The Influence of Rare Earth Metals on the Microstructure and Mechanical Properties of 220 and 356.1 Alloys for Automotive Industry
Abstract
1. Introduction
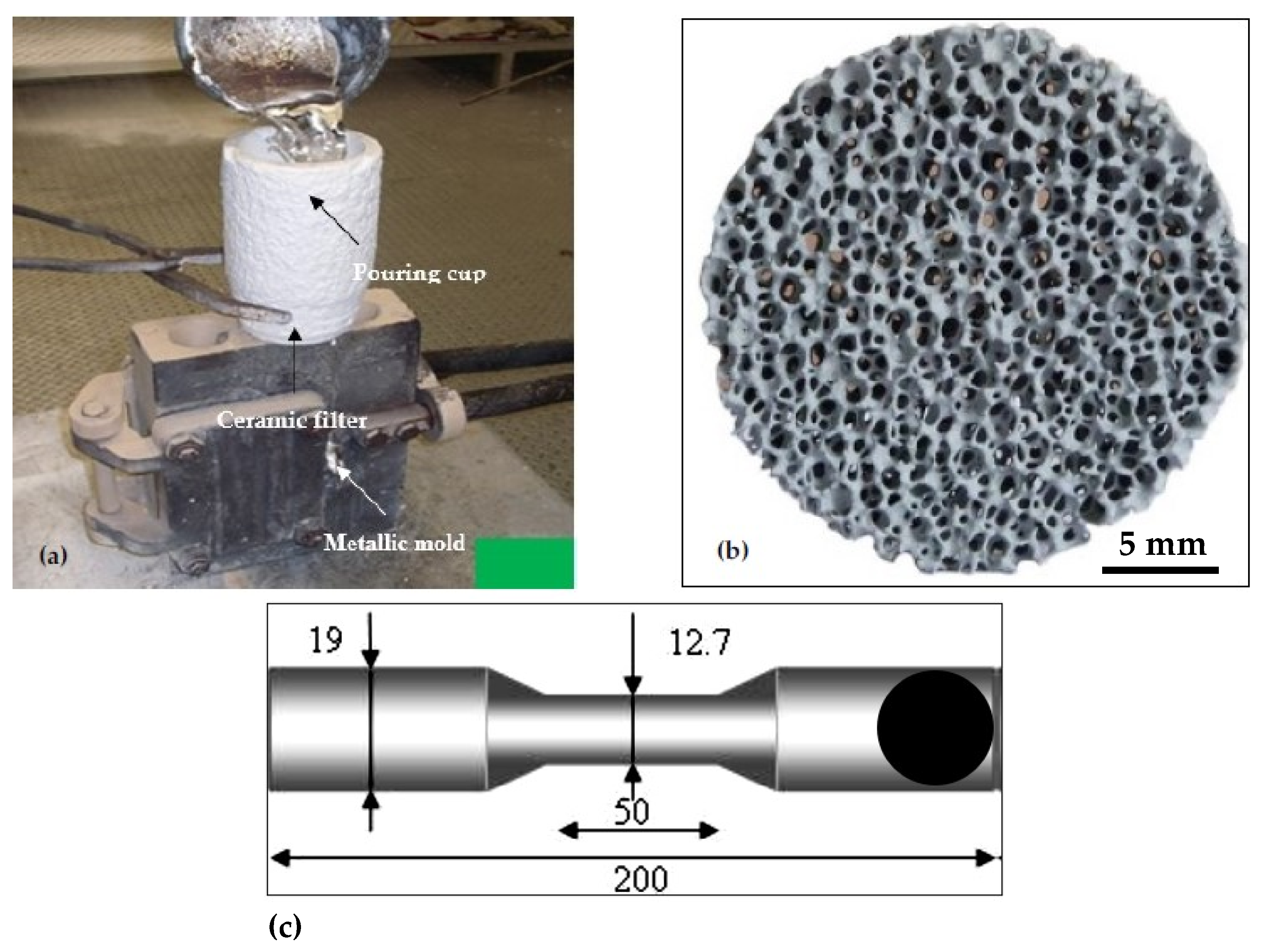
2. Experimental Procedures
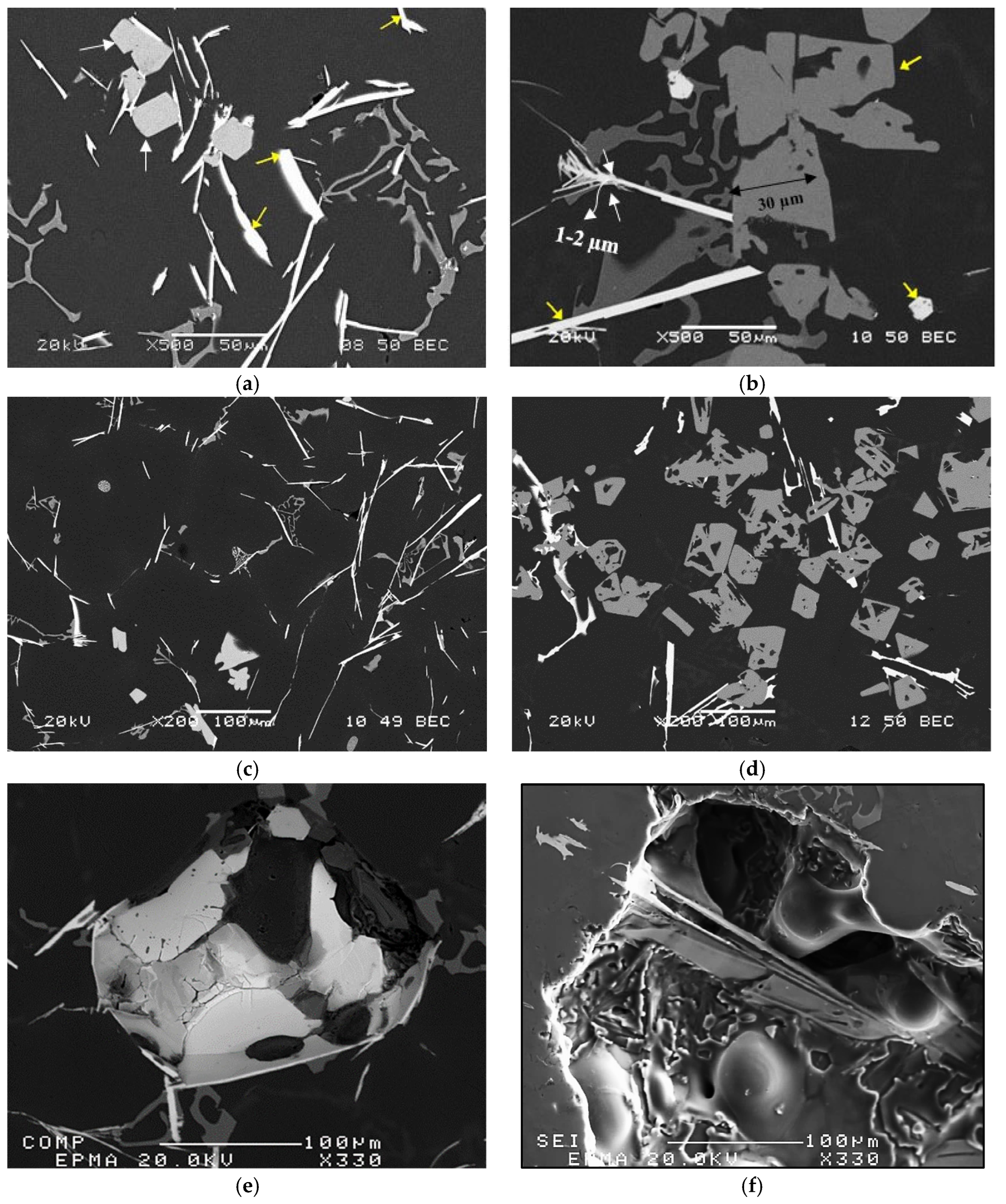
3. Results and Discussion
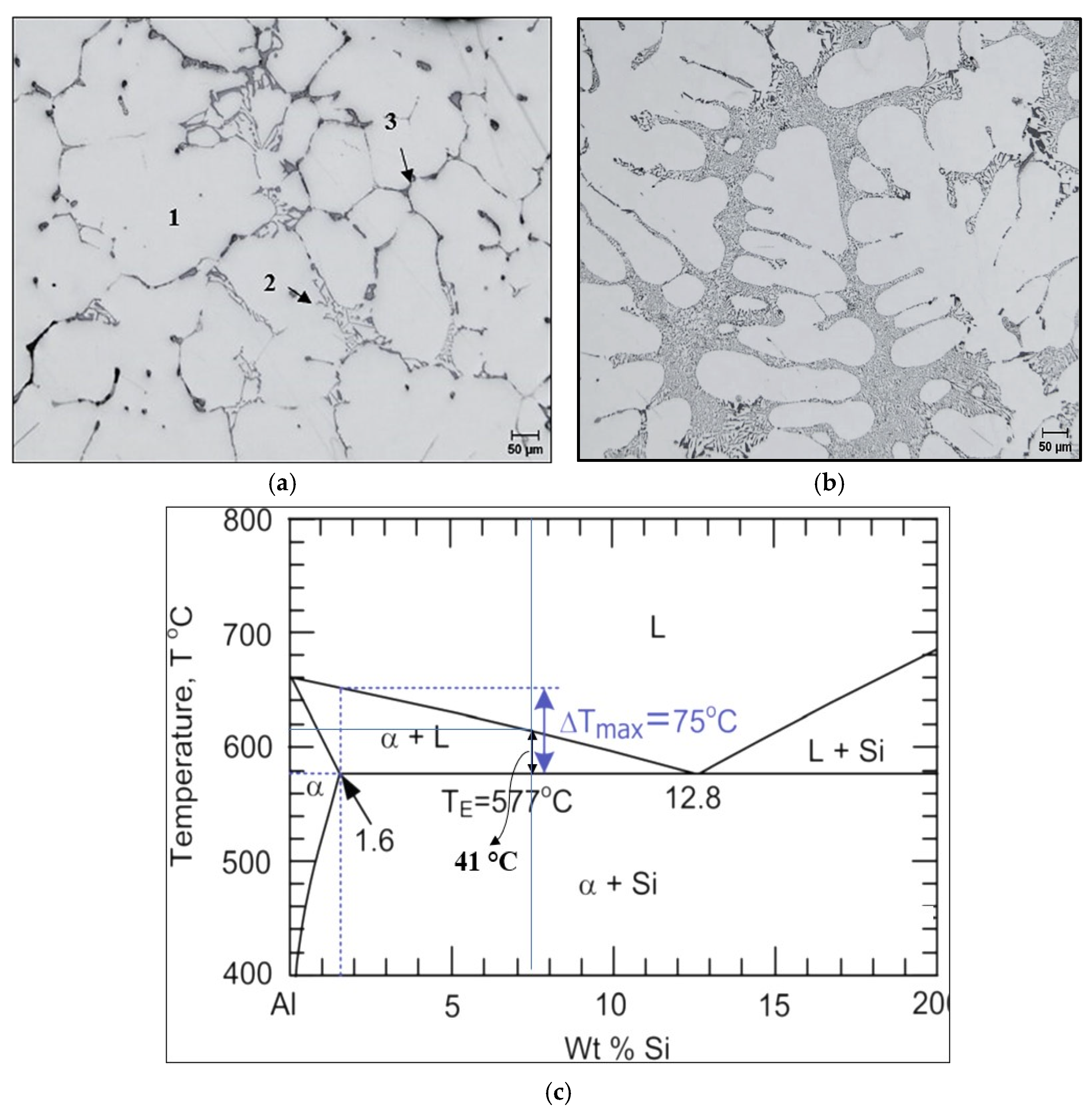
3.1. Metallurgical Background-Solidification Rate ~0.35 °C/s
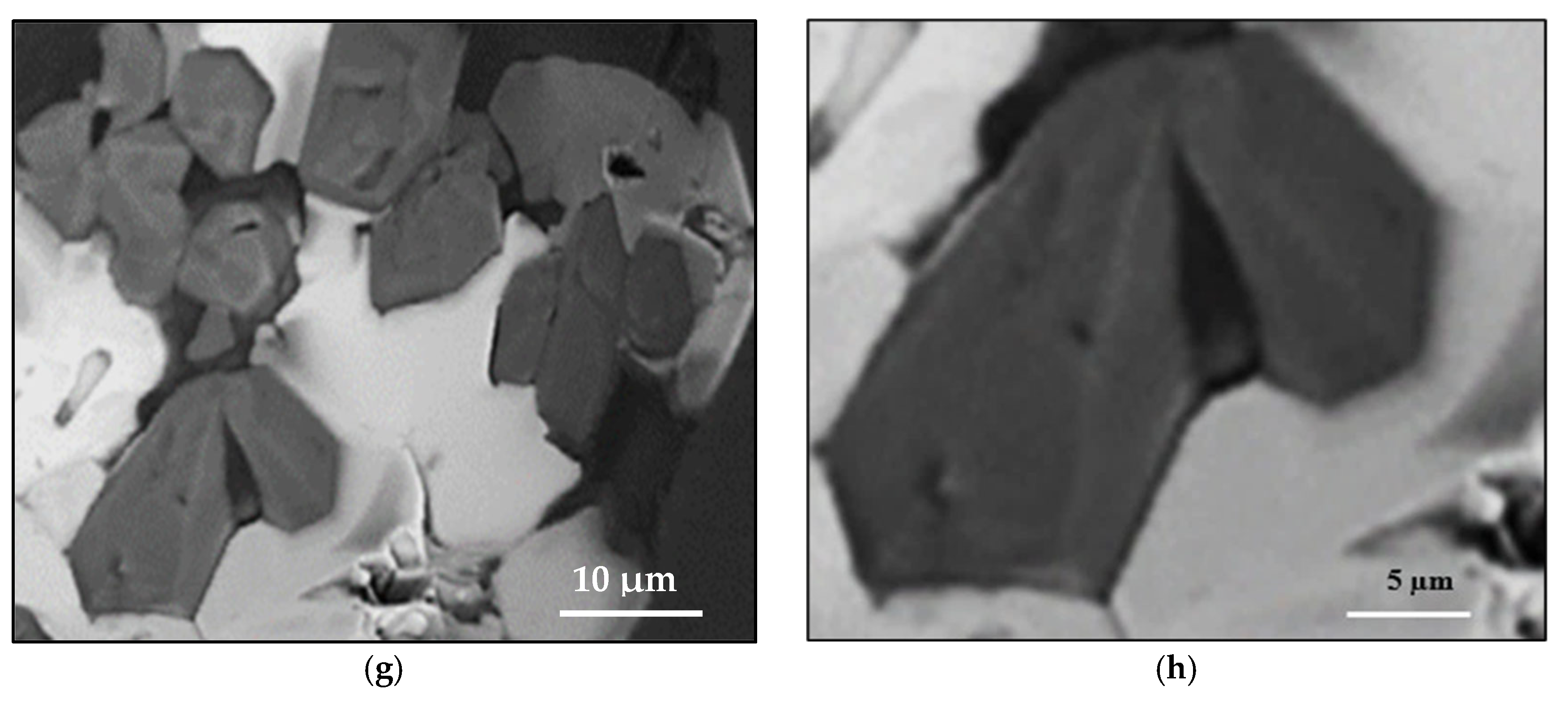
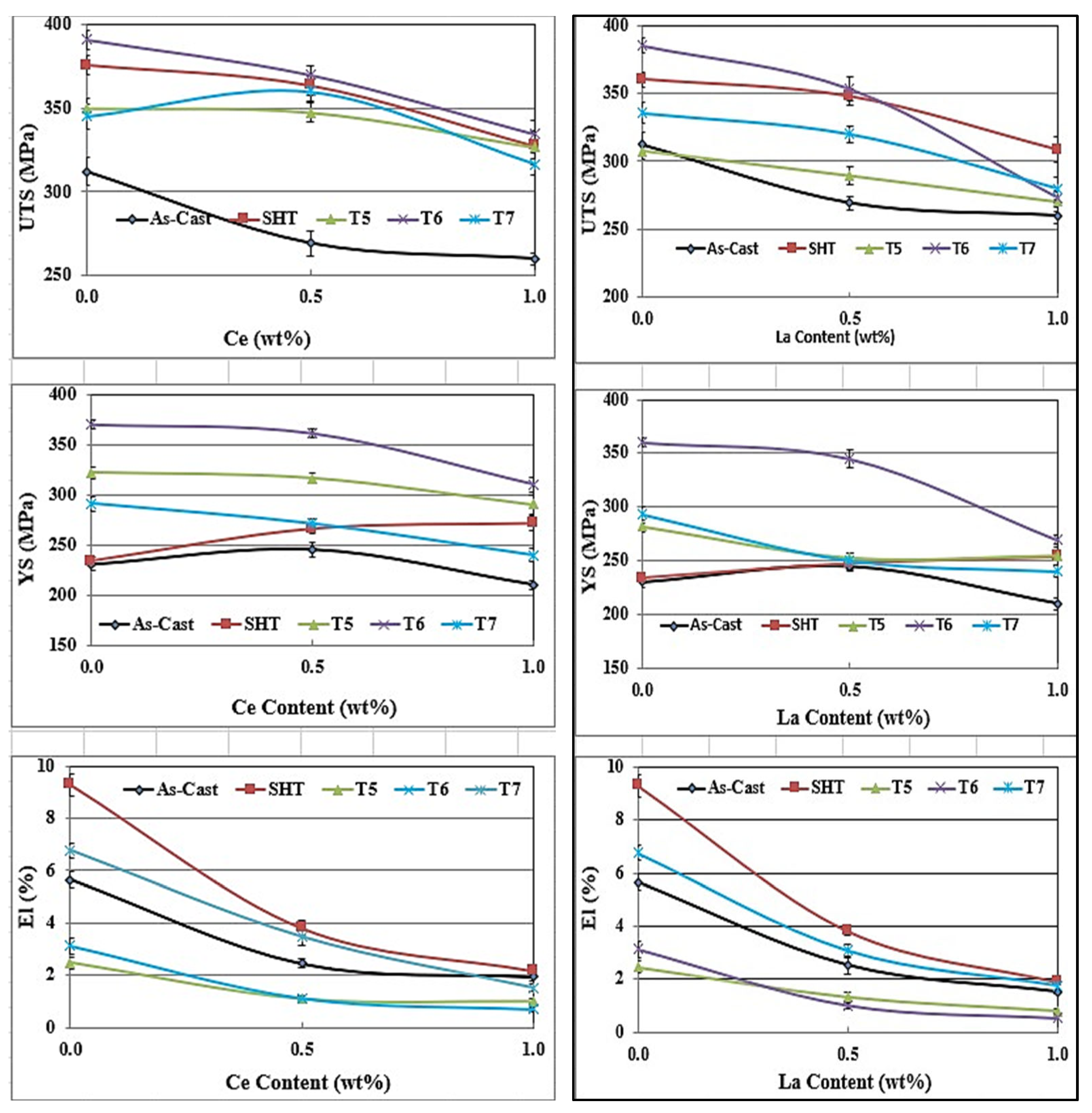
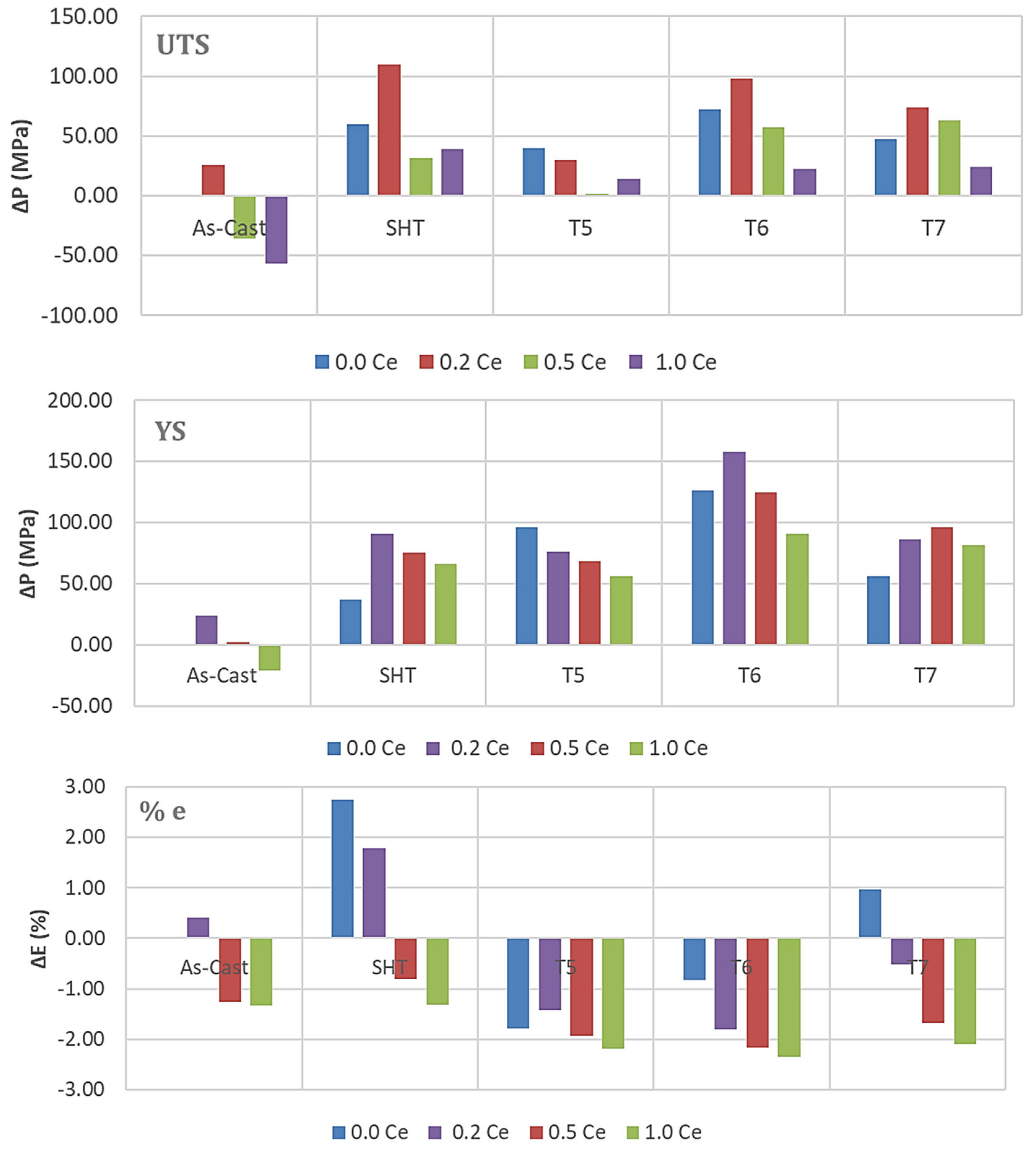
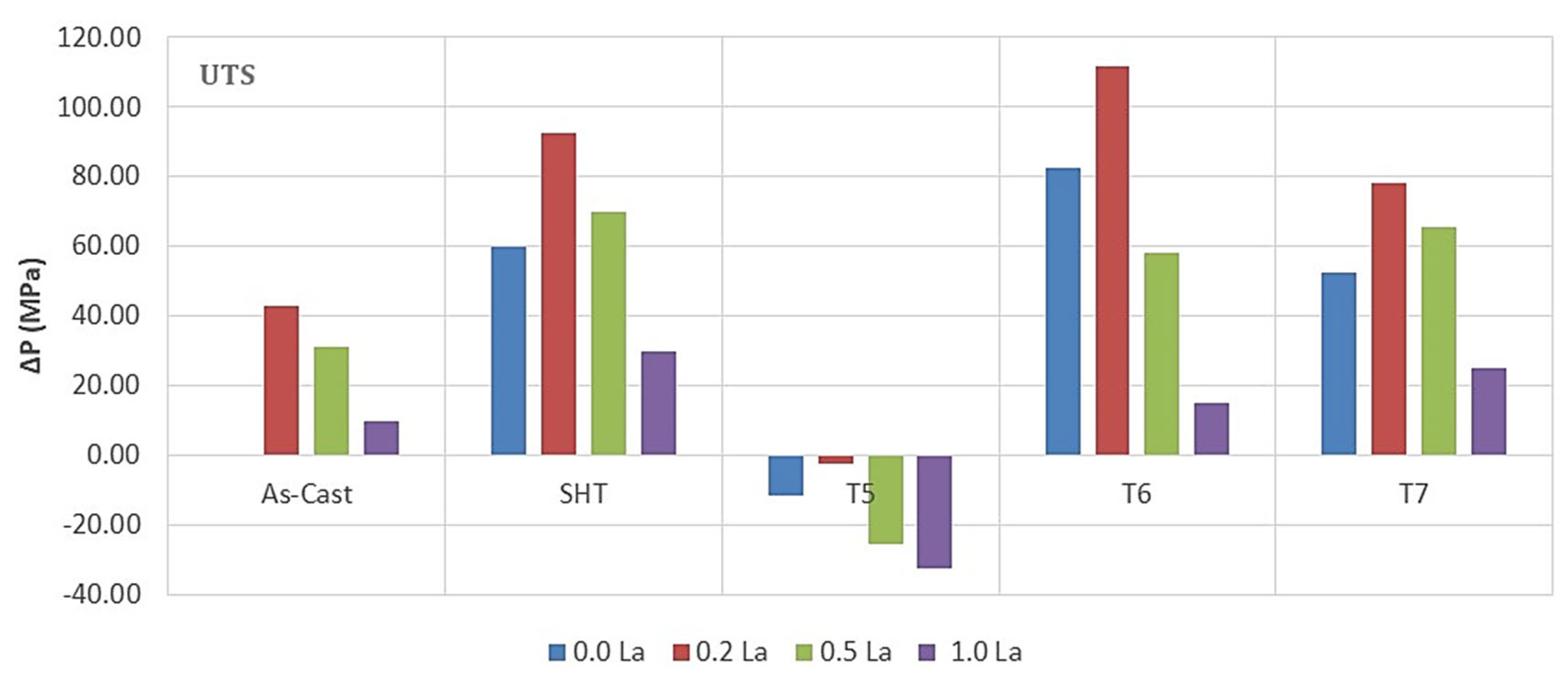
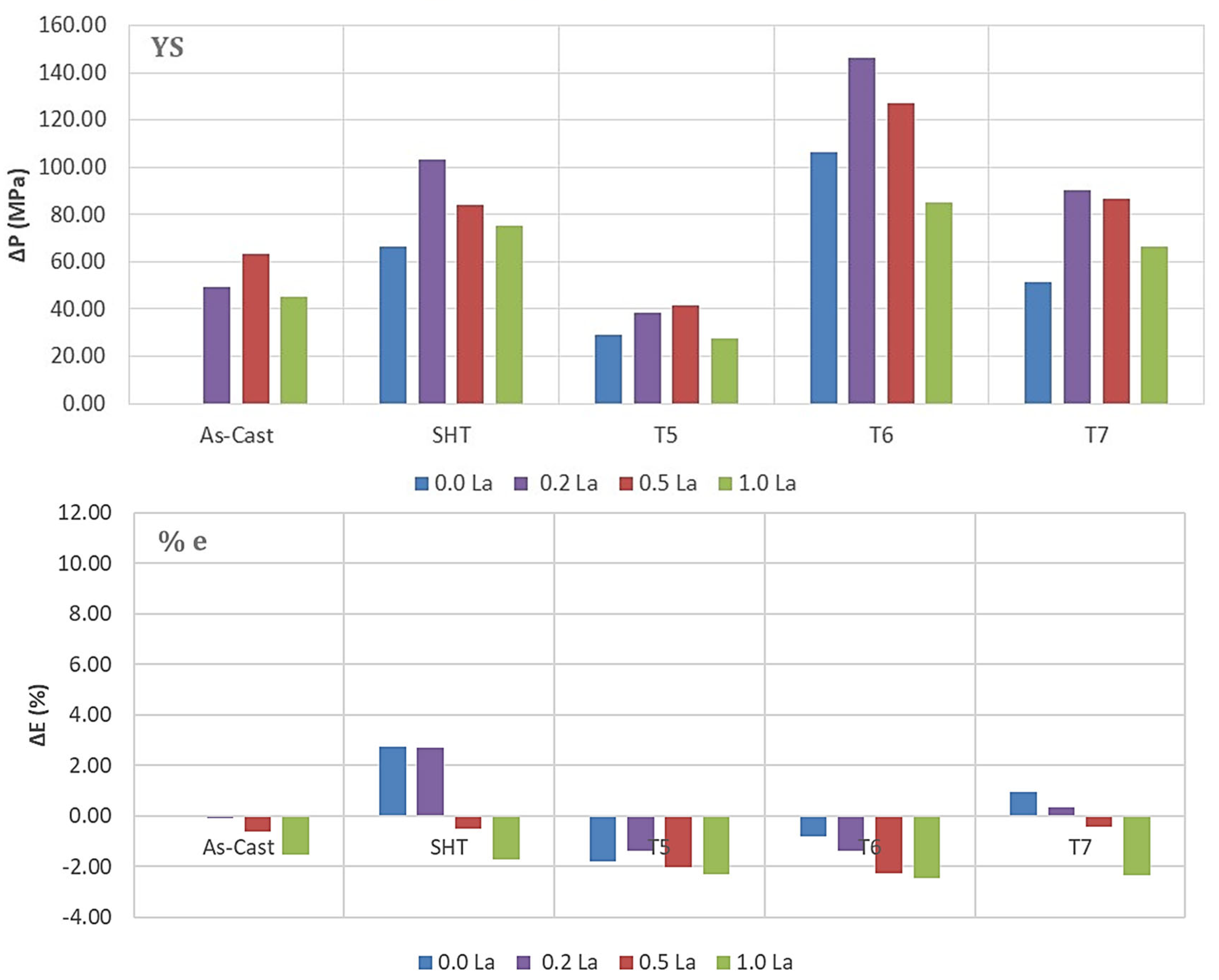
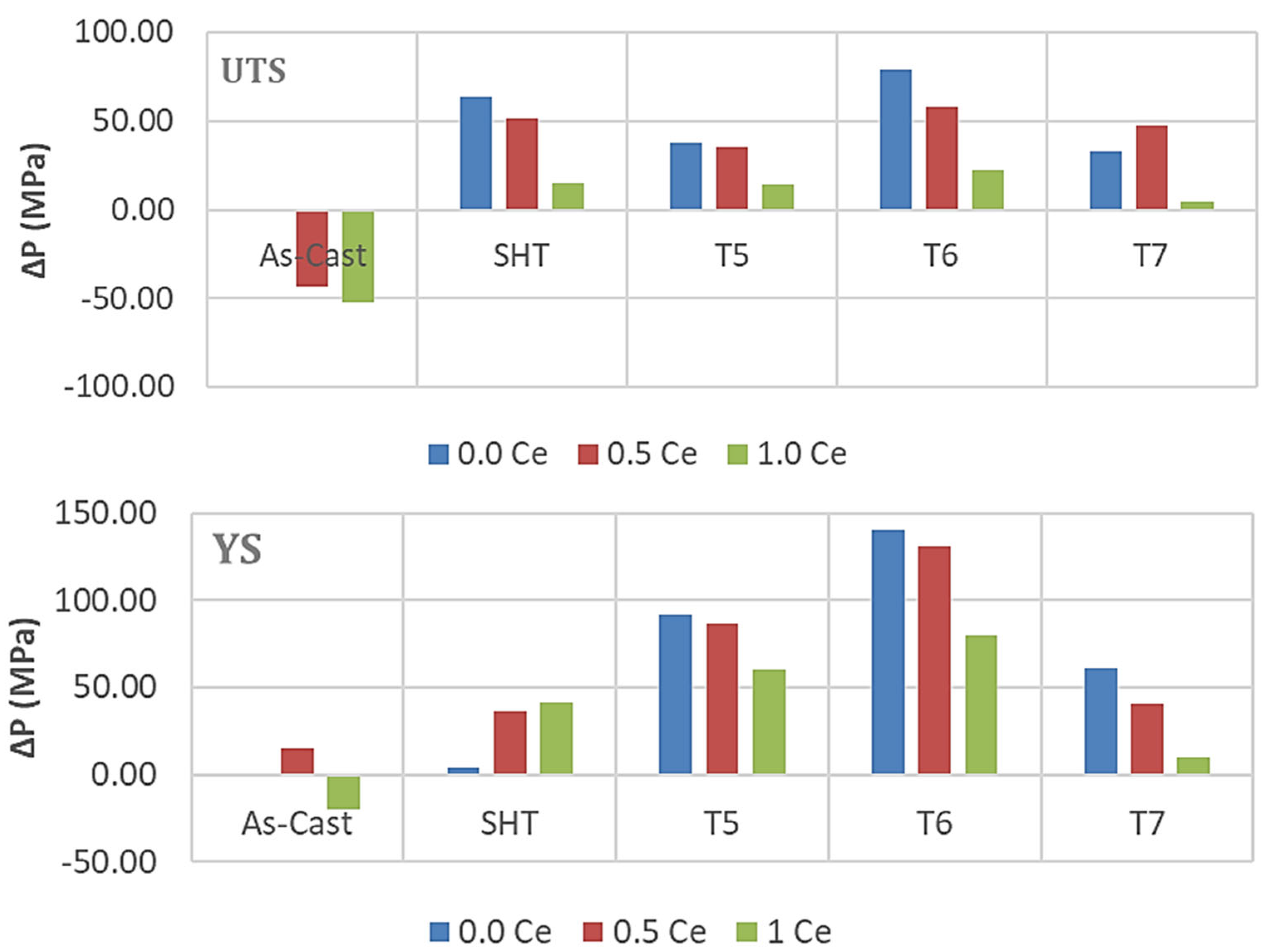
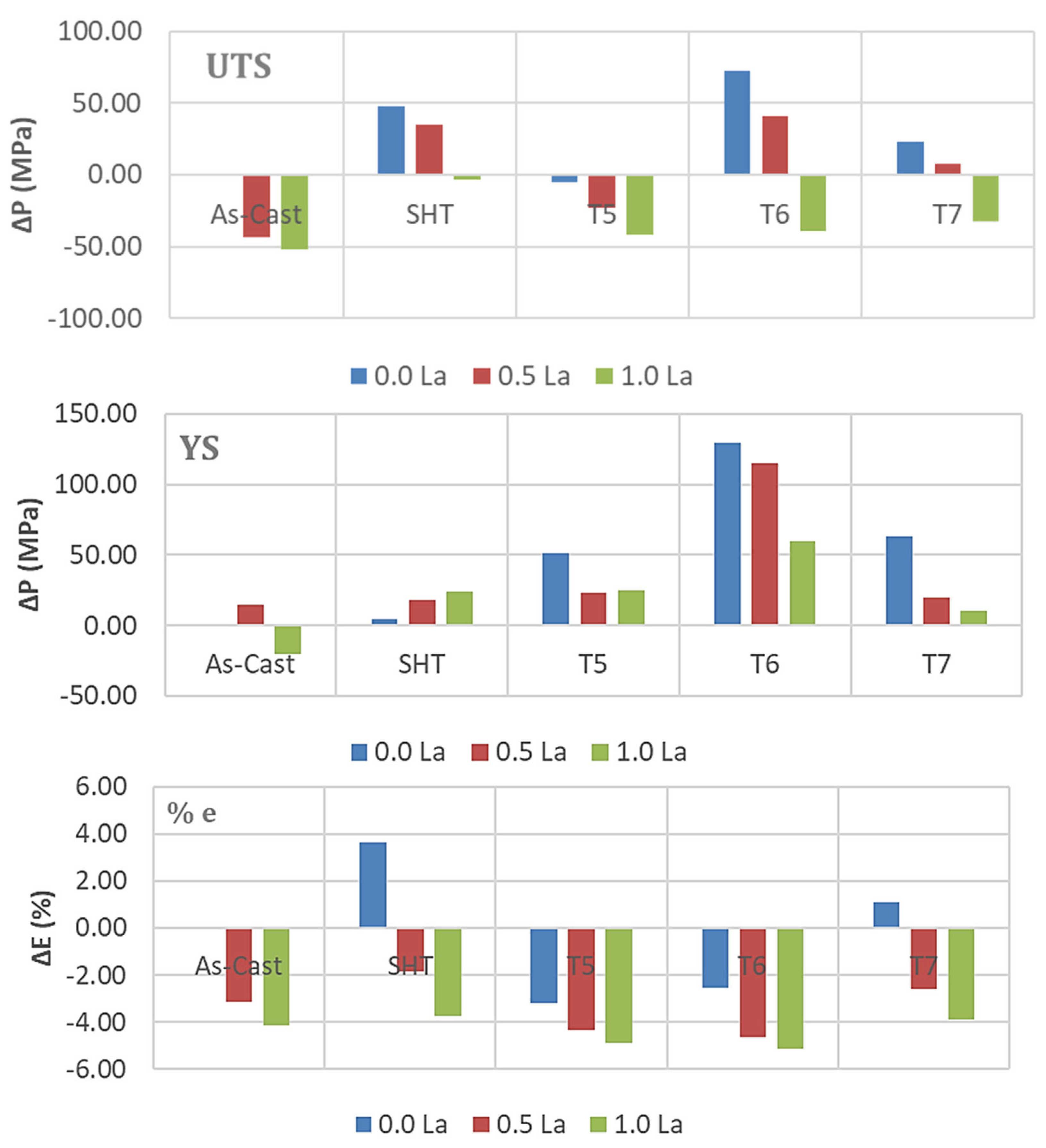
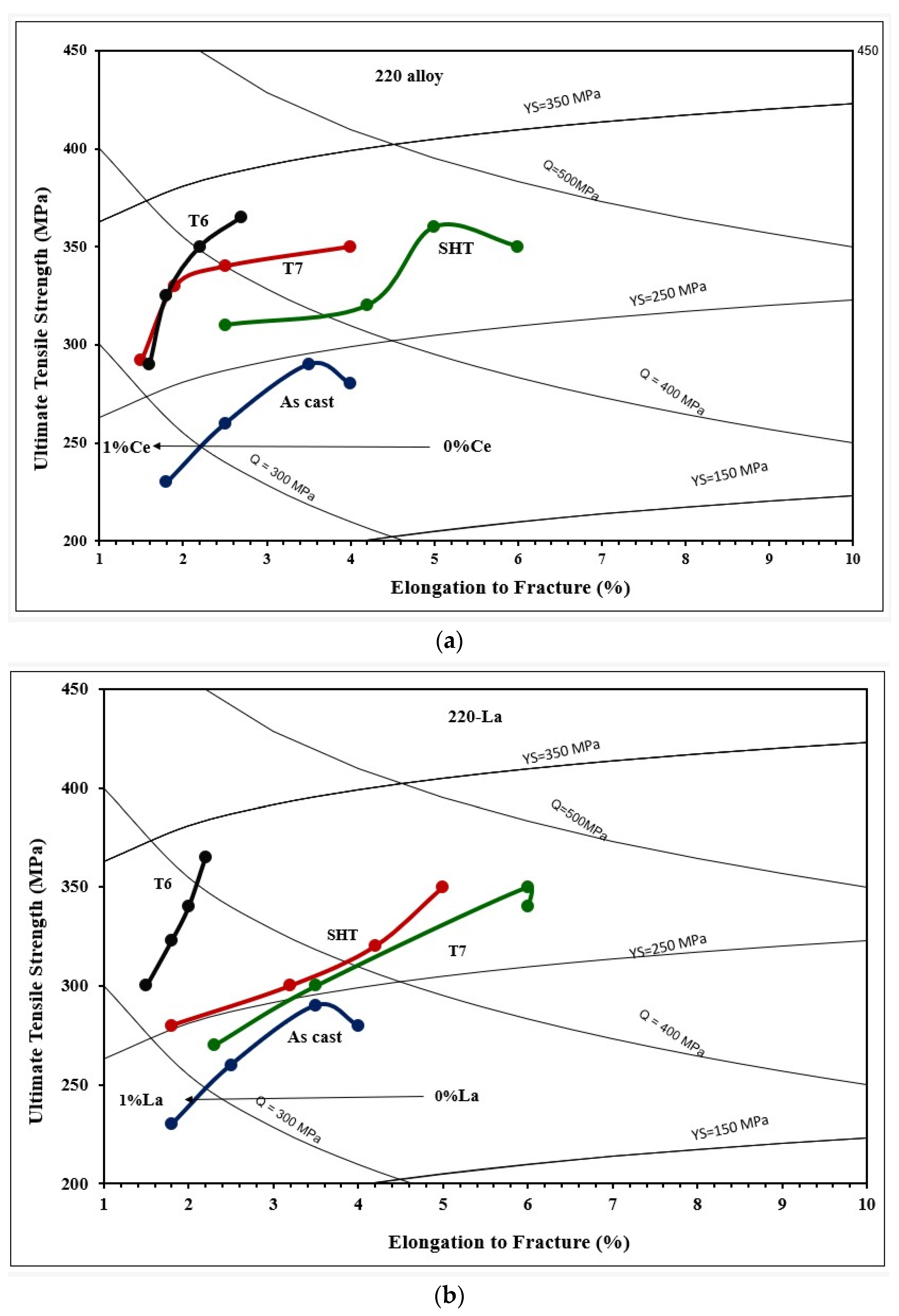
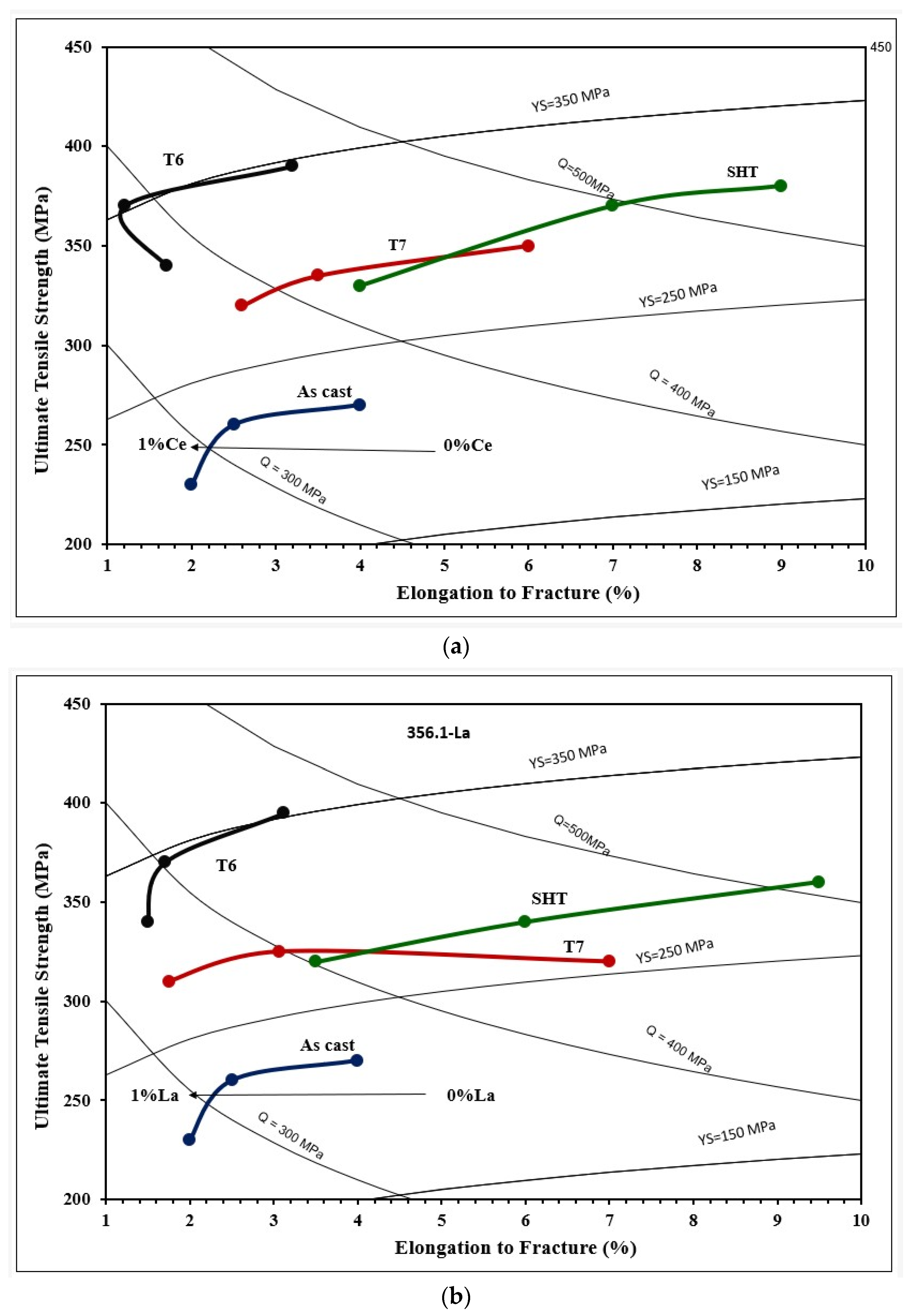
3.2. Tensile Testing Analysis-Solidification Rate About 7 °C/s
4. Conclusions
- The effectiveness of RE as a grain refiner or eutectic Si modifier depends mainly on the purity of the used RE, i.e., free from tramp elements, as well as the concentration of the added RE.
- RE has a moderate grain refining effect caused by constitutional undercooling. When RE is present in the melt in the form of finely dispersed dispersoids, grain refining takes place as well, by heterogeneous nucleation.
- When the molten metal is well degassed and filtered prior to pouring into the mold, grain refining due to precipitation on RE oxides is questionable.
- The addition of 1%La to Sr-modified melt is capable of causing serious demodification if Sr is less than 100 ppm. On the other hand, Ce has no affinity to react with Sr. In other words, REs have no modification effectiveness.
- The volume fraction of insoluble RE depends on the amount of used Ti due to the formation of RE–Ti compounds.
- Due to the thickness of RE platelets (less than 1 µm in Ti-free melts), the exact composition of these platelets is random.
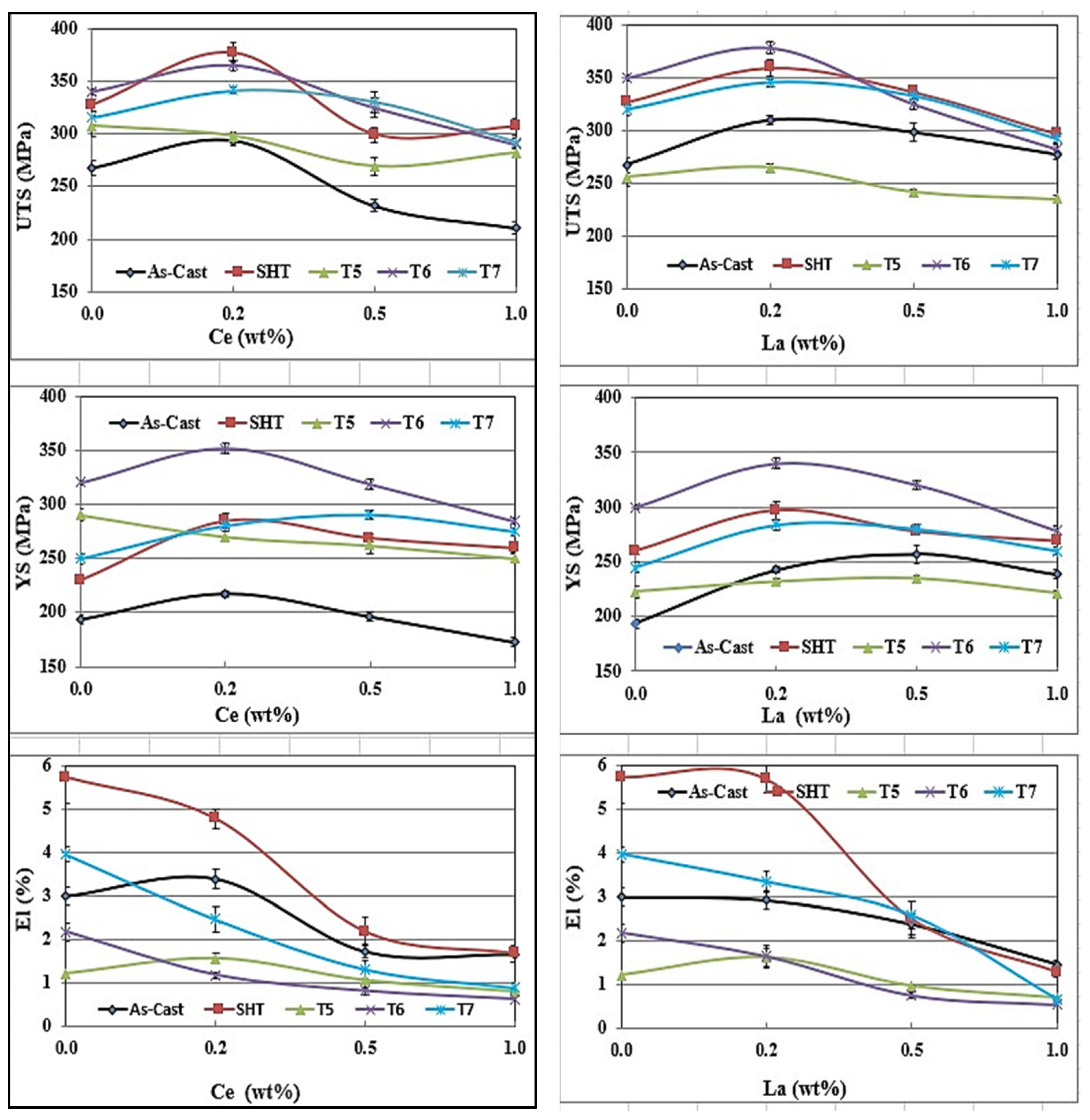
- The addition of 0.2% RE would enhance the alloy strength by about 10–15%. Beyond this concentration, a marked degradation in the alloy strength is likely to take place.
- Among all the applied heat treatments, T6 represents the source of maximum hardening (over 130 MPa), whereas the solutionizing treatment is the best choice for high ductility.
- The addition of 0.2 wt.%RE has a hardening effect caused by the precipitation of RE in the form of dispersoids (300–700 nm).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ford, A.; Huston, D.; Cloutier, J.; Doublier, M.; Schofield, A.; Cheng, Y.; Beyer, E. A national scale mineral potential assessment for carbonatite-related rare earth element mineral systems in Australia. Ore Geol. Rev. 2023, 161, 105658. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z.; Chen, C. Global potential of rare earth resources and demand for rare earths from clean technologies. Minerals 2017, 7, 203. [Google Scholar] [CrossRef]
- Long, K.R.; van Gosen, B.S.; Foley, N.K.; Cordier, D. The Principle Rare Earth Elements Deposits of the United States—A Summary: Of Domestic Deposits and a Global Perspective; Scientific Investigations Report 2010-5220; U.S. Geological Survey: Reston, VA, USA, 2010. [Google Scholar]
- Shen, Y.; Jiang, Y.; Qiu, X.; Zhao, S. Leaching of Light Rare Earth Elements from Sichuan Bastnaesite: A Facile Process to Leach Trivalent Rare Earth Elements Selectively from Tetravalent Cerium. JOM 2017, 69, 1976–1981. [Google Scholar] [CrossRef]
- Xu, D.; Shah, Z.; Cui, Y.; Jin, L.; Peng, X.; Zhang, H.; Sun, G. Recovery of rare earths from nitric acid leach solutions of phosphate ores using solvent extraction with a new amide extractant (TODGA). Hydrometallurgy 2018, 180, 132–138. [Google Scholar] [CrossRef]
- Liu, F.; Porvali, A.; Halli, P.; Wilson, B.P.; Lundström, M. Comparison of Different Leaching Media and Their Effect on REEs Recovery from Spent Nd-Fe-B Magnets. JOM 2020, 72, 806–815. [Google Scholar] [CrossRef]
- Echeverry-Vargas, L.; Ocampo-Carmona, L.M. Recovery of Rare Earth Elements from Mining Tailings: A Case Study for Generating Wealth fromWaste. Minerals 2022, 12, 948. [Google Scholar] [CrossRef]
- Pourbahari, B.; Mirzadeh, H.; Emamy, M. The Effects of Grain Refinement and Rare Earth Intermetallics on Mechanical Properties of As-Cast and Wrought Magnesium Alloys. J. Mater. Eng. Perform. 2018, 27, 1327–1333. [Google Scholar] [CrossRef]
- Czerwinski, F. Cerium in aluminum alloys. J Mater Sci 2020, 55, 24–72. [Google Scholar] [CrossRef]
- Gursoy, O.; Timelli, G. Lanthanides: A focused review of eutectic modification in hypoeutectic Al–Si alloys. J. Mater. Res. Technol. 2020, 9, 8652–8666. [Google Scholar] [CrossRef]
- Cheng, Z.; Yan, H.; Zhang, S.; Zou, X.; Cao, C. Effect of Rare Earth La on Microstructure, Hardness and Corrosion Resistance of A356 Aluminum Alloy. Met. Mater. Int. 2024, 30, 2490–2502. [Google Scholar] [CrossRef]
- Microstructure evolution and strengthening mechanism of A356/Al-X-Ce(Ti, C) system by inoculation treatment. J. Mater. Res. Technol. 2024, 28, 1233–1246. [CrossRef]
- Tsai, Y.-C.; Chou, C.-Y.; Jeng, R.-R.; Lee, S.-L.; Lin, C.-K. Effect of rare earth elements addition on microstructures and mechanical properties of A356 alloy. Int. J. Cast Met. Res. 2011, 24, 83–87. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, F.; Ai, F.; Yan, H. Effects of rare earth Er additions on microstructure development and mechanical properties of diecast ADC12 aluminum alloy. J. Alloys Compd. 2012, 538, 21–27. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Dai, Y.; Li, C. Effects of rare earth elements addition on microstructures, tensile properties and fractography of A357 alloy. Mater. Sci. Eng. A 2014, 597, 237–244. [Google Scholar] [CrossRef]
- Zhang, S.; Su, Y.; Gong, W. Effects of rare earth elements on microstructure and tensile properties of Al-Si-Cu alloy at 250 °C. China Foundry 2021, 18, 474–480. [Google Scholar] [CrossRef]
- Alkahtani, S.A.; Elgallad, E.M.; Tash, M.M.; Samuel, A.M.; Samuel, F.H. Effect of Rare Earth Metals on the Microstructure of Al-Si Based Alloys. Materials 2016, 9, 45. [Google Scholar] [CrossRef]
- Wan, W.; Han, J.; LiI, W.; Wang, J. Study of rare earth element effect on microstructures and mechanical properties of an Al-Cu-Mg-Si cast alloy. Rare Metals 2006, 25, 129–132. [Google Scholar] [CrossRef]
- ASTM B-108; Standard Specification for Aluminum-Alloy Permanent Mold Castings. ASTM: West Conshohocken, PA, USA, 2021.
- Soysal, T. A criterion to find crack-resistant aluminium alloys to avoid solidification cracking. Sci. Technol. Weld. Join. 2021, 26, 99–105. [Google Scholar] [CrossRef]
- Kou, S. A criterion for cracking during solidification. Acta Mater 2015, 88, 366–374. [Google Scholar] [CrossRef]
- Clyne, T.W.; Davies, G.J. Influence of composition on solidification cracking susceptibility in binary alloy systems. Br. Foundrym. 1981, 74, 65–73. [Google Scholar]
- Benyoucef, M.; Clément, N.; Coujou, A. Transmission electron microscope in situ deformation of MC2 superalloy at room temperature. In Fundamental Aspects of Dislocation Interactions; Kostorz, G., Calderon, H.A., Martin, J.L., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 401–406. [Google Scholar]
- Mahmoud, M.G.; Samuel, A.M.; Doty, H.W.; Samuel, F.H. Role of Heat Treatment on the Tensile Properties and Fractography of Al–1.2Si–2.4Cu and Al–8.0Si–2.4Cu Cast Alloys Modified with Ce, La and Sr Addition. Int. J. Met. 2020, 14, 218–242. [Google Scholar] [CrossRef]
- Mahmoud, M.G.; Samuel, A.M.; Doty, H.W.; Samuel, F.H. Effect of the Addition of La and Ce on the Solidification Behavior of Al–Cu and Al–Si–Cu Cast Alloys. Int. J. Met. Cast. 2020, 14, 191–206. [Google Scholar] [CrossRef]
- Li, H.; Huang, D.; Kang, W.; Liu, J.; Ou, Y.; Li, D. Effect of Different Aging Processes on the Microstructure and Mechanical Properties of a Novel Al–Cu–Li Alloy. J. Mater. Sci. Technol. 2016, 32, 1049–1053. [Google Scholar] [CrossRef]
- Khisheh, S.; Azadi, M.; Hendoabadi, V.Z.; Parast, M.S.A.; Winter, G.; Seisenbacher, B.; Gruen, F.; Khalili, K. Influence of T6 heat-treating and over-ageing on out-of-phase thermo-mechanical fatigue behaviors of Al-Si-Cu alloy. Mater. Today Commun. 2022, 33, 104803. [Google Scholar] [CrossRef]
- Cai, Q.; Mendis, C.L.; Chang, I.T.H.; Fan, Z. Effect of short T6 heat treatment on the microstructure and the mechanical properties of newly developed die-cast Al–Si–Mg–Mn alloys. Mater. Sci. Eng. A 2020, 788, 139610. [Google Scholar] [CrossRef]
- Lumley, R. The development of high strength and ductility in high-pressure die-cast Al-Si-Mg alloys from secondary sources. JOM 2019, 71, 382–390. [Google Scholar] [CrossRef]
- Sjölander, E.; Seifeddine, S. Optimization of solution treatment of cast Al-7Si-0.3Mg and Al-8Si-3Cu-0.5Mg alloys. Metall. Mater. Trans. 2014, 45, 1916–1927. [Google Scholar] [CrossRef]
- Kang, H.-J.; Park, J.-Y.; Choi, Y.-S.; Cho, D.-H. Influence of the Solution and Artificial Aging Treatments on the Microstructure and Mechanical Properties of Die-Cast Al–Si–Mg Alloys. Metals 2022, 12, 71. [Google Scholar] [CrossRef]
- Moustafa, M.A.; Samuel, F.H.; Doty, H.W. Effect of Solution Heat Treatment and Additives on the Microstructure of Al-Si (A413.1) Automotive Alloys. J. Mater. Sci. 2003, 8, 4507–4522. [Google Scholar] [CrossRef]
- Royster, D.M. Tensile Properties and Creep Strength of Three Aluminum Alloys Exposed up to 25,000 Hours at 200’ TO 400’ F (370’ TO 480’ K); Langley Research Center Langley Station, Hampton, Va. National Aeronaut Ics and Space Admln Istration: Washington, DC, USA, 1969. [Google Scholar]
- Fracasso, F. Influence of Quench Rate on the Hardness Obtained After Artificial Ageing of an Al-Si-Mg Alloy. Master Thesis, University of Padova, Padova, Italy, 2010. [Google Scholar]
- Colley, L.J. Microstructure-Property Models for Heat Treatment of A356 Aluminum Alloy. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2011. [Google Scholar]
- Gil-Santos, A.; Moelans, N.; Hort, N.; Van der Biest, O. Identification and description of intermetallic compounds in Mg–Si–Sr cast and heat-treated alloys. J. Alloys Compd. 2016, 669, 123–133. [Google Scholar] [CrossRef]
- Drouzy, M.; Jacob, S.; Richard, M. Interpretation of Tensile Results by Means of Quality Index and Probable Yield Strength-Application to Al-Si7 Mg Foundry Alloys-France. Int. Cast Met. J. 1980, 5, 43–50. [Google Scholar]
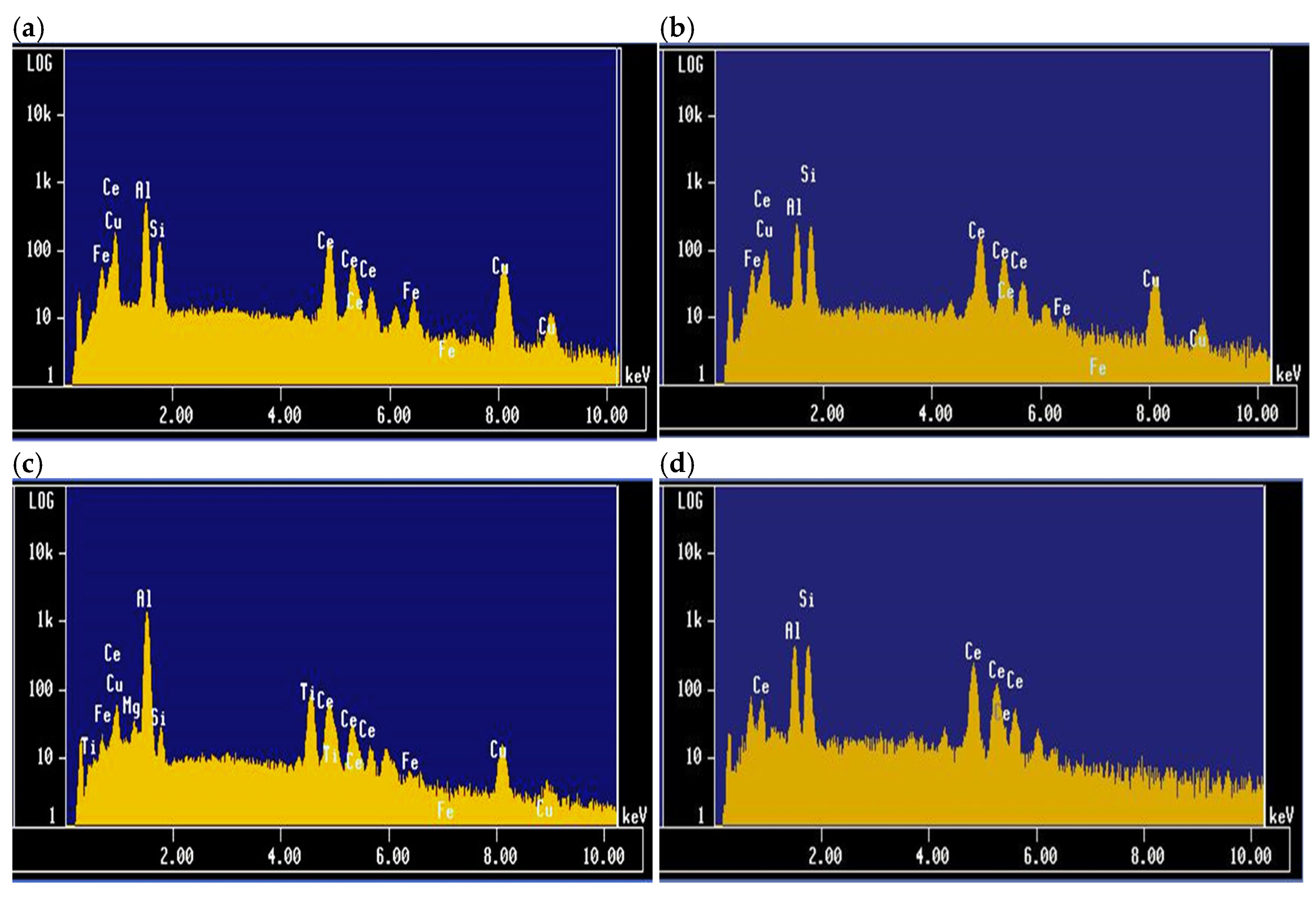

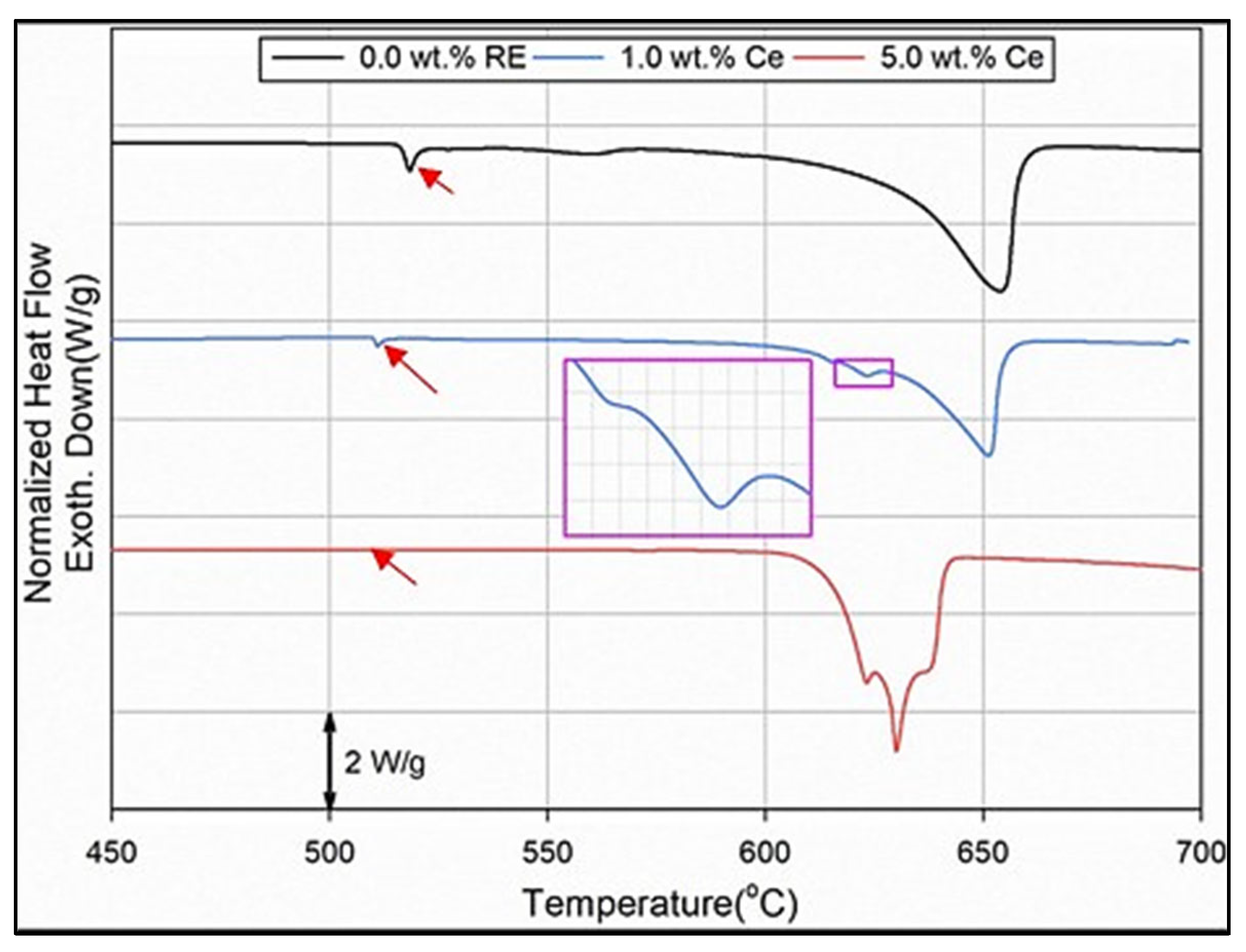

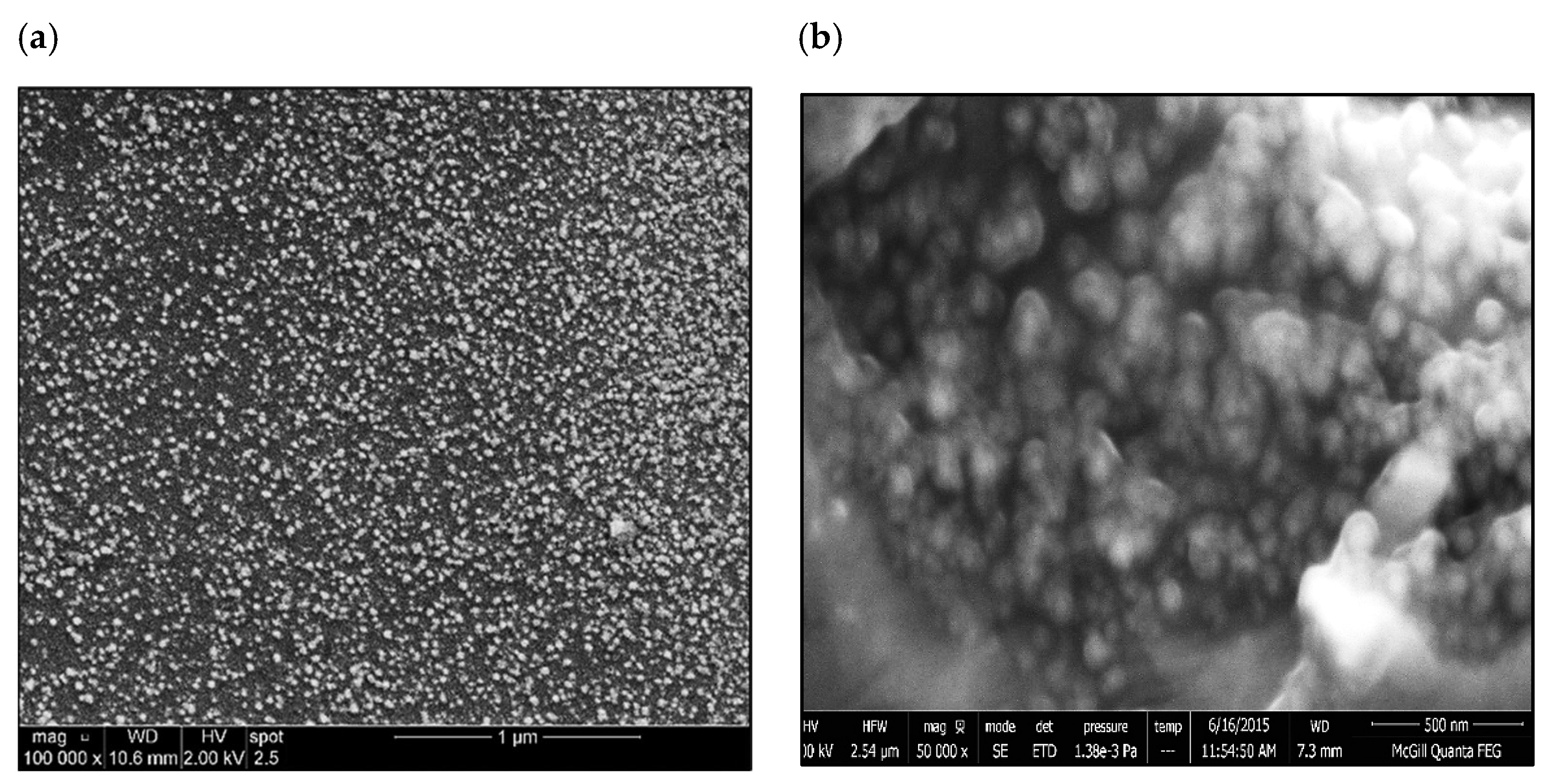
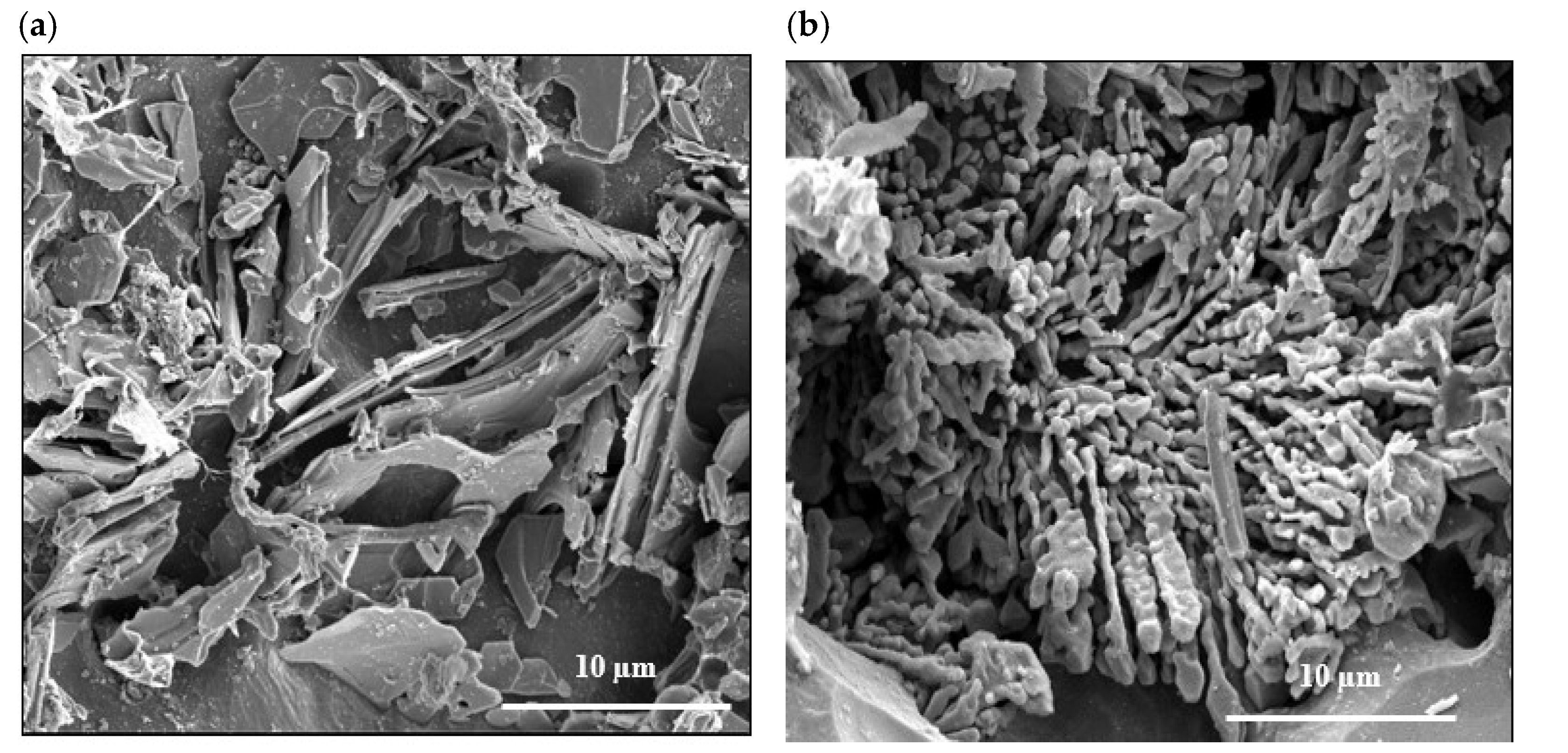
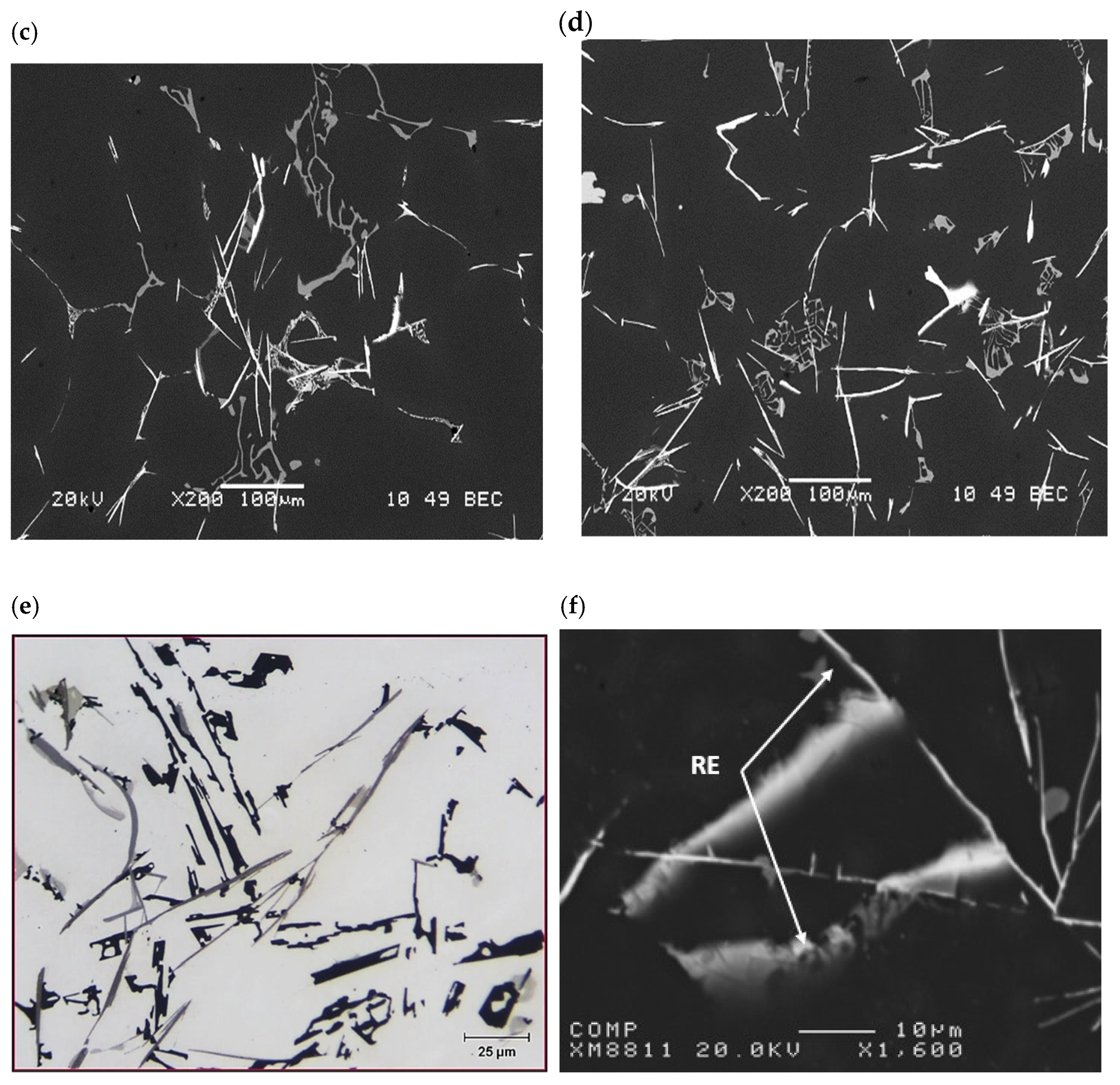

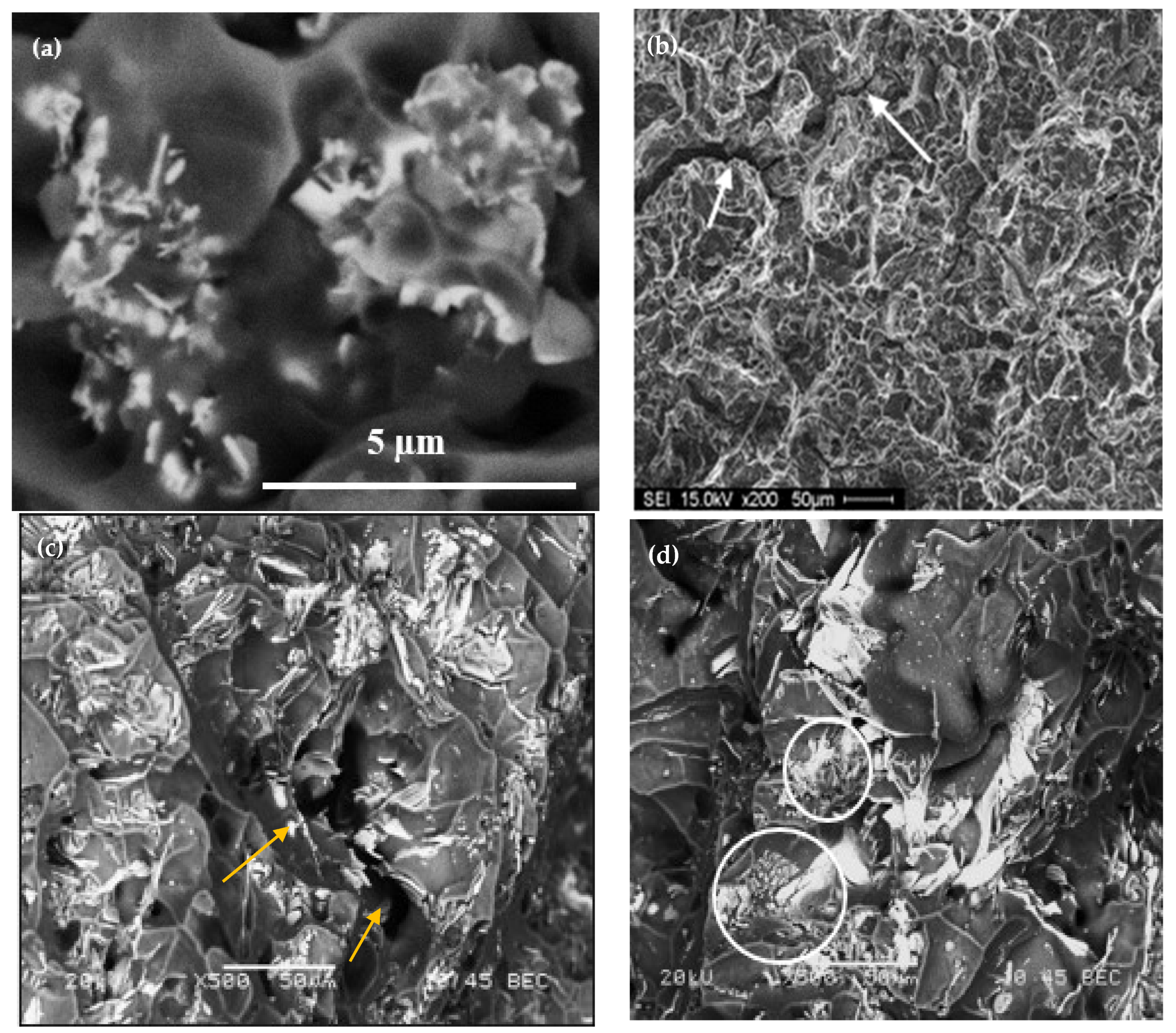
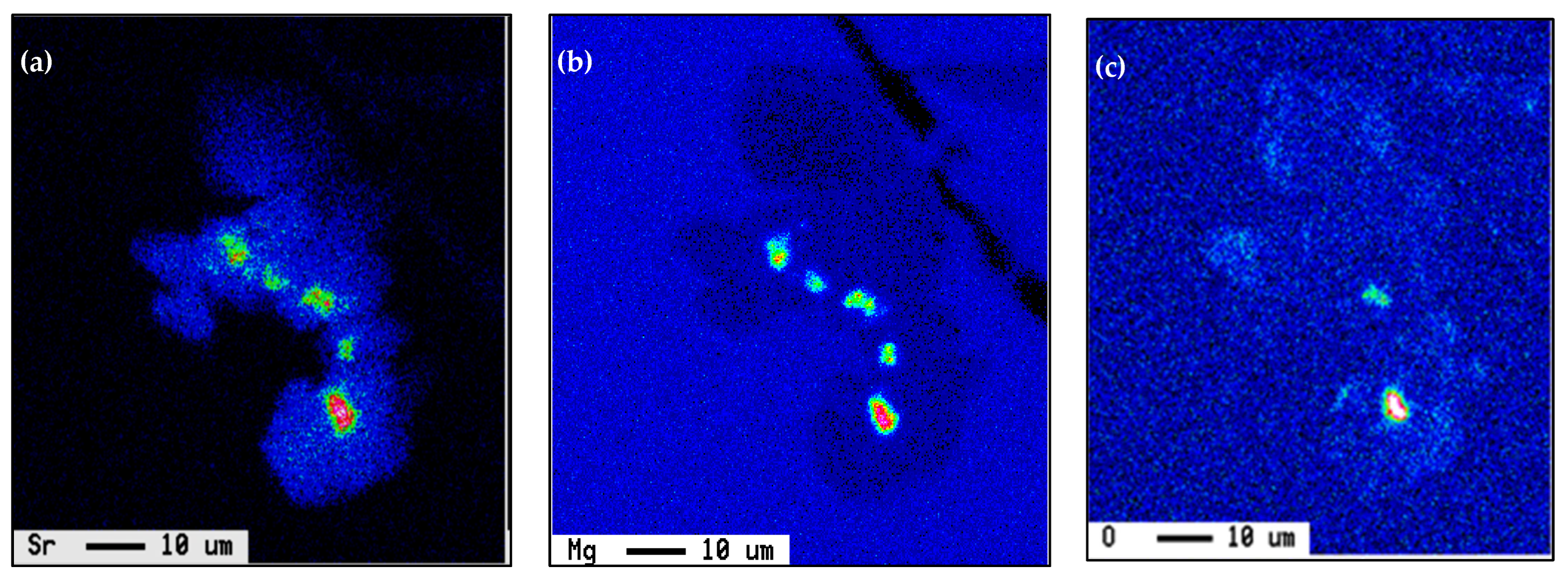
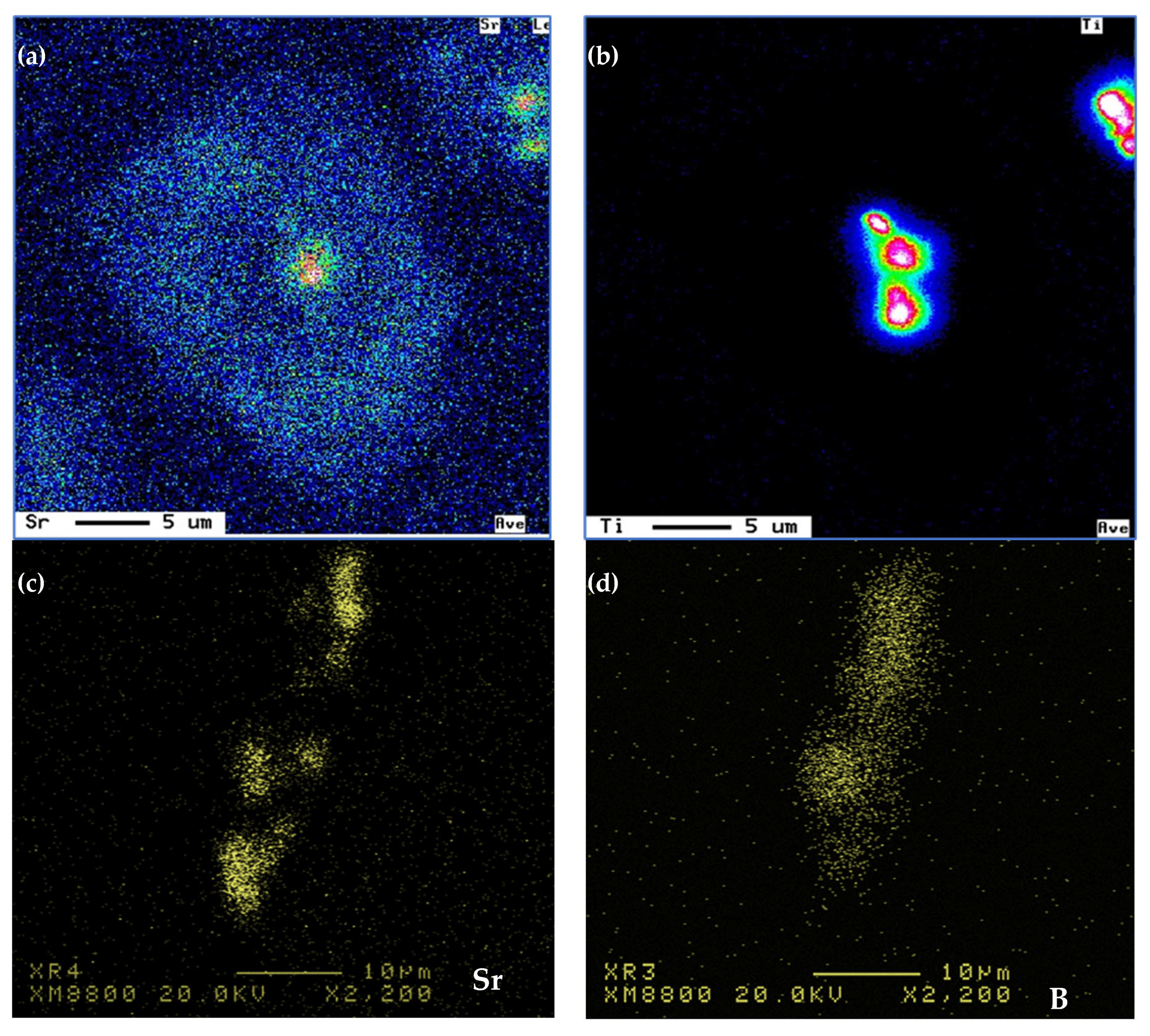

| Chemical Analysis (wt%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Alloy | Elements | |||||||
| Cu | Si | Fe | Mn | Mg | Ti | Zn | Al | |
| 220 | 2.4 | 1.2 | 0.4 | 0.6 | 0.45 | 0.21 | --- | Balance |
| 356.1 | 0.25 | 7.25 | 0.54 | 0.36 | 0.45 | 0.06 | 0.35 | Balance |
| Alloy Type | Condition | Solutionizing Treatment | Quenching Media | Aging Temperature (°C) | Aging Time (h) | |
|---|---|---|---|---|---|---|
| Temp. (°C) | Time (h) | |||||
| 220/356.1 | SHT | 510 | 8 | Warm water (60 °C) | none | none |
| T5 | none | none | 180 | 8 | ||
| T6 | 510 | 8 | 180 | 8 | ||
| T7 | 510 | 8 | 240 | 4 & 50 | ||
| Phase | Composition (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| Al | Ti | Fe | Cu | Si | La | Possible Composition | |
| Gray-1 | 84.75 | 7.215 | 0.014 | 0.374 | 0.358 | 4.343 | Al21Ti2La |
| Gray-2 | 84.27 | 7.317 | 0.013 | 0.344 | 0.496 | 4.330 | Al21Ti2La |
| Gray-3 | 85.22 | 7.428 | 0.009 | 0.258 | 0.333 | 4.320 | Al21Ti2La |
| Gray-5 | 84.32 | 7.730 | 0.012 | 0.451 | 0.408 | 4.384 | Al21Ti2La |
| White-1 | 56.46 | 0.000 | 2.598 | 14.97 | 9.812 | 15.83 | Al11La3(Cu,Fe)4Si2 |
| White-3 | 56.64 | 0.000 | 2.942 | 14.43 | 10.12 | 15.61 | Al11La3(Cu,Fe)4Si2 |
| White-4 | 46.52 | 0.000 | 0.000 | 3.426 | 18.30 | 31.44 | Al5La3Si2 |
| White-4 | 56.14 | 0.000 | 1.541 | 17.57 | 12.02 | 11.91 | Al11La3(Cu,Fe)4Si2 |
| Phase Color | Composition (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| Al | Ti | Fe | Cu | Si | La | Possible Composition | |
| Gray-1 | 85.74 | 6.676 | 0.013 | 0.469 | 0.801 | 3.815 | Al21Ti2La |
| Gray-2 | 85.39 | 6.543 | 0.035 | 0.606 | 0.919 | 3.767 | Al21Ti2La |
| Gray-3 | 85.48 | 6.343 | 0.046 | 0.707 | 0.888 | 3.764 | Al21Ti2La |
| Gray-4 | 85.20 | 6.439 | 0.013 | 0.588 | 1.033 | 3.832 | Al21Ti2La |
| White-1 | 36.57 | 0.000 | 0.000 | 0.290 | 25.93 | 35.23 | AlLaSi |
| White-2 | 34.72 | 0.000 | 0.006 | 0.344 | 27.82 | 35.20 | AlLaSi |
| White-3 | 50.11 | 0.000 | 0.000 | 0.083 | 26.49 | 20.95 | Al2LaSi |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doty, H.W.; El-Hadad, S.; Samuel, E.; Samuel, A.M.; Samuel, F.H. The Influence of Rare Earth Metals on the Microstructure and Mechanical Properties of 220 and 356.1 Alloys for Automotive Industry. Materials 2025, 18, 941. https://doi.org/10.3390/ma18050941
Doty HW, El-Hadad S, Samuel E, Samuel AM, Samuel FH. The Influence of Rare Earth Metals on the Microstructure and Mechanical Properties of 220 and 356.1 Alloys for Automotive Industry. Materials. 2025; 18(5):941. https://doi.org/10.3390/ma18050941
Chicago/Turabian StyleDoty, Herbert W., Shimaa El-Hadad, Ehab Samuel, Agnes M. Samuel, and Fawzy H. Samuel. 2025. "The Influence of Rare Earth Metals on the Microstructure and Mechanical Properties of 220 and 356.1 Alloys for Automotive Industry" Materials 18, no. 5: 941. https://doi.org/10.3390/ma18050941
APA StyleDoty, H. W., El-Hadad, S., Samuel, E., Samuel, A. M., & Samuel, F. H. (2025). The Influence of Rare Earth Metals on the Microstructure and Mechanical Properties of 220 and 356.1 Alloys for Automotive Industry. Materials, 18(5), 941. https://doi.org/10.3390/ma18050941







