Effect of Heat Treatment on the Structure and Properties of Silver-Coated Copper Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Heat Treatment Method of Silver-Coated Copper Powder
2.3. Characterization of Silver-Coated Copper Powder
3. Results and Discussion
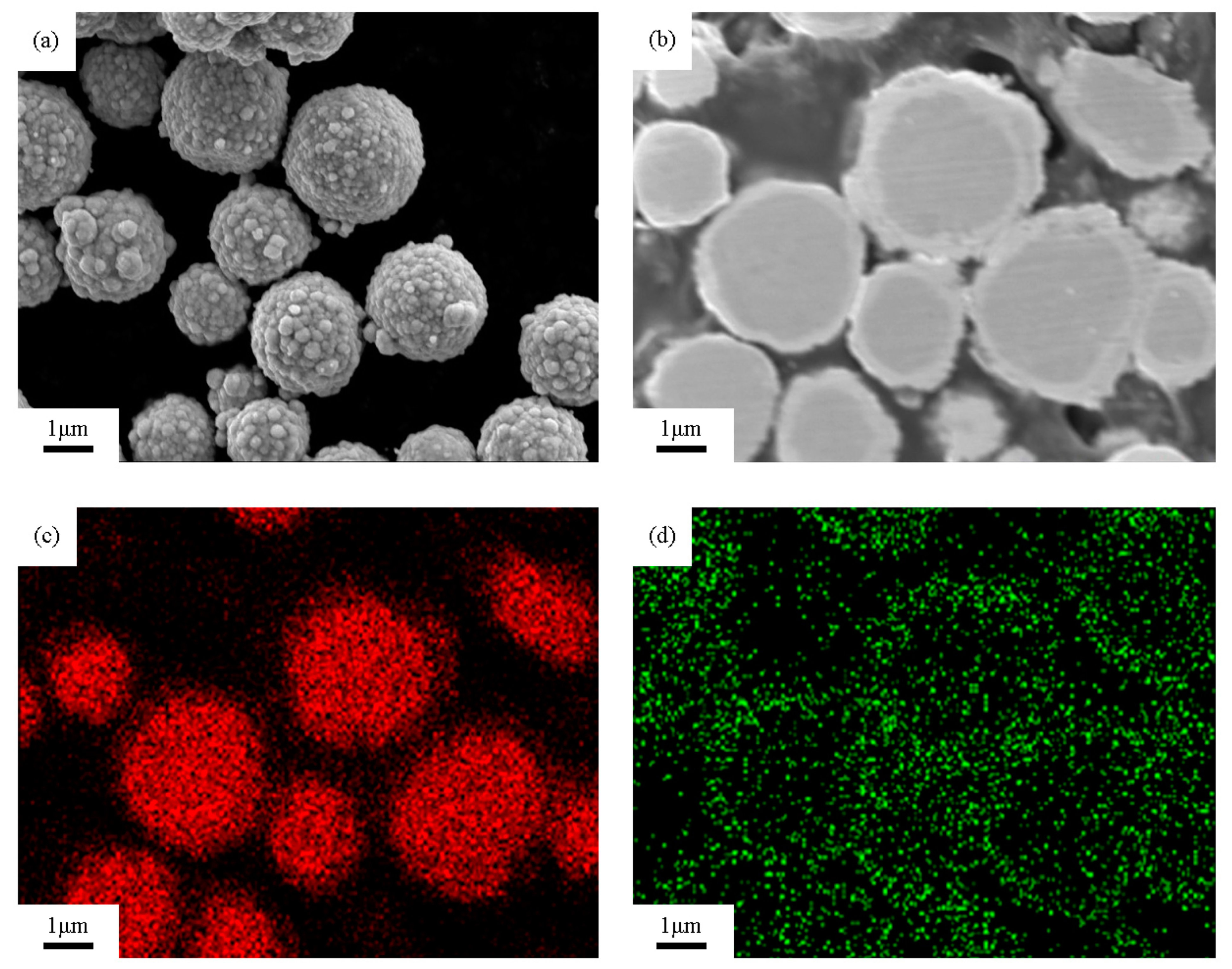
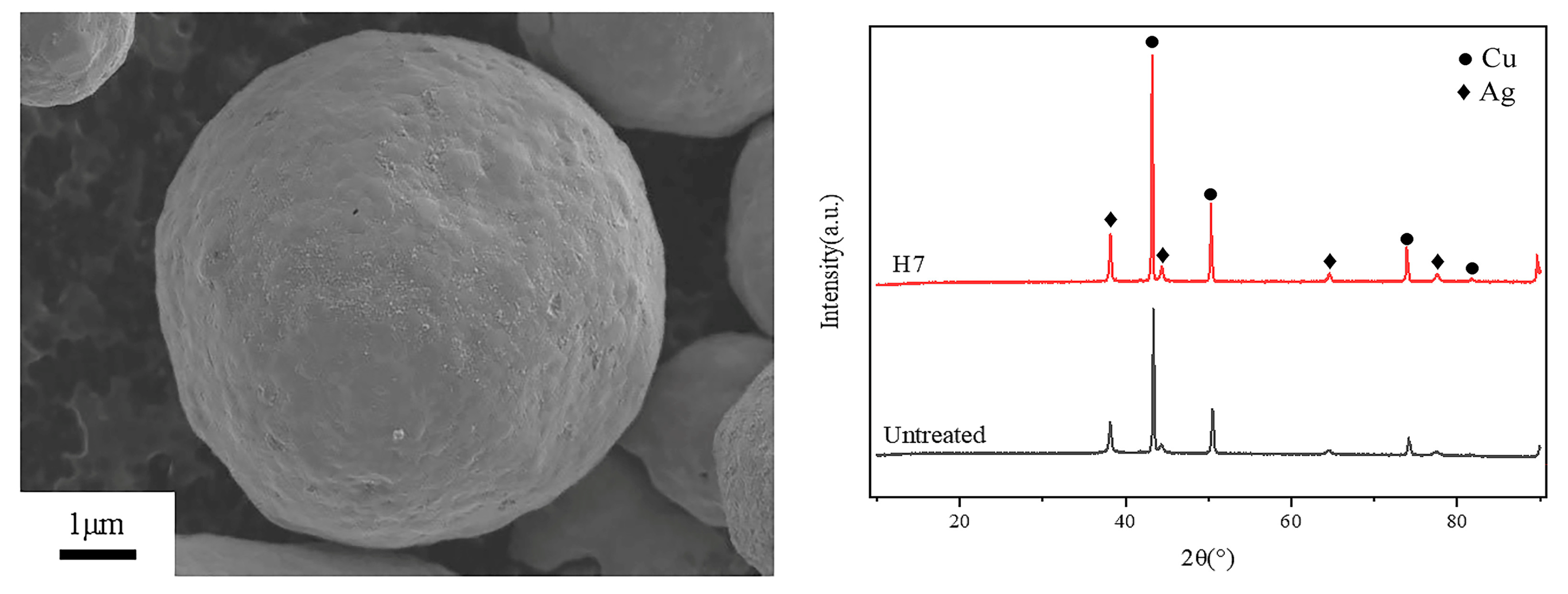
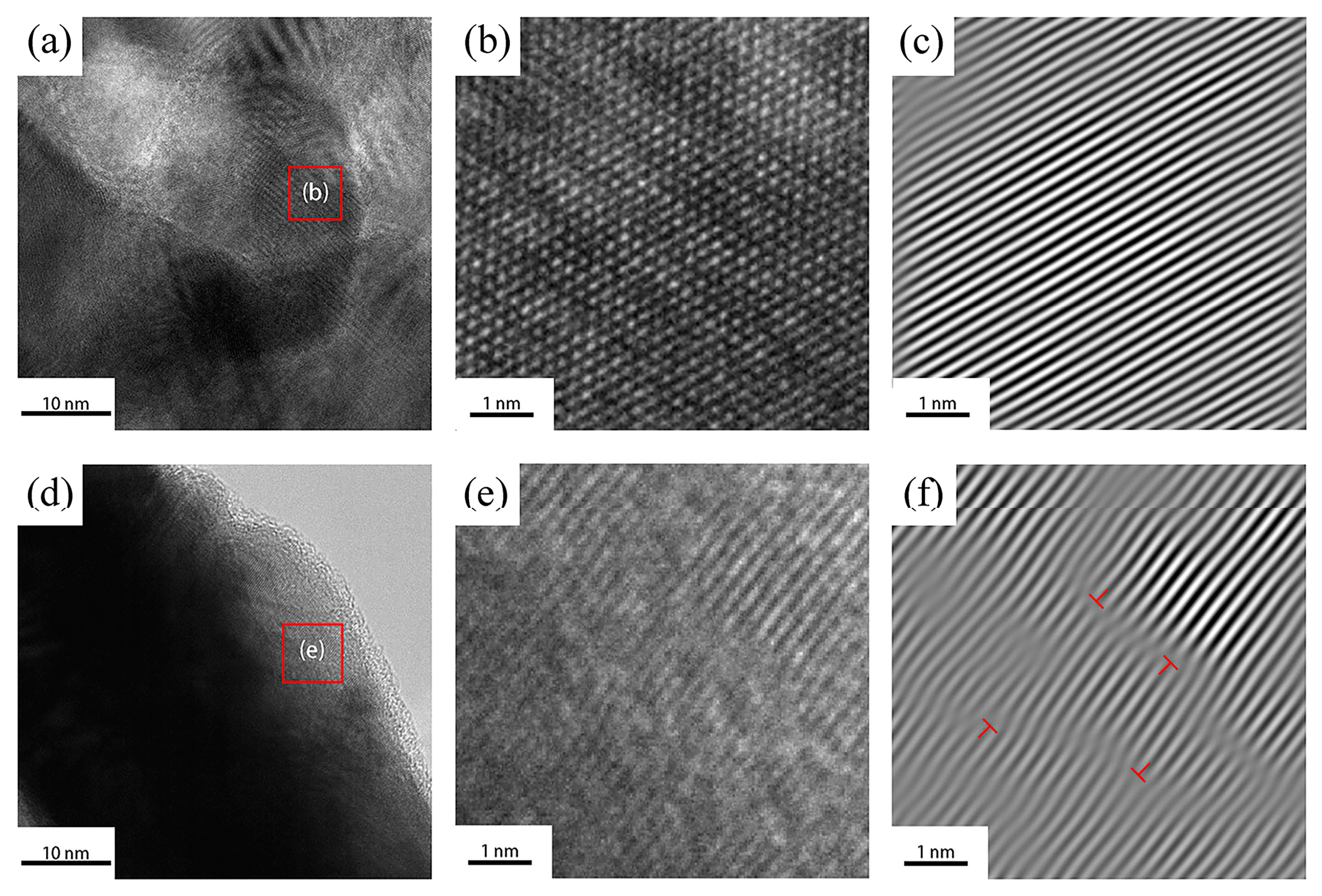
3.1. Coating Morphology Analysis
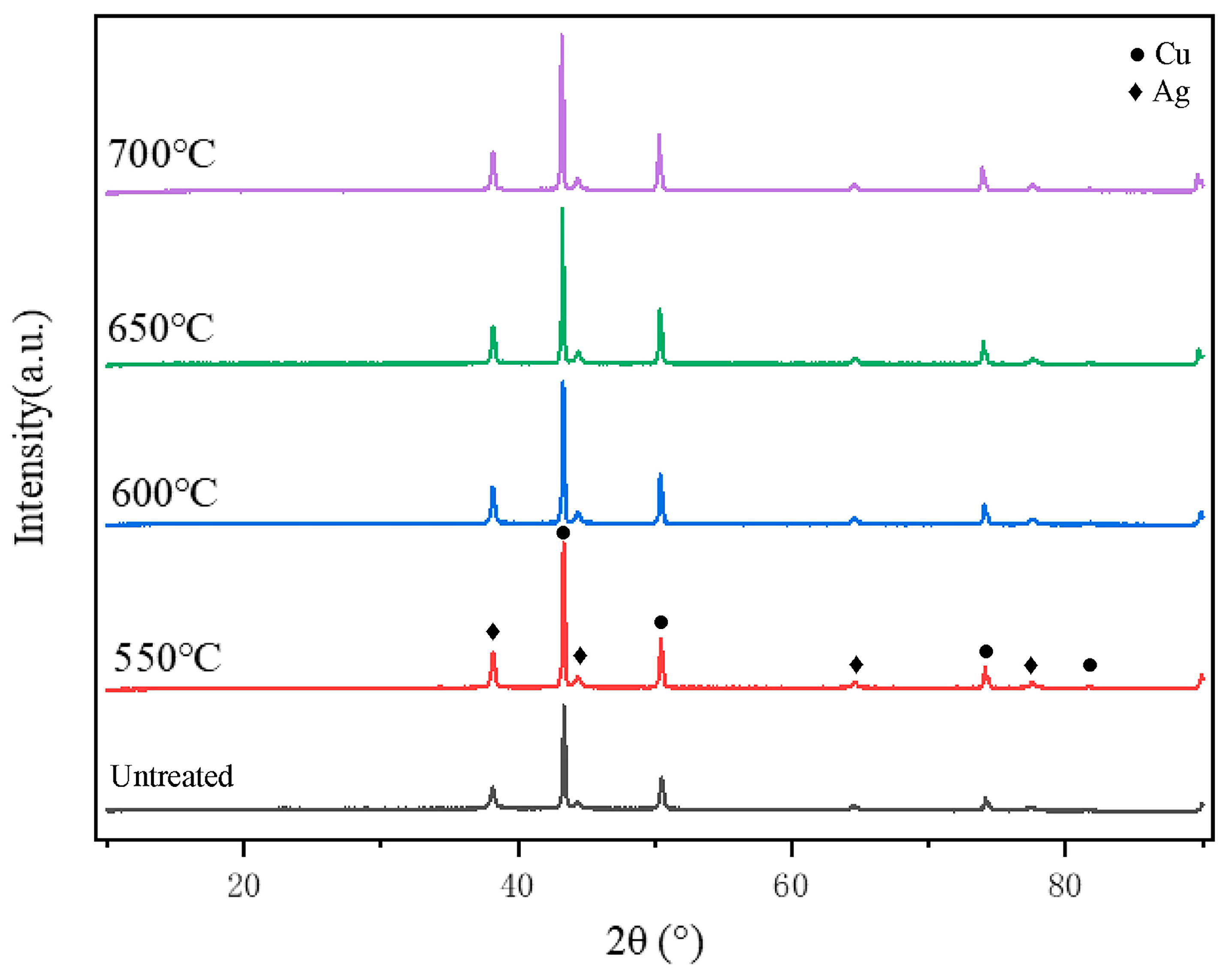
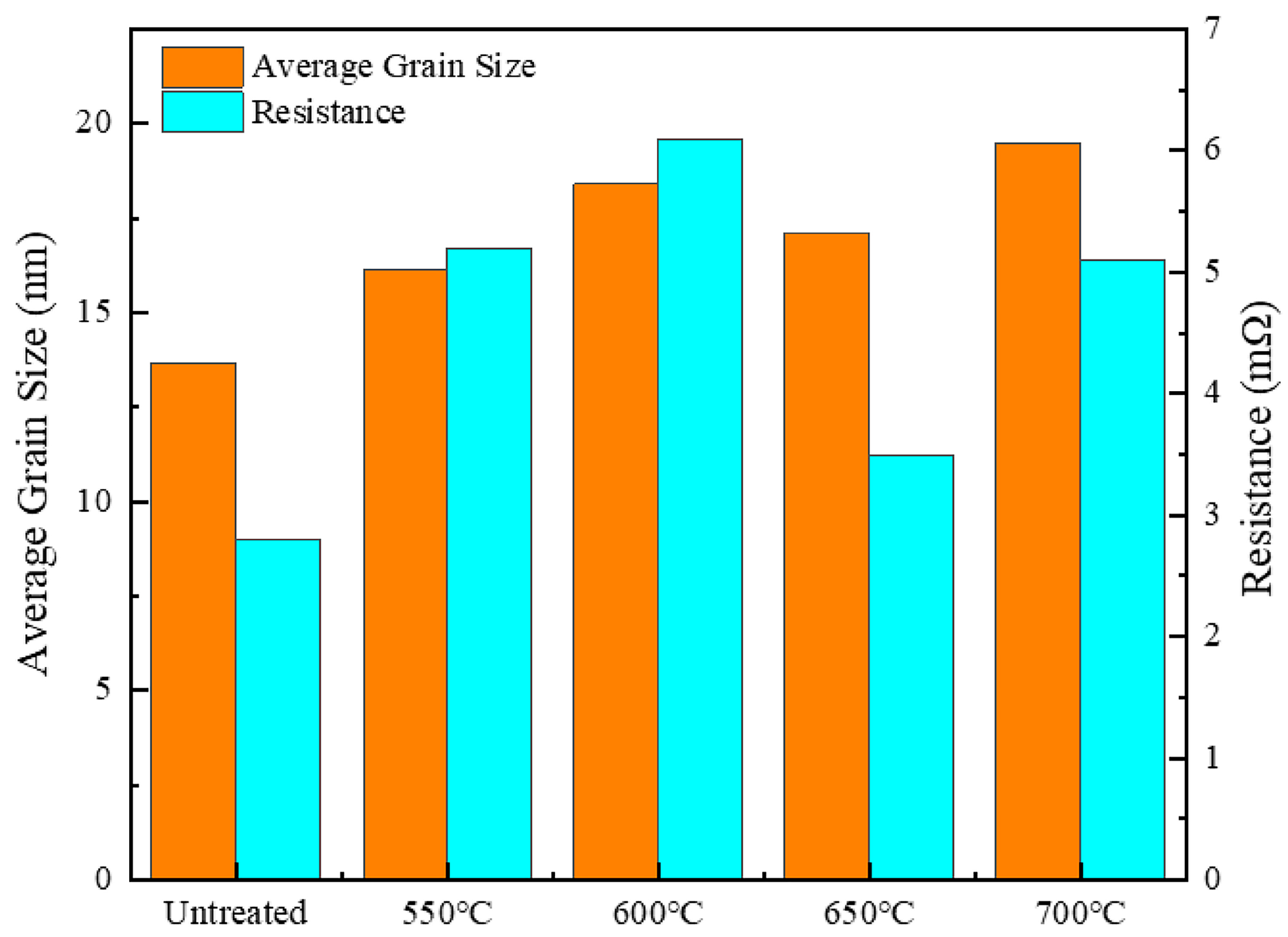
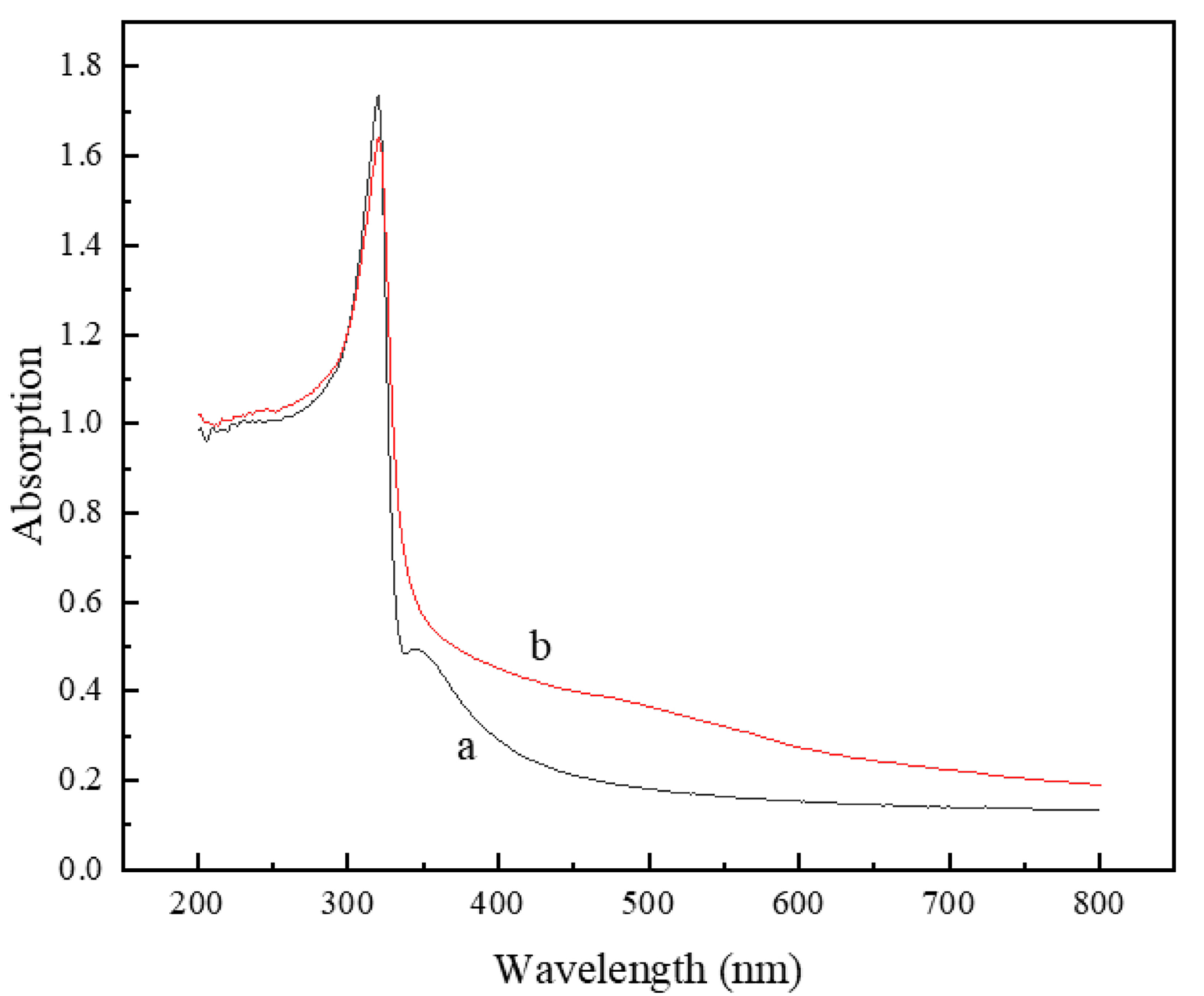
3.2. Effect of Holding Temperature on Silver-Coated Copper Powder
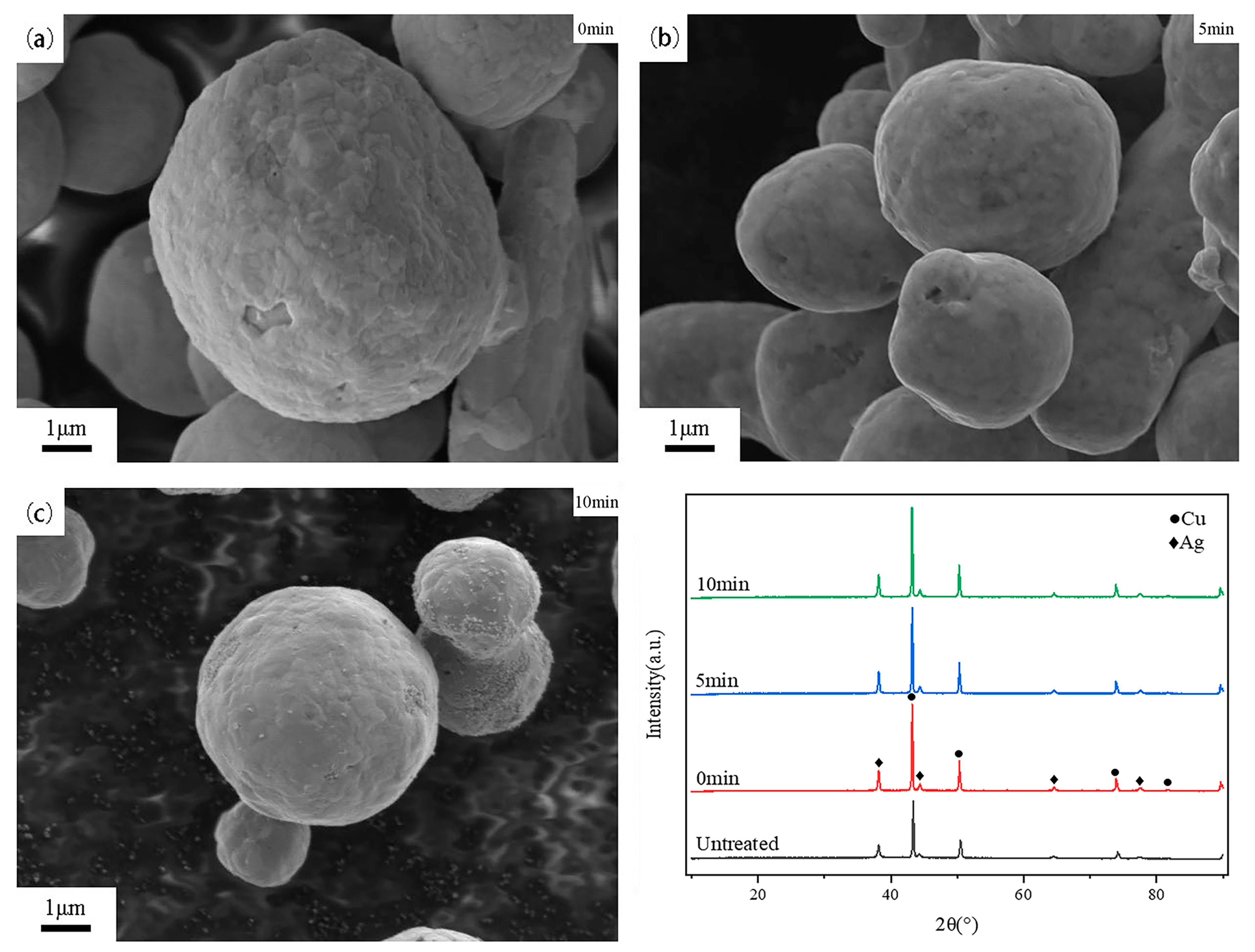
3.3. Effect of Holding Time on Silver-Coated Copper Powder
3.4. Effect of Dual-Temperature Heat Treatment on Silver-Coated Copper Powder
4. Conclusions
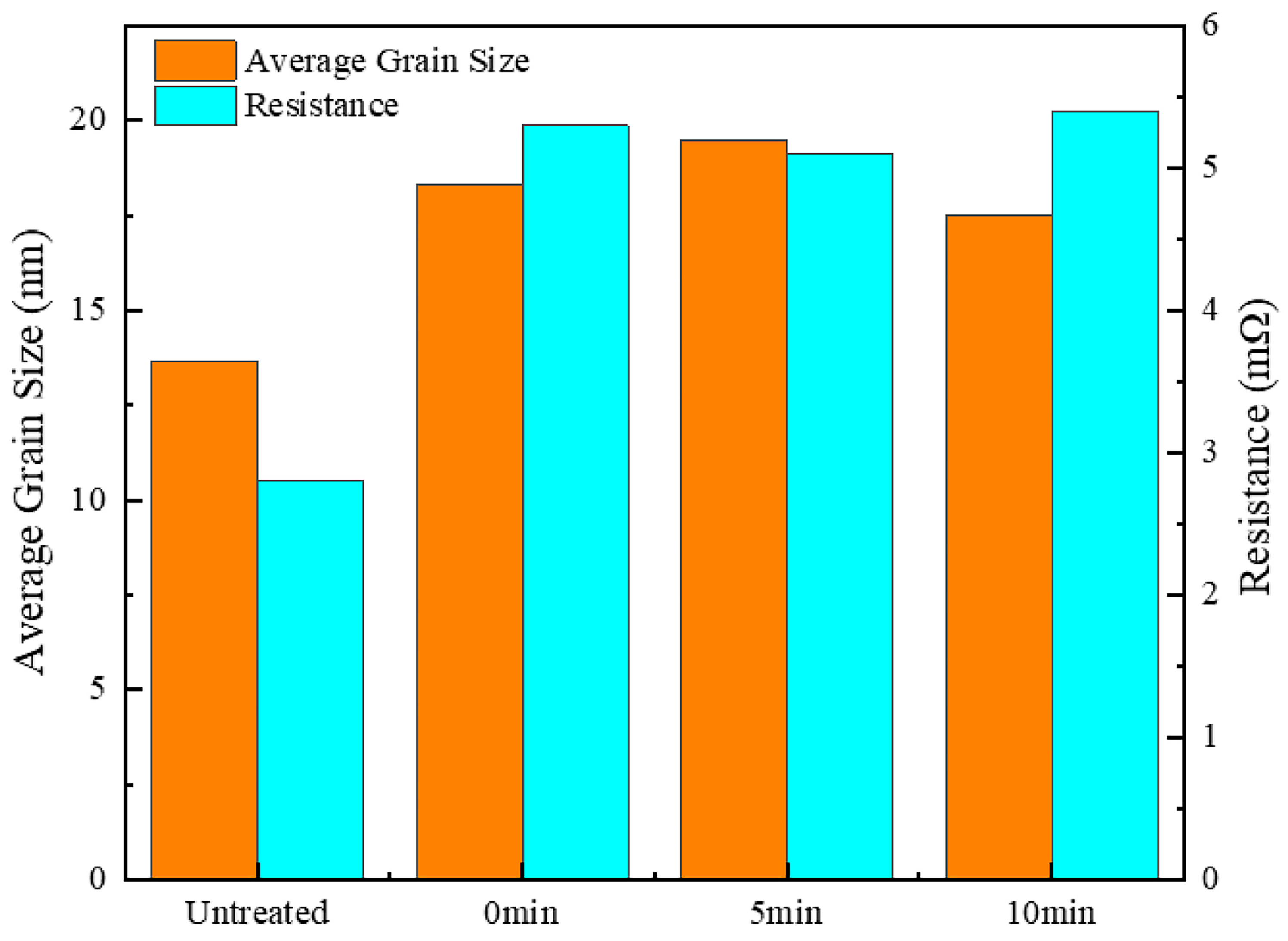
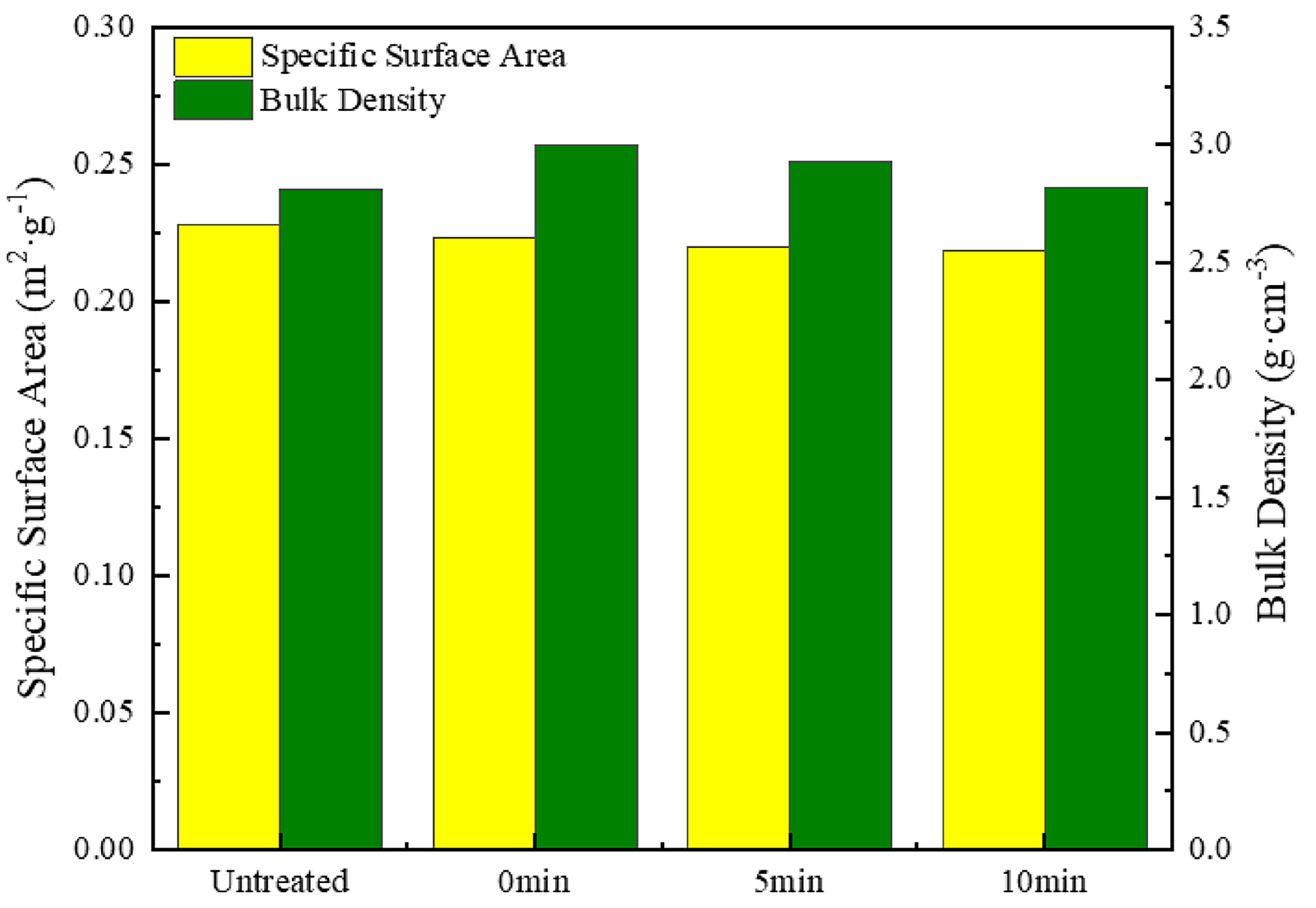
- Surface morphology and smoothness: Heat treatment, particularly the dual-temperature method (600 °C for 5 min followed by 700 °C for 1 min), significantly enhanced the surface flatness and smoothness of the silver-coated copper powder. This process effectively removed surface roughness and pores, resulting in a continuous and uniform silver layer. As a result, the specific surface area decreased from 0.2282 m2/g to 0.2217 m2/g, while the bulk density increased from 2.813 g/cm3 to 2.945 g/cm3, leading to improved fluidity and stability.
- Electrical conductivity: The elimination of dislocation defects and the loss of surface plasmon coupling between silver particles contributed to a decrease in electrical conductivity. As a result, the resistance increased from 2.8 mΩ to 3.2 mΩ. This reduction in conductivity can be attributed to the formation of dislocation defects and changes in grain size during heat treatment, both of which lead to increased electron scattering and hinder the efficient transport of electrons.
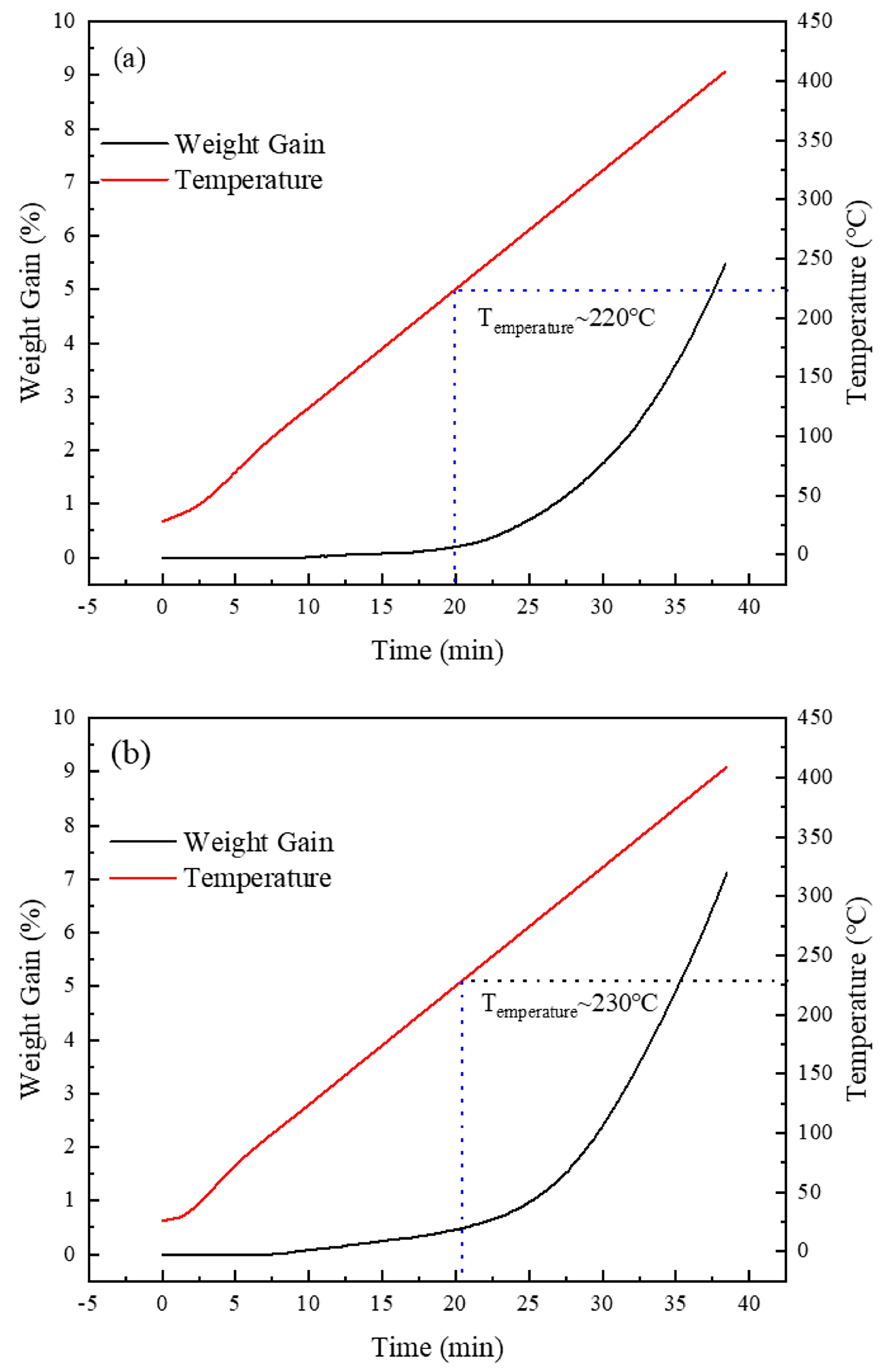
- Oxidation resistance: The initial oxidation temperature increased from 200 °C to 230 °C, indicating an improvement in thermal stability. However, the heat treatment also raised the surface energy and reactivity, which led to an accelerated oxidation rate in the subsequent stages.
- Overall performance and application: The experimental data in this study demonstrate that heat treatment significantly enhances the overall performance of silver-coated copper powder. These findings offer theoretical support for the material’s use in applications such as conductive inks, electronic packaging, and catalytic converters. Future research should focus on further optimizing the heat treatment conditions to achieve an optimal balance between conductivity and other enhanced properties, thereby maximizing the overall performance of silver-coated copper powder.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SEM | scanning electron microscope |
| EDS | energy-dispersive X-ray spectrometer |
| TEM | transmission electron microscope |
| XRD | X-ray diffraction |
| powder diffraction files | |
| FWHM | full width at half maximum |
| HRTEM | high-resolution transmission electron microscopy |
| UV-Vis | ultraviolet–visible absorption spectroscopy |
| IFFT | inverse fast Fourier transform |
| TGA | thermogravimetric analysis |
References
- Guo, W.; Zhang, H.Q.; Zhang, X.Y.; Liu, L.; Peng, P.; Zou, G.S.; Zhou, Y.N. Preparation of nanoparticle and nanowire mixed pastes and their low temperature sintering. J. Alloys Compd. 2017, 690, 86–94. [Google Scholar] [CrossRef]
- Liu, X.; Wu, S.Y.; Chen, B.S.; Ma, Y.Z.; Huang, Y.F.; Tang, S.W.; Liu, W.S. Tuning the electrical resistivity of conductive silver paste prepared by blending multi-morphologies and micro-nanometers silver powder. J. Mater. Sci.-Mater. Electron. 2021, 32, 13777–13786. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Zhang, J.; Fang, C.Q.; Qiu, W.K.; Chen, H.; Liu, H.N.; Wei, Y. Preparation of Low Volatile Organic Compounds Silver Paste Containing Ternary Conductive Fillers and Optimization of Their Performances. Molecules 2022, 27, 8030. [Google Scholar] [CrossRef] [PubMed]
- Scenev, V.; Szalapak, J.; Werft, L.; Hoelck, O.; Jakubowska, M.; von Krshiwoblozki, M.; Kallmayer, C.; Schneider-Ramelow, M. Low-Temperature Processible Highly Conducting Pastes for Printed Electronics Applications. Adv. Eng. Mater. 2022, 24, 2101752. [Google Scholar] [CrossRef]
- Wang, Z.W.; Li, X. Study on the Performance of a Novel Mixed-Particle Silver Paste Sintered at 180 °C. IEEE Trans. Compon. Pack. Manuf. Technol. 2023, 13, 1494–1501. [Google Scholar] [CrossRef]
- Zhang, B.W.; Lu, X.Y.; Ma, H.X.; Wang, D.; Mei, Y.H. Development of Silver Paste With High Sintering Driving Force for Reliable Packaging of Power Electronics. IEEE Trans. Compon. Pack. Manuf. Technol. 2024, 14, 10–17. [Google Scholar] [CrossRef]
- Hamann, L.; Benstetter, G.; Hofer, A.; Mattheis, J.; Haas, M.; Zapf-Gottwick, R. Use of Coated-Metal Particles in Rear Busbar Pastes to Reduce Silver Consumption. IEEE J. Photovolt. 2015, 5, 534–537. [Google Scholar] [CrossRef]
- Jung, D.S.; Lee, H.M.; Kang, Y.C.; Park, S.B. Air-stable silver-coated copper particles of sub-micrometer size. J. Colloid Interface Sci. 2011, 364, 574–581. [Google Scholar] [CrossRef]
- Hsiao, C.H.; Wu, J.S.; Tsai, C.H.; Chen, S.Y.; Song, J.M. The application of Cu-Ag submicron composite particles in microelectronic bonding. In Proceedings of the 2015 International Conference on Electronic Packaging and iMAPS All Asia Conference (ICEP-IAAC), Kyoto, Japan, 14–17 April 2015; pp. 248–251. [Google Scholar]
- Kang, H.S.; Koo, Y.H.; Park, H.D.; Chai, G.S.; Ryoo, S.Y.; Bae, H.B.; Lee, B.C. Manufacturing Method for Core-Shell Metal Nanoparticle Structure Having Excellent Oxidation Stability Using Cu@Ag Core-Shell Nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 8508–8514. [Google Scholar] [CrossRef] [PubMed]
- Snellman, M.; Eom, N.; Ek, M.; Messing, M.E.; Deppert, K. Continuous gas-phase synthesis of core-shell nanoparticles via surface segregation. Nanoscale Adv. 2021, 3, 3041–3052. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Lee, J.-H. Pretreatment Condition of Cu by Ammonium-Based Mixed Solvent and Its Effects on the Fabrication of Ag-Coated Cu Particles. Korean J. Mater. Res. 2016, 26, 109–116. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, F.S.; Zhou, Z.; Zhou, L.Z.; Liu, H. Fabrication of fully covered Cu-Ag core-shell nanoparticles by compound method and anti-oxidation performance. Nanotechnology 2020, 31, 175601. [Google Scholar] [CrossRef]
- Wan, X.Y.; Wang, Y.Y.; Lu, J.L.; Ning, Z.; Li, J.D. Investigation on Stranski-Krastanow (SK) Growth Mode of Ag Coating in Cu/Ag Core-Shell Composites. Coatings 2020, 10, 297. [Google Scholar] [CrossRef]
- Choi, E.B.; Lee, J.H. Submicron Ag-coated Cu particles and characterization methods to evaluate their quality. J. Alloys Compd. 2016, 689, 952–958. [Google Scholar] [CrossRef]
- Hai, H.T.; Takamura, H.; Koike, J. Oxidation behavior of Cu-Ag core-shell particles for solar cell applications. J. Alloys Compd. 2013, 564, 71–77. [Google Scholar] [CrossRef]
- Sun, C.; Lux, S.; Müller, E.; Meffert, M.; Gerthsen, D. Versatile application of a modern scanning electron microscope for materials characterization. J. Mater. Sci. 2020, 55, 13824–13835. [Google Scholar] [CrossRef]
- di, Z.; Yi, C.; Qian, M.; Bin, S.; Lihui, J.; Shun, G. Progress and challenge of electron probe microanalysis technique. Acta Petrol. Sin. 2019, 35, 261–274. [Google Scholar] [CrossRef]
- Lyman, C. Short Courses for Scanning Electron Microscopy and X-ray Microanalysis. Microsc. Microanal. 2013, 19 (Suppl. S2), 294–295. [Google Scholar] [CrossRef]
- Alzoubi, F.Y.; Ahmad, A.A.; Aljarrah, I.A.; Migdadi, A.B.; Al-Bataineh, Q.M. Localize surface plasmon resonance of silver nanoparticles using Mie theory. J. Mater. Sci. Mater. Electron. 2023, 34, 2128. [Google Scholar] [CrossRef]
- Leger, P.E.; Fabregue, N.; Jeandin, M.; Ducos, M. Influence of powder characteristics on the microstructure and bond strength of cold-sprayed aluminum coating. In Proceedings of the IEEE Intelligent Transportation Systems Conference 2016, Rio de Janeiro, Brazil, 1–4 November 2016; pp. 578–584. [Google Scholar]
- Yu, X.; Sun, H.; Qian, Z.; Li, W.; Li, W.; Huang, F.; Li, J.; Gan, G. Effect of Silver Powder Microstructure on the Performance of Silver Powder and Front-Side Solar Silver Paste. Materials 2024, 17, 445. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xie, K.; Lin, Q.; Cao, R.; Qiu, F. Experimental determination of surface energy for high-energy surface: A review. Adv. Colloid Interface Sci. 2023, 315, 102905. [Google Scholar] [CrossRef]
- Gritsenko, V.V.; Bogatyrenko, S.I.; Minenkov, A.A.; Kryshtal, A.P. Size Evolution of Solid State Area on the Ag-Cu Phase Diagram. In Proceedings of the 7th IEEE International Conference Nanomaterials—Application and Properties (NAP), Odessa, Ukraine, 10–15 September 2017. [Google Scholar]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Pinal, R.; Carvajal, M.T. Integrating Particle Microstructure, Surface and Mechanical Characterization with Bulk Powder Processing. KONA Powder Part. J. 2020, 37, 195–213. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, C.M.; Cai, J.X.; Wang, J.; Liu, K.Q.; Cheng, X.W. Synthesis and characterization of activated alumina with high thermal stability by a low-heat solid-phase precursor method. Microporous Mesoporous Mater. 2022, 337, 111921. [Google Scholar] [CrossRef]
- Yang, W.; Hu, S.; Zhu, W.; Li, M. Research progress of low-temperature sintering nano silver paste. Trans. China Weld. Inst. 2022, 43, 137–146. [Google Scholar]
- Ding, C.; Xu, J.; Shan, D.; Guo, B. Research Progress on Metallic Nanolamellar Composites. Mater. China 2022, 41, 808–818. [Google Scholar]
- Zhang, C.; Zhang, J.; Dou, X.Y.; Zhu, Y. Connection of Absorption and Raman Enhancement Characteristics of Different Types of Ag Nanoparticles. Spectrosc. Spectr. Anal. 2021, 41, 1816–1820. [Google Scholar]
- Lee, C.H.; Choi, E.B.; Lee, J.H. Characterization of novel high-speed die attachment method at 225 °C using submicrometer Ag-coated Cu particles. Scr. Mater. 2018, 150, 7–12. [Google Scholar] [CrossRef]
- Choi, E.B.; Lee, J.H. Dewetting behavior of Ag in Ag-coated Cu particle with thick Ag shell. Appl. Surf. Sci. 2019, 480, 839–845. [Google Scholar] [CrossRef]
- Yang, G.N.; Wang, P.Y.; Liu, Y.; Lu, S.Z.; Luo, B.; Lai, T.; Ta, S.; Lin, T.Y.; Luo, J.Y.; Zhang, Y.; et al. Effect of Ag coating on the oxidation resistance, sintering properties, and migration resistance of Cu particles. J. Alloys Compd. 2022, 923, 166271. [Google Scholar] [CrossRef]


| Ingredients | HCl/(mol·L−1) | Ag(NH3)2+/(mol·L−1) | VC/(mol·L−1) | pH | Temperature/(°C) | Dropping Speed/(mL·min−1) |
|---|---|---|---|---|---|---|
| Numeric | 0.01 | 0.02 | 0.14 | 9–11 | 50 | 8–10 |
| Parameter | Atmosphere | Heating Speed/(°C·min−1) | Cooling Rate/(°C·min−1) | Nitrogen Flow Rate/(mL·min−1) | Internal Pressure/(kPa) |
|---|---|---|---|---|---|
| Numeric | Nitrogen | 10 | 10 | 30 | 202.65 |
| Heat Treatment Conditions | |
|---|---|
| H0 | Untreated |
| H1 | Hold at 550 °C for 5 min |
| H2 | Hold at 600 °C for 5 min |
| H3 | Hold at 650 °C for 5 min |
| H4 | Hold at 700 °C for 0 min |
| H5 | Hold at 700 °C for 5 min |
| H6 | Hold at 700 °C for 10 min |
| H7 | Hold at 600 °C for 5 min, then heat to 700 °C and hold for 0 min |
| Property | Particle Size/(μm) | Resistance/(mΩ) | Specific Surface Area/(m2·g−1) | Bulk Density/(g·cm−3) | Theoreticsal Silver Content/(%) |
|---|---|---|---|---|---|
| Numeric | 3–5 | 2.8 | 0.2282 | 2.813 | 15 |
| Property | Resistance/(mΩ) | Average Grain Size/(nm) | Specific Surface Area/(m2·g−1) | Bulk Density/(g·cm−3) |
|---|---|---|---|---|
| Untreated | 2.8 | 13.68 | 0.2282 | 2.813 |
| H7 | 3.2 | 20.33 | 0.2217 | 2.945 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Zhu, X.; Li, X.; Chen, J.; Yang, N. Effect of Heat Treatment on the Structure and Properties of Silver-Coated Copper Powder. Materials 2025, 18, 940. https://doi.org/10.3390/ma18050940
Yang B, Zhu X, Li X, Chen J, Yang N. Effect of Heat Treatment on the Structure and Properties of Silver-Coated Copper Powder. Materials. 2025; 18(5):940. https://doi.org/10.3390/ma18050940
Chicago/Turabian StyleYang, Bingzhe, Xiaoyun Zhu, Xiang Li, Junquan Chen, and Nan Yang. 2025. "Effect of Heat Treatment on the Structure and Properties of Silver-Coated Copper Powder" Materials 18, no. 5: 940. https://doi.org/10.3390/ma18050940
APA StyleYang, B., Zhu, X., Li, X., Chen, J., & Yang, N. (2025). Effect of Heat Treatment on the Structure and Properties of Silver-Coated Copper Powder. Materials, 18(5), 940. https://doi.org/10.3390/ma18050940





