Abstract
Ultra-High-Performance Concrete (UHPC) is being increasingly utilized in major engineering projects due to its excellent mechanical properties, strong durability, and superior overall performance. Nevertheless, the widespread use of premium cementitious materials leads to high expenses and a substantial environmental impact. In this work, crushed recycled paste was calcined at 600 °C for two hours to produce calcined recycled fine powder (RFP) with varying hydration reactivity. UHPC was produced using the RFP in place of some of the cement. Chemical activation was accomplished by adding a composite activator system made up of Ca(OH)2, Na2SO4, Na2SiO3·9H2O, and K2SO4 in order to further improve the performance of UHPC. Particle size, viscosity, fluidity, mechanical properties, and hydration products were analyzed to establish the best activator type and dosage, as well as the ideal activation procedure for recycled fine powder. By mass replacement of cementitious materials, when 15.0% of the calcined recycled fine powder was added, the compressive strength of UHPC reached 149.1 MPa, a 23.2% increase over reference UHPC without calcined recycled fine powder. The results show that the calcined recycled fine powder ground for 60 min exhibits the highest activity. More hydrated products were formed in UHPC as a result of the addition of Ca(OH)2. The compressive strength peaked at 162.2 MPa at an incorporation rate of 1.5%, which is 8.8% higher than UHPC without an activator.
1. Introduction
China’s increased infrastructure development has produced a significant amount of waste concrete. The solidified mortar of the leftover concrete is ground to produce the recycled fine powder [1]. Recycled fine powder has some potential reactivity because it contains a considerable number of hydrated products and a specific percentage of un-hydrated cement particles [2,3,4,5,6]. The potential reactivity of recycled fine powder can be successfully triggered to some degree through procedures like chemical activation, mechanical grinding, and heat treatment.
The activation effects of thermal treatment, chemical activation, and their combination of recycled fine powder were examined by Tian Qing et al. [7]. Their research showed that while calcination modifies the mineral structure of the original components in the powder, chemical activation produces an alkaline environment and reactive ions. It was demonstrated that both techniques may give the recycled fine powder hydraulic action. Kang Xiaoming et al. [8] examined the activation effects on recycled fine powder using a variety of techniques, such as thermal treatment at 600 °C and 800 °C, and the activators Ca(OH)2 and Na2SiO3·9H2O. According to the study, heat activation at 800 °C produced the best results, followed by chemical activation with Ca(OH)2. The ideal temperature for activating recycled fine powder through calcination is 600 °C, according to research by Liu Yanni et al. [9]. The effects of combining chemical and physical activation techniques on the reactivity of recycled fine powder were examined by Xiong Kexing et al. [10]. The study showed that this method was considerably more effective than the single activation method, increasing the reactivity index of recycled fine powder by 14.7%.
UHPC has drawn a lot of attention and widespread use in recent years due to its exceptional qualities, which include great strength and durability. However, a lot of cementitious materials, including cement and ultra-fine powders, are used in the manufacturing of UHPC [11,12]. As a result, problems like high cost and significant environmental impact prevent its widespread use. Thus, it is crucial for its application to develop low-carbon UHPC preparation methods without substantially compromising important features [13,14,15]. In accordance with China’s “dual carbon” rules, the activation treatment of recycled fine powder and its application in the creation of UHPC not only increases the resource utilization level of waste concrete but also lowers the amount of cement utilized.
The impacts of composite fine powder and aeolian sand on the mechanical characteristics of UHPC were examined by Liu Chao et al. [16]. According to their research, the best mechanical qualities of UHPC were obtained by substituting 10.0% of cement with composite fine powder and 30.0% of river sand with aeolian sand. The impact of Ca(OH)2 on the UHPC using recycled and waste tuff fine powder was examined by Daosheng Sun et al. [17]. Their findings showed that activating with Ca(OH)2 increased the reactivity of both recovered fine powder and discarded tuff fine powder. Alkali-activated UHPC was created by X.Y. Zhang et al. [18] by replacing the slag in cementitious materials with recycled fine powder. The findings showed that when the water-to-binder ratio was between 0.27 and 0.29 and the recycled fine powder content was between 10.0% and 30.0%, the UHPC demonstrated good rheological and toughened properties.
The activation of recycled fine powder was restricted to regular concrete in earlier research. The amount of recycled fine powder that could be substituted for cement was not maximized since the activation of the recycled fine powder in UHPC was either not taken into consideration or only one activation method was used. Previous studies have identified 600 °C as the optimal calcination temperature [19], which aligns with findings reported by other researchers. This study uses recycled fine powder that was activated by calcination at 600 °C for two hours and then mechanically ground for use in UHPC preparation in order to overcome the problem of low reactivity in recycled fine powder. Chemical activators were also used to improve UHPC’s characteristics. Microstructural testing was used to examine the differences in hydration products of the resulting UHPC.
2. Materials and Methods
2.1. Materials
Cement, silica fume, quartz sand, and superplasticizer are the primary components of UHPC. Taizhou Yangwan Hailuo Cement Co., Ltd., Taizhou, China, is the supplier of P·II 52.5 Portland cement. The Lingshou Tuyun Mineral Products Processing Plant in Shijiazhuang, China, provided silica fume with a SiO2 level of 98.0%. Fengyang Shengli Quartz Sand Co., Ltd., Chuzhou, China, provided two sizes of quartz sand: 425 μm to 212 μm and 212 μm to 106 μm. Polycarboxylate super-plasticizer from Sobute New Materials Co., Ltd., Nanjing, China, has a solid content of 51.5%. Figure 1 and Figure 2 display the particle size distributions of quartz sand, silica fume, and cement.
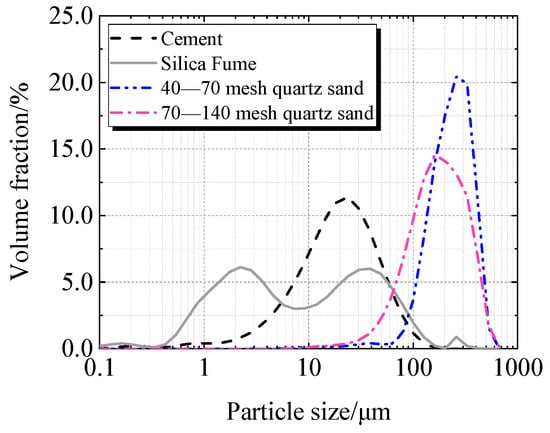
Figure 1.
Particle size distribution of raw material.
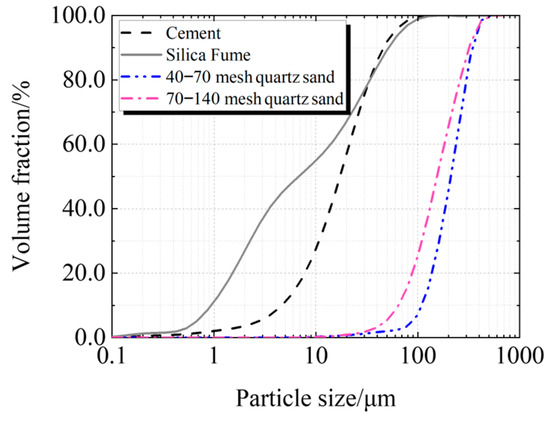
Figure 2.
Cumulative particle size distribution of raw material.
The following process was used to create the recycled fine powder: First, long-term outdoor natural curing was applied to cement paste blocks with a water-to-cement ratio of 0.5. In order to obtain coarse recycled particles, these solid cement paste blocks were crushed before the experiment and sieved through a sieve with a 1 mm size. After that, the coarse recycled particle was heated at a rate of 10 °C per minute until it reached 600 °C in a high-temperature furnace (SX2-12-12A)(Shengwang Instrument Factory, Shaoxing, China). It was then kept at that temperature for 120 min, and after the power was turned off, it was allowed to cool gradually for 6 h to room temperature within the furnace. In order to produce calcined recycled fine powders of various fineness, the recycled powder was ultimately ground for 30, 60, 90, and 120 min, respectively. Figure 3 and Figure 4 display the XRD and TG-DTG of recycled fine powder with and without calcination (reference specimen).
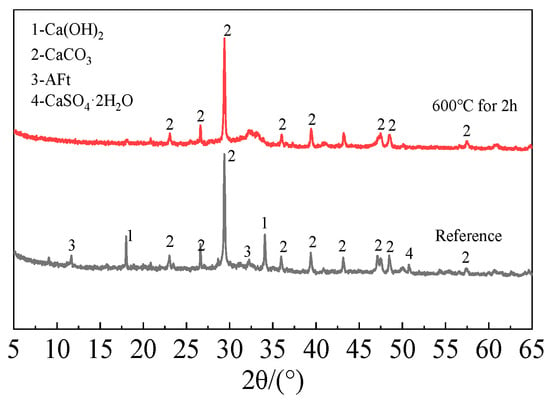
Figure 3.
XRD pattern of recycled fine powder.
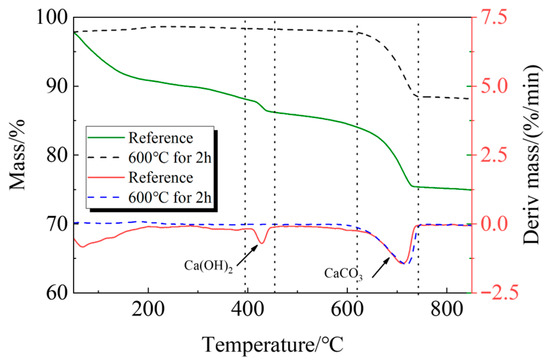
Figure 4.
TG-DTG of recycled fine powder.
All of the chemical activators, Ca(OH)2, Na2SO4, Na2SiO3·9H2O, and K2SO4, are analytical reagents. Sinopharm Chemical Reagent Co., Ltd., Shanghai, China, supplied Ca(OH)2 and Na2SO4. Tianjin Kemiou Chemical Reagent Co., Ltd., located in Tianjin, China, provided the Na2SiO3·9H2O and K2SO4.
2.2. Mix Proportion and Preparation Process
The fundamental mix proportion of UHPC was determined through single-factor experiments. An initial mix proportion was determined based on previous research, with the following composition: mass:cement:silica fume:quartz:sand:water:superplasticizer = 45.0%:5.0%:50.0%:10.0%:0.5% (a 50:50 blend of quartz sand with particle sizes of 425–212 μm and 212–106 μm). Subsequently, the silica fume content was varied by replacing cement in different proportions to evaluate the flowability and compressive strength of UHPC. The optimal silica fume content was determined to be 12.5%, which provided satisfactory fluidity and the highest compressive strength. Following this, while maintaining the silica fume content at 12.5%, the quartz sand content was varied to assess its influence on fluidity and compressive strength, leading to the identification of the optimal quartz sand content. This stepwise approach, where each subsequent raw material’s optimal content was determined based on previously established optimal contents of other materials, was systematically applied, ultimately yielding the fundamental UHPC mix proportion presented in Table 1. Depending on the composition, 5.0%, 10.0%, 15.0%, and 20.0% of the cement was replaced with recycled fine powder that had been processed for varying amounts of time [20]. Doses of 0.5%, 1.0%, 1.5%, and 2.0% of the total mass of binder were used to introduce chemical activators.

Table 1.
Basic mix proportion of UHPC (kg·m−3).
The following stages were taken in the UHPC preparation process: All of the cementitious materials and quartz sands were added to the mixer and combined for one minute. Next, half of the mass of the water and chemical admixture solution was added and mixed for five minutes. Finally, the residue solution was added to the mixer and stirred for an additional five minutes before being discarded.
2.3. Test Methods
The following steps were included in the mechanical grinding process: Fill a ball mill (type: WZM-5L) with 500 g of recycled fine powder, the ceramic grinding balls occupied one-third of the mill jar’s volume, set the grinding time, at a rotational speed of 100 r/min, run it for the specified amount of time, and then gather the sample for testing. An intelligent laser particle size analyzer (type: Bettersize 2000) (Bettersize Instruments Ltd., Dandong, China.) was used to examine the particle size distribution of recycled fine powder with varying grinding times.
The study examined the compressive strength of hardened UHPC as well as the fluidity and viscosity of fresh UHPC. Fresh UHPC was evaluated for fluidity in accordance with GB 2419-2005 [21].
The following steps were involved in the viscosity measuring procedure: The fresh UHPC was poured into a 500 mL beaker and placed under a digital viscometer (Type: DV-2) (Shanghai Fangrui Instrument Co., Ltd., Shanghai, China). The viscometer’s rotor was submerged in the fresh UHPC to a predetermined depth, and the viscosity value was recorded every minute after the viscometer ran. The final result was the average of three consecutive measurements.
The toughened UHPC was subjected to a strength test in compliance with GB/T 17671-2021 [22]. Before being demolded, prism samples measuring 40 mm by 40 mm by 160 mm were created and kept in a conventional curing room for a day. After that, the samples were steam cured at 90 °C for 48 h, and their compressive strength was measured once they had cooled to room temperature [23].
The conductivity and pH tests were conducted with reference to the literature [24], using a conductivity and pH meter (model HI1131). The procedure involved filling a 500 mL beaker with 500 g of distilled water and a magnetic stir bar. After that, the beaker was placed on a water bath with a magnetic thermostat. The thermometer, pH electrode, and conductivity electrode were all submerged in the beaker. The conductivity and pH meter were turned on after 10.0 g of the test powder was quickly added to the beaker, after the water temperature reached 25 °C. Up until the test was finished after 24 h, the conductivity and pH meter gathered data every 10 s.
The dried UHPC paste samples were crushed, powdered, and run through an 80 μm-opening sieve for the TG-DTG and X-ray diffraction tests. A D8 Advance polycrystalline X-ray diffractometer from the German company Bruker AXS (Karlsruhe, Germany) was used for the X-ray diffraction test. Diffraction patterns were recorded between 5° and 65° at a step size of 5°/min. A PerkinElmer Pyris 1 TGA from Springfield, IL, USA was used to perform the TG-DTG test in a nitrogen environment at 10 °C per minute to 850 °C.
3. Results and Discussion
3.1. Effect of Grinding Time on Properties of UHPC Mixed Recycled Fine Powder
Table 2, Figure 5 and Figure 6 display the particle characteristics of calcined recycled fine powder milled for various times. Table 2 shows that the calcined recycled fine powder’s particle size gradually decreases as the grinding time increases. The D10, D50, and D90 decrease when the grinding time exceeds 30 min, which is more noticeable when the grinding time exceeds 60 min. Figure 5 and Figure 6 show that the proportion of particles sized between 1 μm to 100 μm increases significantly with different grinding times.

Table 2.
D10, D50, and D90 of calcined recycled fine powder ground for different times.
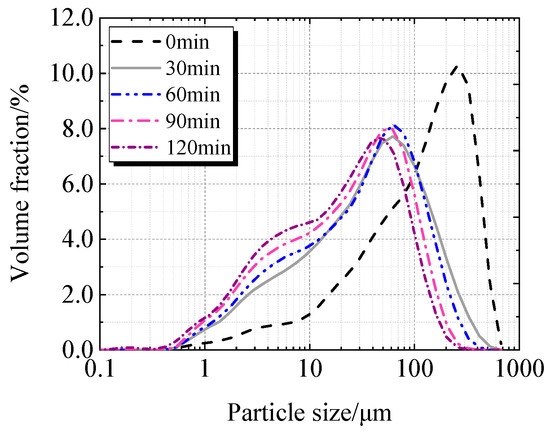
Figure 5.
Particle size distribution of calcined recycled fine powder ground for different times.
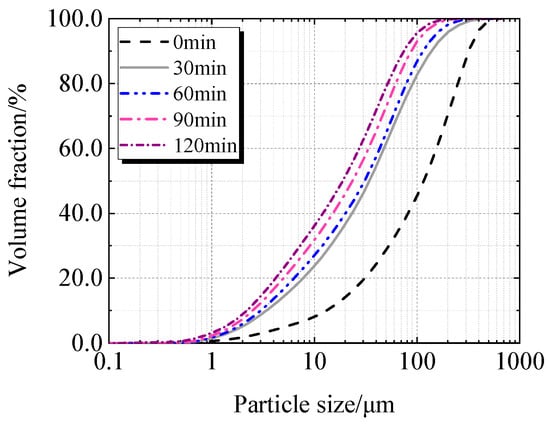
Figure 6.
Cumulative particle size distribution of calcined recycled fine powder ground for different times.
The viscosity, fluidity, and compressive strength of UHPC comprising calcined recycled fine powder milled at various times are shown in Figure 7, Figure 8 and Figure 9. Figure 7 illustrates how the viscosity of UHPC progressively rises as the amount of calcined recycled fine powder increases. The UHPC, including calcined recycled fine powder ground for 120 min shows the greatest viscosity of 20,962.8 mPa·s when the content of calcined recycled fine powder reaches 20.0%. This is 353.5% greater than the reference UHPC without calcined recycled fine powder. The increased specific surface area and improved water absorption capacity of the ground recycled fine powder are responsible for the increase in viscosity of UHPC.
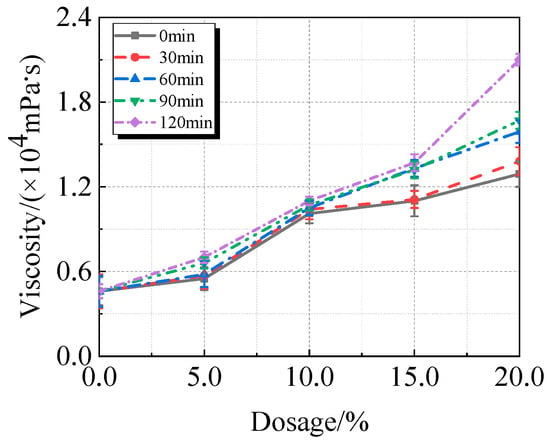
Figure 7.
Viscosity of UHPC mixed with calcined recycled fine powder ground for different durations.
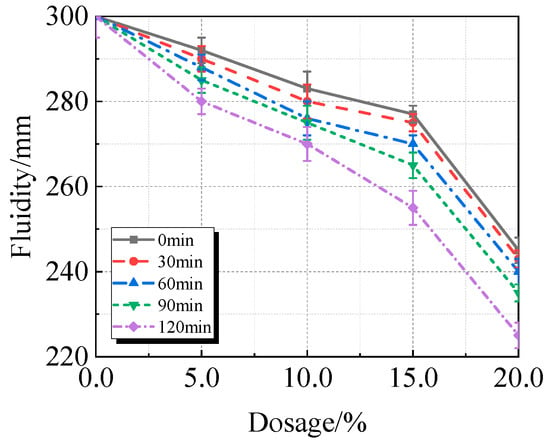
Figure 8.
Fluidity of UHPC mixed with calcined recycled fine powder ground for different durations.
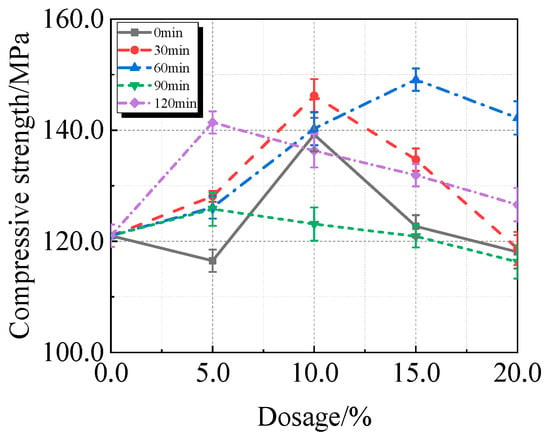
Figure 9.
Compressive strength of UHPC mixed with calcined recycled fine powder ground for different durations.
According to Figure 8, the fluidity of UHPC sharply declines between 15.0% and 20.0% of calcined recycled fine powder that is ground at the same time. As the grinding time increases, the fluidity of UHPC decreases with the same amount of calcined recycled fine powder. The decrease in UHPC fluidity is caused by both the longer grinding time and the higher concentration of calcined recycled fine powder. In comparison to the reference UHPC without calcined recycled fine powder, the UHPC containing 20.0% calcined recycled fine powder ground for 120 min exhibits a fluidity reduction of roughly 25.0%. The fundamental mechanism is that when recycled fine powder is calcined, hydration products such as AFt and Ca(OH)2 break down [25,26]. When these compounds come into contact with water, they rehydrate, which uses up mixing water. Additionally, the increased specific surface area of the particles from longer grinding times increases water demand, which raises the viscosity of UHPC and reduces its fluidity. The water can be consumed by the rehydration of these compounds when they come into contact with water. Additionally, the increased specific surface area of the particles due to the longer grinding time increases the water demand, which raises the viscosity and consequently decreases the fluidity of UHPC.
The compressive strength of UHPC is significantly increased by the addition of calcined recycled fine powder, as shown in Figure 9. A calcined recycled fine powder concentration of 15.0% and a grinding duration of 60 min yield the maximum compressive strength, reached 149.1 MPa, which is 23.2% greater than that of the reference UHPC without calcined recycled fine powder.
Following calcination, the recycled fine powder’s Si and Al structures disintegrate, changing from a stable to a metastable state [27]. By increasing the amount of cementitious materials in the hydration products and promoting the hydration reaction, synergistic interaction with volcanic ash and silicon fume [28]; Furthermore, the material particle size distribution is continuous, structural pores may be successfully filled, and the quartz sand is successfully contained in the cement–silica fume–calcined recycled fine powder ternary cementitious system [29], this transition improves the mechanical characteristics of UHPC that incorporates calcined recycled fine powder.
3.2. Effect of Activators on Properties of UHPC Mixed with Recycled Fine Powder
The viscosity, fluidity, and compressive strength of UHPC with varying concentrations of Ca(OH)2, Na2SO4, Na2SiO3·9H2O, or K2SO4, along with 15% calcined recycled fine powder milled for 60 min, are displayed in Figure 10, Figure 11 and Figure 12.
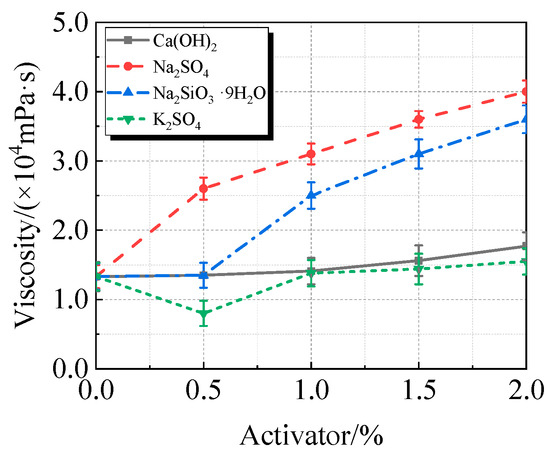
Figure 10.
Viscosity of calcined recycled fine powder UHPC mixed with different dosages of activators.
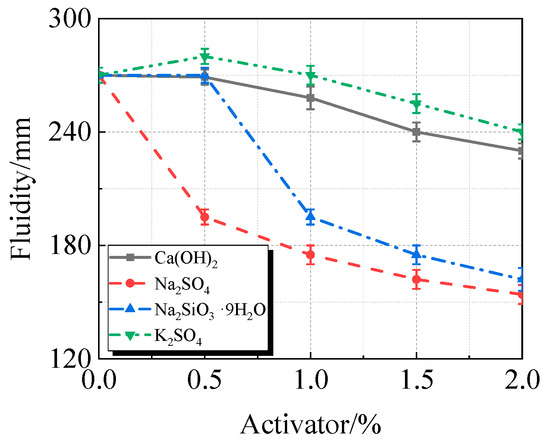
Figure 11.
Fluidity of calcined recycled fine powder UHPC mixed with different dosages of activators.
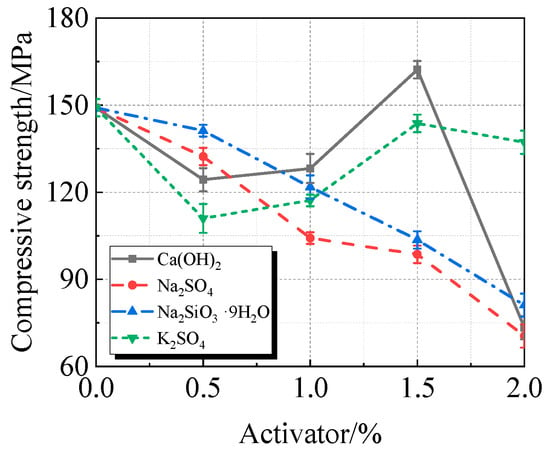
Figure 12.
Compressive strength of calcined recycled fine powder UHPC mixed with different dosages of activators.
The addition of activators typically increases the viscosity and decreases the fluidity of UHPC containing calcined recycled fine powder, as seen in Figure 10 and Figure 11. At a 2.0% dosage, Na2SO4 had the most noticeable effect, increasing viscosity by 200.4% and decreasing fluidity by 43.0%. K2SO4, on the other hand, has the least impact on viscosity, increasing viscosity by 16.5% and decreasing fluidity by 11.1% at a dosage of 2.0%. In terms of viscosity and fluidity, the effects of Ca(OH)2 and Na2SiO3·9H2O fall between those of Na2SO4 and K2SO4.
Figure 12 illustrates how Ca(OH)2 significantly increases the compressive strength of UHPC that incorporates calcined recycled fine powder. When compared to the reference UHPC without any activator, the most significant improvement is seen at an ideal dosage of 1.5%, where the compressive strength reached 162.2 MPa, yielding an 8.8% increase in strength.
Ca(OH)2 enhances the UHPC’s alkaline environment using calcined recycled fine powder, encouraging cementitious material hydration. Concurrently, the Ca2+ takes part in the system’s ion exchange, resulting in the formation of C-S-H gel and other hydration products [30]. The mechanical property is further improved by these goods’ further development of an interwoven network structure.
3.3. Electrical Conductivity and pH Value
The electrical conductivity and pH of the UHPC solution with varying concentrations of calcined recycled fine powder crushed for 60 min are displayed in Figure 13 and Figure 14. As the dosage of the calcined recycled fine powder increases, the UHPC solution’s pH and electrical conductivity both drop, as Figure 13 and Figure 14 demonstrate. This pattern implies that the recycled fine powder’s ability to dissolve ions is lower than that of cement.
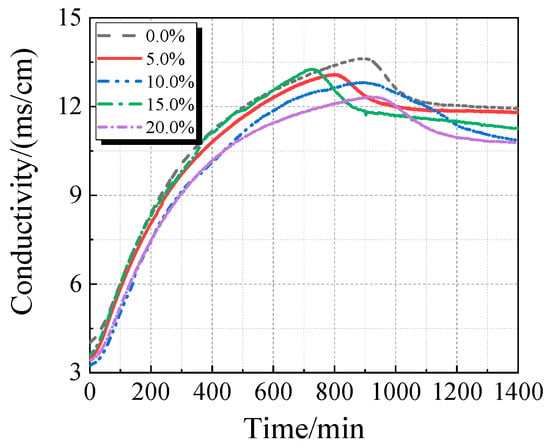
Figure 13.
Conductivity of UHPC solution mixed with different contents of calcined recycled fine powder that was ground for 60 min.
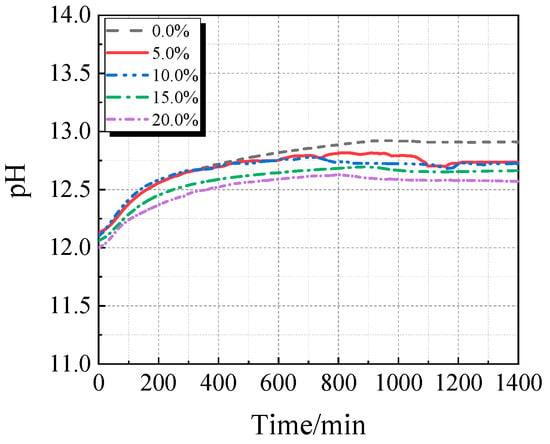
Figure 14.
pH of UHPC solution mixed with different contents of calcined recycled fine powder that was ground for 60 min.
The electrical conductivity and pH value of the UHPC solution with calcined recycled fine powder and varying Ca(OH)2 dosages are displayed in Figure 15 and Figure 16. When Ca(OH)2 is added, the solution’s electrical conductivity drops, as seen in Figure 15. Ca2+ is released from the calcined recycled fine powder when it is combined with distilled water, increasing the electrical conductivity. But when Ca(OH)2 is added, the common-ion effect suppresses the dissolution of Ca2+ from the recycled powder [31], which lowers conductivity. Because Ca(OH)2 is naturally alkaline, as Figure 16 illustrates, the pH of the solution rises as the dosage of Ca(OH)2 increases.
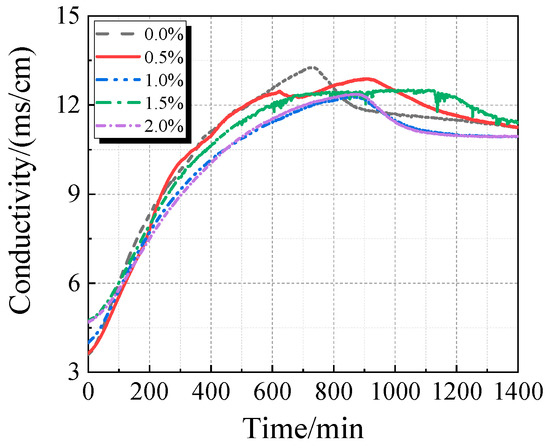
Figure 15.
Conductivity of calcined recycled fine powder UHPC solution mixed with different dosages of Ca(OH)2.
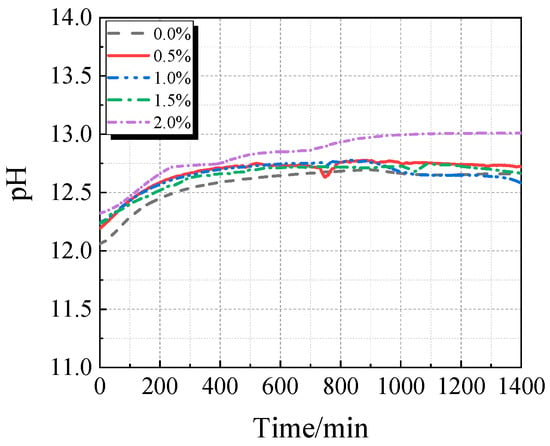
Figure 16.
pH of calcined recycled fine powder UHPC solution mixed with different dosages of Ca(OH)2.
3.4. XRD Analysis
The XRD patterns of UHPC with varying amounts of calcined recycled fine powder crushed for 60 min with and without Ca(OH)2 are displayed in Figure 17. As the amount of calcined recycled fine powder increases, as seen in Figure 17a, the diffraction peaks of Ca(OH)2 and CaCO3 increase while those of C3S and C2S decrease. This suggests that the induction of calcined recycled powder can facilitate the hydration process. However, as the content of calcined recycled fine powder reaches 20.0%, the peaks of C3S and C2S become more prominent, suggesting a slowdown in the hydration rate. The high-water requirement of calcined recycled fine powder is the cause of this phenomenon. Because UHPC has a low water-to-binder ratio by nature, adding too much calcined recycled fine powder causes competition for water, which negatively impacts cement hydration. The addition of Ca(OH)2 weakens the diffraction peaks of C3S and C2S in the hydrated UHPC, as seen in Figure 17b, suggesting that Ca(OH)2 facilitates the hydration procedure. Nevertheless, the diffraction peaks of C3S and C2S rise when the Ca(OH)2 dose approaches 2.0%, indicating that an overly alkaline environment limits the hydration process [32], which ultimately results in a decrease in the compressive strength of UHPC. This is consistent with previous studies on the compressive strength patterns of UHPC with calcined recycled fine powder. The appropriate incorporation of calcined recycled fine powder helps the formation of hydration products, and the addition of Ca(OH)2 creates an alkaline environment conducive to hydration, further promoting the hydration process of the system, increasing the system’s pH, and enhancing the rate of ion dissolution.
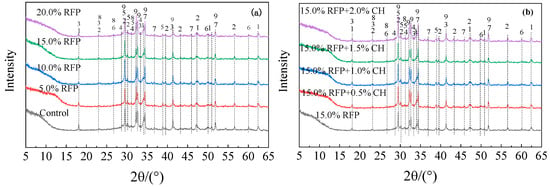
Figure 17.
XRD pattern of hydrated UHPC specimen mixed with calcined recycled fine powder with and without of Ca(OH)2, (a) Recycled fine powder calcined and ground for 60 min, (b) Ca(OH)2 activated recycled fine powder. 1—Ca(OH)2 (Calcium hydroxide), 2—CaCO3 (Calcium carbonate), 3—Aft (Alunite), 4—CaSO4·2H2O (Gypsum), 5—C2S (Dicalcium silicate), 6—SiO2 (Quartz), 7—C2AS (Calcium alumina feldspar), 8—AFm, 9—C3S (Calcium silicate).
3.5. TG-DTG Analysis
The breakdown of hydration products like C-S-H occurs in the first stage between 105 °C and 380 °C; the breakdown of Ca(OH)2 occurs in the second stage between 380 °C and 450 °C; and the breakdown of CaCO3 occurs in the third stage between 615 °C and 710 °C [33,34].
The TG-DTG of UHPC, including 0.0% to 20.0% calcined recycled fine powder milled for 60 min, is displayed in Figure 18 and Figure 19. Between 105 °C and 380 °C, the hydrated UHPC specimens containing calcined recycled fine powder show more mass loss than those without the powder, suggesting that the powder encourages the development of hydration products. In line with the findings in Figure 17a, the mass loss decreased when the dosage of calcined recycled fine powder surpassed 15.0%, indicating a reduction in hydration products. The TG-DTG curves of UHPC with 15.0% calcined recycled fine powder and 0.0% to 2.0% Ca(OH)2 are displayed in Figure 20 and Figure 21. The hydrated UHPC specimens show more mass loss between 105 °C and 380 °C at a Ca(OH)2 dosage of 1.5% than the specimens without Ca(OH)2 addition. It suggests that adding 1.5% Ca(OH)2 encourages UHPC hydration, resulting in the production of more hydration products. In line with the findings shown in Figure 17b, a decrease in mass loss was seen when the dosage of Ca(OH)2 exceeded 1.5%, suggesting a decrease in hydration products.
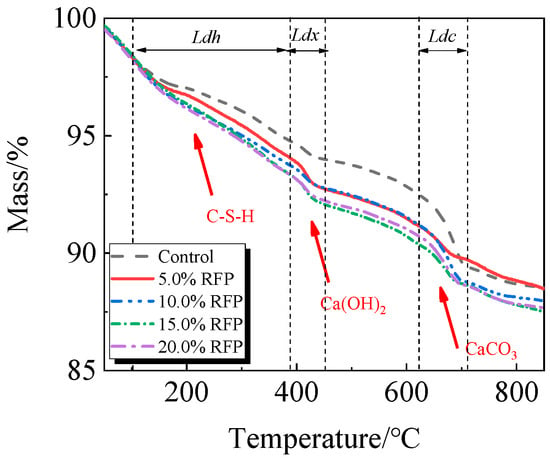
Figure 18.
TG of hydrated UHPC mixed with calcined recycled fine powder.
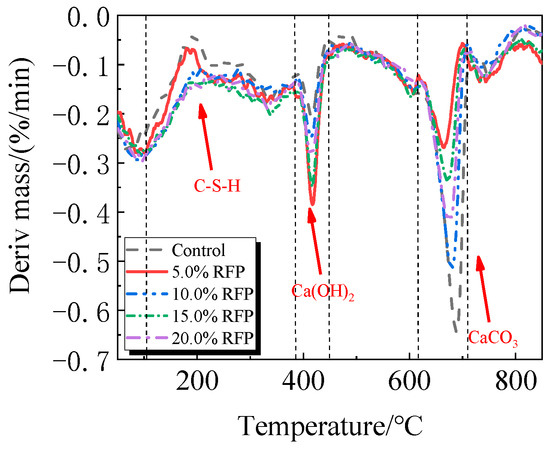
Figure 19.
DTG of hydrated UHPC mixed with calcined recycled fine powder.
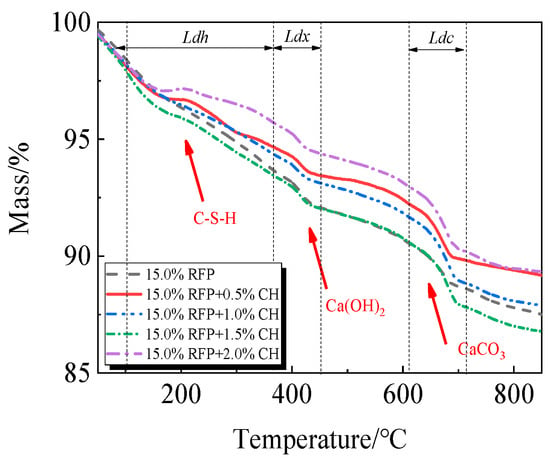
Figure 20.
TG of UHPC mixed with different dosages of Ca(OH)2 and 15.0% calcined recycled fine powder.
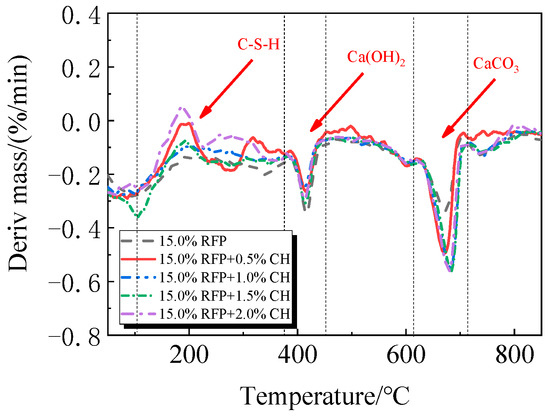
Figure 21.
DTG of UHPC mixed with different dosages of Ca(OH)2 and 15.0% calcined recycled fine powder.
3.6. Degree of Hydration Analysis
Compressive strength and the degree of hydration of cement-based materials are favorably connected. Equations (1) and (2) can be used to determine the degree of hydration of UHPC based on the TG curve [35].
where Wb and α represent the chemically bound water content and the degree of hydration of the specimen, respectively, and Ldh, Ldx, and Ldc represent the mass loss resulting from the breakdown of C-S-H gel, Ca(OH)2, and CaCO3, respectively.
The chemically bound water content and degree of hydration of UHPC, including 0.0% to 20.0% calcined recycled fine powder crushed for 60 min, are displayed in Figure 22 and Figure 23. According to the findings, a larger dosage of calcined recycled fine powder increases the amount of chemically bonded water and the degree of hydration when the dose ranges from 0.0% to 15.0%. Nevertheless, going over the 15.0% dosage has a negative impact on the hydration process.
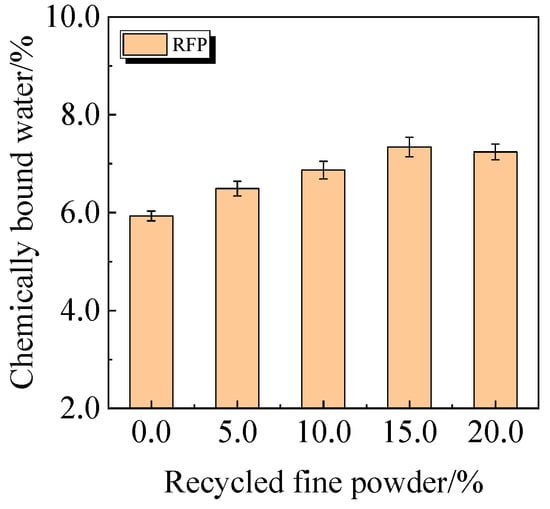
Figure 22.
Chemically bound water of UHPC mixed with calcined recycled fine powder.
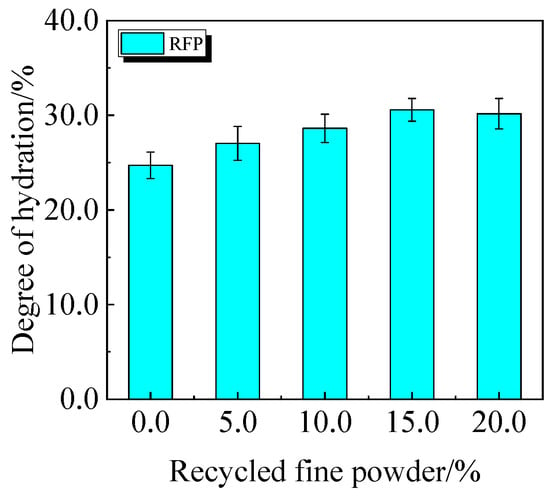
Figure 23.
The hydration degree of UHPC mixed with calcined recycled fine powder.
The chemically bound water content and degree of hydration of UHPC, including 15.0% calcined recycled fine powder milled for 60 min with the addition of 0.0% to 2.0% Ca(OH)2, are displayed in Figure 24 and Figure 25. The hydration of the calcined recycled fine powder is much improved by the addition of 1.5% Ca(OH)2, as the figures demonstrate. The addition of calcined recycled fine powder and Ca(OH)2 improves the hydration process and the creation of hydration products, resulting in an overall increase in the degree of hydration. This investigation strongly supports the earlier XRD and compressive strength patterns.
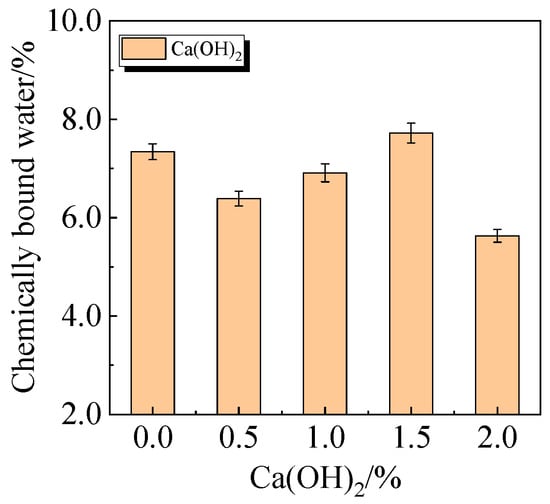
Figure 24.
Chemically bound water of UHPC mixed with different dosages of Ca(OH)2 and 15.0% calcined recycled fine powder.
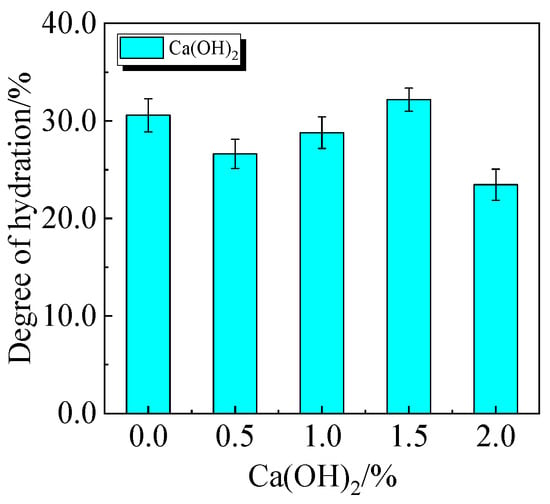
Figure 25.
The hydration degree of UHPC mixed with different dosages of Ca(OH)2 and 15.0% calcined recycled fine powder.
3.7. SEM Analysis
To better analyze the structural impact of coupled activated recycled fine powder on UHPC, it was compared with the control group. As shown in Figure 26 and Figure 27, the incorporation of calcined recycled fine powder and calcium hydroxide makes the UHPC structure more compact. Therefore, it can be said that the coupled activated recycled fine powder has a significant enhancement effect on the hydration of UHPC, resulting in higher compressive strength [36].
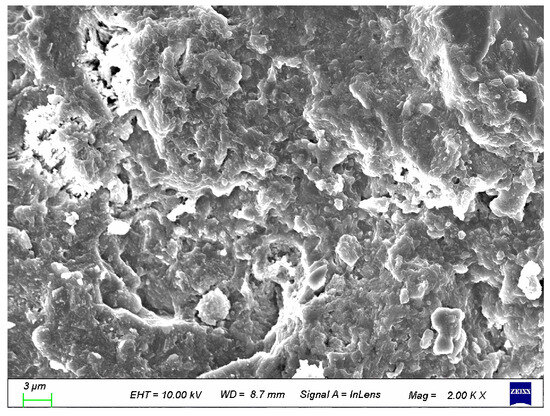
Figure 26.
SEM of control group.
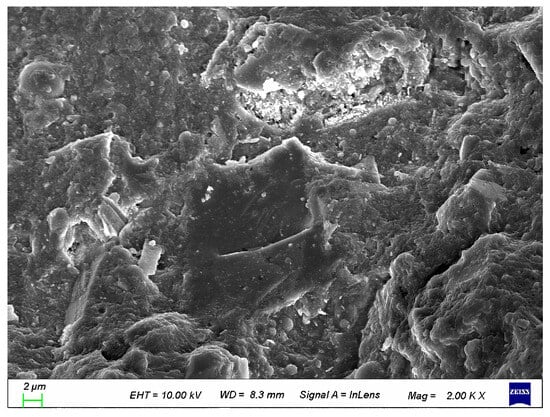
Figure 27.
SEM of UHPC mixed with 2.0% of Ca(OH)2 and 15.0% calcined recycled fine powder.
3.8. Carbon Emission of UHPC Mixed Calcined Recycled Fine Powder
The carbon emission factors of the different raw materials utilized in UHPC are displayed in Table 3 [37]. Due to their low dose levels during UHPC synthesis and negligible effect on the overall carbon footprint, carbon emissions from chemical admixtures and activators are regarded as negligible.

Table 3.
Carbon emission factors of raw materials for UHPC (kg·kg−1).
The study’s recycled fine powder needed to be calcined and then ground. No appreciable CO2 emissions are produced since the calcination temperature was set at 600 °C, which is lower than the CaCO3 breakdown temperature. Electricity use is the main cause of carbon emissions during the calcination process. For high-temperature calcination, the carbon emission factor was 0.0622 kg·kg−1 [38], but for ball mill operation, it was 0.0161 kg·kg−1 [39]. As a result, 0.0973(0.0190 + 0.0161 + 0.0622) kg·kg−1 is the total carbon emission factor for the calcination and grinding of recycled fine powder. In comparison to the reference UHPC without the powder, UHPC with 15.0% calcined recycled fine powder shows a 12.9% decrease in carbon emissions, according to the data shown in Table 1 and Table 3. As a result, using calcined recycled fine powder in UHPC improves compressive strength and lowers carbon emissions, both of which support sustainable growth.
4. Conclusions
600 °C calcined recycled fine powder was ground and chemically activated to create ultra-high-performance concrete (UHPC). Analysis was performed on the calcined recycled fine powder grinding process, the choice of chemical activators, and their effectiveness. The following is a summary of the conclusions.
- (1)
- The best qualities are shown by the recycled fine powder that was calcined at 600 °C for two hours and then milled for sixty minutes. UHPC shows a decrease in fluidity and an increase in viscosity when the dosage of the calcined recycled fine powder increases. UHPC reaches its maximum compressive strength at the ideal dosage of 15.0%, which is a 23.2% improvement over the reference UHPC without any recycled fine powder [40].
- (2)
- The activators Ca(OH)2, Na2SO4, Na2SiO3·9H2O, and K2SO4 cause the UHPC containing calcined recycled fine powder to become more viscous and less fluid. Of them, Na2SO4 has the strongest effect and K2SO4 the least; the effects of Ca(OH)2 and Na2SiO3·9H2O are between those of K2SO4 and Na2SO4. Ca(OH)2 can efficiently boost the strength of UHPC; at a dosage of 1.5%, the maximum strength is reached, leading to an 8.8% increase in compressive strength above reference UHPC.
- (3)
- UHPC hydration is enhanced by calcined recycled fine powder; nevertheless, the degree of hydration decreases when the dosage is above 15.0% [41]; The UHPC with 1.5% Ca(OH)2 shows the greatest amount of hydration products at the 15.0% dosage of calcined recycled fine powder; nevertheless, hydration is negatively impacted by dosages over 1.5% Ca(OH)2. Moreover, the carbon emissions linked to cement usage are successfully decreased by partially substituting calcined recycled fine powder for cement.
Author Contributions
C.L.: Visualization, Validation, Resources, Methodology, Writing—Original Draft. M.Z.: Methodology, Formal analysis, Data curation, Writing—Review and Editing. C.Y.: Formal analysis, Conceptualization, Investigation, Writing—Review and Editing, Validation. D.Y. and N.S.: Investigation, Conceptualization, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the support of the National Natural Science Foundation of China Youth Fund Project, Number (52308534).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ma, S.L.; Yin, J.L.; Wu, W.D.; Zeng, Q.L.; Zhao, Y.H. A review of alkali-activated cementitious materials based on construction and demolition waste-derived recycled fine powder. Concrete 2023, 11, 170–174+180. [Google Scholar]
- Likes, L.; Markandeya, A.; Haider, M.M.; Bollinger, D.; McCloy, J.S.; Nassiri, S. Recycled concrete and brick powders as supplements to Portland cement for more sustainable concrete. J. Clean. Prod. 2022, 364, 132651. [Google Scholar] [CrossRef]
- Ortega, J.M.; Letelier, V.; Solas, C.; Moriconi, G.; Climent, M.Á.; Sánchez, I. Long-term effects of waste brick powder addition in the microstructure and service properties of mortars. Constr. Build. Mater. 2018, 182, 691–702. [Google Scholar] [CrossRef]
- Yuan, C.F.; Chen, Y.; Wang, S.B. Study on the influence and mechanism of activation methods on the activity of recycled brick powder. J. Zhengzhou Univ. (Eng. Sci. Ed.) 2022, 43, 97–103+110. [Google Scholar] [CrossRef]
- Li, Z.; Bian, Y.; Zhao, J.; Wang, Y.; Yuan, Z. Recycled concrete fine powder (RFP) as cement partial replacement: Influences on the physical properties, hydration characteristics, and microstructure of blended cement. J. Build. Eng. 2022, 62, 105326. [Google Scholar] [CrossRef]
- Tang, Y.; Xiao, J.; Liu, Q.; Xia, B.; Singh, A.; Lv, Z.; Song, W. Natural gravel-recycled aggregate concrete applied in rural highway pavement: Material properties and life cycle assessment. J. Clean. Prod. 2022, 334, 130219. [Google Scholar] [CrossRef]
- Tian, Q.; Qu, M.J.; Yao, T.S. The coupling of chemical excitation and heat treatment excites the reactive excitation of recycled powders. Bull. Chin. Ceram. Soc. 2023, 42, 1400–1408. [Google Scholar] [CrossRef]
- Tang, X.M.; Li, Y.; Fan, Y.H. Study on the effects of different excitation methods on the properties of recycled powder. Bull. Chin. Ceram. Soc. 2019, 38, 1135–1139. [Google Scholar] [CrossRef]
- Liu, Y.N.; Wu, X.R.; Wang, G.; Zhao, M.Y.; Tong, Y. Effect of roasting process on microstructure and activity index of recycled powder. China Powder Sci. Technol. 2024, 30, 113–120. [Google Scholar] [CrossRef]
- Xiong, K.X.; Zhan, B.G.; Wang, C. Study on physicochemical joint activation of waste concrete recycled powder. J. Hefei Univ. Technol. (Nat. Sci. Ed.) 2024, 47, 1275–1280+1287. [Google Scholar]
- Zheng, Q.; Wu, D.; Zhang, M.; Yu, J.; Wang, X.P. Design and performance studies of red mud-based ultra-high performance concrete. J. Qingdao Univ. Technol. 2024, 45, 1–7+15. [Google Scholar]
- Liu, C.J. Research on the performance and application of a low-carbon waste cement. Cement 2023, 5, 10–15. [Google Scholar] [CrossRef]
- Wu, N.; Ji, T.; Huang, P.; Fu, T.; Zheng, X.; Xu, Q. Use of sugar cane bagasse ash in ultra-high performance concrete (UHPC) as cement replacement. Constr. Build. Mater. 2022, 317, 125881. [Google Scholar] [CrossRef]
- Ding, M.; Yu, R.; Feng, Y.; Wang, S.; Zhou, F.; Shui, Z.; Gao, X.; He, Y.; Chen, L. Possibility and advantages of producing an ultra-high performance concrete (UHPC) with ultra-low cement content. Constr. Build. Mater. 2021, 273, 122023. [Google Scholar] [CrossRef]
- Wei, J.; Cen, K. Empirical assessing cement CO2 emissions based on China’s economic and social development during 2001–2030. Sci. Total Environ. 2019, 653, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, X.; Liu, H.W.; Hu, T.F. Effect of aeolian sand and recycled composite powder on mechanical properties of ultra-high performance concrete. Acta Mater. Compos. Sin. 2022, 39, 5415–5422. [Google Scholar] [CrossRef]
- Sun, D.; Hu, H.; Ma, R.; Hu, X.; Ding, Y.; Wang, A.; Liu, K. Preparation and hydration of low-carbon UHPC with high fraction of activated tuff and recycled fine powders. J. Build. Eng. 2024, 90, 109396. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Fan, M.X.; Zhou, Y.X.; Ji, D.D.; Li, J.H.; Yu, R. Development of a sustainable alkali activated ultra-high performance concrete (A-UHPC) incorporating recycled concrete fines. J. Build. Eng. 2023, 67, 105986. [Google Scholar] [CrossRef]
- Tong, D.B.; Chen, M.; Feng, L.; Lu, C.; Li, W.P.; Guo, J.; Zhang, M. Experimental study on recycled pervious concrete for ecological revetment engineering in small and medium-sized rivers. J. Water Resour. Archit. Eng. 2024, 22, 181–189. [Google Scholar]
- Zhang, C.; Chen, Z.T.; Li, F.Y. Preparation and Performance Regulation of Green Recycled Micro-UHPC. J. Wuhan Univ. Technol. 2025, 47, 7–13. [Google Scholar]
- GB/T 2419-2005; National Technical Committee for Cement Standardization. Method for Determining the Flowability of Cement Mortar. China Standards Press: Beijing, China, 2005.
- GB/T 17671-2021; National Building Materials Industry Bureau. Method for Testing Mortar Strength of Cement (ISO Method). China Standards Press: Beijing, China, 2021.
- Mao, X.; Yang, D.Y.; Cao, Z.L.; Zhao, J.; Chen, L.X.; Wang, T.Z. Research on the performance improvement of self-compacting ultra-high-performance concrete based on image method. Bull. Chin. Ceram. Soc. 2024, 43, 1398–1409. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.S.; Zhao, Y.Z. Effects of Aluminium Salts on the Properties and Hydration of Potassium Magnesium Phosphate Cement. J. Chin. Ceram. Soc. 2025, 53, 1205–1213. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, Y.; Xie, J.; Chen, W.; Xue, Z.; Zhao, G.; Li, Y.; Li, J.; Yang, J. Compressive behaviour and microstructures of concrete incorporating pretreated recycled powder/aggregates:The coupling effects of calcination and carbonization. J. Build. Eng. 2023, 68, 106158. [Google Scholar] [CrossRef]
- Li, J.; Feng, Y.; Zhong, H.; Zhang, B.; Wang, J.; Zhang, B.; Xie, J. Effect of the Pretreatment on the Properties of Cement-Based Recycled Powder. Coatings 2024, 14, 107. [Google Scholar] [CrossRef]
- Li, X.; Liang, L. Research on the activation and properties of recycled concrete powder. Concrete 2023, 11, 105–108+113. [Google Scholar]
- Marvila, M.T.; de Azevedo, A.R.; de Matos, P.R.; Monteiro, S.N.; Vieira, C.M. Materials for Production of High and Ultra-High Performance Concrete: Review and Perspective of Possible Novel Materials. Materials 2021, 14, 4304. [Google Scholar] [CrossRef]
- Chen, J.S.; Qiao, M.; Wu, Q.Y. Effect and mechanism of different chemical activators on the activity of regenerated powder. Bull. Chin. Ceram. Soc. 2023, 42, 1409–1417. [Google Scholar] [CrossRef]
- Chen, T.; Gao, X.; Ren, M. Effects of Autoclave Curing and Fly Ash on Mechanical Properties of Ultra-High Performance Concrete. Constr. Build. Mater. 2018, 158, 864–872. [Google Scholar] [CrossRef]
- Zhang, X.L. Research on Grinding Characteristics and Powder Properties Evaluation of Limestone Coarse Aggregate Waste Concrete. Master’s Thesis, Guangxi University, Nanning, China, 2016; pp. 39–41. [Google Scholar]
- Fang, J.L.; Lu, W.X.; Xu, C.X. Research progress on the activation technology and mechanism of fly ash activity. J. Shanghai Univ. (Nat. Sci. Ed.) 2002, 3, 255–260. [Google Scholar]
- Deboucha, W.; Leklou, N.; Khelidj, A.; Oudjit, M.N. Hydration development of mineral additives blended cement using thermogravimetric analysis (TGA): Methodology of calculating the degree of hydration. Constr. Build. Mater. 2017, 146, 687–701. [Google Scholar] [CrossRef]
- Luo, X.; Jiang, X.; Chen, Q.; Huang, Z. An assessment method of hydration degree of Rice husk ash blended cement considering temperature effect. Constr. Build. Mater. 2021, 304, 124534. [Google Scholar] [CrossRef]
- Chen, L.; Wei, M.; Lei, N.; Li, H. Effect of chemical–thermal activation on the properties of recycled fine powder cementitious materials. Case Stud. Constr. Mater. 2024, 20, e02956. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Chen, W. Effects of Recycled Micro Powder and Mineral Admixtures on the Mechanical Properties and Microstructure of Concrete. Mater. Rev. 2024, 38, 182–187. [Google Scholar]
- Xia, C.C.; Xu, H.; Zhou, Z.; Zhai, W.Q.; He, Z.H. Performance and carbon emission analysis of recycled fine powder self-leveling mortar. Bull. Chin. Ceram. Soc. 2025, 44, 1477–1485. [Google Scholar] [CrossRef]
- Shi, J.; Li, J.; Hai, Y. Research on carbon source analysis and carbon emission accounting of cement industry. Cement 2023, 11, 20–23. [Google Scholar] [CrossRef]
- Yu, X.H.; Li, X.L.; Ma, R.; Shun, H.D.; Su, Z.P. Research on carbon emissions of prefabricated high-ductility recycled micronized concrete structures based on LCA theory. Mater. Rev. 2024, 38, 89–95. [Google Scholar]
- Yang, F.; Ma, Y.; Li, L.; Liu, S.; Hai, R.; Zhu, Z. Early-Age Behaviour of Portland Cement Incorporating Ultrafine Recycled Powder: Insights into Hydration, Setting, and Chemical Shrinkage. Materials 2024, 17, 5551. [Google Scholar] [CrossRef]
- Deng, M.; Xie, X.; Zhuo, J.; He, Y.; Wang, K. Experimental Study on the Strength and Hydration Products of Cement Mortar with Hybrid Recycled Powders Based Industrial-Construction Residue Cement Stabilization of Crushed Aggregate. Materials 2023, 16, 4233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.