Abstract
Novel structures of an near-infrared (NIR) tetraarylazadipyrromethene (aza-BODIPY) series have been prepared. We designed the core structure containing two amido groups at the para-position of the aromatic rings. The amido group was incorporated to secure insensitivity to pH and to ensure a bathochromic shift to the NIR region. Forty members of aza-BODIPY compounds were synthesized by substitution of the acetyl group with commercial amines on the alpha bromide. The physicochemical properties and photostability were investigated and the fluorescence emission maxima (745~755 nm) were found to be in the near infrared (NIR) range of fluorescence.
1. Introduction
There is an imperative need for the development of new and effective dyes that emit fluorescence in the near-infrared (NIR) region (i.e., 700~1000 nm) for in vivo biological applications [1,2,3,4]. Fluorophores having absorption/emission profiles in the NIR regions are very useful, due to deep penetration of the NIR light, significant reduction of the background signal, low light scattering, as well as being a cheap illumination light source. So far, several scaffolds have been developed as NIR fluorophores such as squarine, quinone and cyanine. As well as having the most extensively developed NIR dye, our research group has also reported synthesis of novel cyanine libraries and their application for fluorescence and SERS in vivo animal imaging [5,6,7,8,9]. NIR dyes with good photostability have been identified, especially from the cyanine libraries, which would benefit in vivo bioimaging.
Since O’Shea and co-workers reported the interesting structures of NIR aza-BODIPY and studied their spectral properties [10], aza-BODIPY compounds had only been prepared in an ad hoc manner, which limited their potential as NIR imaging probes [11]. The diversity-oriented fluorescence library approach (DOFLA) provides a convenient way to generate a large number of fluorescent libraries [12]. Based on previous studies, we intend to construct a fluorescent library in theNIR region, utilizing the superior photophysical properties of aza-BODIPY compounds. Here, we report the synthesis and physicochemical characterization of novel aza-BODIPY NIR derivatives.
2. Experimental Section
2.1. Materials & Method
All reactions were performed in oven-dried glassware under a positive pressure of nitrogen. Unless otherwise noted, starting materials and solvents were purchased from Aldrich and Acros organics and used without further purification. Analytical TLC was carried out on Merck 60 F254 silica gel plate (0.25 mm layer thickness) and visualization was done with UV light. Column chromatography was performed on Merck 60 silica gel (230–400 mesh). NMR spectra were recorded on a Bruker Avance 300 MHz NMR spectrometer. Chemical shifts are reported as δ in units of parts per million (ppm) and coupling constants are reported as a J value in Hertz (Hz). Masses of all the compounds were determined by LC-MS of Agilent Technologies with an electrospray ionization source. The Phenomenex C18 (50 mm × 4.6 mm, 5 µm) column was used for purity confirmation with 0.1% TFA containing acetonitrile/water gradient (5% to 100% in 10 min) elution conditions. Spectroscopic measurements were performed on a fluorometer and a UV/Vis instrument, Synergy 4 of bioteck company and Gemini XS fluorescence plate reader. The slit width was 1 nm for both excitation and emission. Relative quantum efficiencies were obtained by comparing the areas under the corrected emission spectra. The following equation was used to calculate quantum yield.
where Φst is the reported quantum yield of the standard, I is the integrated emission spectrum, A is the absorbance at the excitation wavelength, and η is the refractive index of the solvents used. The subscript × denotes unknown and st denotes the standard. Tetraphenyl-aza-bodipy was used as standard.
Φx = Φst(Ix/Ist)(Ast/Ax)(ηx2/ηst2)
Photobleaching experiments in DMSO solutions were performed by irradiating the samples with light from a 365 nm high-power 30 mW, UV lamp. The photodegradation profiles were obtained by monitoring the fluorescence spectra.
2.2. Synthesis
Synthesis of Compound 2. To an EtOH solution (100 mL) of the p-Boc-aminoacetophenone (1 g, 4.25 mmol) and benzaldehyde (1 equiv), 4 M NaOH (0.5 mL) was added. The mixture solution was heated at 60 °C for 20 h. After cooling to room temperature, the solvent was removed in vacuo, and the oily residue obtained was neutralized with 1 M HCl and partitioned between EtOAc (250 mL) and H2O (250 mL). The organic layer was separated, dried over sodium sulfate, and evaporated under reduced pressure. The obtained product was purified on silica with EtOAc and Hexane (1.2 g, 87%).
1H NMR (300 MHz, CDCl3) δ 8.01 (d, J = 8.7 Hz, 2H), 7.80 (d, J = 15.6 Hz, 1H), 7.66–7.63 (m, 2H), 7.56–7.39 (m, 6H), 6.77 (s, 1H), 1.53 (s, 9H); 13C NMR (75 MHz, CDCl3) δ 188.8, 152.1, 144.2, 142.7, 135.0, 132.7, 130.4, 130.1, 128.9, 128.4, 121.8, 117.5, 81.3, 28.3; ESI-MS m/z (M + H) calcd: 324.2, found 324.2.
Synthesis of Compound 3. A solution of chalcone (1 g, 3.1 mmol), nitromethane (20 equiv) and KOH (0.2 equiv) in EtOH (100 mL) was heated at 60 °C for 12 h. After cooling to room temperature, the solvent was removed in vacuo, and the residue was partitioned between EtOAc (150 mL) and H2O (150 mL). The organic layer was separated, dried over sodium sulfate, and evaporated under reduced pressure. The obtained product was purified by column chromatography (1.1 g, quantitative).
1H NMR (300 MHz, CDCl3) δ 7.90 (d, J = 8.73 Hz, 2H), 7.48 (d, J = 8.73 Hz, 2H), 7.37–7.30 (m, 5H), 6.79 (s, 1H), 4.87 (dd, J = 6.4, 12.4 Hz, 1H), 4.71 (dd, J = 8.1, 12.4, 1H), 4.27–4.22 (m, 1H), 3.45–3.40 (m, 2H), 1.56 (s, 9H); 13C NMR (75 MHz, CDCl3) δ 195.3, 152.0, 143.3, 139.1, 130.8, 129.5, 128.9, 127.8, 127.4, 117.4, 81.4, 79.5, 41.1, 39.3, 28.2; ESI-MS m/z (M + H) calcd: 385.2, found 385.2.
Synthesis of Compound 4. Compound 3 (1 g, 2.6 mmol), and ammonium acetate (35 equiv) in 1-butanol (150 mL) were heated at 120 °C for 6 h. The reaction was cooled to room temperature, solvent was removed in vacuo and the crude product was purified by column chromatography on silica-gel eluting with 1% MeOH in CH2Cl2 to yield compound 4 as blue-black solid.
1H NMR (300 MHz, DMSO-d6) δ 9.79 (s, 2 H), 8.09 (d, J = 7.23 Hz, 4H), 8.00 (d, J = 8.7 Hz, 4H), 7.72 (d, J = 8.7 Hz, 4H), 7.59 (s, 2H), 7.49–7.38 (m, 6H), 1.53 (s, 18H); ESI-MS m/z (M + H) calcd: 680.3, found 680.2.
Synthesis of Compound 5. Compound 4 (0.3 g, 0.44 mmol) was dissolved in dry CH2Cl2 (50 mL), treated with DIEA (10 equiv) and BF3 diethyletherate (15 equiv), and stirred under nitrogen for 24 h. The reaction mixture was quenched with sat. NaHCO3 solution (10 mL). The organic layer was separated, dried over sodium sulfate, and evaporated under reduced pressure. Purification by column chromatography on silica eluting with 1%~2% of MeOH in CH2Cl2 yielded the product as a dark blue solid.
1H NMR (300 MHz, DMSO-d6) δ 8.16 (d, J = 7.4 Hz, 4H), 8.05 (d, J = 8.7 Hz, 4H), 7.54–7.42 (m, 8H), 6.71 (d, J = 8.8 Hz, 4H), 6.35 (s, 4H); ESI-MS m/z (M + H) calcd: 528.2, found 528.2.
Synthesis of Compound 6. Compound 5 (0.2 g, 0.38 mmol) was dissolved in acetonitrile (100 mL), sat. NaHCO3 solution (1 mL) and bromoacetyl bromide (5 equiv) were added and stirred for 10 min. The solvent was removed in vacuo, and partitioned between EtOAc (250 mL) and saturated NaHCO3 solution (100 mL). The organic layer was separated, dried over sodium sulfate, and evaporated under reduced pressure. The obtained product was used for further synthesis without purification.
1H NMR (300 MHz, DMSO-d6) δ 10.8 (s, 2H), 8.20–8.07 (m, 8H), 7.86–7.78 (m, 4H), 7.64–7.39 (m, 8H), 4.34 (s, 4H); ESI-MS m/z (M + Na) calcd: 791.0, found 790.8.
General synthetic procedure for AZA derivatives; A solution of compound 6 (20 mg, 26 µmol), DIEA (5 equiv) and building block amine (5 equiv) was stirred for 5 h at room temperature. The solvent of the reaction mixture was removed in vacuo, and the residue was partitioned between EtOAc (5 mL) and H2O (5 mL). The separated organic layer was washed with saturated citric acid solution and sat. NaHCO3 solution. The crude product was purified by preparative thin layer column chromatography on silica eluting with 10% MeOH in CH2Cl2 to yield the final compounds (see supporting information; Table S1 for the summary of properties and Figure S1 for the representative LC-MS data).
Characteristics of AZA396; AZA396 was synthesized according to the general synthetic procedure. 1H-NMR (DMSO-d6) δ 8.16 (m, 8H), 7.86 (m, 5H, ), 7.54 (m, 9H), 7.32 (d, J = 7.9, 5H), 7.17 (d, J = 7.9, 5H), 3.86 (s, 4H), 2.50 (s, 4H), 2.29 (s, 6H); 13C-NMR (DMSO-d6) δ 168.95, 168.90, 136.60, 134.43, 129.02, 128.91, 128.74, 128.62, 51.46, 20.73; ESI-MS m/z (M + H) calcd: 850.4, found 850.4.
3. Results and Discussion
3.1. Synthesis
The tetraphenyl-substituted aza-BODIPY (TPAB) chromophore has maximum fluorescence emission at 695 nm which is slightly shorter than the NIR region. One of the reported NIR aza-BODIPY scaffolds is O’Shea’s amine containing structure. It has a large bathochromic shift by conjugation of n→π* of the lone pair of electrons of the amine, however this amine also affects both the quantum yield and the pH sensitive properties. In order to develop a new NIR aza-BODIPY having both low pH sensitivity and good physicochemical properties, we designed an amido substituted aza-BODIPY (Figure 1). The n→π* conjugation can be partially removed by the resonance effect of the amide bond and the moderate hypsochromic shift can be achieved from O’Shea’s structure. The synthesis of aza-BODIPY started from the diaryl α,β-unsaturated ketone (chalcone, 2), which was prepared by the Claisen–Schmidt condensation using benzaldehyde and Boc protected 4′-aminoacetophenone (1) with NaOH as a base. Michael addition of nitromethane to the chalcone yielded the 1,3-diaryl-4-nitrobutan-1-one (3) with diethylamine or KOH as base in almost quantitative yield after aqueous workup and this intermediate was then used for the next step without further purification. Condensation with ammonium acetate in refluxing butanol rendered azadipyrromethene (4) via a cascade event where pyrrole and the corresponding nitrosopyrrole were formed in situ following subsequent condensation. The obtained mixture was purified by chromatography with hexane/dichloromethane. Subsequent complexation of the azadipyrromethene with boron trifluoride gave the aza-BODIPY (5) in good yield. Simultaneously, Boc deprotection was completed by excess BF3 etherate. The key intermediate 6 was prepared by bromoacetylation. As a final step, 40 aza-BODIPY compounds (named as AZA) were synthesized by substitution of the bromide with commercial amines at room temperature (Scheme 1).
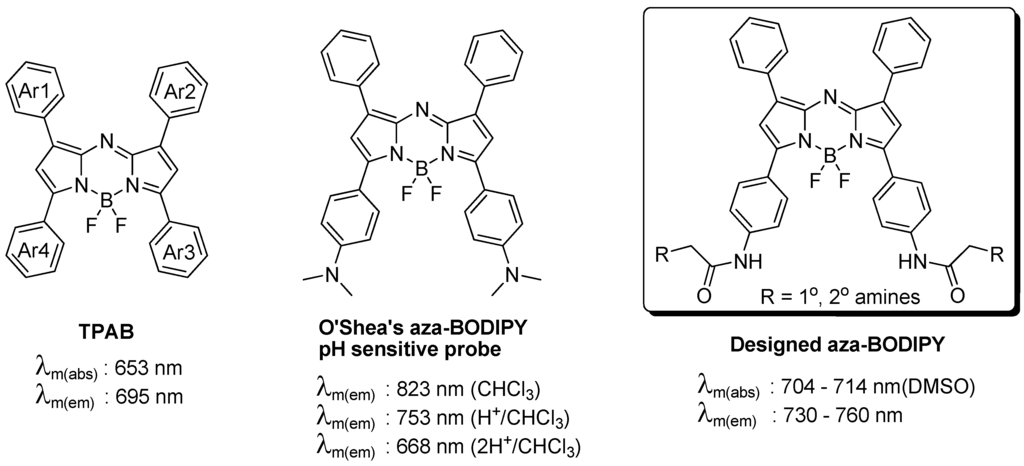
Figure 1.
Tetraarylazadipyrromethene (aza-BODIPY) structures and the new designed scaffold.
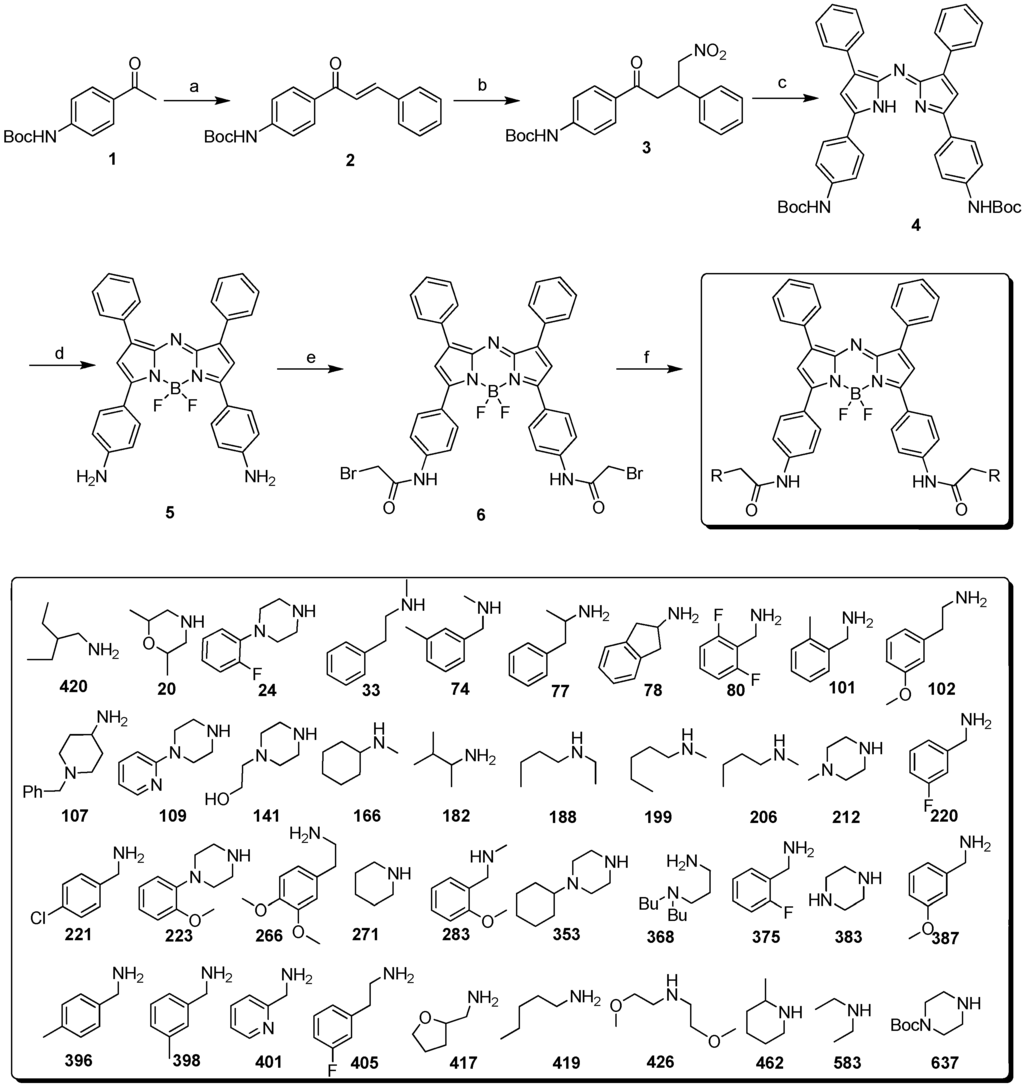
Scheme 1.
(a) Benzaldehyde, KOH; EtOH, 60 °C (b) nitromethane, diethylamine or KOH, EtOH, 60 °C; (c) ammonium acetate, n-BuOH, reflux (d) BF3-etherate, DIEA; in DCM (e) BrAcCl, acetonitrile, saturated aqueous NaHCO3 (f) 1° and 2° amines, DIEA, DMF, RT.
An interesting result was observed during the optimization of Scheme 1. Without the protection of the amino group in compound 1 or with other protective groups such as triphenylmethyl, each intermediate step could be prepared successfully, except for the BF3 complexed product (Scheme 2). This may be explained by steric interference of the trityl group [13]. However, the steric interaction cannot explain the case of free amino and Boc-amino-azadipyrromethene. Presumably, Boc is coordinated by boron as an unstable cyclic intermediate and this observation can be supported by the fact that when step d in Scheme 1 was quenched by methanol, a methylcarbamate product was obtained on one side of the amine (Scheme 2).
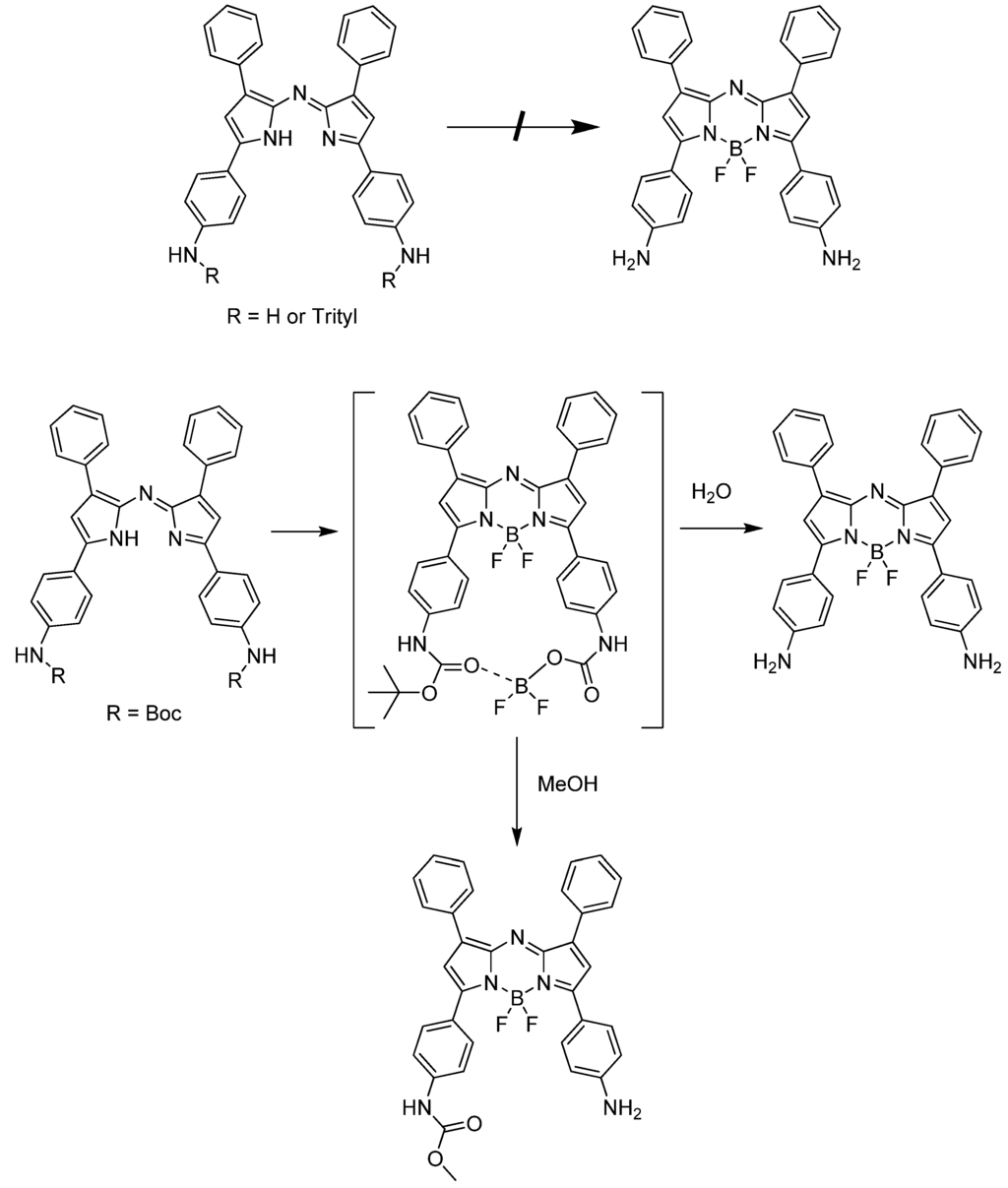
Scheme 2.
Modified test route for the preparation of aza-BODIPY.
3.2. Spectroscopic Properties
The new AZA compounds were dissolved in dimethylsufoxide, and their spectroscopic properties were studied. The wavelengths of the absorption maxima are observed in the range of 704 to 714 nm, while the fluorescence emission maxima are in the range of 745 to 755 nm. The fluorescence quantum yields (QYs) of the dyes are also distributed in a similar range (0.1~0.3) (Figure 2).
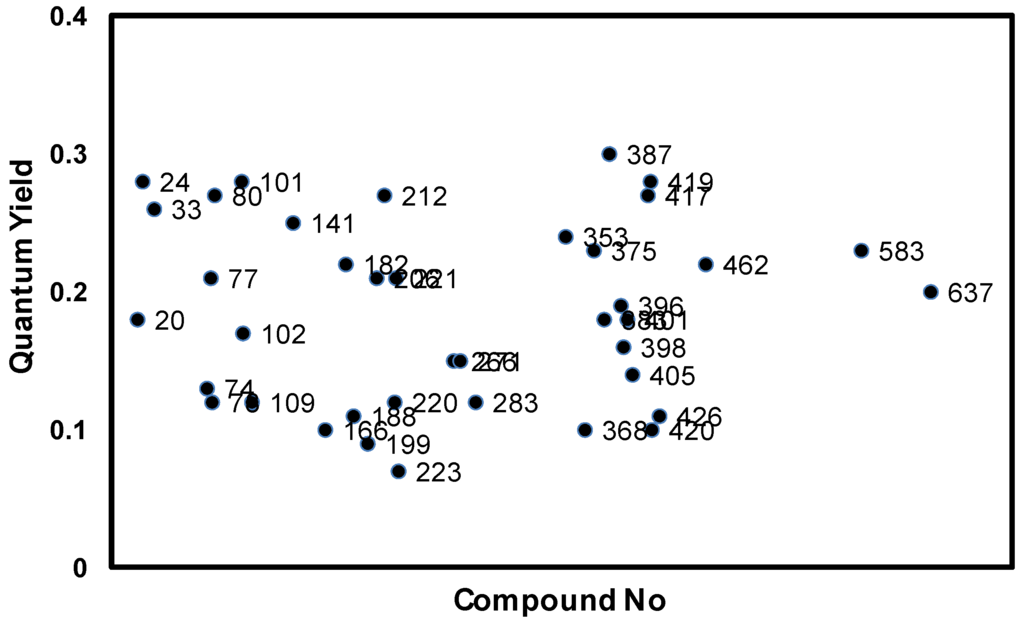
Figure 2.
Quantum yield distribution of AZA compounds; quantum yields (QYs) are measured in 10 μM DMSO solution and calculated using tetraphenyl-substituted aza-BODIPY (TPAB) as reference. Numbers correspond to the amine numbers in Scheme 1.
We picked out one representative compound named AZA396 (λabs: 707 nm; λflu: 754 nm; Quantum yield: 0.19; ε: 909925 M−1 cm−1) and studied its properties further (Figure 3).
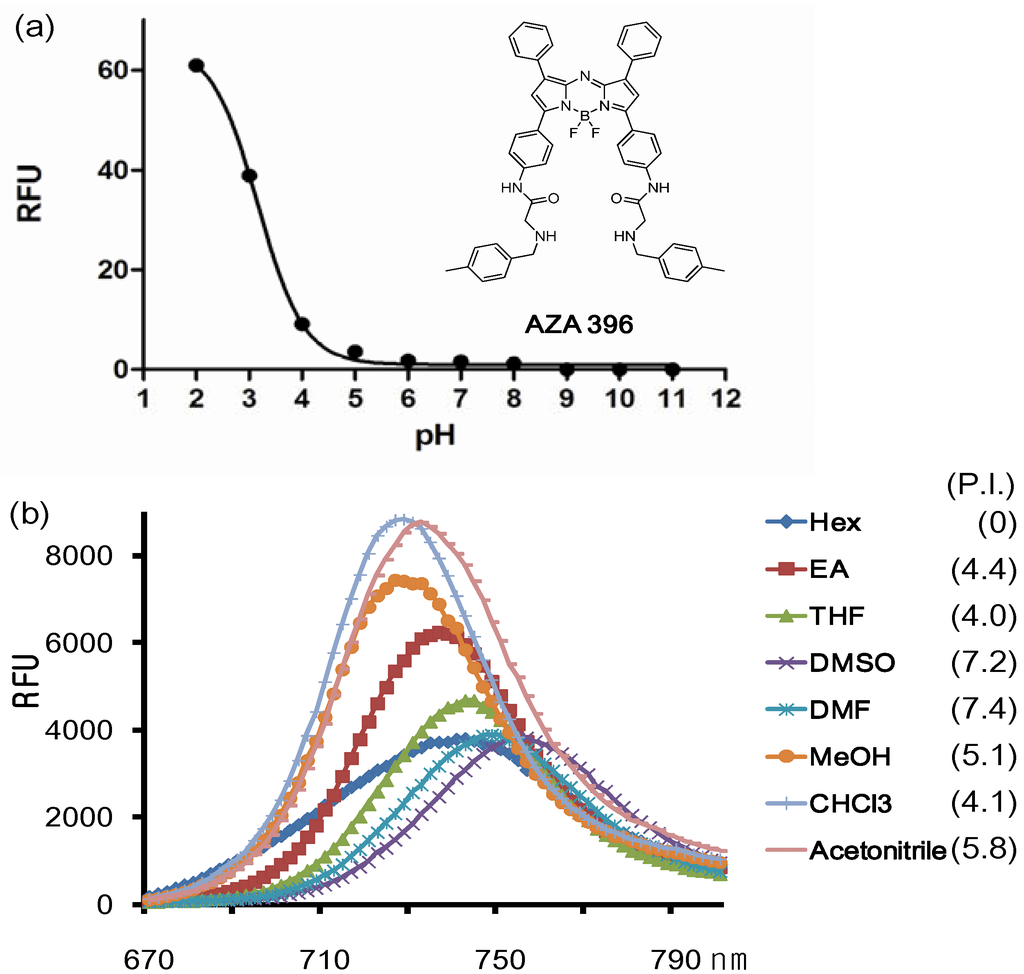
Figure 3.
(a) Fluorescence changes of the representative compound AZA396 with pH and (b) solvents (numbers are polarity index values).
The conjugation of the Ar3 and Ar4 rings of the aza-BODIPY chromophore with the amido group renders a dramatic bathochromic shift which is located in the middle of the values of TPAB and O’Shea’s aza-BODIPY shown in Figure 1. The emission maximum of the probe was affected by solvent and polarity. The maximum emission peaks were distributed in a small range of 25 nm and also the full width at half the maximum peak height had almost the same value (40 nm). Interestingly, the intensity was slightly different depending on solvent but there was no clear connection between intensity and solvent properties except for the dramatic difference between organic solvent and in aqueous media (based on comparison between Figure 3a and Figure 3b; F(organic solvents)/F(aqueous media) > 1000). In aqueous solution, due to the high lipophilicity, AZA396 exhibited almost no emission intensity at high pH, but its fluorescent intensity was slightly turned on at low pH (pH = 2 ~ 4) without a bathochromic shift. Presumably, the photoinduced electron transfer (PET) effect of the amido group did not change at low pH and dye disaggregation was induced by protonation of the secondary amines.
3.3. Photostability
We further investigated the photostability of the dyes in dimethylsulfoxide under continuous illumination with a high power UV lamp at 365 nm. The photodegradation profiles for 10 representative dyes are shown in Figure 4. Photodegradation was measured by the difference in fluorescence intensity before and after 80 min irradiation. In fact, after 80 min of illumination, most of the dyes were still emitting significant fluorescence emission. Average degradation percentage was 32% (lowest 5% and highest 56%). The most photostable compound, AZA396 was further compared with two control compounds (i.e., TPAB and BodipyFl as positive and negative references) by measuring their photodegradation at 10 min intervals (Figure 5).
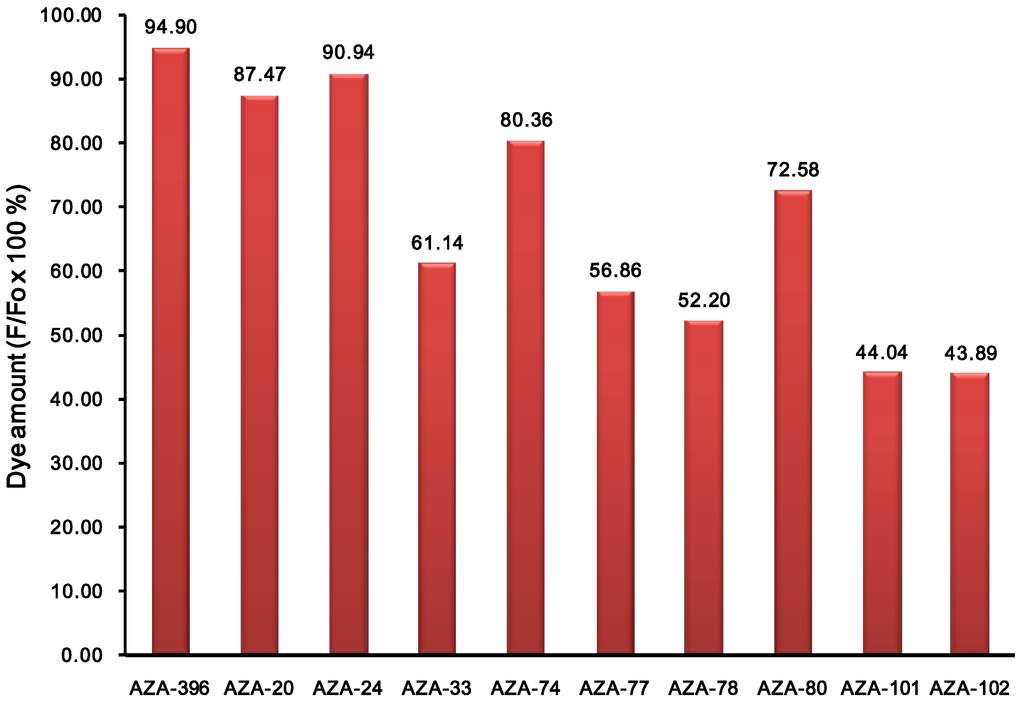
Figure 4.
Photostability test of representative compounds in DMSO solution (at 50 μM). Photostability was measured by fluorescence intensity (F) retention at 80 min under irradiation of UV with a 30 mW lamp at 365 nm. Fo is the initial fluorescent intensity. Compound ID numbers correspond to the building block amines.
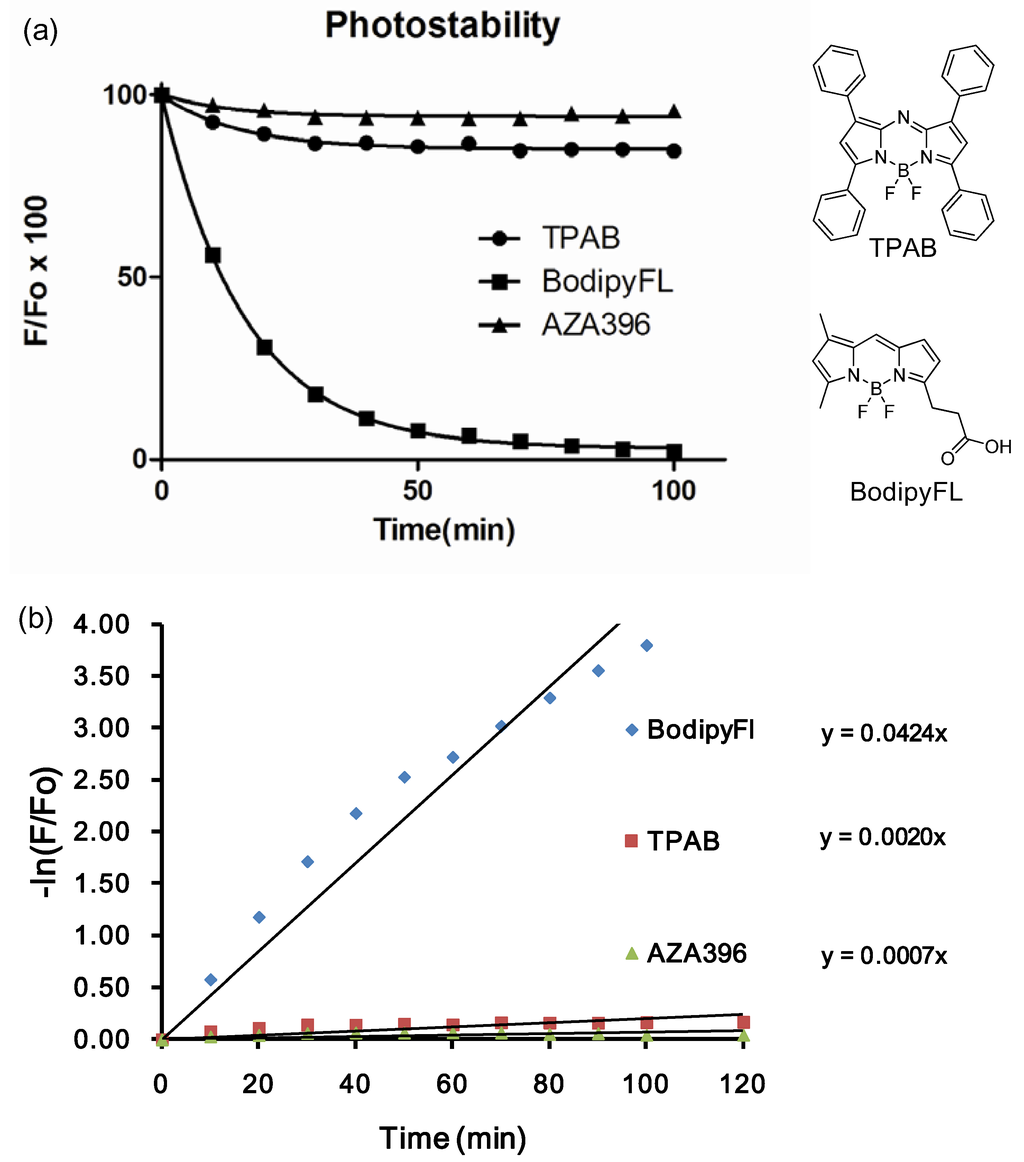
Figure 5.
(a) Photostability test of AZA396 compare with standards compounds tetraphenyl aza-bodipy (TPAB) and BodipyFl acid in DMSO; (b) Values are fitted to a non-linear regression one-phase exponential decay. Rate constants of dye photodecomposition were acquired from the plots of −ln(F/F0) vs. time, considering ln(F/F0) = k*t as a pseudo-first order rate equation.
To compare the degradation rate between AZA396 and control compounds, values were fitted to a non-linear regression. About 60-fold rate constant differences between BodipyFl and AZA396 reflected the superior photostability of the AZA compound making, it particularly suitable for long-duration measurements (Figure 5b).
4. Conclusions
In summary, novel structures of an aza-BODIPY series have been prepared and developed as NIR imaging probes. The photophysical properties of the new aza-BODIPY derivatives were evaluated and showed high photostability and acceptable fluorescence quantum yields.
Specifically, AZA396 can be developed with the potential to be exploited and adapted to suit a diverse range of NIR bio-imaging applications.
Supplementary Materials
Supplementary File 1Acknowledgments
This study was supported by intramural funding from A*STAR (Agency for Science, Technology and Research, Singapore) Biomedical Research Council and a Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2010-T2-1-025).
References
- Stephens, D.J.; Allan, V.J. Light microscopy techniques for live cell imaging. Science 2003, 300, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Lippincott-Schwartz, J.; Patterson, G.H. Development and use of fluorescent protein markers in living cells. Science 2003, 300, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Rieder, C.L.; Khodjakov, A. Mitosis through the microscope: Advances in seeing inside live dividing cells. Science 2003, 300, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Samanta, A.; Ha, H.H.; Chang, Y.T. Solid phase synthesis of ultra-photostable cyanine NIR dye. RSC Adv. 2011, 1, 573–575. [Google Scholar] [CrossRef]
- Maiti, K.K.; Samanta, A.; Vendrell, M.; Soh, K.S.; Olivo, M.; Chang, Y.T. Multiplex cancer cell detection by SERS nanotags with cyanine and triphenylmethine Raman reporters. Chem. Commun. 2011, 47, 3514–3516. [Google Scholar] [CrossRef]
- Samanta, A.; Vendrell, M.; Das, R.; Chang, Y.T. Development of photostable near-IR cyanine dyes. Chem. Commun. 2010, 46, 7406–7408. [Google Scholar] [CrossRef]
- Samanta, A.; Vendrell, M.; Yun, S.W.; Guan, Z.; Xu, Q.H.; Chang, Y.T. A photostable near-infrared protein labeling dye for in vivo imaging. Chem. Asian J. 2011, 6, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Maiti, K.K.; Soh, K.S.; Liao, X.; Vendrell, M.; Dinish, U.S.; Yun, S.W.; Bhuvaneswari, R.; Kim, H.; Rautela, S.; et al. Ultrasensitive near-infrared Raman reporters for SERS-based in vivo cancer detection. Angew. Chem. Int. Ed. 2011, 50, 6089–6092. [Google Scholar] [CrossRef]
- McDonnell, S.O.; O’Shea, D.F. Near-infrared sensing properties of dimethlyamino-substituted BF2-azadipyrromethenes. Org. Lett. 2006, 8, 3493–3496. [Google Scholar] [CrossRef] [PubMed]
- Jokic, T.; Borisov, S.M.; Saf, R.; Nielsen, D.A.; Kuhl, M.; Klimant, I. Highly photostable near-infrared fluorescent pH indicators and sensors based on BF2-chelated tetraarylazadipyrromethene dyes. Anal. Chem. 2012, 84, 6723–6730. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Vendrell, M.; Chang, Y.T. Diversity-oriented optical imaging probe development. Curr. Opin. Chem. Biol. 2011, 15, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Bandichhor, R.; Wu, L.; Burgess, K. Functionalized BF2 Chelated Azadipyrromethene Dyes. Tetrahedron 2008, 64, 3642–3654. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).