Ethylene Propylene Diene Monomer-Based Composites Resistant to the Corrosive Action of Acetic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Laboratory Tests and Analysis
2.2.1. Behavior of Swelling and Mass Changes
2.2.2. Behavior of Cross-Link Density
2.2.3. Behavior of Mechanical Properties
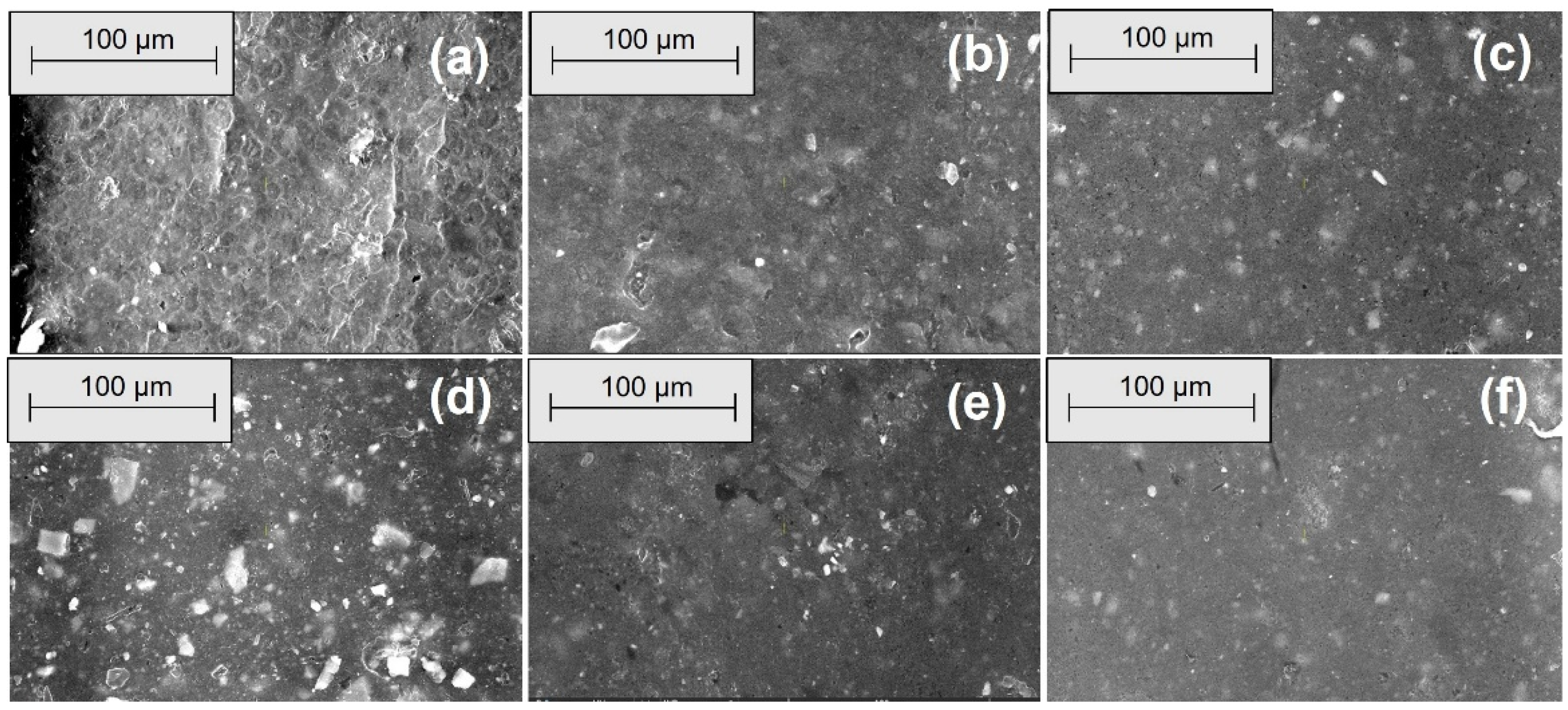
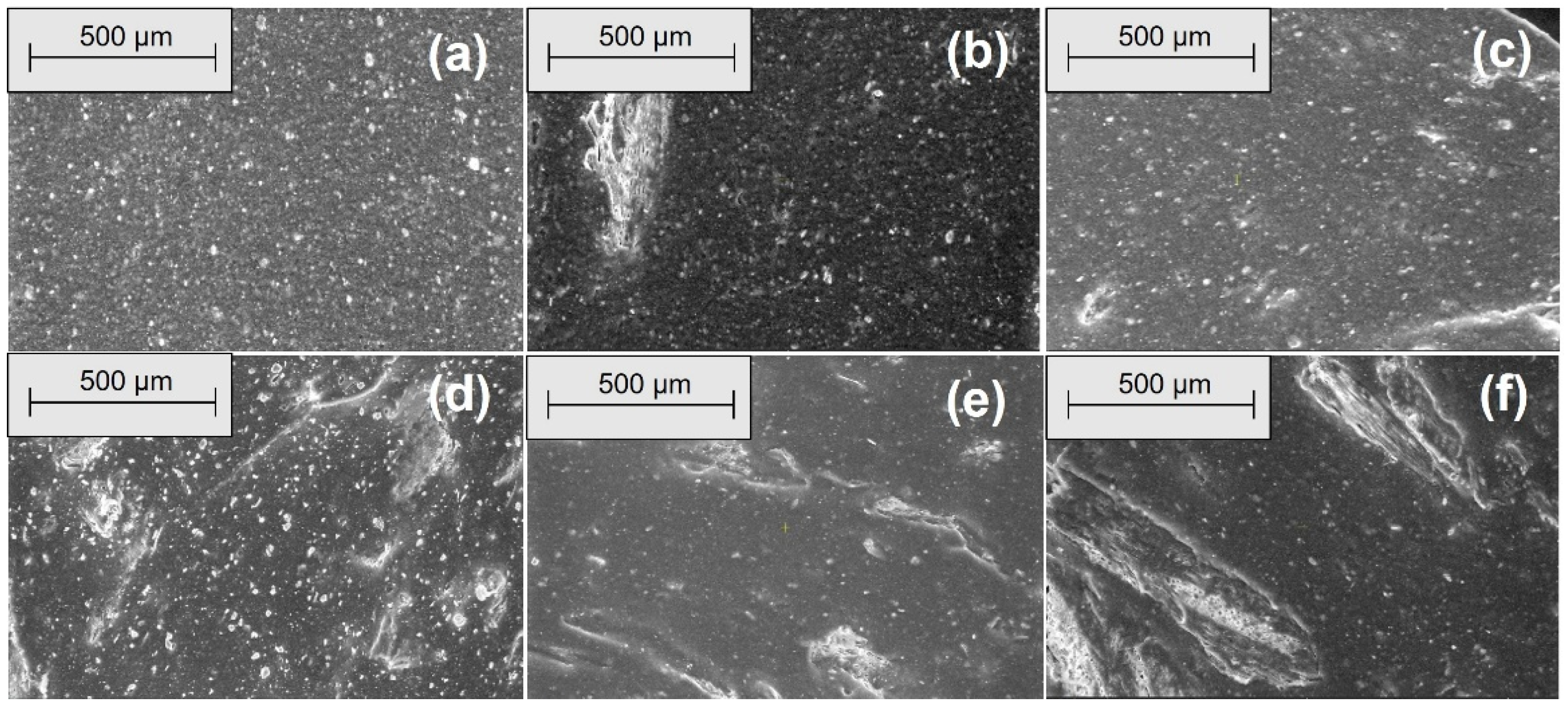
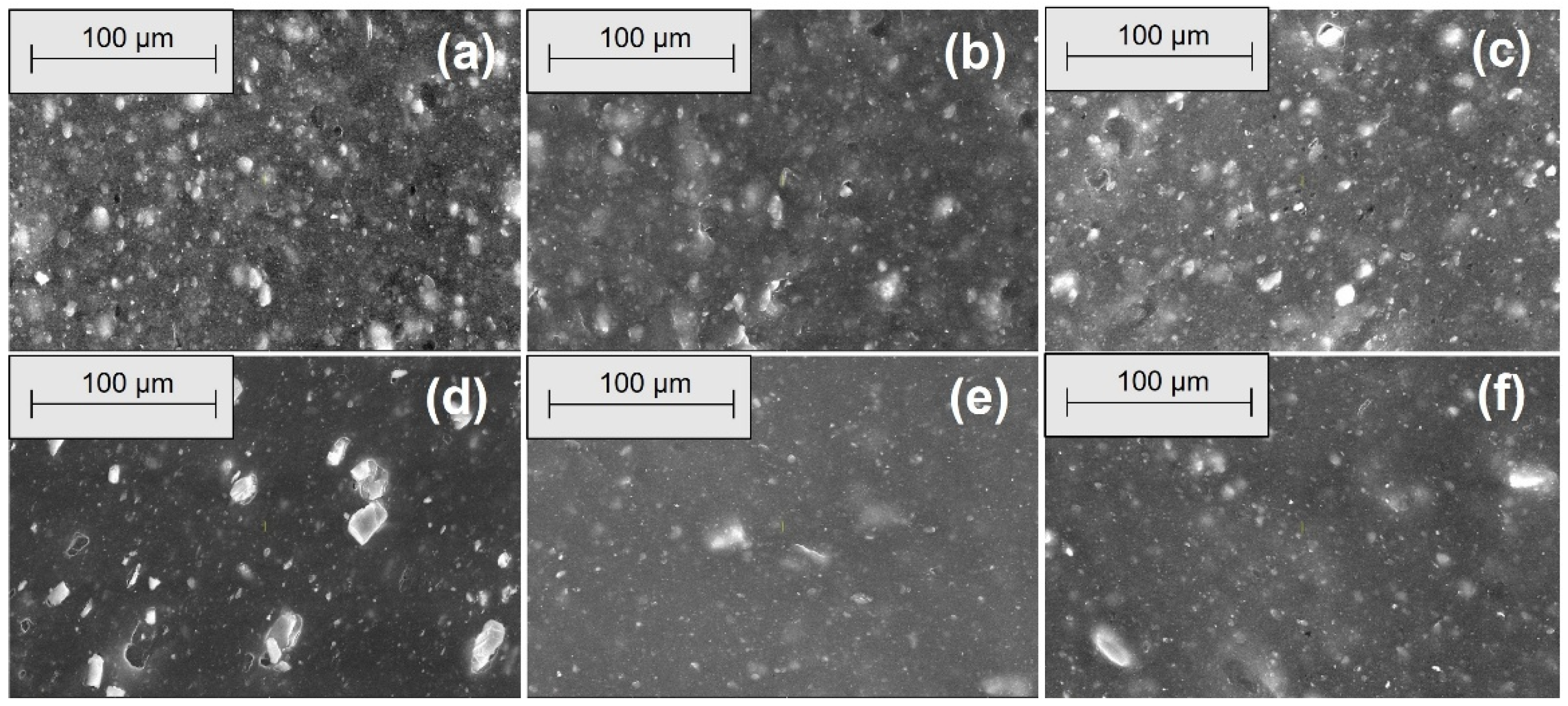
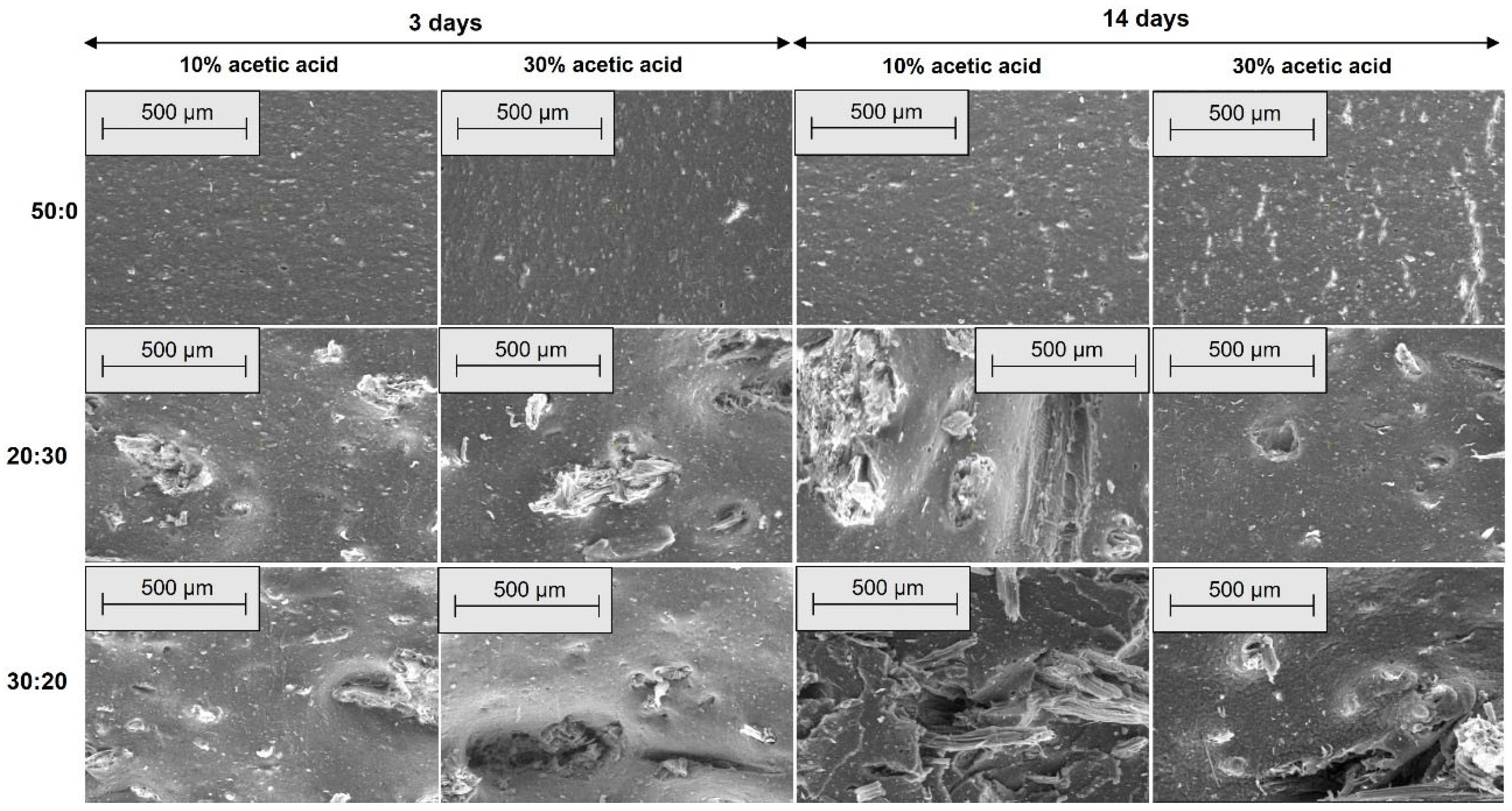
2.2.4. Morphological Investigations by Scanning Electron Microscopy (SEM)
2.2.5. Structural Investigations by Fourier Transform Infrared Spectroscopy (FTIR)
2.2.6. Thermogravimetric and Differential Scanning Calorimetry (TG/DSC) Analysis
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Swelling and Mass Loss Due to the Acetic Acid Exposure
3.2. Cross-Link Density Modification Due to the Acetic Acid Exposure
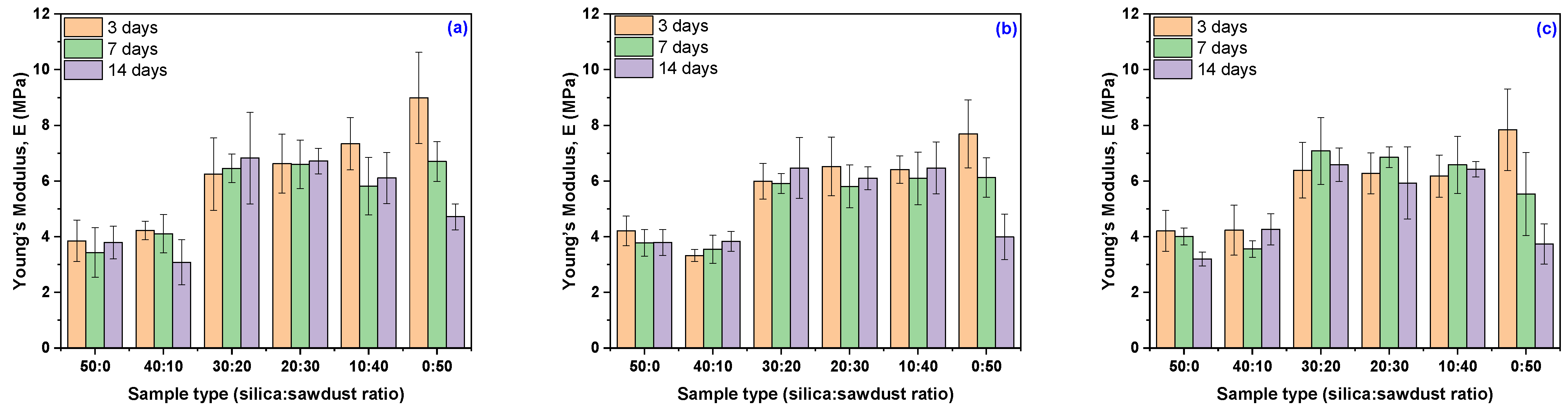
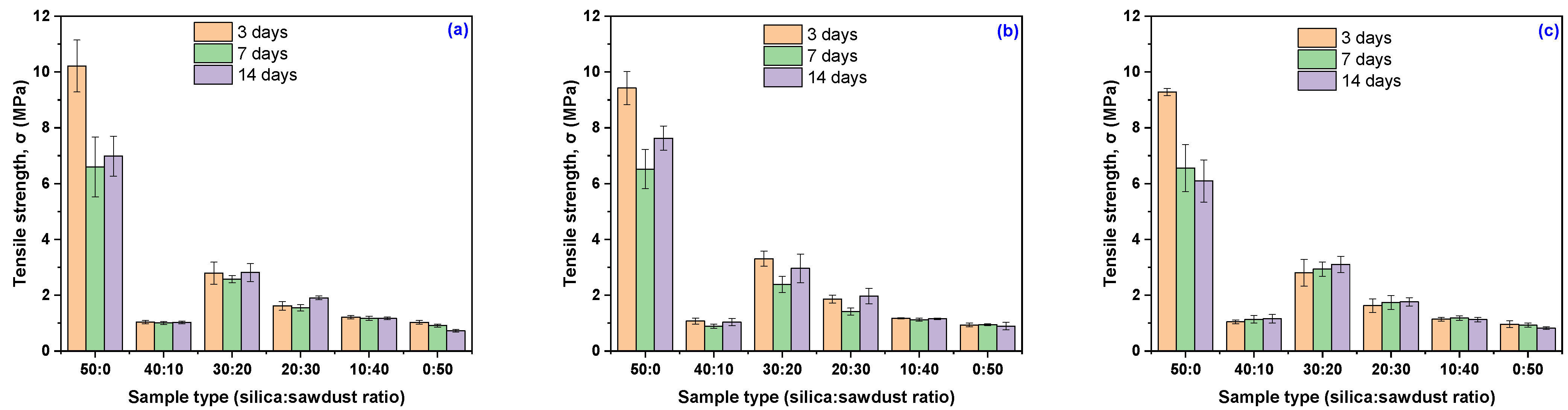
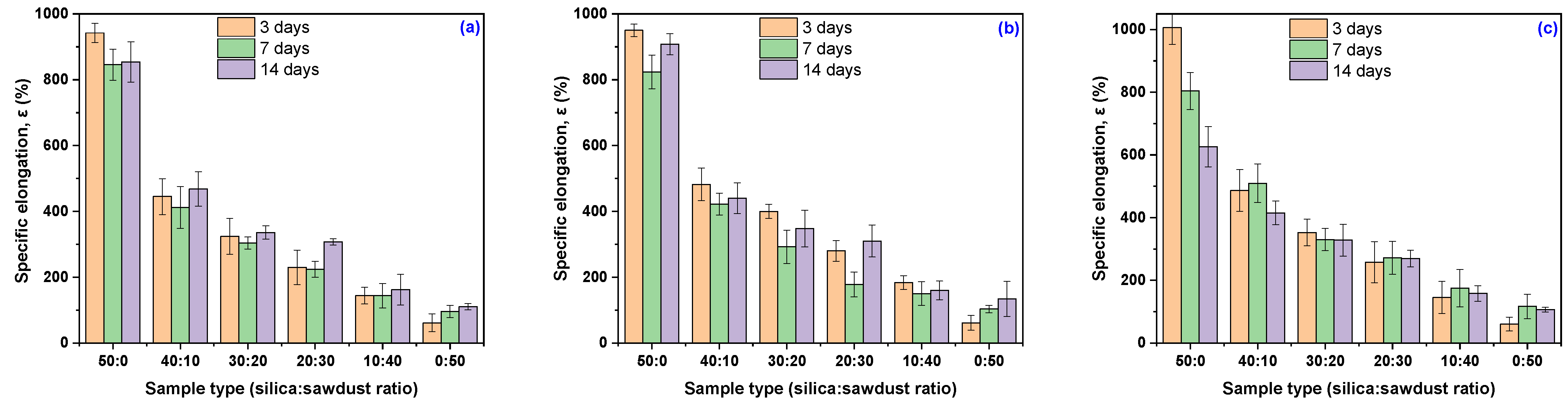
3.3. Mechanical Characteristics Modification Due to the Acetic Acid Exposure
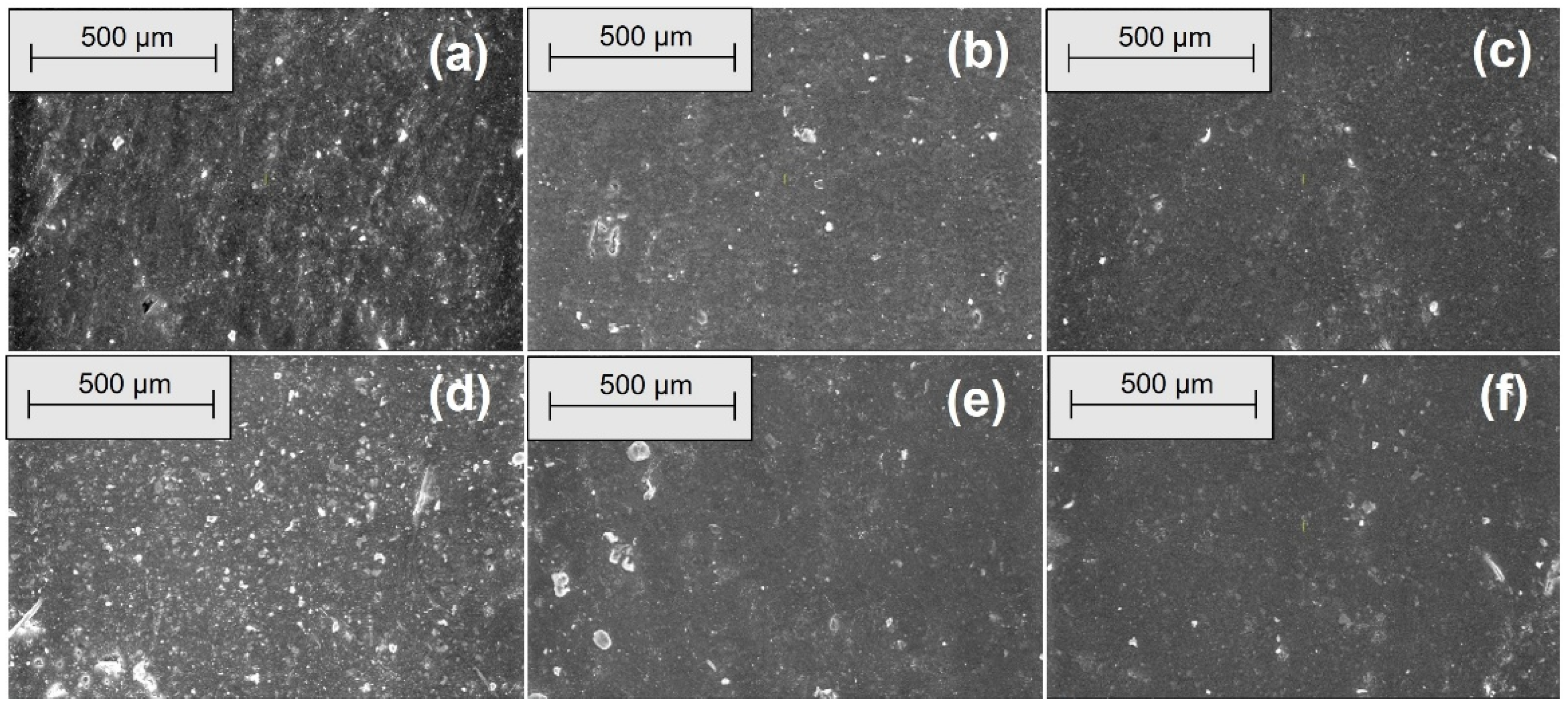
3.4. Morphological Modifications due to the Acetic Acid Exposure
3.5. Structural Modifications due to the Acetic Acid Exposure
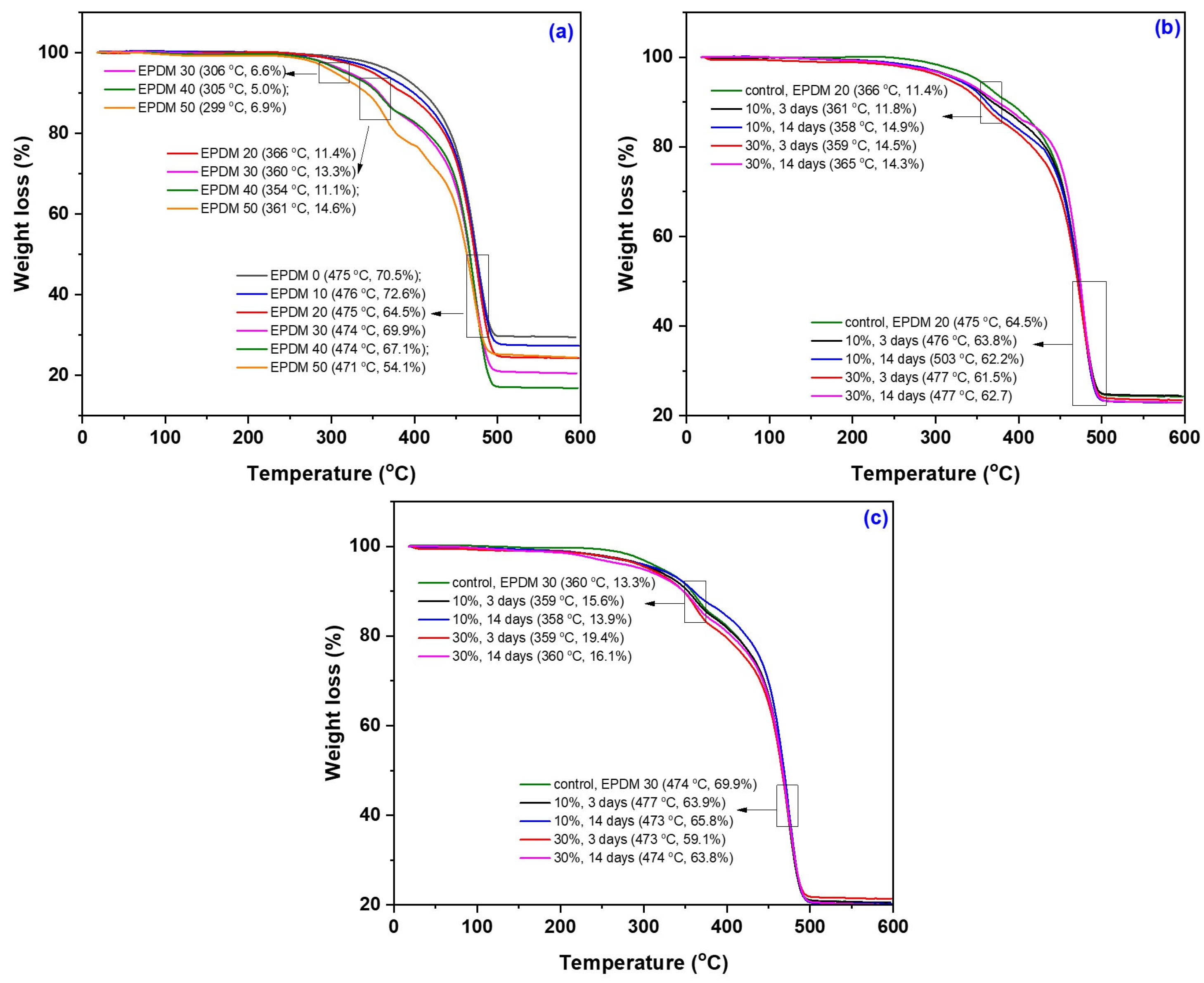
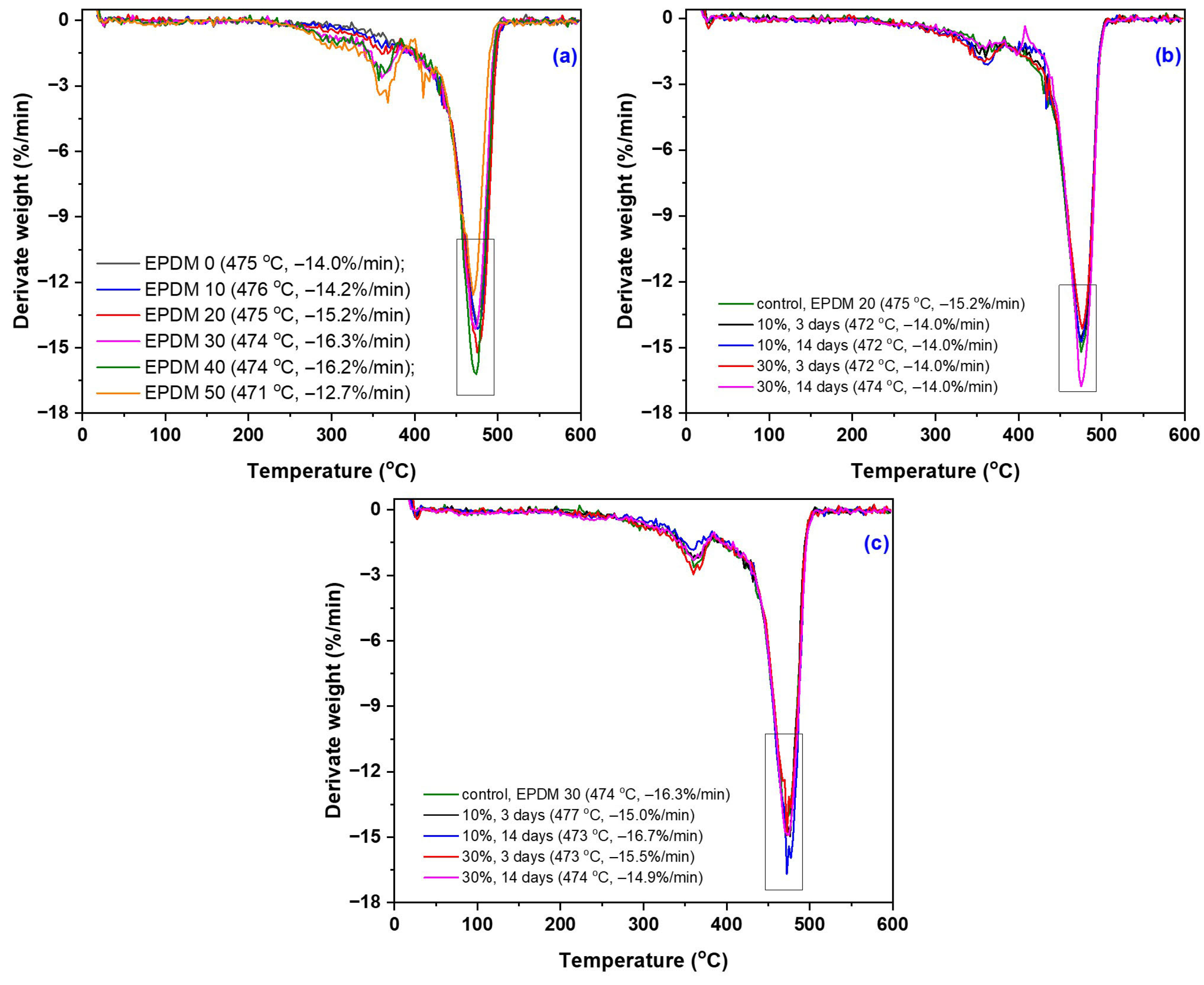
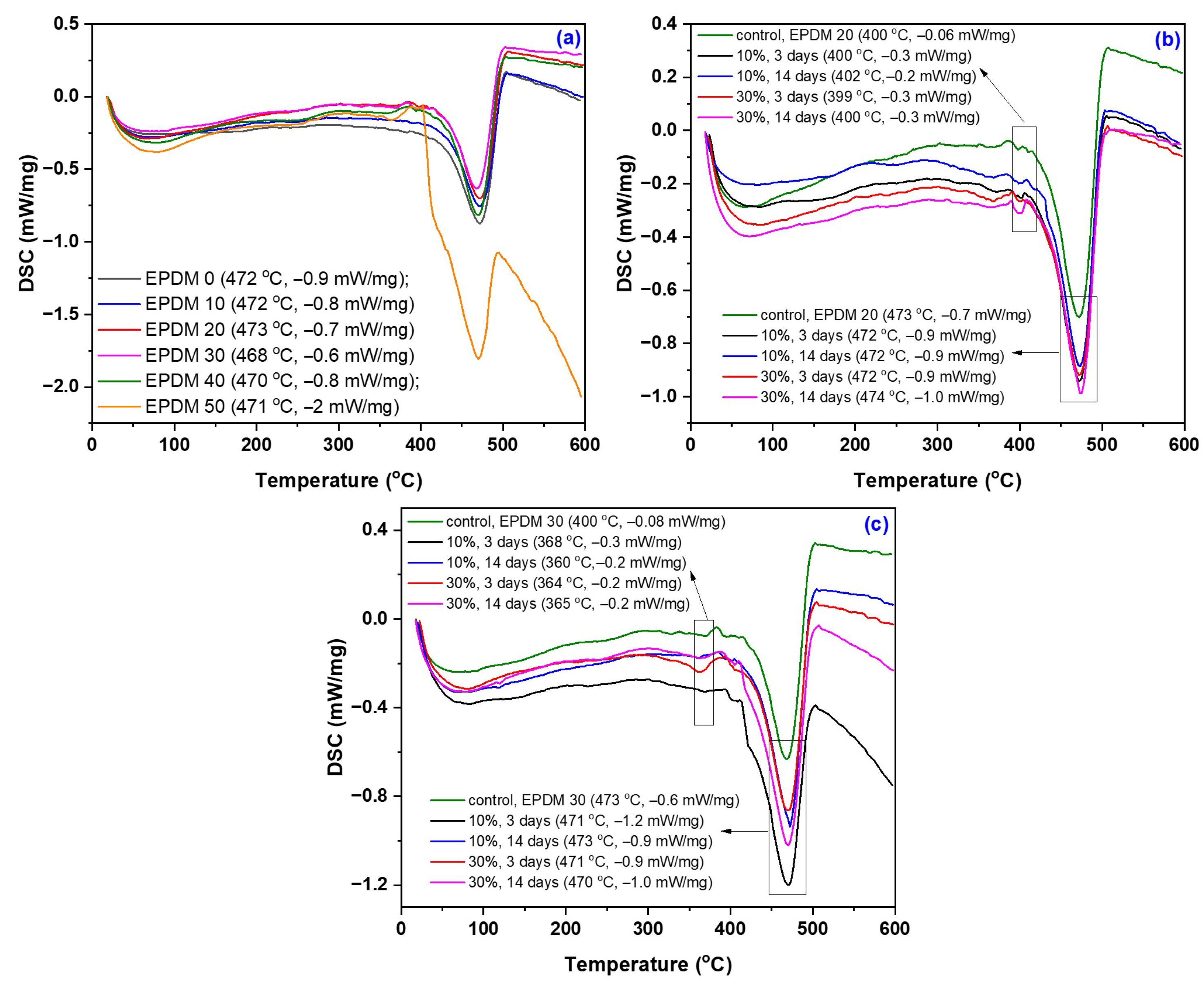
3.6. TGA/DSC Modifications due to the Acetic Acid Exposure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lima Costa, N.; Toshiyuki Hiranobe, C.; Pina Cardim, H.; Dognani, G.; Sanchez, J.C.; Carvalho, J.A.J.; Barrera Torres, G.; Lataro Paim, L.; Ferreira Pinto, L.; Pina Cardim, G.; et al. A Review of EPDM (Ethylene Propylene Diene Monomer) Rubber-Based Nanocomposites: Properties and Progress. Polymers 2024, 16, 1720. [Google Scholar] [CrossRef]
- Nazir, M.T.; Phung, B.T.; Sahoo, A.; Yu, S.; Zhang, Y.; Li, S. Surface Discharge Behaviors, Dielectric and Mechanical Properties of EPDM based Nanocomposites containing Nano-BN. Appl. Nanosci. 2019, 9, 1981–1989. [Google Scholar] [CrossRef]
- Malas, A.; Das, C.K. Carbon black clay hybrid nanocomposites based upon EPDM elastomer. J. Mater. Sci. 2012, 47, 2016–2024. [Google Scholar] [CrossRef]
- Ghase, K.S.; El-Marsafy, S.M.; Abadeer, E.F.; Samir, A.M. Thermal and Mechanical Characteristics of EPDM Composites. Aust. J. Basic. Appl. Sci. 2012, 6, 23–30. Available online: https://www.researchgate.net/publication/290078888_Thermal_and_mechanical_characteristics_of_EPDM_composites (accessed on 15 September 2025).
- Pal, K.; Panwar, V.; Bahadur, J. Chaper 10—Rubber blend nanocomposites. In Progress in Rubber Nanocomposites; Thomas, S., Maria, H.J., Eds.; Elsevier: London, UK, 2017; pp. 319–348. [Google Scholar] [CrossRef]
- Xu, X.; Wang, G.; Yan, H.; Yao, X. Constitutive relationship of fabric rubber composites and its application. Compos. Struct. 2023, 304, 116302. [Google Scholar] [CrossRef]
- Mujkanovic, A.; Vasiljevic, L.; Ostojic, G. Non-black fillers for elastomers. In Proceedings of the 13th International Research/Expert Conference—Trends in the Development of Machinery and Associated Technology (TMT), Hammamet, Tunisia, 16–21 October 2009; Available online: https://www.researchgate.net/publication/351480731 (accessed on 15 September 2025).
- Stelescu, M.D.; Manaila, E.; Georgescu, M.; Nituica, M. New Materials Based on Ethylene Propylene Diene Terpolymer and Hemp Fibers Obtained by Green Reactive Processing. Materials 2020, 13, 2067. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.M.; Amer, H.A.; Atia, M.K.; Rabie, A.M. Effect of Gamma Radiation on the Physicomechanical Characters of EPDM Rubber/Modified Additives Nanocomposites. J. Vinyl Addit. Technol. 2017, 23, E188–E200. [Google Scholar] [CrossRef]
- Jahed, M.; Naderi, G.; Hamid Reza Ghoreishy, M. Microstructure, Mechanical, and Rheological Properties of Natural Rubber/Ethylene Propylene Diene Monomer Nanocomposites Reinforced by Multi-wall Carbon Nanotubes. Polym. Compos. 2018, 39, E745–E753. [Google Scholar] [CrossRef]
- Ismail, H.; Che Mat, N.S.; Othman, N. Curing Characteristics, Tear, Fatigue, and Aging Properties of Bentonite-filled Ethylenepropylene-diene (EPDM) Rubber Composites. J. Vinyl Addit. Technol. 2018, 24, E77–E84. [Google Scholar] [CrossRef]
- Razak, J.; Mohamad, N.; Mahamood, M.A.; Jaafar, R.; Othman, I.S.; Ismail, M.M.; Tee, L.K.; Junid, R.; Mustafa, Z. On the Preparation of EPDM-g-MAH Compatibilizer via Melt-Blending Method. J. Mech. Eng. Sci. 2019, 13, 5424–5440. [Google Scholar] [CrossRef]
- Craciun, G.; Manaila, E.; Ighigeanu, D.; Stelescu, M.D. A Method to Improve the Characteristics of EPDM Rubber Based Eco-Composites with Electron Beam. Polymers 2020, 12, 215. [Google Scholar] [CrossRef]
- Restrepo-Zapata, N.C.; Osswald, T.A.; Hernández-Ortiz, J.P. Vulcanization of EPDM Rubber Compounds with and without Blowing Agents: Identification of Reaction Events and TTT-Diagram Using DSC Data. Polym. Eng. Sci. 2015, 55, 2073–2088. [Google Scholar] [CrossRef]
- Khalaf, A.; Helaly, F.; El Sawy, S. Improvement Properties of EPDM Rubber Using Hybrid Chitin/Clay Filler for Industrial Products. Egypt. J. Chem. 2020, 63, 131–144. [Google Scholar] [CrossRef]
- Manaila, E.; Airinei, A.; Stelescu, M.D.; Sonmez, M.; Alexandrescu, L.; Craciun, G.; Pamfil, D.; Fifere, N.; Varganici, C.-D.; Doroftei, F.; et al. Radiation Processing and Characterization of Some Ethylene-Propylene-Diene Terpolymer/Butyl (Halobutyl) Rubber/Nanosilica Composites. Polymers 2020, 12, 2431. [Google Scholar] [CrossRef]
- Mirjalili, F.; Chuah, L.; Salahi, E. Mechanical and Morphological Properties of Polypropylene/Nano α-Al2O3 Composites. Sci. World J. 2014, 2014, 718765. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, J.; Parathodika, A.R.; Hu, G.-H.; Naskar, K. Functional rubber composites based on silica-silane reinforcement for green tire application: The state of the art. Funct. Compos. Mater. 2022, 3, 7. [Google Scholar] [CrossRef]
- Hao, N.; Li, L.; Tang, F. Shape matters when engineering mesoporous silica based nanomedicines. Biomater. Sci. 2016, 4, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Li, L.; Tang, F. Roles of particle size, shape and surface chemistry of mesoporous silica nanomaterials on biological systems. Int. Mater. Rev. 2017, 62, 57–77. [Google Scholar] [CrossRef]
- Uhrlandt, S.; Blume, A. Unique production process and silica structure. Rubber World 2003, 228, 43–45. [Google Scholar]
- Okel, T.A.; Waddell, W.H. Silica Properties/Rubber Performance Correlation. Carbon Black-Filled Rubber Compounds. Rubber Chem. Technol. 1994, 67, 217–236. [Google Scholar] [CrossRef]
- Karim, J.; Ahmad, A.; Abdullah, I.; Dahlan, H.M. Efects of pH on mechanical and morphological studies of silica flled polyvinyl chloride-50% epoxi-dized natural rubber (PVC-ENR50) nanocomposite. J. Sol-Gel Sci. Technol. 2012, 62, 7–12. [Google Scholar] [CrossRef]
- Schaefer, D.W.; Rieker, T.; Agamalian, M.; Lin, J.S.; Fischer, D.; Sukumaran, S.; Chen, C.; Beaucage, G.; Herd, C.; Ivie, J. Multilevel structure of reinforcing silica and carbon. J. Appl. Crystallogr. 2020, 33, 587–591. [Google Scholar] [CrossRef]
- Suryawanshi, C.N. Study of Factors Influencing Structure of Precipitated Silica. Master’s Thesis, University of Cincinnati, Cincinnati, OH, USA, 2003. [Google Scholar]
- Mujkanovic, A.; Petrovski, P.; Rizvanovic, M.; Busatlic, I. Silica Synthesized from Diluted Sodium Silicate Solutions, By-products of Various Industrial Processes, as Reinforcing Filler for Elastomer Compounds. In Proceedings of the B.E.N.A. (Balkan Environmental Association)—Conference Life Quality and Capacity Building in the frame of a Safe Environment, Katerini, Greece, 17–20 March 2009. [Google Scholar]
- Wolf, S. Chemical aspects of rubber reinforcement by fllers. Rubber Chem. Technol. 1996, 69, 325–346. [Google Scholar] [CrossRef]
- Inphonlek, S.; Bureewong, N.; Kotchapradit, S.; Ruksakulpiwat, Y.; Ruksakulpiwat, C. Synergistic Effects of Hybrid Bio-Fillers and Modified Natural Rubber on Natural Rubber Composite Properties. Polymers 2025, 17, 632. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Manaila, E.; Craciun, G.; Dumitrascu, M. New Green Polymeric Composites Based on Hemp and Natural Rubber Processed by Electron Beam Irradiation. Sci. World J. 2014, 2014, 684047. [Google Scholar] [CrossRef]
- Manaila, E.; Stelescu, M.D.; Craciun, G.; Surdu, L. Effects of benzoyl peroxide on some properties of composites based on hemp and natural rubber. Polym. Bull. 2014, 71, 2001–2022. [Google Scholar] [CrossRef]
- Manaila, E.; Stelescu, M.D.; Doroftei, F. Polymeric composites based on natural rubber and hemp fibers. Iran. Polym. J. 2015, 24, 135–148. [Google Scholar] [CrossRef]
- Manaila, E.; Craciun, G.; Ighigeanu, D. Water Absorption Kinetics in Natural Rubber Composites Reinforced with Natural Fibers Processed by Electron Beam Irradiation. Polymers 2020, 12, 2437. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Airinei, A.; Manaila, E.; Craciun, G.; Fifere, N.; Varganici, C. Property correlations for composites based on ethylene propylene diene rubber reinforced with flax fibers. Polym. Test. 2017, 59, 75–83. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Airinei, A.; Manaila, E.; Fifere, N.; Craciun, G.; Varganici, C.; Doroftei, F. Exploring the effect of electron beam irradiation on the properties of some EPDM-flax fiber composites. Polym. Compos. 2019, 40, 315–327. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Manaila, E.; Craciun, G.; Chirila, C. Development and Characterization of Polymer Eco-Composites Based on Natural Rubber Reinforced with Natural Fibers. Materials 2017, 10, 787. [Google Scholar] [CrossRef]
- Manaila, E.; Stelescu, M.D.; Craciun, G.; Ighigeanu, D. Wood Sawdust/Natural Rubber Ecocomposites Cross-Linked by Electron Beam Irradiation. Materials 2016, 9, 503. [Google Scholar] [CrossRef]
- Manaila, E.; Craciun, G.; Ighigeanu, D.; Lungu, I.B.; Dumitru, M.; Stelescu, M.D. Electron Beam Irradiation: A Method for Degradation of Composites Based on Natural Rubber and Plasticized Starch. Polymers 2021, 13, 1950. [Google Scholar] [CrossRef] [PubMed]
- Manaila, E.; Stelescu, M.D.; Craciun, G. Degradation Studies Realized on Natural Rubber and Plasticized Potato Starch Based Eco-Composites Obtained by Peroxide Cross-Linking. Int. J. Mol. Sci. 2018, 19, 2862. [Google Scholar] [CrossRef] [PubMed]
- Manaila, E.; Craciun, G.; Lungu, I.B.; Grivei, M.D.D.; Stelescu, M.D. Degradation by Electron Beam Irradiation of Some Elastomeric Composites Sulphur Vulcanized. Materials 2023, 16, 2152. [Google Scholar] [CrossRef]
- Cristaldi, G.; Latteri, A.; Recca, G.; Cicala, G. Composites Based on Natural Fibre Fabrics. In Woven Fabric Engineering; Dubrovski, P.D., Ed.; Sciyo: Rijeka, Croatia, 2010; pp. 318–342. Available online: http://www.intechopen.com (accessed on 2 June 2025).
- Begum, K.; Islam, M.A. Natural fiber as a substitute to synthetic fiber in Polymer Composites: A Review. Res. J. Eng. Sci. 2013, 2, 46–53. Available online: https://www.isca.me/IJES/Archive/v2/i4/10.ISCA-RJEngS-2013-010.pdf (accessed on 15 September 2025).
- Mohd Nazif, N. Composites from Polypropylene (PP) Reinforced with Oil Palm Empty Fruit Bunch (OPEFB) Fibre. Ph.D. Thesis, Faculty of Chemical and Natural Resources Engineering, University of Malaysia Pahang, Pahang, Malaysia, 2011. [Google Scholar]
- Fakhrul, T.; Mahbub, R.; Islam, M.A. Properties of wood sawdust and wheat Flour Reinforced Polypropylene Composites. J. Mod. Sci. Technol. 2013, 1, 135–148. [Google Scholar]
- Fu, S.Y.; Feng, X.Q.; Lauke, B.; Mai, Y.W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. Available online: https://www.researchgate.net/publication/239730719_Properties_of_Wood_Sawdust_and_Wheat_Flour_Reinforced_Polypropylene_Composites (accessed on 15 September 2025). [CrossRef]
- Karmarkar, A.; Chauhan, S.S.; Modak, J.M.; Chanda, M. Mechanical properties of wood–fiber reinforced polypropylene composites: Effect of a novel compatibilizer with isocyanate functional group. Compos. Part. A Appl. Sci. Manuf. 2007, 38, 227–233. [Google Scholar] [CrossRef]
- Rahman, M.R.; Hasan, M.; Huque, M.M.; Islam, M.N. Physico-Mechanical Properties of Jute Fiber Reinforced Polypropylene Composites. J. Reinf. Plast. Compos. 2010, 29, 445–455. Available online: https://www.researchgate.net/publication/223460026_Physico-Mechanical_Properties_of_Jute_Fiber_Reinforced_Polypropylene_Composites (accessed on 15 September 2025). [CrossRef]
- Rahman, M.R.; Islam, M.N.; Huque, M.M. Influence of fiber treatment on the mechanical and Morphological Properties of Sawdust Reinforced Polypropylene Composites. J. Environ. Polym. Degr 2010, 18, 443–450. Available online: https://www.researchgate.net/publication/227246789_Influence_of_Fiber_Treatment_on_the_Mechanical_and_Morphological_Properties_of_Sawdust_Reinforced_Polypropylene_Composites (accessed on 15 September 2025). [CrossRef]
- ISO 175/2010; Plastics–Methods of Test for the Determination of the Effects of Immersion in Liquid Chemicals, Edition 3. International Organization for Standardization: Geneva, Switzerland, 2010.
- Colom, X.; Carrillo-Navarrete, F.; Saeb, M.R.; Marin, M.; Formela, K.; Canavate, J. Evaluation and rationale of the performance of several elastomeric composites incorporating devulcanized EPDM. Polym. Test. 2023, 121, 107976. [Google Scholar] [CrossRef]
- ASTM D6814-02; Standard Test Method for Determination of Percent Devulcanization of Crumb Rubber Based on Crosslink Density. ASTM International: West Conshohocken, PA, USA, 2014.
- Lopez-Manchado, M.A.; Herrero, B.; Arroyo, M. Preparation and characterization of organoclay nanocomposites based on natural rubber. Polym. Int. 2003, 52, 1070–1077. [Google Scholar] [CrossRef]
- Chenal, J.M.; Chazeau, L.; Guy, L.; Bomal, Y.; Gauthier, C. Molecular weight between physical entanglements in natural rubber: A critical parameter during strain-induced crystallization. Polymer 2007, 48, 1042–1046. [Google Scholar] [CrossRef]
- DIN EN ISO 37:2017; Rubber, Vulcanized or Thermoplastic–Determination of Tensile Stress-Strain Proprieties, Sixth Edition. International Organization for Standardization: Geneva, Switzerland, 2017.
- SR EN ISO 11358:20022; Plastics. Thermogravimetry (TG) of Polymers. Part 1: General Principles. International Organization for Standardization: Bucharest, Romania, 2022.
- SR EN ISO 11357-1:2023; Plastics. Differential Scanning Calorimetry (DSC). Part 1: General Principles. International Organization for Standardization: Bucharest, Romania, 2023.
- Pickering, K.L.; Aruan Efendy, M.G.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- John, M.J.; Thomas, S. Biofibres and biocomposites. Carbohydr. Polym. 2008, 71, 343–364. [Google Scholar] [CrossRef]
- Kalia, S.; Kaith, B.S.; Kaur, I. Pretreatments of natural fibers and their application as reinforcing material in polymer composites—A review. Polym. Eng. Sci. 2009, 49, 1253–1272. [Google Scholar] [CrossRef]
- Biagiotti, J.; Puglia, D.; Kenny, J.M. A Review on Natural Fibre Based Composites—Part I: Structure, Processing and Properties of Vegetable Fibres. J. Nat. Fibers 2004, 1, 37–68. [Google Scholar] [CrossRef]
- Puglia, D.; Biagiotti, J.; Kenny, J.M. A Review on Natural Fibre-Based Composites—Part II: Application of Natural Reinforcements in Composite Materials for Automotive Industry. J. Nat. Fibers 2005, 1, 23–65. [Google Scholar] [CrossRef]
- Stamboulis, A.; Baillie, C.; Peijs, T. Effects of environmental conditions on mechanical and physical properties of flax fibres. Compos. Part A Appl. Sci. Manuf. 2001, 32, 1105–1115. [Google Scholar] [CrossRef]
- Baena, L.M.; Zuleta, E.C.; Calderón, J.A. Evaluation of the Stability of Polymeric Materials Exposed to Palm Biodiesel and Biodiesel–Organic Acid Blends. Polymers 2018, 10, 511. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, Q.L.; Tu, Z.K.; Tu, W.M.; Wan, Z.M.; Pan, M.; Zhang, H.N. Degradation of silicone rubbers with different hardness in various aqueous solutions. Polym. Degrad. Stab. 2014, 109, 122–128. [Google Scholar] [CrossRef]
- Mallakpour, S.E.; Hajipour, A.R.; Mahdavian, A.R.; Zadhoush, A.; Ali-Hosseini, F. Microwave assisted oxidation of polyethylene under solid-state conditions with potassium permanganate. Eur. Polym. J. 2001, 37, 1199–1206. [Google Scholar] [CrossRef]
- Ferede, E. Evaluation of Mechanical and Water Absorption Properties of Alkaline-Treated Sawdust-Reinforced Polypropylene Composite. J. Eng. 2020, 2020, 3706176. [Google Scholar] [CrossRef]
- Jakob, M.; Mahendran, A.R.; Gindl-Altmutter, W.; Bliem, P.; Konnerth, J.; Mueller, U.; Veigel, S. The strength and stiffness of oriented wood and cellulose-fibre materials: A review. Prog. Mater. Sci. 2022, 125, 100916. [Google Scholar] [CrossRef]
- Sejati, P.S.; Akong, F.O.; Fradet, F.; Gerardin, P. Understanding the thermoplasticization mechanism of wood via esterification with fatty acids: A comparative study of the reactivity of cellulose, hemicelluloses and lignin. Carbohydr. Polym. 2024, 324, 121542. [Google Scholar] [CrossRef]
- Xu, E.; Wang, D.; Lin, L. Chemical Structure and Mechanical Properties of Wood Cell Walls Treated with Acid and Alkali Solution. Forests 2020, 11, 87. [Google Scholar] [CrossRef]
- Darko, C. The link between swelling ratios and physical properties of EPDM rubber compound having different oil amounts. J. Polym. Res. 2022, 29, 325. [Google Scholar] [CrossRef]
- Valentin, J.L.; Carretero-González, J.; Mora-Barrantes, I.; Chasse, W.; Saalwachter, K. Uncertainties in the determination of crosslink density by equilibrium swelling experiments in natural rubber. Macromolecules 2008, 41, 4717–4729. [Google Scholar] [CrossRef]
- El Shafee, E.; Naguib, H.F. Water sorption in cross-linked poly (vinyl alcohol) networks. Polymer 2003, 44, 1647–1653. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Giri, A. Water sorption behaviour of highly swelling (carboxy methylcellulose-g-polyacrylamide) hydrogels and release of potassium nitrate as agrochemical. Carbohydr. Polym. 2003, 53, 271–279. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, J.W.; Lee, D.Y.; Seo, K.H. Correlation between the crosslink characteristics and mechanical properties of natural rubber compound via accelerators and reinforcement. Polymers 2020, 12, 2020. [Google Scholar] [CrossRef] [PubMed]
- Dijkhuis, K.A.; Noordermeer, J.W.; Dierkes, W.K. The relationship between crosslink system, network structure and material properties of carbon black reinforced EPDM. Eur. Polym. J. 2009, 45, 3302–3312. [Google Scholar] [CrossRef]
- Liu, Q.K.; Li, J.; Jiang, Y.C.; Cong, C.A.B.; Xu, L.X.; Zhang, Y.; Meng, X.Y.; Zhou, Q. Effect of crosslinked structure on the chemical degradation of EPDM rubber in an acidic environment. Polym. Degrad. Stab. 2021, 185, 109475. [Google Scholar] [CrossRef]
- Liu, Q.K.; Li, J.; Cong, C.B.; Cui, H.M.; Xu, L.X.; Zhang, Y.; Meng, X.Y.; Zhou, Q. Thermal and thermo-oxidative degradation of tetrafluoroethylene-propylene elastomer above 300 °C. Polym. Degrad. Stab. 2020, 177, 109180. [Google Scholar] [CrossRef]
- Cong, C.B.; Liu, Q.K.; Li, J.; Meng, X.Y.; Zhou, Q. The effect of peroxide crosslinking on the synergistic crosslink of double bond and nitrile group of nitrile rubber in H2S environment. Polym. Test. 2019, 76, 298–304. [Google Scholar] [CrossRef]
- Zirnstein, B.; Schulze, D.; Schartel, B. Mechanical and Fire Properties of Multicomponent Flame Retardant EPDM Rubbers Using Aluminum Trihydroxide, Ammonium Polyphosphate, and Polyaniline. Materials 2019, 12, 1932. [Google Scholar] [CrossRef]
- Jaya, H.; Noriman, N.Z.; AbdulKadir, H.K.; Dahham, O.S.; Muhammad, N.; Latip, N.A.; Aini, A.K. The Effects of Wood Sawdust Loading on Tensile and Physical Properties of Up/Pf/Wsd Composites. In Proceedings of the International Conference on Materials Engineering and Science, Kuala Lumpur, Malaysia, 6–7 August 2018; Dahham, O.S., Zulkepli, N.N., Dahham, S.S., Hamzah, R., AbdRahim, S.Z., Eds.; Book Series IOP Conference Series-Materials Science and Engineering. IOP Publishing: Bristol, UK, 2018; Volume 454, p. 012193. [Google Scholar] [CrossRef]
- Zailan, F.D.; Chen, R.S.; Shahdan, D.; Ahmad, S. Effect of conductive polyaniline in thermoplastic natural rubber blends on the mechanical, thermal stability, and electrical conductivity properties. J. Appl. Polym. Sci. 2019, 136, 47527. [Google Scholar] [CrossRef]
- Faez, R.; de Paoli, M.-A. A conductive rubber based on EPDM and polyaniline: I. Doping method effect. Eur. Polym. J. 2001, 37, 1139–1143. [Google Scholar] [CrossRef]
- Horisawa, S.; Sunagawa, M.; Tamai, Y.; Matsuoka, Y.; Miura, T.; Terazawa, M. Biodegradation of non ligno cellulosic substances II: Physical and chemical properties of sawdust before and after use as artificial soil. J. Wood Sci. 1999, 45, 492–497. [Google Scholar] [CrossRef]
- Panshin, A.J.; de Zeeuw, C. Textbook of Wood Technology: Structure, Identification, Properties, and Uses of the Commercial Woods of the United States and Canada (McGraw-Hill Series in Forest Resources); McGraw-Hill College: New York, NY, USA, 1980; pp. 120–125. ISBN 10-0070484414/13-978-0070484405. [Google Scholar]
- Sjöström, E. Wood Chemistry: Fundamental and Applications, 2nd ed.; Academic Press, Inc.: London, UK; San Diego, CA, USA, 1993; pp. 1–20. ISBN 9780080925899. [Google Scholar]
- Gunasekaran, S.; Natarajan, R.K.; Kala, A. FTIR spectra and mechanical strength analysis of some selected rubber derivatives. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 68, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Kumutha, K.; Alias, Y. FTIR spectra of plasticized grafted natural rubber–LiCF3SO3 electrolytes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 64, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Owen, N.L.; Thomas, D.W. Infrared studies of “hard” and “soft” woods. Appl. Spectrosc. 1989, 43, 451–455. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and Quantitative Analysis of Lignocellulosic Biomass Using Infrared Techniques: A Mini-Review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef]
- Fackler, K.; Stevanic, J.S.; Ters, T.; Hinterstoisser, B.; Schwanninger, M.; Salmén, L. FT-IR imaging microscopy to localise and characterise simultaneous and selective white-rot decay within spruce wood cells. Holzforschung 2011, 65, 411–420. [Google Scholar] [CrossRef]
- Aarthi, R.; Ramalingam, S.; Periandy, S.; Senthil Kannan, K. Molecular structure-associated pharmacodynamic investigation on benzoyl peroxide using spectroscopic and quantum computational tools. J. Taibah Univ. Sci. 2018, 12, 104–122. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Matei, A.; Manciulea, I. Composites acrylic copolymers-wood waste. In Proceedings of the 4th International Conference Advanced Composite Materials Engineering (COMAT 2012), Brasov, Romania, 18–20 October 2012; pp. 597–600. [Google Scholar]
- Bodirlau, R.; Teaca, C.A. Fourier transform infrared spectroscopy and thermal analysis of lignocellulose fillers treated with organic anhydrides. Rom J. Phys. 2009, 54, 93–104. Available online: http://www.nipne.ro/rjp/2009_54_1-2/0093_0105.pdf (accessed on 15 September 2025).
- Liang, C.Y.; Marchessault, R.H. Infrared spectra of crystalline polysaccharides. I. Hydrogen bonds in native celluloses. J. Polym. Sci. Polym. Chem. 1959, 37, 385–395. [Google Scholar] [CrossRef]
- Pang, A.L.; Ismail, H. Tensile properties, water uptake and thermal properties of polypropylene/waste pulverized tire/kenaf (PP/WPT/KNF) composites. BioRes 2013, 8, 806–817. Available online: https://bioresources.cnr.ncsu.edu/resources/tensile-properties-water-uptake-and-thermal-properties-of-polypropylenewaste-pulverized-tirekenaf-ppwptknf-composites/ (accessed on 15 September 2025).
- Mohebby, B. Application of ATR Infrared Spectroscopy in Wood Acetylation. J. Agric. Sci. Technol. 2008, 10, 253–259. Available online: https://jast.modares.ac.ir/article-23-4042-en.pdf (accessed on 15 September 2025).
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous cellulose-structure and characterization. Cell Chem. Technol. 2011, 45, 13–21. Available online: http://www.cellulosechemtechnol.ro/pdf/CCT1-2(2011)/p.13-21.pdf (accessed on 15 September 2025).
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; JohnWiley & Sons, Ltd.: Sydney, Australia, 2004; pp. 72–74. [Google Scholar]
- Bodîrlău, R.; Teacă, C.A.; Spiridon, I. Preparation and characterization of composites comprising modified hardwood and wood polymers/poly(vinyl chloride). BioRes 2009, 4, 1285–1304. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Airinei, A.; Manaila, E.; Craciun, G.; Fifere, N.; Varganici, C.; Pamfil, D.; Doroftei, F. Effects of mechanical, thermal and surface properties of some EPDM/butyl rubber composites. Polymers 2018, 10, 1206. [Google Scholar] [CrossRef]
- Mokkothu, T.H.; Luyt, A.S.; Messori, M. Reinforcement of EPDM rubber with in situ generated silica particles in presence of a coupling agent via a sol-gel route. Polym. Test. 2014, 33, 97–106. [Google Scholar] [CrossRef]
- Schwanninger, M.; Stefkeb, B.; Hinterstoisserb, B. Qualitative assessment of acetylated wood with infrared spectroscopic methods. J. Near Infrared Spectrosc. 2011, 19, 349–357. [Google Scholar] [CrossRef]
- Subroto, E.; Cahyana, Y.; Indiarto, R.; Rahmah, T.A. Modification of Starches and Flours by Acetylation and Its Dual Modifications: A Review of Impact on Physicochemical Properties and Their Applications. Polymers 2023, 15, 2990. [Google Scholar] [CrossRef]
- Rahim, A.; Siswo Huto, G.; Rahman, N.; Bohari, H.S.A. Structure and Functional Properties of Arenga Starch by Acetylation with Different Concentrations of Acetic Anhydride. Asian J. Sci. Res. 2019, 12, 220–228. [Google Scholar] [CrossRef]
- Otemuyiwa, I.O.; Aina, A.F. Physicochemical Properties and In-Vitro Digestibility Studies of Microwave Assisted Chemically Modified Breadfruit (Artocarpus altilis) Starch. Int. J. Food Prop. 2021, 24, 140–151. [Google Scholar] [CrossRef]
- Chi, H.; Xu, K.; Wu, X.; Chen, Q.; Xue, D.; Song, C.; Zhang, W.; Wang, P. Effect of Acetylation on the Properties of Corn Starch. Food Chem. 2008, 106, 923–928. [Google Scholar] [CrossRef]
- Stefke, B.; Windeisen, E.; Schwanninger, M.; Hinterstoisser, B. Determination of the weight percentage gain and of the acetyl group content of acetylated wood by means of different infrared spectroscopic methods. Anal. Chem. 2008, 80, 1272–1279. [Google Scholar] [CrossRef]
- Faix, O. Classification of lignins from different botanical origins by FTIRspectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Chrissafis, K.; Paraskevopoulos, K.M.; Triantafyllidis, K.S.; Antonakou, E.V. Investigation of thermal degradation mechanism of an aliphatic polyester using pyrolysis-gas chromatographyemass spectrometry and a kinetic study of the effect of the amount of polymerization catalyst. Polym. Degrad. Stab. 2007, 92, 525–536. Available online: https://pure.kfupm.edu.sa/en/publications/investigation-of-thermal-degradation-mechanism-of-an-aliphatic-po (accessed on 15 September 2025). [CrossRef]
- Miedzianowska, J.; Masłowski, M.; Strzelec, K. Thermoplastic Elastomeric Composites Filled with Lignocellulose Bioadditives. Part 1: Morphology, Processing, Thermal and Rheological Properties. Materials 2020, 13, 1598. [Google Scholar] [CrossRef]
- Khuntia, T.; Biswas, S. Mechanical, viscoelastic, and flammability properties of polymer composites reinforced with novel Sirisha bark filler. J. Ind. Text. 2022, 51, 5887S–5909S. [Google Scholar] [CrossRef]
- Rosu, D.; Rosu, L.; Varganici, C.D. The thermal stability of some semi-interpenetrated polymer networks based on epoxy resin and aromatic polyurethane. J. Anal. Appl. Pyrolysis 2013, 100, 103–110. [Google Scholar] [CrossRef]



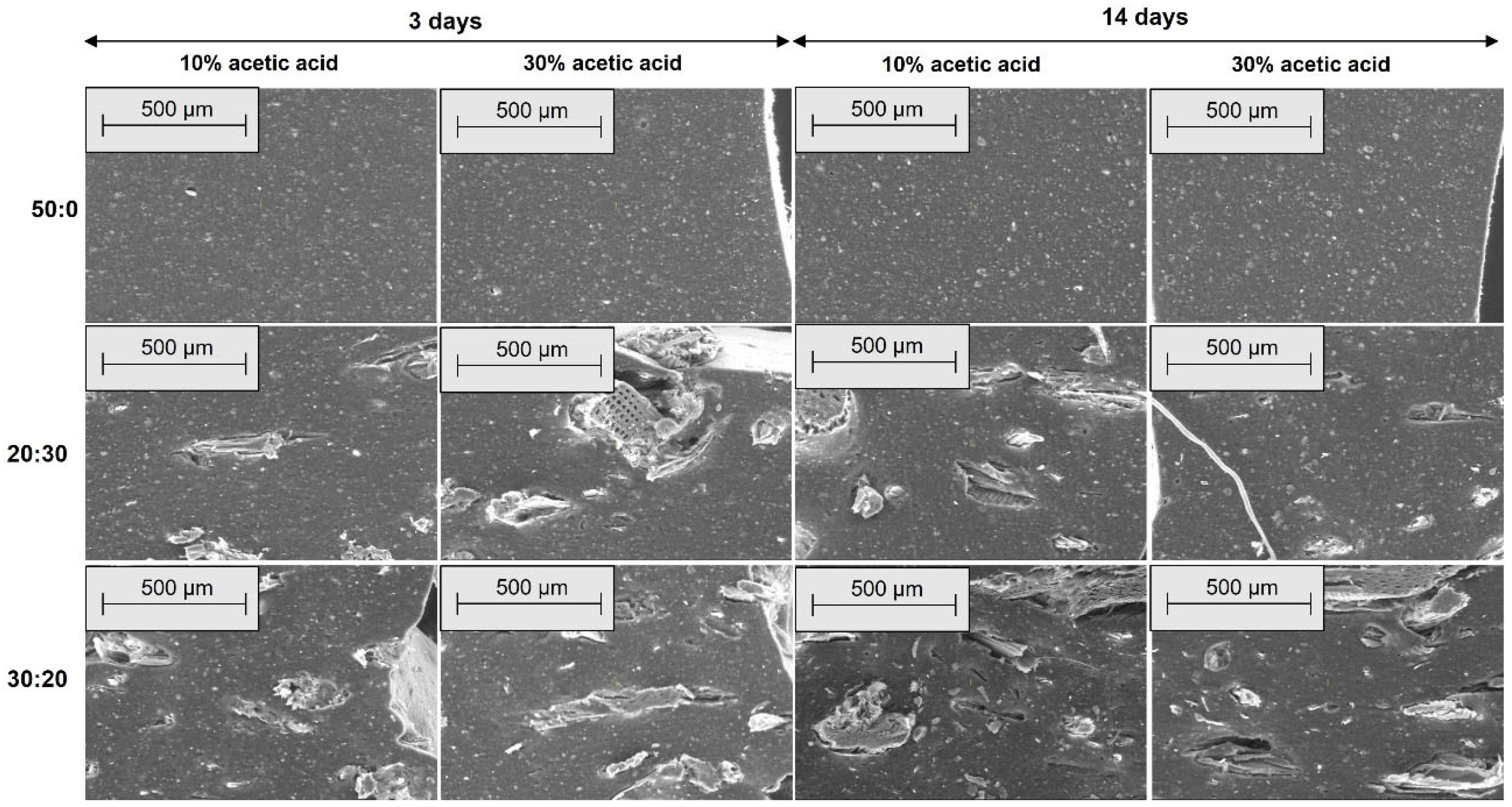
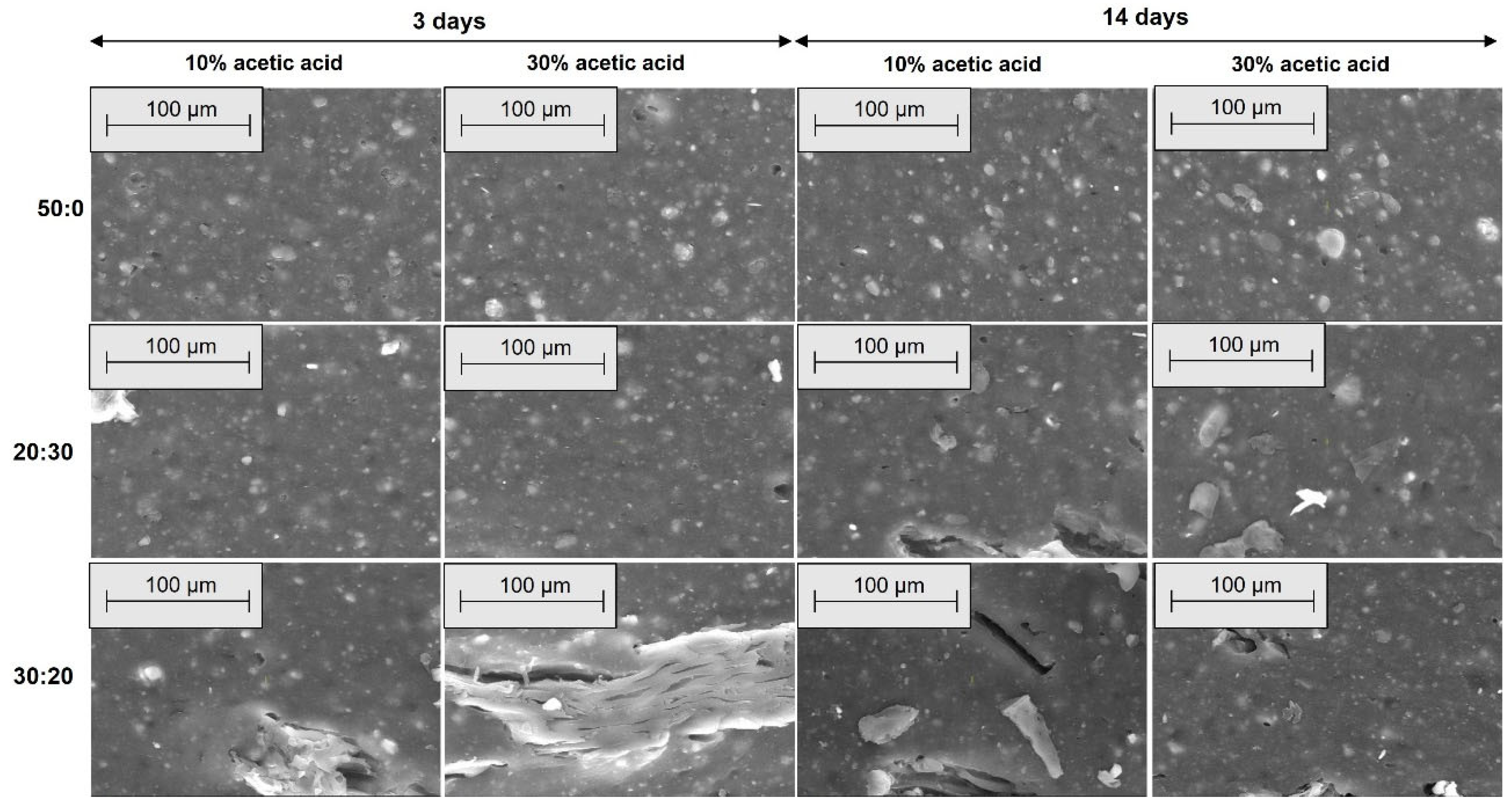

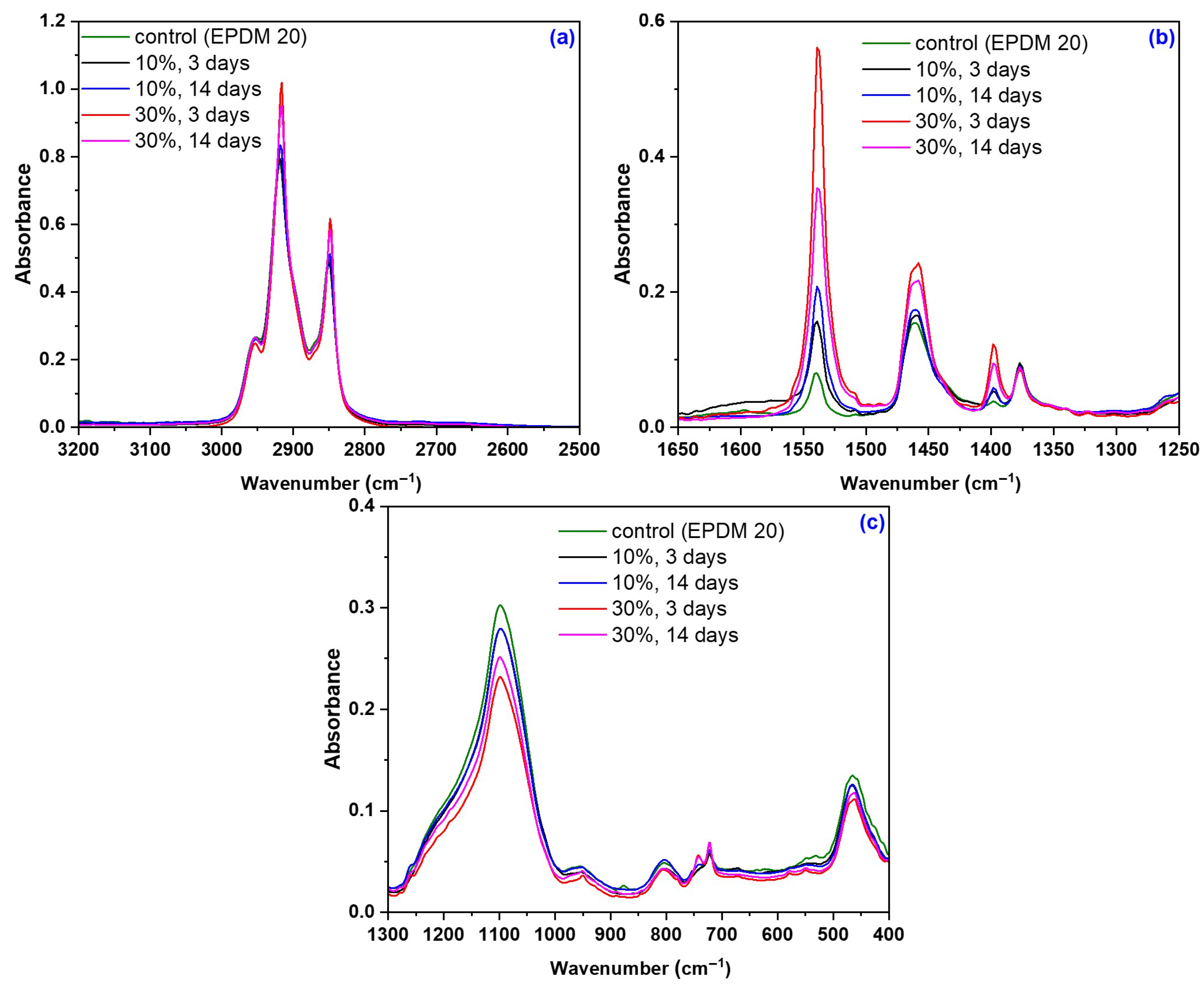
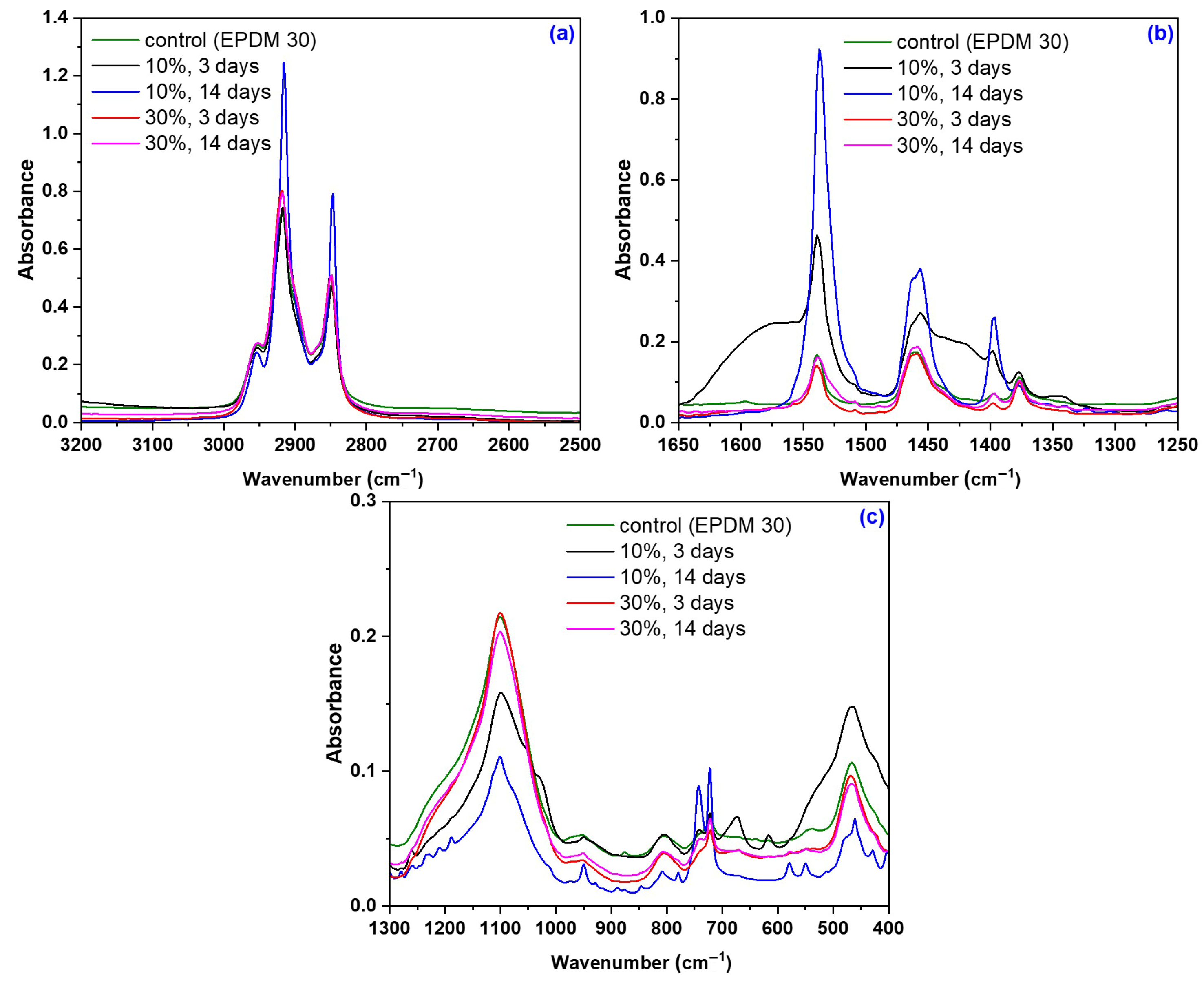
| Mixture Code/Recipe (phr) | EPDM 0 | EPDM 10 | EPDM 20 | EPDM 30 | EPDM 40 | EPDM 50 |
|---|---|---|---|---|---|---|
| EPDM Keltan 512 (EPDM-ENB, ~60% ethylene) | 100 | 100 | 100 | 100 | 100 | 100 |
| Filler (silica) | 50 | 40 | 30 | 20 | 10 | 0 |
| Filler (wood sawdust) | 0 | 10 | 20 | 30 | 40 | 50 |
| Plasticizer, paraffinic oils | 40 | 40 | 30 | 30 | 20 | 25 |
| ZnO | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 |
| Stearine | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| PEG 4000 | 3 | 3 | 3 | 3 | 3 | 3 |
| TiO2 | 4 | 4 | 4 | 4 | 4 | 4 |
| Antioxidants, IPPD (4010) | 1 | 1 | 1 | 1 | 1 | 1 |
| Vulcanizing accelerator, dicyclopentadiene (DCP) | 3 | 3 | 3 | 3 | 3 | 3 |
| Vulcanizing agent, dicumyl peroxide (DCP) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Sample Code | Vrf | Vro/Vrf | Solvent Uptake | Cross-Link Density | Young’s Modulus (E) | Tensile Strength (σ) | Specific Elongation (ε) |
|---|---|---|---|---|---|---|---|
| EPDM 0 | - | 1.505 ± 0.137 | 0.1729 ± 0.0062 e | 5.03 ± 0.71 cd | 6.25 ± 0.19 a | 976 ± 21 a | |
| EPDM 10 | 0.2872 | 0.4500 | 0.630 ± 0.043 | 1.6168 ± 0.0206 d | 4.12 ± 0.52 d | 3.54 ± 0.57 b | 584 ± 72 b |
| EPDM 20 | 0.3402 | 0.3799 | 0.446 ± 0.005 | 3.6900 ± 0.1649 a | 6.72 ± 1.19 bc | 3.08 ± 0.45 b | 330 ± 25 c |
| EPDM 30 | 0.3449 | 0.3748 | 0.391 ± 0.028 | 2.8949 ± 0.12978 b | 6.92 ± 0.87 b | 1.60 ± 0.15 c | 246 ± 36 d |
| EPDM 40 | 0.2991 | 0.4322 | 0.537 ± 0.034 | 2.2149 ± 0.13008 c | 8.13 ± 0.71 ab | 1.50 ± 0.05 c | 298 ± 43 cd |
| EPDM 50 | 0.2948 | 0.4384 | 0.486 ± 0.027 | 2.1974 ± 0.10904 c | 9.12 ± 1.47 a | 1.02 ± 0.05 c | 109 ± 32 e |
| F value | 582.9998 | 18.56968 | 189.03578 | 290.88586 | |||
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Sample Code | 3 Days | 7 Days | 14 Days | |||
|---|---|---|---|---|---|---|
| Swelling | Mass Change | Swelling | Mass Change | Swelling | Mass Change | |
| Acetic acid concentration 10% | ||||||
| EPDM 0 | +13.57 ± 1.51 | −0.48 ± 0.29 | +12.46 ± 1.69 | −1.39 ± 0.39 | +12.38 ± 0.43 | −1.76 ± 0.31 |
| EPDM 10 | +16.17 ± 2.11 | −0.36 ± 0.17 | +21.11 ± 1.46 | −0.69 ± 0.32 | +29.89 ± 0.88 | −0.52 ± 0.19 |
| EPDM 20 | +21.73 ± 1.16 | −0.43 ± 0.20 | +24.73 ± 0.64 | −0.75 ± 0.36 | +28.82 ± 1.01 | −1.23 ± 0.33 |
| EPDM 30 | +23.28 ± 1.39 | +0.42 ± 0.29 | +31.92 ± 2.20 | +0.60 ± 0.29 | +57.20 ± 1.31 | +1.16 ± 0.34 |
| EPDM 40 | +22.21 ± 1.23 | +0.56 ± 0.21 | +33.34 ± 0.77 | +1.47 ± 0.15 | +60.17 ± 3.22 | +1.95 ± 0.36 |
| EPDM 50 | +60.21 ± 3.17 | +2.58 ± 0.37 | +121.93 ± 5.42 | +5.12 ± 0.25 | +306.31 ± 5.86 | +7.78 ± 0.36 |
| Acetic acid concentration 20% | ||||||
| EPDM 0 | +11.65 ± 0.38 | −0.67 ± 0.38 | +15.02 ± 0.48 | −1.33 ± 0.34 | +11.94 ± 0.58 | −2.39 ± 0.26 |
| EPDM 10 | +15.27 ± 1.04 | −0.47 ± 0.01 | +21.49 ± 0.68 | −0.66 ± 0.01 | +29.05 ± 2.64 | −0.30 ± 0.12 |
| EPDM 20 | +19.29 ± 1.58 | −0.59 ± 0.17 | +25.12 ± 1.35 | −0.63 ± 0.25 | +29.75 ± 0.75 | −1.60 ± 0.43 |
| EPDM 30 | +20.53 ± 0.60 | +0.51 ± 0.36 | +33.05 ± 0.86 | +0.65 ± 0.23 | +60.09 ± 1.27 | +1.30 ± 0.21 |
| EPDM 40 | +22.52 ± 1.16 | +0.73 ± 0.46 | +37.01 ± 1.42 | +1.56 ± 0.23 | +66.36 ± 1.51 | +2.01 ± 0.62 |
| EPDM 50 | +66.92 ± 4.31 | +3.38 ± 0.36 | +128.94 ± 6.07 | +6.54 ± 0.27 | +338.19 ± 12.37 | +8.16 ± 0.81 |
| Acetic acid concentration 30% | ||||||
| EPDM 0 | +10.58 ± 0.98 | −0.45 ± 0.22 | +12.94 ± 0.98 | −1.51 ± 0.42 | +12.27 ± 1.65 | −2.80 ± 0.66 |
| EPDM 10 | +15.13 ± 1.25 | −0.48 ± 0.02 | +20.95 ± 1.03 | −0.65 ± 0.43 | +28.49 ± 0.85 | −0.62 ± 0.31 |
| EPDM 20 | +17.78 ± 0.65 | −0.60 ± 0.14 | +25.05 ± 1.03 | −1.10 ± 0.27 | +25.12 ± 2.41 | −2.38 ± 0.58 |
| EPDM 30 | +21.45 ± 0.35 | +0.81 ± 0.22 | +37.26 ± 1.07 | +1.16 ± 0.28 | +64.97 ± 1.50 | −0.47 ± 0.48 |
| EPDM 40 | +24.45 ± 1.19 | +1.54 ± 0.16 | +40.29 ± 1.53 | +1.62 ± 0.12 | +74.23 ± 1.39 | +2.33 ± 0.43 |
| EPDM 50 | +70.93 ± 4.31 | +4.92 ± 0.51 | +151.05 ± 8.51 | +7.68 ± 0.41 | +398.90 ± 24.25 | +8.39 ± 0.28 |
| Sample Code | 3 Days | 7 Days | 14 Days | |||
|---|---|---|---|---|---|---|
| Cross-Link Density | Cross-Link Density Modification | Cross-Link Density | Cross-Link Density Modification | Cross-Link Density | Cross-Link Density Modification | |
| Acetic acid concentration 10% | ||||||
| EPDM 0 | 0.1981 ± 0.0118 fA | +14.57 | 0.2099 ± 0.0089 eA | +21.40 | 0.1423 ± 0.0121 eAB | −17.70 |
| EPDM 10 | 2.1041 ± 0.0110 eA | +30.14 | 2.1410 ± 0.0351 dA | +32.42 | 1.5915 ± 0.0232 dAB | −1.56 |
| EPDM 20 | 3.8544 ± 0.1753 bA | +4.46 | 3.5258 ± 0.0731 aA | −4.45 | 3.3327 ± 0.1850 aA | −9.68 |
| EPDM 30 | 3.4299 ± 0.0564 cA | +18.48 | 3.2086 ± 0.1413 bA | +10.84 | 2.9252 ± 0.0826 bA | +1.05 |
| EPDM 40 | 2.7109 ± 0.1184 dA | +22.39 | 2.4496 ± 0.0368 cA | +10.60 | 2.3462 ± 0.0489 cA | +5.93 |
| EPDM 50 | 4.1766 ± 0.0597 aA | +90.07 | 3.6865 ± 0.0969 aA | +67.77 | 3.0833 ± 0.1045 abA | +40.32 |
| F value | 740.57522 | 803.01025 | 471.19999 | |||
| p value | <0.0001 | <0.0001 | <0.0001 | |||
| Acetic acid concentration 20% | ||||||
| EPDM 0 | 0.1915 ± 0.0052 cA | +10.76 | 0.1782 ± 0.0117 fB | +3.07 | 0.1405 ± 0.0040 dB | −18.74 |
| EPDM 10 | 2.1456 ± 0.0311 bA | +32.71 | 1.7813 ± 0.0261 eB | +10.17 | 1.4699 ± 0.0173 cB | −9.09 |
| EPDM 20 | 3.8243 ± 0.1425 aA | +3.64 | 3.3944 ± 0.1662 bA | −8.01 | 3.2202 ± 0.0973 aA | −12.73 |
| EPDM 30 | 3.4206 ± 0.0851 abA | +18.16 | 2.9947 ± 0.0844 cA | +3.45 | 2.8531 ± 0.0783 aA | −1.44 |
| EPDM 40 | 2.6775 ± 0.1141 abA | +20.89 | 2.3418 ± 0.0345 dB | +5.73 | 2.2555 ± 0.1510 bA | +1.83 |
| EPDM 50 | 4.0036 ± 1.3812 aA | +82.20 | 3.7101 ± 0.1539 aA | +68.84 | 2.9614 ± 0.2692 aA | +34.77 |
| F value | 18.62194 | 498.89353 | 220.45892 | |||
| p value | <0.0001 | <0.0001 | <0.0001 | |||
| Acetic acid concentration 30% | ||||||
| EPDM 0 | 0.1903 ± 0.0048 eA | +10.06 | 0.1798 ± 0.0081 fB | +3.99 | 0.1681 ± 0.0127 eA | −2.78 |
| EPDM 10 | 1.9235 ± 0.0214 dB | +18.97 | 1.7099 ± 0.0315 eB | +5.76 | 1.6414 ± 0.1148 dA | +1.52 |
| EPDM 20 | 3.8045 ± 0.2803 aA | +3.10 | 3.4708 ± 0.0775 bA | −5.94 | 3.2546 ± 0.1391 aA | −11.80 |
| EPDM 30 | 3.2787 ± 0.12006 bA | +13.26 | 2.9609 ± 0.0654 cA | +2.28 | 2.7508 ± 0.0409 bA | −4.98 |
| EPDM 40 | 2.6716 ± 0.1161 cA | +20.62 | 2.2151 ± 0.0538 dC | +0.01 | 2.0789 ± 0.1853 cA | −6.14 |
| EPDM 50 | 4.0434 ± 0.1647 aA | +84.01 | 3.7786 ± 0.1814 aA | +71.96 | 2.8274 ± 0.2206 bA | +28.67 |
| F value | 275.29075 | 671.96274 | 190.98597 | |||
| p value | <0.0001 | <0.0001 | <0.0001 | |||
| Sample Code | 3 Days | 7 Days | 14 Days | |||
|---|---|---|---|---|---|---|
| Young’s Modulus | Young’s Modulus Variation | Young’s Modulus | Young’s Modulus Variation | Young’s Modulus | Young’s Modulus Variation | |
| Acetic acid concentration 10% | ||||||
| EPDM 0 | 3.85 ± 0.75 | −23.46 | 3.43 ± 0.89 | −31.81 | 3.79 ± 0.59 | −24.73 |
| EPDM 10 | 4.22 ± 0.33 | +2.48 | 4.10 ± 0.68 | −0.34 | 3.08 ± 0.82 | −25.25 |
| EPDM 20 | 6.25 ± 1.30 | −7.00 | 6.45 ± 0.51 | −3.96 | 6.82 ± 1.64 | +1.55 |
| EPDM 30 | 6.63 ± 1.06 | −4.30 | 6.60 ± 0.87 | −4.71 | 6.72 ± 0.46 | −3.02 |
| EPDM 40 | 7.34 ± 0.94 | −9.74 | 5.81 ± 1.03 | −28.54 | 6.11 ± 0.92 | −24.90 |
| EPDM 50 | 8.98 ± 1.64 | −1.47 | 6.70 ± 0.72 | −26.52 | 4.71 ± 0.47 | −48.31 |
| Acetic acid concentration 20% | ||||||
| EPDM 0 | 4.20 ± 0.53 | −16.46 | 3.78 ± 0.48 | −24.93 | 3.79 ± 0.46 | −20.32 |
| EPDM 10 | 3.32 ± 0.22 | −19.28 | 3.55 ± 0.51 | −13.89 | 3.83 ± 0.36 | −13.61 |
| EPDM 20 | 5.99 ± 0.64 | −10.87 | 5.91 ± 0.36 | −11.97 | 6.47 ± 1.09 | +5.42 |
| EPDM 30 | 6.52 ± 1.05 | −5.78 | 5.81 ± 0.77 | −16.15 | 6.10 ± 0.41 | −0.95 |
| EPDM 40 | 6.41 ± 0.49 | −21.13 | 6.09 ± 0.95 | −25.07 | 6.47 ± 0.93 | −19.07 |
| EPDM 50 | 7.69 ± 1.22 | −15.64 | 6.13 ± 0.71 | −32.80 | 3.99 ± 0.82 | −39.27 |
| Acetic acid concentration 30% | ||||||
| EPDM 0 | 4.21 ± 0.73 | −16.34 | 4.01 ± 0.31 | −20.32 | 3.20 ± 0.25 | −36.46 |
| EPDM 10 | 4.23 ± 0.90 | +2.72 | 3.56 ± 0.30 | −13.61 | 4.26 ± 0.56 | +3.55 |
| EPDM 20 | 6.39 ± 1.00 | −4.91 | 7.08 ± 1.20 | +5.42 | 6.58 ± 0.61 | −2.00 |
| EPDM 30 | 6.28 ± 0.73 | −9.33 | 6.86 ± 0.37 | −0.95 | 5.92 ± 1.30 | −14.44 |
| EPDM 40 | 6.17 ± 0.76 | −24.08 | 6.58 ± 1.03 | −19.07 | 6.42 ± 0.27 | −21.08 |
| EPDM 50 | 7.84 ± 1.47 | −14.02 | 5.54 ± 1.49 | −39.27 | 3.74 ± 0.73 | −59.02 |
| Sample Code | 3 Days | 7 Days | 14 Days | |||
|---|---|---|---|---|---|---|
| Tensile Strength | Tensile Strength Variation | Tensile Strength | Tensile Strength Variation | Tensile Strength | Tensile Strength Variation | |
| Acetic acid concentration 10% | ||||||
| EPDM 0 | 10.21 ± 0.93 | +63.53 | 6.59 ± 1.07 | +5.57 | 6.98 ± 0.72 | +11.82 |
| EPDM 10 | 1.04 ± 0.06 | −70.73 | 1.00 ± 0.05 | −71.75 | 1.02 ± 0.04 | −71.11 |
| EPDM 20 | 2.79 ± 0.40 | −9.36 | 2.57 ± 0.13 | −16.50 | 2.81 ± 0.32 | −8.64 |
| EPDM 30 | 1.62 ± 0.16 | +0.75 | 1.55 ± 0.11 | −3.62 | 1.90 ± 0.07 | +18.45 |
| EPDM 40 | 1.21 ± 0.06 | −19.31 | 1.17 ± 0.07 | −21.97 | 1.17 ± 0.05 | −22.37 |
| EPDM 50 | 1.02 ± 0.07 | +0.63 | 0.91 ± 0.06 | −10.22 | 0.72 ± 0.05 | −28.92 |
| Acetic acid concentration 20% | ||||||
| EPDM 0 | 9.42 ± 0.59 | +50.88 | 6.52 ± 0.70 | +4.42 | 7.63 ± 0.43 | +22.13 |
| EPDM 10 | 1.07 ± 0.11 | −69.79 | 0.89 ± 0.07 | −74.87 | 1.03 ± 0.13 | −70.83 |
| EPDM 20 | 3.31 ± 0.27 | +7.54 | 2.38 ± 0.29 | −22.68 | 2.96 ± 0.52 | −3.83 |
| EPDM 30 | 1.86 ± 0.14 | +15.84 | 1.41 ± 0.13 | −11.97 | 1.97 ± 0.28 | +22.57 |
| EPDM 40 | 1.17 ± 0.02 | −22.10 | 1.12 ± 0.05 | −25.57 | 1.15 ± 0.03 | −23.30 |
| EPDM 50 | 0.93 ± 0.07 | −8.33 | 0.94 ± 0.03 | −7.21 | 0.89 ± 0.14 | −12.34 |
| Acetic acid concentration 30% | ||||||
| EPDM 0 | 9.28 ± 0.13 | +48.58 | 6.56 ± 0.85 | 5.03 | 6.09 ± 0.76 | −2.43 |
| EPDM 10 | 1.04 ± 0.06 | −70.56 | 1.13 ± 0.14 | −67.99 | 1.15 ± 0.16 | −67.46 |
| EPDM 20 | 2.80 ± 0.48 | −9.03 | 2.94 ± 0.25 | −4.65 | 3.10 ± 0.29 | +0.80 |
| EPDM 30 | 1.63 ± 0.25 | +1.37 | 1.74 ± 0.25 | +8.35 | 1.76 ± 0.15 | +9.85 |
| EPDM 40 | 1.14 ± 0.06 | −24.37 | 1.18 ± 0.08 | −21.70 | 1.12 ± 0.08 | −25.17 |
| EPDM 50 | 0.96 ± 0.12 | −5.67 | 0.93 ± 0.08 | −8.72 | 0.82 ± 0.05 | −19.37 |
| Sample Code | 3 Days | 7 Days | 14 Days | |||
|---|---|---|---|---|---|---|
| Specific Elongation | Specific Elongation Variation | Specific Elongation | Specific Elongation Variation | Specific Elongation | Specific Elongation Variation | |
| Acetic acid concentration 10% | ||||||
| EPDM 0 | 942 ± 29 | −3.48 | 846 ± 47 | −13.32 | 854 ± 61 | −12.50 |
| EPDM 10 | 445 ± 54 | −23.80 | 412 ± 64 | −29.45 | 468 ± 52 | −19.86 |
| EPDM 20 | 324 ± 55 | −1.82 | 304 ± 18 | −7.88 | 336 ± 21 | +1.82 |
| EPDM 30 | 230 ± 52 | −6.50 | 224 ± 24 | −8.94 | 308 ± 10 | +25.00 |
| EPDM 40 | 144 ± 25 | −51.68 | 144 ± 37 | −51.81 | 162 ± 47 | −45.64 |
| EPDM 50 | 61 ± 27 | −43.50 | 96 ± 19 | −11.84 | 110 ± 10 | +1.23 |
| Acetic acid concentration 20% | ||||||
| EPDM 0 | 950 ± 19 | −2.66 | 824 ± 51 | −15.57 | 908 ± 32 | −6.97 |
| EPDM 10 | 482 ± 50 | −17.47 | 423 ± 33 | −27.65 | 440 ± 47 | −24.66 |
| EPDM 20 | 400 ± 22 | +21.21 | 293 ± 51 | −11.36 | 348 ± 56 | +5.30 |
| EPDM 30 | 280 ± 32 | +13.82 | 178 ± 38 | −27.64 | 310 ± 48 | +26.02 |
| EPDM 40 | 184 ± 21 | −38.26 | 150 ± 36 | −49.66 | 160 ± 28 | −46.31 |
| EPDM 50 | 61 ± 22 | −43.68 | 104 ± 11 | −4.66 | 134 ± 53 | +23.31 |
| Acetic acid concentration 30% | ||||||
| EPDM 0 | 1006 ± 53 | +3.07 | 804 ± 59 | −17.62 | 626 ± 64 | −35.86 |
| EPDM 10 | 487 ± 67 | −16.67 | 510 ± 62 | −12.67 | 415 ± 38 | −28.94 |
| EPDM 20 | 353 ± 43 | +6.82 | 330 ± 35 | +0.00 | 328 ± 51 | −0.61 |
| EPDM 30 | 258 ± 65 | +4.88 | 272 ± 53 | +10.57 | 270 ± 26 | +9.76 |
| EPDM 40 | 146 ± 51 | −51.14 | 175 ± 60 | −41.14 | 158 ± 25 | −46.98 |
| EPDM 50 | 61 ± 22 | −44.33 | 117 ± 39 | +7.44 | 106 ± 8 | −2.22 |
| Wavelength Band (cm−1) | Assignment |
|---|---|
| 2953, 2918, 2849 | -C-H stretching vibration (2800–3000 cm−1) [86,87]. |
| 1539 | Aromatic skeletal vibrations caused by lignin and C=O stretch in non-conjugated ketones, carbonyls, and in ester groups [88]. |
| 1461 | -CH2 bending and rocking vibrations [86,87]. |
| 1398 | Stretching and bending vibrations of -CH2 bonds, associated with the amount of the crystalline structure of the cellulose [89,90]. |
| 1377 | -CH3 bending vibration [86,87]. |
| 1098 | -C-O stretching vibrations [91] |
| 953, 890 | -O-O stretching vibration [91] |
| 803 | -C-O bonds in cellulose [89,90] |
| 722 | -CH2 bending and rocking vibrations [86,87]. |
| Sample Code and Treatment Conditions | W1 (%) | T1 (°C) | W2 (%) | T2 (°C) | W3 (%) | T3 (°C) | TDSC (°C) |
|---|---|---|---|---|---|---|---|
| EPDM 0 EPDM 10 EPDM 20 EPDM 30 EPDM 40 EPDM 50 | - - - 6.6 5.0 6.9 | - - - 306 305 299 | - - 11.4 13.3 11.1 14.6 | - - 366 360 354 361 | 70.5 72.6 64.5 69.9 67.1 54.1 | 475 476 475 474 474 471 | 472 472 473 468 470 471 |
| EPDM 20: 10% AA, 3 days EPDM 20: 10% AA, 14 days EPDM 20: 30% AA, 3 days EPDM 20: 30% AA, 14 days | - - - - | - - - - | 11.8 14.9 14.5 14.3 | 361 358 359 365 | 63.8 62.2 61.5 62.7 | 476 503 477 477 | 472 472 472 474 |
| EPDM 30: 10% AA, 3 days EPDM 30: 10% AA, 14 days EPDM 30: 30% AA, 3 days EPDM 30: 30% AA, 14 days | - - - - | - - - - | 15.6 13.9 19.4 16.1 | 359 358 359 360 | 63.9 65.8 59.1 63.8 | 477 473 473 474 | 471 473 471 470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manaila, E.; Lungu, I.B.; Dumitru, M.; Manea, M.M.; Craciun, G. Ethylene Propylene Diene Monomer-Based Composites Resistant to the Corrosive Action of Acetic Acid. Materials 2025, 18, 4557. https://doi.org/10.3390/ma18194557
Manaila E, Lungu IB, Dumitru M, Manea MM, Craciun G. Ethylene Propylene Diene Monomer-Based Composites Resistant to the Corrosive Action of Acetic Acid. Materials. 2025; 18(19):4557. https://doi.org/10.3390/ma18194557
Chicago/Turabian StyleManaila, Elena, Ion Bogdan Lungu, Marius Dumitru, Maria Mihaela Manea, and Gabriela Craciun. 2025. "Ethylene Propylene Diene Monomer-Based Composites Resistant to the Corrosive Action of Acetic Acid" Materials 18, no. 19: 4557. https://doi.org/10.3390/ma18194557
APA StyleManaila, E., Lungu, I. B., Dumitru, M., Manea, M. M., & Craciun, G. (2025). Ethylene Propylene Diene Monomer-Based Composites Resistant to the Corrosive Action of Acetic Acid. Materials, 18(19), 4557. https://doi.org/10.3390/ma18194557







