Abstract
Modeling the structure not only of whole metal products, but also of the protective coatings with which they are coated, brings a number of economic benefits through more resistant coatings and coatings that can be produced by simplifying manufacturing technology or reducing material consumption in the process. This paper presents the results of a study of dip metallization in zinc baths with Ti additions. Both steel and cast iron substrates were coated and similar results were obtained. The obtained coatings were subjected to SEM analysis with chemical composition studies, TEM characterization with selected area electron diffraction (SAED), and corrosion studies. Particle models of the elementary phases present in the zinc coating made with CaRine 3.0 software were presented and used for phase analysis. It emerged that coatings obtained in zinc baths with the addition of Ti are characterized by a more varied microstructure, the occurrence of phase separations to which Ti segregates, and higher corrosion resistance than classical zinc coatings. The higher corrosion resistance is prompted not only by the Ti content in the intermetallic phases, but also by the observed nanostructure favorably located in the alloy layer.
1. Introduction
Current trends in technology development focus on optimizing and increasing the quality of manufactured components. In the case of steel and cast iron, which are characterized by widespread use in many industries, it is necessary to apply protective anti-corrosion coatings due to their high propensity to corrode [1]. Dip galvanizing is an excellent solution both economically and in terms of providing corrosion protection [2]. However, it is a protection that has a lifespan of several decades depending on the process variety used and the additives that enrich the liquid zinc used in the HDG process.
The corrosion protection of galvanized coatings is based on the Fe-Zn intermetallic phases formed in the coating. The alloy layer of the coating usually consists of sublayers of gamma, delta, and dzeta phases in various proportions. These phases differ in chemical composition and structure, and thus in their properties. The phase equilibrium system along with the phase designation is shown in Figure 1 [3,4]. A schematic of the coating structure is shown in Figure 2. Table 1 contains the characteristics of Fe-Zn intermetallic phases.
The shaping of zinc coatings, and consequently their properties, are affected by all the parameters of the process conducted. Researchers aim to optimize the process in all respects, such as by maximizing the resistance of coatings while reducing the cost of the process [5,6,7,8,9,10]. Numerical methods also find application in process optimization [11,12,13,14]. In order to reduce the thickness of the zinc coating, multi-component baths with synergistic effects are used [15,16,17,18].

Table 1.
Characterization of intermetallic phases in zinc coatings [6,7].
Table 1.
Characterization of intermetallic phases in zinc coatings [6,7].
| Phase | Chemical Formula | Crystal Lattice System | Number of Atoms in an Elementary Cell |
|---|---|---|---|
| αFe | Fe | BCC | -- |
| Γ | Fe3Zn10 | BCC | 52 |
| Γ1 | Fe5Zn21 | FCC | 408 |
| δ | FeZn10 | Hexagonal | 555 |
| ζ | FeZn13 | Single slope layout | 28 |
| η(Zn) | Zn | HCP | 6 |
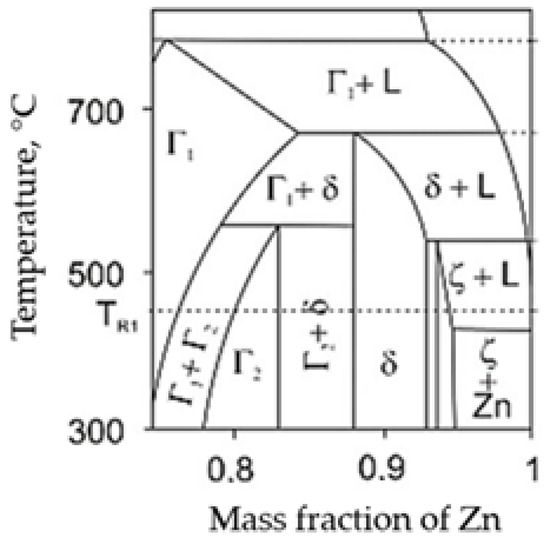
Figure 1.
Part of the Fe-Zn phase diagram [3,4] from the zinc-rich side.
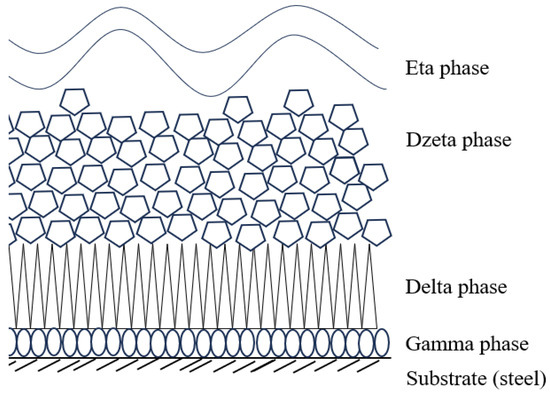
Figure 2.
Phase Fe-Zn arrangement in the coating [19].
In one study, the authors tested the addition of Ti to the zinc bath. The researchers noted the existence of new phases in such a variation of the process: Γ2-Fe2TiZn22 and TiZn15 [20]. By adding Ti to the process, the reactivity of the steel is lowered, resulting in thinner zinc coatings. However, the additional phases that are formed can deteriorate the coating’s appearance by increasing roughness. There are papers on coatings obtained by the HDG (Hot Dip Galvanizing) process with the addition of Ti [21,22,23,24,25], but none of them show the structure of the elementary cells of intermetallic phases by TEM.
The use of Ti additives in the zinc bath has also been aimed at limiting the growth of the zinc coating over time, so that zinc waste in the process is reduced, and the obtained coatings can be thinner. The problem of intensive zinc coating growth mainly affects alloys containing silicon and phosphorus [26,27]. Sandelin steels, where the Si content is in the <0.1% Si range, are particularly sensitive to these elements [28]. To reduce coating growth for steels in the Sandelin range, a solution was developed by adding Ni to the zinc bath. This addition has a negligible effect in forming coatings on high-silicon steels [29]. Zinc coatings with Ni obtained on Sandelin steel have a similar structure to the coating obtained on low-silicon steel [5]. An Ni content above 0.06% in the zinc bath significantly changes the equilibrium in the Fe-Zn-Ni system. The delta phase in such a variation of the process can crystallize directly from the liquid already at 450 °C [30]. In the structure of such a coating, a mixture region of δ and η phases can be distinguished. Separations of the ζ phase are formed as loose crystals at the boundary of the δ phase, without forming a continuous layer [31]. A zinc bath with Ti added, prepared for testing, can produce similar effects as the bath with Ni added, but limits the growth of the coating over the entire range of Si content. Analysis of the Fe-Zn-Ti and Fe-Zn-Ni triple systems, which show great similarity, leads to such conclusions. The systems are presented in Figure 3.
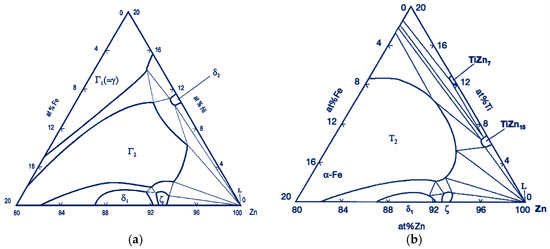
Figure 3.
Triple phase equilibrium system at 450 °C (a) Fe-Zn-Ni [32]; (b) Fe-Zn-Ti [33].
The purpose of this study was to thoroughly analyze the phases present in zinc coatings obtained in baths with varying Ti contents by transmission electron microscopy. The admixture in the tin bath can either create new phases in the coating or solutionally strengthen existing phases, depending on the amount. Usually these effects will exist simultaneously.
2. Materials and Methods
An experimental study of immersion metallization in a zinc bath with a titanium addition was carried out. The substrate subjected to coating was steel and cast iron of EN-GJS-500-7 grade with the chemical composition shown in Table 2. The substrate to be metallized was properly prepared by pretreatment of mechanical cleaning, followed by acid etching and fluxing in an aqueous ZnCl2 salt solution, which was dried in a stream of boiling air to get rid of moisture from the surface. A schematic of the surface preparation is shown in Figure 4.

Table 2.
Chemical characteristics of the substrate used in the experiment.
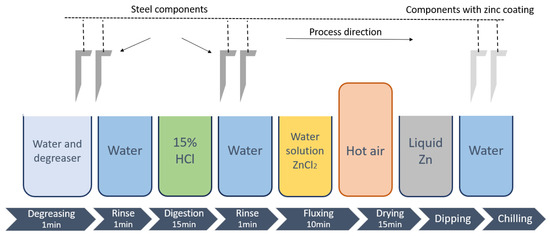
Figure 4.
Schematic diagram of the process with the sequence of surface preparation steps.
The experimental baths were prepared in a PTE-900 type furnace with a non-reactive ceramic crucible installed. The liquid bath zinc was obtained from pure ZnII and from recycled aircraft sheet metal. Three zinc baths were prepared with the compositions shown in Table 3. The bath without a Ti addition was the reference in the results of the obtained zinc coatings. While running the process, a similar technological problem was observed as observed for the Ni-containing baths.

Table 3.
Concentration of additive for zinc bath.
Microstructural characterization in bright-field (BF) mode was performed using a TECNAI G2 SuperTWIN FEG transmission electron microscope (TEM). Phase analysis was carried out using selected area electron diffraction patterns (SADP).
Corrosion tests were performed using cyclic voltammetry in a 3.5% aqueous NaCl solution. The cyclic voltammetry method involves polarizing the test sample linearly with a time-varying potential. At the same time, the current flowing through the circuit is recorded depending on the electrode potential against the reference electrode. During the test, the working electrode is immersed in the solution together with the reference electrode and connected by an electrolytic key. An important factor in this method is the temperature at which the test is conducted, as it affects the course of the electrode reactions. For this reason, the same thermostatic conditions were maintained for all samples. The chronoamperometric curve consists of a cathodic part, lying in the range of negative potentials, and an anodic part, lying in the range of positive potentials. The change in the direction of electrode polarization occurs after the cathodic or anodic peak current. The test carried out using this method allowed the number of current peaks indicating the number of stages of the process to be determined.
3. Results and Discussion
3.1. SEM Analysis of Obtained Zinc Coatings
In order to reveal the microstructure of the obtained zinc coatings, a metalographic scan was made on the cross-section of the galvanized samples. The samples were ground and then polished until a metallographic flash was obtained. The finished smears were etched with Villel’s reagent for 1 min to highlight the interfacial boundaries in the structure, then washed in ethanol and dried.
SEM microanalyses were performed using a JEOL 500LV microscope (JEOL Ltd., Akishima, Japan). This made it possible to capture the microstructures of the coatings obtained in the experiment more accurately. The morphology of the resulting phases was analyzed using SEM imaging, and the chemical composition was determined using an X-ray microanalysis attachment with an EDS detector. Results of the analysis are shown in Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10 and Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9.
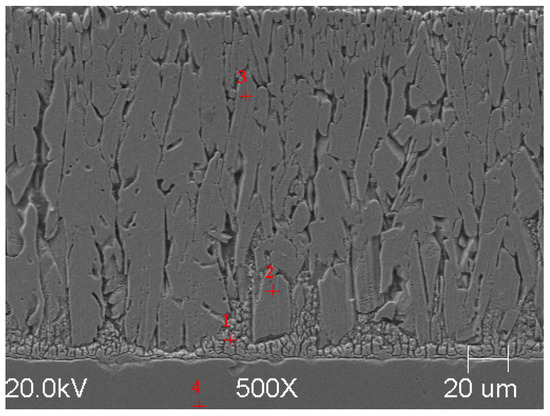
Figure 5.
Resulting coating microstructure after 180 s immersion in Bath 0 at 450 °C.
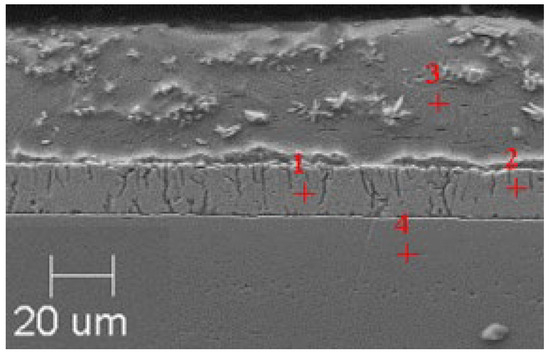
Figure 6.
Resulting coating microstructure after 180 s immersion in Bath 0 at 550 °C.
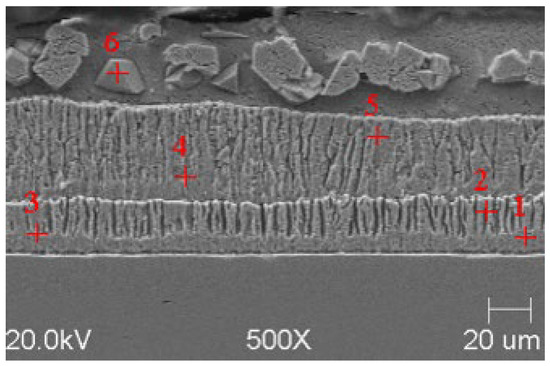
Figure 7.
Resulting coating microstructure after 180 s immersion in Bath B at 450 °C.
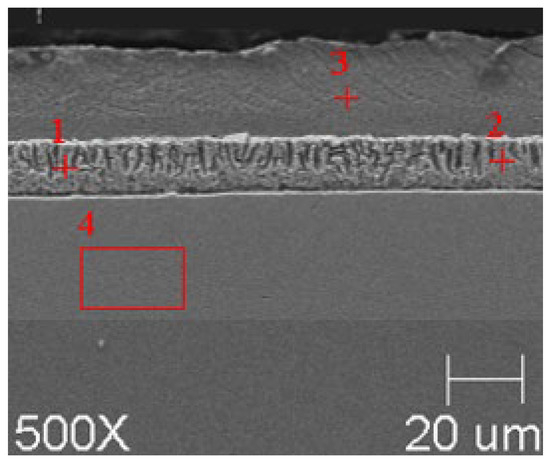
Figure 8.
Resulting coating microstructure after 180 s immersion in Bath B at 550 °C.
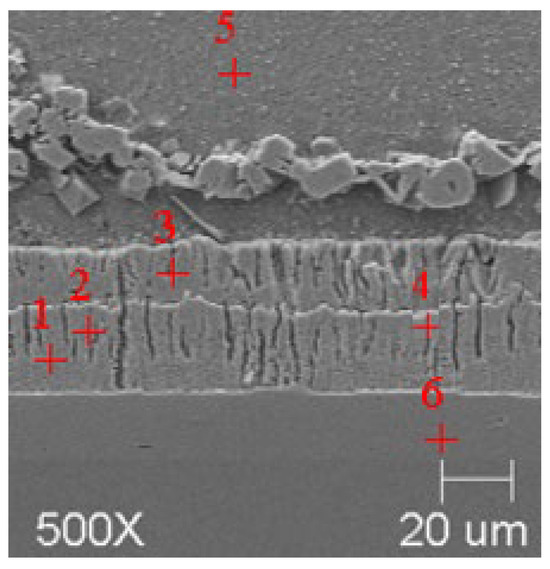
Figure 9.
Resulting coating microstructure after 180 s immersion in Bath C at 450 °C.
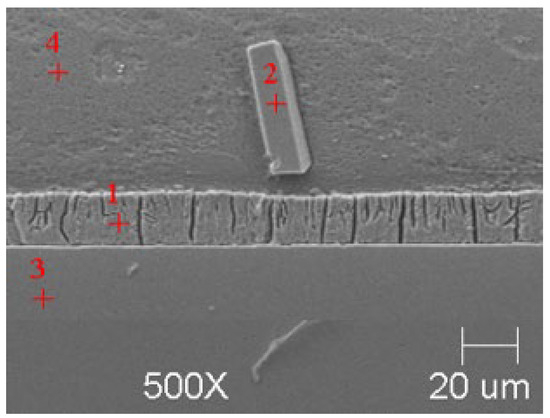
Figure 10.
Resulting coating microstructure after 180 s immersion in Bath C at 550 °C.

Table 4.
Summary of the chemical composition analysis for Bath 0 at 450 °C.

Table 5.
Summary of the chemical composition analysis for Bath 0 at 550 °C.

Table 6.
Summary of the chemical composition analysis for Bath B at 450 °C.

Table 7.
Summary of the chemical composition analysis for Bath B at 550 °C.

Table 8.
Summary of the chemical composition analysis for Bath C at 450 °C.

Table 9.
Summary of the chemical composition analysis for Bath C at 550 °C.
Applying the same process variation with the addition of Ti to cast iron yields similar structural effects in the coating (Figure 11). This is all the more interesting because cast iron is usually characterized by a greater coating thickness due to the composition of the substrate. In this study, a similar coating structure was obtained for both steel and cast iron. A temperature of 425 °C was used for cast iron due to experiments conducted as part of another series of studies. The photos are presented to show the versatility of the process for substrates made of various Fe-C alloys.
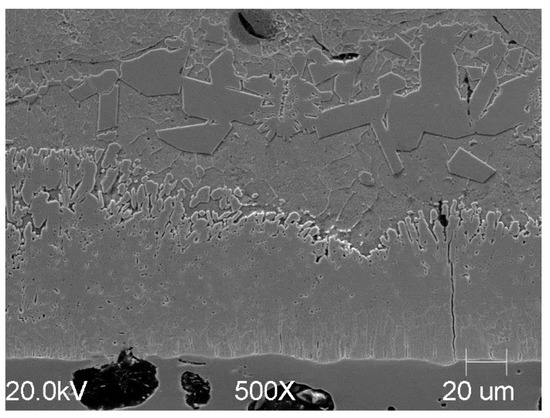
Figure 11.
Microstructural view of the coating formed on a cast iron substrate in Bath B at 425 °C after around 300 s of immersion.
The results obtained from the microstructure images of zinc coatings demonstrate the importance of both the process temperature and the chemical composition of the zinc bath. A varied microstructure of coatings will also be characterized by varied anti-corrosive and mechanical properties.
Changing the chemical composition of a zinc bath by introducing a Ti additive leads to a change in the composition and structure of the coating obtained in such a bath by dip metallization. The coatings obtained in the Ti-enriched bath are characterized by separations of the phase into which Ti segregates, as confirmed by EDS analysis.
The chemical compositions of the various intermetallic phases in the coatings were similar. An enrichment of the layers with the addition of Ti was observed. No anomalies were observed in the proportion of Fe and Zn on the cross-section of the coating. The closer to the substrate, the higher the Fe content of the layer.
3.2. Preparation Samples of Zinc Coatings for TEM Analysis
The use of a Tecnai G2 F20 transmission electron microscope enabled the identification, with high probability, of the phases present in the material. This analysis requires the preparation of sufficiently thin samples, the performance of which in the case of heterogeneous materials poses many difficulties. The thin foils for testing were prepared using the focused ion beam (FIB) technique on a Thermo Fisher Scientific SCIOS 2 Dual-Beam microscope due to its high accuracy and precision in cutting. Figure 12 shows the locations from which thin foils were collected for TEM observation, marked with red rectangles.
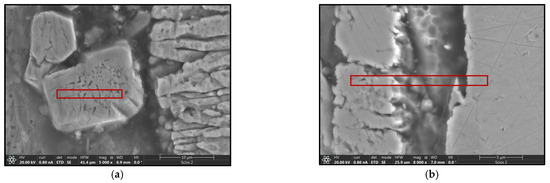
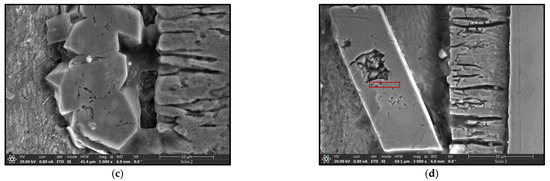
Figure 12.
SEM images of coatings with marked areas for TEM analysis. (a) B450; (b) B550; (c) C450; (d) C550.
The thin foils taken for testing were 100 nm thick and approximately 10 μm × 10 μm in size. The finished preparations are shown in Figure 13. Due to the different phase composition within the sampled films, the obtained preparations were cracked.
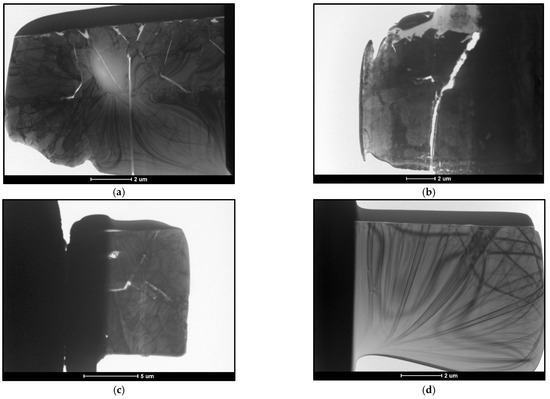
Figure 13.
Thin foils prepared for testing TEM. (a) B450; (b) B550; (c) C450; (d) C550.
The samples prepared in this way were subjected to TEM studies structure characterization. The result of this action can be obtained by SADP. The image of the diffraction grating was analyzed, the parameters of the lattice were determined, and the particle with the best representation was matched. The particle models of the elementary phases present in the zinc coating are shown in Figure 14.

Figure 14.
Phase particle model: (a) gamma; (b) delta; (c) dzeta acquired with CaRine 3.0 software.
Using CaRine 3.0 software, it is possible to construct models of phase elementary particles and determine the complexity of their structure. The largest difference in the number of atoms of an elementary cell is shown by the delta phase, where there can be even more than 500 atoms. Such large elementary particles are hard to identify unambiguously even by TEM.
3.3. Phase Analysis Using SADP Obtained from Prepared Samples
In the illustrations of the elementary particle models of the various phases occurring in the zinc coating, one can see a significant difference between the levels of complexity of the particle structure. The crystal structure of the elementary cell, the number of atoms, and the lattice parameters have a key effect on the properties of the phases present.
The particle models should be confronted with the diffraction grids of the tested materials to determine the fit of the results to the models. The microstructure characterization of zinc coatings along with the phase analysis using SADP are presented in Figure 15 and Figure 16.
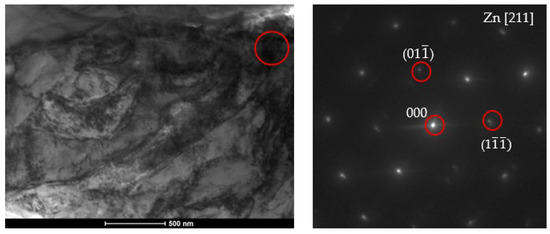
Figure 15.
Bright field (BF) TEM image with phase analysis using SADP from region marked from the B450 sample at the site of the η (Zn) phase.
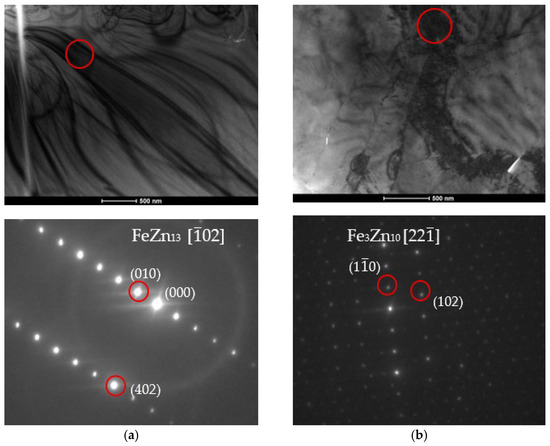
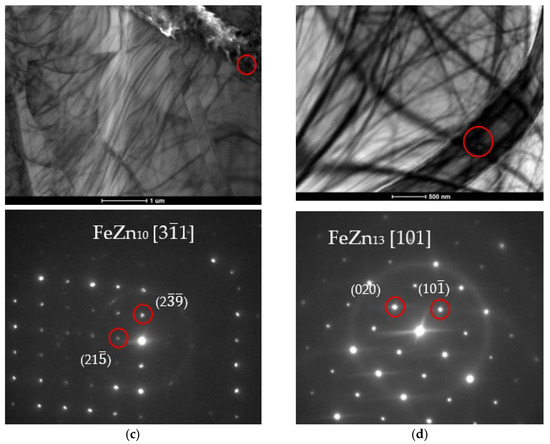
Figure 16.
Bright field (BF) TEM images with phase analysis performed using SADP confirming the presence (a) FeZn13; (b) Fe3Zn10; (c) FeZn10; (d) FeZn13 phases.
The crystal lattice parameters can be read from the photographs. The axis value is greater than three, which should disqualify the measurements. However, it is worth noting that these coefficients assume that only Zn and Fe atoms are present in the particle. In the case of the tested coatings, Ti atoms must be taken into account, as they deform and push apart the crystal lattice, as evidenced by measurements of parameters slightly above the norm.
TEM analysis allows for highly accurate determination of the type of phase being examined, along with its crystal lattice parameters. As the size of the unit cell increases, accurate analysis becomes more difficult. Intermetallic phases occurring in zinc coatings have varied chemical compositions and unit cell structures. For example, the cell of a pure zinc layer is relatively simple compared to the δ phase cell, which can have over 500 atoms in the unit cell. An additional complication in the case of the tested coatings was the addition of Ti to the zinc bath. Ti showed segregation into precipitates resembling the ζ phase in terms of structure and morphology. This phase can be considered Ti-enriched. The Ti content in these precipitates will affect both the mechanical properties of the coating and its corrosion resistance.
The authors [33] observed similar results for the tested coatings obtained by continuous galvanizing in zinc baths without the addition of Ti. The ζ and δ phases were observed and identified, while no Γ2 phase precipitates were observed. Regardless of the method used to obtain the zinc coating, the same intermetallic phases are observed, which are defined in the Fe-Zn phase equilibrium diagram. When alloying additives are used in the zinc bath, the morphology of the phases may differ, as may their proportions in the alloy layer. However, these are always the same Fe-Zn intermetallic phases [33].
The researchers conducted an experiment in which the intermetallic phases Γ1 and Γ2 were additionally obtained. The diffraction results for these phases are presented. The structure of all phases in the study revealed various types of lattice defects, which appear, among other things, as a result of non-equilibrium process conditions [34].
3.4. Observed Nanostructure
Nanostructural separations are of particular importance for both the mechanical and corrosion resistance of the materials obtained. The nanostructure observed in the zinc coatings studied was located between the separations of the phase to which Ti segregated. This barrier (separations between phases containing titanium + nanostructure between them) ensures increased corrosion protection in the obtained coating, as confirmed by the corrosion tests described below. The nanostructure is shown in Figure 17.
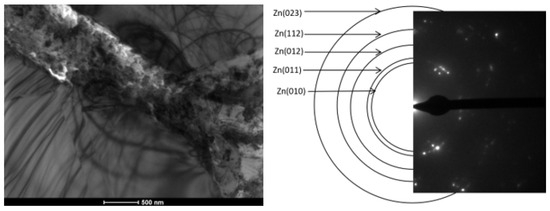
Figure 17.
Bright field (BF) TEM image with phase analysis carried out using SADP from the place marked in BF TEM.
The Zn nanostructure is likely to form during rapid cooling of the coatings after metallization as a result of rapid atomic segregation. This is evidenced by the location of this structure between the Ti-enriched ζ phase precipitates.
3.5. Corrosion Tests of Coatings with Ti Additives
The corrosion tests carried out confirmed the increased resistance of coatings with Ti additives compared to reference samples obtained in baths without Ti additives. The results of cyclic voltammetry are shown in Table 10 and Figure 18. Based on the results of the peak currents, it was possible to calculate the corrosion rate according to the following formula:
Explanation: k—const. 3.27 × 10−3, [(mm × g)/(μA × cm × y)]
EW—molar mass/2, g/mol
—density, g/cm3
icorr—peak current, [μA/cm2]

Table 10.
Results of corrosion tests.
Table 10.
Results of corrosion tests.
| 450 °C | 550 °C | ||||||
|---|---|---|---|---|---|---|---|
| %Ti | Ecorr | icorr | CR | Ecorr | icorr | CR | |
| 0 | 0 | −1.373 | 0.013 | 1.95 × 10−4 | −1.382 | 0.004 | 5.99 × 10−5 |
| B | 0.05 | −1.3749 | 0.005 | 7.49 × 10−5 | −1.367 | 0.002 | 2.99 × 10−5 |
| C | 0.1 | −1.389 | 0.008 | 1.20 × 10−4 | −1.36 | 0.002 | 2.99 × 10−5 |
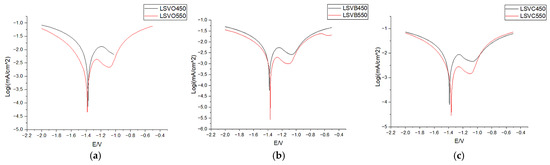
Figure 18.
Chronovoltamperometric curves obtained in the study depending on chemical composition: (a) bath without additives; (b) B—0.05% Ti; (c) C—0.1% Ti.
The high-temperature HDG process produced coatings with greater corrosion resistance than those conducted at 450 °C. Greater corrosion resistance is associated with longer durability of steel protection by zinc coating. Increasing this resistance is an important factor in guiding the development of the technologies being designed. Such effects are achieved through process modifications (using different temperatures and reagents for preparation–treatment) as well as by adding additives to the zinc bath. In the presented studies, the additive was Ti, and it can be clearly concluded that it improves the corrosion resistance of zinc coatings. However, the concentration of Ti in the bath must be controlled, as too high a concentration leads to the formation of coatings with an unattractive appearance.
4. Conclusions
The TEM method makes it possible to determine the arrangement of the crystal lattice of the various precipitates present in the zinc coating. Through TEM analysis, it was possible to identify the nanostructure present in the zinc coating.
The corrosion rate of a Ti-enriched coating can be up to an order of magnitude lower compared to a pure zinc coating. This indicates a much higher corrosion resistance. Such an effect is achieved both by strengthening Fe-Zn intermetallic phases with titanium and by the presence of nanostructures.
The coating obtained by the described process is formed in a similar way on both iron and steel. However, for cast iron, this solution may be crucial in limiting the growth of the zinc coating on this type of substrate.
Author Contributions
Conceptualization, K.B.-K. and D.K.; methodology, A.B., M.J.-S. and D.K.; formal analysis, K.B.-K. and M.J.-S.; investigation, K.B.-K.; resources, K.B.-K.; writing—original draft preparation, K.B.-K.; writing—review and editing, A.B., M.J.-S. and D.K.; supervision, A.B. and D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by AGH and The APC was funded by AGH.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article material. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research was conducted as part of subvention from the AGH University of Krakow in order to maintain research potential.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yu, Z.; Hu, J.; Meng, H. A Review of Recent Developments in Coating Systems for Hot-Dip Galvanized Steel. Front. Mater. 2020, 7, 74. [Google Scholar] [CrossRef]
- Kopyciński, D.; Guzik, E. Nowe możliwości kształtowania budowy powłoki ochronnej w procesie cynkowania zanurzeniowego. Ochr. Przed Korozją 2007, 11, 426–430. [Google Scholar]
- Hong, M.; Saka, H. Transmission electron microscopy of the iron-zinc δ1 intermetallic phase. Scr. Mater. 1997, 36, 1423–1429. [Google Scholar] [CrossRef]
- Hong, M.H.; Saka, H. Plasticity and grain boundary structure of δ1k and δ1p intermetallic phases in the FeZn system. Acta Mater. 1997, 45, 4225–4230. [Google Scholar] [CrossRef]
- Kania, H.; Mendala, J.; Kozuba, J.; Saternus, M. Development of Bath Chemical Composition for Batch Hot-Dip Galvanizing—A Review. Materials 2020, 13, 4168. [Google Scholar] [CrossRef]
- Henryk, K.; Mariola, S. Benefits and Limitations of the Use of Pb, Sn and Bi Alloying Elements in Hot Dip Galvanizing Bath: A Review. J. Mater. Eng. Perform. 2023, 32, 5680–5688. [Google Scholar] [CrossRef]
- Verma, A.R.B.; van Ooij, W.J. High-temperature batch hot-dip galvanizing. Part 2. Comparison of coatings formed in the temperature range 520–555 °C. Surf. Coatings Technol. 1997, 89, 143–150. [Google Scholar] [CrossRef]
- Strutzenberger, J.; Faderl, J. Solidification and Spangle Formation of Hot-Dip-Galvanized Zinc Coatings. Met. Mater. Trans. A 1998, 29, 631–646. [Google Scholar] [CrossRef]
- Peng, S.; Xie, S.K.; Lu, J.T.; Zhang, L.C. Surface characteristics and corrosion resistance of spangle on hot-dip galvanized coating. J. Alloys Compd. 2017, 728, 1002–1008. [Google Scholar] [CrossRef]
- Cai, X.F.; Huang, Y.Z.; Li, Y.G.; Zhao, L.N. Production Process and Technology Development of Hot-Dip Galvanizing. Appl. Mech. Mater. 2014, 488–489, 61–65. [Google Scholar] [CrossRef]
- Vantadori, S.; Zanichelli, A.; Ronchei, C.; Scorza, D.; Di Cocco, V.; Iacoviello, F. Numerical Simulation of Traditional and Technological Zinc-Based Coatings: Part I. Adv. Eng. Mater. 2022, 24, 2101619. [Google Scholar] [CrossRef]
- Wołczyński, W.; Pogoda, Z.; Garzeł, G.; Kucharska, B.; Sypień, A.; Okane, T. Part I. Thermodynamic and kinetic aspects of the hot DIP (Zn)-Coating formation. Arch. Metall. Mater. 2014, 59, 1223–1233. [Google Scholar] [CrossRef]
- Wołczyński, W.; Pogoda, Z.; Garzeł, G.; Kucharska, B.; Sypień, A.; Okane, T. Part II. Model for the protective coating formation during hot dip galvanizing. Arch. Metall. Mater. 2014, 59, 1394–1404. [Google Scholar] [CrossRef]
- Wołczyński, W.; Kucharska, B.; Garzeł, G.; Sypień, A.; Pogoda, Z.; Okane, T. Part III. Kinetics of the (Zn)-Coating deposition during stable and meta-stable solidifications. Arch. Metall. Mater. 2015, 60, 199–207. [Google Scholar] [CrossRef]
- Hu, D.; Song, S.; Zhang, Z.; Wang, L. Experimental study on corrosion resistance of Zn–Al–Mg alloy coating of high-strength steel wires for bridge cables. Anti-Corros. Methods Mater. 2023, 70, 459–468. [Google Scholar] [CrossRef]
- García, F.; Salinas, A.; Nava, E. The role of Si and Ti additions on the formation of the alloy layer at the interface of hot-dip Al–Zn coatings on steel strips. Mater. Lett. 2006, 60, 775–778. [Google Scholar] [CrossRef]
- Kania, H.; Liberski, P. Synergistic influence of Al, Ni, Bi and Sn addition to a zinc bath upon growth kinetics and the structure of coatings. IOP Conf. Ser. Mater. Sci. Eng. 2012, 35, 012004. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Tu, H.; Wu, C.; Su, X.; Wang, J. Effect of Ti on the growth of the Fe–Al layer in a hot dipped Zn–6Al–3Mg coating. Surf. Coat. Technol. 2015, 275, 90–97. [Google Scholar] [CrossRef]
- Shibli, S.M.A.; Meena, B.N.; Remya, R. A review on recent approaches in the field of hot dip zinc galvanizing process. Surf. Coat. Technol. 2015, 262, 210–215. [Google Scholar] [CrossRef]
- Gloriant, T.; Reumont, G.; Perrot, P. The Fe±Zn±Ti System at 450 °C. Int. J. Mater. Res. 1997, 88, 539–544. [Google Scholar]
- Takáts, V.; Hakl, J.; Csik, A.; Bereczki, H.F.; Lévai, G.; Godzsák, M.; Török, T.I.; Kaptay, G.; Vad, K. Ti oxidation states in Zn(Ti) coating of hot-dip galvanized steels. Surf. Coat. Technol. 2017, 326, 121–125. [Google Scholar] [CrossRef]
- Bellini, C.; Di Cocco, V.; Iacoviello, F.; Mocanu, L.P. Impact of Copper, Tin and Titanium Addition on Bending-Induced Damage of Intermetallic Phases in Hot Dip Galvanizing. Metals 2022, 12, 2035. [Google Scholar] [CrossRef]
- Bellini, C.; Carlino, F.; Natali, S. Analysis of the Al and Ti additions influences on phases generation and damage in a hot dip galvanizing process. Procedia Struct. Integr. 2019, 18, 688–693. [Google Scholar] [CrossRef]
- Natali, S.; Volpe, V.; Zortea, L.; Burattini, C.; Di Cocco, V.; Iacoviello, F. Mechanical and Structural Characterization of Zn-Ti Colored Coatings. Procedia Eng. 2015, 109, 105–112. [Google Scholar] [CrossRef]
- Fortese, G.; Carpinteri, A.; Di Cocco, V.; Iacoviello, F.; Natali, S.; Ronchei, C.; Scorza, D.; Vantadori, S. Improved Zn-based coatings for ipersandelin steel products. Procedia Struct. Integr. 2016, 2, 2263–2268. [Google Scholar] [CrossRef]
- Liberski, P. Antykorozyjne Powłoki Zanurzeniowe; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2013. [Google Scholar]
- Maaß, P.; Peißker, P. Cynkowanie Ogniowe; Agencja Wydawnicza Placet: Warszawa, Poland, 1998. [Google Scholar]
- Tatarek, A.; Liberski, P.; Kania, H.; Podolski, P. Mechanizm tworzenia zanurzeniowej powłoki cynkowej na stopach żelaza zawierających krzem. Inżynieria Mater. 2008, 29, 788–791. [Google Scholar]
- Verma, N.; Sharma, V.; Badar, M.A.; Choubey, N.; Parihar, R.S. Optimization of Zinc Coating Thickness by Unreplicated Factorial Design of Experiments in Hot-Dip Galvanization Process. Int. J. Precis. Eng. Manuf. 2022, 23, 1173–1182. [Google Scholar] [CrossRef]
- Kania, H.; Liberski, P.; Podolski, P.; Mendala, J. Struktura powłok otrzymanych w kąpieli cynkowej zawierającej nikiel na stalach z krzemem. Inżynieria Mater. 2005, 26, 763–765. [Google Scholar]
- Chen, Z.W.; See, J.B. Dross Phases Formed in Galvanizing Baths Containing (0–0.1) wt% Nickel at 450 °C. ISIJ Int. 1993, 33, 307–312. [Google Scholar] [CrossRef]
- Reumont, G.; Perrot, P.; Foct, J. Thermodynamic study of the galvanizing process in a Zn-0.1%Ni bath. J. Mater. Sci. 1998, 33, 4759–4768. [Google Scholar] [CrossRef]
- Kao, F.H.; Li, W.C.; Chen, C.Y.; Huang, C.Y.; Yang, J.R.; Wang, S.H. Cross-sectional observation of the intermetallic phase in a galvannealed steel. Mater. Sci. Eng. A 2009, 499, 45–48. [Google Scholar] [CrossRef]
- Hong, M.H.; Kato, T.; Saka, H. Identification of the Fe-Zn Intermetallic Phases by TEM. Tetsu-to-hagané 1997, 83, 311–316. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).