Abstract
Myrcianthes hallii (O. Berg) McVaugh (Myrtaceae) is a plant native to Ecuador, traditionally used for its antiseptic properties. The composition of the hydro-methanolic extract of this plant was determined by submitting it to ultra-high performance liquid chromatography (UHPLC) hyphenated to heated-electrospray ionization mass spectrometry and UV detection. The presence of antimicrobial components prompted us to test the extract against methicillin-resistant and methicillin-susceptible Staphylococcus aureus, multidrug-resistant and susceptible Escherichia coli, Pseudomonas aeruginosa, Enterococcus spp. and Streptococcus pyogenes strains. The chromatographic analysis led to the identification of 38 compounds, including polyphenols and organic acids, and represents the first chemical characterization of this plant. The extract showed modest antibacterial activity against all tested bacteria, with the exception of E. coli which was found to be less sensitive. Whilst methicillin-resistant strains usually display resistance to several drugs, no relevant differences were observed between methicillin-susceptible and resistant strains. Considering its long-standing use in folk medicine, which suggests the relative safety of the plant, and the presence of many known antibacterial polyphenolic compounds responsible for its antibacterial activity, the results show that M. hallii extract could be used as a potential new antiseptic agent. Moreover, new anti-infective biomaterials and nanomaterials could be designed through the incorporation of M. hallii polyphenols. This prospective biomedical application is also discussed.
1. Introduction
The Myrtaceae family includes about 30 genera and 1500 species found in the Neotropics. In Ecuador there are 15 native genera and about 200 species. Native species are mainly grown for their edible fruits and wood and some are used as medicinal plants due to their biological properties [1]. In recent years, increasing interest in herbal medicine has been registered in developed and developing countries, and much attention has been paid to natural antibacterial substances for use in alternative therapies against conventionally resistant infections or as new antiseptic agents. In the last decade, many species of Myrtaceae (including those from the Myrtus, Eucalyptus, Psidium, and Syzygium genera) have been studied for their antimicrobial properties [2,3,4,5]. Myrcianthes is a genus of Myrtaceae that includes shrubs and small trees. At present, 38 species of Myrcianthes are known to be distributed across several countries of Central and South America, from Mexico to Chile, including Ecuador [6]. Although Myrcianthes is closely related to the large genus Eugenia L. recent studies have confirmed its individuality and role as a sister group to the rest of the Eugenia clades [7]. Thanks to recent taxonomic research [8,9], new species belonging to the Myrcianthes genus have been described. Myrcianthes hallii (O. Berg) McVaugh is a medicinal and aromatic species commonly known in Ecuador as “arrayán” [10,11]. It grows as a shrub or a tree up to 8 m high. Leaves and branchlets are nearly glabrous, flowers are tetramerous and the hypanthium is characteristically densely pale and strigose. This species is recorded in Peru, Ecuador, Venezuela and possibly in Colombia [12,13]. In Ecuador M. hallii is found both native and cultivated, growing in the Andean region from 2500 to 3000 metres above mean sea level (MAMSL), and mainly in the provinces of Azuay, Bolivar, Carchi, Chimborazo, Imbabura, Loja and Pichincha [11,13]. Its leaves and berries are used in both traditional medicine and in cosmetics and foods, as a culinary herb or spice. In traditional medicine, arrayán is consumed as an infusion and a decoction for its antiseptic, haemostatic, and balsamic properties. Moreover, dried ground leaves applied to wounds aid healing and are used in baths, vapors and massages for their stimulating and tonic properties. Green leaves are used to clean teeth and gums, with the added effect of whitening teeth naturally. Finally, fresh leaves macerated in olive oil seem to prevent hair loss [10,11,14]. The chemical compositions of plants belonging to the Myrtaceae family (such as Eucalyptus and Myrtus) have been widely studied. Eucalyptus species are rich sources of biologically active terpenoids, tannins, flavonoids and phloroglucinol derivatives [3,15,16]. In Myrtus communis L. (myrtle) the presence of different polyphenolic classes is reported, including phenolic acids, flavonoids, galloyl derivatives and hydrolysable tannins [2,17,18]. Some of the main polyphenols found in myrtle are also present in green tea (catechin and gallocatechin derivatives) which is widely recognized as an excellent source of powerful nutraceuticals [17,18]. A recent study on leaf extract obtained from Myrcianthes cisplatensis (Cambess.) O. Berg, reported the presence of α-methyl-1-(2′,4′,6′-trimethoxyphenyl)-1-propanone, known as conglomerone, which shows antibacterial activity against methicillin-sensitive and resistant Staphylococcus aureus strains [19].
Although studies reporting the composition of essential oils or the biological activity of extracts do exist for several species of Myrcianthes (M. pungens (O. Berg) D. Legrand, M. cisplatensis (Cambess.) O. Berg, M. pseudomato (D. Legrand) McVaugh, M. fragrans (Sw.) McVaugh, M. rhopaloides (Kunth) McVaugh, M. osteomeloides (Rusby) McVaugh, and M. coquimbensis (Barnéoud) Landrum et Grifo) [19,20,21,22,23,24,25,26], the available information concerning M. hallii is particularly limited. Therefore, in view of the fact that M. hallii is commonly employed as an antibacterial agent in Ecuadorian folk medicine, and therefore is likely safe and effective, and the importance of finding new anti-infective extracts to be loaded into or coated onto biomaterials, the aims of this study were to investigate the chemical composition of M. hallii and its antibacterial activity against Gram positive and Gram negative multidrug-resistant strains.
2. Results
2.1. UHPLC-PDA-hESI-MSn Analysis of LMW Fraction of MHE
The acidic hydro-methanolic leaf extract of M. hallii (MHE) was submitted to dialysis, using a membrane with a nominal molecular weight cut-off (MWCO) of 3500 Da. A low molecular weight (LMW) fraction, suitable for UHPLC-PDA-hESI-MSn analysis, was obtained. The chromatographic profile, acquired at 280 nm, is reported in Figure 1. Thirty-eight compounds were identified based on their chromatographic behaviour and mass spectra, in comparison with the literature. Table 1 summarizes the identified compounds, their retention times and m/z values for the parent ion and fragment ions.
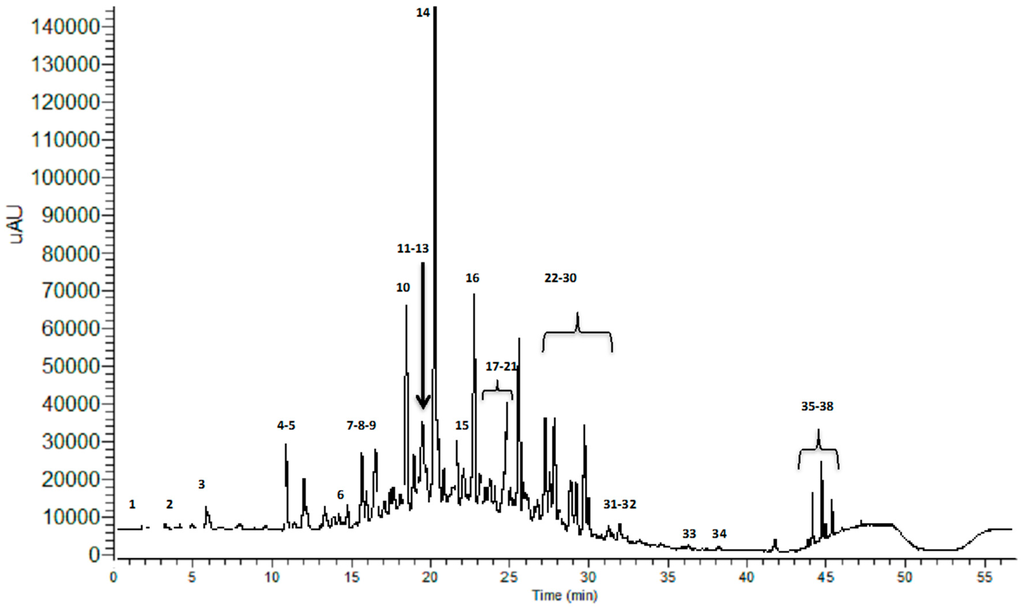
Figure 1.
UHPLC-UV chromatographic profile of the LMW fraction of MHE, registered at 280 nm.

Table 1.
MS and MSn data of the compounds identified in LMW fraction of MHE.
The analysis shows the presence of 29 flavonoids, consisting of (a) 5 flavan-3-ols (gallocatechin, catechin, epigallocatechin, epicatechin and epigallocatechin gallate); (b) 7 condensed tannin derivatives (three isomers of procyanidin dimer, procyanidin-gallate, and three isomers of procyanidin-digallate); (c) 12 flavonols (myricetin 3-O-galactoside/myricetin 3-O-glucoside, myricetin 3-O-arabinoside, myricetin 3-O-rhamnoside, quercetin, quercetin hexosyl-gallate, quercetin 3-O-rhamnoside, quercetin 3-O-galactoside/quercetin 3-O-glucoside, quercetin 3-O-arabinose, acylated myricetrin, kaempferol 3-O-glucoside, aromadendrin-rhamnoside, and cypellogin A or B); (d) a flavanone derivative (pinobanksin 3-O-butyrate); (e) a flavone derivative (apigenin-hexoside) and (f) 3 anthocyanin derivatives (cyanidin-dihexoside, cyanidin-3-O-rutinoside, and cyanidin-3-glucoside/cyaniding-3-galactoside). Moreover, in MHE a phenolic acid (gallic acid), 5 hydrolysable tannins (hexahydroxydiphenoyl-glucose, hexahydroxydiphenoyl-galloylglucose, digalloylglucose, trigalloylglucose, and monogalloyl-quinic acid), and 3 organic acids (quinic acid, malic acid and gluconic acid) were detected.
In further detail, five flavan-3-ols were identified in MHE. Peaks 9 and 13 were identified as gallocatechin and epigallocatechin, respectively, by comparing their MS2 spectra with literature data (MW 306). Their parent ions (m/z 305) produced characteristic fragment ions at m/z 261, 221, 219, 179, 167, and 165, generated by the cleavage of the A ring, the heterocyclic ring fission, and the retro-Diels-Alder fission occurring during the fragmentation process as reported by Dou et al. [27]. It was possible to identify the epimers by comparison with commercial standards. Peaks 15 and 18, which both showed a parental ion at m/z 289, were assigned to catechin and epicatechin respectively (MW 290). Their MS2 spectra showed the presence of ions at m/z 245, 205, 203, and 137, generated by the cleavage of ring A and the retro-Diels-Alder fission occurring during the fragmentation process [27]. It was again possible to identify the epimers by comparison with commercial standards. Peak 19, which produced a molecular ion [M–H]− at m/z 457, was identified as epigallocatechin-3-gallate (MW 458) since it produced fragment ions at m/z 305 and 169 corresponding to the deprotonated ion of epigallocatechin and gallic acid [27]. It was possible to identify this compound by comparison with the commercial standard.
Twelve flavonols were identified in MHE: four myricetin derivatives, five quercetin derivatives, a quercetin aglycone, and two kaempferol derivatives. Four myricetin derivatives were identified in MHE due to their MS and MS/MS spectra. Peaks 21, 23, 24, 32 were assigned to myricetin derivatives due to the presence of the aglycone at m/z 317, in their MS2 spectra. Peak 21 was identified as myricetin glucoside or galactoside (MW 480) due to the parental ion at m/z 479 producing the MS2 fragment [M–162]−; peak 24 was assigned to myricetin rhamnoside (MW 464) due to the loss of 146 Da from the molecular ion at m/z 463, according to Faria et al. [28]. Peak 23 was identified as myricetin arabinoside (MW 450), since it produced the MS2 ion at m/z 317 from the molecular ion at m/z 449, due to the loss of [M–132]− corresponding to the arabinoil moiety. Peak 32 was assigned to acylated myricitrin (MW 506). Its molecular ion at m/z 505 produced a base peak at m/z 316, corresponding to the myricetin aglycone, and a fragment at m/z 463, due to the loss of the acylic moiety. In MHE five quercetin derivatives were identified, because all peaks presented the characteristic fragment ions of quercetin aglycone (e.g., MSn data at m/z 300, 301, 179). Peaks 25, 27, 30 were assigned to quercetin glucoside or galactoside (MW 464), quercetin arabinoside (MW 434), and quercetin rhamnoside (MW 448), since they yielded the fragments [M–162]−, [M–132]−, [M–146]−, respectively. Peak 22 was identified as quercetin hexosylgallate (MW 616), since the molecular ion [M–H]− at m/z 615 produced a fragment at m/z 463, corresponding to the loss of a galloyl group, and a fragment at m/z 301 due to the loss of a hexosyl group, corresponding to the aglycone [15]. Peak 34 may correspond to Cypellogin A or B (MW 630), which is a quercetin glucoside or galactoside acylated with an oleuropeic acid residue [29]; in fact, the compound yielded a base peak at m/z 301 corresponding to the aglycone. Quercetin (MW 302) was identified as peak 35 due to the presence of the molecular ion [M–H]− at m/z 301. With regards to kaempferol derivatives, peak 28 was assigned to kaempferol 3-O-glucoside (MW 448) by observing the MS2 data closely related to the glycosylation position. According to Ablajan et al. [30], peak 28 showed a MS2 spectrum typical of a 3-O-glucosyl derivative. It produced a base peak at m/z 284, related to the homolytic cleavage of deprotonated flavonoid glycosides, a fragment at m/z 255 more abundant than that registered at m/z 257 and a fragment at m/z 327 that is not present in 7-O-glucosyl derivatives. Peak 31 was identified as aromadendrin rhamnoside (MW 434), since the molecular ion [M–H]− at m/z 433 produced the fragment [M–146]− corresponding to the aglycon at m/z 287.
Regarding flavanone and flavone derivatives, peak 38 was assigned to pinobanksin 3-O-butyrate, the parent ion (m/z 343) producing fragment ions as reported by Chua et al. [31]. Peak 37 was identified as apigenin-hexoside, since it produced a molecular ion [M–H]− at m/z 431, and yielded a fragment ion at m/z 269 corresponding to apigenin aglycone due to the loss of a hexosyl group. Three anthocyanidin derivatives were identified in MHE. Peaks 8, 10, 29 showed the presence of the cyanidin aglycone at m/z 287 in their MS2 spectra. Peak 8 was assigned to cyanidin-dihexoside (MW 610), since the parent ion (m/z 611) produced fragment ions at m/z 449 and m/z 287, corresponding to the loss of the sugar molecules linked to the aglycone. Peak 10 was identified as cyanidin-3-rutinoside thanks to the pseudomolecular ion [M + H]+ at m/z 595 and its MS2 fragments at m/z 433, caused by the loss of one of the sugar molecules linked to the aglycone, and m/z 287, corresponding to the cyaniding aglycone (MW 594). Peak 29, which produced a molecular ion [M + H]+ at m/z 449 and yielded a fragment ion at m/z 287 corresponding to the loss of the hexosyl moiety on MS2, may be assigned to the glucoside or galactoside derivative of cyanidin (MW 448).
With regards to benzoic acids, gallic acid (MW 170) was identified as peak 4 in MHE, since it produced a base peak at m/z 125 corresponding to the loss of a carboxyl group [M–H–CO2]−.
As far as tannins are concerned, condensed tannins and hydrolizable tannins (gallotannins and ellagitanninis) were detected. Seven condensed tannin compounds were detected in MHE. Three peaks (12, 14, 16) showed molecular ions at m/z 577 with the same MS2 fragmentation pattern (m/z 425, 407, 289), but different retention times: consequently they can be considered to be three isomers of procyanidin (MW 578) [32]. Peak 20 produced a molecular ion [M–H]− at m/z 729 and the most significant MS2 fragments at m/z 577, corresponding to the loss of a galloyl group, and at m/z 289, corresponding to the monomer: for these reasons peak 20 was assigned to a monogalloyl procyanidin dimer. Peaks 7, 11, 17 produced a molecular ion [M–H]− at m/z 881, which could correspond to procyanidin digallate, and the same MS2 fragmentation spectra. The main fragments produced were at m/z 729 (corresponding to the loss of a galloyl group), at m/z 711 (corresponding to the loss of a molecule of gallic acid), at m/z 577 (corresponding to procyanidin), and at m/z 289 (corresponding to the monomer (−)-epicatechin or (−)-catechin). Consequently, peaks 7, 11, and 17 were assigned to three different isomers of procyanidin digallate. With regards to hydrolizable tannins, peaks 3 and 5 were assigned to hesahydroxydiphenoyl-glucose and hesahydroxydiphenoyl-galloylglucose, respectively. Peak 3 was identified as hesahydroxydiphenoyl-glucose (MW 480), since it produced a molecular ion at m/z 481 and a MS2 fragment ion at m/z 301, corresponding to the loss of a glucose unit as reported in the literature [33]. Peak 5 produced a molecular ion at m/z 633, an intense fragment ion at m/z 301 suggesting the loss of a galloylglucose unit, and a fragment ion at m/z 481, corresponding to the molecular ion of hesahydroxydiphenoyl-glucose, due to the loss of a galloyl unit. Through comparison with data from the literature [33], the fragmentation pattern of this molecule suggests that the galloyl moiety is directly linked to the glucose unit, so the compound was identified as hesahydroxydiphenoyl-galloylglucose (MW 634). Peak 33 was assigned to digalloylglucose (MW 484), since it provided the typical MS2 fragment ions at m/z 331 [M–H–152]− and m/z 169 [M–H–162]−, corresponding to the sequential loss of the galloyl and the glucosyl moiety, respectively [33]. Moreover, peak 36 was identified as trigalloylglucose (MW 636), since the molecular ion at m/z 635 produced a base peak at m/z 483, due to the loss of a galloyl group, leading to m/z typical of digalloyl glucose, and a fragment at m/z 465 due to the loss of gallic acid. Finally, peak 6 was assigned to monogalloyl quinic acid (MW 344). It showed a molecular ion at m/z 343 and a MS2 spectrum characterized by the presence of fragment ions at m/z 169 and 125. The neutral loss of 174 Da corresponds to quinic acid.
Finally, some organic acids (quinic acid, malic acid and gluconic acid) were identified in MHE. Peak 1 was identified as quinic acid (MW 192), with a retention time of approximately 2 min and MS2 fragments at m/z 173 [M–H–H2O]−, corresponding to the loss of a water molecule, and at m/z 127 [M–H–CO–2H2O]−, corresponding to the loss of the carboxylic moiety and two water molecules. Peak 2, showing a retention time of 3.10 min and a MS2 fragment at m/z 115 [M–H–H2O]−, was identified as malic acid. Peak 26 yielded to MS2 fragments at m/z 179 [M–H–H2O]−, due to the loss of a water molecule, and at m/z 135 [M–H–CO–H2O]− representing the 60% of the base peak, due to the loss of the carboxylic group and a water molecule. Thus, peak 26 was identified as gluconic acid (MW 198) [34].
2.2. Antibacterial Activity of MHE
The antibacterial activity of MHE obtained from M. hallii dried leaves was tested against ten MRSA and MSSA, ten E. coli, ten P. aeruginosa, ten Enterococcus spp, and ten S. pyogenes strains. MHE showed antibacterial activity against all the tested S. aureus (MIC range 0.007–0.0019 gr/mL), P. aeruginosa (MIC range 0.125–0.062 gr/mL), S. pyogenes (MIC range 0.007–0.0039 gr/mL) and Enterococcus spp. (MIC range 0.0039–0.0019 gr/mL). MHE was found to be less active against E. coli strains (MIC range 0.25 > 0.5 gr/mL) (Table 2 and Table 3 and Figure 2). Our data showed that MHE extract can inhibit bacterial strains irrespectively of their mechanisms of resistance. No significant differences were observed between strains carrying well-known mechanisms of resistance and susceptible ones, because one-dilution differences in this kind of analysis are taken for granted.

Table 2.
In vitro antibacterial cumulative activity of MHE against ten S. aureus, ten E. coli, ten P. aeruginosa, ten Enterococcus spp., and ten S. pyogenes strains.

Table 3.
MIC (mg/mL) of MHE against ten S. aureus, ten S. pyogenes, five E. faecalis, five E. faecium, ten P. aeruginosa, and ten E. coli strains. Frequencies (%) of the different bacterial species within MIC classes are shown.
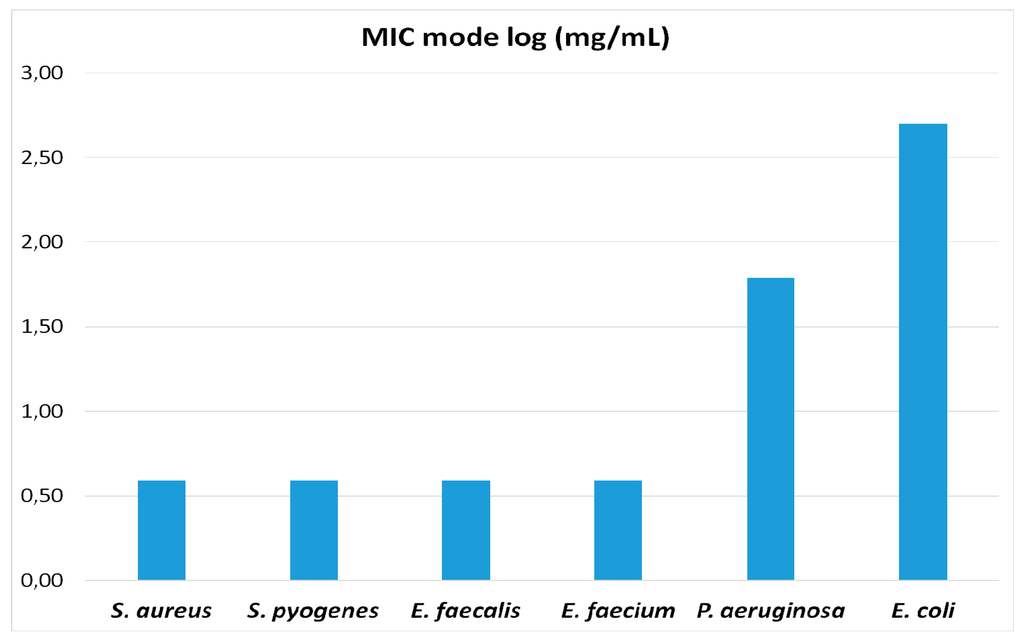
Figure 2.
MIC values within groups follow a modal distribution. MIC mode values (expressed as log10) for the different bacterial species assayed are represented in the figure. The four Gram-positive species (S. aureus, S. pyogenes, E. faecalis, E. faecium) exhibit equal values of MIC mode, while the MIC mode values against the two Gram-negative species (P. aeruginosa and E. coli) are much higher (E. coli > P. aeruginosa).
3. Discussion
This research represents the first report on the isolation and identification of polyphenols and organic acids from M. hallii to date. UHPLC-PDA-hESI-MSn analysis revealed the presence of thirty-eight compounds, belonging to flavonoids, phenolic acids, tannins and organic acids. Some of them have been previously identified in extracts obtained from plants and fruits belonging to the Myrtaceae family, whilst for others, this is the first report of their identification in a Myrtaceae species.
In MHE extract, 29 flavonoids were identified, consisting of flavan-3-ols, condensed tannin derivatives, flavonols, a flavanone and a flavone derivative and anthocyanin derivatives. Flavan-3-ols were also detected in the methanolic extract of the bark of Tristaniopsis callobuxus Brongn. & Gris, by Bellosta et al. [35], which showed the presence of gallocatechin and epigallocatechin. Catechin was also detected in the methanolic extracts obtained from the air-dried leaves of Myrtus communis L. (myrtle) and Eucalyptus globulus Labill [16,18]. Amongst Myrtaceae, flavonol derivatives were also detected in the methanolic extracts of Eucalyptus globulus. For myrtle, infusions and leaf methanolic extracts were both shown to contain myricetin and quercetin derivatives [2,18]. In the methanolic extract obtained from the fruits of Myrciaria vexator McVaugh, Dastmalchi et al. identified the following flavonols, quercitrin (quercetin 3-rhamnoside), quercetin 3-glucoside, and myricetin [36]. Moreover, in the fruits of Myrcianthes pungens (O. Berg) D. Legrande, De Mello Andrade et al. showed the presence of quercitrin [24]. Regarding anthocyanins, in the fruits of Myrciaria dubia (Kunth) McVaugh, a plant native of Amazonian rainforest, cyanidin-3-glucoside was identified as the main pigment [37]. The other two cyanidin-derivatives identified in MHE have never been reported in plants belonging to Myrtaceae family. Condensed tannins, such as procyanidin, were detected in many species belonging to Eucalyptus and Eugenia genera [38]. Ellagitannis have only been found in dicotyledoneous angiosperms and Myrtaceae are indicated as being rich in ellagitannins. In support of this fact, ellagitannis were detected in Callistemon lanceolatus Sweet, Eucalyptus alba Reinw. Ex Blume, Eugenia grandis Wight, Kunzea ambigua (Sm.) Druce, Melaleuca squarrosa Sm., Pimenta dioica (L.) Merr., Siphoneugena densiflora, Syzygium aqueum (Burm. f.) Alston, and Syzygium aromaticum (L.) Merr & L.M. Perry [39]. In MHE, gallic acid, hydrolysable tannins and organic acids have been detected. Gallic acid is a secondary metabolite of plants, mainly formed from 3-dehydroshikimic acid through the shikimic acid pathway [40]. It is widely distributed in many different families of higher plants, both in the free state and as a part of more complex molecules such as ester derivatives or polymers. Amongst Myrtaceae, gallic acid was detected in the methanolic extract obtained from the leaves of Eucalyptus globulus [16], the fruits of Rhodomyrtus tomentosa (Aiton) Hassk. [41], the methanolic extract of the bark of Tristaniopsis callobuxus [35], leaf infusions or methanolic extract obtained from Myrtus communis [2,18] and the seed and fruit extracts of Syzygium cumini (L.) Skeels [42]. Quinic acid has been identified in many plants in the Myrtaceae family, such as Syzygium cumini and Myrtus communis [17,43].
Amongst the thirty-eight compounds occurring in M. hallii leaf extract, many of them possess well-documented antibacterial activity [44]. For example, the in vitro antibacterial activity of catechin derivatives has been known since the 1990s and has been demonstrated against different strains, such as Streptococcus mutans, E. coli, Clostridium perfringes and Bacillus cereus [45,46,47]. Moreover, flavonols, especially myricetin, quercetin and kaempferol derivatives, are characterized by a remarkable antibacterial activity against both Gram-positive and Gram-negative bacteria, such as S. aureus, Lactobacillus acidophilus, Porphyromonas gingivalis and Prevotella melaninogenica [48]. Other categories of compounds with known antibacterial activity are gallotannins and procyanidins. The former showed antibacterial activity against Gram-positive food-borne bacteria (i.e., Clostridium botulinum, Bacillus subtilis, B. cereus) [49]; the latter, especially those derived from berries, were found to be active against E. coli, S. mutans and oxacillin-resistant S. aureus [50]. On this basis, we evaluated MHE activity against different strains of S. aureus, P. aeruginosa, S. pyogenes, Enterococcus spp. and E. coli. To the best of our knowledge, no data are available on the antibacterial activity of M. hallii against drug resistant bacteria. Although MHE was found to be as rich in polyphenolic components that could exert antibacterial activity, our results showed that the antibacterial activity of MHE against S. aureus, P. aeruginosa, S. pyogenes and Enterococcus spp. strains is modest but appreciable, especially against Enterococcus spp. MHE was shown to be much less active against E. coli strains. Moreover, our data shows that, whilst methicillin-resistant strains usually display resistance to several drugs, no relevant differences were observed between methicillin-susceptible and resistant strains. Our results agree with earlier studies carried out on other species belonging to the Myrcianthes genus. The ethanol extract obtained from Myrcianthes discolor (Kunth) McVaugh dried leaves showed antibacterial activity against a S. aureus strain isolated from laryngitis samples, but did not show any activity against two E. coli strains isolated from urinary tract infection samples [51,52]. Moreover, M. cisplatensis showed antibacterial activity against methicillin-sensitive and resistant S. aureus strains [19]. According to the results obtained from UHPLC-PDA-hESI-MSn analysis, the appreciable antibacterial activity of MHE against S. aureus, P. aeruginosa, S. pyogenes, and especially Enterococcus spp., could be explained by the wide spectrum of polyphenols identified in the extract, which act as antimicrobial substances via different mechanisms of action (MOA). In fact, many MOA are ascribed to polyphenols, such as cytoplasmatic membrane damage, inhibition of nucleic acids, cell walls and cell membrane synthesis. Moreover, in addition to their direct antibacterial activity, a growing body of evidence suggests that polyphenols may interfere with some bacterial virulence factors such as enzymes, toxins and signal receptors [44].
The search for natural antimicrobial compounds is incited by the need to thwart the increasing bacterial resistance to antibiotics. In the biomedical field, this microbial antibiotic-resistance leads to a growing need for new, effective anti-infective materials for the prevention and delay of implant- and device-associated infections [53]. Anti-infective biomaterials have become a primary strategy to achieve this end. Natural polyphenols are interesting candidates for new antimicrobial agents to be loaded into or coated onto biomaterials. The development of nanoparticles carrying bioactive compounds with antimicrobial activity has been the target of investigations over the past years. In particular, several technologies have been developed at the nanoscale, such as nanoparticles, nanofibers, and nanocapsules, providing targeted delivery of polyphenols for therapeutic uses [54]. Recently, polyphenol delivery systems with antimicrobial activity have been described, focusing on nanoparticles based on chitosan as the main structural and functional material [55]. Polyphenols, ubiquitously expressed in plants have been shown to exert anticancer and immunomodulatory properties along with their anti-inflammatory and antimicrobial activities. However, some issues have been raised regarding the use of free polyphenols as medical drugs due to their fast metabolism and excretion in the human body. Indeed, this behavior might restrict or hamper their in vivo bioactive effects [56]. Therefore, a successful strategy for the use of M. hallii polyphenols as antimicrobial agents in the biomedical field may be require their administration in a form linked to or incorporated with nanomaterials able to support their controlled and prolonged release.
4. Materials and Methods
4.1. Chemicals and Materials
LC-MS grade methanol, acetonitrile and formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Millipore grade water was obtained with a Milli-Q water purification system (Millipore Corporation, Billerica, MA, USA). Filtration membranes (0.22 and 0.45 μm, cellulose acetate/cellulose nitrate mixed esters) were purchased from Millipore (Millipore Corporation). (−)-Epicatechin, (−)-catechin, (−)-epigallogatechin, (−)-gallocatechin and (−)-epigallocatechin-3-gallate were purchased by PhytoLab GmbH & Co.KG (Vestenbergsgreuth, Germany).
4.2. Plant Material
M. hallii was collected in July 2013 at Quito, Pichincha Province, Ecuador. Leaves were isolated manually from aerial parts and dried at room temperature for 3 weeks. Desiccated specimens were identified as M. hallii (O. Berg) McVaugh (syn. Eugenia halli Berg, Amyrsia halli (Berg) Kausel). Sample specimens have been deposited at the Herbarium of the University of Pavia (Department of Earth and Environmental Sciences, University of Pavia, Pavia, Italy) for future reference with the code: Quito (Pichincha, Ecuador), 26/07/2013, F. Bracco (PAV).
4.3. Extraction and Dialysis
Two 10 g aliquots of MHE were separately taken from the dried and finely ground leaves of M. hallii in 100 mL of H2O/methanol solution (70:30; % v/v) containing formic acid (0.1%, v/v), prepared in the dark, under constant stirring at room temperature for 24 h. Both extracts were filtered on a paper filter, gathered and freeze-dried. The residue was weighted and then reconstituted to 20 mL with Millipore-grade water and subdivided into two 10 mL aliquots. The first one was subjected to microbiological assays, the second was submitted to dialysis. Dialysis was performed using a Spectra/Por® Biotech Regenerated Cellulose membrane (Spectrum Europe B.V., Breda, The Netherlands) with a nominal molecular weight cut-off (MWCO) of 3500 Da. An aliquot (10 mL) of MHE was submitted to dialysis in 1000 mL of Millipore-grade water for 24 h at 4 °C under constant stirring. The dialysate (low molecular weight fraction, LMW) was freeze-dried and the dry residue was assessed and dissolved in 10 mL of Millipore-grade water and subjected to microbiological assays and to UHPLC-PDA-hESI-MSn analysis, following filtration through 0.45 and 0.22 μm filters.
4.4. UHPLC-PDA-hESI-MSn Analysis
The experiment was performed using a Jasco X-LC system (Jasco, Easton, MD, USA) equipped with a quaternary pump, an UHPLC photodiode array detector (PDA) and a linear ion trap mass spectrometer LTQ-XL (Thermo Scientific, Waltham, MA, USA) through an h-ESI source. Separation was achieved on a Purospher® STAR RP-18e (5 μm) LiChroCART® 250-4 (250 × 4 mm2 i.d., 5 μm) with its corresponding guard column (both from Merck KGaA, Darmstadt, Germany). The mobile phase consisted of A (0.1% formic acid in water) and B (acetonitrile) at a flow rate of 1 mL/min with an injection volume of 5 μL. Gradient elution was carried out using the following timetable: 98% A/2% B 0–5 min, 60% A/40% B 5–40 min, 0% A/100% B 40–45 min, 0% A/100% B 45–47 min, 98% A/2% B 47–52 min, 98% A/2% B 52–57 min. The resulting total run time was 57 min, including column reconditioning. The sample tray was set at 4 °C and the column oven temperature was set at 24 °C. The chromatograms were recorded at λ 280, 220, 366 and 520 nm); spectral data were acquired in the range of 200–650 nm for all peaks. The ion trap operated in data dependent, full scan (80–1500 m/z), zoom scan and MSn mode. To obtain the fragment ions a collision energy of 35% and an isolation of 2 m/z were applied; the voltage was kept at 3 kV for negative ionization and 5 kV for the positive one, the temperature of the capillary tube was 275 °C with a sheath gas flow rate of 45 arbitrary units and an auxiliary gas flow rate of 20 arbitrary units, while the ionization chamber was maintained at 100 °C. ThermoFisher Scientific Excalibur 2.0 software (SR2, Thermo Electron Corporation 1998–2006, Waltham, MA, USA) was used for data acquisition and processing. Three independent assays were performed to analyze the sample (filtered through a cellulose acetate/cellulose nitrate mixed esters membrane, 0.22 μm) and no relevant variations attributable to the nature of the detected fragments or their relative intensities were observed.
4.5. Bacterial Strains and Growth Conditions
The bacteria were recent clinical isolates, belonging to the Institute of Microbiology (University of Genova) collection. They comprised of: (i) ten S. aureus strains, including five methicillin-resistant (MRSA) and five methicillin-susceptible (MSSA) strains. Of the MRSA strains, three were multi-resistant (resistant to at least three classes of antibiotics); (ii) ten multi-resistant Escherichia coli strains; (iii) ten Pseudomonas aeruginosa strains; (iv) ten vancomycin-resistant and susceptible Enterococcus faecalis and Enterococcus faecium strains; and (v) ten group A streptococci (Streptococcus pyogenes) strains, which remain universally susceptible to penicillin. All isolates were identified at the species level using clinical methods and an API STAPH, API20E, API NE and API STREP system (bioMèrieux, Marcy l’Etoile, France) for S. aureus, E. coli, P. aeruginosa, Enterococcus spp. and S. pyogenes respectively. The antibiotype was determined using the disk diffusion test, as according to the latest Clinical and Laboratory Standards Institute (CLSI) guidelines [57]. Strains were cultured in Mueller-Hinton Broth, Mueller-Hinton agar, MacConkey agar and Columbia blood agar (Biolife, Milan, Italy) at 37 °C.
4.6. Susceptibility (MIC Determination)
The Minimum Inhibitory Concentration (MIC) of plant extract was determined using the broth microdilution method, following the CLSI guidelines [58]. In brief, exponentially growing bacteria (5 × 105 cells per mL, final inoculum) were added to the various concentrations of plant extract, 2-fold serially diluted in 96-well microtitre plates of Mueller-Hinton broth or cation-adjusted Mueller Hinton broth with 5% of lysed horse blood for S. pyogenes. The following concentrations (gr/mL) of extracts were used: 0.5, 0.25, 0.125, 0.006, 0.031, 0.015, 0.007, 0.0039, 0.019, 0.00097, 0.00048. After 18–24 h of incubation at 37 °C, the concentration at which the plant extract prevented visible bacterial growth was identified as the MIC. All tests were performed in triplicate and executed three times. S. aureus ATCC 29213, E. coli ATCC 25922 and P. aeruginosa ATCC 27853 were added as control strains.
5. Conclusions
In conclusion, this study represents the first attempt to phytochemically characterize of M. hallii leaf extract and demonstrate its antimicrobial activity against drug resistant bacteria. The extract exhibits antimicrobial activity against S. aureus, P. aeruginosa, S. pyogenes, and Enterococcus spp. strains due to the presence of many different classes of polyphenolic compounds that possess antibacterial activity. The data reported in this paper reveal that M. hallii is a potential source of polyphenols with antimicrobial properties and shows the great potential of this species, not just for pharmaceutical applications, but also for biomedical, food and cosmetic applications.
Author Contributions
Maria Daglia and Anna Marchese designed the research, were responsible for the correctness of the chemical and microbiological analyses, respectively, and contributed to writing the manuscript; Patricia Chavez Carvajal collected the plant material and performed the sample preparation; Francesco Bracco identified the collected plant material as M. hallii (O. Berg) McVaugh and deposited sample specimens at the Herbarium of the University of Pavia; Arianna Di Lorenzo and Davide Gozzini performed the chemical analysis; Erika Coppo performed the microbiogical tests; Arianna Di Lorenzo and Patricia Chavez Carvajal contributed to the writing of the introduction and chemical analysis section; Giuseppe Zanoni and Seyed Mohammad Nabavi revised the final version; Carla Renata Arciola contributed to the critical analysis of data and to the discussion, and she revised the final version. All authors approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MHE | Myrcianthes hallii extract |
| LMW | low molecular weight |
| MRSA | methicillin-resistant Staphylococcus aureus |
| MSSA | methicillin-susceptible Staphylococcus aureus |
| CLSI | Clinical and Laboratory Standards Institute |
| MIC | Minimum inhibitory concentration |
| MOA | mechanism of action |
References
- León-Yánez, S.; Valencia, R.; Pitman, N.; Endara, L.; Ulloa Ulloa, C.; Navarrete, H. (Eds.) Libro Rojo Delas Plantas Endémicas del Ecuador, 2nd ed.; Publicaciones del Herbario QCA, Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2011.
- Messaoud, C.; Laabidi, A. Myrtus communis L. Infusions: The effect of infusion time on phytochemical composition, antioxidant, and antimicrobial activities. J. Food Sci. 2012, 77, C941–C947. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kokubo, R. Antimicrobial activities of eucalyptus leaf extracts and flavonoids from Eucalyptus maculata. Lett. Appl. Microbiol. 2004, 39, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Metwally, A.M.; Omar, A.A. Phytochemical investigation and antimicrobial activity of Psidium guajava L. leaves. Pharmacogn. Mag. 2010, 6, 212–218. [Google Scholar] [PubMed]
- Chandrasekaran, M.; Venkatesalu, V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J. Ethnopharmacol. 2004, 91, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Royal Botanical Gardens. Available online: http://www.kew.org/ (accessed on 18 November 2015).
- Mazine, F.F.; Castro Souza, V. A preliminary phylogenetic analysis of Eugenia (Myrtaceae: Myrteae), with a focus on Neotropical species. Kew Bull. 2014, 69, 94–97. [Google Scholar] [CrossRef]
- Proença, C.E.B.; Jennings, L.V.S. Two new species of Myrtaceae (Myrteae) from northern South America. Brittonia 2011, 63, 46–50. [Google Scholar] [CrossRef]
- Sobral, M.; Grippa, C.R. Fourteen new species and two taxonomic notes on Brazilian Myrtaceae. Phytotaxa 2012, 50, 19–50. [Google Scholar] [CrossRef]
- Ministerio de, S.P.; Programa de, A.S.S.E. Medicina Tradicional Andina y Plantas Curativas, 1st ed.; Centro de Orientamiento Educativo COE, Ed.; Ministerio de Salud: Quito, Ecuador, 2008; p. 84. [Google Scholar]
- White, A. Herbs of Ecuador: Medicinal Plants; Libri, M., Ed.; Edicions Libri Mundi: Quito, Ecuador, 1985; pp. 59–62. [Google Scholar]
- Tropicos®. Available online: http://www.tropicos.org/ (accessed on 10 November 2015).
- Holst, B.K. Myrtaceae. In Catalogue of the Vascular Plants of Ecuador; Jørgensen, P.M., León-Yánez, S., Eds.; Missouri Botanical Garden: St. Louis, MO, USA, 1999; p. 621. [Google Scholar]
- Ulloa, C. Usos de plantas en los Andes Centrales. In Botánica Económica de Los Andes Centrales; Moraes, M., Øllgard, B., Eds.; Universidad Mayor de San Andres: La Paz, Bolivia, 2006; pp. 313–328. [Google Scholar]
- Boulekbache-Makhlouf, L.; Meudec, E. Qualitative and semi-quantitative analysis of phenolics in Eucalyptus globulus leaves by high-performance liquid chromatography coupled with diode array detection and electrospray ionization mass spectrometry. Phytochem. Anal. 2013, 24, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.; Freire, C.S. Characterization of phenolic components in polar extracts of Eucalyptus globulus Labill. bark by high-performance liquid chromatography–mass spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Amakura, Y. Polyphenolic compounds isolated from the leaves of Myrtus communis. J. Nat. Med. 2008, 62, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Wannes, W.A.; Mhamdi, B. Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food Chem. Toxicol. 2010, 48, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, E.; Barneche, S. Identification of a bioactive compound from Myrcianthes cysplatensis. Pharmacogn. J. 2011, 3, 18–20. [Google Scholar] [CrossRef]
- Andrade, J.M.; Aboy, A.L. Phenolic composition in different genotypes of guabiju fruits (Myrcianthes pungens) and their potential as antioxidant and antichemotactic agents. J. Food Sci. 2011, 76, C1181–C1187. [Google Scholar] [CrossRef] [PubMed]
- Arze, J.B.L.; Jean, F.I. Essential oils from bolivia. VII. Myrtaceae: Myrcianthes osteomeloides (Rusby) McVaugh and Myrcianthes pseudomato (Legrand) McVaugh. J. Essent. Oil Res. 2005, 17, 64–65. [Google Scholar] [CrossRef]
- Cole, R.A.; Haber, W.A. Leaf essential oil composition of three species of Myrcianthes from Monteverde, Costa Rica. Chem. Biodivers. 2008, 5, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Dalla Nora, C.; Dal-Ri Müller, C. Effect of processing on the stability of bioactive compounds from red guava (Psidium cattleyanum Sabine) and guabiju (Myrcianthes pungens). J. Food Compos. Anal. 2014, 34, 18–25. [Google Scholar] [CrossRef]
- De Mello Andrade, J.M.; Marin, R. Comparison of the fatty acid profiles of edible native fruit seeds from Southern Brazil. Int. J. Food Prop. 2012, 15, 815–822. [Google Scholar] [CrossRef]
- Marin, R.; Apel, M.A. Volatile components and antioxidant activity from some myrtaceous fruits cultivated in Southern Brazil. Lat. Am. J. Pharm. 2008, 27, 172–177. [Google Scholar]
- Toloza, A.C.; Zygadlo, J. Bioactivity of Argentinean essential oils against permethrin-resistant head lice, Pediculus humanus capitis. J. Insect Sci. 2010, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Lee, V.S.Y. Identification and comparison of phenolic compounds in the preparation of Oolong tea manufactured by semifermentation and drying processing. J. Agric. Food Chem. 2007, 55, 7462–7468. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.F.; Marques, M.C. Identification of bioactive compounds from jambolão (Syzygium cumini) and antioxidant capacity evaluation in different pH conditions. Food Chem. 2011, 126, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Kasajima, N.; Ito, H. Cypellogins A, B and C, acylated flavonol glycosides from Eucalyptus cypellocarpa. Chem. Pharm. Bull. 2005, 53, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Ablajan, K.; Abliz, Z. Structural characterization of flavonol 3,7-di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2006, 41, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.S.; Rahaman, N.L. Antioxidant activity of three honey samples in relation with their biochemical components. J. Anal. Methods Chem. 2013, 2013, 313798. [Google Scholar] [CrossRef] [PubMed]
- Callemien, D.; Collin, S. Use of RP-HPLC-ESI(–)-MS/MS to differentiate various proanthocyanidin isomers in lager beer extracts. J. Am. Soc. Brew. Chem. 2008, 66, 109–115. [Google Scholar]
- Sandhu, A.K.; Gu, L. Antioxidant capacity, phenolic content and profiling of phenolic compounds in the seeds, skin and pulp of Vitis rotundifolia (muscadine grapes) as determined by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2010, 58, 4681–4692. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, S.C.; Castilho, P.C. Analysis of phenolic compounds from different morphological parts of Helichrysum devium by liquid chromatography with on-line UV and electrospray ionization mass spectrometric detection. Rapid Commun. Mass Spectrom. 2009, 23, 3939–3953. [Google Scholar] [CrossRef] [PubMed]
- Bellosta, S.; Dell’Agli, M. Inhibition of metalloproteinase-9 activity and gene expression by polyphenolic compounds isolated from the bark of Tristaniopsis calobuxus (Myrtaceae). Cell. Mol. Life Sci. 2003, 60, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Dastmalchi, K.; Flores, G. Edible Myrciaria vexator fruits: Bioactive phenolics for potential COPD therapy. Bioorg. Med. Chem. 2012, 20, 4549–4555. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, C.F.; Cuevas, E. Determination of anthocyanins from Camu-camu (Myrciaria dubia) by HPLC-PDA, HPLC-MS, and NMR. J. Agric. Food Chem. 2005, 53, 9531–9535. [Google Scholar] [CrossRef] [PubMed]
- Haron, N.W.; Moore, D.M. Distribution and taxonomic significance of flavonoids in the genus Eugenia (Myrtaceae). Biochem. Syst. Ecol. 1992, 20, 266–268. [Google Scholar] [CrossRef]
- Takashi Yoshida, T.; Amakura, Y. Structural features and biological properties of ellagitannins in some plant families of the order Myrtales. Int. J. Mol. Sci. 2010, 11, 79–106. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Di Lorenzo, A. Gallic acid and related compounds as neuroprotective agents: You are what you eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.N.H.; Herent, M.-F. Piceatannol, a potent bioactive stilbene, as major phenolic component in Rhodomyrtus tomentosa. Food Chem. 2013, 138, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.K.; Zaman, N.M. Comparative evaluation of the medicinal activities of methanolic extract of seeds, fruit pulps and fresh juice of Syzygium cumini in vitro. J. Coast. Life Med. 2013, 1, 300–308. [Google Scholar]
- Neungchamnong, N.; Ingkaninan, K. On-line characterization of phenolic antioxidants in fruit wines from family Myrtaceae by liquid chromatography combined with electrospray ionization tandem mass spectrometry and radical scavenging detection. Food Sci. Technol. 2009, 42, 297–302. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, S.N.; Shimura, M. Preventive effect of green tea polyphenols against dental caries in conventional rats. Biosci. Biotechnol. Biochem. 1992, 56, 592–594. [Google Scholar] [CrossRef]
- Diker, K.S.; Akan, M. The bacterial activity of tea against Campylobacter jejuni and Campylobacter coli. Lett. Appl. Microbiol. 1991, 12, 34–35. [Google Scholar] [CrossRef]
- Isogai, E.; Isogai, H. Protective effect of Japanese green tea extract on gnotobiotic mice infected with an Escherichia coli O157:H7 strain. Microbiol. Immunol. 1998, 42, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Hamilton, V.E.S. Aggregation of Staphylococcus aureus following treatment with the antibacterial flavonol galangin. J. Appl. Microbiol. 2007, 103, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Engels, C.; Schieber, A. Inhibitory spectra and modes of antimicrobial action of gallotannins from mango kernels (Mangifera indica L.). Appl. Environ. Microbiol. 2011, 77, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Côté, J.; Caillet, S. Bioactive compounds in cranberries and their biological properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, R.W.; Glenn, A. Antibacterial Activity of Medicinal Plants of Northern Peru—Part II. Arnaldoa 2009, 16, 93–103. [Google Scholar]
- Bussmann, R.W.; Glenn, A. Antibacterial activity of medicinal plants of Northern Peru—Can traditional applications provide leads for modern science? Indian J. Tradit. Knowl. 2010, 9, 742–753. [Google Scholar]
- Campoccia, D.; Montanaro, L. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]
- Ciurana, J.; Rodriguez, C.A. Trends in nanomaterials and processing for drug delivery of polyphenols for cancer and other treatments. Curr. Drug Targets 2015. epub head of print. [Google Scholar] [CrossRef]
- Madureira, A.R.; Pereira, A. Current state on the development of nanoparticles for use against bacterial gastrointestinal pathogens. Focus on chitosan nanoparticles loaded with phenolic compounds. Carbohydr. Polym. 2015, 130, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.; Benvenuto, M. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests f or Bacteria That Grow Aerobically, Approved Standard—Tenth Edition; CLSI Document M07-A10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standard-Twelfth Edition; CLSI Document M02-A12; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).