Abstract
As a metallurgical solid waste rich in active calcium oxide, magnesium slag (MS) is endowed with significant carbon dioxide sequestration potential due to its inherent properties, providing a feasible path for the simultaneous solution of waste residue disposal and carbon dioxide emission reduction. However, current research has neither clarified the kinetic mechanism (core theoretical support for carbon dioxide sequestration industrialization) nor systematically evaluated the life cycle environmental impacts of MS’s two carbonation routes (direct or indirect leaching carbonation). To address this, this study explores kinetic laws via the single-factor control variable method, and combines life cycle assessment (LCA) to fill the gap, providing key theoretical support for process optimization and engineering promotion. Kinetic results show indirect carbon dioxide sequestration (ICDS) forms an inert silicon-rich layer (core-shrinkage model, mixed control, 28.4 kJ/mol activation energy), while direct carbon dioxide sequestration (DCDS) involves dual-layer formation and pore blockage (mixed control, 14.0 kJ/mol). The ICDS achieves a higher reaction rate of 89%, compared to 63% for the DCDS. In life cycle assessments, DCDS demonstrates outstanding overall environmental sustainability, particularly excelling in carbon dioxide sequestration and acidification control, while ICDS exhibits significant environmental drawbacks (such as high carbon dioxide emissions and ecological toxicity). However, ICDS possesses advantages such as high feedstock utilization and strong synthesis capabilities for high-value-added products. Through targeted optimization, its environmental indicators can be reduced in the future, making it suitable for specific scenarios like high-end calcium carbonate production and resource utilization of low-grade magnesium slag.
1. Introduction
As the third most widely used metal after steel and aluminum, magnesium, with its light weight, high strength, and excellent corrosion resistance, plays a crucial role in emerging industries such as new energy vehicles, aerospace, and electronic devices [1]. China dominates the global magnesium production, accounting for over 85% of the total output, among which the Pidgeon process is the main smelting technology [2]. However, the rapid development of the magnesium industry is accompanied by two pressing challenges: the massive generation of magnesium slag (MS) and the escalating pressure of carbon dioxide (CO2) emission reduction. Approximately 6–8 tons of MS are produced per ton of metallic magnesium [3]. The stockpiling of MS occupies a large amount of valuable land resources, and the slag tends to pulverize in the natural environment. Its fine particles are prone to being scattered by the wind, causing air pollution, affecting air quality, and endangering human respiratory health [4]. The massive emission and accumulation of MS have become a key bottleneck restricting the sustainable development of the magnesium industry.
As an industrial by-product, MS has emerged as an ideal material for CO2 sequestration due to its chemical similarity to steel slag and high reactivity with CO2 [5,6]. Alkaline in nature and rich in magnesium and calcium silicates, MS can form stable carbonates through wet carbonation, achieving the dual benefits of waste resource utilization and greenhouse gas emission reduction [7]. Recent studies have shown that MS can achieve a carbon dioxide sequestration capacity of up to 22.14% (mass fraction) through carbonation. The reaction products-mainly calcium carbonate (calcite and aragonite) and highly polymerized calcium silicate hydrate (C-S-H) gels-not only fix CO2 but also enhance the mechanical properties of the material [8]. The carbonation mechanism involves the dissolution of active MgO and CaO phases in MS, followed by carbonate precipitation that densifies the microstructure and improves compressive strength. This allows MS to replace up to 30% (mass fraction) of clinker in cement formulations without compromising performance [6].
From an environmental perspective, the MS carbonation process aligns with the concept of the circular economy by converting waste into value-added products. For example, ladle furnace slag (LFS)-another by-product of the steel industry-can absorb 8% (mass fraction) of CO2 and immobilize heavy metals such as lead (Pb) through carbonate encapsulation, reducing leaching concentrations by four orders of magnitude [9]. Similarly, MS-based materials for mine backfilling exhibit a CO2 sequestration rate of 14.55% (mass fraction), along with improved rheological properties and early strength development (reaching 8.854 MPa after 7 days of carbonation curing) [7]. These advancements highlight the scalability of MS carbonation, especially in industrial scenarios where waste resources and CO2 emissions intersect.
Despite significant progress in understanding the carbonation efficiency and product formation of MS through mineral carbonation [10], critical gaps remain in quantifying carbonation kinetics and evaluating the environmental footprint of the process. Existing studies have confirmed that mechanochemical activation and optimization of carbonation conditions can enhance the reactivity of calcium/magnesium silicates in MS, doubling CO2 absorption [11]. However, the temporal dynamic characteristics of the carbonation process (such as reaction rate and intermediate phase evolution) in both direct carbonation and indirect leaching carbonation pathways have not been systematically quantified. For instance, early exposure of the MgO-SiO2 system to CO2 accelerates the formation of hydrated magnesium hydroxycarbonate (HMHC) but reduces the content of M-S-H gels, indicating a trade-off between carbonation rate and material stability [12]. Yet, the differences in such laws between the two MS carbonation pathways lack in-depth analysis. In addition, although MS-based materials demonstrate excellent CO2 sequestration capacity and mechanical properties [7], the life cycle assessment (LCA) of their entire chain-including energy input, CO2 emissions from pretreatment (e.g., grinding), and long-term stability of carbonation products-remains insufficiently explored. Notably, innovative technologies such as thermochemical coupling have achieved an energy efficiency of 47.6% in CO2 mineralization [7,13,14,15], providing new ideas for reducing the energy consumption of MS carbonation processes. However, the large-scale integration of such technologies with direct/indirect MS carbonation still requires verification.
The existence of these aforementioned gaps has hindered the large-scale application of MS carbonation technology in fields such as construction and mining. Addressing the core issues of insufficient systematic quantification of the kinetic mechanisms of direct carbonation and indirect leaching carbonation, as well as the unclear full-chain environmental impacts in LCA, this study aims to achieve “high-efficiency CO2 sequestration of MS coupled with low-CO2 emission” and conducts two key research tasks: firstly, systematically investigate the kinetic characteristics of both direct carbonation and indirect leaching carbonation processes, clarifying the rate-determining steps of reaction rate. Establish kinetic models for the two carbonation pathways to quantify the correlation between CO2 sequestration efficiency and reaction rate under different conditions. Secondly, construct a full-LCA framework for the MS carbonation process, covering the entire stages of raw material pretreatment, carbonation reaction, and product application, to evaluate its energy consumption, carbon dioxide emissions, and environmental sustainability. This study innovatively integrates kinetic mechanism quantification and full-chain LCA for the first time, systematically clarifying the rate-determining steps of two carbonation pathways and their environmental footprint differences. Unlike previous studies that only focus on carbonation efficiency, this work realizes the dual breakthrough of ‘theoretical mechanism + environmental impact’ evaluation, providing a comprehensive decision-making basis for industrial application of MS carbon sequestration technology.
2. Materials and Methods
2.1. Experimental Raw Materials
The raw material utilized in this study is the MS generated during the production of metallic magnesium via the Pidgeon process at a non-ferrous metal smelter located in Yulin, Shaanxi Province, China. Raw MS was first ground in a ball mill for 5–8 min to produce a fine powder, then screened through a 200-mesh sieve to control particle size below 74 microns. The screened slag powder was thoroughly mixed and dried for subsequent analysis and experimentation. The chemical composition of the MS used in the experiments is shown in Table 1.

Table 1.
Chemical composition analysis of MS.
2.2. Experimental Method
Mineral carbonation achieves permanent CO2 sequestration by reacting CO2 with calcium- and magnesium-rich silicate minerals or industrial wastes (e.g., steel slag, fly ash) to form stable carbonates. Its technical routes are mainly classified into the indirect and direct methods.
2.2.1. Indirect Method
The indirect CO2 sequestration (ICDS) first leaches calcium and magnesium ions from MS via chemical extraction (e.g., acid or alkali dissolution), then reacts these ions with CO2 in a separate reactor to precipitate as carbonates. The process consists of two steps: “dissolution and precipitation”. In the ICDS experiments of this study, ammonium chloride solution was used as the leaching agent, mixed and stirred with MS powder at a liquid-to-solid ratio of 4:1. For the calcium ion leaching stage, five groups of experimental temperatures were set: 30 °C, 50 °C, 70 °C, 90 °C, and 100 °C. The calcium ion precipitation experiments were uniformly conducted at room temperature, reacting for a certain time under an atmosphere of 99.9% pure CO2. Due to the short reaction time and high precipitation rate of calcium ions in calcium chloride solutions, this process is not a controlling step in indirect carbonation. Therefore, the kinetic analysis of indirect carbonation focuses solely on the leaching process. The leaching efficiency of calcium ions from the MS can be equated to the slag’s CO2 sequestration capacity. In this study, ethylenediaminetetraacetic acid (EDTA) titration was employed to analyze the calcium ion leaching efficiency of the two CO2 sequestration methods. The reaction rate of CaO in MS is denoted by X and calculated using Formula (1).
Among these, : reaction rate of CaO in MS, %; m0: mass of MS, g; w0: calcium content in MS, %; V: volume of calcium leachate, L; w1: calcium content in calcium leachate, g/L.
2.2.2. Direct Method
The direct CO2 sequestration (DCDS) involves directly introducing CO2 into a single reactor to induce carbonation reactions with minerals or wastes, which can be divided into two forms: gas–solid phase direct carbonation and aqueous phase direct carbonation. For the DCDS experiments, purified water was used as the leaching agent, mixed and stirred with MS powder at a liquid-to-solid ratio of 15:1. Three groups of experimental temperatures were set: 30 °C, 60 °C, and 90 °C, with reactions carried out for a certain time under an atmosphere of 99.9% pure CO2.
The reaction rate of calcium oxide in MS was determined by thermogravimetric analysis (TGA). To eliminate interference from inherent crystalline water in the mineral, samples directly after solid CO2 fixation were first heated to 500 °C and held at this temperature for 12 h to remove crystalline water. The samples were then weighed and their mass recorded. The samples were then heated to 1000 °C, held at this temperature for 12 h, and weighed again. Since the decomposition temperature range of CaCO3 primarily spans between 600 °C and 900 °C, mass loss within this temperature range during the latter stage was attributed to the decomposition of CaCO3 in the samples [16]. The amount of CO2 directly absorbed per 100 g of MG was calculated and converted to the reaction rate of CaO using Equation (2):
: reaction rate of CaO in MS, %; w0: calcium content in MS, %; m500 and m1000 denote the mass of the direct carbonation product after sintering and drying at 500 °C and 1000 °C, g. Here, 56 and 44 represent the molecular weights of CaO and CO2, respectively.
2.3. Material Characterization Methods
To determine the elemental composition of the MS samples, quantitative analysis was performed on the preprocessed MS powder using an X-ray fluorescence spectrometer (Model: ZSX100e) equipped with a silicon drift detector (SDD). To identify the phase composition of the MS samples, XRD analysis was conducted on the preprocessed MS powder. The diffractometer (Model: Bruker D8) was coupled with an X’celerator detector using a copper (Cu) Kα X-ray source. The scanning range was 10° to 90°, with a scanning speed of 3.5 °/min. X-ray diffraction patterns were analyzed using JADE software (v.6.5). A field emission scanning electron microscope (SEM) model Quanta250FEG, FEI, Hillsboro, OR, USA was employed to analyze the microstructure of the MS samples. The samples were sputter-coated with a thin layer of gold (thickness: ~10 nm) to improve conductivity, and then observed under an acceleration voltage of 15 kV. TGA was performed on the MS samples subjected to indirect and direct carbonation using a thermogravimetric analyzer (Model: Q600) with a maximum measurement range of 1350 °C, a sensitivity of 0.1 ug. Mass loss was continuously recorded from 25 °C to 1000 °C at a heating rate of 5 °C/min. The thermogravimetric curves of the direct carbon sequestration products in this study are shown in Figure 1.
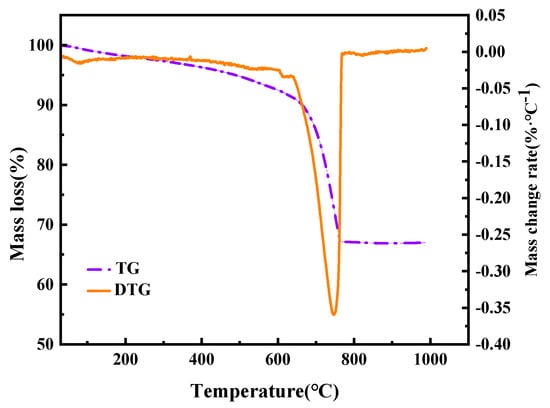
Figure 1.
The thermogravimetric curves of indirect CO2 sequestration products at 60 °C.
2.4. Life Cycle Assessment Methodology
Life cycle impact assessment (LCA) was performed with SimaPro 10.1.0.4 software, adopting the TRACI 2.2 method for impact characterization. This method quantifies potential environmental impacts across multiple categories, including global warming potential (GWP), acidification potential (AP), eutrophication potential (EP), ozone depletion potential (ODP), by applying region-specific characterization factors. This method covers the impact categories closely related to carbon sequestration and industrial waste utilization, and is suitable for both scientific research and industrial scenarios.
2.5. Visualization of Research Approach
This study established a systematic research framework comprising “dual-path process comparison, kinetic mechanism analysis, and full life cycle assessment.” First, experiments on direct carbonation and indirect carbonation were conducted using MS as raw material. The direct method involved carbonation by introducing CO2 gas directly into the MS slurry, whereas the indirect method utilized an ammonium chloride solution to leach calcium ions, followed by carbonation through the injection of CO2 gas. Second, to identify rate-limiting steps and mechanisms, this study conducted in-depth kinetic analyses of both pathways, calculating and evaluating kinetic equations and rate-influencing factors for each carbonation method. Finally, the Life Cycle Assessment (LCA) methodology was applied to quantify key indicators, such as Global Warming Potential (GWP), under both carbonation pathways, enabling a comprehensive environmental comparison and optimization. The research framework is illustrated in Figure 2.
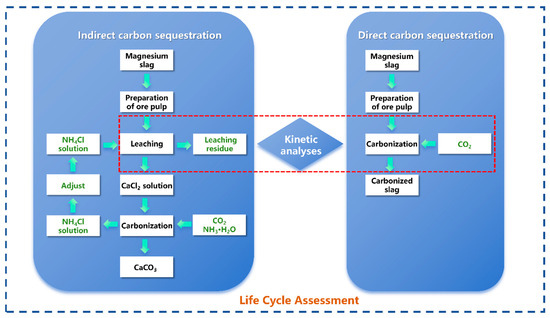
Figure 2.
Research framework diagram.
3. Results and Discussion
3.1. Magnesium Slag Analysis
Table 1 indicates that the slag primarily consists of CaO, SiO2, and MgO, with relatively low Fe2O3 and Al2O3 content. Calcium, as the primary reactive element in mineral carbonation, its content level serves as an intuitive indicator to evaluate the carbonation potential of a mineral. With a CaO content as high as 56.66%, the MS is rich in calcium-bearing minerals, which makes it an ideal raw material for mineral carbonation-based CO2 sequestration and endows it with high utilization value. Phase analysis of the MS was conducted using X-ray diffraction (XRD), results are shown in Figure 3a. In MS, calcium exists predominantly in the form of crystalline mineral phases, including β-dicalcium silicate (β-Ca2SiO4), γ-dicalcium silicate (γ-Ca2SiO4), calcium sulfate pentoxide (Ca3SO5), along with magnesium oxide (MgO) and calcium oxide (CaO) as associated components. SEM-EDS analysis was performed on the MS samples to characterize their microtopography and elemental composition. The analytical results are presented in Figure 3b. These results indicate that, the particle size of the MS is non-uniform and has an irregular granular morphology, which is consistent with the result of sieving analysis (less than 74 μm). The main elements present were Ca, Si, O, and Mg.
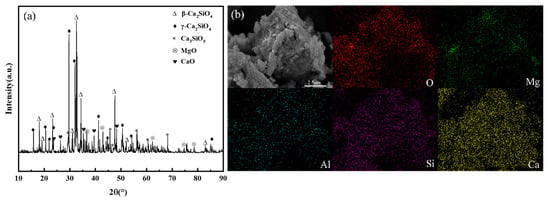
Figure 3.
X-ray diffraction pattern and SEM-EDS image of MS sample (a) X-ray (b) SEM-EDS.
3.2. Dynamics Research
Temperature is a key factor influencing the choice of indirect and direct carbonation processes. The experimental temperature range of 30–100 °C encompasses both typical industrial leaching operating temperatures and the solubility range of CO2 in water. In the ICDS leaching process, Figure 4a shows that temperature significantly affects the reaction rate of CaO. As the temperature increases, the reaction rate of CaO rises. Within the same reaction time, higher temperatures yield a greater reaction rate of CaO. During the process of DCDS, Figure 4b reveals a stepwise effect of temperature on the reaction rate of CaO. The results indicate that the reaction rate of CaO in the DCDS process does not increase linearly with rising temperature but instead follows an initial increase followed by a decrease. Within the 30 °C to 60 °C temperature range, the reaction rate of CaO increases with temperature, indicating that the reaction rate accelerates with rising temperature, consistent with the Arrhenius equation. Reaction rate of CaO peaks at 60 °C. When temperatures exceed 60 °C (i.e., upon further heating to 90 °C), the reaction rate of CaO actually declines. This occurs because further temperature increases significantly reduce CO2 solubility, counteracting the acceleration effect of temperature on reaction rate.
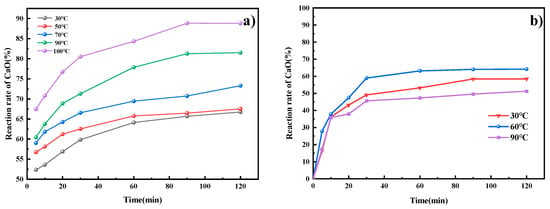
Figure 4.
The effect of temperature on indirect and direct CO2 sequestration processes. (a) ICDS; (b) DCDS.
To gain an in-depth understanding of the reaction pathways of the two carbon dioxide sequestration processes, reaction kinetic modeling was performed. In most solid–liquid reactions, the heterogeneous reaction kinetics of various ores can be described by the classical shrinking core model [17,18,19]. This model states that the reaction rate of the leaching process is mainly controlled by chemical reaction, intraparticle diffusion through the product layer, and mixed control. Furthermore, the reaction process is typically dominated by one of its steps. The primary reaction-controlling processes may include the following three types.
If the solid–liquid reaction process is controlled by chemical reactions, the reaction kinetic equation can be simplified to
If intra-product-layer diffusion limits the leaching process, the reaction kinetics equation can be simplified to
If the reaction process is controlled by a combination of interfacial chemical reactions and diffusion within the product layer, the reaction kinetics equation can be simplified to
In this equation, represents the CO2 sequestration capacity (%); ki (i = 1,2,3) denotes the kinetic constant of the leaching reaction control model; and t signifies the leaching time (minutes).
By substituting the X at different temperatures into Equations (3)–(5), kinetic models for the reaction rate of CaO in the ICDS and the DCDS at various temperatures were obtained. These models are shown in Figure 5a–c, The apparent rate constants k and correlation coefficients R2 at different temperatures obtained from the figure are shown in Table 2 and Table 3. It can be concluded that the correlation coefficients in Figure 5c and Figure 6c are larger, and their fitting effects are optimal. Therefore, it can be determined that both CO2 sequestration methods are more consistent with the hybrid model.
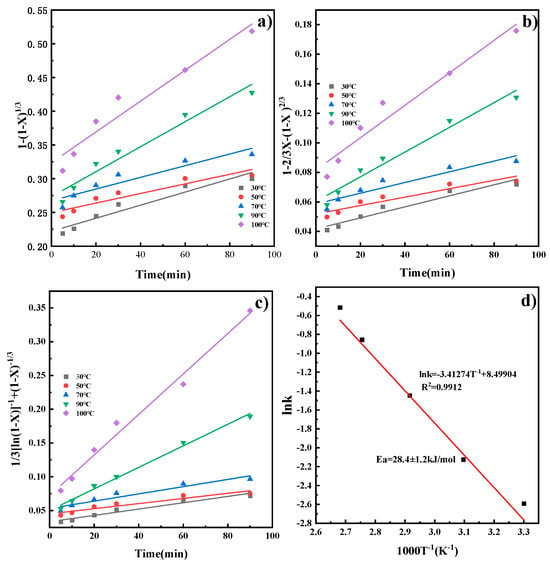
Figure 5.
Kinetic model of ICDS reaction process and Arrhenius diagram (a) chemical reaction; (b) internal diffusion reaction; (c) chemical reaction and diffusion mixed reaction; (d) Arrhenius plot.

Table 2.
Apparent rate constants and correlation coefficients of ICDS at different temperatures.

Table 3.
Apparent rate constants and correlation coefficients of DCDS at different temperatures.
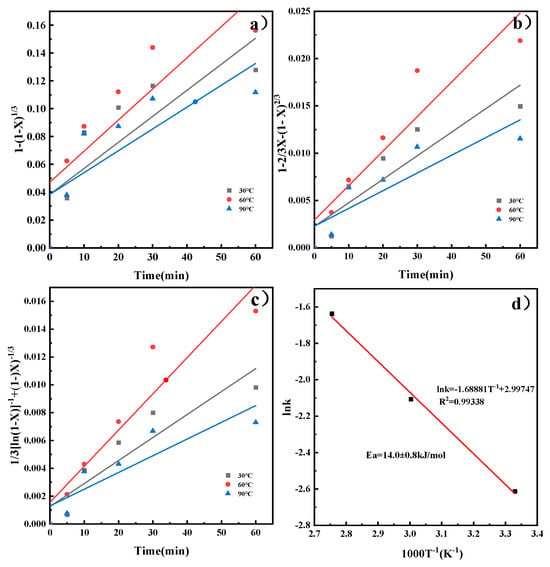
Figure 6.
Kinetic model of DCDS reaction process and Arrhenius diagram, (a) chemical reaction; (b) internal diffusion reaction; (c) chemical reaction and diffusion mixed reaction; (d) Arrhenius plot.
The reaction rate constants were calculated from Figure 4a,b and Figure 5a–c were used to determine the activation energy according to the Arrhenius equation [20,21,22]:
Here, k is the reaction rate constant, A is the frequency factor (a pre-exponential factor in the Arrhenius equation), Ea is the apparent activation energy (in kilojoules per mole), R is the gas constant (typically 8.314 J/(mol·K)), and T is the absolute temperature (in Kelvin).
Substituting the leaching reaction rate constant at each reaction temperature T into Equation (6) for linear fitting yields the results shown in Figure 5d and Figure 6d. The plots reveal a linear relationship between lnk and 1000/T. Calculating the slope of the line yields apparent activation energies of 28.3 ± 1.2 kJ/mol and 14.0 ± 0.8 kJ/mol, respectively. The activation energy was calculated with a standard deviation of ±1.2 kJ/mol (ICDS) and ±0.8 kJ/mol (DCDS) using linear fitting of the Arrhenius equation, which reflects the reliability of the kinetic data. Activation energies for processes controlled by external diffusion or chemical reactions typically exceed 42 kJ/mol. Chemical reaction- and diffusion-mixed processes have activation energies from 12 to 42 kJ/mol. Processes controlled by internal diffusion show lower activation energies, generally between 4 and 12 kJ/mol [23]. This indicates that the calcium leaching process in indirect carbonation is under the combined control of chemical reaction and diffusion. The direct carbonation process is also governed by the same combined control mechanism, which is consistent with the results obtained from the previous analysis.
3.3. Study on Reaction Mechanism
In the ICDS leaching process, the dynamic process of calcium leaching is illustrated in Figure 7a. During the initial stage, ion exchange occurs immediately upon contact between the ammonium chloride solution and MS. Calcium ions detach from the MS lattice and enter the solution, causing the calcium concentration to rise rapidly. The reaction is concentrated on the surface of the MS. During the intermediate stage, calcium ion depletion on the slag surface reduces leaching agent contact probability. Concurrently, partial calcium ion side reactions or re-adsorption occur, slowing the growth of the calcium concentration. In the late stage, calcium concentration stabilizes as the reaction approaches equilibrium, with nearly all leachable calcium ions entering the solution. During the reaction, a silicon-rich solid layer forms on the magnesia slag surface, impeding ion diffusion.
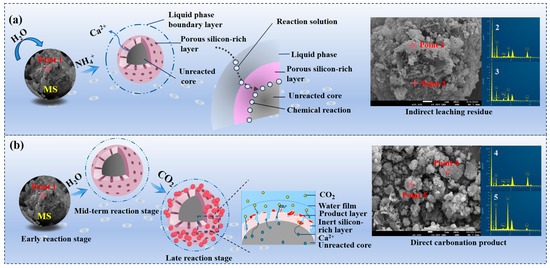
Figure 7.
Carbonation reaction core contraction model (a) ICDS; (b) DCDS.
The DCDS process is based on the coupling of “two-step reactions + two-layer diffusion,” as illustrated in Figure 7b. During the initial reaction phase, the three-phase interface forms and ion dissolution initiates. After mixing MS with water to form a slurry and introducing CO2, the reaction commences at the “MS surface-water phase-CO2 bubble” three-phase interface. CO2 dissolves and ionizes (forming H2CO3 and H+, (HCO3)−, (CO3)2−), lowering the aqueous phase pH. The porous structure of the magnesia slag surface provides pathways for contact. CaO hydrate to form Ca(OH)2 and then dissolves into Ca2+. Directional dissolving of Ca2+ forms a silicate-rich layer. Increased (CO3)2− concentration in the aqueous phase creates a mobile boundary between the silicate-rich layer and unreacted MS. H+ reacts with calcium silicates (e.g., Ca2SiO4) in the MS, causing Ca2+ to diffuse out of the lattice into the aqueous phase; inert SiO2 accumulates, forming a nanoscale “inert silica-rich layer” that gradually thickens and densifies, initially impeding ion transport. Calcium carbonate precipitates, and the product layer grows. Ca2+ diffuses to the “silicon-rich layer-aqueous phase” interface, reacting with (CO3)2− to form a “product layer-silicon-rich layer” moving boundary.
During the mid-to-late reaction stages, diffusion resistance is coupled and controlled by both layers. The silicate-rich layer has extended pores and ion adsorption. The dense product layer is dominated by lattice diffusion with low coefficients. Together, they form a superimposed resistance that makes displacement reaction kinetics the rate-controlling factor. When ion transport rates fall below a threshold, the reaction slows. Ultimately, unreacted MS becomes encapsulated by both layers, and the reaction stops. The CO2 carbonation rate depends on the equilibrium between the thicknesses, porosity, and diffusion coefficients of the layers.
Table 4 presents the elemental distributions of raw MS, indirect leaching residue, and direct carbonation residue.

Table 4.
EDS elemental composition of MS before and after CO2 sequestration.
The EDS data intuitively reflect the elemental migration during ICDS and DCDS, consistent with the reaction mechanisms. Raw MS (Point 1) mainly contains O (67.7 at%), Ca (21.7 at%), Si (8.0 at%), and minor Mg (2.4 at%) without C, with Ca-containing phases as active components. After ICDS leaching (Points 2, 3), Ca content drops to 6.95~14.76 at %, Si rises to 21.86~26.65 at % (silicon-rich layer formation), and no C is detected. Following DCDS treatment (points 4 and 5), the presence of C confirms CO2 sequestration. Meanwhile, Ca and Si exhibit irregular, opposite distributions, indicating that the carbonation slag consists of CaCO3 products and unreacted SiO2.
3.4. Comparison of CO2 Sequestration Capacity
To more intuitively compare the CO2 sequestration efficiency of the two methods, the CO2 sequestration capacity is defined as the mass of CO2 absorbed by 100 g of MS, calculated using Formula (7).
: CO2 sequestration capacity of 100 g MS, g/100 g. Under the optimal process conditions of the two carbon sequestration pathways (100 °C for the indirect pathway and 60 °C for the direct pathway), the experimental results are shown in Figure 8. The carbonation level of ICDS continuously increased throughout the entire period and then tended to stabilize. It steadily rose from 29.89% at 5 min to 39.46% at 90 min, and then increased further to 39.62% at 120 min, with almost no significant change, indicating obvious signs of saturation in the ICDS reaction. In contrast, the DCDS rate increased rapidly in the early stage and then stabilized over time. A significant growth was observed within the initial 60 min, increasing from 12.39% at 5 min to 28.13% at 60 min. After 60 min, the growth slowed down and stabilized at approximately 28% at 90 min (28.51%) and 120 min (28.48%), demonstrating that the DCDS reaction gradually reached saturation after 60 min.
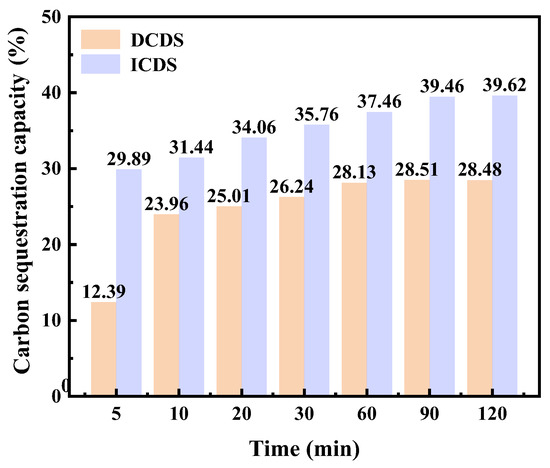
Figure 8.
Comparative Analysis of CO2 sequestration capacity of DCDS and ICDS for MS.
Furthermore, the initial carbonation level of ICDS (29.89%) was higher than that of DCDS (12.39%), and it maintained a growth advantage throughout the entire process. This indicates that the ICDS capacity of MS is significantly greater than its DCDS capacity.
3.5. Life Cycle Assessment
TRACI 2.2 (Tool for the Reduction and Assessment of Chemical and other Environmental Impacts) is a life cycle impact assessment (LCA) methodology developed by the U.S. Environmental Protection Agency (U.S. EPA, Washington, DC, USA). As a mid-point level assessment model, it converts resource consumption and pollutant emissions into indicator values for several environmental impacts by assigning characterization factors to inventory data. In this study, SimaPro 10.1.0.4 was used to establish two CO2 sequestration process routes (DCDS and ICDS) for MS, and a LCA was conducted using the TRACI 2.2 methodology [24,25,26,27]. The process considered in this study is detailed in Figure 9, including the inputs, outputs, and a clear demarcation between the background and foreground systems. The functional calculation unit was 1 ton of MS for the entire CO2 sequestration life cycle process.
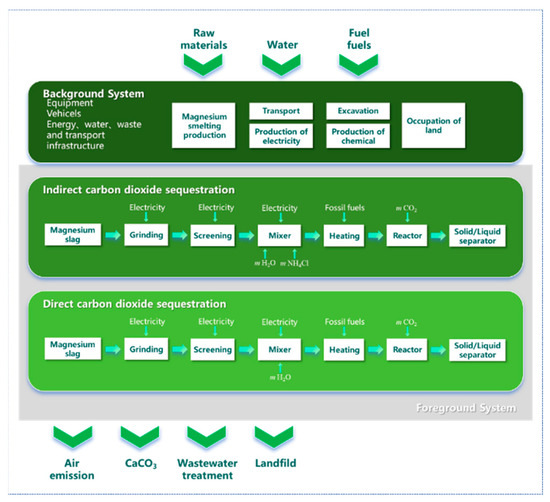
Figure 9.
Life cycle assessment (LCA) boundaries.
This study conducts a comparative assessment of the environmental contributions of ICDS and DCDS across ten distinct dimensions. The objective is to systematically and comprehensively evaluate the actual performance of both technological routes, propose feasible recommendations for process improvement, and provide a valuable reference for optimizing project environmental performance. Specific numerical results are presented in Table 5 and Table 6, while a direct visual comparison of the data is illustrated in Figure 10.

Table 5.
LCA data by DCDS from MS.

Table 6.
LCA data by ICDS from MS.
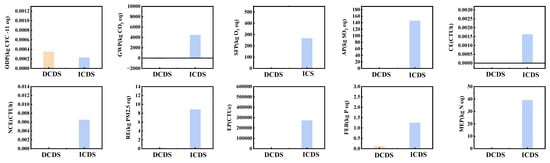
Figure 10.
Comparison of characterization data from LCA between DCDS and ICDS processes.
The DCDS and ICDS exhibit stark differences in core environmental impact dimensions, with global warming potential (GWP) being the most pronounced. DCDS demonstrates significant CO2 sequestration benefits, while ICDS generates substantial CO2 emissions, creating a stark contrast. DCS has a climate change potential of −162.32 kg CO2 eq (negative values indicate CO2 sequestration), primarily stemming from the direct carbonization process itself, which alone achieves −284.85 kg CO2 eq of CO2 sequestration. This fully offsets emissions from tap water (84.59 kg CO2 eq), electricity (38.63 kg CO2 eq), and wastewater (14.81 kg CO2 eq). In contrast, the ICDS process exhibits a climate change potential of 4477.83 kg CO2 eq, with 88% of these emissions derived from ammonia usage (3949.68 kg CO2 eq). Ammonium chloride (1477.77 kg CO2 eq) and electricity (47.89 kg CO2 eq) further contribute to the emission burden. The difference in CO2 emissions between these two components reaches 4640 kg CO2 eq, equivalent to the emissions from burning 2 tons of standard coal.
Regarding acidification impacts, the acidification potential (AP) of DCS is only 0.53 kg SO2 eq, representing an extremely low environmental burden. In contrast, ICDS exhibits an AP of 146.48 kg CO2 eq, nearly 276 times that of DCDS. Approximately 75% of this acidification contribution stems from the ICDS process itself (110.52 kg SO2 eq), with acidic reagents used in the indirect carbonation process being the primary cause. In freshwater eutrophication potential (FEP), ICDS also significantly exceeds DCDS. Its potential of 1.26 kg P eq is 10.5 times that of DCDS (0.12 kg P eq). Ammonia usage (1.17 kg P eq, accounting for 93%) is the primary driver of aquatic eutrophication. The difference in ecotoxicity is even more striking. ICDS’s ecotoxicity potential (EP, 273,802.2 CTUe) is 146 times that of DCDS (1869.11 CTUe). Ammonia’s high ecotoxicity profile (290,191.53 CTUe, accounting for 99% of the total) makes it a critical threat to ecosystems. In terms of smog formation potential (SFP), ICS’s potential of 267.32 kg O3 eq is 49 times that of DCDS (5.47 kg O3 eq). As a key precursor to photochemical smog, ammonia contributes 89% of the smog formation load (237.15 kg O3 eq). Overall, DCDS demonstrates significant advantages across all five core environmental impact dimensions, exhibiting particularly qualitative superiority in CO2 sequestration and acidification control. Conversely, ICDS’s extensive use of ammonia reagents results in multiple environmental impact indicators exceeding standards, incurring higher environmental costs.
Beyond core environmental impacts, DCDS and ICDS also exhibit distinct characteristics in other dimensions such as ozone depletion potential (ODP), human health, and marine eutrophication (ME), with particularly significant differences observed in certain indicators. Regarding ODP, both processes exhibit extremely low impacts. DCDS has an ODP of 0.00035 kg CFC-11 eq, while ICDS is 0.66 times that of DCDS at 0.00023 kg CFC-11 eq. In this dimension, the risk of stratospheric ozone depletion from both is negligible. In the human health impact dimension, the differences between the two processes are polarized. Regarding carcinogenicity (CE), DCDS exhibits a slight negative contribution (−5.17 × 10−6 CTUh). In contrast, ICS generates a significant positive contribution (0.0016 CTUh), with a negative impact intensity 309 times that of DCDS. Ammonia usage (contribution: 0.0016 CTUh) is the primary driver of ICDS’s dramatically elevated carcinogenic risk. For non-carcinogenic effects (NCE), ICDS’s potential (0.0065 CTUh) also far exceeds DCDS (5.98 × 10−5 CTUh), reaching 109 times higher. Here, ammonia’s high non-carcinogenic toxicity (contribution: 0.0072 CTUh) is the dominant factor. Regarding respiratory effects (RE), ICDS exhibits a PM2.5 equivalent impact potential of 8.87 kg PM2.5 eq, which is 59 times the value for DCDS (0.15 kg PM2.5 eq). The ICDS technology itself (contributing 3.92 kg PM2.5 eq) is the primary driver of this significant increase in respiratory hazards. The most pronounced disparity emerged in marine eutrophication (ME), where ICDS exhibited a nitrogen equivalent potential of 39.09 kg N eq, 782 times that of DCDS (0.05 kg N eq). ICDS emissions (contributing 34.90 kg N eq) became the primary driver of ME, while DCDS demonstrated extremely low environmental impact in this dimension. Overall, except for ODP, where both impacts are negligible, ICDS exhibits higher environmental risks than DCDS across dimensions, including human health (CE, NCE), respiratory effects, and marine eutrophication (MEP). The disparities in MEP and CE are particularly pronounced, with the core cause directly linked to the use of ammonia reagents in ICDS processes.
As can be seen from Figure 11 on Greenhouse Gas Emissions (GWP), the emission contributions of specific materials such as ammonium chloride are clearly distinguishable in the GWP-ICDS chart. Indirect carbon emissions from ammonia-related processes represent a high-emission segment of the ICDS process. Optimizing the production processes of ammonia-based materials can achieve highly efficient emission reductions. Indirect embodied carbon emissions involve upstream or supporting links in the industrial chain, including electricity and supplementary cementitious materials (e.g., calcium carbonate). Compared with the control of direct embodied carbon emissions, emission reduction measures in the indirect embodied carbon dimension are more flexible and can deliver cross-link emission reduction benefits through industrial chain collaboration. The control of direct embodied carbon emissions mostly focuses on real-time optimization at the production end, with relatively weaker continuity of long-term benefits.
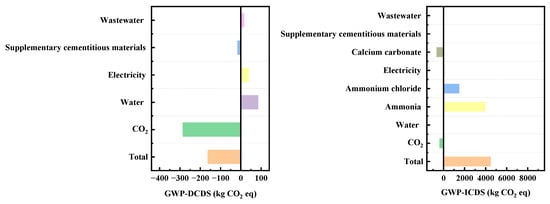
Figure 11.
Analysis of GWP indicators for DCDS and ICDS processes.
Although ICDS demonstrates superior efficiency compared to the DCDS, its associated environmental impacts are significantly more pronounced. Consequently, the selection of an appropriate CO2 sequestration technology in practical applications warrants careful deliberation. The analysis presented in the radar chart (Figure 12) indicates that the ICDS exhibits a substantially broader profile and more extensive implications across multiple critical environmental dimensions. In nine categories, including ecotoxicity, freshwater eutrophication, and marine eutrophication, the impact factors of ICDS significantly exceed those of DCDS. This signifies that the ICDS plays a dominant role in the progression of these environmental issues.
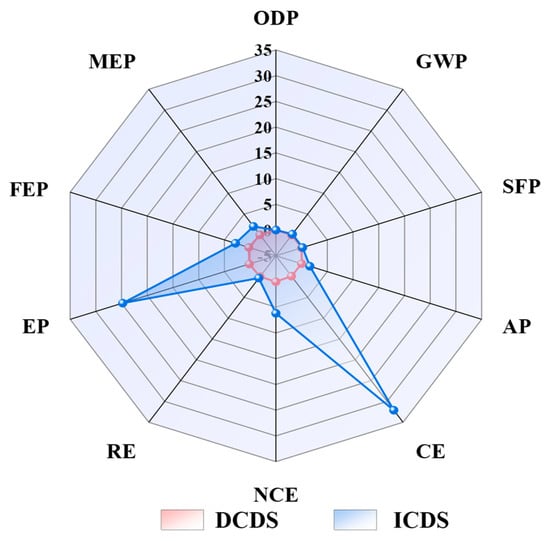
Figure 12.
Comparison of normalized data between DCDS and ICDS. (GWP: global warming potential; AP: acidification potential; FEP: freshwater eutrophication potential; EP: ecotoxicity potential; SFP: smog formation potential; ODP: ozone depletion potential; MEP: marine eutrophication; CE: carcinogenicity; NCE: non-carcinogenic effects; RE: respiratory effects).
3.6. Comparison and Evaluation of DCDS and ICDS Processes
The DCDS process demonstrates comprehensive environmental sustainability advantages over the ICDS process, achieving a qualitative leap, particularly in climate change mitigation (CO2 sequestration) and acidification control. It represents a more viable and environmentally friendly option. However, relying on its core advantages of high raw material utilization rates, high product added value, and process flexibility, ICDS is also highly worthy of promotion in specific scenarios. Its “leaching-purification-carbonization” staged process overcomes mass-transfer limitations, achieving conversion rates of active components in MS that are over 30% higher than those in DCDS, making it particularly suitable for low-activity, high-impurity MS. Furthermore, ICDS enables the directional synthesis of high-value-added calcium carbonate with a purity exceeding 95%, whose economic value is 5–8 times higher than that of DCDS-derived products, thus conferring superior economic feasibility. The system also exhibits far greater flexibility than DCDS, as its operational parameters can be readily adjusted to accommodate raw materials with varying compositions.
To mitigate the environmental drawbacks of the ICDS process, three targeted optimization measures can be adopted to reduce its environmental footprint, as detailed below: First, anhydrous ammonia can be replaced with industrial waste lye, or over 90% of ammonia can be recycled; this approach can eliminate 88% of the primary CO2 emission sources. Second, the leaching pH can be adjusted and optimized to the range of 6.0–7.0, and dedicated wastewater treatment units can be incorporated to cut ecotoxicity by 95%. Third, conventional grid electricity can be substituted with renewable energy sources. After the implementation of these optimization strategies, the process’s global warming potential (GWP) can be reduced to below −50 kg CO2 eq, while ecotoxicity and other key environmental indicators can approach the levels of the direct carbonation system (DCDS). A core advantage of the ICDS process is its exceptional suitability for three specific scenarios: high-end calcium carbonate production, low-quality MS valorization, and co-disposal of industrial solid wastes. In summary, the optimized ICDS process achieves the goals of “advanced technology, economic viability, and environmental controllability”, rendering it a preferable alternative to DCDS for targeted applications.
4. Conclusions
This study addresses the key gaps in MS CO2 sequestration that involve unclear kinetic mechanisms and the lack of systematic life cycle assessment (LCA) for direct CO2 sequestration (DCDS) and indirect CO2 sequestration (ICDS), and it provides critical support for process optimization and engineering application through kinetic analysis and LCA.
First, the kinetic mechanisms of the two processes were clarified: ICDS follows a shrinking core model under mixed control (activation energy: 28.4 ± 1.2 kJ/mol) with a reaction rate of 89%, forming a silicon-rich inert layer. DCDS involves double-layer formation and pore blocking under mixed control (activation energy: 14.0 ± 0.8 kJ/mol) with a lower reaction rate of 63%. This reveals ICDS’s advantage in active component conversion and DCDS’s merit of low initiation energy consumption, filling the theoretical gap for MS CO2 sequestration industrialization. Second, LCA results differentiated the two processes: DCDS demonstrates excellent overall environmental sustainability, especially in CO2 sequestration and acidification control, making it an eco-friendly route for waste disposal and CO2 reduction; ICDS, despite severe environmental drawbacks (e.g., high CO2 emissions and ecotoxicity), boasts high raw material utilization and capacity for high-value-added calcium carbonate synthesis, suiting scenarios like low-grade MS resource utilization and high-end calcium carbonate production. Third, targeted optimizations (ammonia recycling, leaching pH regulation, renewable energy substitution) can reduce ICDS’s environmental load to near DCDS levels, achieving a balance of technical advancement, economic feasibility, and environmental controllability. Thus, DCDS is prioritized for large-scale eco-oriented projects, while optimized ICDS excels in high-value and low-quality MS disposal scenarios.
This study lays a theoretical foundation for MS carbonation process selection and promotion. Future work should focus on large-scale economic evaluation and long-term environmental monitoring to advance lab-to-industry translation, supporting the synergistic achievement of metallurgical solid waste valorization and dual-carbon goals.
Author Contributions
Conceptualization, Y.W. and Y.B.; Methodology, Z.L., Y.W., C.Z. and L.Z.; Investigation, Z.L., Y.W., H.D. and C.Z.; Resources, H.D.; Data curation, Z.L., H.D. and C.Z.; Writing—original draft, Z.L., H.D. and C.Z.; Writing—review & editing, Y.W. and Y.B.; Visualization, H.D. and L.Z.; Supervision, Y.W., Y.B. and L.Z.; Project administration, Y.B. and L.Z.; Funding acquisition, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Liaoning Province Key R&D Program (2024) [ZX20250018].
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guo, Y.; Sun, M.; Zhang, W.; Wang, L. Machine Learning in Enhancing Corrosion Resistance of Magnesium Alloys: A Comprehensive Review. Metals 2023, 13, 1790. [Google Scholar] [CrossRef]
- He, J.; Che, Y. Pidgeon Process. In The ECPH Encyclopedia of Mining and Metallurgy; Kuangdi, X., Ed.; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.; Chen, Z.; Cheng, F.; Guo, Y. Chemical, Mineralogical, and Morphological Characteristics of Pidgeon Magnesium Slag. Environ. Eng. Sci. 2016, 33, 290–297. [Google Scholar] [CrossRef]
- Wang, X.-B.; Yan, X.; Li, X.-Y. Environmental risks for application of magnesium slag to soils in China. J. Integr. Agric. 2020, 19, 1671–1679. [Google Scholar] [CrossRef]
- Ye, J.; Liu, S.; Zhao, Y.; Li, Y.; Fang, J.; Zhang, H.; Guan, X. Development of Ultrafine Mineral Admixture from Magnesium Slag and Sequestration of CO2. Buildings 2023, 13, 204. [Google Scholar] [CrossRef]
- Zhu, M.; Zhai, R.; Zhu, M.; He, J. Evaluating Alkali Activation in Magnesium Slag Carbonization and Its Mechanism. Crystals 2024, 14, 847. [Google Scholar] [CrossRef]
- Fang, Z.; Gao, Y.; He, W.; Zhu, M.; Xia, L.; Yang, P.; Liu, D.; He, J. Carbonation curing of magnesium-coal slag solid waste backfill material: Study on properties of flow, mechanics and carbon sequestration. Case Stud. Constr. Mater. 2024, 20, e03204. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Wang, Q.; Liu, Z.; Wang, F. Preparation of ultra-high strength carbonated compacts via accelerated carbonation of magnesium slag. J. CO2 Util. 2024, 83, 102829. [Google Scholar] [CrossRef]
- Xu, B.; Yi, Y. Immobilization of lead (Pb) using ladle furnace slag and carbon dioxide. Chemosphere 2022, 308, 136387. [Google Scholar] [CrossRef]
- Baris, K.; Ozarslan, A.; Sahin, N. The Assesment for CO2 Sequestration Potential by Magnesium silicate Minerals in Turkey: Cases of Orhaneli-Bursa and Divrigi-Sivas Regions. Energy Explor. Exploit. 2008, 26, 293–309. [Google Scholar] [CrossRef]
- Ke, X.; Baki, V.A.; Skevi, L. Mechanochemical activation for improving the direct mineral carbonation efficiency and capacity of a timber biomass ash. J. CO2 Util. 2022, 68, 102367. [Google Scholar] [CrossRef]
- Mármol, G.; Fernández-Jiménez, A.; Blanco-Varela, M.-T.; García-Lodeiro, I. Carbonation and Phase Evolution in MgO-SiO2 Cements: Impact on Strength. Molecules 2025, 30, 1072. [Google Scholar] [CrossRef]
- Xu, C.; Cai, J.; Wang, Z.; Ni, M.; Cen, K.; Zhang, Y. United Conversion Process Coupling CO2 Mineralization with Thermochemical Hydrogen Production. Environ. Sci. Technol. 2019, 53, 12091–12100. [Google Scholar] [CrossRef]
- Eikeland, E.; Blichfeld, A.B.; Tyrsted, C.; Jensen, A.; Iversen, B.B. Optimized carbonation of magnesium silicate mineral for CO2 storage. ACS Appl. Mater. Interfaces 2015, 7, 5258–5264. [Google Scholar] [CrossRef]
- Onyekwena, C.C.; Li, Q.; Wang, Y.; Alvi, I.H.; Li, W.; Hou, Y.; Zhang, X.; Zhang, M. Dredged marine soil stabilization using magnesia cement augmented with biochar/slag. J. Rock Mech. Geotech. Eng. 2023, 16, 1000–1017. [Google Scholar] [CrossRef]
- Abe, M.; Tanaka, S.; Noguchi, M.; Yamasaki, A. Investigation of Mineral Carbonation with Direct Bubbling into Concrete Sludge. ACS Omega 2021, 6, 15564–15571. [Google Scholar] [CrossRef]
- Sanda, O.; Ehinmitola, O.E.; Taiwo, E.A. Leaching Kinetics of Spent 6F22 Dry Cells Roast Residue in Nitric and Hydrochloric Acids Using a Modified Shrinking Core Model. Chem. Afr. 2023, 7, 479–489. [Google Scholar] [CrossRef]
- Zhong, Y.; Shi, T.; Chen, Q.; Yang, X.; Xu, D.; Zhang, Z.; Wang, X.; Zhong, B. Leaching calcium from phosphogypsum desulfurization slag by using ammonium chloride solution: Thermodynamics and kinetics study. Chin. J. Chem. Eng. 2020, 28, 208–215. [Google Scholar] [CrossRef]
- Ye, X. Bound states and vortex core shrinking effects in iron-based superconductors. Phys. C Supercond. 2013, 48, 6–9. [Google Scholar] [CrossRef]
- Zhu, S.; Hu, J.; Zhang, C.; Li, S.; Chen, W. Process Optimization and Kinetics of Titanium Leaching from Mechanically Activated Titanium-Bearing Blast Furnace Slag. J. Sustain. Metall. 2022, 9, 230–239. [Google Scholar] [CrossRef]
- Mattila, H.; Grigaliūnaitė, I.; Zevenhoven, R. Chemical kinetics modeling and process parameter sensitivity for precipitated calcium carbonate production from steelmaking slags. Chem. Eng. J. 2012, 19, 77–89. [Google Scholar] [CrossRef]
- Yan, F.; Luo, K.; Ye, J.; Zhang, W.; Chen, J.; Ren, X.; Liu, Z.; Li, J. Leaching kinetics and dissolution model of steel slag in NaOH solution. Constr. Build. Mater. 2024, 434, 743–750. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Wang, S.; Xie, F. Study on the kinetics and mechanism of ultrasonic-microwave synergistic enhancement for leaching indium from zinc oxide dust. Chem. Pap. 2024, 78, 3667–3685. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Y.; Liu, H.; Boczkaj, G.; Cao, Y.; Wang, C. Carbon dioxide sequestration by industrial wastes through mineral carbonation: Current status and perspectives. J. Clean. Prod. 2024, 43, 42–58. [Google Scholar] [CrossRef]
- Li, X.; Mehdizadeh, H.; Ling, T.-C. Environmental, economic and engineering performances of aqueous carbonated steel slag powders as alternative material in cement pastes: Influence of particle size. Sci. Total Environ. 2023, 90, 166210. [Google Scholar] [CrossRef] [PubMed]
- Baciocchi, R.; Costa, G.; DI Gianfilippo, M.; Polettini, A.; Pomi, R.; Stramazzo, A. Thin-film versus slurry-phase carbonation of steel slag: CO2 uptake and effects on mineralogy. J. Hazard. Mater. 2015, 28, 302–313. [Google Scholar] [CrossRef]
- Ghasemi, S.; Costa, G.; Zingaretti, D.; Bäbler, M.U.; Baciocchi, R. Comparative Life-cycle Assessment of Slurry and Wet Accelerated Carbonation of BOF Slag. Energy Procedia 2017, 11, 5393–5403. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.