1. Introduction
The treatment of an infected root canal has been based on nonspecific elimination of intraradicular microorganisms through the application of broad-spectrum antimicrobial approaches [
1]. Nevertheless, it has been shown that it is nearly impossible to obtain complete elimination of microorganisms in the root canal system [
2,
3]. Therefore, the continued development of treatments that can effectively penetrate dentin to eliminate root canal infection is a priority in clinical endodontic research.
Currently, the most frequent intracanal medicaments employed to treat infected root canals include calcium hydroxide (Ca(OH)
2), potassium iodine (KI), and chlorhexidine (CHX). The efficacy of root canal disinfectants can be influenced by several factors such as pH, serum proteins, collagen, and dentin among others [
4,
5,
6]. However,
in vitro studies have demonstrated that CHX is more effective in eliminating bacteria from internal dentinal tubules in comparison to the other disinfectants when dispersed in liquid or hydrogel systems [
7,
8]. Besides its proven antimicrobial activity and non-toxicity at low dosages, CHX provides substantivity to dentin tissues, which may offer protection against microbial colonization for extended periods of time after treatment [
7,
9]. These attributes make CHX a potent disinfectant in root canal treatment. Dentin permeability and the complex anatomy of the root canal, however, impose challenges to the penetration and subsequent action of these disinfectants. Gomes
et al. [
10] demonstrated
in vitro that medicaments containing 2% CHX were able to diffuse into the dentin, reaching the external root surface. The information on the length of activity time of the various agents in the root canal is limited.
Nanoparticles in recent years have been employed in several clinical applications [
11]. In endodontics, nanoparticles have been suggested to act as irrigants [
12], incorporated into intracanal medicaments [
13] or root canal sealers [
14,
15]. Nanoparticle technology for drug delivery includes nanoencapsulation, which is the coating of a substance within another material, typically a polymer based system. It aims to maximize the therapeutic efficacy while minimizing undesirable side effects due to the control of the drug bioavailability and release [
16,
17]. There is scarce data in the current literature on the synthesis, characterization, and application of nanoencapsulated medicaments that are typically employed in root canal treatment. Shrestha and Kishen [
18] evaluated the effect of rose bengal-functionalized chitosan nanoparticles associated with photodynamic therapy over monospecies bacteria/biofilms and assessed their antibiofilm efficacy on a multispecies biofilm grown on dentin. Shrestha
et al. [
19] examined the ability of the temporally controlled release of bovine serum albumin from chitosan nanoparticles to regulate the alkaline phosphatase activity in stem cells from apical papilla.
Nanoparticles produced by drug nanoencapsulation have specific characteristics such as size, release pattern, and activity, which are factors determined by the synthetic method employed, polymer system of choice, and polymer molecular weight. Therefore, protocols should be conducted to achieve proper nanoencapsulation of medicaments, taking into account the stability of the system or release profile to achieve the desirable antiseptic activity inside a specific target or tissue. Provided the anatomical complexity of the root canal, permeability of dentin, and limited penetration of medicaments in the dentin, the goal of this study is to develop and characterize a novel CHX-encapsulated system for root canal applications. In this work, the biodegradable and biocompatible block copolymer of choice was poly(ethylene glycol)–block–poly(l-lactide) (PEG–b–PLA) to create CHX loaded nanoparticles. PEG–b–PLA bilayer nanoparticles have advantages for drug delivery, such as small size and hydrophobic and hydrophilic functionalities in the polymer backbone that improves in vivo half-life. These polymeric nanoparticles were characterized for size, morphology, and drug loading proficiency. The nanoparticles were found to be small enough to penetrate dentin tubules, dispersed well in a hydrogel matrix used as a carrier system, and enhanced bacterial inhibition over longer periods of time.
3. Discussion
The present study discussed the synthesis, characterization, and antimicrobial effectiveness of a new drug delivery system designed to allow for extended release of CHX inside the root canal system. It was hypothesized that nanoparticles prepared with an appropriate size and a controlled CHX-release profile could carry the medicament deep into dentin tubules, allowing for sustained inhibition of bacteria in the root canal system.
Chlorhexidine has been used previously in hydrogel form and in liquid formulations in root canal treatment. CHX is well-known to be rapidly released from poly(lactic acid) microparticles [
20]. Therefore, in order to enhance bacterial elimination from infected root canal systems and to create an environment that is ideal for periapical healing, it is highly desirable to extend the release period of CHX
in situ beyond the delivery systems currently in use. Nanoencapsulation can provide better control while further extending the release period of the medicament in dentin tissues. The antibacterial activity of CHX in different concentrations and preparation forms has been extensively tested. In endodontics, CHX has been employed as an intracanal medicament in a 2% concentration, alone or associated with calcium hydroxide [
21]. It should also be emphasized that, the higher the drug concentration, the higher its side effects.
The bacterial activity of CHX loaded poly(ε-caprolactone) nanocapsules and CHX digluconate against
S. epidermis was studied in [
22]. The CHX carrier system was observed to improve drug targeting of bacteria, further reducing bacterial growth onto skin in relation to CHX digluconate. In the present study, CHX was encapsulated in a poly(ethylene glycol)–
block–poly(
l-lactide) (“PEG–
b–PLA”). The choice of PEG–
b–PLA is optimal as poly(
l-lactic acid) has long been known for its biocompatibility and biodegradation properties [
23]. It is a common choice to safely encapsulate bioactive drugs and control their release [
24]. PEG has been copolymerized with PLA to create a block polymer and facilitate drug release due to its dual nature, hydrophilic-hydrophobic, which can increase pore formation in the nanoparticles along with a rise in the rate of polymer degradation. PLA microspheres degrade through a hydrolytic chain cleavage reaction affecting both the surface and the bulk properties of spheres while exhibiting no serious health risks [
24]. All these properties make the copolymer a viable candidate to both encapsulate and release CHX. The PEG–
b–PLA nanoparticles were prepared through the oil-in-water emulsion technique. The goal of this methodology was to synthesize nanoparticles smaller than 1 µm in order to facilitate penetration of the nanoparticles deep into the tubules. The encapsulation process resulted in nanoparticles with a size ranging much smaller than the diameter of dentin tubules [
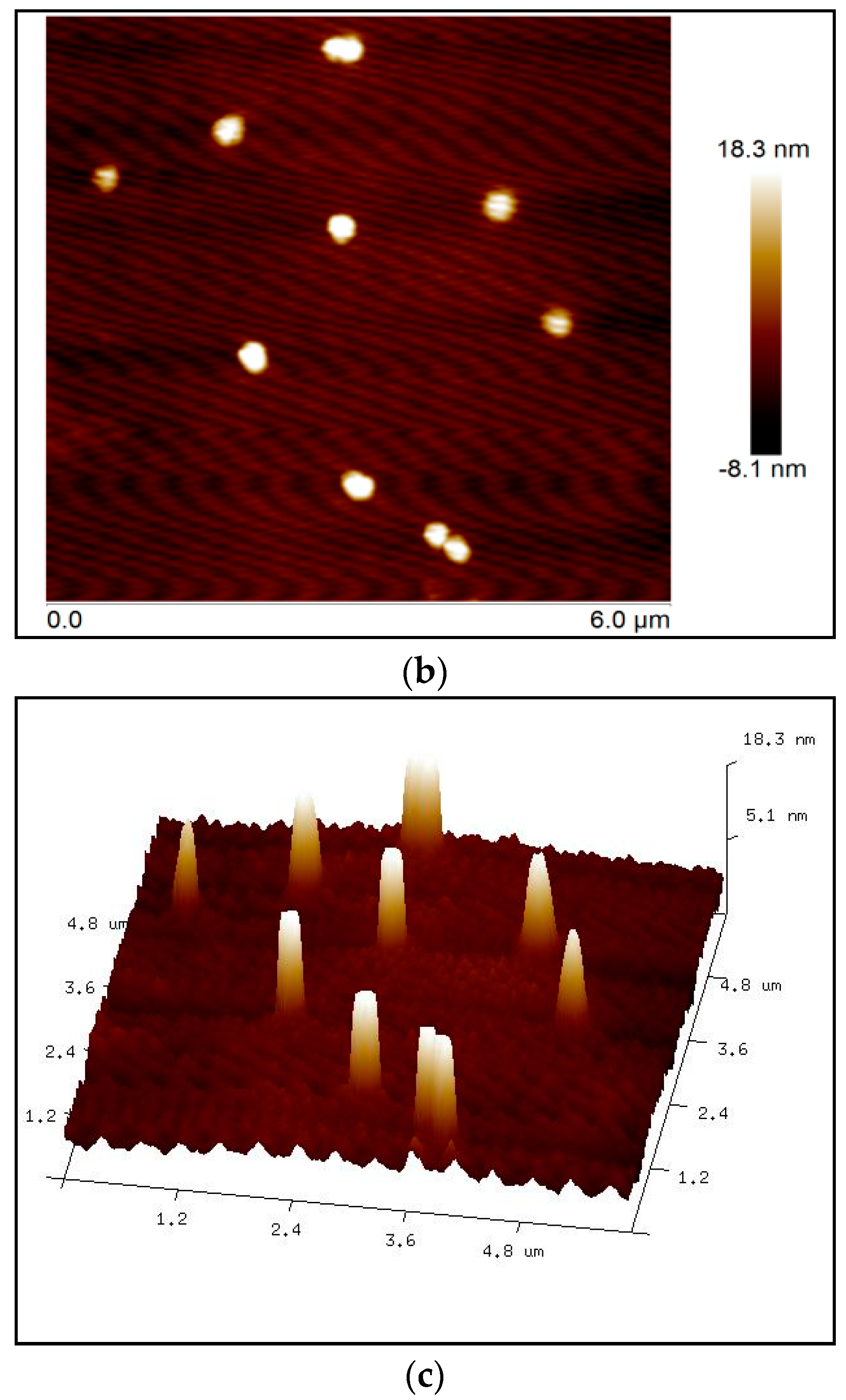
25]. Atomic force microscopy revealed that the size of the nanoparticles ranged from 300 to 500 nm (
Figure 4), which was verified by dynamic light scattering (DLS).
The PEG–
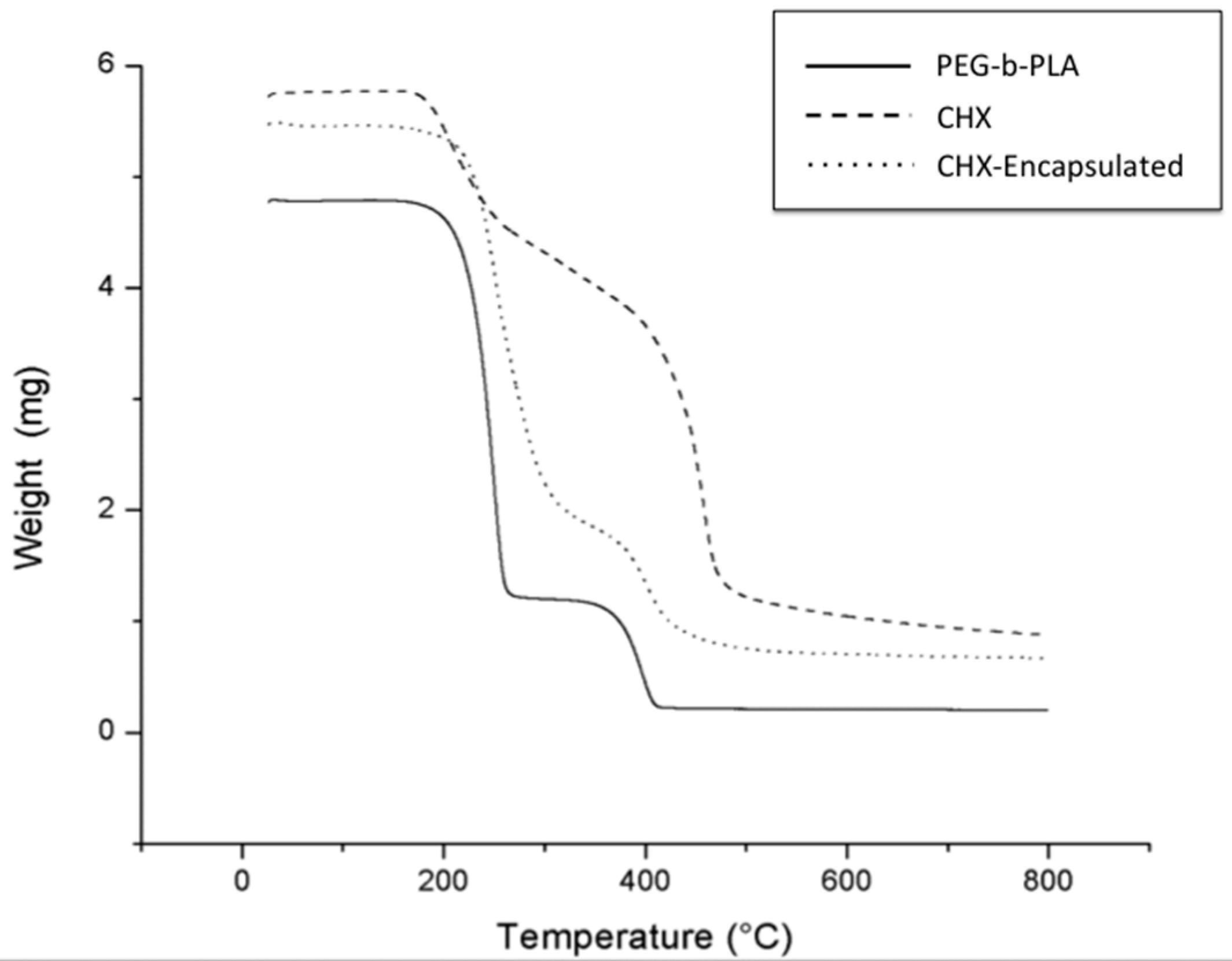
b–PLA nanoparticles prepared through the oil-in-water emulsion technique resulted in high material yields and drug encapsulation efficiencies as high as 80%. TGA revealed that CHX was incorporated into the polymer as demonstrated by weight changes of decomposition reactions of the CHX-containing nanoparticles in comparison to starting materials—CHX and the PEG–
b–PLA block copolymer (
Figure 2).
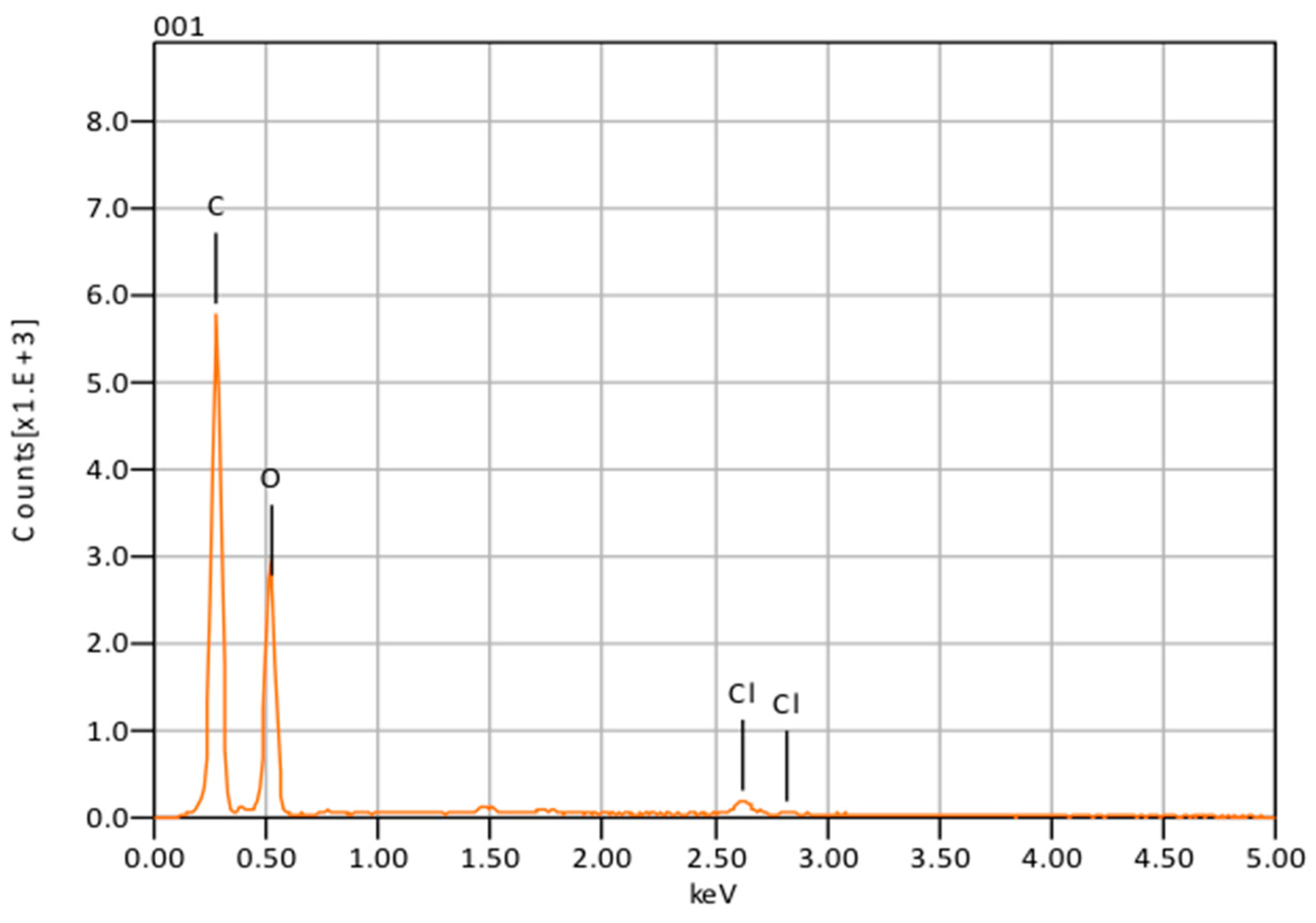
The presence of chlorine peaks (
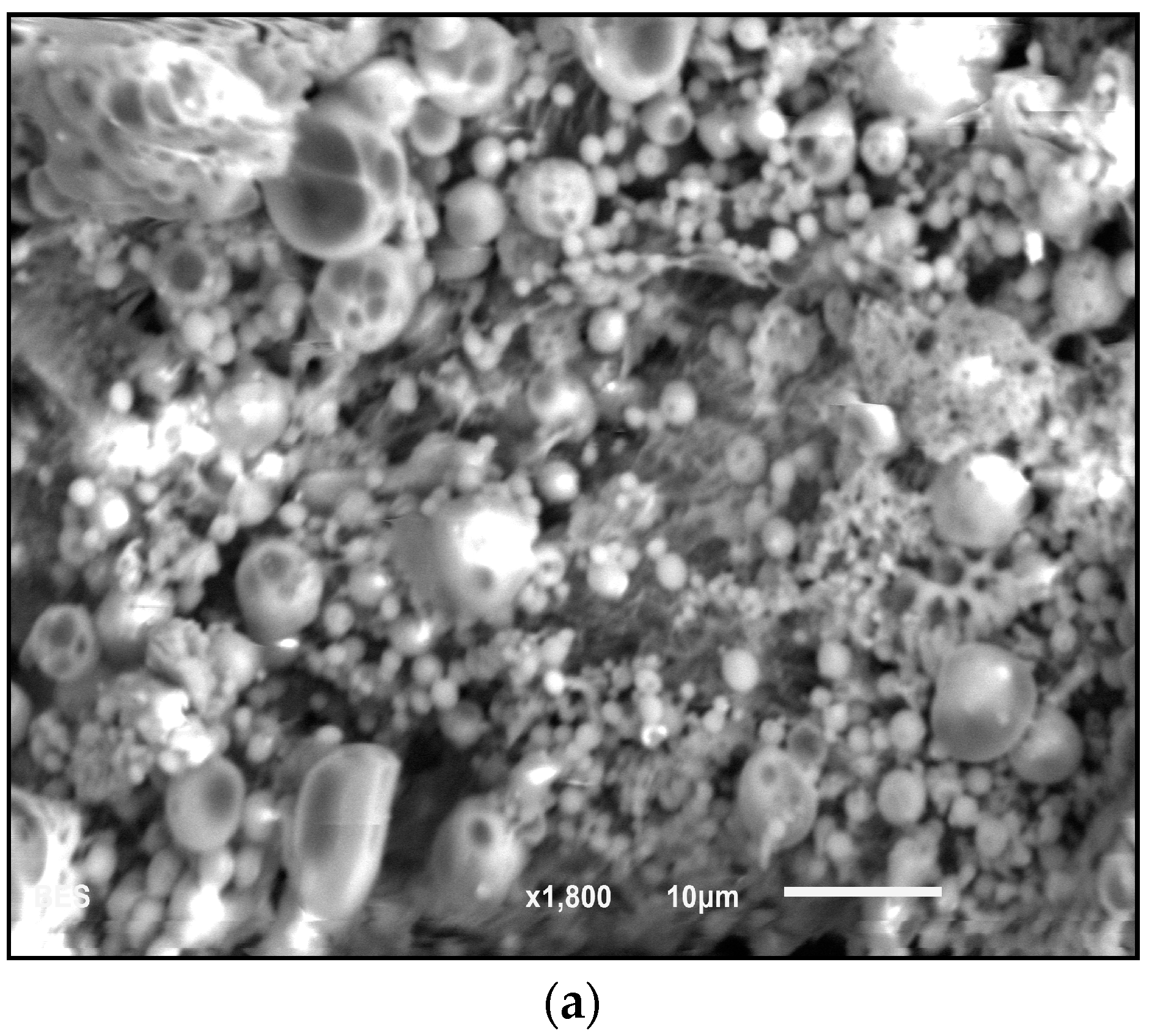
Figure 3) in the EDS spectra are evidence of CHX-encapsulated nanoparticles. As this element is characteristic of CHX and not the polymer, there is evidence to suggest that the CHX is being absorbed on the surface of the nanoparticles and encapsulated. Although SEM showed that the nanoparticles can form large clumps during the lyophilization process, these were significantly reduced when the nanoparticles were dispersed in a hydrogel matrix made out of 1% Natrosol™ (hydroxyethyl cellulose) in water.
Nanoparticle disperssion within the hydrogel matrix consists of polymer chains from the hydrogel forming weak bonds with many nanoparticles. This in turn produces a loosely interlinked network of polymers and nanoparticles [
26]. Since each connection point is rather weak, the bonds breakdown under mechanical stress, for instance, when injected through syringes [
27]. When the shear forces subside, the polymers and nanoparticles form new connections with the walls of dentinal tubules and within the hydrogel itself. Now, the high water content and large pore sizes of this hydrogel will be sufficient to trigger hydrolysis of the backbone ester groups in PLA and the oxidation of the ether backbone in PEG, thereupon diffusing the chlorhexidine.
Microorganisms may penetrate inside dentin to different extents and may survive inside the tubules, even after the use of the currently employed disinfection protocols. In a clinical study, Siqueira
et al. [
28] demonstrated that chemomechanical preparations with 2.5% NaOCl as irrigant significantly reduced the number of bacteria in the canal, but allowed for microbial recovery through cultivation in more than one-half of all cases. Authors also reported that a seven-day intracanal dressing with Ca(OH)
2/camphorated paramonochlorophenol (CPMC) paste significantly increased the number of culture-negative cases. However, positive cultures were still obtained. Furthermore,
Enterococcus faecalis, a facultative anaerobe that can be isolated from persistent/secondary root canal infections, has demonstrated to be resistant to calcium hydroxide, especially when in biofilms. They can adapt to harsh environmental changes and can colonize dentinal tubules where they remain protected from medicaments. Upadya
et al. [
29] showed that
E. faecalis biofilms were considerably more resistant to Ca(OH)
2 solutions than free-floating cells. A fraction from the biofilm cells persisted viable even after 24-h exposure to a saturated Ca(OH)
2 solution. It was pointed out that the increased resistance might be attributed to the biofilm structure or extrapolimeric substance. Therefore, other antimicrobial agents, with different mechanisms of action should be employed to enhance the root canal system disinfection, especially in areas that are difficult to reach with conventional instrumentation and currently employed intracanal medicament. Chlorhexidine digluconate is water-soluble and readily dissociates at physiologic pH releasing the positively charged chlorhexidine component. The bactericidal effect of the drug is due to the cationic molecule binding to extra microbial complexes and negatively charged microbial cell walls, thereby altering the cells’ osmotic equilibrium [
30,
31]. Chlorhexidine with a concentration of 2%, in both liquid or gel presentations, has been employed as an auxiliary chemical substance during root canal preparation or as a final irrigant [
32,
33,
34]. Several
in vitro studies assessed the properties of CHX as an intracanal medicament [
10,
35,
36]. However, there are few clinical studies that employed CHX hydrogel as an intracanal medicament. Gama
et al. [
37] evaluated the incidence of postoperative pain after intracanal dressings with either 0.12% chlorhexidine digluconate gel or a calcium hydroxide/camphorated paramonochlorophenol/glycerin paste. Therefore, this study intended to develop a nanoparticle–hydrogel matrix system to be employed as an intracanal medicament that would carry CHX, allowing for its continuous release inside the root canal system.
Few studies have assessed the antimicrobial effect of antimicrobial-containing nanoparticles over endodontic pathogens, especially
Enterococcus faecalis. The effect of rose bengal-functionalized chitosan nanoparticles over
E. faecalis cells and multispecies biofilm structures was assessed by Shrestha and Kishen [
18]. Despite the quantification of the CHX release from nanoparticles assessed through analytical methods [
38,
39,
40], no study has evaluated the residual antimicrobial effect produced by suspended nanoparticles over time, especially against
E. faecalis cells. In the present study,
in vitro studies in
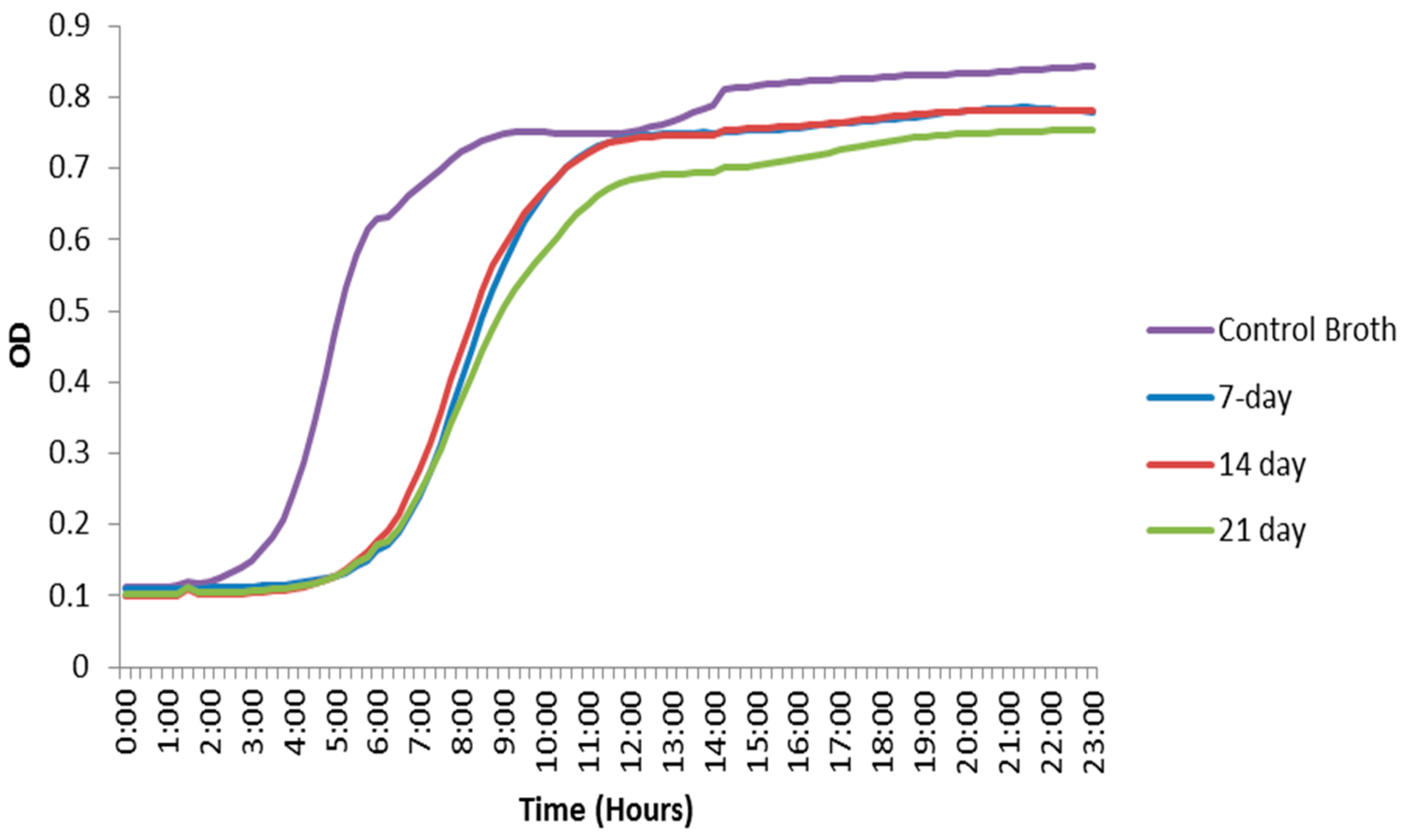
E. faecalis were performed to investigate bacterial growth inhibition in the presence of CHX-encapsulated nanoparticles. The experiment was performed at different time points to investigate the length of antibacterial activity and effectiveness of the synthesized delivery system. The initial goal was to maintain CHX release for a minimum period of 7 to 14 days, which corresponds to the period that an intracanal medicament (such as calcium hydroxide paste) is often kept between appointments. The synthesized nanoparticles were immersed in both PBS and BHI (brain–heart infusion) broth. Nanoparticles removed from the PBS and placed on bacterial lawns showed a zone of inhibition after being immersed for as long as 21 days. These zones of inhibition demonstrate that CHX both elutes into the solution and remains in the particles after eluting for up to three weeks. They also provide evidence of the area where bacteria will not come into contact with the material even after eluting the drug into a solution. The broth containing the nanoparticles demonstrated a lag in the growth phase of
E. faecalis bacteria as well as a decrease in total cell density during this lag phase, indicating inhibition and demonstrating that CHX was indeed diffusing from the nanoparticles. The diffusion of CHX is directed by chemical potential gradients arising by osmotic pressure. In addition to diffusion, CHX could be released by erosion of the polymer matrix, which leads to pore formation [
41].
The initial burst release behavior of the nanoparticles could be attributed to the CHX absorbed on the surface of the nanoparticles, with subsequent releases corresponding to the encapsulated portion. Both results (ZOI and OD) suggest that the nanoparticles demonstrate a bacteriostatic effect for at least three weeks. The zone of inhibition tests showed that CHX was eluting from the hydrophobic core of the PEG–b–PLA nanoparticles into the PBS solution, while some CHX still remained in the nanoparticles and were still capable of antimicrobial action. Furthermore, the bacterial growth curves using the OD data demonstrated that eluting CHX into bacterial broth did have an effect on the E. faecalis bacteria’s growth curve. However, the size of the zones of inhibition decreased over time. Eventually, the release of CHX reached equilibrium, and the nanoparticles that had been immersed for 14 days in PBS showed similar-sized zones as those immersed for 21 days. This suggests that hydrolysis or enzymatic cleavage of the PEG–b–PLA backbone reached the hydrophobic core of the nanoparticle, which causes bulk erosion, hence releasing the remaining CHX during the time period between 14 and 21 days. Additionally, the bacterial growth curves in the broth eventually reached their growth phase after the initial lag and had a final OD similar to the control, albeit slightly lower.
The intent of this nanoparticle-hydrogel matrix system was to develop an intracanal medicament that allows for the distribution of the nanoparticles through the lateral tubules and accessory canals that are exposed during the root canal procedure. The nanoparticle–hydrogel matrix system should not remain in the root canal itself after the endodontist does adequate irrigations prior to canal filling. There have been studies on hydrogel formulations and their ability to insert into narrow and convoluted locations, such as in the inter-tubular dentin matrix [
13,
42,
43]. Thus, we are using this adhesive property of hydrogels in order to deposit and fasten the nanoparticles to the walls of dentinal tubules and accessory canals.
Control PEG–
b–PLA nanoparticles (synthesized without the addition of CHX) showed no ZOI, indicating that no reactive oxygen species (ROS) were introduced during the synthesis of the nanoparticles. ROS are partially reduced metabolites of oxygen, including hydrogen peroxide and hydroxyl radical, which can result from polymer degradation [
44]; this in turn causes additional oxidative stress in many pathological pathways making them toxic to cells [
45]. Free radical generation in the cell culture containing the nanoparticles can also arise from the cellular uptake of low molecular weight polymer chains that result from their degradation [
46]. This likewise leads to cytotoxicity due to the stimulation of ROS and/or the accumulation of polymer degradation products inside the cell. During the 21-day antimicrobial effectiveness study of PEG–
b–PLA nanoparticles synthesized without the addition of CHX, no inhibitory diameter was detected, indicating that, even if the polymer is degrading, cells are not being affected by its degradation products or triggering ROS production inside cells. The results from the control in this study are of significant importance in the design of nano-carrier systems for endodontic drug delivery, thus validating the well-known biodegradability and biocompatibility of nanoparticles made out of PEG–
b–PLA and making this a safe and suitable system of CHX delivery inside dentinal tubules.
The physiochemical properties of the PEG–b–PLA nanoparticles will need to be further tuned in order to enhance bacterial inhibition over longer periods of time. This will allow for prolonged release in the dentin tubules to better ensure the success of a root canal procedure. Future studies will include increasing the concentration of nanoparticles in medium and assessing the nanoparticle hydrogel matrix delivery network inside the root canal system.
4. Materials and Methods
4.1. Materials
Poly(ethylene glycol) methyl ether [average molecular weight (Mn) = 2000 g/mol], l-lactide, toluene, tin (II) 2-ethylhexanoate, chlorhexidine, poly(vinyl alcohol) (PVA) [87%–90% hydrolyzed, average Mw = 30,000–70,000 g/mol], dichloromethane (DCM), 1x phosphate buffer saline (PBS), and pentane were used as received without further purification. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
4.2. Nanoparticles Synthesis
The process of preparing bilayer nanoparticles comprised two steps: (1) polymer synthesis followed by (2) encapsulation of the drug.
4.2.1. Polymer Synthesis
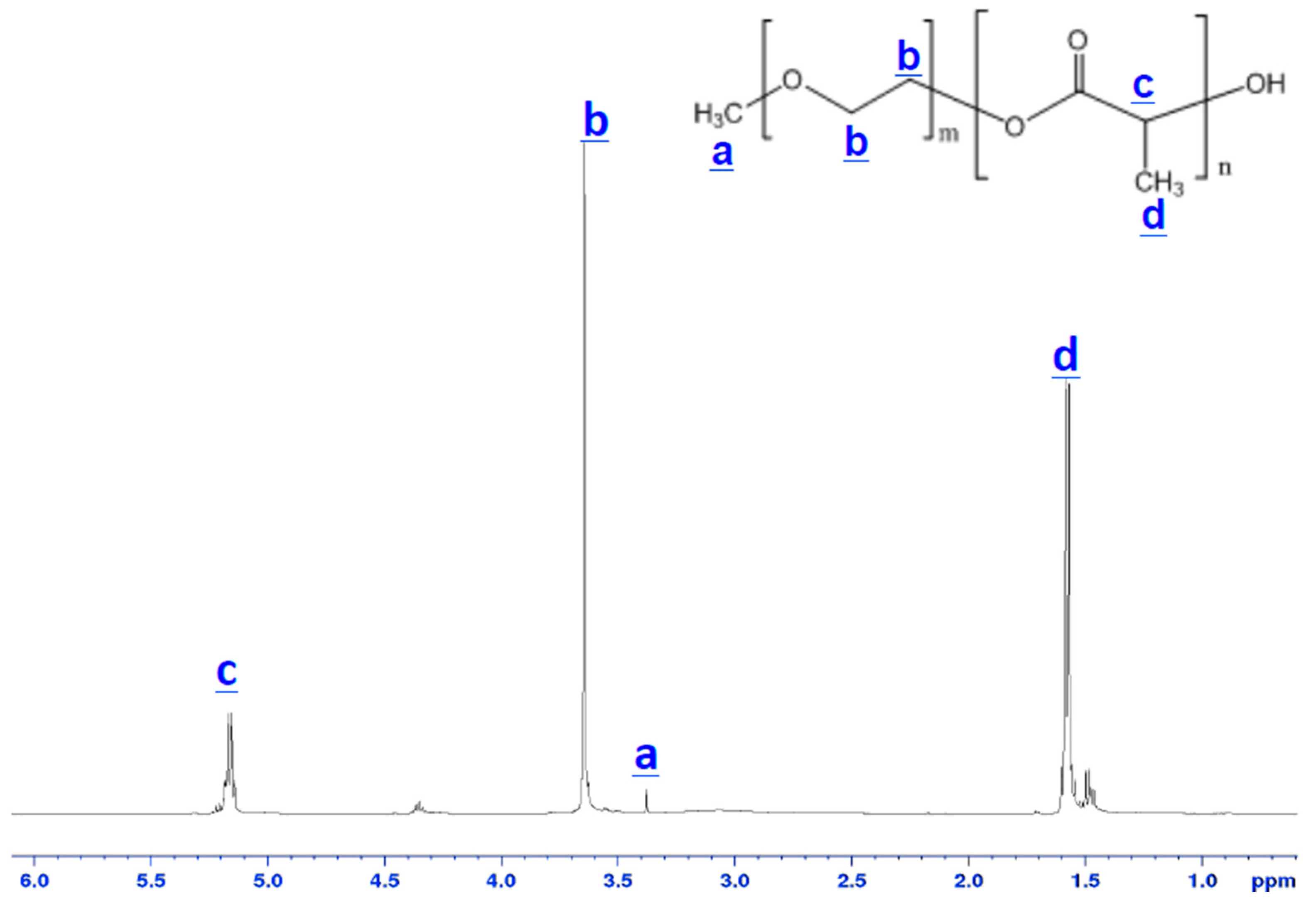
Poly(ethylene glycol)–block–poly(l-lactide) (PEG–b–PLA) was synthesized in house by introducing poly(ethylene glycol) methyl ether (Mn = 2000) and l-lactide (Lactide-(3S)-cis-3,6-Dimethyl-1,4-dioxane-2,5-diene) into toluene distilled over sodium benzyl phenol at 25 °C. The mixture was subsequently placed under vacuum to remove moisture. Tin (II) 2-ethylhexanoate was added as a catalyst during continuous stirring at 100 °C for 5 h. The resulting polymer was precipitated in pentane and air-dried at room temperature overnight. The chemicals used were purchased from Sigma-Aldrich (St. Louis, MO, USA). The number-average molecular weight of the block copolymer was determined by 1H NMR (ADVANCE III 500 MHz, Bruker, Santa Barbara, CA, USA). Samples temperature was regulated for all measurements and was set at 25 °C.
4.2.2. Encapsulation Process
An oil-water-emulsion-evaporation method was carried out for encapsulation of chlorhexidine (CHX). The oil/organic phase consisted of PEG–b–PLA and CHX in a 5:1 ratio, respectively, dissolved in dichloromethane (DCM). The water phase was a 1% w/v solution of poly(vinyl alcohol) and deionized water. The organic and water phases were combined and emulsified using ultra-sonication for 1 min (Branson Ultrasonics Corporation, Bransonic CPX3800H, Danbury, CT, USA). The resulting emulsion was stirred for 2 h at 25 °C at atmospheric pressure to allow the organic solvent to evaporate. Finally, the particles were centrifuged, washed, and freeze-dried.
4.3. Characterization
The starting and final products were characterized using multiple techniques: (1) encapsulation efficiency and (2) microscopy studies of nanoparticle morphology and size.
4.4. Encapsulation Efficiency
Encapsulation efficiency was found using quantitative measurement of mass change from dehydration, decomposition, and oxidation with time and temperature of the initial polymer (PEG–b–PLA), CHX, and CHX-encapsulated nanoparticles. Mass changes, from their physiochemical reactions, were measured using thermogravimetric analysis (TGA, Metler Toledo TGA1, Greifensee, Switzerland). This instrument detects changes in weight that occur in a material with increasing temperature (25 °C to 800 °C). A heating rate of 5 °C per minute was applied with about 5 mg of sample used for each run. The temperatures at which the polymers and CHX characteristic mass dropped were recorded and compared. For the CHX curve, the temperature at which a significant mass loss percentage occurred was recorded (CHXTemp) and used as a baseline temperature. As the percentage loss of CHX at this temperature was determined earlier, the percentage of CHX lost in the nanoparticles could be found as well. This percentage was used to find the amount of CHX in the encapsulated nanoparticles and subsequently used to find encapsulated efficiency of the polymer (Equation (1)). The polymer curve was used to ensure that the polymer mass loss temperature did not overlap with CHXTemp. CHX was detected when characteristic changes in the thermal decomposition temperatures were observed for the CHX-encapsulated polymer in comparison to the starting materials (pure PEG–b–PLA and pure CHX).
4.5. Particle Morphology and Composition
The morphology and size of polymer and encapsulated nanoparticles were observed with scanning electron microscopy (SEM, JEOL JSM-6010LA, Peabody, MA, USA) and atomic force microscopy (AFM, Bruker, Bioscope Catalyst, Santa Barbara, CA, USA). For the SEM analysis, a thin layer of nanoparticles was deposited on a metallic stub. The composition of the materials was measured with energy dispersive X-ray spectroscopy (EDS, JEOL JSM-6010LA, Peabody, MA, USA), which enabled detection of the individual elements of the nanoparticles. For AFM analysis, CHX-encapsulated nanoparticles were dispersed in a hydrogel solution (1% Natrosol™ hydroxyethyl cellulose in water) and sonicated for about 30 h (Branson Ultrasonics Corporation, Bransonic CPX3800H, Danbury, CT, USA) to disrupt clumps. A drop of solution containing nanoparticles was deposited on a glass slide and was allowed to dry forming a thin film. The AFM analysis was performed by using the quantitative nanomechanics method (QNM) for compositional mapping, which, besides determining morphological features, enables quantitative measurement of nanoscale material properties. The nanoparticle’s size and size distribution were also investigated by dynamic light scattering (DLS) technique, using a non-invasive backscatter optics (NIBS) (Zetasizer Nano ZS, Malvern, Worcestershire, UK), after suspending 2 mg of the nanoparticles in 10 mL of deionized water.
4.6. Antimicrobial Effectiveness
The antimicrobial effect of the encapsulated nanoparticles was tested against Enterococcus faecalis OG1RF. CHX-encapsulated nanoparticles were immersed in phosphate buffer saline (PBS) and agitated for set time periods (1 h, 7 days, 14 days and 21 days). Then, the nanoparticles were filtered from the solution using vacuum filtration and air-dried for approximately 24 h. E. faecalis overnight broth culture was spread onto BHI (brain-heart infusion) agar plates and ~1 mg of dried CHX-encapsulated nanoparticles filtered previously from the PBS were placed onto the bacterial lawns in a roughly circular formation to test for zones of growth inhibition (ZOI) around the nanoparticles. The bacterial lawn plates were incubated for 24 h at 37 °C, and the ZOIs were observed. These experiments were performed with nanoparticles that were immersed in a PBS solution for 1 h, 7 days, 14 days and 21 days to investigate the potency of bacterial inhibition and whether any drug burst release was present. Digital calipers, which are measurement tools, were used to determine the diameter of the ZOI and the diameter of a batch of nanoparticles placed on the plates. As the mass, shape, and area of nanoparticles placed on the plate were variable (nanoparticles charge made placement difficult on the plate surface), the relative diameter of an assembled group of nanoparticles was measured and compared to the diameter of the ZOI. Therefore, if the ratio increased, that meant the ZOI decreased, as this would signify that the zone was closer to the nanoparticle mass. Three trials of this test were performed.
CHX-encapsulated nanoparticles were also immersed in BHI broth for varied periods (7 days, 14 days and 21 days). These time periods represent how long the nanoparticles would be active inside dentinal tubules. The nanoparticles were filtered out, and the remaining broth was kept. The broth was then inoculated with E. faecalis at an initial optical density at 600 nm (OD600) of ~0.001. Afterwards, 200 µL of the inoculated broth was aliquoted in triplicate into a 96-well plate and incubated at 37 °C for 24 h. The OD600 was monitored with a Monochromater-based Multi-Mode Microplate Reader (Synergy Mx, Winooksi, VT, USA) every 15 min during the 24-h incubation period. The OD data was used to generate bacterial growth curves made by averaging triplicates from three trials together, not including clear outliers obtained during the experiments. The mean values of intermediate OD readings when the control broth was entering the growth phase were analyzed statistically with one-way ANOVA (analysis of variance) method at a 5% significance level. The OD readings of the broth that had contained the CHX nanoparticles were compared to the control to verify that the broth with CHX nanoparticles was indeed causing a delay in the growth phase.