Aluminum Extractions by the Alkali Method Directly from Alkali-Acid (NaOH-HCl) Chemical Deashing of Coals
Abstract
1. Introduction
2. Materials and Methods
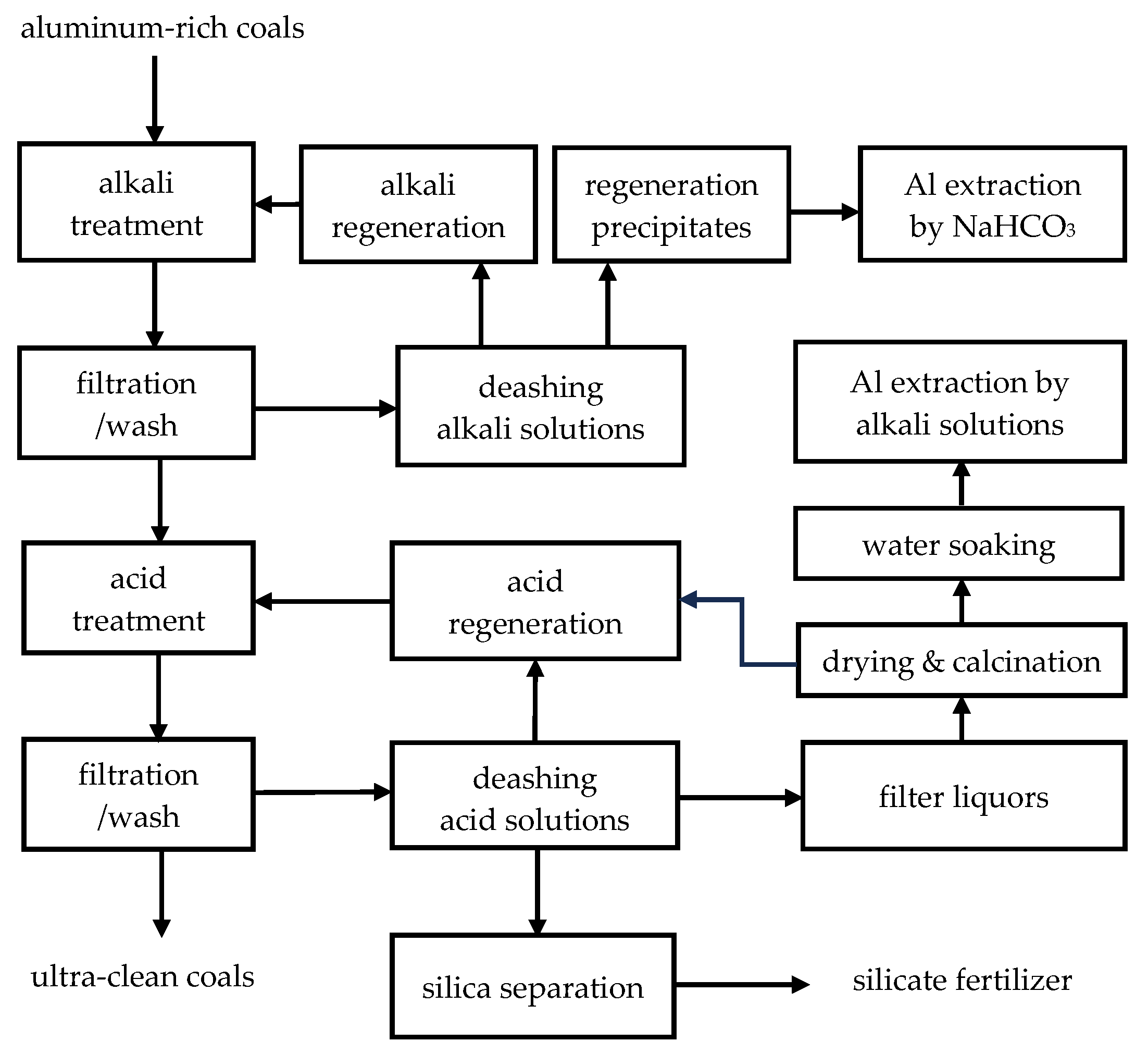
2.1. Preparation of the Deashing Alkali and Acid Solutions of Coals
2.2. Aluminum Extractions from the Deashing Alkali and Acid Solutions of Coals
2.3. Chemicals and Instrumental Analysis
3. Results and Discussions
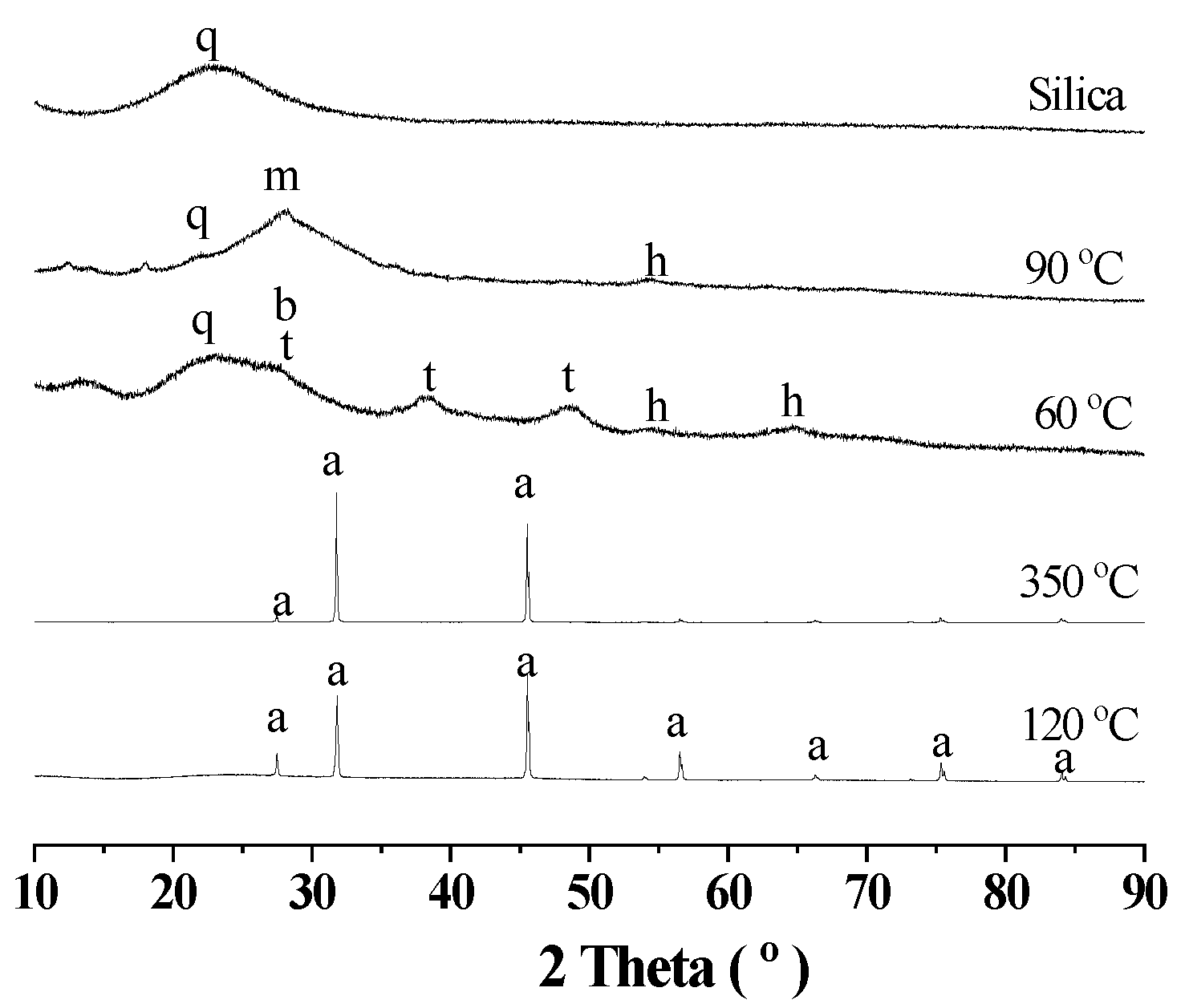
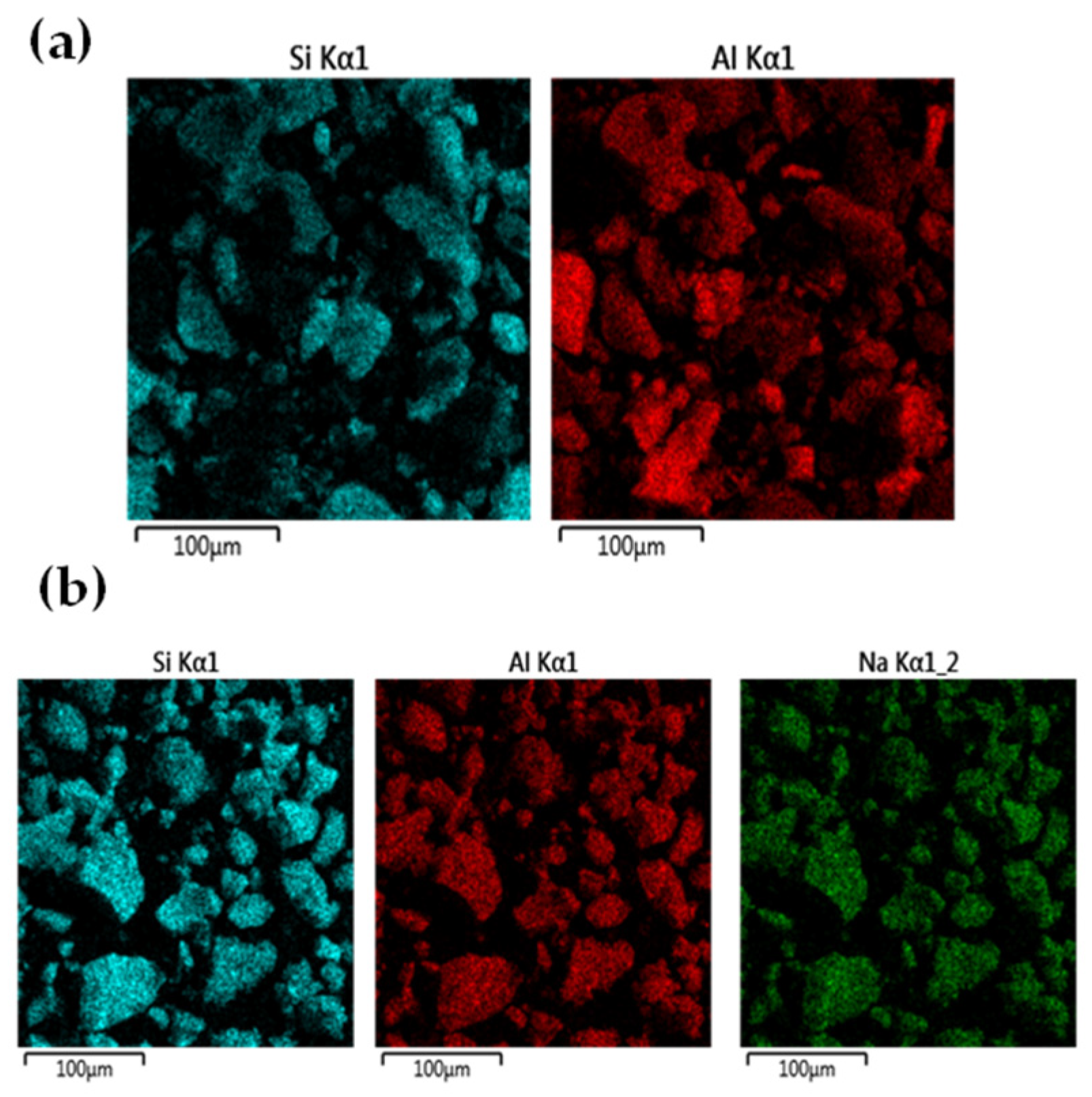
3.1. Aluminum Extraction from the Deashing Alkali Solutions of Coals
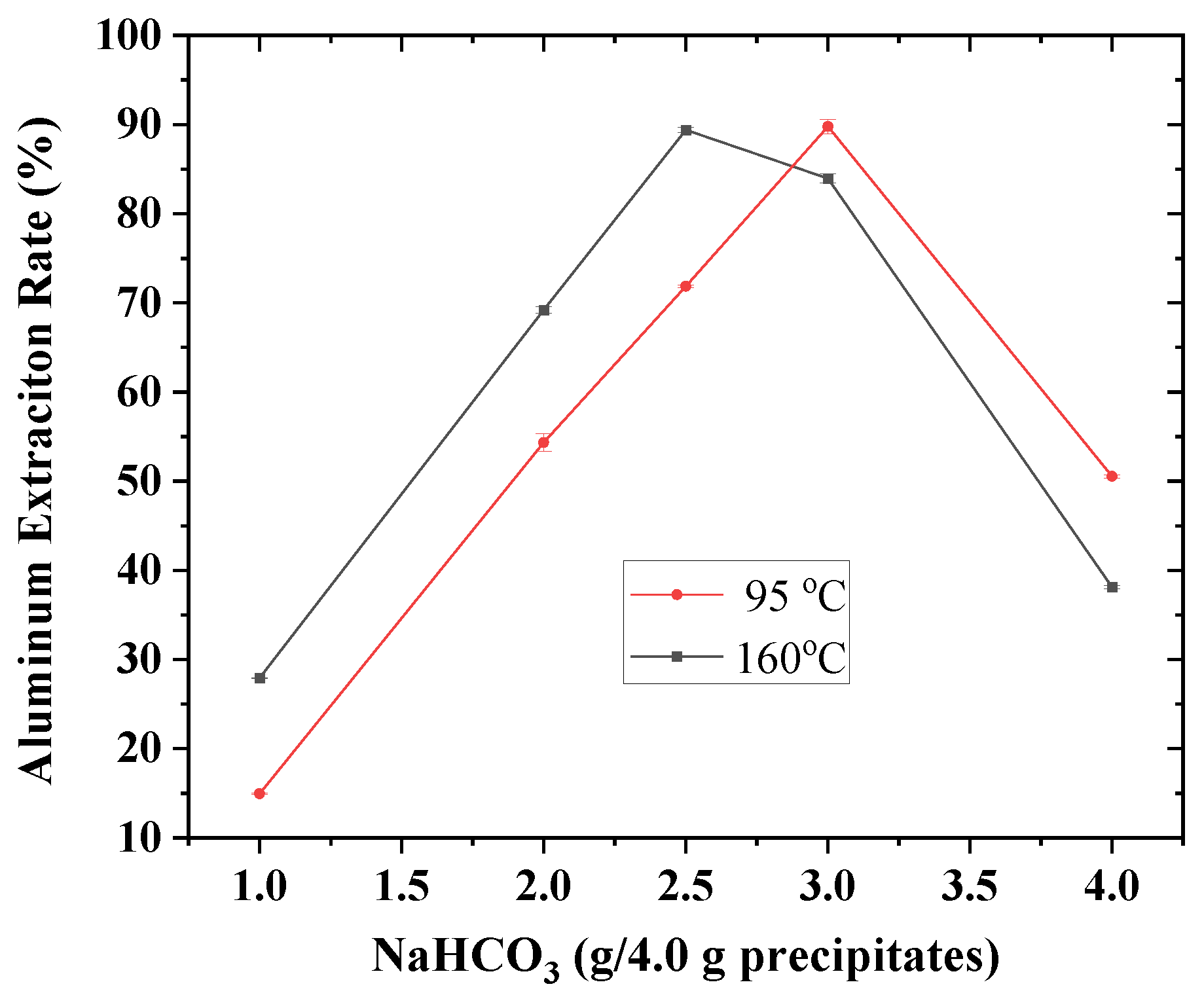
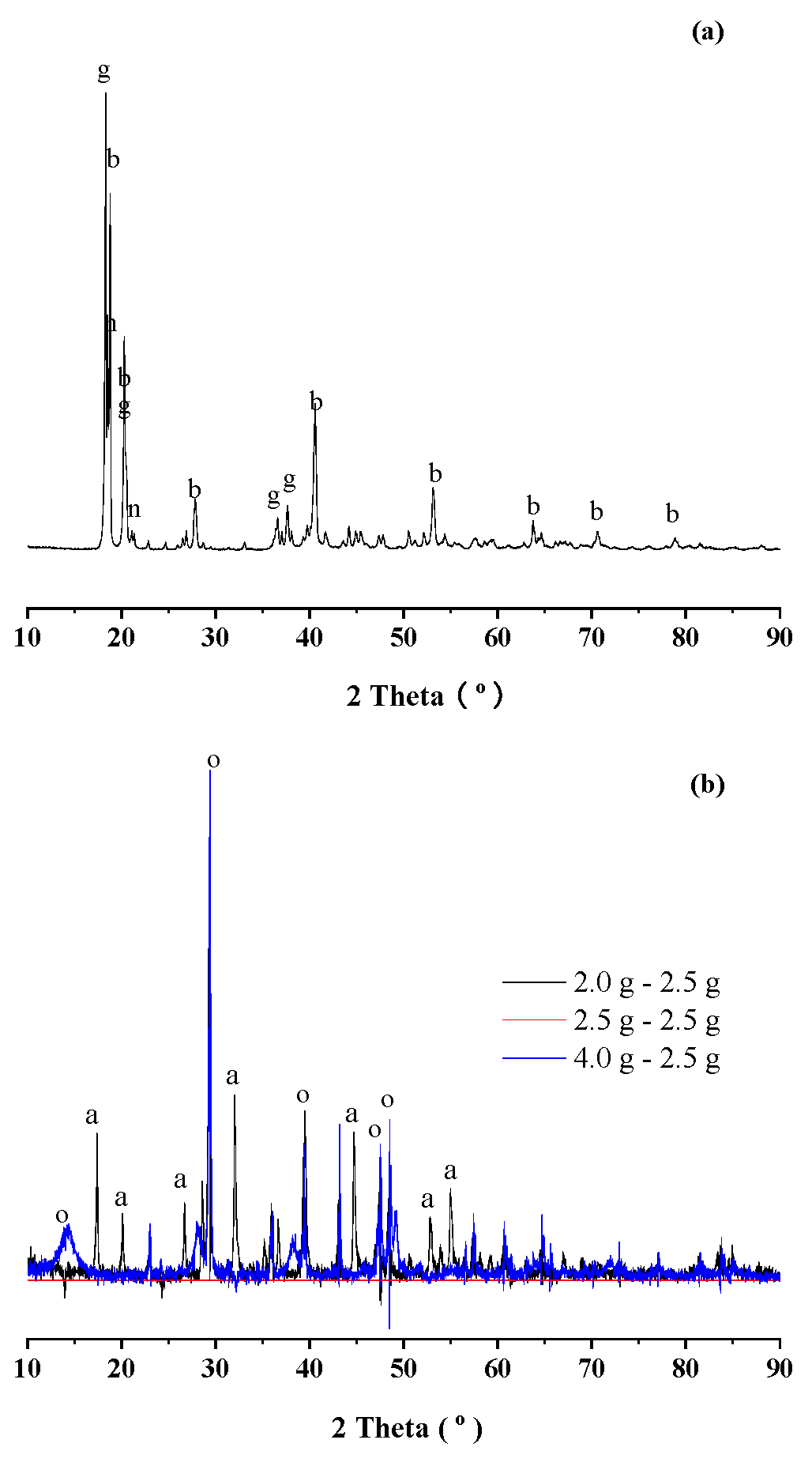
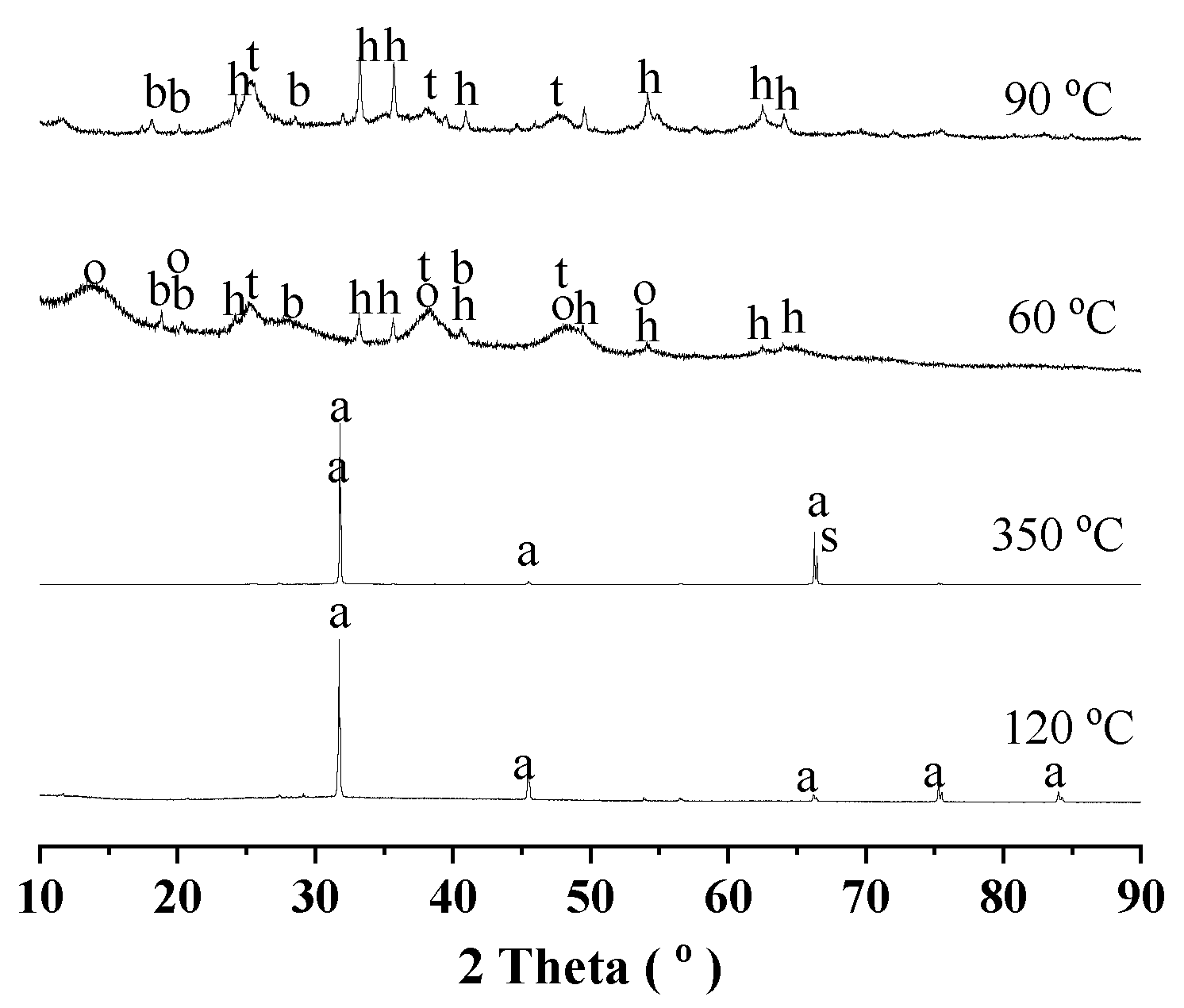
3.2. Route One for Aluminum Extractions from the Deashing Acid Solutions of Coals
3.3. Route Two for Aluminum Extractions from the Deashing Acid Solutions of Coals
3.4. Defining Aluminum Extractions in the Context of Deashing Auminum-Rich Coals (ARCs)
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, L. Calcination of aluminum chloride hexahydrate (ach) for alumina production: Implications for alumina extraction from aluminum rich fly ash (ARFA). Arch. Metall. Mater. 2018, 63, 235–240. [Google Scholar] [CrossRef]
- Essary, J. Extraction of Alumina from Mineral Resources Found at New Mexico Mine Sites. Master’s Thesis, New Mexico Institute of Mining and Technology, Socorro, NM, USA, May 2025. [Google Scholar]
- Park, K.Y.; Jeong, J. Manufacture of low-soda alumina from clay. Ind. Eng. Chem. Res. 1996, 35, 4379–4385. [Google Scholar] [CrossRef]
- Akhmadiyeva, N.; Abdulvaliyev, R.; Gladyshev, S.; Kassymzhanova, A. Hydrochemical method for the production of alumina from nepheline using effective calcium reagents. Processes 2024, 12, 1355. [Google Scholar] [CrossRef]
- Wang, M.L.; Xie, F.; Pi, J.Q.; Zhao, Z.Q.; Wang, Y.L. Oxidative Bayer digestion of sedimentary bauxite aiming at the extraction of alumina from chlorite. JOM 2025, 77, 3197–3204. [Google Scholar] [CrossRef]
- Zhao, B.; Guo, Y.; Wang, W.; Wan, X.; He, S.; Wang, T. Study on experimental parameters of alkali-assisted extraction of aluminum from fly ash. Materials 2025, 18, 1568. [Google Scholar] [CrossRef] [PubMed]
- Valeev, D.; Bobylev, P.; Osokin, N.; Zolotova, I.; Rodionov, I.; Salazar-Concha, C.; Verichev, K. A review of the alumina production from coal fly ash, with a focus in Russia. J. Clean. Prod. 2022, 363, 132360. [Google Scholar] [CrossRef]
- Yadav, V.K.; Gnanamoorthy, G.; Yadav, K.K.; Ali, I.H.; Bagabas, A.A.; Choudhary, N.; Yadav, S.; Suriyaprabha, R.; Islam, S.; Modi, S.; et al. Utilization of incense stick ash in hydrometallurgy method for extracting oxides of Fe, Al, Si, and Ca. Materials 2022, 15, 1879. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, W.; Chen, A.; Jin, F.; Jiang, X.; Liu, H.; Xiao, Y. Method for Removing Ash from Solid Carbonaceous Material. International Patent Application No. PCT/CN2019/108066, 26 September 2019. [Google Scholar]
- Zhao, L. Alumina and silica extraction and byproduct development directly from chemical deashing of coals. Minerals 2022, 12, 179. [Google Scholar] [CrossRef]
- Brooks, P.; Waugh, A.B.; Clark, K.N.; Weir, S.B. Process for Demineralising Coal. International Patent Application No. PCT/AU2003/001409, 23 October 2003. [Google Scholar]
- Li, Y.; Zhang, Y.; Yang, C.; Zhang, Y. Precipitating sandy aluminum hydroxide from sodium aluminate solution by the neutralization of sodium bicarbonate. Hydrometallurgy 2009, 98, 52–57. [Google Scholar] [CrossRef]
- Suss, A.; Senyuta, A.; Kravchenya, M.; Smirnov, A.; Panov, A. The quality of alumina produced by the hydrochloric acid process and potential for improvement. In Proceedings of the The International Committee for Study of Bauxite, Alumina & Aluminium (ICSOBA), Dubai, United Arab Emirates, 29 November 2015; Volume 44, pp. 1–8. [Google Scholar]
- Hartman, M.; Trnka, O.; Šolcová, O. Thermal decomposition of aluminum chloride hexahydrate. Ind. Eng. Chem. Res. 2005, 44, 6591–6598. [Google Scholar] [CrossRef]
- Valeev, D.; Shoppert, A.; Mikhailova, A.; Kondratiev, A. Acid and acid-alkali treatment method of Al-chloride solution obtained by the leaching of coal fly ash to produce sandy grade alumina. Metals 2020, 10, 585. [Google Scholar] [CrossRef]
- Sriramoju, S.K.; Suresh, A.; Lingam, R.K.; Dash, P.S. Mechanism of a coal chemical-leaching process and recovery of spent chemicals: A pilot-scale study. Int. J. Coal Prep. Util. 2017, 37, 293–302. [Google Scholar] [CrossRef]
- Liu, G.; Li, X.; Peng, Z.; Zhang, C.; Liu, X. Reaction behavior between calcium oxide or calcium hydroxide and aluminate solution with heavy caustic soda. Chin. J. Nonferr. Met. 2000, 10, 266–269. [Google Scholar] [CrossRef]
- Tan, D.; Ma, H.; Zou, D.; Li, G. An experimental study of extracting aluminum hydroxide from high caustic sodium aluminate solution. Appl. Chem. Ind. 2008, 37, 1320–1324. [Google Scholar] [CrossRef]
- Zhao, L. Spectroscopic characterizations of silicate fertilizers prepared by chemical deashing of coals. Heliyon 2024, 10, e32318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cui, W.; Hu, J.Z.; Wang, H.-W.; Prange, M.P.; Wan, C.; Jaegers, N.R.; Zong, M.; Zhang, H.; Pearce, C.I.; et al. Transformation of gibbsite to boehmite in caustic aqueous solution at hydrothermal conditions. Cryst. Growth Des. 2019, 19, 5557–5567. [Google Scholar] [CrossRef]
- Bi, S. Production Technology of Alumina; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
- Nicol, K. The Direct Injection Carbon Engine; CCC243; IEA Clean Coal Centre: London, UK, 2014. [Google Scholar]
- Reid, I. Non-Energy Uses of Coal; CCC/291; IEA Clean Coal Centre: London, UK, 2018. [Google Scholar]
- Nalbandian, H. Non-Fuel Uses of Coal; CCC/236; IEA Clean Coal Centre: London, UK, 2014. [Google Scholar]
- Kong, J.; Su, Z.; Dong, C.; Chen, Q.; Pan, G. Overview of coals as carbon anode materials for sodium-ion batteries. Clean Energy 2024, 8, 197–218. [Google Scholar] [CrossRef]
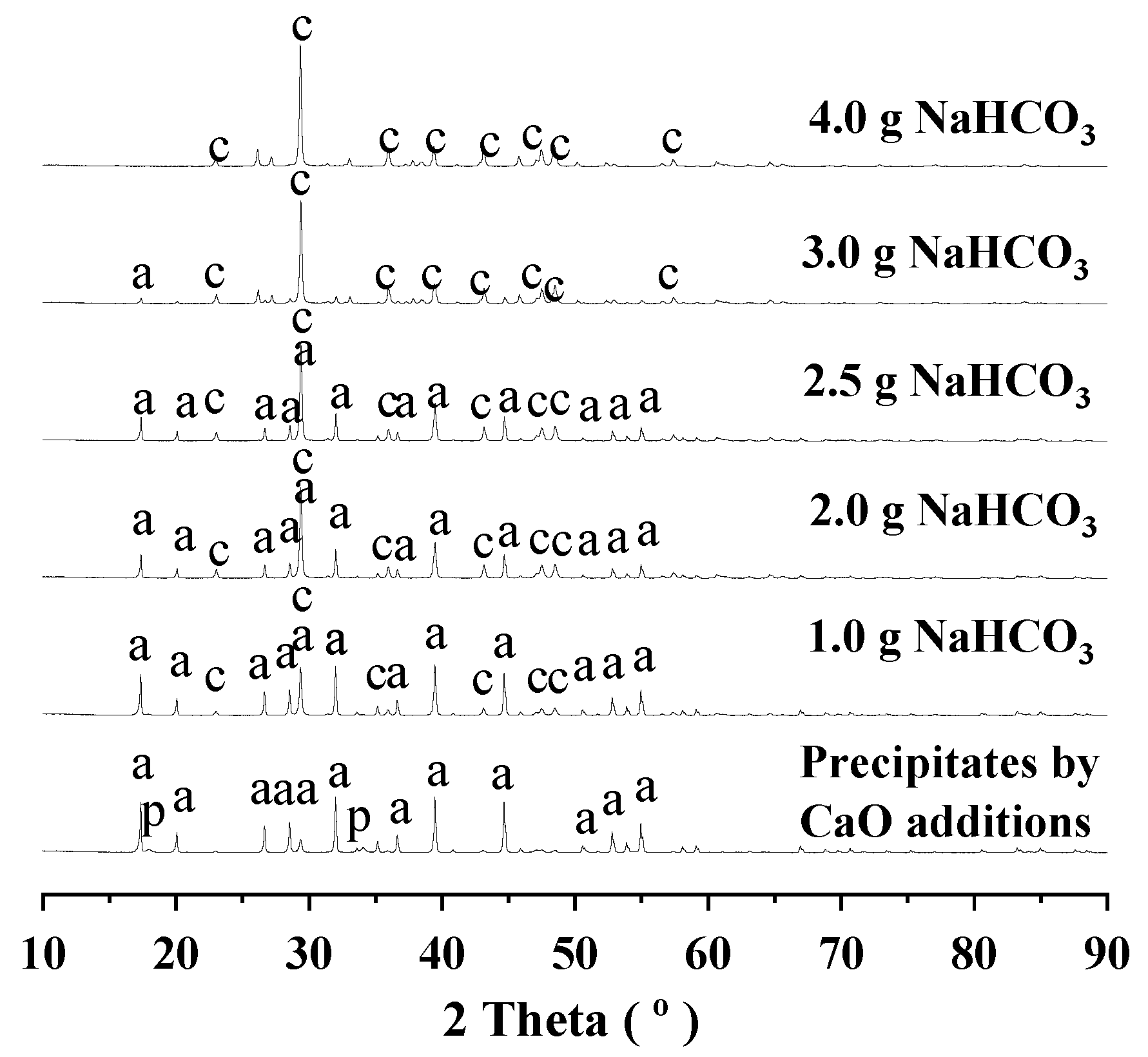
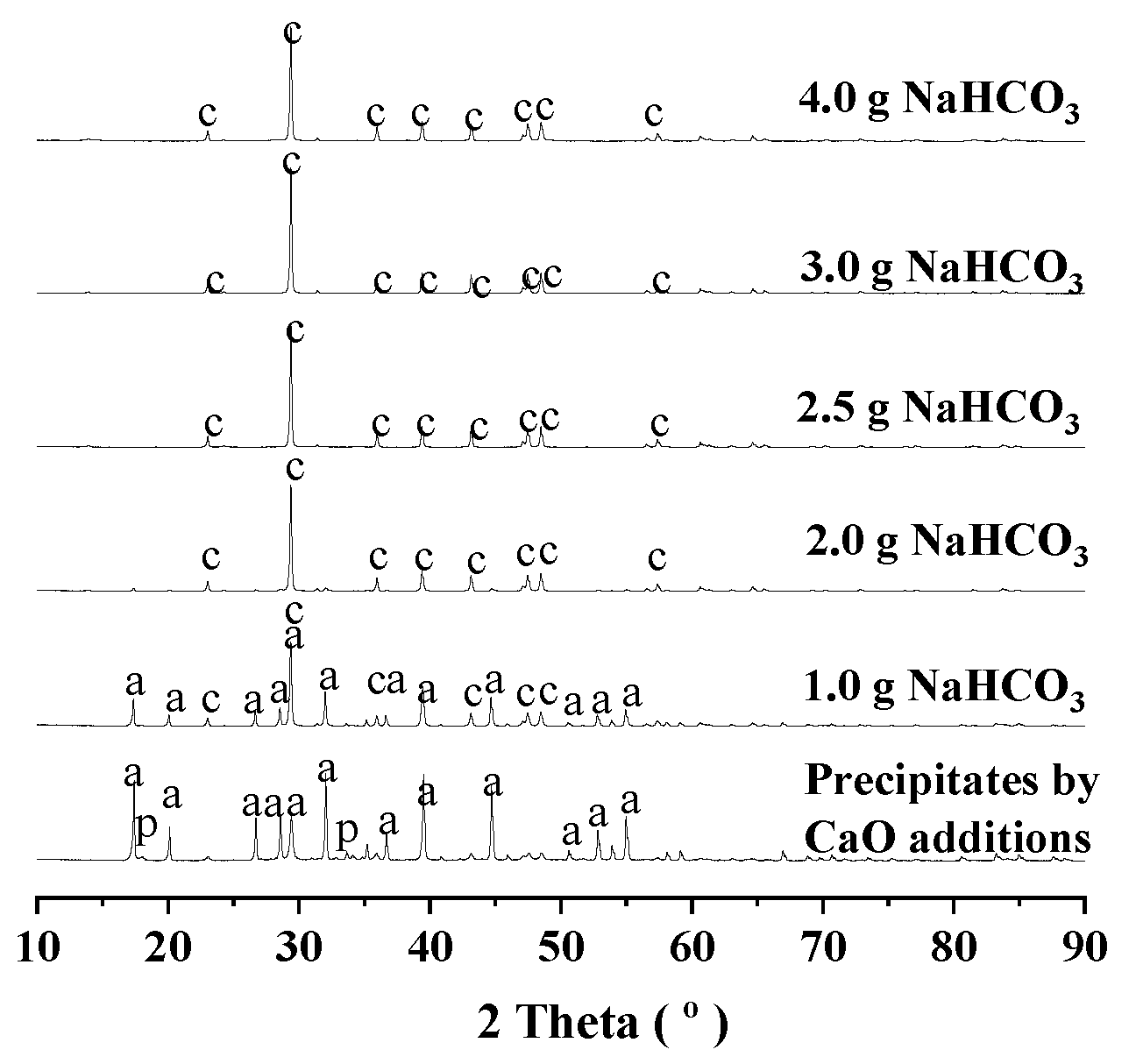

| Sample | Ash wt% | Na2O wt% | MgO wt% | Al2O3 wt% | SiO2 wt% | SO3 wt% | K2O wt% | CaO wt% | TiO2 wt% | Fe2O3 wt% |
|---|---|---|---|---|---|---|---|---|---|---|
| Raw Coals | 27.47 | 1.2 | 0.4 | 47.0 | 33.3 | 2.7 | 0.6 | 5.1 | 3.8 | 5.0 |
| Alkali-treated Coals | 27.42 | 26.2 | 0.4 | 26.1 | 28.7 | 4.3 | 0.5 | 5.0 | 3.3 | 4.7 |
| Acid-treated Coals | 0.46 | 1.9 | 0.4 | 13.7 | 48.9 | 3.7 | 0.2 | 10.3 | 10.0 | 7.1 |
| Na2O wt% | MgO wt% | Al2O3 wt% | SiO2 wt% | P2O5 wt% | SO3 wt% | CaO wt% | Fe2O3 wt% |
|---|---|---|---|---|---|---|---|
| 0.3 | 1.3 | 24.5 | 5.0 | 0.2 | 0.1 | 68.3 | 0.3 |
| Route One | Na2O wt% | Al2O3 wt% | SiO2 wt% | SO3 wt% | Cl wt% | CaO wt% | TiO2 wt% | Fe2O3 wt% |
|---|---|---|---|---|---|---|---|---|
| 350 °C salts calcination | 29.0 | 20.8 | 15.4 | 0.4 | 30.9 | 1.7 | 1.0 | 1.0 |
| 60 °C filter cakes dried | 0.3 | 45.5 | 46.7 | 0.8 | 0.3 | 1.3 | 2.8 | 2.4 |
| 90 °C filter cakes dried | 18.1 | 32.5 | 42.2 | bdl | bdl | 1.5 | 3.1 | 2.7 |
| 60 °C filter liquors dried | 44.5 | 0.5 | bdl | 0.4 | 52.0 | 2.6 | bdl | bdl |
| 90 °C filter liquors dried | 74.3 | 19.1 | 6.7 | bdl | bdl | bdl | bdl | bdl |
| Na2O wt% | Al2O3 wt% | SiO2 wt% | SO3 wt% | Cl wt% | K2O wt% | TiO2 wt% | Fe2O3 wt% |
|---|---|---|---|---|---|---|---|
| 0.3 | 0.1 | 96.4 | 0.4 | 0.7 | 0.1 | 1.7 | 0.3 |
| Route Two | Na2O wt% | Al2O3 wt% | SiO2 wt% | SO3 wt% | Cl wt% | CaO wt% | TiO2 wt% | Fe2O3 wt% |
|---|---|---|---|---|---|---|---|---|
| 350 °C calcination | 13.0 | 46.5 | bdl | 1.5 | 23.9 | 7.8 | 2.9 | 4.3 |
| 60 °C filter cakes dried | 0.5 | 81.5 | 0.1 | 1.6 | 0.3 | 0.6 | 8.4 | 7.0 |
| 90 °C filter cakes dried | bdl | 4.8 | bdl | bdl | bdl | 3.3 | 41.1 | 50.8 |
| 60 °C filter liquors dried | 39.1 | 0.4 | bdl | 0.6 | 51.4 | 8.5 | bdl | bdl |
| 90 °C filter liquors dried | 76.4 | 23.6 | bdl | bdl | bdl | bdl | bdl | bdl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L. Aluminum Extractions by the Alkali Method Directly from Alkali-Acid (NaOH-HCl) Chemical Deashing of Coals. Materials 2025, 18, 3661. https://doi.org/10.3390/ma18153661
Zhao L. Aluminum Extractions by the Alkali Method Directly from Alkali-Acid (NaOH-HCl) Chemical Deashing of Coals. Materials. 2025; 18(15):3661. https://doi.org/10.3390/ma18153661
Chicago/Turabian StyleZhao, Lijun. 2025. "Aluminum Extractions by the Alkali Method Directly from Alkali-Acid (NaOH-HCl) Chemical Deashing of Coals" Materials 18, no. 15: 3661. https://doi.org/10.3390/ma18153661
APA StyleZhao, L. (2025). Aluminum Extractions by the Alkali Method Directly from Alkali-Acid (NaOH-HCl) Chemical Deashing of Coals. Materials, 18(15), 3661. https://doi.org/10.3390/ma18153661







