Abstract
The application of dense gases in particle formation processes has attracted great attention due to documented advantages over conventional technologies. In particular, the use of dense CO2 in the process has been subject of many works and explored in a variety of different techniques. This article presents a review of the current available techniques in use in particle formation processes, focusing exclusively on those employing dense CO2 as a solute, co-solute or co-solvent during the process, such as PGSS (Particles from gas-saturated solutions®), CPF (Concentrated Powder Form®), CPCSP (Continuous Powder Coating Spraying Process), CAN-BD (Carbon dioxide Assisted Nebulization with a Bubble Dryer®), SEA (Supercritical Enhanced Atomization), SAA (Supercritical Fluid-Assisted Atomization), PGSS-Drying and DELOS (Depressurization of an Expanded Liquid Organic Solution). Special emphasis is given to modifications introduced in the different techniques, as well as the limitations that have been overcome.
1. Dense CO2 in Particle Formation Processes
Particle formation processes using dense gases have emerged within the last two decades as a promising alternative technology to overcome some technical problems and limitations related to the use of conventional methodologies [1,2,3,4,5,6,7]. The most used classical processes such as jet and ball milling, spray-drying, and recrystallization using solvent evaporation or liquid anti-solvent, do comprise several drawbacks like the presence of high shear forces, high temperatures, electrostatic charges and also the contamination of the final product with undesirable organic solvents [8,9]. Dense gas techniques can overcome most of these disadvantages by exploring the “unique” properties of fluids in the vicinity of the critical point. These properties include liquid-like densities, gas-like transport properties and an unusual high compressibility which allows adjustment of the solvent power of the fluid with minor changes in pressure and temperature [8,9,10,11].
Particularly in the case of dense CO2 (the most widely used dense gas) processes can be carried out at mild temperatures, due to CO2 low critical temperature, avoiding thermal degradation of labile compounds. Furthermore, the benign properties of CO2 (non-flammability and relatively low toxicity) and its ready separation from the products make CO2 the elected solvent for processing products for human consumption, which has generated a special interest from the pharmaceutical and food sectors, the main top target industries of this particle formation technology [10].
Depending on the technique, dense CO2 can totally or partially replace the use of harmful organic solvents, which is often highlighted as an important strategy within green chemistry and to enable new, clean technologies [12]. Several green chemistry principles are in fact satisfied, namely in what concerns pollution prevention, lower toxicity and the use of an abundantly available resource [13]. However, as it was pointed out by Beckman in 2004 [10], it is essential to assure that the use of CO2 can originate a product with superior characteristics providing a performance rather than just an environmental advantage, making of this technology an effective alternative to well established industrial processes. In this context, numerous scientific works have been published and the suitability of dense CO2 has been demonstrated both for the precipitation of pure compounds and composites, showing improved performances mainly in terms of reduction of particle size and distribution, as well as in terms of morphology control [14,15,16,17,18,19,20].
CO2 precipitation processes can be divided in two major groups, the first including operations that are driven by the solvent strength of CO2, where CO2 can act as a solvent as in Rapid Expansion from Saturated Solutions (RESS) or as an anti-solvent as in the Supercritical Anti-Solvent process (SAS). Briefly, in the RESS process, the solid substance to be micronized is dissolved in compressed CO2 and then rapidly depressurized through a nozzle with consequent precipitation of the substance due to the large experimented decrease of CO2 solvent power. In the SAS process, the substance of interest is dissolved in a classical solvent and precipitates when contacted with dense CO2 as a result of the supersaturation attained due to the large solubility of CO2 in most organic solvents. For the SAS process, different methodologies based on different mixing models between solution and SCF were subsequently developed as Gaseous Anti-solvent (GAS), Aerosol Solvent Extraction (ASES), and Solution Enhanced Dispersion by Supercritical Fluids (SEDS) [14].
The second group comprises all the operations that do not depend on CO2 solvent power but instead take advantage of the great volume expansion and the large cooling effect produced when CO2 is depressurized from operating conditions to ambient pressure as in Particles from Gas Saturated Solutions (PGSS) and subsequent developed processes, CPF (Concentrated Powder Form), CAN-BD (Carbon dioxide Assisted Nebulization with a Bubble Dryer®), SEA (Supercritical Enhanced Atomization), SAA (Supercritical Fluid-Assisted Atomization), PGSS drying and DELOS (Depressurization of an Expanded Liquid Organic Solution).
This review will be focused exclusively in this second group in which CO2 can be used as solute, co-solute or co-solvent.
2. The Particles from Gas Saturated Solution (PGSS) Technique
The PGSS technique was patented [21] by Weidner and co-workers in 1994 and presented [22] in the Third International Symposium on Supercritical Fluids in Strasbourg in the same year. It is considered one of the most attractive CO2 based micronization processes because it does not rely on the solvent strength of CO2, it employs relatively low operating pressures and can totally eliminate the need for organic solvents [9]. A schematic diagram of a typical PGSS process is presented in Figure 1. The process consists in dissolving the compressed gas into the molten material in a stirred high pressure reactor until saturation is reached. The gas-saturated solution formed which can typically contain between 5–50 wt % of the compressed gas is then expanded through a nozzle and solid particles are formed due to the extremely rapidly temperature decrease caused by the fluid expansion that is commonly known as the Joule-Thomson effect [20].
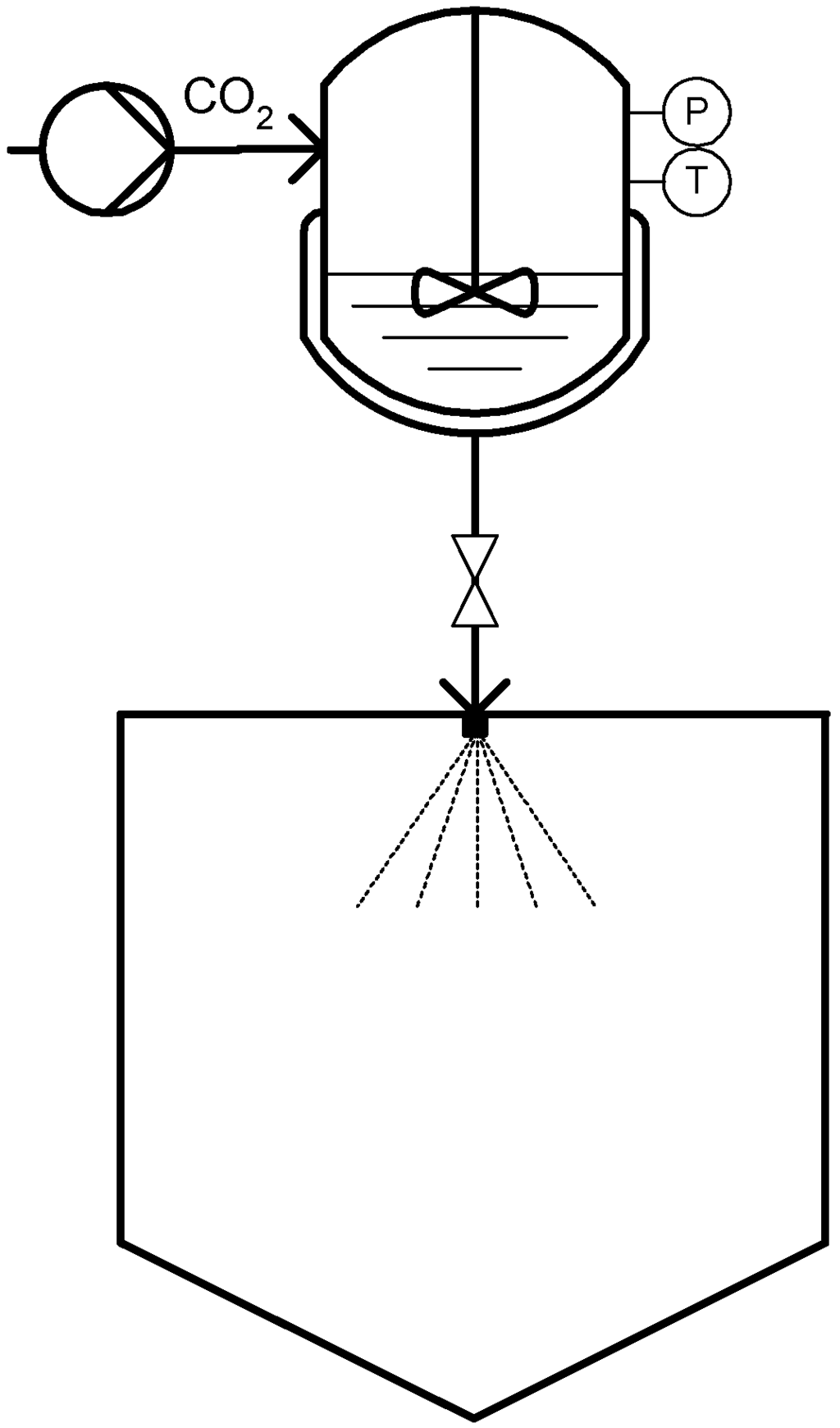
Figure 1.
Particles from Gas Saturated Solution (PGSS) technique.
The PGSS process can also be operated in a continuous mode in which the solute of interest is fed in the molten state via a pump and at the same time pressurized CO2 is introduced into that pipe. Intensive mixing between the two streams is achieved in a static mixer. After the mixing zone, the mixture is expanded through a nozzle [20,23]. A schematic diagram of a PGSS process in continuous mode is presented in Figure 2.
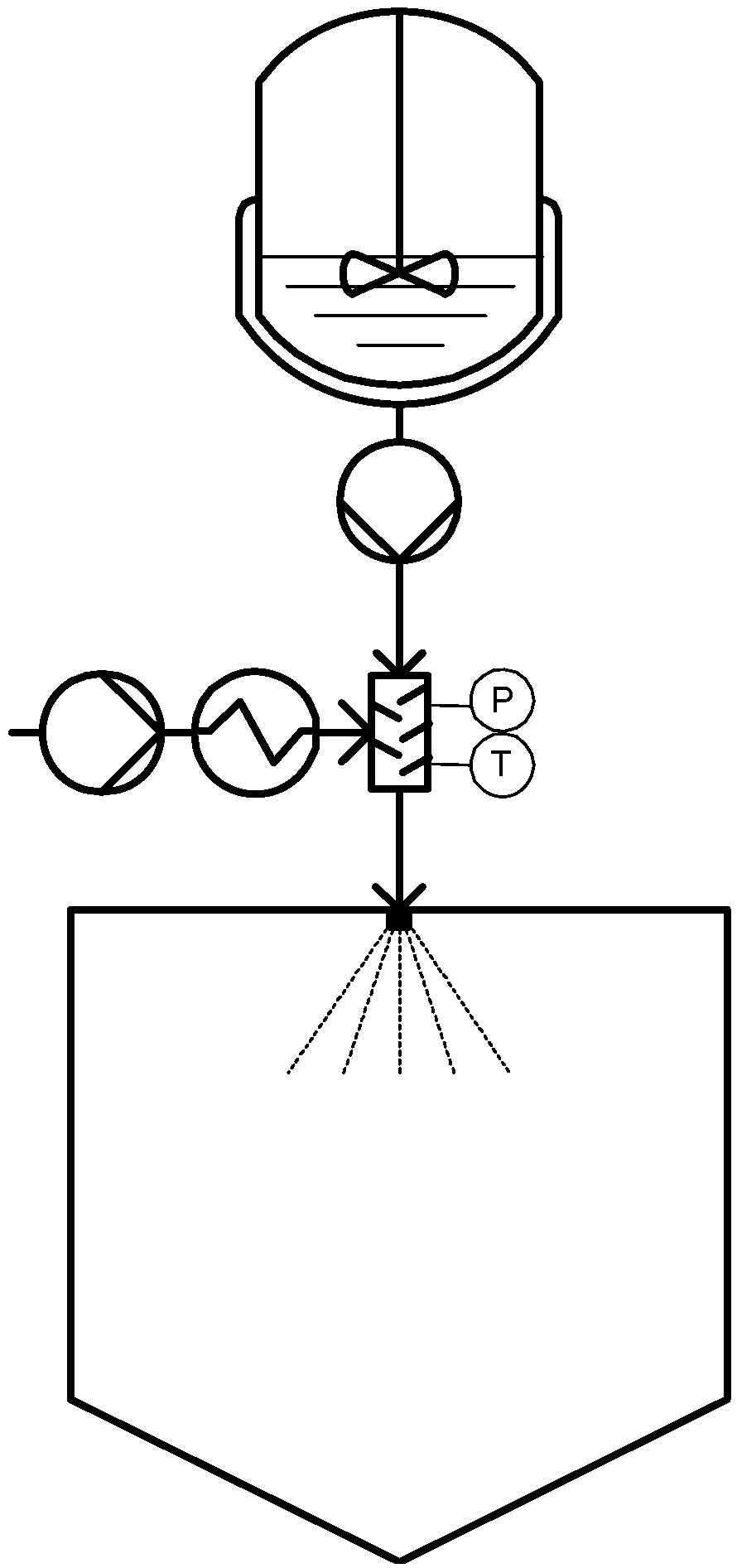
Figure 2.
Continuous PGSS technique.
This process is especially suitable for processing polymers and lipids in which CO2 has a large solubility. Moreover, since it has a melting point depression effect, substances can be sprayed, which, under classical conditions can hardly be sprayed or even not be sprayed at all [20]. The extent of melting point depression experimented by each substance depends on the amount of CO2 that solubilizes into the substance and is caused by molecular interactions between dissolved CO2 and the substance of interest [6]. Determination of solid-liquid transitions in pressurized systems is essentially as it gives information on the pressure needed to melt the substance to be micronized and form a liquid phase at a given temperature [24,25].
The first PGSS reported application was for the generation of powders from Polyethylene glycols (PEGs) [26]. PEG is a widely used hydrophilic polymer due to its biocompatibility and non-toxicity; it is used as a carrier material in the development of pharmaceutical and cosmetic formulations and was used by Weidner and co-workers to improve understanding on how process parameters influence final product properties. For this purpose, dependencies of particle size distribution, morphology and bulk density on process parameters like pre-expansion pressure, pre-expansion temperature and gas to product ratio (GTP) were studied [20]. The authors found out that smaller particles are formed with increasing pressure and GTP ratios and that, for higher GTP ratios, the pressure influence is less pronounced. Particle morphologies are strongly influenced by pre-expansion temperature and can actually be tailor made in a range between 3 to 500 µm with bulk densities from about 90 kg/m3 up to 600 kg/m3, by applying different operating conditions [19,27]. The technique concept has already proven it feasibility even at the economical level (often considered as the major obstacle to SCF industrial application) and reached the industrial scale, which is a big advantage over other technologies that are still under development [20]. Nevertheless, some fundamental issues still require further research in order to build theoretical models, for example, for the mixing process under pressure, the spray generation in the nozzle and the solidification kinetics of the substance [20].
Main limitations of the PGSS process is that the solute has to be melted, which can be problematic for heat sensitive materials [1,8]. In order to overcome this limitation, the technique has also been applied to process suspensions of active substances in low melting polymers or other carriers to produce composite particles mainly containing bioactive compounds [18,28,29,30] and also for coating applications [31,32,33,34,35,36]. Different strategies can be used to improve the process performance depending on the difficulties experimented and that are in most of the cases inherent to the systems under investigation. Hao et al. [37] reported the use of a nitrogen back pressure to suppress the loss of CO2 from the PDLLA/CO2 liquefied mixture and in that way slow down the rate of polymer solidification to achieve the production of fine particles. The authors have also cooled the collection chamber with liquid nitrogen to prevent aggregation of the newly formed microparticles. In addition, Salmasso et al. [38,39] described a variation of the PGSS technique that was reported by the authors as the Gas Assisted Melting Atomization (GAMA) process, in which the introduction of a co-axial air injection device in the typical PGSS precipitation vessel facilitated the yield of insulin-loaded solid lipid submicron particles and avoided agglomeration.
3. Role of Dense CO2 in PGSS Related Techniques
3.1. Solute: CPF and CPCSP
After Weidner patented the PGSS technique, several modifications were introduced in the process by the former and by other authors in order to extend its applications and/or to overcome the main process limitations.
In 1997, CPF® (Concentrated Powder Form) was proposed by Weidner [40,41] which allows for the generation of powders containing an unusually high content of liquids. In the CPF process, a liquid substance is contacted with the dense gas and depressurized through a nozzle. Instead of solid particles, a dispersed spray of fine droplets is formed during the expansion step of the gas-saturated liquid. A powdered carrier material is then blown into that spray by means of an inert gas, which binds the droplets, so that a free flowing powder that can contain 90 wt % (or more) of liquid is formed [20]. Figure 3 represents a schematic diagram of a CPF process.
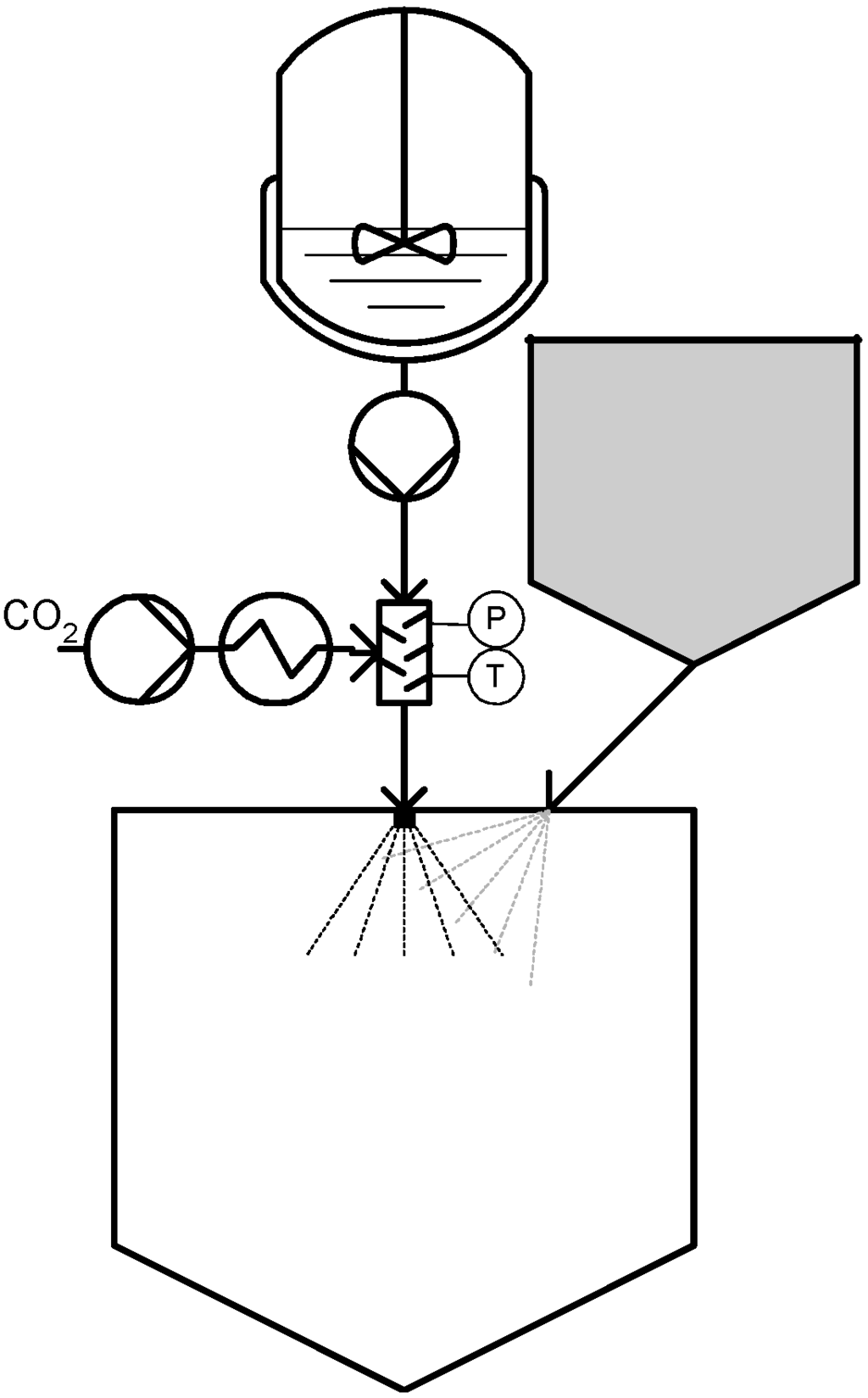
Figure 3.
Concentrated Powder Form (CPF®).
It should be noted that, unlike original patented PGSS, precipitation does not occur at any point of the process, instead, particles are formed either by infiltration of droplets into porous materials or by agglomeration of non-porous material, depending on the carrier used. This process has been applied to more than 100 liquids and 60 different solid carriers and is already being performed at an industrial scale with a capacity of 300 kg/h of powder production. According to Weidner, powders produced on this scale are mainly liquid extracts of essential oils which are converted by the CPF® process into an easily dosable powder with standardized quality and a long shelf life [20].
Another PGSS-derived process was proposed by Weidner in 1999 as an alternative technique for the manufacture of powder coatings [42]. The conventional technique involves high temperature stress of the coating system composed by two polymers, the binder and the hardener, as well as long residences times which may cause a premature reaction that would destroy the product [43]. In the Continuous Powder Coating Spraying Process (CPCSP), the binder and hardener are melted in separate vessels to avoid premature reaction of the polymers as illustrated in Figure 4. Both melted components are then admitted to a static mixer and are homogenized with compressed CO2. Due to dissolved carbon dioxide, the melting point of the mixture decreases and thus, it is possible to perform homogenization at very low temperatures and using very short residence times and in this manner avoid reaction. The solution formed in the mixer is expanded afterwards via a nozzle into a spray tower. Due to CO2 volume expansion and consequent drastic temperature decrease, a fine powder coating is formed. With a blower, the gas is removed from the spray tower and by means of a cyclone and a filter the fine particles are separated from the gas [43].
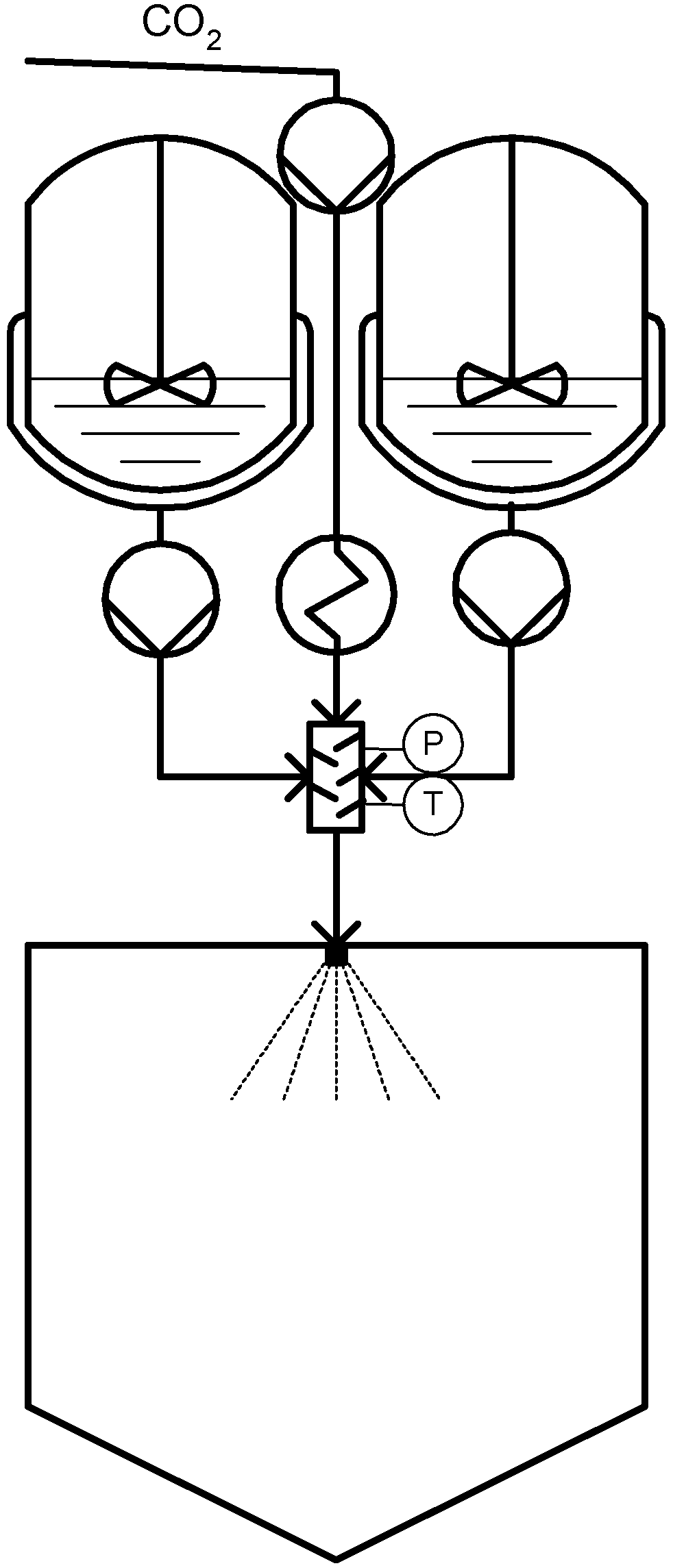
Figure 4.
Continuous Powder Coating Spraying Process (CPCSP).
Weidner et al. [43] have effectively applied this technology to low melting polyester powder coatings with an average particle size of less than 40 μm, which is not possible to achieve by manufacturing the coating powders using a conventional process.
3.2. Co-Solute (and Propellant): CAN-BD®, SAA, SEA and PGSS-Drying
Sievers et al. [44,45] proposed a modification to the PGSS technique which allowed expanding the process application to the use of any compound that is water soluble, greatly increasing the range of substances that can be processed. This patented process, known as Carbon Dioxide Assisted Nebulization with a Bubble Dryer (CAN-BD)®, involves the mixing of a liquid stream (generally aqueous although it can also be organic) containing the drug and any excipients or stabilizers (typically 1 to 10% of total solids dissolved) and a stream of a compressed CO2 to generate a gas-liquid emulsion or solution. This solution is then decompressed through a flow restrictor forming a primarily aerosol of microbubbles and microdroplets which are further break by the expansion of dissolved CO2 in the liquid. Because CO2 is one of the most soluble gases in water (1.6 mole % at 63 °C and 100 bar), its use enhances the expansion process [46,47,48]. This aerosol is introduced into a classical spray tower to be dried by means of a conventional drying gas (air or nitrogen).
This process generates particles less than 5 μm in diameter by rapidly drying of aerosolized solutions at relatively low temperatures (typically between 5 °C and 65 °C) [48]. It is claimed that the use of CO2 facilitates the formation of extremely fine droplets that dry faster than aerosol formed with conventional methods. In this way the temperature required to dry the nebulized solutions are lower than those required in the spray-drying technique thus making this technique more suitable for processing thermally labile compounds [9].
Different variants for contacting the liquid solution with CO2 are described by the inventors. The most cited variation, schematically presented in Figure 5, is the dynamic approach in which the biphasic mixture of the compressed gas and the aqueous solution is generated in a low-dead-volume (<1 µL) mixing tee. Alternatively, a static approach is described in which the compressed gas is introduced in a vessel containing an aqueous solution and mixed. The mixture is then decompressed through the restrictor [14,47].
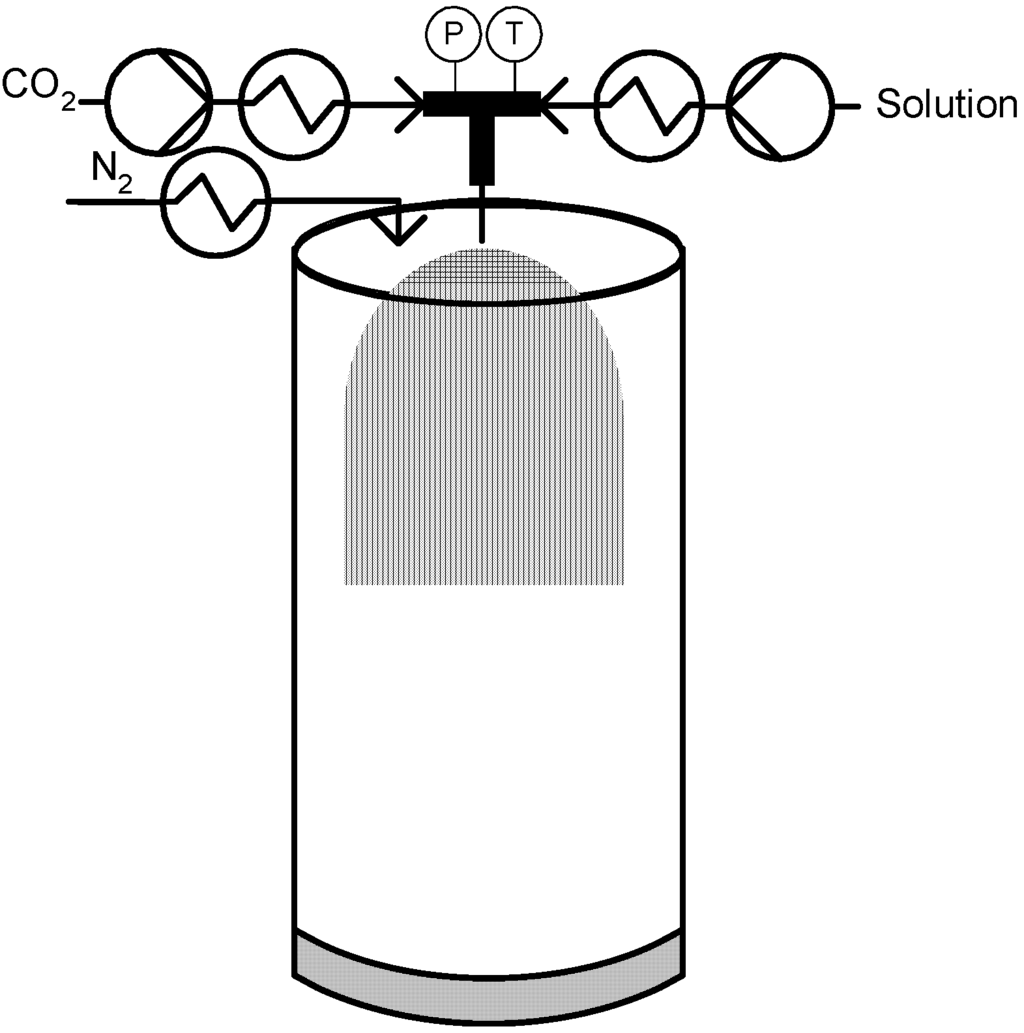
Figure 5.
Carbon Dioxide Assisted Nebulization with a Bubble Dryer (CAN-BD)®.
This technique seems to have a lot of advantages since it is very simple and can be applied to a large range of substances, namely proteins and pharmaceuticals. Applications of this technique were extensively revised by Perrut in 2001 [14], by Cor Peters in 2003 [15] and by Sievers in 2008 [48]. Since original patent in 1997, Sievers and co-workers have proposed other applications and also some modifications in order to make CAN-BD process more versatile. The first extended application was the use of this technique in the production of powders of drug alcoholic solutions [49,50]. An additional modification was later proposed which uses a low-volume mixing cross instead of a tee that allowed mixing two liquid solutions (a water-based and an organic solvent-based solution) with scCO2 in order to generate composite particles [51]. More recently this technique has been applied by Sievers to the micronization of vaccines [52,53].
In 2002, Reverchon and co-workers introduced some modifications to the process developed by Sievers, particularly to the dynamic process, which allow improving the efficiency of mixing between CO2 and the liquid solution [54]. As mentioned by several authors, in the dynamic variant of the CAN-BD process, it is not clear to which extent fluid dissolution occurs and if saturation is in fact achieved due to the extremely short contact time in the tee [5,14,55]. Sievers showed by the acidity of the droplets formed, that at least some dissolution occurs and stated that the dispersion is not at all as efficient when CO2 is substituted by nitrogen or air at similar pressure [14].
In this context, Reverchon [56] proposed a different process setup to the one described by Sievers. The process intends also to deal with compounds that are water soluble but with several modifications. Main alteration proposed is the use of a saturator instead of a micrometric volume tee to obtain the mixing between the aqueous stream and the dense gas. The saturator loaded with stainless steel perforated saddles was design to provide a contacting surface and a residence time sufficient to allow the dissolution of SC-CO2 in the liquid solution up to the saturation conditions at the pressure and temperature of processing [54].
The other modification introduced was the elimination of the capillary tube to avoid the problem of pressure drop and possible capillary blockage; instead, the liquid solution formed on the contraction device is sent to a thin wall injector. The injector produces a spray that forms the droplets in the precipitator, where a flow of heated N2 is introduced to facilitate the evaporation of the liquid solvent [54]. Figure 6 illustrates a schematic diagram of the SAA process.
Reverchon mentions that experiments were immediately successful using not only water but also organic solvents and that the SAA technique provides a good control over particle size producing microparticles sizing between 0,5 and 5 µm. The process parameter that mainly controlled the particle size in the SAA process was the concentration of the liquid solution [54]. The main applications of the SAA process were published by Reverchon and co-workers to the micronization of superconductors, ceramics and catalysts precursors, cyclodextrins and several drug compounds and also antibiotics using different liquid solvents [9,14]. More recently, the process was adapted to produce polymer particles and also drug-polymer composite particles [57].
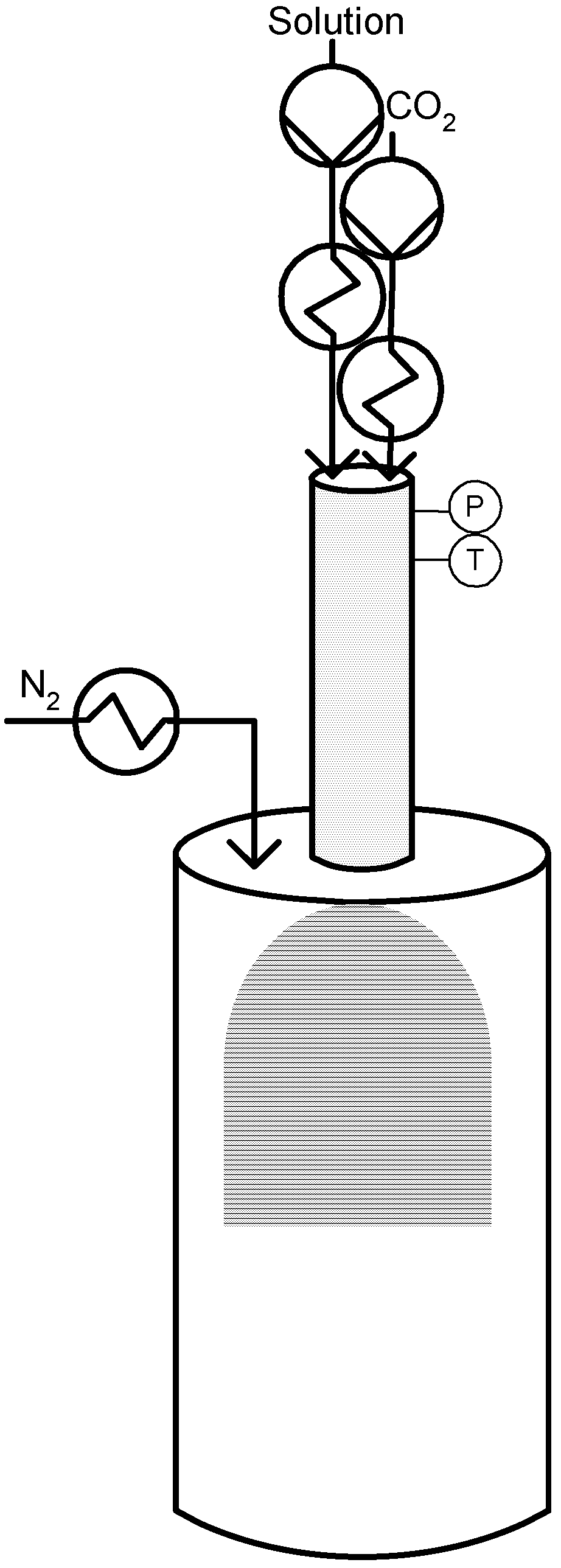
Figure 6.
Supercritical Assisted Atomization (SAA).
As in the CAN-BD process, in the SAA, atomization takes place in two steps. In a first step, primary droplets are produced at the outlet of the injector and in a second step this droplets are divided in secondary droplets by the CO2 expansion from the inside of primary droplets. These secondary droplets are rapidly dried by warm N2 and solid particles are formed on the basis of a one droplet-one particle mechanism [58]. The main differences between CAN-BD and SAA processes are the region where the mixing is achieved and the extent of solubilization of scCO2 in the liquid solution where the solute is dissolved [6]. In both processes, CO2 acts as a co-solute during the process although it role is actually to assist the spraying of the liquid solutions by atomizing the solution which may not succeed if an eventual anti-solvent effect occurs. On the other hand, if the liquid massively solubilizes in the fluid phase, the remaining liquid solution saturates and solid precipitation starts in the contactor and the process fails. Thus, vapor liquid equilibrium data of the binary (CO2 + liquid solvent) systems of interest is an essential information in order to properly select operating conditions (P and T) that can guarantee a limited solubility of CO2 in the liquid and a small solubility of liquid in CO2 [50]. In 2008, Cai et al. [59] introduced a hydrodynamic cavitation mixer (HCM) in the SAA process in order to improve mass transfer between CO2 and liquid solution. The SAA-HCM process was successfully used to micronized levofloxacin hydrochloride and the influences of several process parameters were investigated and reported by the authors.
Another variation of the CAN-BD process was explored by Rodrigues et al. [60] The Supercritical Enhanced Atomization (SEA) process is also based on the supercritical fluids ability to enhance liquid jet dispersion into fine droplets when depressurized simultaneously with liquid solutions [61,62]. The authors emphasize that, in contrast to SAA, this process setup is not intended to saturate the solution with the supercritical fluid, which is also not likely to happen in the CAN-BD process. The main difference between this and the CAN-BD process is the utilization of a co-axial nozzle with a pre-expansion mixing chamber instead of the micrometric volume tee. The pre-expansion mixing chamber allows the mixing of both fluids at selected conditions of pressure and temperature prior to its depressurization into a precipitation vessel at atmospheric pressure [60]. An interesting feature of this setup is that the precipitation mechanism can be switch from atomization and droplet drying to anti-solvent precipitation, just by properly selecting the operational conditions of the mixture in the mixing chamber. When conditions are selected in a way that CO2 acts as an anti-solvent, the process is similar to a SEDS process. This setup was used by the authors to investigate the different morphologies that can be obtained for the micronization of lysozyme by changing the governing mechanism for precipitation from spray drying (spherical particles) to anti-solvent (production of fibers was favored). The authors further report that lysozyme activity was severely affected when an anti-solvent effect was observed. Finally, due to the high gas/liquid ratios involved, the setup makes no use of a secondary gas flow inside the precipitator for solvent drying as used by both CAN-BD and SAA techniques [60].
In addition, in a different approach called PGSS-drying [63], particles do not need to be dried by means of a flow of heated N2 as in CAN-BD and SAA techniques. Instead, the solvent is removed from the spray tower together with the gas and a free flowing powder precipitates at low temperatures (30–60 °C), in an inert oxygen-free atmosphere, which is particularly important when processing sensitive substances. As in the original PGSS process the liquid solution to be dried is intensively mixed with scCO2 using a static mixer at the desired temperature and pressure, except that, at this stage, despite the dissolution of some CO2 in the liquid solution, also a considerable amount of the solvent is extracted into the gas. This biphasic mixture is then sprayed through a nozzle into the spray tower where fine droplets are formed (due to CO2 expansion because of atmospheric conditions) and, in addition, evaporation of the residual solvent takes place (due to pre-selected temperature conditions in the spray tower) [20]. In order to achieve an efficient evaporation of the solvent in the spray tower, temperature has to be carefully selected in a way that both solvent and gas form a homogeneous phase which will then be exhausted by a blower from the spray tower. To achieve this homogeneous gaseous phase, post-expansion temperature must therefore be at least higher than the dew point of the binary system gas and solvent [64,65]. If the liquid solvent used is a mixture then phase equilibrium data of the multi-component system is necessary for determining suitable conditions for successfully performing the precipitation [66]. At the end of the process, a free flowing powder is collected at the bottom of the spray tower. As for the original patented PGSS process, the authors used PEG as a model substance to analyze the fundamentals of the process, discuss mass and energy balances, phase equilibrium conditions, mass transfer rates and atomization mechanisms [67]. Furthermore, a detailed experimental analysis of the influence of different process and design parameters (temperature, pressure, flow rates, design of the static mixer used to put into contact aqueous solution and CO2) has been carried out [68].
Although very promising, there are not many published applications of this technique, which has been explored by the inventors, mainly for industrial purposes [20]. The PGSS-drying was patented by Weidner in 2000, but first scientific publication of this process was only later reported by the author and co-workers in 2008 for the drying of aqueous green tea extracts [66]. It is to be expected that, in the next few years, several other applications will emerge, since besides being very promising, this technique already exists at a pilot stage and on an industrial scale, providing a basis for the demonstration of its technical and economic feasibility for industrial applications [20]. Very recently Cocero and co-workers have applied the PGSS-drying technique to encapsulate an essential oil, lavandin, in n-octenyl succinic modified starches [69].
3.3. Co-Solvent: DELOS
Ventosa et al. developed the DELOS (Depressurization of an Expanded Liquid Organic Solution) process [70]. In this case the compressed gas (e.g., CO2) is used to saturate an organic solution of the solute of interest, forming a volumetric gas expanded liquid solution. This solution is further expanded through a non-returning valve to atmospheric pressure experiencing a large temperature decrease due to pressure reduction and consequent CO2 expansion, which causes the precipitation of submicron or micron-sized particles with a narrow particle distribution [71]. A schematic diagram is shown in Figure 7.
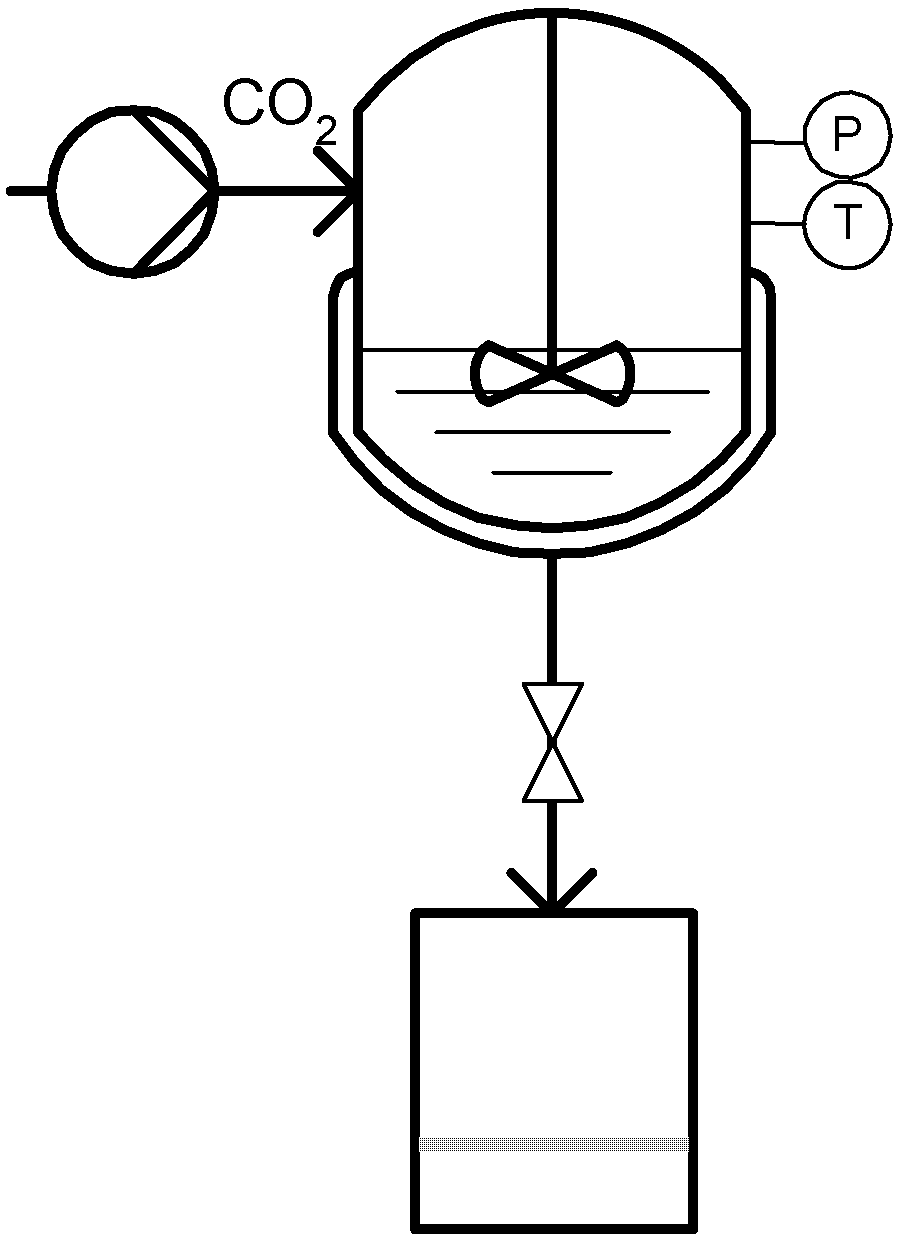
Figure 7.
Depressurization of an Expanded Liquid Organic Solution (DELOS).
The inventors describe the process in three sequence stages comprising initially the dissolution of the solute of interest in a conventional organic solvent at atmospheric pressure and operating temperature with a concentration bellow the saturation limit. The next step consists of pressurizing the organic solution by adding dense CO2 until working pressure is reached. An expanded liquid solution is formed with a certain molar fraction of dissolved gas, which the inventors term the working composition. The operative pressure should not exceed the critical point of the CO2/solvent mixture. Finally, the gas expanded solution is depressurized over a non-returning valve to atmospheric pressure, keeping the upstream pressure constant with N2. Particles are collected after cleaning of the precipitates with CO2.
It should be noted that, in this process, the compressed gas acts as a co-solvent being completely miscible at a given pressure and temperature with the organic solution of the solute to be crystallized [71]. An undesired anti-solvent effect of CO2 is therefore a possibility to be considered at stage 2, as if it occurs; the solute will precipitate in the saturator and not in the expansion vessel [15]. The solute concentration at this stage should therefore remain below the saturation limit in the expanded solution. The knowledge of the solute solubility behavior in CO2 expanded solvent is, however, of crucial importance to successfully employ the DELOS process [72]. This process can only be applied to substances for which the anti-solvent effect of CO2 is small [8].
Using the DELOS process, Ventosa et al. [71,72] have obtained particles less than 5 μm with a narrow size distribution and a high degree of cristalinity of a colorant powder, (1,4-bis-(n-butylamino)-9,1) anthraquinone. The authors found out that the size of the particles is a function of the magnitude of temperature decrease in the third stage [9], which is ruled by the working composition of the ternary mixture before depressurization [73]. The greater the decrease in temperature experienced by the solution, the smaller the particles obtained. For this specific substance, the authors also found that the DELOS process was more efficient than the GAS process [72]. In fact, smaller particles with a narrower size distribution were obtained which the authors explained to be due to the fact that the system solute/acetone/CO2 exist in one phase liquid solution for a wide range of CO2 molar fractions and added that, in systems with this behavior, the DELOS process is an alternative to the GAS process. The authors do not mention the existence of stirring in the high pressure vessel, actually they even refer the possibility of introducing CO2 through the bottom of the vessel in order to ensure faster CO2 dissolution rate and add that the design of the stirring system is not important because the characteristic of the particles produced do not depend on the mixing efficiency.
A modification to the DELOS process, called DELOS-SUSP [74] patented in 2006 and first reported by Cano-Sarabia et al. [75] in 2008, consists of depressurizing the gas expanded liquid solution into another solvent that acts as a crystallization interruption agent [5]. The authors used this process in the preparation of unilamellar cholesterol vesicles. Briefly, the gas-expanded liquid solution was depressurized from the working pressure to the atmospheric one through a non-return valve over a pumped aqueous solution with 1 wt % surface-active compound. The aqueous solution flow rate was adjusted to fix the cholesterol/surfactant ratio in the final vesicular system. Stable and structurally well-defined uniform spherically shaped, unilamellar rich cholesterol nanovesicles dispersed in an aqueous phase were formed by this process showing physicochemical characteristics unachievable by conventional mixing technologies [75].
All the techniques described in this review are based on the PGSS concept of expanding (or spraying) a gas saturated solution through a restriction device (e.g., a nozzle) and were developed based on one or more limitations with the intention of expanding the applicability of the original patented PGSS technique. Table 1 summarizes the main aspects of different PGSS-related techniques, in which CO2 plays different roles and that have been developed since Weidner and co-workers patented the process in 1994.

Table 1.
Main aspects of different PGSS-based techniques.
| Technique | CO2 role | Liquid solvents | Pre-requisites | Equilibrium measurements | Saturation | Precipitation | Drying |
|---|---|---|---|---|---|---|---|
| PGSS | Solute | NA | Melted or substances that experiment a mp depression effect under CO2 conditions | S-L-G equlibrium | High pressure reactor with mixing or in a static mixer | Spray tower | NA |
| CPF | The solute to be powderized is a liquid | VLE of the binary system (solute + CO2) | High pressure reactor with mixing | NA (Infiltration occur in a spray tower) | |||
| CPCSP | Melted or substances that experiment a mp depression effect under CO2 conditions | S-L-G equlibrium | Static mixer | Spray tower | |||
| CAN-BD | Co-solute (aerosolization aid) | Water and alcohol | Limited solubility between scCO2 and the liquid solvent | VLE of the binary system (solvent + CO2) | CO2 solubilisation occur in a low volume tee | Spar tower | With N2 |
| SEA | CO2 solubilisation occur in a pre-expansion mixing chamber | High pressure vessel equipped with a filter | NA | ||||
| SAA | Packed tower | Spay tower | With N2 | ||||
| PGSS-drying | Static mixer | Spray tower | With CO2 (T must be higher than the dew point of the binary system gas and solvent) | ||||
| DELOS | Co-solvent | Organic solvents | CO2 acts as a co-solvent | VLE of the ternary system (solute + CO2 +organic solvent) | Autoclave | High pressure vessel equipped with a filter | With CO2 |
5. Applications of PGSS and PGSS-based Techniques
PGSS and related techniques have been successfully applied to a large range of different substances underlining its enormous versatility. Applications of PGSS and PGSS-based techniques were extensively revised by Perrut in 2001 [14] and by Cor Peters in 2003 [15]. For the CAN-BD and SAA techniques, published applications have further been revised by Sievers in 2008 [48]. The intention of this review is to provide a compilation of all applications published until 2010 in the form of tables (Table 2, Table 3, Table 4 and Table 5) divided by type of process and listing the substances to be micronized in alphabetic order.

Table 2.
Substances atomized by the PGSS, CPF, CPCSP and PGSS drying techniques.
| Substance | Technique | References |
|---|---|---|
| Anthocyanin extracts/silica | CPF | Vatai et al. (2008) [76] |
| Caffeine/glyceryl monostearate | PGSS | de Sousa et al. (2007) [77] |
| Caffeine/glyceryl monostearate/cutine/TiO2 | PGSS | Garcia-Gonzalez et al. (2009) [78] |
| trans-Chalcone | PGSS | de Sousa et al. (2009) [79] |
| Citrus flavour | CPF | Gruner et al. (2003) [80] |
| Citric acid/PEG | PGSS | Weidner et al. (1996) [26] |
| Cyclosporine | PGSS | Tandya et al. (2006) [81] |
| Coatings systems (acrylic coatings, polyester-epoxy systems, low-melting polyester coatings) | CPCSP | Weidner et al. (2001) [43] |
| Cocoa butter | PGSS | Letourneau et al. (2005) [82] |
| Cocoa powder | PGSS | Perva-Uzunalic et al. (2008) [83] |
| Cilantro(Coriandrum sativum)/PEG | Choi et al. (2009) [84] | |
| Cydia pomonella granulovirus | PGSS | Pemsel et al. (2010) [85] |
| Felodipine, Felofipine/lactose, Felodipine/PEG4000 | PGSS | Kerc et al. (1999) [86] |
| Fenofibrate, Fenofibrate/PEG4000 | PGSS | Kerc et al. (1999) [86] |
| Glutathione/glyceryl monostearate/cutine/TiO2 | PGSS | Garcia-Gonzalez et al. (2009) [78] |
| Glyceryl monostearate | PGSS | de Sousa et al. (2007) [77] |
| Green tea extracts (Aqueous) | PGSS-drying | Meterc et al.(2008) [64] |
| hgH/PLGA/PLA | PGSS | Jordan et al. (2010) [87] |
| rh-gH/ Phosphatidylcholine/PEG/Tristearin | PGSS | Salmasso et al. (2009) [88] |
| Hydrogenated palm oil | PGSS | Li et al. (2005) [89] |
| Insulin/tristearin, Tween-80, phosphatidylcholine, PEG, Insulin/tristearin, dioctyl sulfosuccinate and phosphatidylcholine | PGSS | Salmaso et al. (2009) [38] |
| Ketoprofen/glyceryl monostearate/cutine/TiO2 | PGSS | Garcia-Gonzalez et al. (2009) [78] |
| Lavandin essential oil/(OSA)-starch | PGSS-drying | Varona et al. (2010) [69] |
| Lavandin essential oil/PEG | PGSS | Varona et al. (2010) [69] |
| Lysozyme/P(DLLA) | PGSS | Whitaker et al. (2005) [90] |
| Monostearate | PGSS | Mandzuka et al. (2008) [91] |
| Nifedipine, Nifedipine/PEG 4000 | PGSS | Kerc et al. (1999) [86], Sencar-Bozic et al. (1997) [28] |
| Polybutylenterephthalate, Polybutylenterephthalate /zinc oxide, Polybutylenterephthalate/bentonite | PGSS | Pollak et al. (2010) [92] |
| Poly (DL-lactic acid) | PGSS | Hao et al. (2004) [37] |
| Poly (ethylene glycol) | PGSS | Hao et al. (2005) [93], Nalawade et al. (2007) [94] |
| Poly (ethylene glycol) aqueous solution | PGSS-drying | Martin et al. (2010) [68] |
| Precirol | PGSS | Calderone et al. (2007) [95] |
| Rapeseed 70 | PGSS | Manuklu et al. (2007) [96] |
| PEGylated Ribonuclease/ Triestearin/Phosphatidylcholine/PEG | PGSS | Vezzu et a. (2010) [97] |
| Ribonuclease A/P(DLLA) | PGSS | Whitaker et al. (2005) [90] |
| Theophylline/hydrogenated palm oil | PGSS | Rodrigues et al. (2004) [30] |
| TiO2-PLA, TiO2-PS-b-PMMA-co-PGMA | PGSS | Matsuyama et al. (2007) [98] |
| Triacetyl-β-cyclodextrin | PGSS | Nunes et al. (2010) [99] |
| Tristearate | PGSS | Mandzuka et al. (2008) [91], Mandzuka et al. (2010) [100] |
| Vegetable oil emulsion/cellulose | CPF | Wehowski et al. (2008) [101] |
| YNS3107/PEG400/PEG4000/Polaxamer 407 | PGSS | Brion et al. (2009) [102] |

Table 3.
Substances atomized with the CAN-BD and SEA processes.
| Substance | Liquid solvent | References |
|---|---|---|
| Albuterol sulfate | Water | Sievers et al. (1998, 2000, 2001) [47,103,104] |
| Alpha-1-antitrypsin | Water | Cape et al. (2008) [48] |
| Amphotericin B | Ethanol | Sievers et al. (2003) [50] |
| Anti-CD4 | Water | Cape et al. (2008) [48] |
| Betamethasone-17,21-dipropionate | Ethanol | Villa et al. (2005) [51] |
| Budesonide | Ethanol | Sievers et al. (2003) [50] |
| Cromolyn sodium | Water | Sievers et al. (2000) [47] |
| Doxycycline | Water | Sievers et al. (2003) [105] |
| Glutathione | Water | Sievers et al. (1999) [46] |
| Myo-inositol | Water | Huang et al. (2003) [49] |
| HBsAg (Hepatitis B surface antigen protein)/Albumin hydroxide | Water | Sievers et al. (2007) [52] |
| Iron oxides mixture (Fe3O4 and FeO) | Water | Sievers et al. (1999) [46] |
| Lactate dehydrogenase (LDH) | Water | Sellers et al. (2001) [106], Sievers et al. (2001) [104] |
| Lactose | Water | Sievers et al. (2000) [47], Villa et al. (2005) [51] |
| Lactose/Betamethasone | Water/Ethanol | Villa et al. (2005) [51] |
| Lactose/( Betamethasone/Stearic acid) | Water/Ethanol | Villa et al. (2005) [51] |
| Lactose/Palmitic acid | Water/Ethanol | Villa et al. (2005) [51] |
| Lysozyme | Water | Sellers et al. (2001) [106], Sievers et al. (2001) [104] |
| Mannitol | Water | Huang et al. (2003) [49] |
| Measles Vaccine virus, live-attenuated | Water | Sievers et al. (2007) [52], Burger et al. (2008) [53] |
| Naproxen | Water | Sievers et al. (2003) [50] |
| Ovalbumin/trehalose | Water | Sievers et al. (2001) [104], Sievers et al. (2003) [50] |
| Palmitic acid | Ethanol | Villa et al. (2005) [51] |
| rhDNase | water | Sievers et al. (1999) [46] |
| Rifampin | Ethyl acetate | Sievers et al. (2007) [52] |
| Sacharin(SAC)-Aspirin, SAC-Caffeine, SAC-Carbamazeoine, SAC-Indomethacin, SAC-Sulfamethazine, SAC-Theophylline (Cocrystals) | Ethanol | Padrela et al. (2009) [61], Padrela et al. (2010) [62] |
| Sodium chloride | Water | Sievers et al. (2001) [104], Sievers et al. (2003) [50], Villa et al. (2005) [51] |
| Sodium chloride/Palmitic acid | Water/acetone | Villa et al. (2005) [51] |
| Sodium chloride/PLGA | Water/acetone | Villa et al. (2005) [51] |
| Tobramycin sulfate | Water | Sievers et al. (1998) [103] |
| Trypsinogen | Water | Cape et al. (2008) [48] |
| Yttrium oxide phosphors (Y2O3:Eu, Y2O3:Tb) | Water | Xu et al. (1997) [45] |
| Zanamivir (Relenza®) | Water | Sievers et al. (2007) [52] |

Table 4.
Substances atomized with the SAA process.
| Substance | Liquid solvent | References |
|---|---|---|
| Albumin/Gentamicin sulfate | Water | Della Porta et al. (2010) [107] |
| Aluminum sulfate | Water | Reverchon et al. (2002) [54] |
| Amonium chloride | Water | Reverchon et al. (2004) [108] |
| Ampicillin | Water, methanol, ethanol | Reverchon et al. (2002, 2003) [54,109] |
| Ampicillin trihydrate /Chitosan | Water | Reverchon et al. (2007) [110] |
| HPMC/ampicillin trihydrate | Buffer solution | Reverchon et al. (2008) [111] |
| Beclomethasone | Methanol, acetone, methanol/water, acetone/water | Reverchon et al. (2010) [112] |
| Carbamazepine | Methanol | Reverchon et al. (2002) [54] |
| Cefadroxil | Water | Li et al. (2009) [113] |
| Chitosan | 1%acid acetic aqueous solution | Reverchon et al. (2006) [114] |
| Cromolyn Sodium | Water | Reverchon et al. (2007) [115] |
| α-Cyclodextrin | Water | Reverchon et al. (2006) [116] |
| Dexamethasone, Dexamethasone acetate | Acetone, methanol | Reverchon et al. (2002, 2006) [54,117] |
| Erythromycin | Methanol, ethanol, acetone | Reverchon et al. (2003, 2004) [118,119], Li et al. (2007) [120] |
| Ginkgo biloba leaves extract | x | Miao et al. (2010) [121] |
| Griseofulvin | Acetone, acetone/ethanol | Reverchon et al. (2004) [122], Li et al. (2008) [123] |
| HP-beta-CD | Water | Reverchon et al. (2006) [114] |
| HMR1031 (new chemical entity by Aventis Pharma) | Methanol | Reverchon et al. (2005) [124] |
| Levofloxacin hydrochloride | Methanol | Cai et al. (2008) [60] |
| Lysozyme | Water, water/ethanol mixtures | Reverchon et al. 2009 [125] |
| Pigment red 60 | Acetone | Reverchon et al. (2005) [126] |
| PLLA | DCM | Reverchon et al. (2007) [127] |
| PMMA | Acetone | Reverchon et al. (2007) [127] |
| Potassium iodide | Water, methanol | Reverchon et al. (2004) [108] |
| Rifampicine | Methanol | Reverchon et al. (2003) [128] |
| Sodium chloride | Water | Reverchon et al. (2002, 2004) [54,108] |
| Sodium cellulose sulfate | Water | Wang et al. [129] |
| Terbutaline | Water | Reverchon et al. (2003) [130] |
| Tetracycline | Water, water/ethanol | Reverchon et al. (2003) [128,119], Li et al. 2008 [131] |
| Triclabenzadol | Methanol | Reverchon et al. (2002) [54] |
| Yttrium acetate | Water, methanol | Reverchon et al. (2002, 2003) [54,119] |
| Zinc acetate | Methanol | Reverchon et al. (2002) [54] |
| Zirconyl nitrate hydrate | Water | Reverchon et al. (2002) [54] |

Table 5.
Substances atomized with the DELOS process.
| Substance | Liquid solvent | References |
|---|---|---|
| 1,4-bis-(n-butylamino)-9,10-anthraquinone (solventblue35) | Acetone | Ventosa et al. (2001, 2003) [71,72] |
| 1,4-bis-(n-butylamino)-9,10-anthraquinone (solventblue35) | 1,1,1,2-Tetrafluoroethane | Gimeno et al. (2006) [132] |
| 1,3,5,7-Tetraazatricyclo[3.3.1.13,7]decane (hexamethylenetetramine) | 1,1,1,2-Tetrafluoroethane | Gimeno et al. (2006) [132] |
| Acetylsalicylic acid (aspirin) | 1,1,1,2-Tetrafluoroethane | Gimeno et al. (2006) [132] |
| Cholesterol | Cano-Sarabia et al. (2008) [75] | |
| Ibuprofen | Ethanol and Acetone | Munto et al. (2008) [133] |
| Naproxen | ethanol | Munto et al. (2008) [133] |
| Poloxamer F-127 | ethanol | Munto et al. (2008) [134] |
| Stearic acid | Ethyl acetate | Sala et al. (2010) [135] |
6. Conclusions and Future Perspectives
Since Weidner and co-workers patented the PGSS process in 1994, several variations based on the same concept were developed in which dense CO2 plays different roles; as a solute in CPF and CPCSP; as a co-solute for CAN-BD, SEA, SAA and PGSS-drying; and as a co-solvent in the DELOS process. PGSS-derived techniques, besides offering several advantages over conventional processes, are based on the very simple concept of expanding (or spraying) a solution saturated with a dense gas through a restriction device (e.g., a nozzle). The concept has actually proven its feasibility as PGSS is already operating on large scales for producing products for the food industry. Nevertheless, most published papers presented in this review explore applications directed to the pharmaceutical industry, which is in general more conservative when it comes to technological changes. For example, the widely in use spray drying process had only started to be employed by the pharmaceutical industry twenty years after it found its first industrial application in the food industry, for milk drying [136]. It is therefore very likely that, in coming years, PGSS-based techniques will find their way into the pharmaceutical industry. The research road ahead is as important as the one done until this point, but is essentially more demanding. A huge number of publications have evidenced the versatility of these techniques in allowing the processing of several different types of substances and, although the development of new products remains important, it is crucial to understand some process mechanisms that are still not fully understood. Even though some efforts have been done in the past few years, some fundamental issues still require further research in order to better understand the process mechanisms involved. The development of models that can accurately predict the characteristics of the final product constitute the great challenge that scientists in the field have to address, so that the technology can become widespread.
References
- York, P. Strategies for particle design using supercritical fluid technologies. Pharm. Sci. Technol. Today 1999, 2, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Bertucco, A.; Vetter, G. High Pressure Process Technology: Fundamentals and Applications, Industrial Chemistry Library; Elsevier: Amsterdam, The Netherlands, 2001; Volume 9. [Google Scholar]
- Hakuta, Y.; Hayashi, H.; Arai, K. Fine particle formation using supercritical fluids. Curr. Opin. Solid State Mater. Sci. 2003, 7, 341–351. [Google Scholar] [CrossRef]
- Fages, J.; Lochard, H.; Letourneau, J.J.; Sauceau, M.; Rodier, E. Particle generation for pharmaceutical applications using supercritical fluid technology. Powder Technol. 2004, 141, 219–226. [Google Scholar] [CrossRef]
- Martin, A.; Cocero, M.J. Precipitation processes with supercritical fluids: Patents review. Recent Pat. Eng. 2008, 1, 9–20. [Google Scholar]
- Pasquali, I.; Bettini, R. Are pharmaceutics really going supercritical? Int. J. Pharm. 2008, 364, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K. Biodegradable particle formation for drug and gene delivery using supercritical fluid and dense gas. Adv. Drug Deliv. Rev. 2008, 60, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Cocero, M.J. Micronization processes with supercritical fluids: Fundamentals and mechanisms. Adv. Drug Deliv. Rev. 2008, 60, 339–350. [Google Scholar] [CrossRef]
- Foster, N.; Mammucari, R.; Dehghani, F.; Barrett, A.; Bezanehtak, K.; Coen, E.; Combes, G.; Meure, L.; Ng, A.; Regtop, H.L.; Tandya, A. Processing pharmaceutical compounds using dense gas technology. Ind. Eng. Chem. Res. 2003, 42, 6476–6493. [Google Scholar] [CrossRef]
- Beckman, E.J. Supercritical and near-critical CO2 in green chemical synthesis and processing. J. Supercrit. Fluids 2004, 28, 121–191. [Google Scholar] [CrossRef]
- Clifford, T. Fundamentals of Supercritical Fluids; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Leitner, W.; Poliakoff, M. Supercritical fluids in green chemistry. Green Chem. 2008, 10, 730–730. [Google Scholar] [CrossRef]
- Green Chemistry Using Liquid and Supercritical Carbon Dioxide; DeSimone, J.M.; Tumas, W. (Eds.) Oxford University Press: Oxford, UK, 2003.
- Jung, J.; Perrut, M. Particle design using supercritical fluids: Literature and patent survey. J. Supercrit. Fluids 2001, 20, 179–219. [Google Scholar] [CrossRef]
- Shariati, A.; Peters, C. J. Recent developments in particle design using supercritical fluids. Curr. Opin. Solid State Mater. Sci. 2003, 7, 371–383. [Google Scholar] [CrossRef]
- Reverchon, E.; Volpe, M.C.; Caputo, G. Supercritical fluid processing of polymers: Composite particles and porous materials elaboration. Curr. Opin. Solid State Mater. Sci. 2003, 7, 391–397. [Google Scholar] [CrossRef]
- Yeo, S.D.; Kiran, E. Formation of polymer particles with supercritical fluids: A review. J. Supercrit. Fluids 2005, 34, 287–308. [Google Scholar] [CrossRef]
- Tandya, A.; Mammucari, R.; Dehghani, F.; Foster, N.R. Dense gas processing of polymeric controlled release formulations. Int. J. Pharm. 2007, 328, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cocero, M.J.; Martin, A.; Mattea, F.; Varona, S. Encapsulation and co-precipitation processes with supercritical fluids: Fundamentals and applications. J. Supercrit. Fluids 2009, 47, 546–555. [Google Scholar] [CrossRef]
- Weidner, E. High pressure micronization for food applications. J. Supercrit. Fluids 2009, 47, 556–565. [Google Scholar] [CrossRef]
- Weidner, E.; Knez, Z.; Novak, Z. A Process and Equipment for Production and Fractionation of Fine Particles from Gas Saturated Solutions. World Patent WO 95/21688, 1994. [Google Scholar]
- Weidner, E.; Knez, Z.; Novak, Z. PGSS (Particles from Gas Saturated Solutions)—A new process for powder generation. In Proceedings of the 3rd International Symposium on Supercritical Fluids, Strasbourg, France, 17–19 October 1994; Volume 3, pp. 229–235.
- Lack, E.; Weidner, E.; Knez, Z.; Gruner, S.; Weinreich, B.; Seidlitz, H. Particle generation with supercritical CO2. In Proceedings of the 1st Vienna International Conference: Micro- and Nano-Technology, Vienna, Austria, 9–11 March 2005.
- Knez, Z.; Weidner, E. Particles formation and particle design using supercritical fluids. Curr. Opin. Solid State Mater. Sci. 2003, 7, 353–361. [Google Scholar] [CrossRef]
- Knez, Z.; Skerget, M.; Mandzuka, Z. Determination of S-L phase transitions under gas pressure. J. Supercrit. Fluids 2010, 55, 648–652. [Google Scholar] [CrossRef]
- Weidner, E.; Steiner, R.; Knez, Z. Powder generation from polyethyleneglycols with compressible fluids. High Press. Chem. Eng. 1996, 12, 223–228. [Google Scholar]
- Weidner, E.; Petermann, M.; Knez, Z. Multifunctional composites by high-pressure spray processes. Curr. Opin. Solid State Mater. Sci. 2003, 7, 385–390. [Google Scholar] [CrossRef]
- SencarBozic, P.; Srcic, S.; Knez, Z.; Kerc, J. Improvement of nifedipine dissolution characteristics using supercritical CO2. Int. J. Pharm. 1997, 148, 123–130. [Google Scholar] [CrossRef]
- Howdle, S.M.; Watson, M.S.; Whitaker, M.J.; Popov, V.K.; Davies, M.C.; Mandel, F.S.; Wang, J.D.; Shakesheff, K.M. Supercritical fluid mixing: Preparation of thermally sensitive polymer composites containing bioactive materials. Chem. Commun. 2001, 109–110. [Google Scholar]
- Rodrigues, M.; Peirico, N.; Matos, H.; de Azevedo, E.G.; Lobato, M.R.; Almeida, A.J. Microcomposites theophylline/hydrogenated palm oil from a PGSS process for controlled drug delivery systems. J. Supercrit. Fluids 2004, 29, 175–184. [Google Scholar] [CrossRef]
- Shine, A.D.; Gelb, J. Forming microparticles of material, partic. protein|by swelling and liquefaction of polymer, then releasing pressure, used for e.g., drugs, vaccines, agrochemicals and deodorants. WO9815348, 16 April 1998. [Google Scholar]
- Foster, N.R.; Regtop, H.L.; Dehghani, F.; Tandya, A. Preparation of biologically active micro particles by pulverization for pharmaceutical purposes. WO2003088951, 30 October 2003. [Google Scholar]
- Perrut, M. Prepn. of liq. loaded powder useful in topical compsns. of e.g., sunscreen. WO200205944, 24 January 2002. [Google Scholar]
- Calderone, M.; Rodier, E. Coating of powdery solid active substances, for use in e.g., a pharmaceutical formulation, comprises contacting a mixture (having coating) and individualized particles of active substance. WO2006030112, 23 March 2006. [Google Scholar]
- Calderone, M.; Rodier, E.; Fages, J. Microencapsulation by a solvent-free supercritical fluid process: Use of density, calorimetric, and size analysis to quantify and qualify the coating. Part. Sci. Technol. 2007, 25, 213–225. [Google Scholar] [CrossRef]
- Calderone, M.; Rodier, E.; Lochard, H.; Marciacq, F.; Fages, J. A new supercritical co-injection process to coat microparticles. Chem. Eng. Process. 2008, 47, 2228–2237. [Google Scholar] [CrossRef]
- Hao, J.Y.; Whitaker, M.J.; Wong, B.; Serhatkulu, G.; Shakesheff, K.M.; Howdle, S.M. Plasticization and spraying of poly (dl-lactic acid) using supercritical carbon dioxide: Control of particle size. J. Pharm. Sci. 2004, 93, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, S.; Elvassore, N.; Bertucco, A.; Caliceti, P. Production of solid lipid submicron particles for protein delivery using a novel supercritical gas-assisted melting atomization process. J. Pharm. Sci. 2009, 98, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Bertucco, A.; Caliceti, P.; Elvassore, N. Process for the production of nano-particles. WO2007028421, 2007. [Google Scholar]
- Weidner, E.; Steiner, R.; Dirscherl, H.; Weinreich, B. Verfahren zur Herstellung eines pulverförmigen Produktes aus einem flüssigen Stoff oder Stoffgemisch. European Patent EP 9705484, 6 October 1997. [Google Scholar]
- Petermann, M.; Weidner, E.; Grüner, S.; Weinreich, B. CPF—Concentrated powder form—A high pressure spray agglomeration technique. In Proceedings of the Spray Drying ’01 and Related Processes, Dortmund, Germany, 8–10 October 2001.
- Petermann, M.; Weidner, E.; Blatter, K.; Simmrock, H.U. Manufacture of powder coatings by spraying of gas saturated melts. In Proceedings of the 6th International Symposium on Supercritical Fluids, Versailles, France, 28–30 April 2003.
- Weidner, E.; Petermann, M.; Blatter, K.; Rekowski, V. Manufacture of powder coatings by spraying of gas-enriched melts. Chem. Eng. Technol. 2001, 24, 529–533. [Google Scholar] [CrossRef]
- Sievers, R.E.; Karst, U. Methods for fine particle formation. US Patent 5,639,441, 17 June 1997. [Google Scholar]
- Xu, C.Y.; Watkins, B.A.; Sievers, R.E.; Jing, X.P.; Trowga, P.; Gibbons, C.S.; Vecht, A. Submicron-sited spherical yttrium oxide based phosphors prepared by supercritical CO2-assisted aerosolization and pyrolysis. Appl. Phys. Lett. 1997, 71, 1643–1645. [Google Scholar] [CrossRef]
- Sievers, R.E.; Karst, U.; Milewski, P.D.; Sellers, S.P.; Miles, B.A.; Schaefer, J.D.; Stoldt, C.R.; Xu, C.Y. Formation of aqueous small droplet aerosols assisted by supercritical carbon dioxide. Aerosol Sci. Technol. 1999, 30, 3–15. [Google Scholar] [CrossRef]
- Sievers, R.E.; Milewski, P.D.; Sellers, S.P.; Miles, B.A.; Korte, B.J.; Kusek, K.D.; Clark, G.S.; Mioskowski, B.; Villa, J.A. Supercritical and near-critical carbon dioxide assisted low-temperature bubble drying. Ind. Eng. Chem. Res. 2000, 39, 4831–4836. [Google Scholar] [CrossRef]
- Cape, S.P.; Villa, J.A.; Huang, E.T.S.; Yang, T.H.; Carpenter, J.F.; Sievers, R.E. Preparation of active proteins, vaccines and pharmaceuticals as fine powders using supercritical or near-critical fluids. Pharm. Res. 2008, 25, 1967–1990. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.T.S.; Chang, H.; Liang, C.D.; Sievers, R.E. Fine particle pharmaceutical manufacturing using dense carbon dioxide mixed with aqueous or alcoholic solutions. In Supercritical Carbon Dioxide: Separations and Processes; Gopalan, A.S., Wai, C.M., Jacobs, H.K., Eds.; 2003; Volume 860, pp. 324–338. [Google Scholar]
- Sievers, R.E.; Huang, E.T.S.; Villa, J.A.; Engling, G.; Brauer, P.R. Micronization of water-soluble or alcohol-soluble pharmaceuticals and model compounds with a low-temperature Bubble Dryer (R). J. Supercrit. Fluids 2003, 26, 9–16. [Google Scholar] [CrossRef]
- Villa, J.A.; Huang, E.T.S.; Cape, S.P.; Sievers, R.E. Synthesis of composite microparticles with a mixing cross. Aerosol Sci. Technol. 2005, 39, 473–484. [Google Scholar] [CrossRef]
- Sievers, R.E.; Quinn, B.P.; Cape, S.P.; Searles, J.A.; Braun, C.S.; Bhagwat, P.; Rebits, L.G.; McAdams, D.H.; Burger, J.L.; Best, J.A.; Lindsay, L.; Hernandez, M.T.; Kisich, K.O.; Iacovangelo, T.; Kristensen, D.; Chen, D. Near-critical fluid micronization of stabilized vaccines, antibiotics and anti-virals. J. Supercrit. Fluids 2007, 42, 385–391. [Google Scholar] [CrossRef]
- Burger, J.L.; Cape, S.P.; Braun, C.S.; McAdams, D.H.; Best, J.A.; Bhagwat, P.; Pathak, P.; Rebits, L.G.; Sievers, R.E. Stabilizing formulations for inhalable powders of live-attenuated measles virus vaccine. J. Aerosol. Med. Pulm. Drug Deliv. 2008, 21, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E. Supercritical-assisted atomization to produce micro- and/or nanoparticles of controlled size and distribution. Ind. Eng. Chem. Res. 2002, 41, 2405–2411. [Google Scholar] [CrossRef]
- Charbit, G.; Badens, E.; Boutin, O. Methods of particle production. In Supercritical Fluid Technology for Drug Product Development; York, P., Kompella, U.B., Shekunov, B.Y., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004. [Google Scholar]
- Reverchon, E. Continuous separation of fatty acid mono:glyceride from mixed glyceride(s). WO2003004142-A, 16 January 2003. [Google Scholar]
- Reverchon, E.; Adami, R.; Cardea, S.; Della Porta, G. Supercritical fluids processing of polymers for pharmaceutical and medical applications. J. Supercrit. Fluids 2009, 47, 484–492. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R.; Caputo, G. Supercritical assisted atomization: Performance comparison between laboratory and pilot scale. J. Supercrit. Fluids 2006, 37, 298–306. [Google Scholar] [CrossRef]
- Cai, M.Q.; Guan, Y.X.; Yao, S.J.; Zhu, Z.Q. Supercritical fluid assisted atomization introduced by hydrodynamic cavitation mixer (SAA-HCM) for micronization of levofloxacin hydrochloride. J. Supercrit. Fluids 2008, 43, 524–534. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Li, J.; Padrela, L.; Almeida, A.; Matos, H.A.; de Azevedo, E.G. Anti-solvent effect in the production of lysozyme nanoparticles by supercritical fluid-assisted atomization processes. J. Supercrit. Fluids 2009, 48, 253–260. [Google Scholar] [CrossRef]
- Padrela, L.; Rodrigues, M.A.; Velaga, S.R.; Matos, H.A.; de Azevedo, E.G. Formation of indomethacin-saccharin cocrystals using supercritical fluid technology. Eur. J. Pharm. Sci. 2009, 38, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Padrela, L.; Rodrigues, M.A.; Velaga, S.P.; Fernandes, A.C.; Matos, H.A.; de Azevedo, E.G. Screening for pharmaceutical cocrystals using the supercritical fluid enhanced atomization process. J. Supercrit. Fluids 2010, 53, 156–164. [Google Scholar] [CrossRef]
- Weidner, E.; Kilzer, A.; Petermann, M.; Prob, A.; Lucas, K.; Stepanski, R. Verfahren zur Erzeugung von Polyurethanpartikeln. Deutsches Patent DE 10040551.7, 15 August 2000. [Google Scholar]
- Meterc, D.; Petermann, M.; Weidner, E. Drying of aqueous green tea extracts using a supercritical fluid spray process. J. Supercrit. Fluids 2008, 45, 253–259. [Google Scholar] [CrossRef]
- Meterc, D.; Petermann, M.; Weidner, E. Extraction of green tea and drying with a high pressure spray process. Hem. Ind. 2007, 61, 222–228. [Google Scholar] [CrossRef]
- Martin, A.; Pham, H.M.; Kilzer, A.; Kareth, S.; Weidner, E. Phase equilibria of carbon dioxide plus poly ethylene glycol plus water mixtures at high pressure: Measurements and modelling. Fluid Phase Equilibria 2009, 286, 162–169. [Google Scholar] [CrossRef]
- Martin, A.; Weidner, E. PGSS-drying: Mechanisms and modeling. J. Supercrit. Fluids 2010, 55, 271–281. [Google Scholar] [CrossRef]
- Martin, A.; Huu, M.P.; Kilzer, A.; Kareth, S.; Weidner, E. Micronization of polyethylene glycol by PGSS (Particles from Gas Saturated Solutions)-drying of aqueous solutions. Chem. Eng. Process. 2010, 49, 1259–1266. [Google Scholar] [CrossRef]
- Varona, S.; Kareth, S.; Martin, A.; Cocero, M.J. Formulation of lavandin essential oil with biopolymers by PGSS for application as biocide in ecological agriculture. J. Supercrit. Fluids 2010, 54, 369–377. [Google Scholar] [CrossRef]
- Ventosa, N.; Veciana, J.; Rovira, C.; Sala, S.; Carburos Metàlicos, S.E. Method for Precipitating Finely Divided Solid Particles. US Patent 7,291,295, 23 August 2001. PCT/ES01/00327. [Google Scholar]
- Ventosa, N.; Sala, S.; Veciana, J.; Torres, J.; Llibre, J. Depressurization of an expanded liquid organic solution (DELOS): A new procedure for obtaining submicron- or micron-sized crystalline particles. Cryst. Growth Des. 2001, 1, 299–303. [Google Scholar] [CrossRef]
- Ventosa, N.; Sala, S.; Veciana, J. DELOS process: A crystallization technique using compressed fluids—1. Comparison to the GAS crystallization method. J. Supercrit. Fluids 2003, 26, 33–45. [Google Scholar] [CrossRef]
- Dalvi, S.V.; Mukhopadhyay, M. Parameters controlling supersaturation by DELOS using carbon dioxide. J. Chem. Technol. Biotechnol. 2006, 81, 1267–1270. [Google Scholar] [CrossRef]
- Ventosa, N.; Veciana, J.; Sala, S.; Cano, M. Method for obtaining micro- and nano-disperse systems. Patents ES2265262; WO2006/0799889, 1 February 2007. [Google Scholar]
- Cano-Sarabia, M.; Ventosa, N.; Sala, S.; Patino, C.; Arranz, R.; Veciana, J. Preparation of uniform rich cholesterol unilamellar nanovesicles using CO2-expanded solvents. Langmuir 2008, 24, 2433–2437. [Google Scholar] [CrossRef] [PubMed]
- Vatai, T.; Skerget, M.; Knez, Z.; Kareth, S.; Wehowski, M.; Weidner, E. Extraction and formulation of anthocyanin-concentrates from grape residues. J. Supercrit. Fluids 2008, 45, 32–36. [Google Scholar] [CrossRef]
- De Sousa, A.R.S.; Simplicio, A.L.; de Sousa, H.C.; Duarte, C.M.M. Preparation of glyceryl mono stearate-based particles by PGSS(R)—Application to caffeine. J. Supercrit. Fluids 2007, 43, 120–125. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, C.A.; da Sousa, A.R.S.; Argemi, A.; Periago, A.L.; Saurina, J.; Duarte, C.M.M.; Domingo, C. Production of hybrid lipid-based particles loaded with inorganic nanoparticles and active compounds for prolonged topical release. Int. J. Pharm. 2009, 382, 296–304. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, A.R.S.; Silva, R.; Tay, F.H.; Simplicio, A.L.; Kazarian, S.G.; Duarte, C.M.M. Solubility enhancement of trans-chalcone using lipid carriers and supercritical CO2 processing. J. Supercrit. Fluids 2009, 48, 120–125. [Google Scholar] [CrossRef]
- Grüner, S.; Otto, F.; Weinreich, B. CPF-technology—A new cryogenic spraying process for pulverization of liquid. In Proceedings of the 6th International Symposium on Supercritical Fluids, Versailles, France, 28–30 April 2003.
- Tandya, A.; Dehghani, F.; Foster, N.R. Micronization of cyclosporine using dense gas techniques. J. Supercrit. Fluids 2006, 37, 272–278. [Google Scholar] [CrossRef]
- Letourneau, J.J.; Vigneau, S.; Gonus, P.; Fages, J. Micronized cocoa butter particles produced by a supercritical process. Chem. Eng. Process. 2005, 44, 201–207. [Google Scholar] [CrossRef]
- Perva-Uzunalic, A.; Skerget, M.; Knez, Z. Supercritical fluids for producing cocoa powder. In Proceedings of the 2008 Joint Central European Congress, Cavtat, Croatia, 9–11 April 2008; Volume 1, pp. 211–217.
- Jin-Ah, C.; In-Il, J.; Gio-Bin, L.; Jong-Hoon, R. Preparation of drug-loaded polymeric particles using a PGSS process and their characterization. J. Biosci. Bioeng. 2009, 108, S26. [Google Scholar]
- Pemsel, M.; Schwab, S.; Scheurer, A.; Freitag, D.; Schatz, R.; Schlucker, E. Advanced PGSS process for the encapsulation of the biopesticide Cydia pomonella granulovirus. J. Supercrit. Fluids 2010, 53, 174–178. [Google Scholar] [CrossRef]
- Kerc, J.; Srcic, S.; Knez, Z.; Sencar-Bozic, P. Micronization of drugs using supercritical carbon dioxide. Int. J. Pharm. 1999, 182, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Jordan, F.; Naylor, A.; Kelly, C.A.; Howdle, S.M.; Lewis, A.; Illum, L. Sustained release hGH microsphere formulation produced by a novel supercritical fluid technology: In vivo studies. J. Control. Release 2010, 141, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, S.; Bersani, S.; Elvassore, N.; Bertucco, A.; Caliceti, P. Biopharmaceutical characterisation of insulin and recombinant human growth hormone loaded lipid submicron particles produced by supercritical gas micro-atomisation. Int. J. Pharm. 2009, 379, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rodrigues, M.; Paiva, A.; Matos, H.A.; de Azevedo, E.G. Modeling of the PGSS process by crystallization and atomization. AIChE J. 2005, 51, 2343–2357. [Google Scholar] [CrossRef]
- Whitaker, M.J.; Hao, J.Y.; Davies, O.R.; Serhatkulu, G.; Stolnik-Trenkic, S.; Howdle, S.M.; Shakesheff, K.M. The production of protein-loaded microparticles by supercritical fluid enhanced mixing and spraying. J. Control. Release 2005, 101, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Mandzuka, Z.; Knez, Z. Influence of temperature and pressure during PGSS (TM) micronization and storage time on degree of crystallinity and crystal forms of monostearate and tristearate. J. Supercrit. Fluids 2008, 45, 102–111. [Google Scholar] [CrossRef]
- Pollak, S.; Petermann, M.; Kareth, S.; Kilzer, A. Manufacturing of pulverised nanocomposites-dosing and dispersion of additives by the use of supercritical carbon dioxide. J. Supercrit. Fluids 2010, 53, 137–141. [Google Scholar] [CrossRef]
- Hao, J.Y.; Whitaker, M.J.; Serhatkulu, G.; Shakesheff, K.M.; Howdle, S.M. Supercritical fluid assisted melting of poly(ethylene glycol): A new solvent-free route to microparticles. J. Mater. Chem. 2005, 15, 1148–1153. [Google Scholar] [CrossRef]
- Nalawade, S.P.; Picchioni, F.; Janssen, L. Batch production of micron size particles from poly(ethylene glycol) using supercritical CO2 as a processing solvent. Chem. Eng. Sci. 2007, 62, 1712–1720. [Google Scholar] [CrossRef]
- Calderone, M.; Rodier, E.; Letourneau, J.J.; Fages, J. Solidification of Precirol (R) by the expansion of a supercritical fluid saturated melt: From the thermodynamic balance towards the crystallization aspect. J. Supercrit. Fluids 2007, 42, 189–199. [Google Scholar] [CrossRef]
- Munuklu, P.; Jansens, P.J. Particle formation of an edible fat (rapeseed 70) using the supercritical melt micronization (ScMM) process. J. Supercrit. Fluids 2007, 40, 433–442. [Google Scholar] [CrossRef]
- Vezzu, K.; Borin, D.; Bertucco, A.; Bersani, S.; Salmaso, S.; Caliceti, P. Production of lipid microparticles containing bioactive molecules functionalized with PEG. J. Supercrit. Fluids 2010, 54, 328–334. [Google Scholar] [CrossRef]
- Matsuyama, K.; Mishima, K. Formation of TiO2-polymer composite microparticles by rapid expansion of CO2 saturated polymer suspensions with high shear mixing. J. Supercrit. Fluids 2007, 40, 117–124. [Google Scholar] [CrossRef]
- Nunes, A.V.M.; Almeida, A.P.C.; Marques, S.R.; de Sousa, A.R.S.; Casimiro, T.; Duarte, C.M.M. Processing triacetyl-beta-cyclodextrin in the liquid phase using supercritical CO2. J. Supercrit. Fluids 2010, 54, 357–361. [Google Scholar] [CrossRef]
- Mandzuka, Z.; Skerget, M.; Knez, Z. High Pressure Micronization of Tristearate. J. Am. Oil Chem. Soc. 2010, 87, 119–125. [Google Scholar] [CrossRef]
- Wehowski, M.; Weidner, E.; Kilzer, A. Production of powderous emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2008, 331, 143–149. [Google Scholar] [CrossRef]
- Brion, M.; Jaspart, S.; Perrone, L.; Piel, G.; Evrard, B. The supercritical micronization of solid dispersions by Particles from Gas Saturated Solutions using experimental design. J. Supercrit. Fluids 2009, 51, 50–56. [Google Scholar] [CrossRef]
- Sievers, R.E.; Milewski, P.D.; Sellers, S.P.; Kusek, K.D.; Kleutz, P.G.; Miles, B.A. Supercritical CO2-assisted methods for the production and pulmonary administration of pharmaceutical aerosols. J. Aerosol. Sci. 1998, 29, S1271–S1272. [Google Scholar] [CrossRef]
- Sievers, R.E.; Huang, E.T.S.; Villa, J.A.; Kawamoto, J.K.; Evans, M.M.; Brauer, P.R. Low-temperature manufacturing of fine pharmaceutical powders with supercritical fluid aerosolization in a Bubble Dryer (R). Pure Appl. Chem. 2001, 73, 1299–1303. [Google Scholar] [CrossRef]
- Sievers, R.E.; Clark, G.J.; Villa, A.; Alargov, D.; Rinner, L.; Cape, S.P.; Huang, E.T.S. Micronization of inhalable drugs with liquid carbon dioxide at near ambient conditions. J. Aerosol. Med. 2003, 16, 213. [Google Scholar]
- Sellers, S.P.; Clark, G.S.; Sievers, R.E.; Carpenter, J.F. Dry powders of stable protein formulations from aqueous solutions prepared using supercritical CO2-assisted aerosolization. J. Pharm. Sci. 2001, 90, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Della Porta, G.; Adami, R.; del Gaudio, P.; Prota, L.; Aquino, R.; Reverchon, E. Albumin/Gentamicin Microspheres produced by supercritical assisted atomization: Optimization of size, drug loading and release. J. Pharm. Sci. 2010, 99, 4720–4729. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Spada, A. Crystalline microparticles of controlled size produced by supercritical-assisted atomizatione. Ind. Eng. Chem. Res. 2004, 43, 1460–1465. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G.; Spada, A. Ampicillin micronization by supercritical assisted atomization. J. Pharm. Pharmacol. 2003, 55, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Antonacci, A. Drug-polymer microparticles produced by supercritical assisted atomization. Biotechnol. Bioeng. 2007, 97, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Lamberti, G.; Antonacci, A. Supercritical fluid assisted production of HPMC composite microparticles. J. Supercrit. Fluids 2008, 46, 185–196. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R.; Scognamiglio, M.; Fortunato, G.; Della Porta, G. Beclomethasone Microparticles for Wet Inhalation, Produced by Supercritical Assisted Atomization. Ind. Eng. Chem. Res. 2010, 49, 12747–12755. [Google Scholar] [CrossRef]
- Li, Z.Y.; Jiang, J.Z.; Liu, X.W.; Tang, H.H.; Wei, W. Experimental investigation on the micronization of aqueous cefadroxil by supercritical fluid technology. J. Supercrit. Fluids 2009, 48, 247–252. [Google Scholar] [CrossRef]
- Reverchon, E.; Antonacci, A. Cyclodextrins micrometric powders obtained by supercritical fluid processing. Biotechnol. Bioeng. 2006, 94, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Antonacci, A. Chitosan microparticles production by supercritical fluid processing. Ind. Eng. Chem. Res. 2006, 45, 5722–5728. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R.; Caputo, G. Production of cromolyn sodium microparticles for aerosol delivery by supercritical assisted atomization. AAPS Pharmscitech 2007, 8, 272–280. [Google Scholar] [CrossRef]
- Della Porta, G.; Ercolino, S.F.; Parente, L.; Reverchon, E. Corticosteroid microparticles produced by supercritical-assisted atomization: Process optimization, product characterization, and “in vitro” performance. J. Pharm. Sci. 2006, 95, 2062–2076. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Della Porta, G. Particle design using supercritical fluids. Chem. Eng. Technol. 2003, 26, 840–845. [Google Scholar] [CrossRef]
- Reverchon, E.; Spada, A. Erythromycin micro-particles produced by supercritical fluid atomization. Powder Technol. 2004, 141, 100–108. [Google Scholar] [CrossRef]
- Li, Z.Y.; Jiang, J.Z.; Liu, X.W.; Zhao, S.X.; Xia, Y.J.; Tang, H.H. Preparation of erythromycin microparticles by supercritical fluid expansion depressurization. J. Supercrit. Fluids 2007, 41, 285–292. [Google Scholar] [CrossRef]
- Miao, S.F.; Yu, J.P.; Du, Z.; Guan, Y.X.; Yao, S.J.; Zhu, Z.Q. Supercritical fluid extraction and micronization of ginkgo flavonoids from ginkgo biloba leaves. Ind. Eng. Chem. Res. 2010, 49, 5461–5466. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G.; Spada, A.; Antonacci, A. Griseofulvin micronization and dissolution rate improvement by supercritical assisted atomization. J. Pharm. Pharmacol. 2004, 56, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Jiang, J.Z.; Liu, X.W.; Zhao, S.X.; Xia, Y.J.; Wang, J. Preparation of griseofulvin microparticles by supercritical fluid expansion depressurization process. Powder Technol. 2008, 182, 459–465. [Google Scholar] [CrossRef]
- Della Porta, G.; de Vittori, C.; Reverchon, E. Supercritical assisted atomization: A novel technology for microparticles preparation of an asthma-controlling drug. AAPS Pharmscitech 2005, 6, E421–E428. [Google Scholar] [CrossRef] [PubMed]
- Adami, R.; Osseo, L.S.; Reverchon, E. Micronization of Lysozyme by Supercritical Assisted Atomization. Biotechnol. Bioeng. 2009, 104, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Adami, R.; de Marco, I.; Laudani, C.G.; Spada, A. Pigment Red 60 micronization using supercritical fluids based techniques. J. Supercrit. Fluids 2005, 35, 76–82. [Google Scholar] [CrossRef]
- Reverchon, E.; Antonacci, A. Polymer microparticles production by supercritical assisted atomization. J. Supercrit. Fluids 2007, 39, 444–452. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G. Micronization of antibiotics by supercritical assisted atomization. J. Supercrit. Fluids 2003, 26, 243–252. [Google Scholar] [CrossRef]
- Wang, Q.; Guan, Y.X.; Yao, S.J.; Zhu, Z.Q. Microparticle formation of sodium cellulose sulfate using supercritical fluid assisted atomization introduced by hydrodynamic cavitation mixer. Chem. Eng. J. 2010, 159, 220–229. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G. Terbutaline microparticles suitable for aerosol delivery produced by supercritical assisted atomization. Int. J. Pharm. 2003, 258, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Jiang, J.Z.; Liu, X.W.; Xia, Y.J.; Zhao, S.X.; Jian, W. Preparation of tetracycline microparticles suitable for inhalation administration by supercritical fluid expansion depressurization. Chem. Eng. Process. 2008, 47, 1317–1322. [Google Scholar] [CrossRef]
- Gimeno, M.; Ventosa, N.; Sala, S.; Veciana, J. Use of 1,1,1,2-tetrafluoroethane (R-134a)-expanded liquids as solvent media for ecoefficient particle design with the DELOS crystallization process. Cryst. Growth Des. 2006, 6, 23–25. [Google Scholar] [CrossRef]
- Munto, M.; Ventosa, N.; Sala, S.; Veciana, J. Solubility behaviors of ibuprofen and naproxen drugs in liquid “CO2-organic solvent” mixtures. J. Supercrit. Fluids 2008, 47, 147–153. [Google Scholar] [CrossRef]
- Munto, M.; Ventosa, N.; Veciana, J. Synergistic solubility behaviour of a polyoxyalkylene block co-polymer and its precipitation from liquid CO2-expanded ethanol as solid microparticles. J. Supercrit. Fluids 2008, 47, 290–295. [Google Scholar] [CrossRef]
- Sala, S.; Elizondo, E.; Moreno, E.; Calvet, T.; Cuevas-Diarte, M.A.; Ventosa, N.; Veciana, J. Kinetically driven crystallization of a pure polymorphic phase of stearic acid from CO2-expanded solutions. Cryst. Growth Des. 2010, 10, 1226–1232. [Google Scholar] [CrossRef]
- Spray Drying: A Review; Pharmainfo.net: Regina, Canada, 2009. Available online: http://www.pharmainfo.net/reviews/spray-drying-review (accessed on 28 September 2009).
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).