Investigation of Stress Distribution and Fatigue Performance in Restored Teeth Using Different Thicknesses of Adhesive Materials and Different Restorative Materials: 3D Finite Element Analysis (FEM)
Highlights
- Low-modulus materials increased stress and fractures in enamel and dentin.
- High-modulus materials failed earlier due to higher internal stress transfer.
- Thicker adhesive layers reduced stress within the adhesive interface.
- Bulk-fill composites delayed restoration fracture but stressed dental tissues. 5. Hybrid composites fractured earliest among all tested restorative materials.
Abstract
1. Background
2. Methods
3. Results
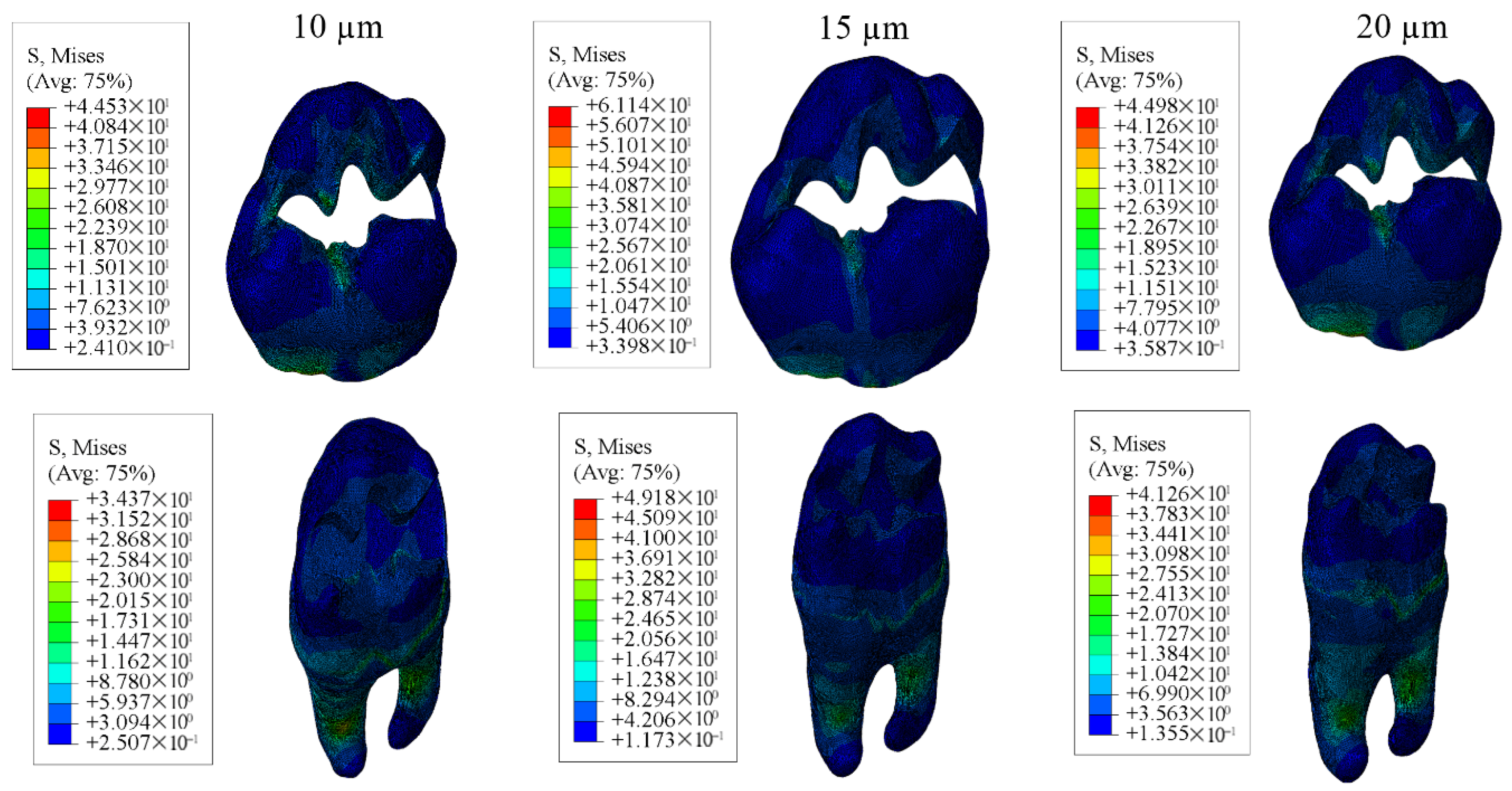
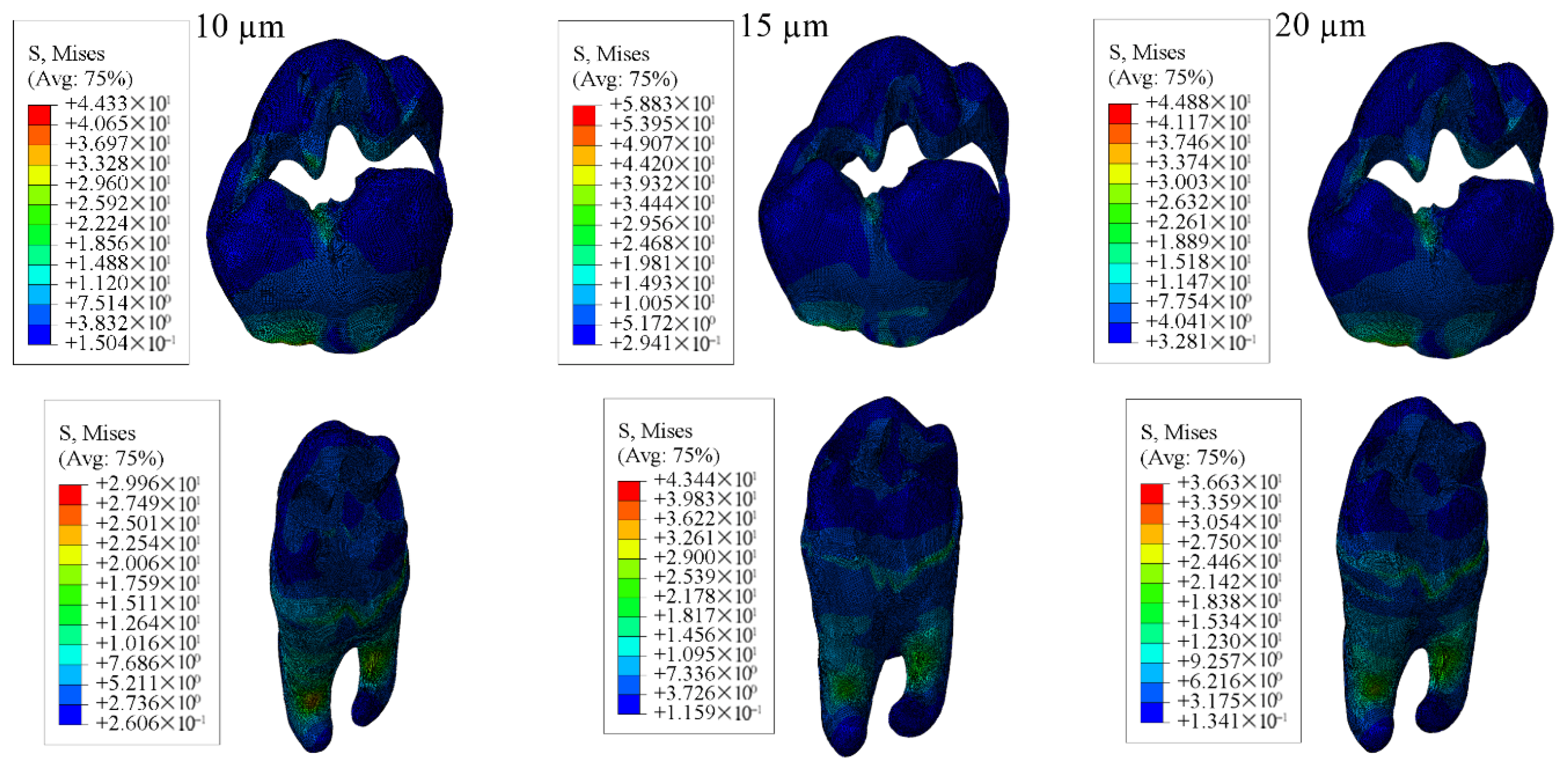
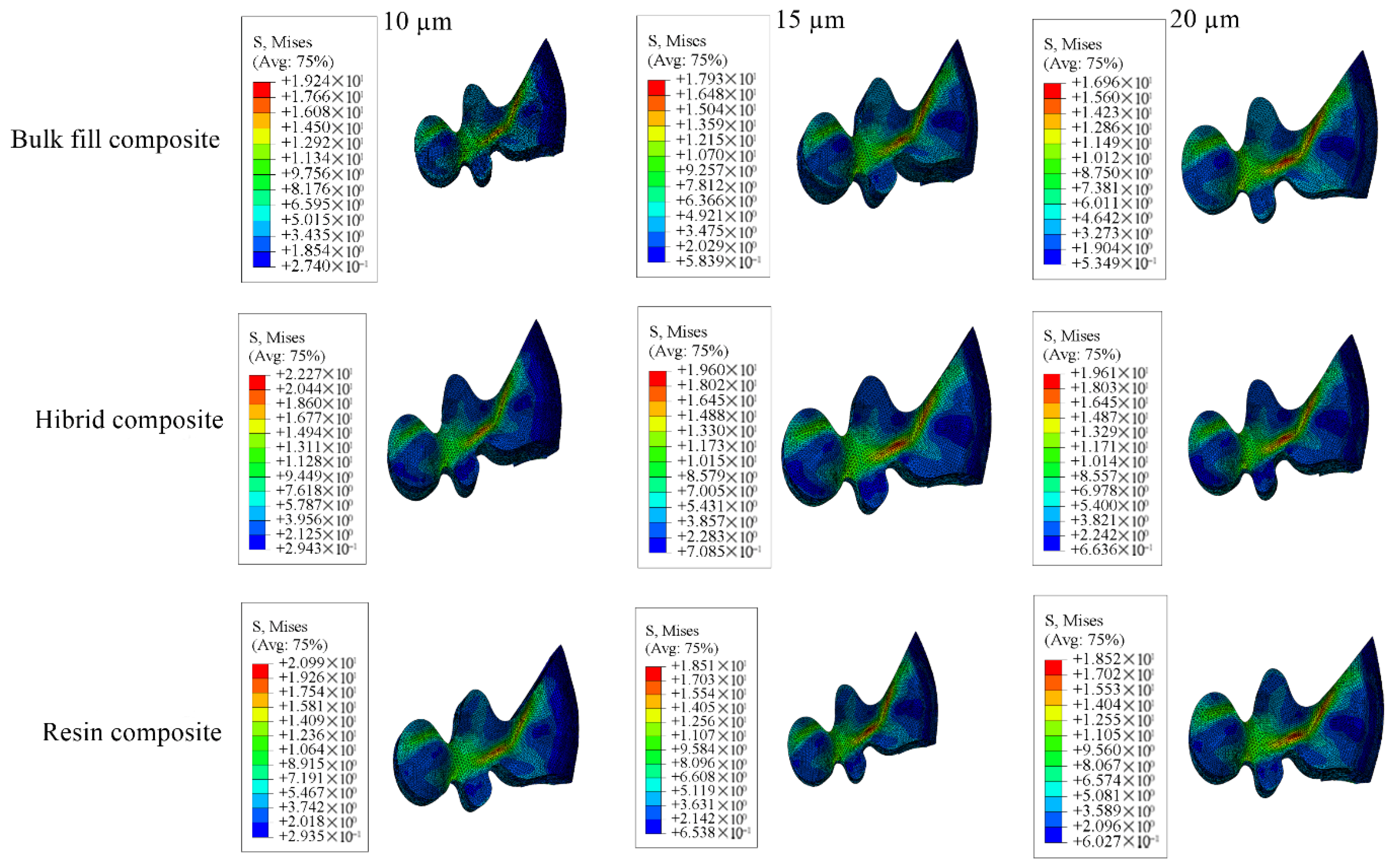
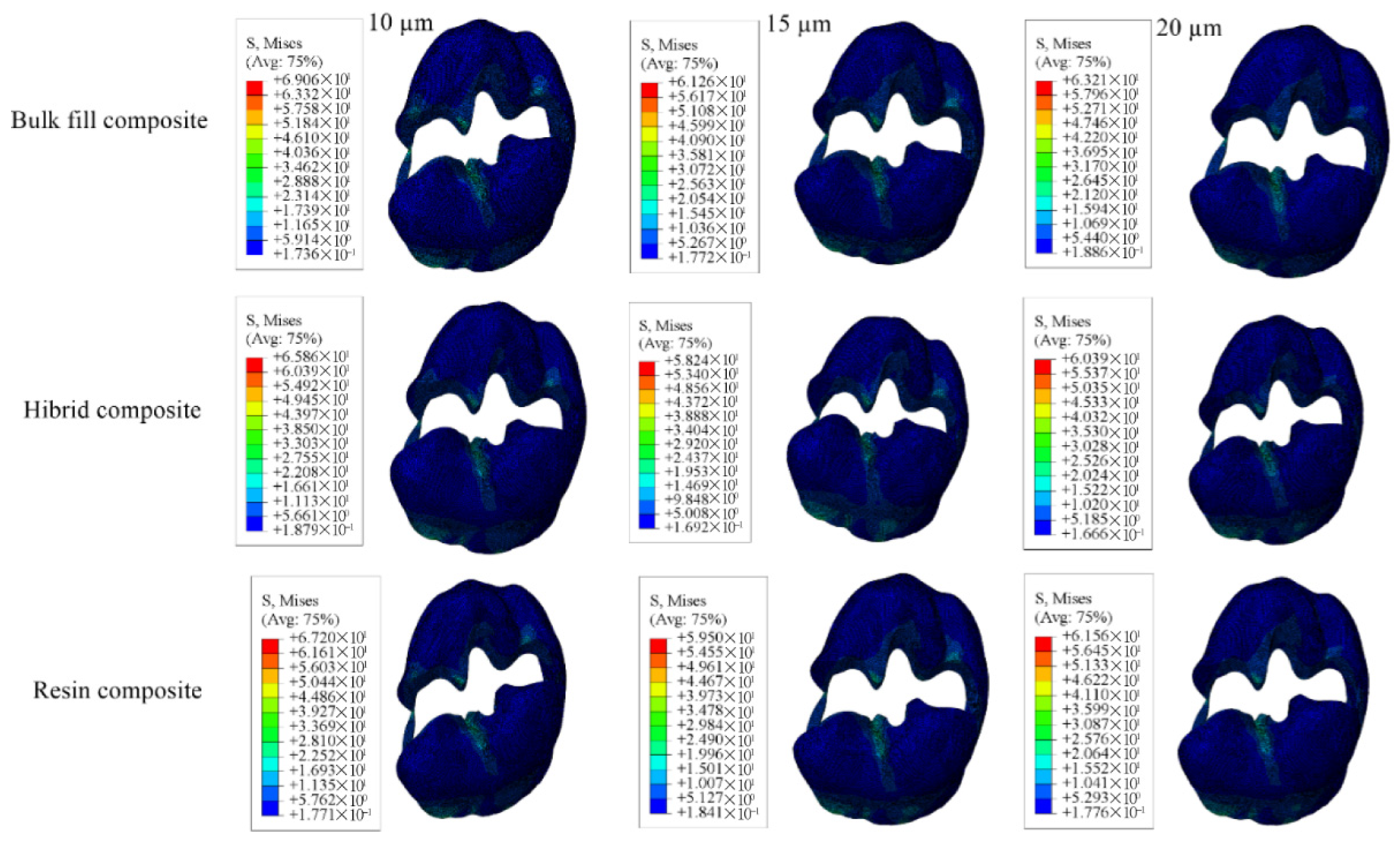
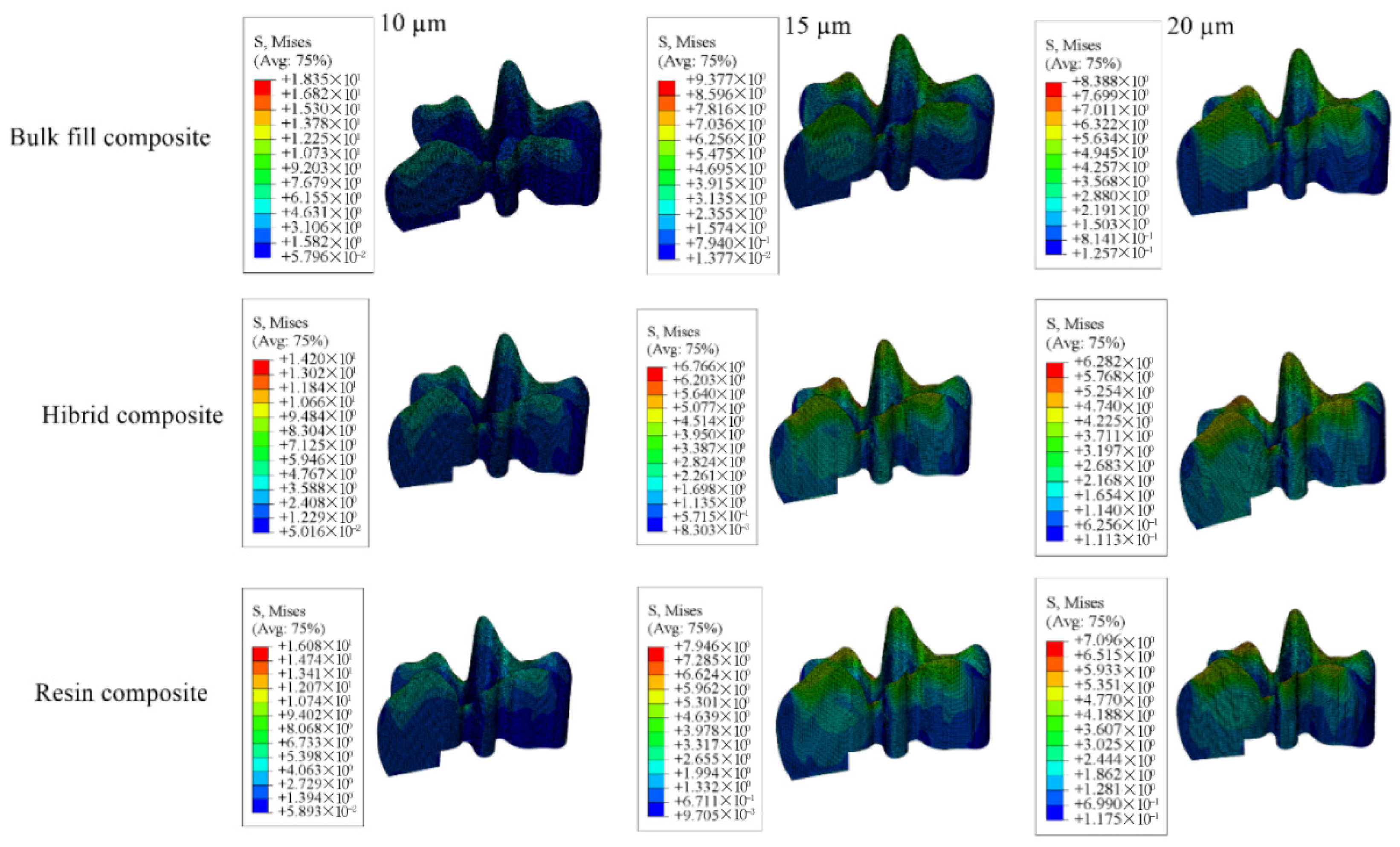
3.1. Results on Stress Distribution
3.1.1. Results of the Model with Class II Disto-Occlusal Cavity
3.1.2. Results of the Model with Class II Mesio-Occlusal Cavity
3.1.3. Results of the Model with Class II Mesio-Occluso-Distal (MOD) Cavity
3.1.4. Results for the Model with Class I Occlusal Cavity
3.2. Results of Fracture Lifespan
3.2.1. Results of the Model with Class II Disto-Occlusal Cavity
3.2.2. Results of the Model with Class II Mesio-Occlusal Cavity
3.2.3. Results of the Model with Class II MOD Cavity
3.2.4. Results of the Model with Class I Occlusal Cavity
4. Discussion
5. Conclusions
- The use of materials with a low Young’s modulus—indicating greater flexibility—resulted in significantly higher stress accumulation in the enamel and dentin, which, in turn, led to earlier fracture of these tissues. Conversely, stiffer materials with a higher Young’s modulus generated lower stress values in these regions. Thus, the stress in enamel and dentin was inversely related to the elastic modulus of the restorative material.
- Restorative materials with a higher Young’s modulus exhibited earlier structural failure compared to those with lower stiffness, indicating a trade-off between rigidity and internal stress tolerance.
- Increasing the thickness of the adhesive layer led to a reduction in stress accumulation within the adhesive itself, suggesting that a thicker adhesive layer may provide a protective buffering effect against stress concentration.
Clinical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DICOM | Digital Imaging and Communications in Medicine |
| STL | Standard Tessellation Language |
| FEA | Finite Element Analysis |
| CBCT | Cone-Beam Computed Tomography |
| Gpa | Gigapascal |
| Mpa | Megapascal |
References
- Pitts, N.B. Are We Ready to Move from Operative to Non-Operative/Preventive Treatment of Dental Caries in Clinical Practice? Caries Res. 2004, 38, 294–304. [Google Scholar] [CrossRef]
- Featherstone, J.D.B. The Science and Practice of Caries Prevention. J. Am. Dent. Assoc. 2000, 131, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, R.V.; Axmann, D.; Geis-Gerstorfer, J. Dynamic visco-elastic properties of dental composite resins. Dent. Mater. 2006, 22, 258–267. [Google Scholar] [CrossRef]
- Yamanel, K.; Caglar, A.; Gülsahi, K.; Ozden, U.A. Effects of different ceramic and composite materials on stress distribution in inlay and onlay cavities: 3-D finite element analysis. Dent. Mater. J. 2009, 28, 661–670. [Google Scholar] [CrossRef]
- Kaya, İ.; Erol, A.; Çobanoğlu, N. Evaluation of the Effect of Background Color on the Color Change of Composite Resins of Different Translucencies. Necmettin Erbakan Üniversitesi Diş Hekim. Derg. 2024, 88–96. [Google Scholar] [CrossRef]
- Gönder, H.Y.; Öz, C. Bulk-fill kompozit rezinler. Necmettin Erbakan Üniversitesi Diş Hekim. Derg. 2020, 2, 117–123. [Google Scholar] [CrossRef]
- Pineda-Vélez, E.; Yadalam, P.K.; Ardila, C.M. Efficacy of the finite element analysis in assessing the effects of light curing on the mechanical properties of direct restorative composites: A systematic review. J. Clin. Exp. Dent. 2024, 16, e1411–e1421. [Google Scholar] [CrossRef] [PubMed]
- Seydibeyoğlu, M.Ö.; Dogru, A.; Wang, J.; Rencheck, M.; Han, Y.; Wang, L.; Seydibeyoğlu, E.A.; Zhao, X.; Ong, K.; Shatkin, J.A.; et al. Review on Hybrid Reinforced Polymer Matrix Composites with Nanocellulose, Nanomaterials, and Other Fibers. Polymers 2023, 15, 984. [Google Scholar] [CrossRef]
- Özmen, B.; Mutlu, B.; Elgörmüş, A.M. Evaluation of the Effect of Different Bonding Systems and Restorative Materials on Shear Bond Strength in the Repair of High-Viscosity Glass Ionomer Cement. Necmettin Erbakan Üniversitesi Diş Hekim. Derg. 2024, 6, 13–21. [Google Scholar] [CrossRef]
- Liu, Y.; Tjäderhane, L.; Breschi, L.; Mazzoni, A.; Li, N.; Mao, J.; Pashley, D.H.; Tay, F.R. Limitations in Bonding to Dentin and Experimental Strategies to Prevent Bond Degradation. J. Dent. Res. 2011, 90, 953–968. [Google Scholar] [CrossRef]
- Rueggeberg, F.A.; Margeson, D.H. The Effect of Oxygen Inhibition on an Unfilled/Filled Composite System. J. Dent. Res. 1990, 69, 1652–1658. [Google Scholar] [CrossRef]
- Magne, P. Immediate Dentin Sealing: A Fundamental Procedure for Indirect Bonded Restorations. J. Esthet. Restor. Dent. 2006, 17, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Meira, J.B.C.; Boaro, L.C.C.; Xavier, T.A. Adhesion to tooth structure: A critical review of macro mechanical aspects. J. Dent. 2010, 38, 9–20. [Google Scholar]
- Ausiello, P.; Franciosa, P.; Martorelli, M.; Watts, D.C. Numerical fatigue 3D-FE modeling of indirect composite-restored posterior teeth. Dent. Mater. 2011, 27, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S. The Finite Element Method in Engineering; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Magne, P. Efficient 3D finite element analysis of dental restorative procedures using micro-CT data. Dent. Mater. 2007, 23, 539–548. [Google Scholar] [CrossRef]
- Reddy, M.S.; Sundram, R.; Abdemagyd, H.E. Application of finite element model in implant dentistry: A systematic review. J. Pharm. Bioallied Sci. 2019, 11, 85. [Google Scholar] [CrossRef]
- Guler, M.S.; Guler, C.; Cakici, F.; Cakici, E.B.; Sen, S. Finite element analysis of thermal stress distribution in different restorative materials used in class V cavities. Niger. J. Clin. Pract. 2016, 19, 30. [Google Scholar] [CrossRef]
- Jiang, W.; Bo, H.; YongChun, G.; LongXing, N. Stress distribution in molars restored with inlays or onlays with or without endodontic treatment: A three-dimensional finite element analysis. J. Prosthet. Dent. 2010, 103, 6–12. [Google Scholar] [CrossRef]
- Chuang, S.F.; Chang, C.H.; Chen, T.Y.F. Contraction behaviors of dental composite restorations—Finite element investigation with DIC validation. J. Mech. Behav. Biomed. Mater. 2011, 4, 2138–2149. [Google Scholar] [CrossRef]
- Luo, X.; Rong, Q.; Luan, Q.; Yu, X. Effect of partial restorative treatment on stress distributions in non-carious cervical lesions: A three-dimensional finite element analysis. BMC Oral Health 2022, 22, 607. [Google Scholar] [CrossRef]
- Rodrigues, M.P.; Soares, P.B.F.; Gomes, M.A.B.; Pereira, R.A.; Tantbirojn, D.; Versluis, A.; Soares, C.J. Direct resin composite restoration of endodontically-treated permanent molars in adolescents: Bite force and patient-specific finite element analysis. J. Appl. Oral Sci. 2020, 28, e20190544. [Google Scholar] [CrossRef]
- Ausiello, P.; Ciaramella, S.; Fabianelli, A.; Gloria, A.; Martorelli, M.; Lanzotti, A.; Watts, D.C. Mechanical behavior of bulk direct composite versus block composite and lithium disilicate indirect Class II restorations by CAD-FEM modeling. Dent. Mater. 2017, 33, 690–701. [Google Scholar] [CrossRef]
- Juloski, J.; Apicella, D.; Ferrari, M. The effect of ferrule height on stress distribution within a tooth restored with fibre posts and ceramic crown: A finite element analysis. Dent. Mater. 2014, 30, 1304–1315. [Google Scholar] [CrossRef]
- Çulhaoğlu, A.K.; Terzioğlu, H. Comparing Static, Dynamic and Impact Loading Behavior of Biomimetic Porous Dental Implants with Conventional Dental Implants (3D Finite Element Analysis). Selcuk. Dent. J. 2020, 7, 471–480. [Google Scholar] [CrossRef]
- Homaei, E.; Jin, X.Z.; Pow, E.H.N.; Matinlinna, J.P.; Tsoi, J.K.H.; Farhangdoost, K. Numerical fatigue analysis of premolars restored by CAD/CAM ceramic crowns. Dent. Mater. 2018, 34, e149–e157. [Google Scholar] [CrossRef]
- Mutluay, M.M.; Yahyazadehfar, M.; Ryou, H.; Majd, H.; Do, D.; Arola, D. Fatigue of the resin–dentin interface: A new approach for evaluating the durability of dentin bonds. Dent. Mater. 2013, 29, 437–449. [Google Scholar] [CrossRef]
- Nalla, R.K.; Kinney, J.H.; Marshall, S.J.; Ritchie, R.O. On the in vitro Fatigue Behavior of Human Dentin: Effect of Mean Stress. J. Dent. Res. 2004, 83, 211–215. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, R.A.M.; Pereira, M.D.; Furtado, F.; Prado, G.P.R.; Mestriner, W.; Ferreira, L.M. Masticatory efficiency and bite force in individuals with normal occlusion. Arch. Oral Biol. 2014, 59, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Beitzel, D.; Mutluay, M.; Tay, F.R.; Pashley, D.H.; Arola, D. On the durability of resin–dentin bonds: Identifying the weakest links. Dent. Mater. 2015, 31, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.; Das, U. Comparison of fracture resistance of teeth restored with ceramic inlay and resin composite: An in vitro study. Indian J. Dent. Res. 2011, 22, 877. [Google Scholar] [CrossRef]
- Ausiello, P.; Ciaramella, S.; Garcia-Godoy, F.; Gloria, A.; Lanzotti, A.; Maietta, S.; Martorelli, M. The effects of cavity-margin-angles and bolus stiffness on the mechanical behavior of indirect resin composite class II restorations. Dent. Mater. 2017, 33, e39–e47. [Google Scholar] [CrossRef] [PubMed]
- Eliguzeloglu, E.; Eraslan, O.; Omurlu, H.; Eskitascıoglu, G.; Belli, S. Effect of Hybrid Layer and Thickness on Stress Distribution of Cervical Wedge-Shaped Restorations. Eur. J. Dent. 2010, 4, 160–165. [Google Scholar] [CrossRef]
- Opdam, N.J.M.; Bronkhorst, E.M.; Loomans, B.A.C.; Huysmans, M.C.D.N.J.M. 12-year Survival of Composite vs. Amalgam Restorations. J. Dent. Res. 2010, 89, 1063–1067. [Google Scholar] [CrossRef]
- Ulusoy, M.; Aydın, A.K. Diş Hekimliğinde Hareketli Bölümlü Protezler; Ankara Üniversitesi Diş Hekimliği Yayınları: Ankara, Türkiye, 2010. [Google Scholar]
- Ausiello, P.; Ciaramella, S.; Garcia-Godoy, F.; Martorelli, M.; Sorrentino, R.; Gloria, A. Stress distribution of bulk-fill resin composite in class II restorations. Am. J. Dent. 2017, 30, 227–232. [Google Scholar]
- Yaman, S.D.; Şahin, M.; Aydin, C. Finite element analysis of strength characteristics of various resin based restorative materials in Class V cavities. J. Oral Rehabil. 2003, 30, 630–641. [Google Scholar] [CrossRef]
- Yasin Gönder, H.; Mohammadi, R.; Harmankaya, A.; Burak Yüksel, İ.; Seda Gültekin, D. Investigation of the Effects of Adhesive Materials of Different Types and Thicknesses on Dental Tissue Stress via FEM Analysis. BioMed Res. Int. 2022, 2022, 8493909. [Google Scholar] [CrossRef]
- Gönder, H.Y.; Mohammadi, R.; Harmankaya, A.; Yüksel, İ.B.; Fidancıoğlu, Y.D.; Karabekiroğlu, S. Teeth Restored with Bulk–Fill Composites and Conventional Resin Composites; Investigation of Stress Distribution and Fracture Lifespan on Enamel, Dentin, and Restorative Materials via Three-Dimensional Finite Element Analysis. Polymers 2023, 15, 1637. [Google Scholar] [CrossRef]
- Ausiello, P.; Apicella, A.; Davidson, C.L.; Rengo, S. 3D-finite element analyses of cusp movements in a human upper premolar, restored with adhesive resin-based composites. J. Biomech. 2001, 34, 1269–1277. [Google Scholar] [CrossRef]
- Belli, S.; Erdemir, A.; Yildirim, C. Evaluation of the fracture resistance of premolars restored with different materials. J. Endod. 2005, 31, 116–119. [Google Scholar]
- Fonseca, R.B.; Fernandes-Neto, A.J.; Correr-Sobrinho, L.; Soares, C.J. The influence of cavity preparation design on fracture strength and mode of fracture of laboratory-processed composite resin restorations. J. Prosthet. Dent. 2007, 98, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Ordinola-Zapata, R.; Ye, N.; Xu, H.; Fok, A.S.L. Fatigue analysis of restored teeth longitudinally cracked under cyclic loading. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2022, 38, 204–213. [Google Scholar]
- Lohbauer, U.; Belli, R.; Ferracane, J.L. Factors involved in mechanical fatigue degradation of dental resin composites. J. Dent. Res. 2013, 92, 584–591. [Google Scholar] [CrossRef] [PubMed]
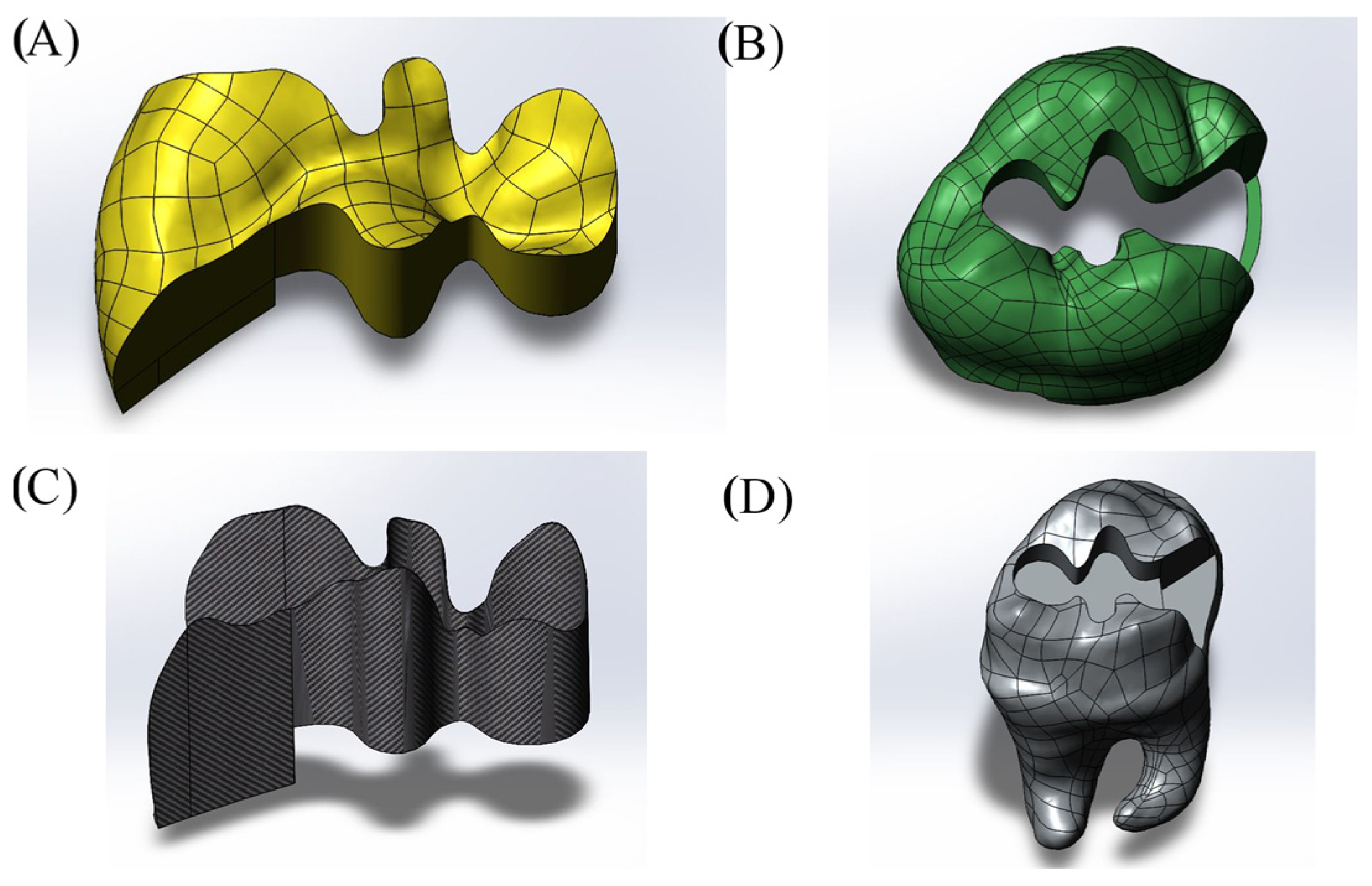

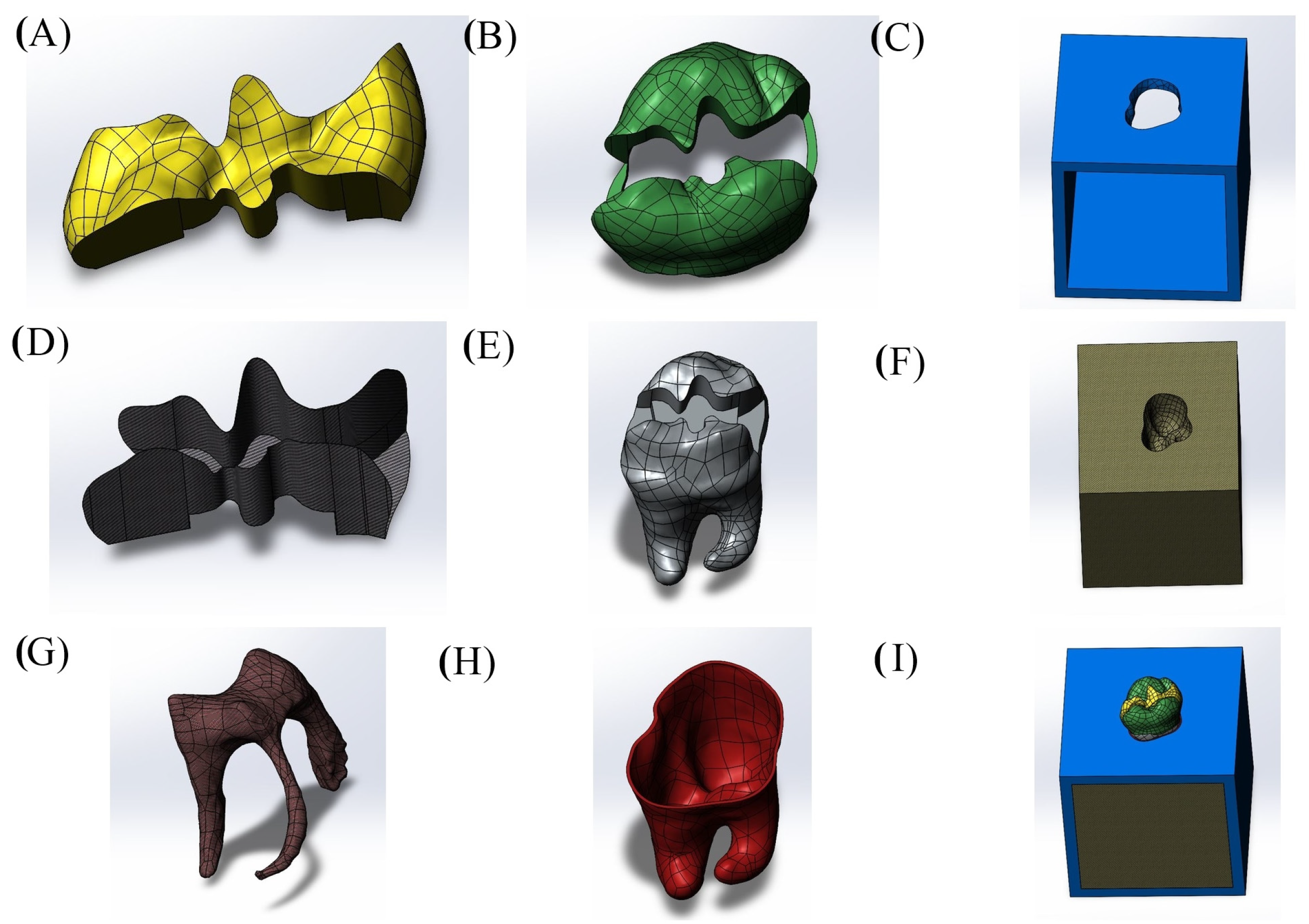

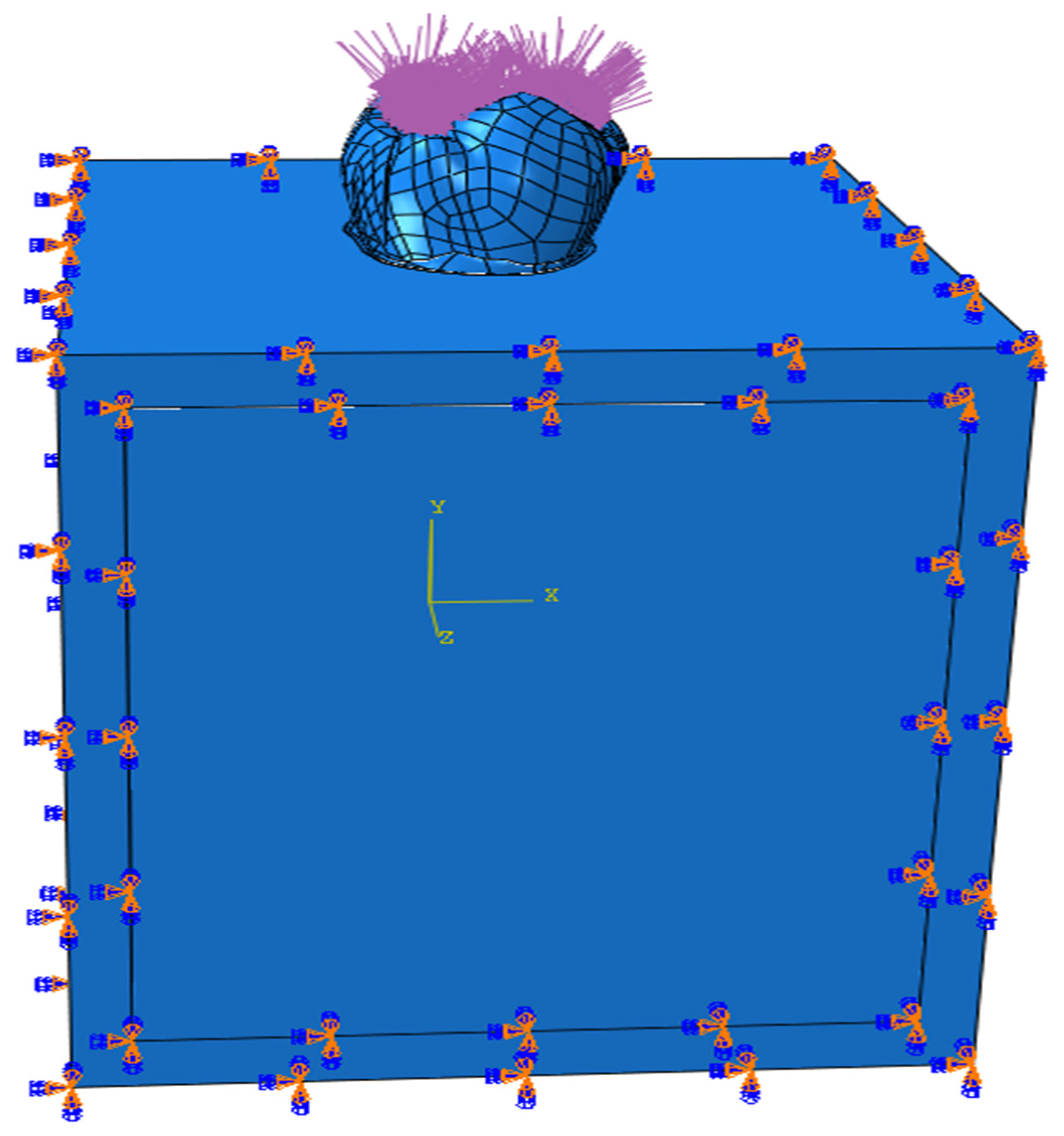







| Young’s Modulus (Mpa) | Poisson’s Ratio | Compressive Strength (MPa) | Flexural Strength (MPa) | Shear Strength (MPa) | Fracture Toughness (Mpa m1/2) | Microhardness (HV) | |
|---|---|---|---|---|---|---|---|
| Enamel | 84.1 | 0.33 | 384 | 11.5 | 60 | 0.8 | 3–6 |
| Dentin | 18.6 | 0.31 | 297 | 105.5 | 12–138 | 3.08 | 0.13–0.51 |
| Adhesive Material | 1 | 0.24 | - | - | - | - | - |
| Pulp | 0.002 | 0.45 | - | - | - | - | - |
| PDL | 0.0689 | 0.45 | - | - | - | - | - |
| Cortical bone | 13.7 | 0.3 | - | - | - | - | - |
| Spongious bone | 1.37 | 0.3 | - | - | - | - | - |
| Bulk-fill Composite | 12 | 0.25 | 169 | 42 | - | - | - |
| Conventional Composite | 16.6 | 0.24 | 294 | 77 | - | - | - |
| Hybrid Composite | 22 | 0.27 | - | - | - | - | - |
| Material | A (MPa) | B | σf (MPa) | b |
|---|---|---|---|---|
| Enamel | - | - | 310 | −0.111 |
| Dentin | - | - | 247 | −0.111 |
| Bulk-Fill Composite | 54 | −0.020 | - | - |
| Conventional Composite | 84 | −0.035 | - | - |
| Hybrid Composite | 182.8 | −0.056 | - | - |
| Model with 10 µm Thick Adhesive Material | Model with 15 µm Thick Adhesive Material | Model with 20 µm Thick Adhesive Material | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bulk-Fill Composite | Conventional Composite | Hybrid Composite | Bulk-Fill Composite | Conventional Composite | Hybrid Composite | Bulk-Fill Composite | Conventional Composite | Hybrid Composite | |
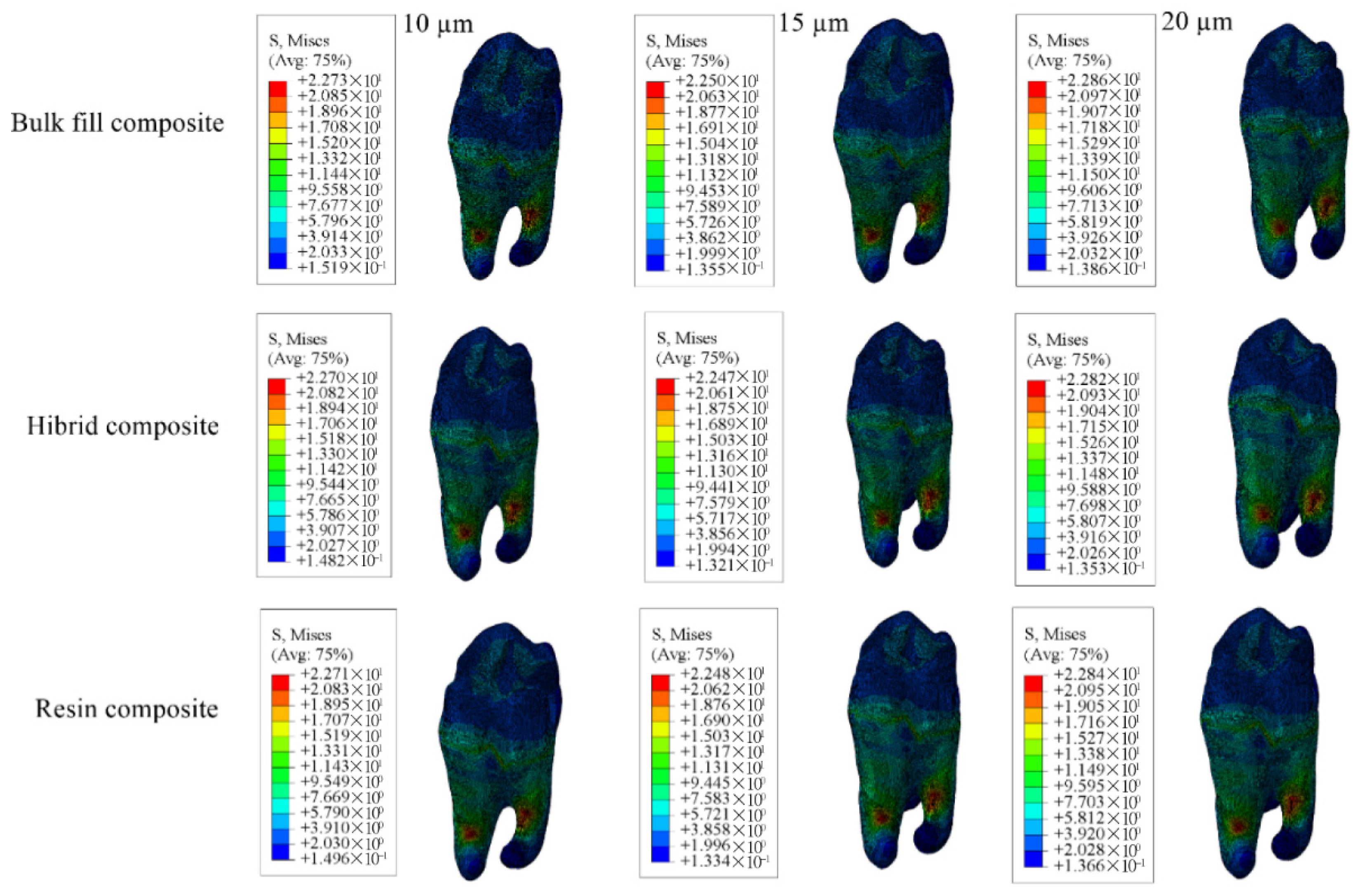
| Enamel | 44.53 | 43.47 | 44.33 | 61.14 | 59.67 | 58.83 | 44.98 | 44.86 | 44.88 |
| Dentin | 34.37 | 31.88 | 29.96 | 49.18 | 46.02 | 43.44 | 41.26 | 38.69 | 36.63 |
| Restorative Material | 19.24 | 20.99 | 22.27 | 17.93 | 18.51 | 19.60 | 16.96 | 18.52 | 19.61 |
| Adhesive Material | 17.29 | 14.83 | 13.08 | 10.24 | 10.04 | 10.06 | 9.981 | 9.938 | 9.854 |
| Model with 10 µm Thick Adhesive Material | Model with 15 µm Thick Adhesive Material | Model with 20 µm Thick Adhesive Material | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bulk-Fill Composite | Conventional Composite | Hybrid Composite | Bulk-Fill Composite | Conventional Composite | Hybrid Composite | Bulk-Fill Composite | Conventional Composite | Hybrid Composite | |
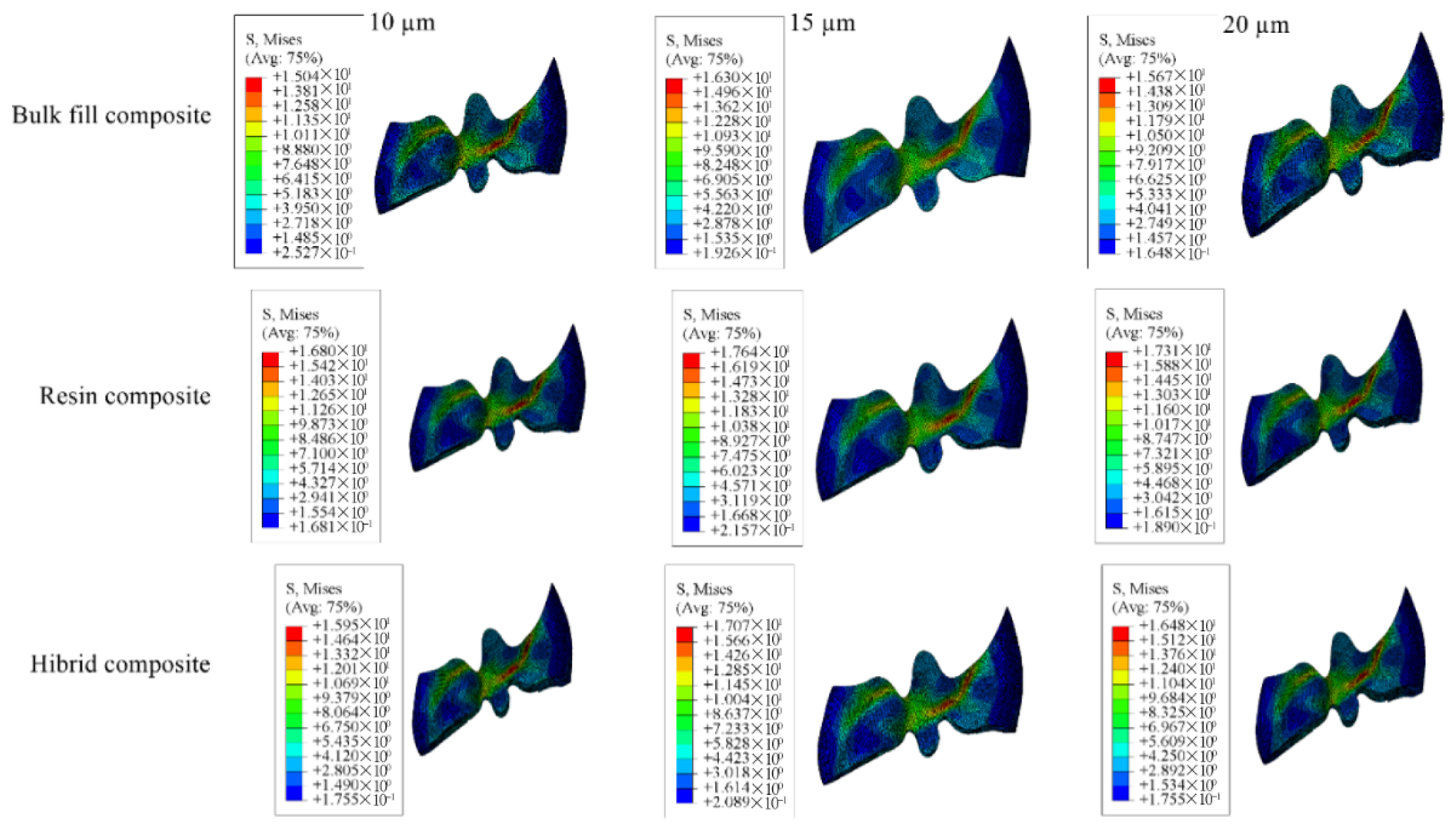
| Enamel | 69.06 | 67.20 | 65.86 | 61.26 | 59.50 | 58.24 | 63.21 | 61.56 | 60.39 |
| Dentin | 28.11 | 27.62 | 27.31 | 27.45 | 26.95 | 26.59 | 30.69 | 30.13 | 29.68 |
| Restorative Material | 14.70 | 15.90 | 16.84 | 14.80 | 16.21 | 17.18 | 15.55 | 16.66 | 17.47 |
| Adhesive Material | 18.35 | 16.08 | 14.20 | 9.377 | 7.946 | 6.766 | 8.388 | 7.096 | 6.282 |
| Model with 10 µm Thick Adhesive Material | Model with 15 µm Thick Adhesive Material | Model with 20 µm Thick Adhesive Material | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bulk-Fill Composite | Conventional Composite | Hybrid Composite | Bulk-Fill Composite | Conventional Composite | Hybrid Composite | Bulk-Fill Composite | Conventional Composite | Hybrid Composite | |
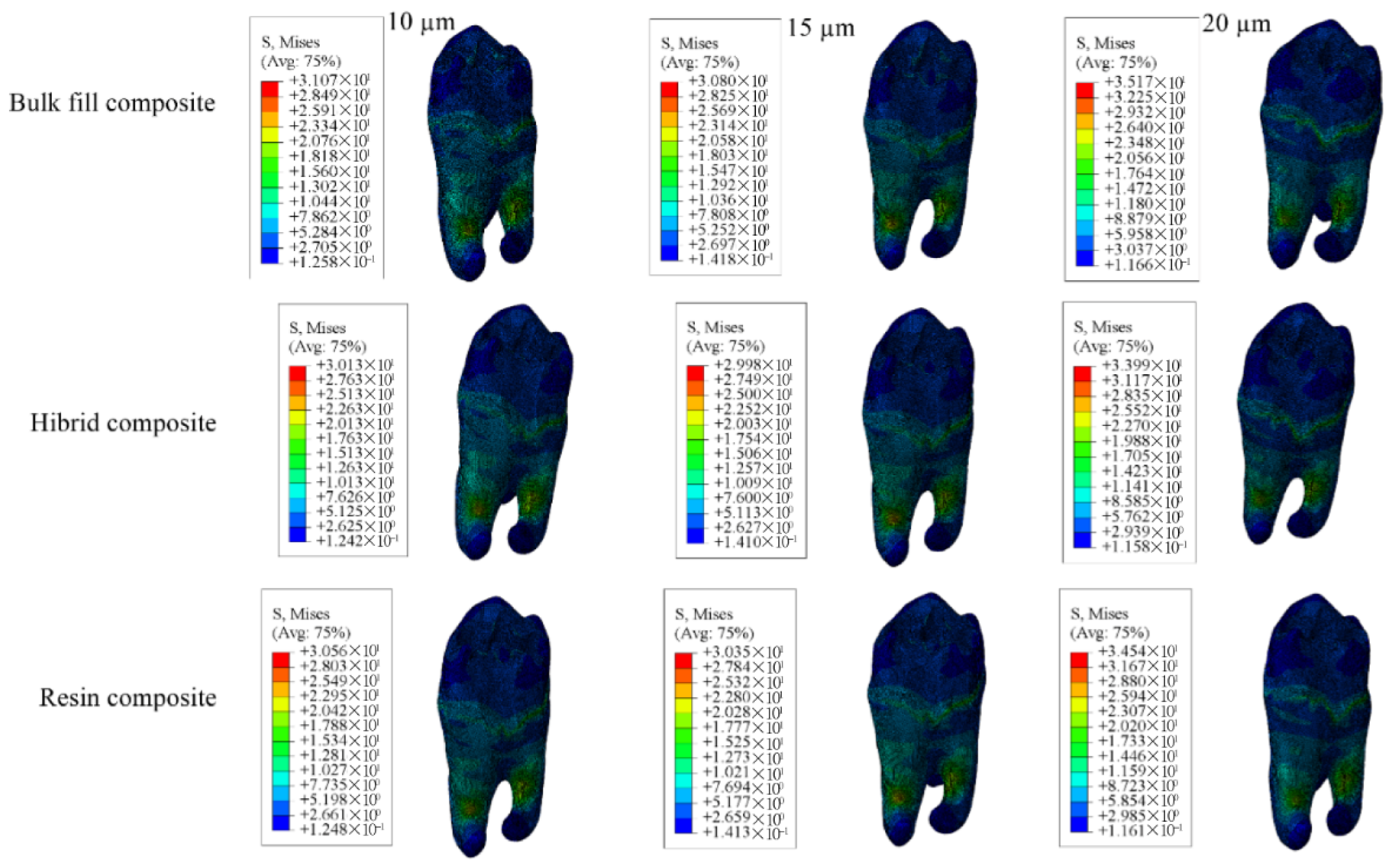
| Enamel | 71.79 | 69.04 | 67.14 | 71.08 | 68.42 | 66.58 | 66.13 | 63.80 | 62.19 |
| Dentin | 22.73 | 22.71 | 22.70 | 22.50 | 22.48 | 22.47 | 22.86 | 22.84 | 22.82 |
| Restorative Material | 15.04 | 15.95 | 16.80 | 16.30 | 17.07 | 17.64 | 15.67 | 16.48 | 17.31 |
| Adhesive Material | 33.06 | 30.42 | 27.16 | 9.715 | 8.067 | 6.765 | 8.920 | 7.680 | 6.761 |
| Model with 10 µm Thick Adhesive Material | Model with 15 µm Thick Adhesive Material | Model with 20 µm Thick Adhesive Material | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bulk-Fill Composite | Conventional Composite | Hybrid Composite | Bulk-Fill Composite | Conventional Composite | Hybrid Composite | Bulk-Fill Composite | Conventional Composite | Hybrid Composite | |
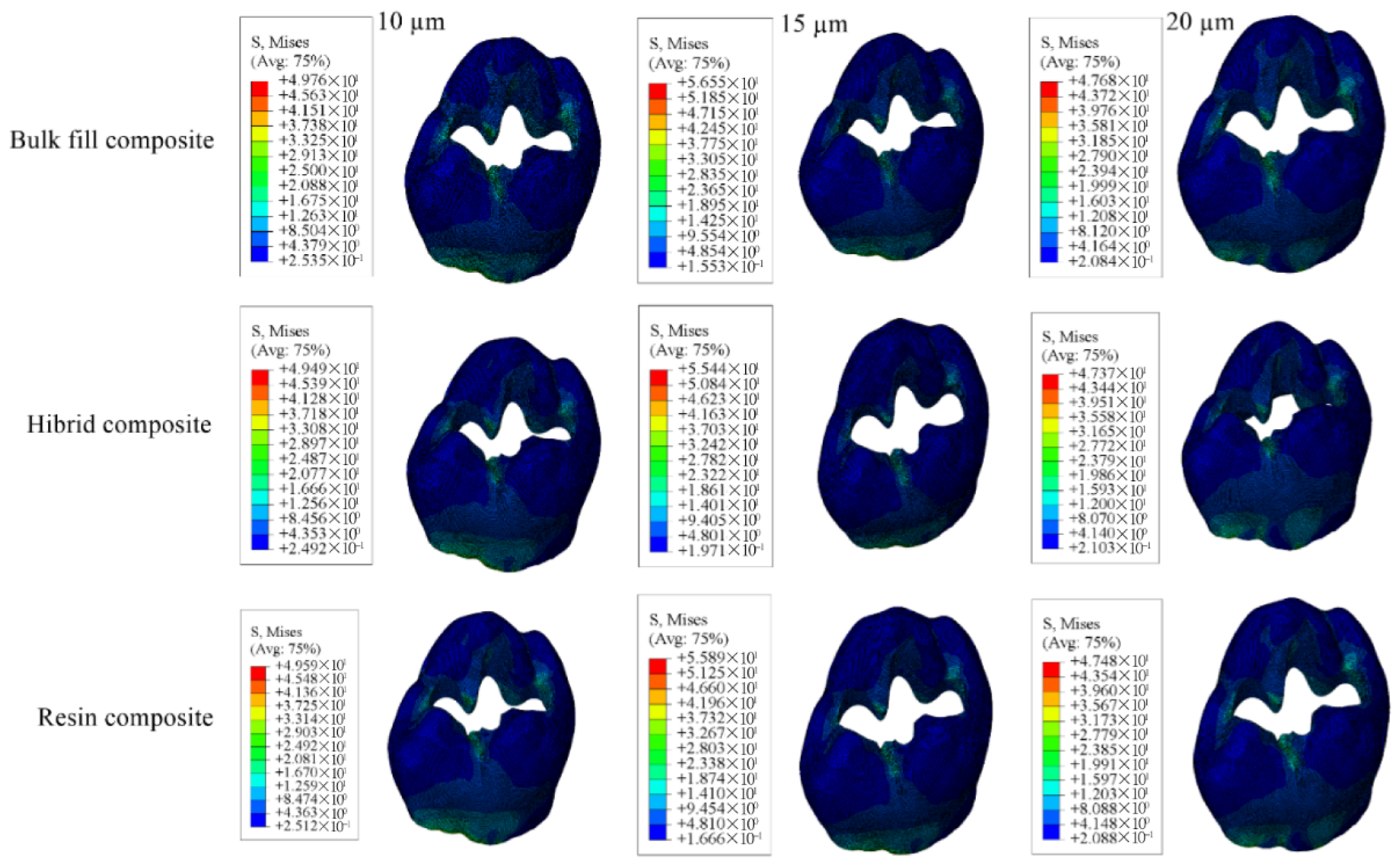
| Enamel | 49.76 | 49.59 | 49.49 | 56.55 | 55.89 | 55.44 | 47.68 | 47.48 | 47.37 |
| Dentin | 31.07 | 30.56 | 30.13 | 30.80 | 30.35 | 29.98 | 35.17 | 34.54 | 33.99 |
| Restorative Material | 19.24 | 16.32 | 17.33 | 17.39 | 16.23 | 17.25 | 14.90 | 16.45 | 17.52 |
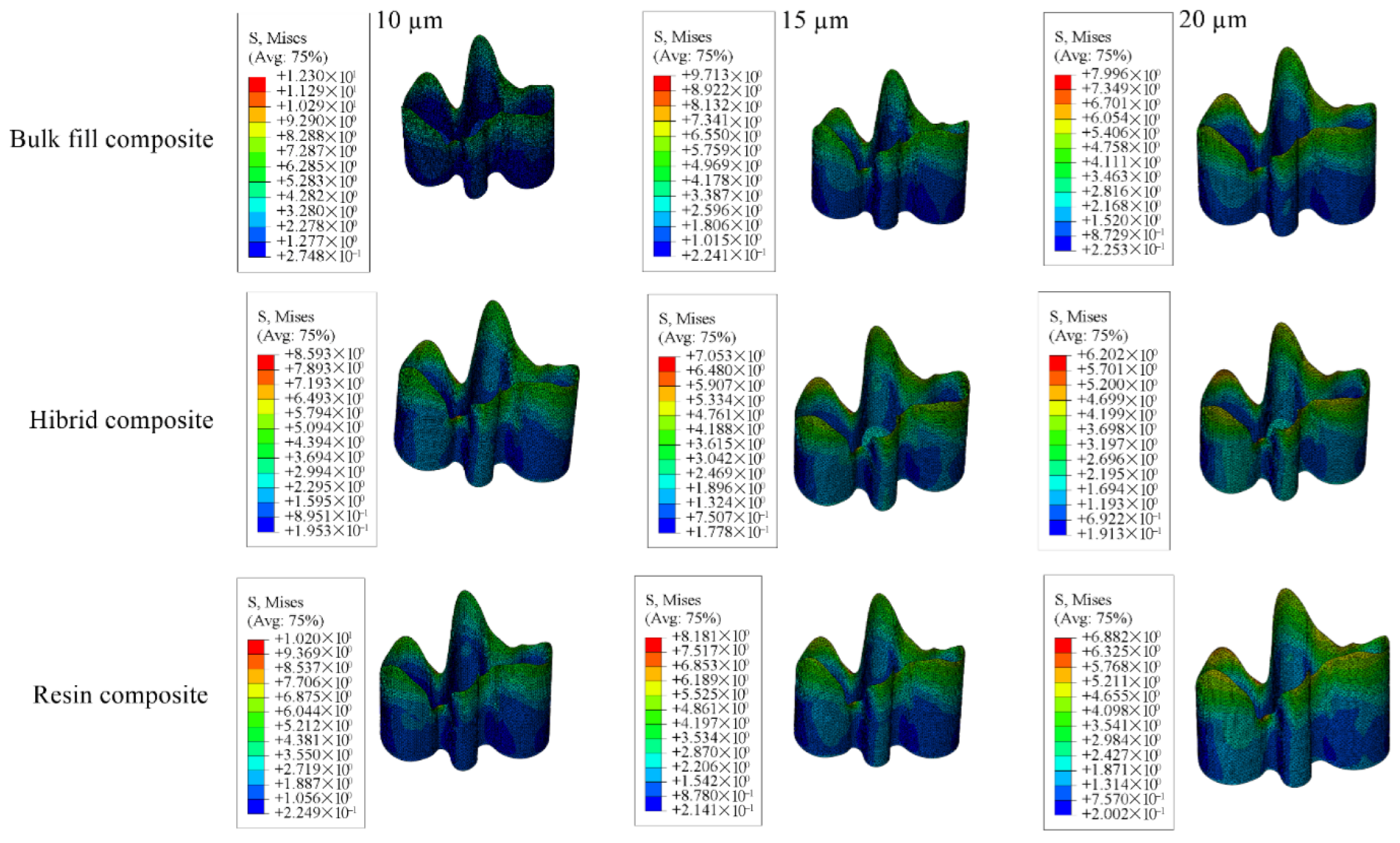
| Adhesive Material | 12.30 | 10.20 | 8.593 | 9.713 | 8.181 | 7.053 | 7.996 | 6.882 | 6.202 |
| Model with 10 µm Thick Adhesive Material | Model with 15 µm Thick Adhesive Material | Model with 20 µm Thick Adhesive Material | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bulk-Fill Composite | Conventional Composite | Hybrid Composite | Bulk-Fill Composite | Conventional Composite | Hybrid Composite | Bulk-Fill Composite | Conventional Composite | Hybrid Composite | |
| Enamel | 5.382 × 109 | 5.486 × 109 | 5.528 × 109 | 2.3768 × 108 | 1.211 × 109 | 3.474 × 108 | 4.987 × 109 | 5.095 × 109 | 5.067 × 109 |
| Dentin | 7.441 × 109 | 1.5507 × 1010 | 2.830 × 109 | 2.106 × 108 | 4.088 × 108 | 7.249 × 108 | 1.210 × 109 | 2.277 × 109 | 3.889 × 109 |
| Restorative Material | 5.921 × 1037 | 9.621 × 1025 | 6.378 × 1021 | 5.142 × 1039 | 6.529 × 1027 | 3.999 × 1017 | 7.867 × 1040 | 5.922 × 1027 | 9.016 × 1022 |
| Model with 10 µm Thick Adhesive Material | Model with 15 µm Thick Adhesive Material | Model with 20 µm Thick Adhesive Material | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bulk-Fill Composite | Conventional Composite | Hybrid Composite | Bulk-Fill Composite | Conventional Composite | Hybrid Composite | Bulk-Fill Composite | Conventional Composite | Hybrid Composite | |
| Enamel | 6.802 × 107 | 8.976 × 107 | 1.102 × 108 | 2.280 × 108 | 3.056 × 108 | 3.776 × 108 | 1.667 × 108 | 2.167 × 108 | 2.628 × 108 |
| Dentin | 5.037 × 1010 | 5.959 × 1010 | 6.639 × 1010 | 6.325 × 1010 | 7.541 × 1010 | 8.575 × 1010 | 2.177 × 1010 | 2.599 × 1010 | 3.003 × 1010 |
| Restorative Material | 6.242 × 1043 | 3.132 × 1029 | 9.761 × 1023 | 5.345 × 1044 | 1.286 × 1029 | 5.976 × 1023 | 2.482 × 10−42 | 6.565 × 1028 | 4.629 × 1023 |
| Model with 10 µm Thick Adhesive Material | Model with 15 µm Thick Adhesive Material | Model with 20 µm Thick Adhesive Material | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bulk-Fill Composite | Conventional Composite | Hybrid Composite | Bulk-Fill Composite | Conventional Composite | Hybrid Composite | Bulk-Fill Composite | Conventional Composite | Hybrid Composite | |
| Enamel | 4.657 × 107 | 6.962 × 107 | 9.291 × 107 | 5.132 × 107 | 7.553 × 107 | 9.955 × 107 | 1.075 × 108 | 1.546 × 108 | 2.003 × 108 |
| Dentin | 3.853 × 1011 | 3.882 × 1011 | 3.897 × 1011 | 4.218 × 1011 | 4.250 × 1011 | 4.266 × 1011 | 3.632 × 1011 | 3.659 × 1011 | 3.688 × 1011 |
| Restorative Material | 1.502 × 1043 | 2.254 × 1029 | 9.235 × 1023 | 2.089 × 1041 | 3.360 × 1028 | 4.022 × 1023 | 1.404 × 1042 | 8.768 × 1028 | 5.504 × 1023 |
| Model with 10 µm Thick Adhesive Material | Model with 15 µm Thick Adhesive Material | Model with 20 µm Thick Adhesive Material | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bulk-Fill Composite | Conventional Composite | Hybrid Composite | Bulk-Fill Composite | Conventional Composite | Hybrid Composite | Bulk-Fill Composite | Conventional Composite | Hybrid Composite | |
| Enamel | 1.817 × 109 | 1.878 × 109 | 1.916 × 109 | 5.052 × 108 | 5.686 × 108 | 6.189 × 108 | 2.742 × 109 | 2.858 × 109 | 2.924 × 109 |
| Dentin | 1.918 × 1010 | 2.250 × 1010 | 2.578 × 1010 | 2.096 × 1010 | 2.415 × 1010 | 2.718 × 1010 | 5.759 × 109 | 6.864 × 109 | 8.022 × 109 |
| Restorative Material | 4.278 × 1037 | 1.130 × 1029 | 5.078 × 1023 | 6.939 × 1039 | 1.349 × 1029 | 5.588 × 1023 | 1.744 × 1043 | 9.177 × 1028 | 4.195 × 1023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi, R.; Alkurt Kaplan, S.; Harmankaya, A.; Gönder, H.Y. Investigation of Stress Distribution and Fatigue Performance in Restored Teeth Using Different Thicknesses of Adhesive Materials and Different Restorative Materials: 3D Finite Element Analysis (FEM). Materials 2025, 18, 3888. https://doi.org/10.3390/ma18163888
Mohammadi R, Alkurt Kaplan S, Harmankaya A, Gönder HY. Investigation of Stress Distribution and Fatigue Performance in Restored Teeth Using Different Thicknesses of Adhesive Materials and Different Restorative Materials: 3D Finite Element Analysis (FEM). Materials. 2025; 18(16):3888. https://doi.org/10.3390/ma18163888
Chicago/Turabian StyleMohammadi, Reza, Sinem Alkurt Kaplan, Abdulkadir Harmankaya, and Hakan Yasin Gönder. 2025. "Investigation of Stress Distribution and Fatigue Performance in Restored Teeth Using Different Thicknesses of Adhesive Materials and Different Restorative Materials: 3D Finite Element Analysis (FEM)" Materials 18, no. 16: 3888. https://doi.org/10.3390/ma18163888
APA StyleMohammadi, R., Alkurt Kaplan, S., Harmankaya, A., & Gönder, H. Y. (2025). Investigation of Stress Distribution and Fatigue Performance in Restored Teeth Using Different Thicknesses of Adhesive Materials and Different Restorative Materials: 3D Finite Element Analysis (FEM). Materials, 18(16), 3888. https://doi.org/10.3390/ma18163888






