; Martín-Gil, J.; Acuña-Rello, L. Chitosan-based coatings to prevent the decay of Populus spp. wood caused by Trametes versicolor. Coatings 2018, 8, 415. [Google Scholar] [CrossRef]
- Silva-Castro, I.; Diez, J.; Martín-Ramos, P.; Pinto, G.; Alves, A.; Martín-Gil, J.; Martín-García, J. Application of bioactive coatings based on chitosan and propolis for Pinus spp. protection against Fusarium circinatum. Forests 2018, 9, 685. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Marcucci, M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed. Pharmacother. 2018, 98, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Matei, P.M.; Martin-Ramos, P.; Sanchez-Bascones, M.; Hernandez-Navarro, S.; Correa-Guimaraes, A.; Navas-Gracia, L.M.; Rufino, C.A.; Ramos-Sanchez, M.C.; Martin-Gil, J. Synthesis of chitosan oligomers/propolis/silver nanoparticles composite systems and study of their activity against Diplodia seriata. Int. J. Polym. Sci. 2015, 2015, 864729. [Google Scholar] [CrossRef]
- Costa, C.N.; Teixeira, V.G.; Delpech, M.C.; Souza, J.V.S.; Costa, M.A.S. Viscometric study of chitosan solutions in acetic acid/sodium acetate and acetic acid/sodium chloride. Carbohydr. Polym. 2015, 133, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Rufino, C.; Fernandes-Vieira, J.; Martín-Ramos, P.; Silva-Castro, I.; Fernandes-Correa, M.; Matei, P.M.; Sánchez-Báscones, M.; Ramos-Sánchez, M.C.; Martín-Gil, J. Synthesis of chitosan oligomers composite systems and study of their activity against Bipolaris Oryzae. J. Mater. Sci. Eng. Adv. Technol. 2016, 13, 29–52. [Google Scholar]
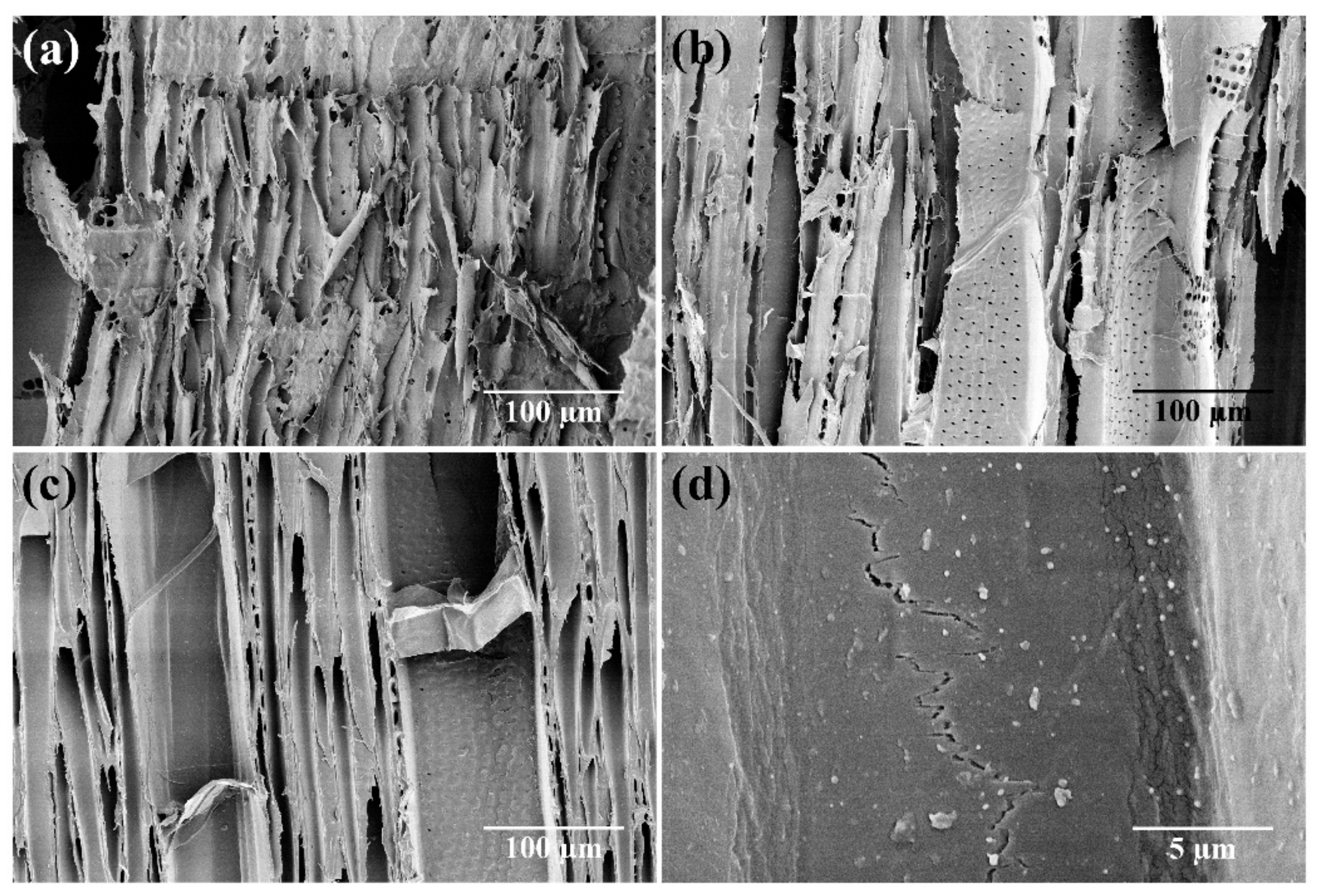
- Schwarze, F.W.M.R. Wood decay under the microscope. Fungal Biol. Rev. 2007, 21, 133–170. [Google Scholar] [CrossRef]
- Akhtari, M.; Arefkhani, M. Study of microscopy properties of wood impregnated with nanoparticles during exposed to white-rot fungus. Agric. Sci. Dev. 2013, 2, 116–119. [Google Scholar]
- Larnøy, E.; Eikenes, M.; Militz, H. Evaluation of factors that have an influence on the fixation of chitosan in wood. Wood Mater. Sci. Eng. 2006, 1, 138–145. [Google Scholar] [CrossRef]
- El-Gamal, R.; Nikolaivits, E.; Zervakis, G.I.; Abdel-Maksoud, G.; Topakas, E.; Christakopoulos, P. The use of chitosan in protecting wooden artifacts from damage by mold fungi. Electron. J. Biotechnol. 2016, 24, 70–78. [Google Scholar] [CrossRef]

| Silver Nanoparticles (ppm) | Chitosan Oligomers (mg/mL) | Propolis (mg/mL) |
|---|---|---|
| 20 | 80 | 40 |
| 15 | 40 | 20 |
| 10 | 20 | 10 |
| 5 | 10 | 5 |
| Exposure Period (weeks) | AgNPs Concentration (ppm) | Weight Loss (%) * | Shapiro–Wilk Test p-Value | Bartlett p-Value | ANOVA p-Value | Homogeneous Groups † | |||
|---|---|---|---|---|---|---|---|---|---|
| 4 | Control | 13.75 ± 1.21 | 0.0532 | 0.6538 | 2.71 × 10−6 | X | |||
| 5 | 8.80 ± 0.09 | 0.8964 | c | ||||||
| 10 | 8.67 ± 0.09 | 0.8290 | b | c | |||||
| 15 | 8.52 ± 0.19 | 0.7587 | b | ||||||
| 20 | 8.33 ± 0.19 | 0.9515 | a | ||||||
| 8 | Control | 17.55 ± 2.26 | 0.7000 | 0.9059 | 0.00135 | X | |||
| 5 | 8.93 ± 0.11 | 0.6224 | c | ||||||
| 10 | 8.73 ± 0.18 | 0.9775 | b | c | |||||
| 15 | 8.59 ± 0.19 | 0.7082 | a | b | |||||
| 20 | 8.43 ± 0.18 | 0.8408 | a | ||||||
| 12 | Control | 23.86 ± 1.95 | 0.5846 | 0.7605 | 0.0542 | X | |||
| 5 | 8.85 ± 0.21 | 0.6889 | a | ||||||
| 10 | 8.74 ± 0.13 | 0.6406 | a | ||||||
| 15 | 8.59 ± 0.19 | 0.9706 | a | ||||||
| 20 | 8.43 ± 0.19 | 0.9854 | a | ||||||
| 16 | Control | 24.79 ± 5.21 | 0.4149 | 0.511 | 0.0291 | X | |||
| 5 | 8.94 ± 0.16 | 0.7192 | b | ||||||
| 10 | 8.81 ± 0.14 | 0.5510 | b | ||||||
| 15 | 8.80 ± 0.13 | 0.7146 | b | ||||||
| 20 | 8.49 ± 0.22 | 0.8678 | a | b | |||||
| Exposure Period (weeks) | Chitosan Oligomers Concentration (mg/mL) | Weight Loss (%) * | Shapiro–Wilk Test p-Value | Bartlett p-Value | ANOVA p-Value | Homogeneous Groups † | |||
|---|---|---|---|---|---|---|---|---|---|
| 4 | Control | 14.54 ± 0.82 | 0.0621 | 0.1393 | 2.71 × 10−7 | X | |||
| 10 | 11.58 ± 1.70 | 0.6750 | c | ||||||
| 20 | 9.34 ± 0.78 | 0.7082 | b | c | |||||
| 40 | 7.91 ± 0.87 | 0.3403 | b | ||||||
| 80 | 4.96 ± 0.63 | 0.3632 | a | ||||||
| 8 | Control | 19.43 ± 3.34 | 0.5128 | 0.9059 | 0.00135 | X | |||
| 10 | 12.33 ± 0.75 | 0.6924 | b | ||||||
| 20 | 9.34 ± 0.78 | 0.6589 | b | ||||||
| 40 | 9.93 ± 0.81 | 0.6982 | a | b | |||||
| 80 | 8.92 ± 1.03 | 0.5524 | a | ||||||
| 12 | Control | 25.86 ± 2.50 | 0.6602 | 0.8604 | 0.0542 | X | |||
| 10 | 22.25 ± 1.72 | 0.9546 | a | ||||||
| 20 | 21.36 ± 2.16 | 0.7794 | a | ||||||
| 40 | 20.12 ± 1.84 | 0.6710 | a | ||||||
| 80 | 17.44 ± 2.47 | 0.8068 | a | ||||||
| 16 | Control | 32.14 ± 3.05 | 0.7002 | 0.8871 | 0.0291 | X | |||
| 10 | 29.95 ± 4.25 | 0.6900 | b | ||||||
| 20 | 21.36 ± 2.16 | 0.5467 | a | b | |||||
| 40 | 23.28 ± 4.27 | 0.8115 | a | b | |||||
| 80 | 19.35 ± 3.34 | 0.8773 | a | ||||||
| Exposure Period (weeks) | Propolis Concentration (mg/mL) | Weight Loss (%) * | Shapiro–Wilk Test p-Value | Bartlett p-Value | ANOVA p-Value | Homogeneous Groups † | |||
|---|---|---|---|---|---|---|---|---|---|
| 4 | Control | 14.212 ± 2.065 | 0.3225 | 0.863 | 7.91 × 10−8 | X | |||
| 5 | 13.742 ± 0.650 | 0.8964 | c | ||||||
| 10 | 12.116 ± 0.565 | 0.4721 | b | ||||||
| 20 | 11.113 ± 0.627 | 0.9877 | b | ||||||
| 40 | 9.775 ± 0.458 | 0.9794 | a | ||||||
| 8 | Control | 19.897 ± 1.801 | 0.505 | 0.5844 | 5.68 × 10−7 | X | |||
| 5 | 17.870 ± 1.383 | 0.829 | c | ||||||
| 10 | 15.416 ± 0.955 | 0.9422 | b | ||||||
| 20 | 12.587 ± 1.561 | 0.8152 | a | ||||||
| 40 | 11.115 ± 0.877 | 0.6586 | a | ||||||
| 12 | Control | 21.036 ± 3.141 | 0.770 | 0.5511 | 0.713 | X | |||
| 5 | 19.892 ± 1.620 | 0.7587 | b | ||||||
| 10 | 18.639 ± 1.321 | 0.8894 | b | ||||||
| 20 | 17.413 ± 1.053 | 0.8444 | a | ||||||
| 40 | 16.288 ± 0.810 | 0.7739 | a | ||||||
| 16 | Control | 28.795 ± 2.841 | 0.2954 | 0.9257 | 0.00014 | X | |||
| 5 | 24.934 ± 2.133 | 0.9515 | c | ||||||
| 10 | 22.456 ± 1.614 | 0.8911 | b | c | |||||
| 20 | 19.439 ± 1.664 | 0.7038 | b | ||||||
| 40 | 17.105 ± 1.690 | 0.2817 | a | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casado-Sanz, M.M.; Silva-Castro, I.; Ponce-Herrero, L.; Martín-Ramos, P.; Martín-Gil, J.; Acuña-Rello, L. White-Rot Fungi Control on Populus spp. Wood by Pressure Treatments with Silver Nanoparticles, Chitosan Oligomers and Propolis. Forests 2019, 10, 885. https://doi.org/10.3390/f10100885
Casado-Sanz MM, Silva-Castro I, Ponce-Herrero L, Martín-Ramos P, Martín-Gil J, Acuña-Rello L. White-Rot Fungi Control on Populus spp. Wood by Pressure Treatments with Silver Nanoparticles, Chitosan Oligomers and Propolis. Forests. 2019; 10(10):885. https://doi.org/10.3390/f10100885
Chicago/Turabian StyleCasado-Sanz, María Milagrosa, Iosody Silva-Castro, Laura Ponce-Herrero, Pablo Martín-Ramos, Jesús Martín-Gil, and Luis Acuña-Rello. 2019. "White-Rot Fungi Control on Populus spp. Wood by Pressure Treatments with Silver Nanoparticles, Chitosan Oligomers and Propolis" Forests 10, no. 10: 885. https://doi.org/10.3390/f10100885
APA StyleCasado-Sanz, M. M., Silva-Castro, I., Ponce-Herrero, L., Martín-Ramos, P., Martín-Gil, J., & Acuña-Rello, L. (2019). White-Rot Fungi Control on Populus spp. Wood by Pressure Treatments with Silver Nanoparticles, Chitosan Oligomers and Propolis. Forests, 10(10), 885. https://doi.org/10.3390/f10100885