Silicon-Based Polymer-Derived Ceramics as Anode Materials in Lithium-Ion Batteries
Abstract
1. Introduction
1.1. Anode Materials in Lithium-Ion Batteries
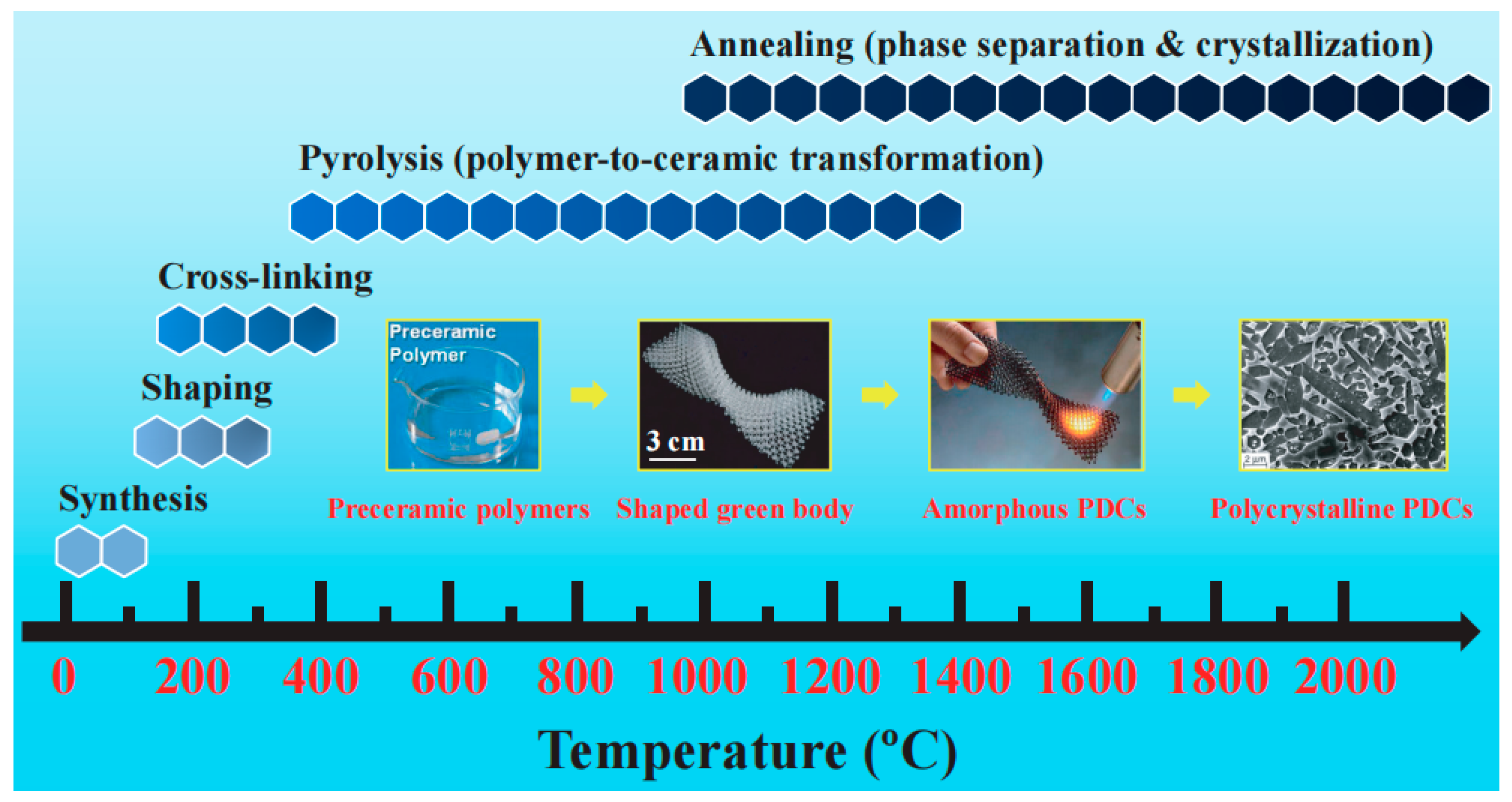
1.2. Si-Based Polymer-Derived Ceramics
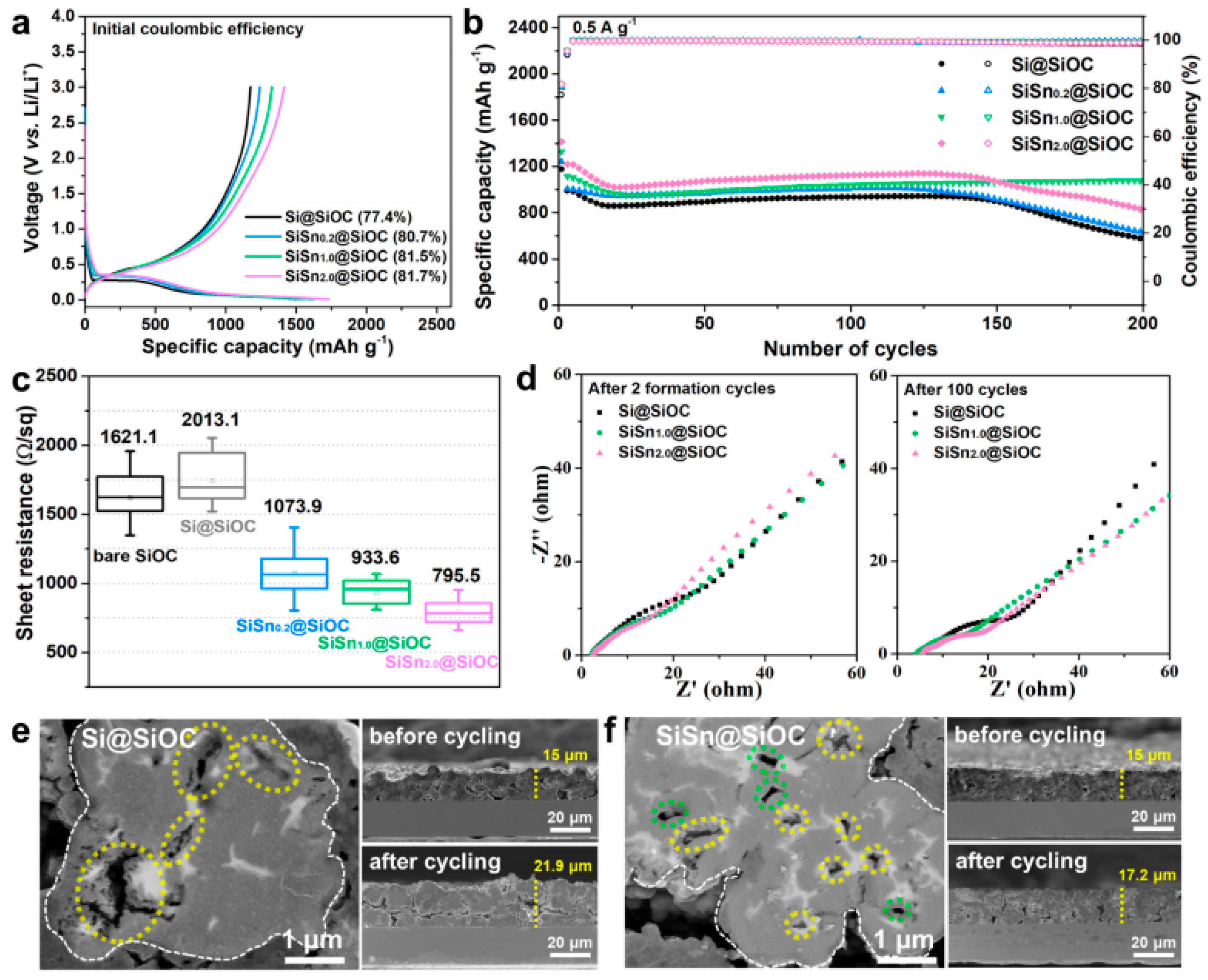
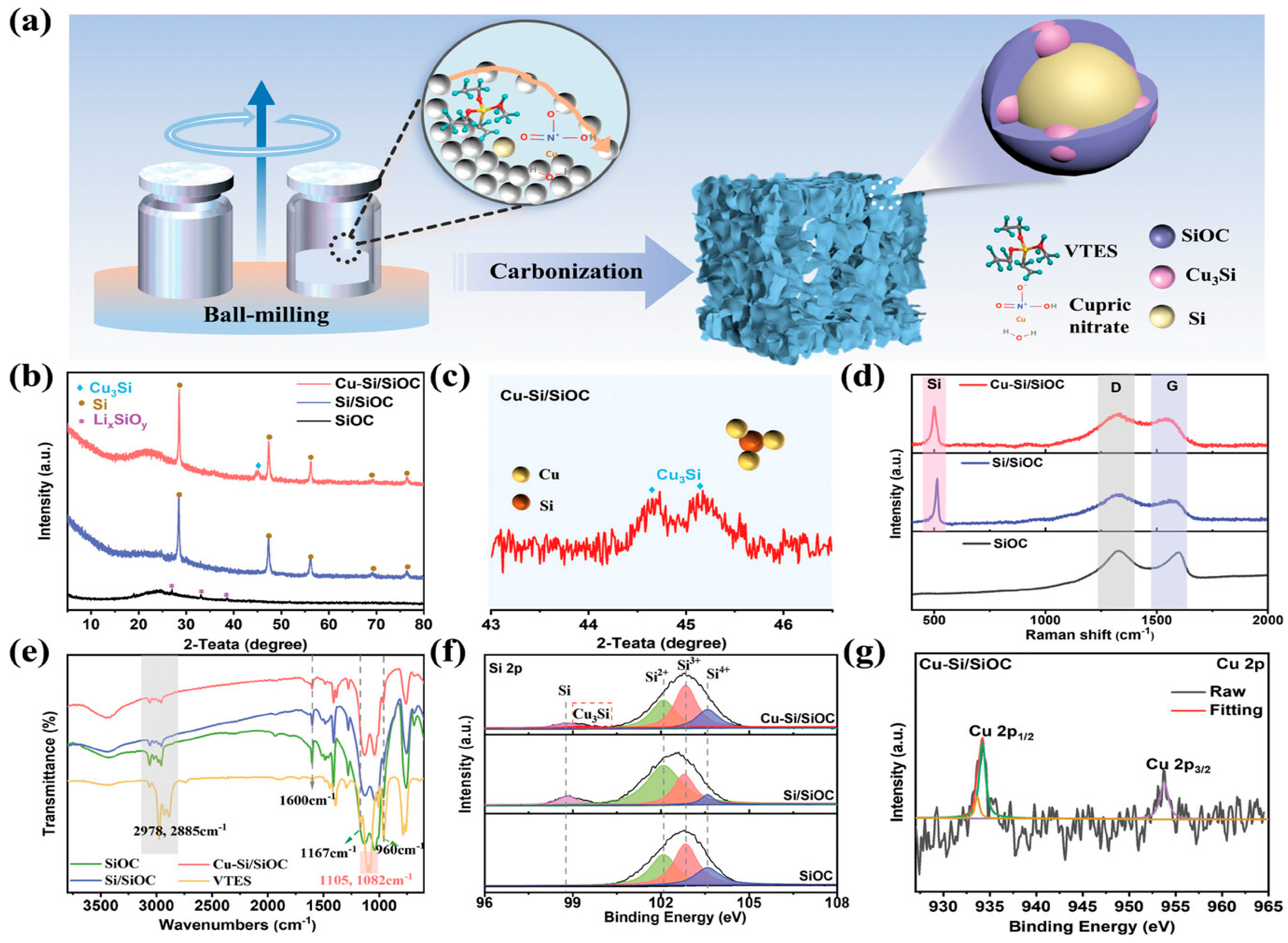
2. SiOC-Based Anode Materials
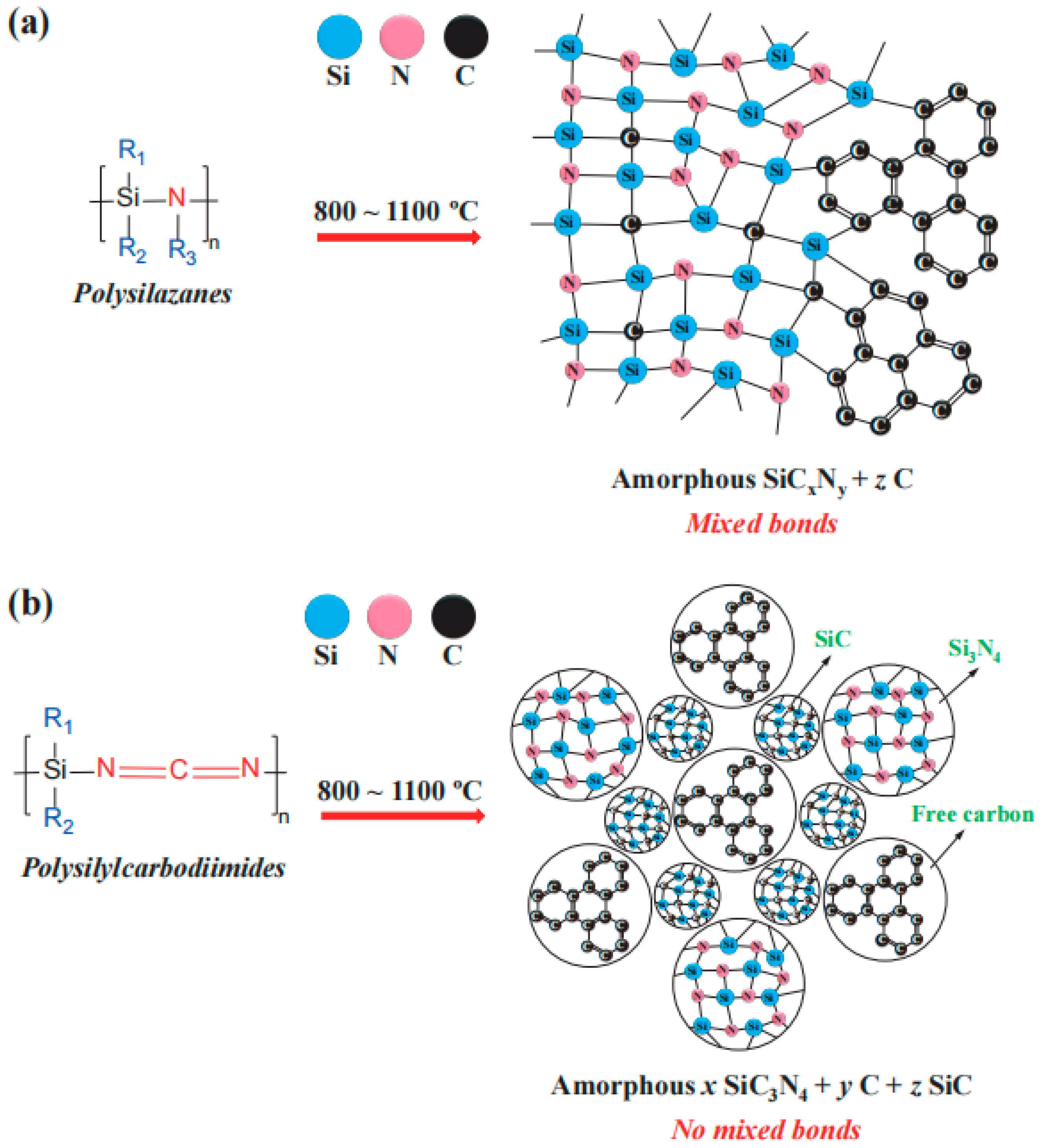
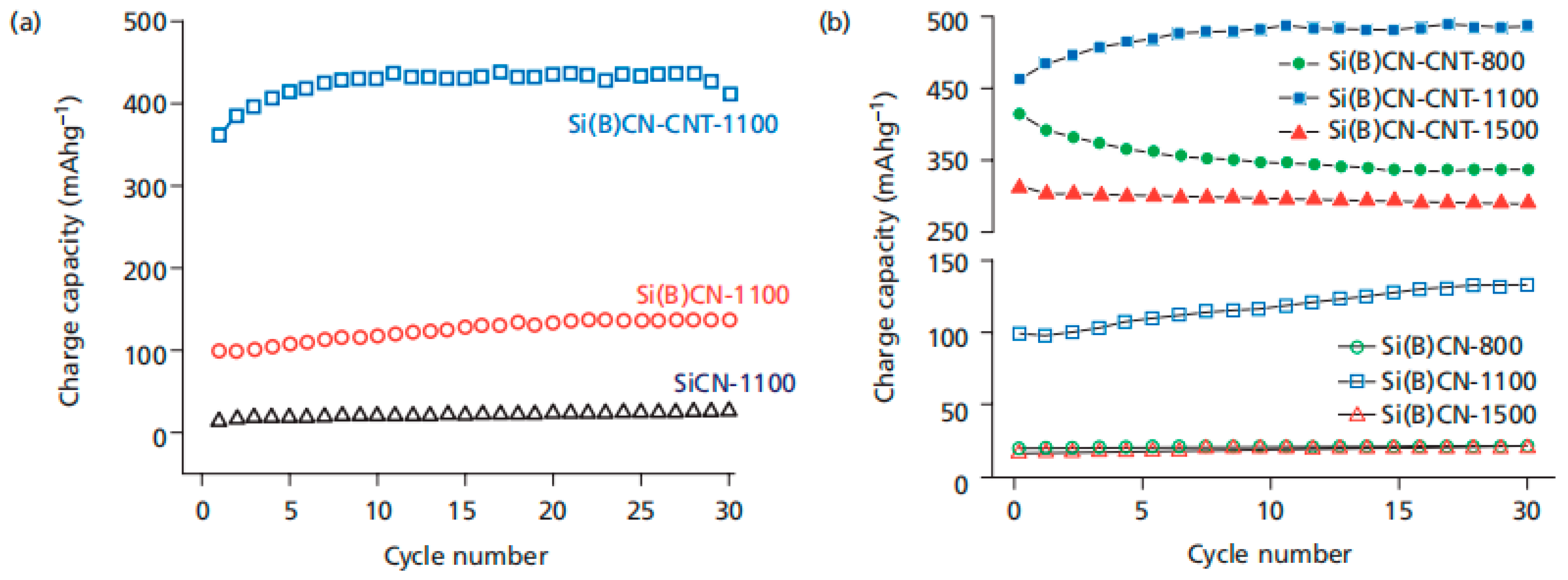
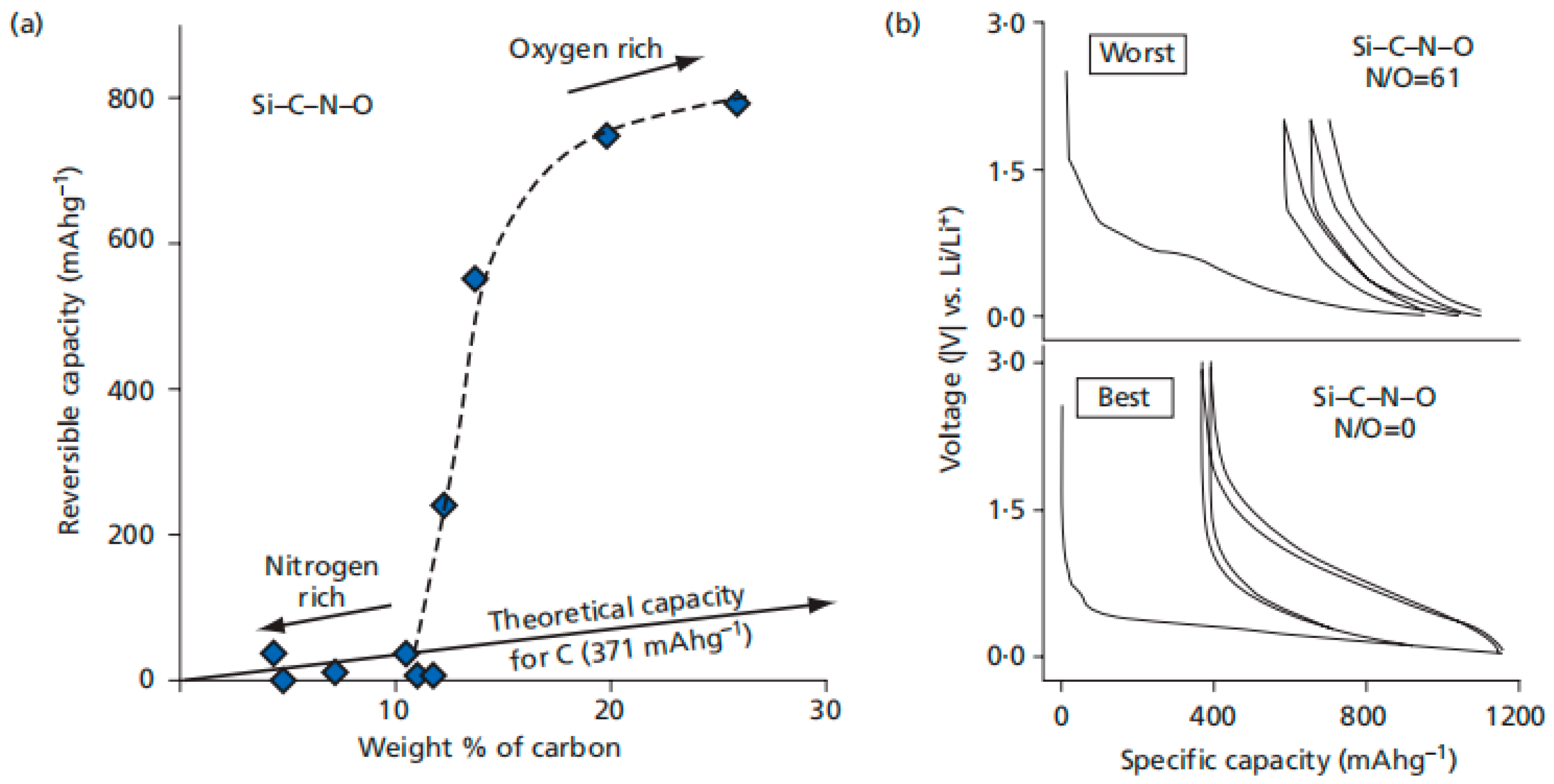
3. SiCN-Based Anode Materials
4. Other Si-Based Anode Materials
5. Mechanism for Lithium-Ion Storage in Si-Based Anode Materials
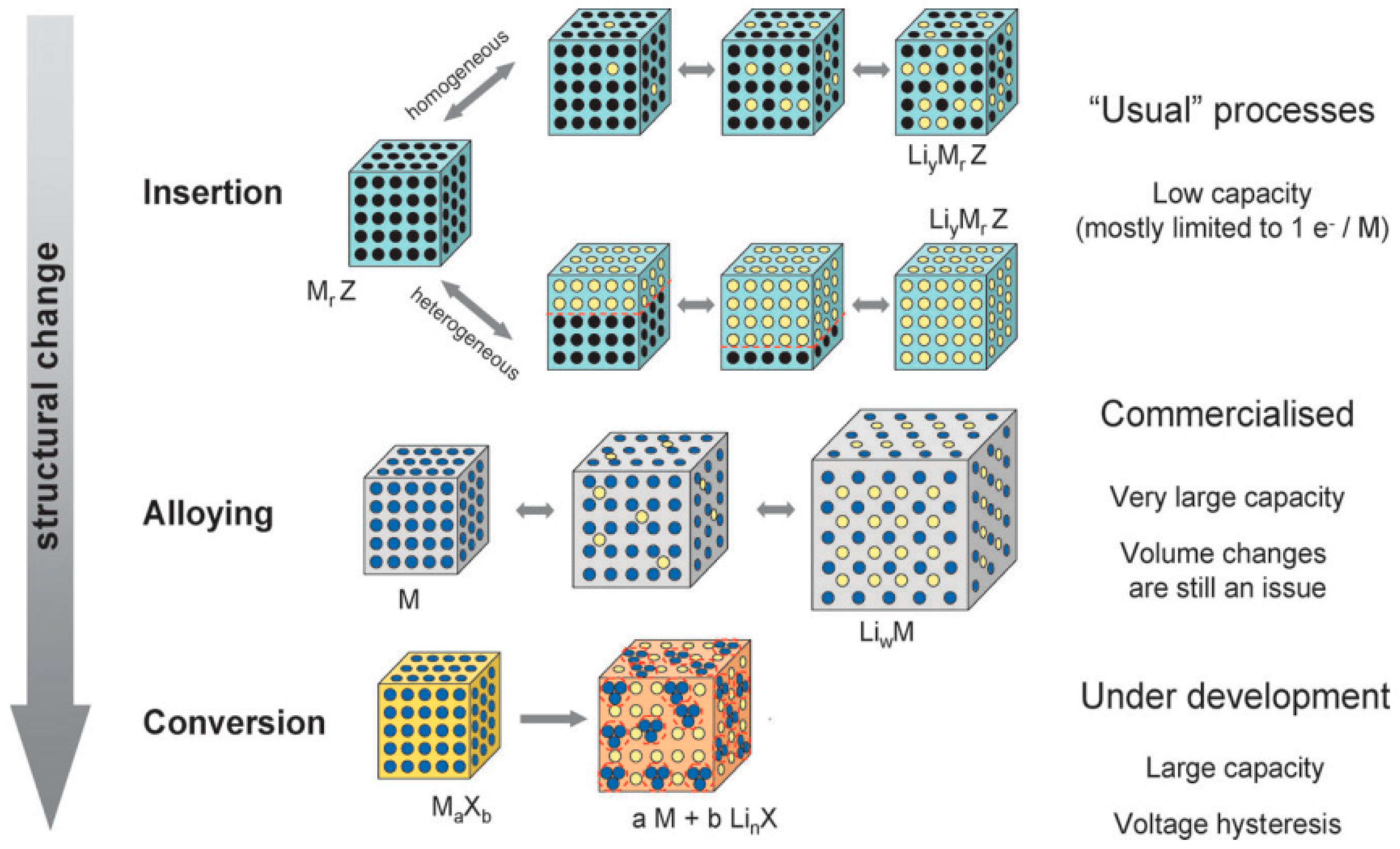
5.1. Intercalation/De-Intercalation Process
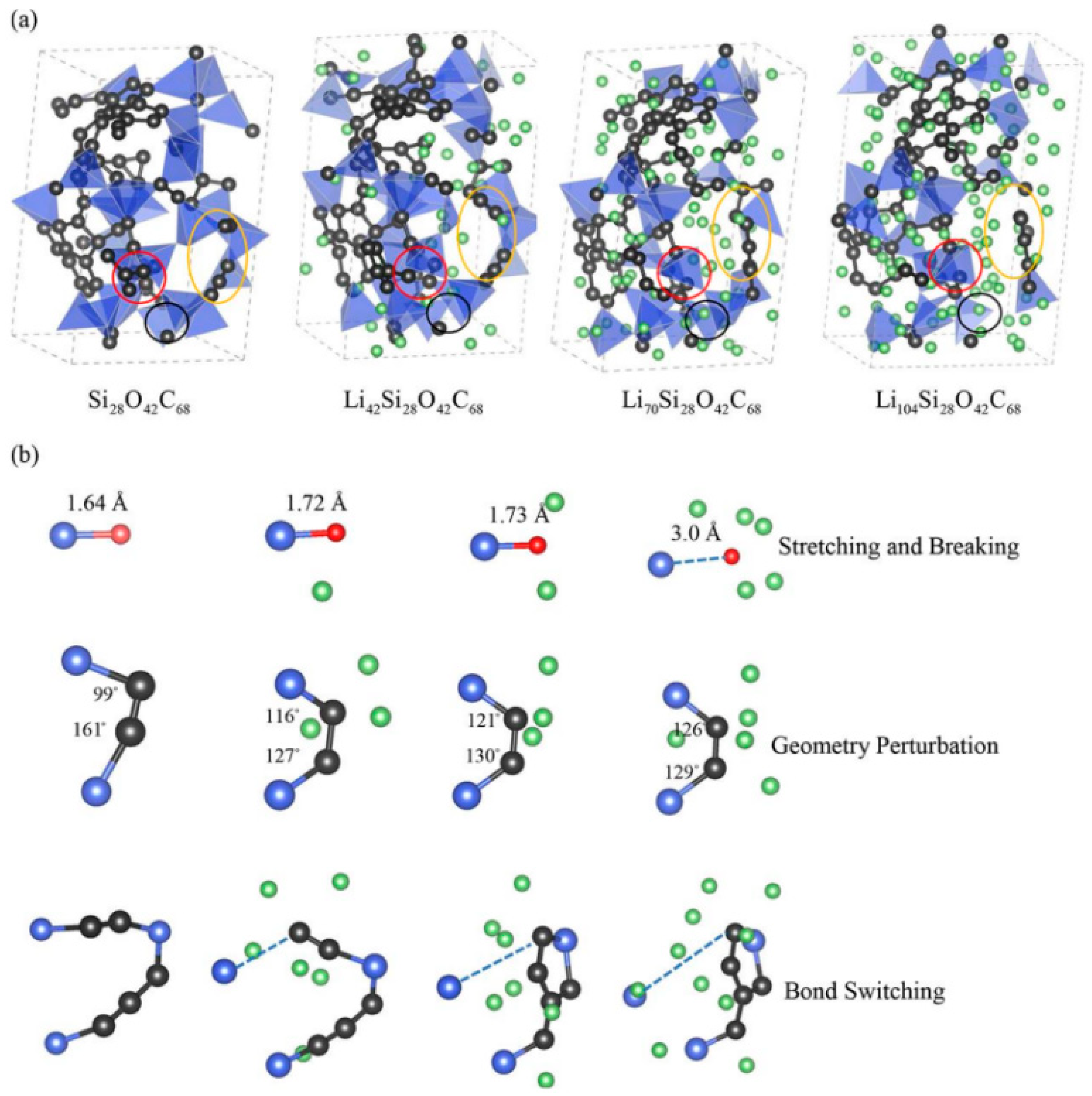
5.2. Phase Transition Behavior
5.3. Structural Influence
6. Role of Free Carbon in Si-Based Anode Materials
6.1. Improvement of Electrical Conductivity
6.2. Enhancement of Structural Stability
6.3. Optimization of Electrochemical Performance
7. Challenges
- (1)
- Capacity Limitation of Silicon-Glass Phases: The intrinsic properties of silicon-based glass phases inherently restrict capacity enhancement. While amorphous structures exhibit suboptimal long-term cycling stability and conductivity, strategic modifications—such as boron (B) and nitrogen (N) doping or composite design—can improve interfacial stability. Notably, the synergistic effects of multi-element doping (e.g., B-N co-doping) remain underexplored and warrant systematic investigation to unlock higher capacity retention.
- (2)
- Low Initial Coulombic Efficiency (ICE): The persistently low ICE (50–70%) severely hinders commercial viability. To bridge this gap, targeted strategies including pre-lithiation techniques (chemical/electrochemical) and surface coatings must be optimized. The trade-offs between ICE improvement and long-term cycling stability require quantitative analysis to establish optimal processing parameters.
- (3)
- Unresolved Interfacial Interactions: Compared to mainstream anode materials, the interactions between PDCs and other battery components (current collectors, binders, electrolytes) lack comprehensive understanding. Specifically, the correlation between particle size distribution and electrochemical performance (e.g., rate capability, SEI formation) remains ambiguous. The role of PDCs–electrolyte interphase dynamics in dictating cycling behavior needs mechanistic clarification.
- (4)
- Complex Storage Mechanisms and Performance Metrics: The capacity storage mechanism involves intricate interfacial reactions and phase transformations, where performance degradation correlates strongly with structural evolution. Key gaps include absence of standardized evaluation protocols for quantifying capacity fade mechanisms, incomplete understanding of how microstructural features (e.g., free carbon domains, Si nanoclusters) evolve during cycling, and lack of consensus on performance benchmarks (e.g., acceptable ICE thresholds, capacity retention rates).
8. Perspectives
- (1)
- Multi-Component Synergistic Design and Molecular Engineering: Develop multi-component PDCs systems through precise molecular-level design to tailor silicon-carbon-heteroatom network structures. Establish structure–property relationships linking microstructure (porosity, interfacial phases) to lithium storage mechanisms (alloying/intercalation/conversion reactions).
- (2)
- Advanced In Situ Characterization: Utilize advanced in situ platforms (e.g., in situ XRD/Raman) to dynamically resolve lithium-ion diffusion pathways within amorphous networks, track phase evolution during cycling, and reveal microscopic capacity fade mechanisms. This will provide theoretical guidance for structural optimization.
- (3)
- Development of Green Scalable Synthesis: Explore low-energy, environmentally benign synthetic routes, prioritizing precursor selection and reaction condition optimization (e.g., low-temperature adaptations of hydrothermal/sol-gel methods), strategies balancing cost control with yield enhancement, bridging the technical gap between lab-scale synthesis and industrial production.
- (4)
- Establishment of Standardized Testing Protocols: Develop unified electrochemical performance evaluation standards, including normalized data reporting formats (e.g., capacity retention rate calculation methods, rate capability testing procedures). Industry-wide adoption of these benchmarks will accelerate the translation from laboratory research to commercialization.
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X. Research on Preparation and Lithium Storage Mechanism of High Capacity C/Si-O-C Composite Anode Materials. Ph.D. Thesis, National University of Defense Technology, Changsha, China, 2014. [Google Scholar]
- Wang, M.; Tang, Y. A Review on the Features and Progress of Dual-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703320. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Gao, J.; Li, J.; Jiang, C. Manufacturing Method for Cathode Materials of Li-Ion Batteries. Energy Storage Sci. Eng. 2018, 7, 888–896. [Google Scholar]
- Zhou, H. Progress of Cathode Materials for High Energy Density Li-Ion Battery. In Proceedings of the 31st National Conference on Chemical and Physical Power Sources, Tianjin, China, 16–18 October 2015. [Google Scholar]
- Baboukani, A.R.; Khakpour, I.; Adelowo, E.; Drozd, V.; Shang, W.; Wang, C. High-Performance Red Phosphorus-Sulfurized Polyacrylonitrile Composite by Electrostatic Spray Deposition for Lithium-Ion Batteries. Electrochim. Acta 2020, 345, 136227. [Google Scholar] [CrossRef]
- Mishra, A.K.; Patial, B.S. A Review on Recent Advances in Anode Materials in Lithium Ion Batteries. Mater. Today Electron. 2024, 7, 100089. [Google Scholar] [CrossRef]
- Marom, R.; Amalraj, S.F.; Leifer, N.; Jacob, D.; Aurbach, D. A Review of Advanced and Practical Lithium Battery Materials. J. Mater. Chem. 2011, 21, 9938–9954. [Google Scholar] [CrossRef]
- Li, H.; Zhou, H. Enhancing the Performances of Li-Ion Batteries by Carbon-Coating: Present and Future. Chem. Commun. 2012, 48, 1201–1217. [Google Scholar] [CrossRef]
- Dimov, N.; Fukuda, K.; Umeno, T.; Kugino, S.; Yoshio, M. Characterization of Carbon-Coated Natural Graphite as a Lithium-Ion Battery Anode Material. J. Electrochem. Soc. 2002, 149, A499. [Google Scholar]
- Chou, C.-Y.; Kim, H.; Hwang, G.S. A Comparative First-Principles Study of the Structure, Energetics, and Properties of Li–M (M = Si, Ge, Sn) Alloys. JPCC 2011, 115, 20018–20026. [Google Scholar] [CrossRef]
- Baggetto, L.; Danilov, D.; Notten, P.H. Honeycomb-Structured Silicon: Remarkable Morphological Changes Induced by Electrochemical (De) Lithiation. Adv. Mater. 2011, 23, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-M.; Kim, J.-H.; Kim, H.; Sohn, H.-J. Li-Alloy Based Anode Materials for Li Secondary Batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef]
- Shi, B. Preparation and Electrochemical Properties of SiOC Ceramics. Ph.D. Thesis, Xiamen University, Xiamen, China, 2021. [Google Scholar]
- Dong, Y.; Slade, T.; Stolt, M.J.; Li, L.; Girard, S.N.; Mai, L.; Jin, S. Low-Temperature Molten-Salt Production of Silicon Nanowires by the Electrochemical Reduction of Casio3. Angew. Chem. Int. Ed. 2017, 56, 14453–14457. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Le, Z.; Chen, G.; Liu, D.; Liu, X.; Wu, H.B.; Xu, P.; Li, X.; Liu, F.; Chang, L.; et al. Graphene Caging Silicon Particles for High-Performance Lithium-Ion Batteries. Small 2018, 14, 1800635. [Google Scholar] [CrossRef]
- Choi, H.; No, P.; Lee, Y.-J.; Choi, J.-H. A Pore-Structured Si Alloy Anode Using an Unzipping Polymer for a Lithium Ion Battery. J. Appl. Electrochem. 2017, 47, 1127–1136. [Google Scholar] [CrossRef]
- Dahn, J.R.; Wilson, A.M.; Xing, W.; Zank, G.A. Electrodes for Lithium Ion Batteries Using Polysilazanes. U.S. Patent 5631106, 20 May 1997. [Google Scholar]
- Wen, Q.; Yu, Z.; Riedel, R. The Fate and Role of in Situ Formed Carbon in Polymer-Derived Ceramics. Prog. Mater Sci. 2020, 109, 100623. [Google Scholar] [CrossRef]
- Vrankovic, D.; Graczyk-Zajac, M.; Kalcher, C.; Rohrer, J.; Becker, M.; Stabler, C.; Trykowski, G.; Albe, K.; Riedel, R. Highly Porous Silicon Embedded in a Ceramic Matrix: A Stable High-Capacity Electrode for Li-Ion Batteries. ACS Nano 2017, 11, 11409–11416. [Google Scholar] [CrossRef]
- Ahn, D.; Raj, R. Cyclic Stability and C-Rate Performance of Amorphous Silicon and Carbon Based Anodes for Electrochemical Storage of Lithium. J. Power Sources 2011, 196, 2179–2186. [Google Scholar] [CrossRef]
- Sanchez-Jimenez, P.E.; Raj, R. Lithium Insertion in Polymer-Derived Silicon Oxycarbide Ceramics. J. Am. Ceram. Soc. 2010, 93, 1127–1135. [Google Scholar] [CrossRef]
- Sujith, R.; Gangadhar, J.; Greenough, M.; Bordia, R.K.; Panda, D.K. A Review of Silicon Oxycarbide Ceramics as Next Generation Anode Materials for Lithium-Ion Batteries and Other Electrochemical Applications. J. Mater. Chem. A 2023, 11, 20324–20348. [Google Scholar] [CrossRef]
- Bhandavat, R.; Singh, G. Improved Electrochemical Capacity of Precursor-Derived Si(B)Cn-Carbon Nanotube Composite as Li-Ion Battery Anode. ACS Appl. Mater. Interfaces 2012, 4, 5092–5097. [Google Scholar] [CrossRef]
- Sang, Z.; Yan, X.; Wen, L.; Su, D.; Zhao, Z.; Liu, Y.; Ji, H.; Liang, J.; Dou, S.X. A Graphene-Modified Flexible SiOC Ceramic Cloth for High-Performance Lithium Storage. Energy Storage Mater. 2020, 25, 876–884. [Google Scholar] [CrossRef]
- Rosa Palacin, M. Recent Advances in Rechargeable Battery Materials: A Chemist’s Perspective. Chem. Soc. Rev. 2009, 38, 2565–2575. [Google Scholar] [CrossRef]
- Fox, A.M.; Vrankovic, D.; Buchmeiser, M.R. Influence of the Silicon–Carbon Interface on the Structure and Electrochem-ical Performance of a Phenolic Resin-Derived Si@C Core–Shell Nanocomposite-Based Anode. ACS Appl. Mater. Interfaces 2021, 14, 761–770. [Google Scholar] [CrossRef]
- Wen, Q.; Qu, F.; Yu, Z.; Graczyk-Zajac, M.; Xiong, X.; Riedel, R. Si-Based Polymer-Derived Ceramics for Energy Conversion and Storage. J. Adv. Ceram. 2022, 11, 197–246. [Google Scholar] [CrossRef]
- Bois, L.; Maquet, J.; Babonneau, F.; Mutin, H.; Bahloul, D. Structural Characterization of Sol-Gel Derived Oxycarbide Glasses. 1. Study of the Pyrolysis Process. Chem. Mater. 1994, 6, 796–802. [Google Scholar] [CrossRef]
- Renlund, G.M.; Prochazka, S.; Doremus, R.H. Silicon Oxycarbide Glasses: Part Ii. Structure and Properties. J. Mater. Res. 1991, 6, 2723–2734. [Google Scholar] [CrossRef]
- Wilson, A.; Reimers, J.; Fuller, E.; Dahn, J. Lithium Insertion in Pyrolyzed Siloxane Polymers. Solid State Ion. 1994, 74, 249–254. [Google Scholar] [CrossRef]
- Pradeep, V.S.; Graczyk-Zajac, M.; Riedel, R.; Soraru, G.D. New Insights in to the Lithium Storage Mechanism in Polymer Derived SiOC Anode Materials. Electrochim. Acta 2014, 119, 78–85. [Google Scholar] [CrossRef]
- Graczyk-Zajac, M.; Vrankovic, D.; Waleska, P.; Hess, C.; Sasikumar, P.V.; Lauterbach, S.; Kleebe, H.-J.; Soraru, G.D. The Li-Storage Capacity of SiOC Glasses with and without Mixed Silicon Oxycarbide Bonds. J. Mater. Chem. A 2018, 6, 93–103. [Google Scholar] [CrossRef]
- Do, K.; Park, C.; Hwang, J.; Kim, S.; Jung, Y.; Lee, S.H.; Lim, H.-D.; Ahn, H. Covalent-Assisted Seeding of Si Nanoparticles into a Dual-Matrix Design toward Advanced Si-Based Li-Ion Batteries. J. Mater. Chem. A 2024, 12, 11062–11074. [Google Scholar] [CrossRef]
- Shen, J.; Ahn, D.; Raj, R. C-Rate Performance of Silicon Oxycarbide Anodes for Li+ Batteries Enhanced by Carbon Nanotubes. J. Power Sources 2011, 196, 2875–2878. [Google Scholar] [CrossRef]
- Ma, M.; Wang, H.; Xiong, L.; Huang, S.; Li, X.; Du, X. Self-Assembled Homogeneous SiOC@ C/Graphene with Three-Dimensional Lamellar Structure Enabling Improved Capacity and Rate Performances for Lithium Ion Storage. Carbon 2022, 186, 273–281. [Google Scholar] [CrossRef]
- Ren, Z.; Mujib, S.B.; Singh, G. High-Temperature Properties and Applications of Si-Based Polymer-Derived Ceramics: A Review. Materials 2021, 14, 614. [Google Scholar] [CrossRef]
- Wang, J.; Jin, S.; He, Z.; Kong, D.; Hu, H.; Feng, X.; Chen, D. Rational Design of Cu3si Interphase for 3d Micron-Sized SiOC-Based Anode to Enable Long-Term Cycling of Lithium-Ion Battery. Adv. Funct. Mater. 2025, 35, 2413540. [Google Scholar] [CrossRef]
- Li, K.; Yuan, G.; Liu, X.; Guo, Y.; Huang, R.; Li, H.; Zhang, H.; Jia, Q.; Xie, Z.; Zhang, S.; et al. On the Practical Applicability of Rambutan-Like SiOC Anode with Enhanced Reaction Kinetics for Lithium-Ion Storage. Adv. Funct. Mater. 2023, 33, 2302348. [Google Scholar] [CrossRef]
- Lin, X.; Dong, Y.; Liu, X.; Chen, X.; Li, A.; Song, H. In-Situ Pre-Lithiated Onion-Like SiOC/C Anode Materials Based on Metallasilsesquioxanes for Li-Ion Batteries. Chem. Eng. J. 2022, 428, 132125. [Google Scholar] [CrossRef]
- Lu, S.; Kan, S.; Yuan, M.; Zhang, X.; Huang, B.; Liu, S. Composite Anode Material for Lithium-Ion Batteries: Fabrication and Applications. Chinese Patent CN104659341B, 10 May 2017. [Google Scholar]
- Mera, G.; Riedel, R. Organosilicon-Based Polymers as Precursors for Ceramics. In Polymer Derived Ceramics: From Nanostructure to Applications; DEStech Publications, Inc.: Lancaster, PA, USA, 2009; pp. 51–89. [Google Scholar]
- Riedel, R.; Mera, G.; Hauser, R.; Klonczynski, A. Silicon-Based Polymer-Derived Ceramics: Synthesis Properties and Applications-a Review Dedicated to Prof. Dr. Fritz Aldinger on the Occasion of His 65th Birthday. J. Ceram. Soc. Jpn. 2006, 114, 425–444. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, N.-N.; Du, G.-X. Preparation and Electrochemical Performance of Polymer-Derived Sicn-Graphite Composite as Anode Material for Lithium Ion Batteries. Int. J. Electrochem. Sci. 2012, 7, 3135–3140. [Google Scholar] [CrossRef]
- Su, D.; Li, Y.L.; Feng, Y.; Jin, J. Electrochemical Properties of Polymer-Derived Sicn Materials as the Anode in Lithium Ion Batteries. J. Am. Ceram. Soc. 2009, 92, 2962–2968. [Google Scholar] [CrossRef]
- Graczyk-Zajac, M.; Mera, G.; Kaspar, J.; Riedel, R. Electrochemical Studies of Carbon-Rich Polymer-Derived Sicn Ceramics as Anode Materials for Lithium-Ion Batteries. J. Eur. Ceram. Soc. 2010, 30, 3235–3243. [Google Scholar] [CrossRef]
- Kaspar, J.; Mera, G.; Nowak, A.P.; Graczyk-Zajac, M.; Riedel, R. Electrochemical Study of Lithium Insertion into Carbon-Rich Polymer-Derived Silicon Carbonitride Ceramics. Electrochim. Acta 2010, 56, 174–182. [Google Scholar] [CrossRef]
- Reinold, L.M.; Yamada, Y.; Graczyk-Zajac, M.; Munakata, H.; Kanamura, K.; Riedel, R. The Influence of the Pyrolysis Temperature on the Electrochemical Behavior of Carbon-Rich Sicn Polymer-Derived Ceramics as Anode Materials in Lithium-Ion Batteries. J. Power Sources 2015, 282, 409–415. [Google Scholar] [CrossRef]
- Reinold, L.M.; Graczyk-Zajac, M.; Fasel, C.; Riedel, R. Prevention of Solid Electrolyte Interphase Damaging on Silicon by Using Polymer Derived Sicn Ceramics. ECS Trans. 2011, 35, 37. [Google Scholar] [CrossRef]
- Feng, Y.; Du, G.-X.; Zhao, X.-J.; Yang, E.-C. Preparation and Electrochemical Performance of Sicn–Cnts Composite Anode Material for Lithium Ion Batteries. J. Appl. Electrochem. 2011, 41, 999–1002. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, C.; Liu, Z.; Wang, W.; Xin, X.; Shen, L.; Zhou, X.; Zhou, J.; Huang, Q. Enhanced Rate Capability of Polymer-Derived Sicn Anode Material for Electrochemical Storage of Lithium with 3-D Carbon Nanotube Network Dispersed in Nanoscale. J. Nanosci. Nanotechnol. 2015, 15, 3067–3075. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, N.; Wei, Y.; Bai, Y. Preparation and Improved Electrochemical Performance of Sicn–Graphene Composite Derived from Poly (Silylcarbondiimide) as Li-Ion Battery Anode. J. Mater. Chem. A 2014, 2, 4168–4177. [Google Scholar] [CrossRef]
- Reinold, L.M.; Graczyk-Zajac, M.; Gao, Y.; Mera, G.; Riedel, R. Carbon-Rich Sicn Ceramics as High Capacity/High Stability Anode Material for Lithium-Ion Batteries. J. Power Sources 2013, 236, 224–229. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, N.; Du, G.; Wei, Y. Porous SiCN-HF Anode Materials for Lithium-Ion Batteries: Fabrication Methods and Applications. Chinese Patent CN103500814A, 26 August 2015. [Google Scholar]
- Chen, Q.; Li, D.; Yang, Z.; Jia, D.; Zhou, Y.; Riedel, R. Bcl3 Modified Tris(Dichloromethylsilylethyl)Borane as a Precursor for Sibcn Ceramics Applied in Lithium-Ion Battery Anodes. Ceram. Int. 2021, 47, 22839–22853. [Google Scholar] [CrossRef]
- Ning, L.J.; Wu, Y.P.; Wang, L.Z.; Fang, S.B.; Holze, R. Carbon Anode Materials from Polysiloxanes for Lithium Ion Batteries. J. Solid State Electrochem. 2005, 9, 520–523. [Google Scholar] [CrossRef]
- Idrees, M.; Batool, S.; Kong, J.; Zhuang, Q.; Liu, H.; Shao, Q.; Lu, N.; Feng, Y.; Wujcik, E.K.; Gao, Q. Polyborosilazane Derived Ceramics-Nitrogen Sulfur Dual Doped Graphene Nanocomposite Anode for Enhanced Lithium Ion Batteries. Electrochim. Acta 2019, 296, 925–937. [Google Scholar] [CrossRef]
- Wang, S.; Hu, X.; Dai, Y. Preparation and Electrochemical Performance of Polymer-Derived Sibcn-Graphene Composite as Anode Material for Lithium Ion Batteries. Ceram. Int. 2017, 43, 1210–1216. [Google Scholar] [CrossRef]
- Wang, J.; Kober, D.; Shao, G.; Epping, J.D.; Görke, O.; Li, S.; Gurlo, A.; Bekheet, M.F. Stable Anodes for Lithium-Ion Batteries Based on Tin-Containing Silicon Oxycarbonitride Ceramic Nanocomposites. Mater. Today Energy 2022, 26, 100989. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, J.; Xia, Q.; Zhang, L. Complexation-Assisted Polymerization for the Synthesis of Functional Silicon Oxycarbonitride with Well-Dispersed Ultrafine Cos as High- Performance Anode for Lithium-Ion Batteries. J. Alloys Compd. 2023, 949, 169824. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, J.; Yan, H.; Duan, X.; Wu, C.; Wang, Y.; Zhang, L. Synthesis of Novel Silicon Oxycarbonitride Anode Material and Controllable Chemical Prelithiation. Adv. New Renew. Energy 2024, 12, 664–670. [Google Scholar]
- Fukui, H.; Ohsuka, H.; Hino, T.; Kanamura, K. A Si-O-C Composite Anode: High Capability and Proposed Mechanism of Lithium Storage Associated with Microstructural Characteristics. ACS Appl. Mater. Inter. 2010, 2, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhao, K. Atomistic Origins of High Capacity and High Structural Stability of Polymer-Derived SiOC Anode Materials. ACS Appl. Mater. Interfaces 2017, 9, 35001–35009. [Google Scholar] [CrossRef]
- Kaspar, J. Carbon-Rich Silicon Oxycarbide (SiOC) and Silicon Oxycarbide/Element (SiOC/X, X = Si, Sn) Nano-Composites as New Anode Materials for Li-Ion Battery Application. Ph.D. Thesis, Technical University of Darmstadt, Darmstadt, Germany, 2014. [Google Scholar]
- Amaral, M.M.; Mujib, S.B.; Zanin, H.; Singh, G. A Perspective on Silicon-Based Polymer-Derived Ceramics Materials for Beyond Lithium-Ion Batteries. J. Phys. Mater. 2023, 6, 021001. [Google Scholar] [CrossRef]
- Francis, A. Progress in Polymer-Derived Functional Silicon-Based Ceramic Composites for Biomedical and Engineering Applications. Mater. Res. Express 2018, 5, 062003. [Google Scholar] [CrossRef]
- Kaspar, J.; Graczyk-Zajac, M.; Choudhury, S.; Riedel, R. Impact of the Electrical Conductivity on the Lithium Capacity of Polymer-Derived Silicon Oxycarbide (SiOC) Ceramics. Electrochim. Acta 2016, 216, 196–202. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, C.; Do, K.; Ahn, H. Maximizing the Utilization of Active Sites through the Formation of Native Nanovoids of Silicon Oxycarbide as Anode Materials in Lithium-Ion Batteries. Energy Storage Mater. 2021, 35, 130–141. [Google Scholar] [CrossRef]
- Wilamowsk, M.; Pradeep, V.S.; Graczyk-Zajac, M.; Riedel, R.; Sorarù, G.D. Tailoring of SiOC Composition as a Way to Better Performing Anodes for Li-Ion Batteries. Solid State Ion. 2014, 260, 94–100. [Google Scholar] [CrossRef]
- Kaspar, J.; Graczyk-Zajac, M.; Riedel, R. Lithium insertion into carbon-rich SiOC ceramics: Influence of pyrolysis temperature on electrochemical properties. J. Power Sources 2013, 244, 450–455. [Google Scholar] [CrossRef]
- Dibandjo, P.; Graczyk-Zajac, M.; Riedel, R.; Pradeep, V.S.; Soraru, G.D. Lithium Insertion into Dense and Porous Carbon-Rich Polymer-Derived SiOC Ceramics. J. Eur. Ceram. Soc. 2012, 32, 2495–2503. [Google Scholar] [CrossRef]
- Ahn, D.; Raj, R. Thermodynamic Measurements Pertaining to the Hysteretic Intercalation of Lithium in Polymer-Derived Silicon Oxycarbide. J. Power Sources 2010, 195, 3900–3906. [Google Scholar] [CrossRef]
- Pradeep, V.; Graczyk-Zajac, M.; Wilamowska, M.; Riedel, R.; Soraru, G. Influence of Pyrolysis Atmosphere on the Lithium Storage Properties of Carbon-Rich Polymer Derived SiOC Ceramic Anodes. Solid State Ion. 2014, 262, 22–24. [Google Scholar] [CrossRef]
- Pradeep, V.; Ayana, D.; Graczyk-Zajac, M.; Soraru, G.; Riedel, R. High Rate Capability of SiOC Ceramic Aerogels with Tailored Porosity as Anode Materials for Li-Ion Batteries. Electrochim. Acta 2015, 157, 41–45. [Google Scholar] [CrossRef]
- Liu, G.; Kaspar, J.; Reinold, L.M.; Graczyk-Zajac, M.; Riedel, R. Electrochemical Performance of Dvb-Modified SiOC and Sicn Polymer-Derived Negative Electrodes for Lithium-Ion Batteries. Electrochim. Acta 2013, 106, 101–108. [Google Scholar] [CrossRef]
- Wilamowska, M.; Graczyk-Zajac, M.; Riedel, R. Composite Materials Based on Polymer-Derived Sicn Ceramic and Disordered Hard Carbons as Anodes for Lithium-Ion Batteries. J. Power Sources 2013, 244, 80–86. [Google Scholar] [CrossRef]
- Li, K.; Yuan, G.; Liu, X.; Xie, Q.; Dong, L.; Li, Z.; Zhang, H.; Xie, Z.; Zhang, S.; Lei, W. Deciphering Fast Lithium Storage Kinetics Via R-Based Self-Derivation Effects in Siloxanes. Energy Storage Mater. 2024, 65, 103194. [Google Scholar] [CrossRef]


| Samples | Free Carbon [wt%] | Crev [mAh∙g−1] | Cirr [mAh∙g−1] | η [%] | Cycling Current [mA g−1] | Capacity Retention | Ref. |
|---|---|---|---|---|---|---|---|
| SiO1.5C3.9 | 44.3 | 640 | 340 | 65 | 14.8 | n.d. | [30] |
| SiO0.51C7.78 | 65.2 | 608 | 259 | 70 | 32.7 | 95% after 40 cycles | [61] |
| SiO0.85C1.99 | 25.9 | 794 | 370 | 68 | 100 | n.d. | [71] |
| SiO0.90C4.40 | 48.5 | 568 | 330 | 63 | 18 | cycling stable | [72] |
| SiO0.98C2.47 | 32.0 | 605 | 325 | 65 | 18 | cycling stable | [31] |
| SiO1.59C3.36 | 43 | 600 | 680 | 47 | 360 | cycling stable | [73] |
| SiO1.18C5.52 | 54.2 | 504.3 | 287.1 | 63.7 | 37 | 68.8% after 60 cycles | [66] |
| SiO0.95C3.72 | 43.6 | 535.9 | 335.8 | 61.5 | 37 | 56.0% after 60 cycles | [66] |
| SiO1.01C2.93 | 36.8 | 434.3 | 273.8 | 61.3 | 37 | 58.7% after 60 cycles | [66] |
| SiO0.93C2.26 | 29.5 | 501.4 | 302.7 | 62.3 | 37 | 47.3% after 60 cycles | [66] |
| SiO0.87C1.62 | 20.6 | 682.5 | 495.8 | 57.9 | 37 | 13.5% after 60 cycles | [66] |
| SiO1.00C1.05 | 11.6 | 706.1 | 375.5 | 65.3 | 37 | 5.2% after 60 cycles | [66] |
| SiO1.40C0.70 | 8.1 | 500.7 | 754.6 | 39. 9 | 37 | 1.5% after 60 cycles | [66] |
| SiC5.35N0.98O0.19 | 57.04 | 383 | 172 | 69 | 18 | cycling stable | [46] |
| SiC3.70N0.69O0.62 | 46.02 | 241 | 291 | 45 | 18 | cycling stable | [45] |
| SiO0.06C1.54N0.74 | 23.2 | 69 | 67 | 50.6 | 18.6 | 127.5% after 114 cycles | [74] |
| SiO0.05C2.22N0.84 | 33.4 | 278 | 199 | 58.3 | 18.6 | 112.9% after 114 cycles | [74] |
| SiO0.10C4.04N0.69 | 49.3 | 374 | 227 | 60.5 | 18.6 | 115.9% after 114 cycles | [74] |
| SiC3.9O0.1N0.8 | 48 | 703 | 375 | 65 | 18 | 89% after 134 cycles | [52] |
| SiC10.59O1.56N0.21 | 69.1 | 570 | 367 | 61 | 18 | cycling stable | [75] |
| SiC3.7O0.1N1.3H0.9 | 48.1 | 674 | 525 | 56 | 18.6 | 68% after 134 cycles | [47] |
| SiC5.3O0.3N1.2H0.2 | 56.0 | 282 | 224 | 56 | 18.6 | 109% after 134 cycles | [47] |
| SiOC-phenyl | 37.5 | 793 | 394 | 67 | 100 | cycling stable | [76] |
| SiOC-propyl | 14.3 | 687 | 660 | 51 | 100 | cycling stable | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Fei, H.; Wang, C.; Ma, H.; Li, X.; Gao, P.; Wen, Q.; Tao, S.; Xiong, X. Silicon-Based Polymer-Derived Ceramics as Anode Materials in Lithium-Ion Batteries. Materials 2025, 18, 3648. https://doi.org/10.3390/ma18153648
Zhang L, Fei H, Wang C, Ma H, Li X, Gao P, Wen Q, Tao S, Xiong X. Silicon-Based Polymer-Derived Ceramics as Anode Materials in Lithium-Ion Batteries. Materials. 2025; 18(15):3648. https://doi.org/10.3390/ma18153648
Chicago/Turabian StyleZhang, Liang, Han Fei, Chenghuan Wang, Hao Ma, Xuan Li, Pengjie Gao, Qingbo Wen, Shasha Tao, and Xiang Xiong. 2025. "Silicon-Based Polymer-Derived Ceramics as Anode Materials in Lithium-Ion Batteries" Materials 18, no. 15: 3648. https://doi.org/10.3390/ma18153648
APA StyleZhang, L., Fei, H., Wang, C., Ma, H., Li, X., Gao, P., Wen, Q., Tao, S., & Xiong, X. (2025). Silicon-Based Polymer-Derived Ceramics as Anode Materials in Lithium-Ion Batteries. Materials, 18(15), 3648. https://doi.org/10.3390/ma18153648








