Laser-Based Crystallization of Chemical Solution Deposited Proton-Conducting Thin Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Precursor Chemistry
2.2. Laser Annealing
2.3. Sample Characterization
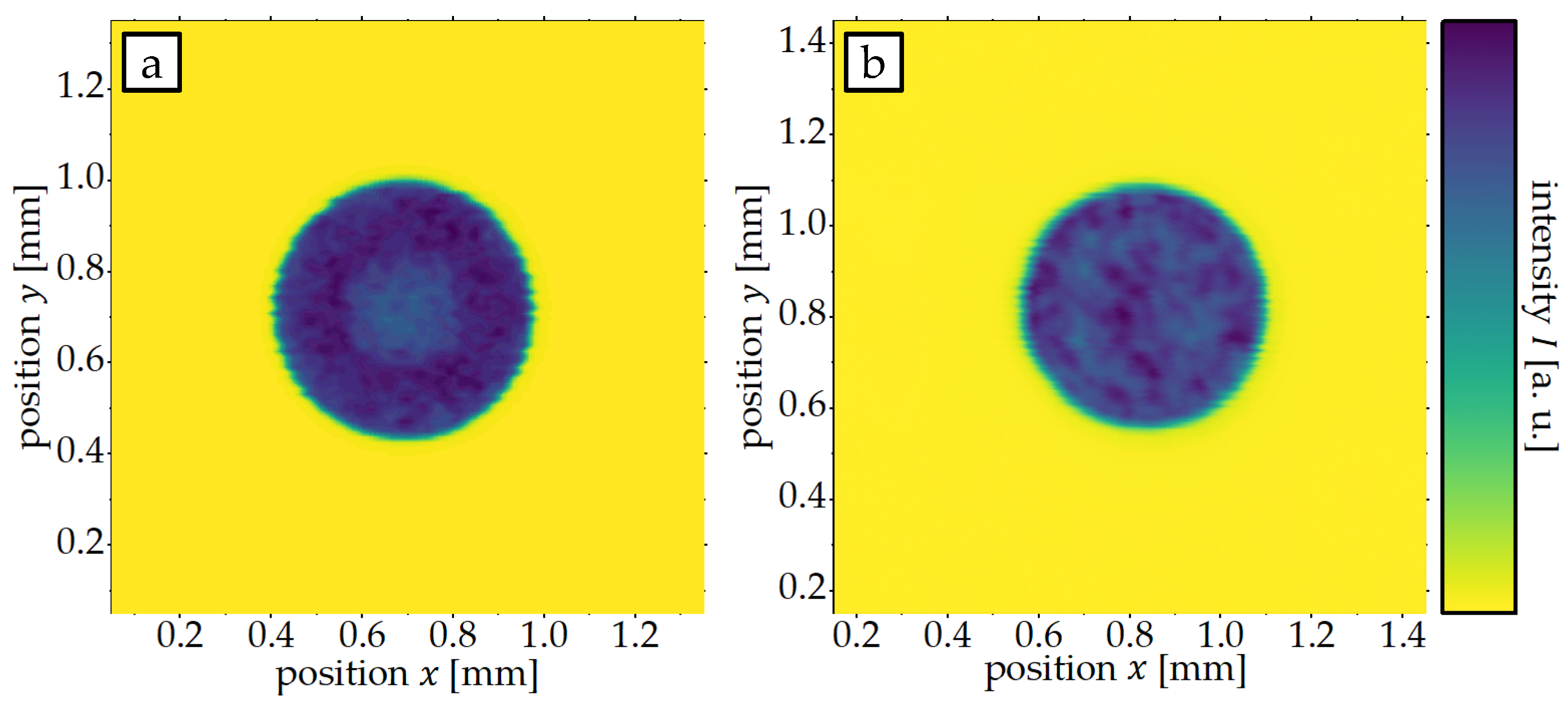
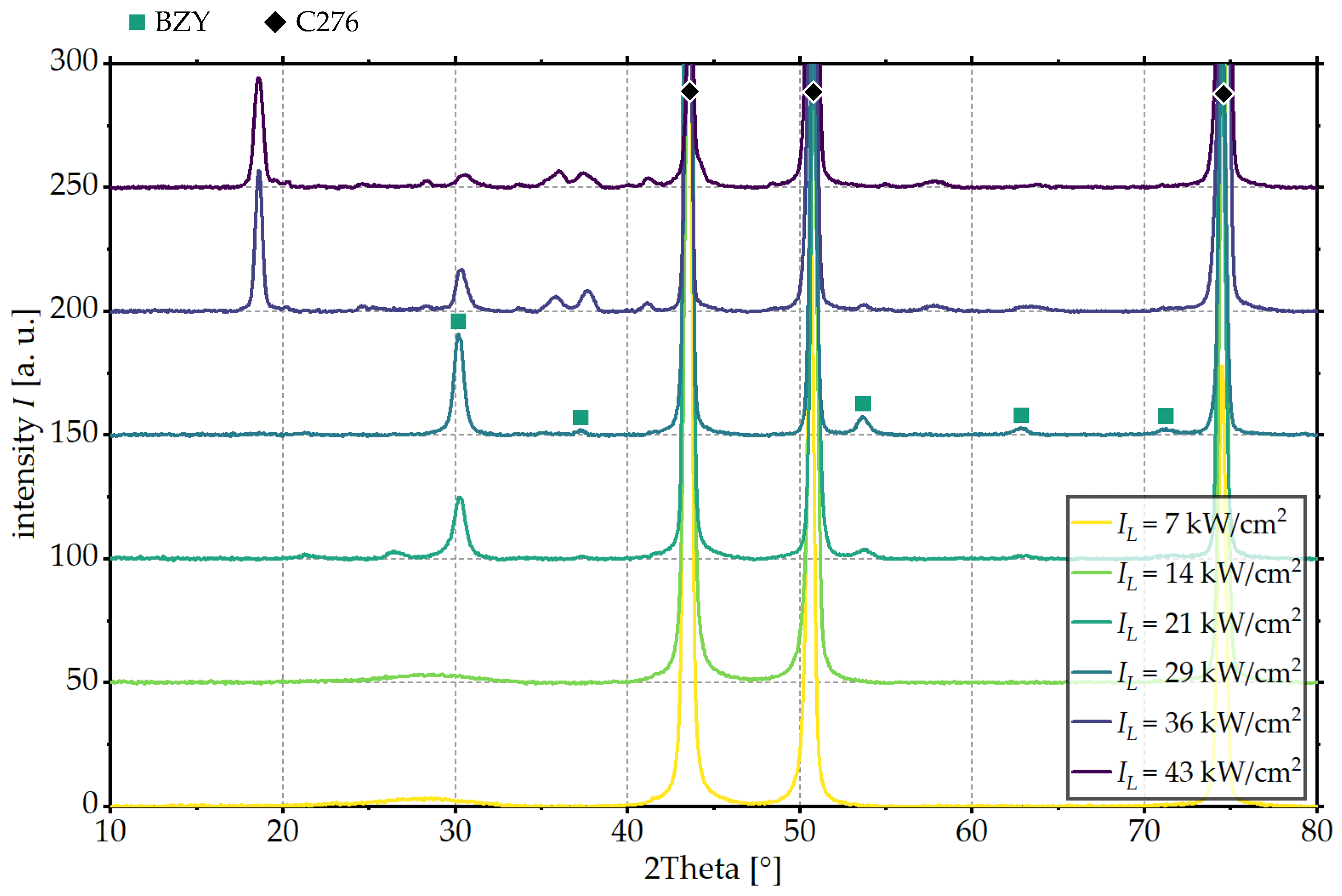
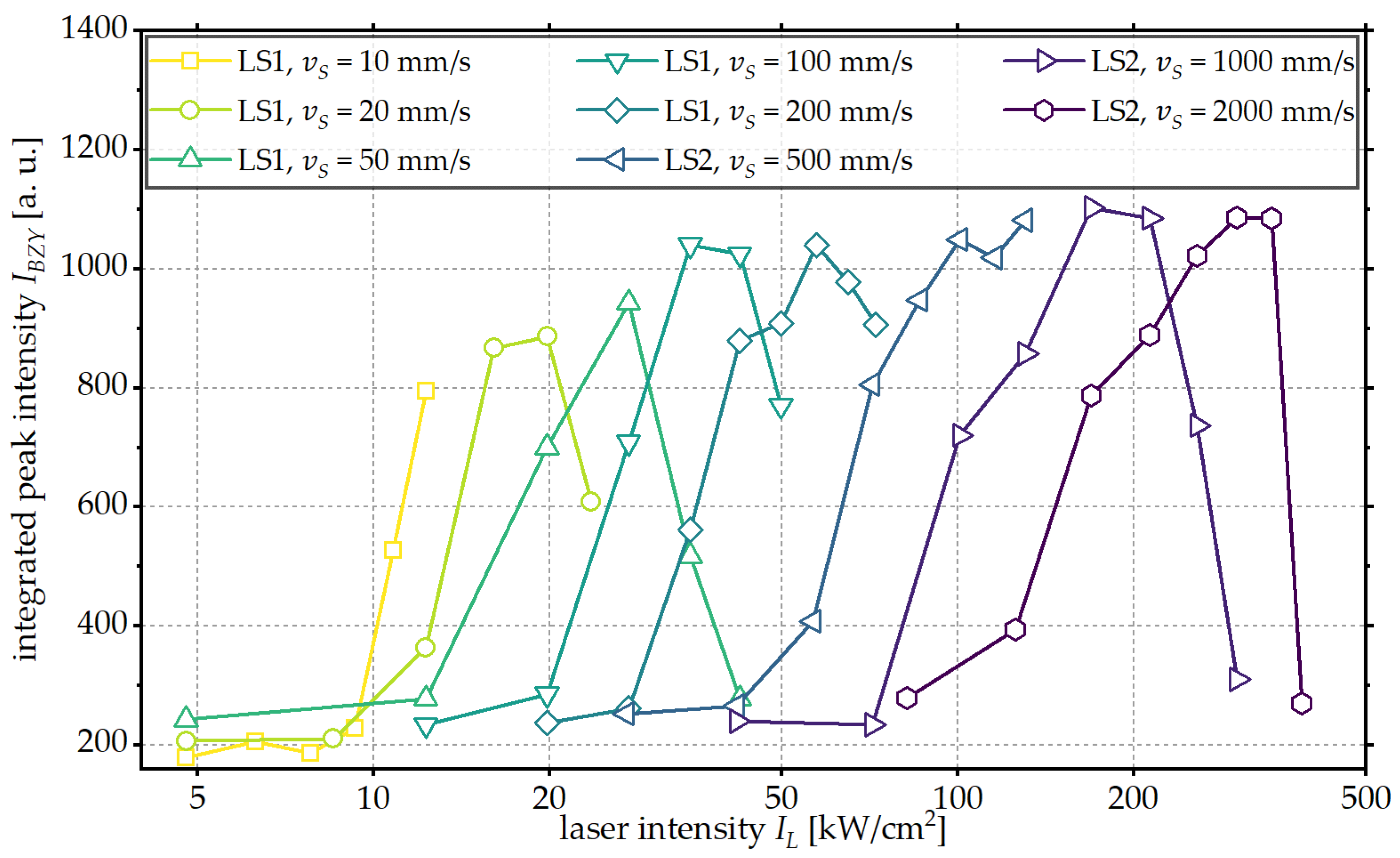
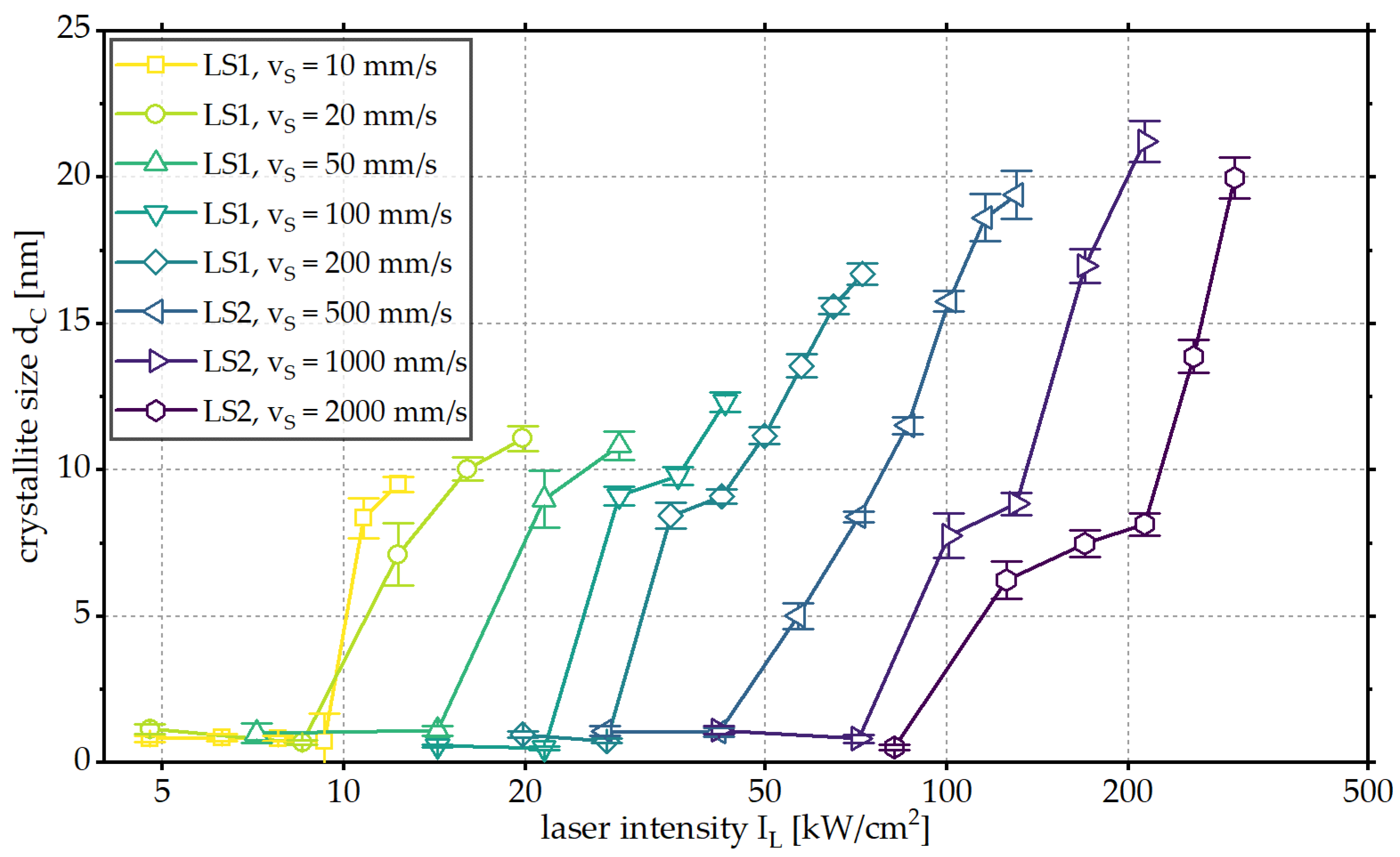
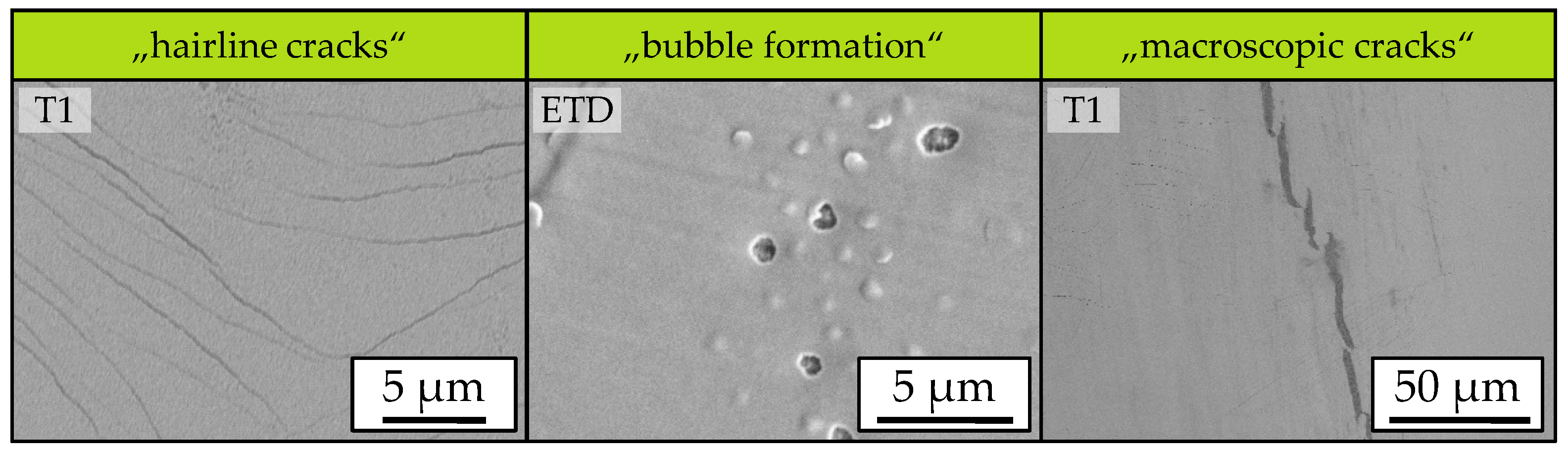
3. Results and Discussion
3.1. Influence of Laser Intensity
3.2. Influence of Scanning Velocity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALD | Atomic Layer Deposition |
| BCY | Yttrium-Doped Barium Cerate |
| BZY | Yttrium-Doped Barium Zirconate |
| CSD | Chemical Solution Deposition |
| CTE | Coefficient of Thermal Extension |
| cw | Continuous Wave |
| EBE | Electron Beam Evaporation |
| EDX | Energy Dispersive X-ray Spectroscopy |
| ETD | Everhart-Thornley Detector |
| LS1 | Laser System 1 |
| LS2 | Laser System 2 |
| MOD | Metallo-Organic Decomposition |
| PG | Power to Gas |
| PLD | Pulsed Laser Deposition |
| PZT | Lead Zirconate Titanate |
| SEM | Scanning Electron Microscopy |
| SOEC | Solid Oxide Electrolyzer Cell |
| SOFC | Solid Oxide Fuel Cell |
| TEM | Transmission Electron Microscopy |
| XPS | X-Ray Photoelectron Spectroscopy |
| XRD | X-Ray Diffraction |
| YSZ | Yttrium-Stabilized Zirconium Oxide |
References
- Thema, M.; Bauer, F.; Sterner, M. Power-to-Gas: Electrolysis and methanation status review. Renew. Sustain. Energy Rev. 2019, 112, 775–787. [Google Scholar] [CrossRef]
- Ozturk, M.; Dincer, I. A comprehensive review on power-to-gas with hydrogen options for cleaner applications. Int. J. Hydrogen Energy 2021, 46, 31511–31522. [Google Scholar] [CrossRef]
- Wulf, C.; Linßen, J.; Zapp, P. Review of Power-to-Gas Projects in Europe. Energy Procedia 2018, 155, 367–378. [Google Scholar] [CrossRef]
- Choudhury, A.; Chandra, H.; Arora, A. Application of solide oxide fuel cell technology for power generation—A review. Renew. Sustain. Energy Rev. 2013, 20, 430–442. [Google Scholar] [CrossRef]
- Golkhatmi, S.Z.; Asghar, M.I.; Lund, P.D. A review on solid oxide fuel cell durability: Latest progress, mechanisms, and study tools. Renew. Sustain. Energy Rev. 2022, 161, 1–34. [Google Scholar] [CrossRef]
- Shi, H.; Su, C.; Ran, R.; Cao, J.; Shao, Z. Electrolyte materials for intermediate-temperature solid oxide fuel cells. Prog. Nat. Sci. Mater. Int. 2020, 30, 764–774. [Google Scholar] [CrossRef]
- Alenazey, F.; Alyousef, Y.; AlOtaibi, B.; Almutairi, G.; Minakshi, M.; Cheng, C.K.; Vo, D.-V.N. Degradation Behaviors of Solid Oxide Fuel Cell Stacks in Steady-State and Cycling Conditions. Energy Fuels 2020, 34, 14864–14873. [Google Scholar] [CrossRef]
- Hussain, S.; Yangping, L. Review of solid oxide fuel cell materials: Cathode, anode, and electrolyte. Energy Transit. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- Salim, K.M.A.; Maelah, R.; Hishamuddin, H.; Amir, A.M.; Ab Rahman, M.N. Two Decades of Life Cycle Sustainability Assessment of Solid Oxide Fuel Cells (SOFCs): A Review. Sustainability 2022, 14, 12380. [Google Scholar] [CrossRef]
- Humayun, M.; Li, Z.; Israr, M.; Khan, A.; Luo, W.; Wang, C.; Shao, Z. Perovskite Type ABO3 Oxides in Photocatalysis, Electrocatalysis, and Solid Oxide Fuel Cells: State of the Art and Future Prospects. Chem. Rev. 2025, 125, 3165–3241. [Google Scholar] [CrossRef]
- Singh, M.; Zappa, D.; Comini, E. Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int. J. Hydrogen Energy 2021, 46, 27643–27674. [Google Scholar] [CrossRef]
- Yatoo, M.A.; Habib, F.; Malik, A.H.; Qazi, M.J.; Ahmad, S.; Ganayee, M.A.; Ahmad, Z. Solid-oxide fuel cells: A critical review of materials for cell components. MRS Commun. 2023, 13, 378–384. [Google Scholar] [CrossRef]
- Shahid, M. Recent advances in protonconducting electrolytes for solid oxide fuel cells. Ionics 2022, 28, 3583–3601. [Google Scholar] [CrossRef]
- Regalado Vera, C.Y.; Ding, H.; Peterson, D.; Gibbons, W.T.; Zhou, M.; Ding, D. A mini-review on proton conduction of BaZrO3 -based perovskite electrolytes. J. Phys. Energy 2021, 3, 32019. [Google Scholar] [CrossRef]
- Islam, S.; Wang, S.; Nolan, A.M.; Mo, Y. First-Principles Computational Design and Discovery of Novel Double-Perovskite Proton Conductors. Chem. Mater. 2021, 33, 8278–8288. [Google Scholar] [CrossRef]
- Hossain, M.K.; Biswas, M.C.; Chanda, R.K.; Rubel, M.H.K.; Khan, M.I.; Hashizume, K. A review on experimental and theoretical studies of perovskite barium zirconate proton conductors. Emergent Mater. 2021, 4, 999–1027. [Google Scholar] [CrossRef]
- Cao, J.; Ji, Y.; Shao, Z. Perovskites for protonic ceramic fuel cells: A review. Energy Environ. Sci. 2022, 15, 2200–2232. [Google Scholar] [CrossRef]
- Han, D.; Liu, X.; Bjorheim, T.S.; Uda, T. Yttrium-Doped Barium Zirconate-Cerate Solid Solution as Proton Conducting Electrolyte: Why Higher Cerium Concentration Leads to Better Performance for Fuel Cells and Electrolysis Cells. Adv. Energy Mater. 2021, 11, 2003149. [Google Scholar] [CrossRef]
- Langnickel, H.; Rautanen, M.; Gandiglio, M.; Santarelli, M.; Hakala, T.; Acri, M.; Kiviaho, J. Efficiency analysis of 50 kWe SOFC systems fueled with biogas from waste water. J. Power Sources Adv. 2020, 2, 100009. [Google Scholar] [CrossRef]
- Vinchhi, P.; Khandla, M.; Chaudhary, K.; Pati, R. Recent advances on electrolyte materials for SOFC: A review. Inorg. Chem. Commun. 2023, 152, 110724. [Google Scholar] [CrossRef]
- Zakaria, Z.; Abu Hassan, S.H.; Shaari, N.; Yahaya, A.Z.; Boon Kar, Y. A review on recent status and challenges of yttria stabilized zirconia modification to lowering the temperature of solid oxide fuel cells operation. Int. J. Energy Res. 2020, 44, 631–650. [Google Scholar] [CrossRef]
- Sata, N.; Han, F.; Zheng, H.; Dayaghi, A.M.; Norby, T.; Stange, M.; Semerad, R.; Costa, R. Development of Proton Conducting Ceramic Cells in Metal Supported Architecture. ECS Trans. 2021, 103, 1779–1789. [Google Scholar] [CrossRef]
- Stange, M.; Dayaghi, A.M.; Denonville, C.; Larring, Y.; Rørvik, P.M.; Haugsrud, R.; Norby, T. Fabrication of Metal-Supported Proton-Conducting Electrolysers with Thin Film Sr- and Ce-Doped BZY Electrolyte. ECS Trans. 2019, 91, 941–949. [Google Scholar] [CrossRef]
- Zheng, H.; Han, F.; Sata, N.; Riegraf, M.; Dayaghi, A.M.; Norby, T.; Costa, R. Metal Supported Proton Conducting Ceramic Cell with Thin Film Electrolyte for Electrolysis Application. ECS Trans. 2021, 103, 693–700. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hernandez-Sanchez, R.; Haile, S.M. High Total Proton Conductivity in Large-Grained Yttrium-Doped Barium Zirconate. Chem. Mater. 2009, 21, 2755–2762. [Google Scholar] [CrossRef]
- Bae, K.; Jang, D.Y.; Choi, H.J.; Kim, D.; Hong, J.; Kim, B.-K.; Lee, J.-H.; Son, J.-W.; Shim, J.H. Demonstrating the potential of yttrium-doped barium zirconate electrolyte for high-performance fuel cells. Nat. Commun. 2017, 8, 14553. [Google Scholar] [CrossRef]
- Subramaniyan, A.; Tong, J.; O’Hayre, R.; Sammes, N.M. Sintering Studies on 20 mol% Yttrium-Doped Barium Cerate. J. Am. Ceram. Soc. 2011, 94, 1800–1804. [Google Scholar] [CrossRef]
- Tong, J.; Clark, D.; Bernau, L.; Subramaniyan, A.; O’Hayre, R. Proton-conducting yttrium-doped barium cerate ceramics synthesized by a cost-effective solid-state reactive sintering method. Solid State Ion. 2010, 181, 1486–1498. [Google Scholar] [CrossRef]
- Yoo, Y.; Lim, N. Performance and stability of proton conducting solid oxide fuel cells based on yttrium-doped barium cerate-zirconate thin-film electrolyte. J. Power Sources 2013, 229, 48–57. [Google Scholar] [CrossRef]
- Hakim, M.; Yoo, C.-Y.; Joo, J.H.; Yu, J.H. Enhanced durability of a proton conducting oxide fuel cell with a purified yttrium-doped barium zirconate-cerate electrolyte. J. Power Sources 2015, 278, 320–324. [Google Scholar] [CrossRef]
- Duan, C.; Huang, J.; Sullivan, N.; O’Hayre, R. Proton-conducting oxides for energy conversion and storage. Appl. Phys. Rev. 2020, 7, 011314. [Google Scholar] [CrossRef]
- Ahmed, I.; Eriksson, S.; Ahlberg, E.; Knee, C.; Götlind, H.; Johansson, L.; Karlsson, M.; Matic, A.; Börjesson, L. Structural study and proton conductivity in Yb-doped BaZrO3. Solid State Ion. 2007, 178, 515–520. [Google Scholar] [CrossRef]
- Mikami, Y.; Sekitani, Y.; Yamauchi, K.; Kuroha, T.; Okuyama, Y. Effect of Transition Element Dissolution on Ytterbium-Doped Barium-Zirconate-Based Protonic Ceramic Fuel Cells. Appl. Energy Mater. 2024, 7, 1136–1148. [Google Scholar] [CrossRef]
- Li, M.; Hua, B.; Luo, J.L.; Jiang, S.P.; Pu, J.; Chi, B.; Jian, L. Carbon-tolerant Ni-based cermet anodes modified by proton conducting yttrium- and ytterbium-doped barium cerates for direct methane solid oxide fuel cells. J. Mater. Chem. A 2015, 3, 21609–21617. [Google Scholar] [CrossRef]
- Kjølseth, C.; Fjeld, H.; Prytz, Ø.; Dahl, P.I.; Estournès, C.; Haugsrud, R.; Norby, T. Space–charge theory applied to the grain boundary impedance of proton conducting BaZr0.9Y0.1O3−δ. Solid State Ion. 2010, 181, 268–275. [Google Scholar] [CrossRef]
- Guo, X.; Waser, R. Electrical properties of the grain boundaries of oxygen ion conductors: Acceptor-doped zirconia and ceria. Prog. Mater. Sci. 2006, 51, 151–210. [Google Scholar] [CrossRef]
- Ricote, S.; Bonanos, N.; Manerbino, A.; Sullivan, N.P.; Coors, W.G. Effects of the fabrication process on the grain-boundary resistance in BaZr0.9Y0.1O3−δ. J. Mater. Chem. A 2014, 2, 16107–16115. [Google Scholar] [CrossRef]
- Tucker, M.C. Progress in metal-supported solid oxide fuel cells: A review. J. Power Sources 2010, 195, 4570–4582. [Google Scholar] [CrossRef]
- Krishnan, V.V. Recent developments in metal-supported solid oxide fuel cells. Wiley Interdiscip. Rev. Energy Environ. 2017, 6, e246. [Google Scholar] [CrossRef]
- Nielsen, J.; Persson, Å.H.; Muhl, T.T.; Brodersen, K. Towards High Power Density Metal Supported Solid Oxide Fuel Cell for Mobile Applications. J. Electrochem. Soc. 2018, 165, F90–F96. [Google Scholar] [CrossRef]
- Bi, L.; Traversa, E. Synthesis strategies for improving the performance of doped-BaZrO3 materials in solid oxide fuel cell applications. J. Mater. Res. 2014, 29, 1–15. [Google Scholar] [CrossRef]
- Jennings, D.; Ricote, S.; Caicedo, J.M.; Santiso, J.; Reimanis, I. The effect of Ni and Fe on the decomposition of yttrium doped barium zirconate thin films. Scr. Mater. 2021, 201, 113948. [Google Scholar] [CrossRef]
- Yan, N.; Zeng, Y.; Shalchi, B.; Wang, W.; Gao, T.; Rothenberg, G.; Luo, J.-l. Discovery and Understanding of the Ambient-Condition Degradation of Doped Barium Cerate Proton-Conducting Perovskite Oxide in Solid Oxide Fuel Cells. J. Electrochem. Soc. 2015, 162, F1408–F1414. [Google Scholar] [CrossRef]
- Babilo, P.; Haile, S.M. Enhanced Sintering of Yttrium-Doped Barium Zirconate by Addition of ZnO. J. Am. Ceram. Soc. 2005, 88, 2362–2368. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, Y.-B.; An, J.; Shim, J.H.; Gür, T.M.; Prinz, F.B. Effect of cation non-stoichiometry and crystallinity on the ionic conductivity of atomic layer deposited Y:BaZrO3 films. Thin Solid Films 2013, 539, 166–169. [Google Scholar] [CrossRef]
- Arab Pour Yazdi, M.; Briois, P.; Georges, S.; Shaula, A.L.; Cavaleiro, A.; Billard, A. Comparison of Structural and Electrical Properties of Barium Zirconate Pellets and Thin Films. J. Electrochem. Soc. 2010, 157, 1582–1587. [Google Scholar] [CrossRef]
- Wallis, J.; Ravkina, O.; Käufer, F.; Mallinckrodt, R.V.; Surkus, A.-E.; Wulff, H.; Wartmann, J.; Kruth, A. Combined magnetron sputtering and laser annealing process for the fabrication of proton conducting thin films. Vacuum 2024, 229, 113582. [Google Scholar] [CrossRef]
- Campos Covarrubias, M.S.; Sriubas, M.; Bockute, K.; Poskaite, A.; Vazgys, R.; Gazda, M.; Laukaitis, G. Properties on Yttrium-Doped/Undoped Barium Cerate and Barium Zirconate Thin Films Formed by E-Beam Vapor Deposition. Appl. Sci. 2022, 13, 6422. [Google Scholar] [CrossRef]
- Stange, M.; Stefan, E.; Denonville, C.; Larring, Y.; Rørvik, P.M.; Haugsrud, R. Development of novel metal-supported proton ceramic electrolyser cell with thin film BZY15–Ni electrode and BZY15 electrolyte. Int. J. Hydrogen Energy 2017, 42, 13454–13462. [Google Scholar] [CrossRef]
- Pergolesi, D.; Fabbri, E.; D’Epifanio, A.; Di Bartolomeo, E.; Tebano, A.; Sanna, S.; Licoccia, S.; Balestrino, G.; Traversa, E. High proton conduction in grain-boundary-free yttrium-doped barium zirconate films grown by pulsed laser deposition. Nat. Mater. 2010, 9, 846–852. [Google Scholar] [CrossRef]
- Schneller, T.; Waser, R.; Kosec, M.; Payne, D. Chemical Solution Deposition of Functional Oxide Thin Films; Springer: Vienna, Austria, 2013; ISBN 978-3-211-99310-1. [Google Scholar]
- Xiao, Y.; Waser, R.; Schneller, T. Microstructure engineering of yttrium-doped barium zirconate thin films via seed layer technique. Surf. Coat. Technol. 2022, 434, 128161. [Google Scholar] [CrossRef]
- Babilo, P.; Uda, T.; Haile, S.M. Processing of yttrium-doped barium zirconate for high proton conductivity. J. Mater. Res. 2007, 22, 1322–1330. [Google Scholar] [CrossRef]
- Imashuku, S.; Uda, T.; Nose, Y.; Awakura, Y. Fabrication and electrical characterization of 15% yttrium-doped barium Zirconate-Nitrate freeze drying method combined with vacuum heating. J. Alloys Compd. 2011, 509, 3872–3879. [Google Scholar] [CrossRef]
- Bi, L.; Fabbri, E.; Sun, Z.; Traversa, E. Sinteractive anodic powders improve densification and electrochemical properties of BaZr0.8Y0.2O3−δ electrolyte films for anode-supported solid oxide fuel cells. Energy Environ. Sci. 2011, 4, 1352. [Google Scholar] [CrossRef]
- Goulart, C.A.; Villas-Boas, L.A.; Morelli, M.R.; Souza, D.P.F.d. Reactive sintering of yttrium-doped barium zirconate BaZr0.8Y0.2O3 without sintering aids. Ceram. Int. 2021, 47, 2565–2571. [Google Scholar] [CrossRef]
- Lagaeva, J.; Medvedev, D.; Demin, A.; Tsiakaras, P. Insights on thermal and transport features of BaCe0.8−Zr Y0.2O3−δ proton-conducting materials. J. Power Sources 2015, 278, 436–444. [Google Scholar] [CrossRef]
- Magrasó, A.; Ballesteros, B.; Rodríguez-Lamas, R.; Sunding, M.F.; Santiso, J. Optimisation of growth parameters to obtain epitaxial Y-doped BaZrO3 proton conducting thin films. Solid State Ion. 2018, 314, 9–16. [Google Scholar] [CrossRef]
- Schneller, T.; Griesche, D. Inkjet Printed Y-Substituted Barium Zirconate Layers as Electrolyte Membrane for Thin Film Electrochemical Devices. Membranes 2019, 9, 131. [Google Scholar] [CrossRef]
- Fink, S.; Lübben, J.; Schneller, T.; Vedder, C.; Böttger, U. Impact of the processing temperature on the laser-based crystallization of chemical solution deposited lead zirconate titanate thin films on short timescales. J. Appl. Phys. 2022, 131, 125302. [Google Scholar] [CrossRef]
- Han, D.; Hatada, N.; Uda, T. Chemical Expansion of Yttrium-Doped Barium Zirconate and Correlation with Proton Concentration and Conductivity. J. Am. Ceram. Soc. 2016, 99, 3745–3753. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frühling, J.; Fink, S.; Schneller, T.; Vedder, C. Laser-Based Crystallization of Chemical Solution Deposited Proton-Conducting Thin Films. Materials 2025, 18, 5235. https://doi.org/10.3390/ma18225235
Frühling J, Fink S, Schneller T, Vedder C. Laser-Based Crystallization of Chemical Solution Deposited Proton-Conducting Thin Films. Materials. 2025; 18(22):5235. https://doi.org/10.3390/ma18225235
Chicago/Turabian StyleFrühling, Jonas, Samuel Fink, Theodor Schneller, and Christian Vedder. 2025. "Laser-Based Crystallization of Chemical Solution Deposited Proton-Conducting Thin Films" Materials 18, no. 22: 5235. https://doi.org/10.3390/ma18225235
APA StyleFrühling, J., Fink, S., Schneller, T., & Vedder, C. (2025). Laser-Based Crystallization of Chemical Solution Deposited Proton-Conducting Thin Films. Materials, 18(22), 5235. https://doi.org/10.3390/ma18225235






