Abstract
The electrochemical reduction of nitrate to ammonia offers a promising solution for both alleviating nitrate pollution in wastewater and providing a sustainable ammonia source for agriculture use. This review focuses on the role of carbon-based catalysts in electrochemical nitrate reduction to ammonia, emphasizing their potential in addressing environmental pollution and supporting sustainable ammonia production. Carbon materials, known for their abundance, affordability, and eco-friendly properties, are central to this process. The review highlights key strategies for enhancing catalytic performance, including heteroatom doping, the development of porous structures, and the integration of metal/metal oxide nanoparticles. Additionally, it addresses significant challenges such as weak nitrate adsorption, slow reaction kinetics, and competition with the hydrogen evolution reaction. Through the integration of advanced material design, mechanistic insights, and innovative engineering strategies, this review provides valuable guidance for the future design of carbon-based catalysts, paving the way for significant advancements in both nitrate removal and sustainable ammonia synthesis.
1. Introduction
The increasing presence of nitrate pollution in water bodies has become a growing environmental concern globally [1]. Nitrate contamination originates mainly from agricultural runoff, industrial effluents, and wastewater discharges, leading to detrimental effects on ecosystems and public health [2,3]. Traditional approaches of nitrate treatment from water, such as biological denitrification and reverse osmosis, are often costly, energy-intensive, and produce undesirable by-products [4,5]. This highlights the urgent need for efficient, sustainable, and economically viable alternatives for nitrate remediation. Recently, the electrochemical nitrate reduction reaction (NO3RR) has emerged as a promising technology to address this challenge, not only for nitrate removal but also for the production of valuable by-products, particularly ammonia (NH3) [6,7,8]. NH3, a key component of fertilizers, has traditionally been synthesized through the energy-intensive Haber–Bosch process. Electrochemical NO3RR, however, offers a potential pathway for “green ammonia” production, aligning with both environmental sustainability goals and the growing demand for ammonia in agriculture.
Among the various catalytic materials explored for NO3RR, carbon-based catalysts have attracted considerable interest because of their unique advantages. Carbon materials are abundant, low-cost, environmentally friendly, and exhibit high electrical conductivity [9,10,11]. Moreover, their structural properties can be easily tailored to enhance their catalytic performance, making them ideal candidates for electrochemical applications [12,13]. These materials can be modified at the atomic and molecular levels, allowing for improved activity, selectivity, and stability in various reactions, including nitrate reduction. The versatility of carbon-based materials such as carbon nanotubes (CNTs), graphene, and various carbon composites further expands their applicability in NO3RR processes, providing ample opportunities for innovation in catalyst design and optimization.
Despite their potential, carbon-based catalysts for NO3RR face several challenges. The weak adsorption of nitrate ions on carbon surfaces hinders efficient reduction, as strong interactions are needed for electron transfer and reduced activation energy. Additionally, slow reaction kinetics lead to low current efficiencies. The competitive hydrogen evolution reaction (HER) further complicates the process, diverting electrons from nitrate reduction and reducing selectivity [14,15,16,17,18]. To improve efficiency, carbon-based catalysts must suppress HER and enhance nitrate reduction by optimizing their electronic structure, surface properties, and reaction conditions. Significant advancements have been achieved in addressing these challenges in recent years. Researchers have developed various strategies to improve the performance of carbon-based catalysts for NO3RR, such as doping with heteroatoms (e.g., nitrogen, sulfur, phosphorus) to modify the electronic properties of the carbon surface, creating porous structures to increase the surface area and improve nitrate adsorption, and designing composite catalysts by integrating carbon materials with metal or metal oxide nanoparticles to boost catalytic activity [19,20,21,22]. These strategies aim to enhance the binding affinity for nitrate ions, accelerate electron transfer, and suppress HER, thereby improving the overall performance of the electrochemical nitrate reduction process.
In this review, we provide an in-depth analysis of the design strategies and mechanistic insights related to carbon-based catalysts for NO3RR. We mainly highlight the unique properties of carbon-based materials in this context and outline the key challenges associated with their use. The review further explores the latest advances in catalyst design, focusing on strategies to improve nitrate adsorption, enhance catalytic kinetics, suppress HER, and improve catalyst durability. By synthesizing the current state of research, this review aims to offer valuable insights into the future direction of carbon-based catalyst development for NO3RR, ultimately contributing to more efficient and sustainable nitrate removal and ammonia production technologies.
2. Experimental Techniques for NO3RR
2.1. Fundamental Electrochemical Cell Configurations
The H-cell setup is widely employed for initial catalyst screening. This configuration consists of two compartments—an anode and a cathode—separated by an ion-exchange membrane to prevent product crossover and oxidation at the anode. The working electrode, coated with the carbon-based catalyst, is submerged in the catholyte. A counter electrode, such as a Pt wire or graphite rod, and a reference electrode, like Ag/AgCl, Hg/HgO, or SCE, complete the three-electrode system, typically controlled by a potentiostat/galvanostat. The catholyte volume is usually small, and the system is often purged with an inert gas before and during experiments to exclude oxygen. While versatile and simple, H-cells may experience significant mass transport limitations, particularly at high current densities.
Flow cells are increasingly utilized for experiments requiring higher reaction rates and enhanced mass transport. In these systems, catalyst-coated electrodes, such as gas diffusion electrodes, are incorporated into a cell where the catholyte or the anolyte is continuously circulated. This design enhances nitrate flux to the catalyst surface, enabling the study of reactions at industrially relevant current densities. Flow cells are critical for evaluating practical feasibility but introduce engineering challenges related to sealing and product separation/analysis.
2.2. Electrochemical Testing Protocols
Carbon materials are typically dispersed in a solvent mixture (e.g., water, isopropanol), binder (e.g., Nafion), and sometimes conductive agents (e.g., carbon black). The resulting ink is drop-cast, spray-coated, or doctor-bladed onto conductive substrates such as carbon paper, carbon cloth, glassy carbon, or Ti foil.
Cyclic voltammetry is used to probe the redox behavior of the catalyst, estimate the electrochemical active surface area, and identify the onset potentials for nitrate reduction and associated reaction kinetics.
Linear sweep voltammetry provides steady-state or pseudo-steady-state current measurements as a function of applied potential, generating polarization curves to assess the activity of different catalysts.
Chronoamperometry (CA) and chronopotentiometry (CP) are employed to quantify reaction products over extended periods. In CA, a fixed potential is applied, while in CP, a constant current density is used. Both methods typically run for 1–2 h for initial screening and longer for stability tests. The electrolyte is sampled periodically for product analysis and yield quantification.
2.3. Key Performance Metrics
The ammonia yield rate is the mass of NH3 produced per unit time and per unit geometric area of the electrode (e.g., mg h−1 cm−2) or per unit mass of catalyst (e.g., mg h−1 mgcat−1).
Faradaic efficiency (FENH3) is the percentage of the total charge passed that is utilized for ammonia production. It is calculated as:
where n is the number of electrons transferred per NH3 molecule (8e−), F is Faraday’s constant, CNH3 is the ammonia concentration, V is the electrolyte volume, Q is the total charge passed, and M is the molecular weight of NH3.
FENH3 = (n·F·CNH3·V/Q·M) × 100%
The selectivity of the product is defined as the ratio of the concentration of the desired product to the degraded concentration of nitrate, expressed as a percentage.
Stability and selectivity over time are assessed through long-term CA/CP experiments with periodic product analysis. Post-mortem catalyst characterization is often conducted to determine the stability and selectivity of the catalyst over extended reaction times.
3. Carbon Material Categories for NO3RR
The selection and design of carbon-based materials are crucial for improving the efficiency of NO3RR. These materials can be broadly classified into two main categories: intrinsic carbon materials and functionalized carbon-based composites. Intrinsic carbon materials, including activated carbon, graphene, and CNTs, exhibit distinctive characteristics such as a high surface area and electrical conductivity, making them advantageous for electrochemical processes. On the other hand, functionalized carbon-based composites incorporate heteroatoms, metals, or metal oxides to enhance catalytic activity, improve nitrate adsorption, and overcome challenges like slow reaction kinetics and hydrogen evolution competition.
3.1. Intrinsic Carbon Materials
Intrinsic carbon materials, including graphene, CNTs, and porous carbon architectures, demonstrate critical advantages for NO3RR applications owing to their superior electrical conductivity, tailorable surface chemistry, and natural abundance. These intrinsic characteristics enable essential functional enhancements in electrocatalytic systems by promoting efficient electron transport mechanisms, optimizing nitrate ion adsorption kinetics, and establishing favorable mass transfer pathways throughout the reduction process.
3.1.1. Graphene/Oxidized Graphene
Graphene, composed of sp2-hybridized carbon atoms in a hexagonal structure, is a highly efficient electron conductor, making it a perfect choice for electrochemical applications [23,24,25]. The flat, two-dimensional structure of graphene facilitates effective electron transfer during the reduction of nitrate. Graphene and graphene oxide are rarely used directly as electrocatalysts in the NO3RR. Instead, their catalytic performance is typically enhanced by introducing defects or modifications to their structure. These modifications can include doping with various elements (such as nitrogen, boron, or sulfur), creating vacancies, or functionalizing the surface with oxygen-containing groups. These approaches help to optimize the electronic properties and increase the catalytic activity of graphene-based materials, making them more effective for nitrate reduction reactions. Recent advancements in this field have focused on controlling the density and type of defects to improve the selectivity and efficiency of the electrochemical process, paving the way for more efficient and sustainable catalysts for NO3RR. The specific research progress will be discussed in the following sections.
3.1.2. Carbon Nanotubes
CNTs are another promising class of carbon-based materials for NO3RR. Their one-dimensional tubular structure induces a confinement effect, which can modulate reaction pathways at the atomic level. CNTs provide high surface area and structural integrity, which are crucial for stabilizing the active sites involved in nitrate reduction [26,27]. The unique confinement effect helps control the adsorption of nitrate ions, thereby guiding the reaction toward the desired product. Additionally, CNTs exhibit excellent conductivity, which further enhances the overall electrochemical performance of the catalyst. The researchers discovered that pristine multi-walled carbon nanotubes (MWCNTs) displayed significant electrocatalytic performance for the reduction of NO3− to NH3 in a near-neutral solution, while single-walled CNTs showed even greater activity and selectivity (Figure 1a–c). Different from the conventional belief that heteroatom doping improves electrocatalytic performance, the introduction of oxygen and nitrogen groups to MWCNTs actually decreased their activity for NO3− reduction. These results suggest that carbon serves as the active site for NO3RR [28].
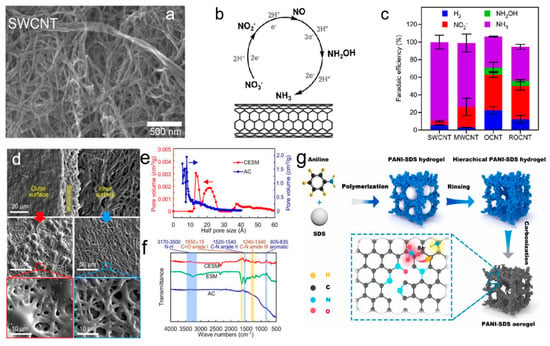

Figure 1.
(a) SEM images of CNT. (b) Potential reaction pathway for the reduction of NO3− to NH3. (c) Distribution of products for various CNTs at –0.85 V in a solution of 0.1 M PB + 0.4 M KNO3. Reproduced from ref. [28] copyright (2022), with permission from the American Chemical Society. (d–f) Characteristics of CESM and AC: (d) SEM images; (e) pore size distribution for different carbon materials; (f) FTIR spectra for various carbon materials. Reproduced from ref. [29] copyright (2025), with permission from Elsevier. (g) Schematic of the synthesis process for N-C-1000 catalysts derived from hydrogels. Reproduced from ref. [30] copyright (2025), with permission from Elsevier.
3.1.3. Porous Carbon
Porous carbon materials, such as activated carbon and carbon aerogels, are known for their high surface area and tunable pore structures. These materials offer significant advantages in terms of nitrate ion enrichment and mass transport optimization. The hierarchical porous structure of these materials facilitates the diffusion of nitrate ions and provides abundant active sites for adsorption, which is critical for improving the efficiency of NO3RR [30,31]. Chen et al. developed an ultrathin porous electrode from eggshell bio-waste for the electrosorption of anionic species [29]. The carbonized eggshell membrane (CESM) displayed a hierarchical pore structure and, despite its low surface area, showed a significantly higher ion adsorption capacity than activated carbon (Figure 1d–f). CESM exhibited the highest selectivity for NO3− over competing anions. Density functional theory (DFT) calculations revealed that nitrogen-containing functional groups in a porous structure played a key role in NO3− adsorption. These findings highlighted the potential of porous biomaterials for selective NO3− electrodes and suggested pathways for enhancing their performance through activation or functionalization.
3.2. Functionalized Carbon-Based Composites
Functionalization of carbon materials through heteroatom doping or the incorporation of metal catalysts has emerged as an effective strategy to enhance their catalytic performance for NO3RR. These modifications can significantly alter the electronic properties and surface characteristics of the carbon materials, facilitating better nitrate adsorption and electron transfer while suppressing unwanted reactions like HER.
3.2.1. Heteroatom-Doped Carbon
Doping carbon materials with heteroatoms such as nitrogen (N), boron (B), phosphorus (P), and sulfur (S) introduces functional groups that modify the electronic structure and enhance the reactivity of the material. N doping, in particular, has been widely studied for its ability to induce charge redistribution and create more favorable sites for nitrate adsorption. For example, pyridinic N sites are known to promote the adsorption of NO3−, while graphitic N can enhance conductivity and improve the overall electrochemical performance (Figure 1g) [30,32,33]. B and P doping can disrupt the inherent inertness of carbon through activating the surface and enabling more effective stabilization of intermediate species like nitrite [34,35,36]. In Jiang et al.’s study on B and O dual-doped carbon nitride nanotubes (B/O-CNNTs) synthesized via a copolymerization process, the dual doping of B and O not only altered the coordination environment and electronic structure of the carbon nitride framework, but also introduced frustrated Lewis pairs, which were crucial for activating chemisorbed nitrate (Figure 2a–c). The B/O-CNNTs achieved maximum Faradaic efficiencies of 96% at −1.1 V vs. RHE for NO3RR, with a corresponding ammonia yield of 211.4 μg h−1 mg−1. The unique 1D nanotubular structure, carbon vacancies, and the synergistic effect of B/O doping enhanced interactions among reactants and boosted electron transfer rates [37]. These modifications helped to break the carbon material’s inertness and optimized its catalytic properties for NO3RR. Heteroatom-doped carbon materials are commonly utilized as catalyst supports, as the incorporation of heteroatoms alongside metal species creates a coordinated environment that can modulate the d-band structure of metal. This interaction effectively fine-tunes the electronic properties of the metal, enhancing catalytic performance.
3.2.2. Defective Carbon
The introduction of defects in carbon materials, such as vacancies or edge defects, is another promising approach to enhance catalytic performance. These defects create active sites that can serve as favorable adsorption points for nitrate ions and facilitate electron transfer during the reduction reaction. Vacancies, such as five-membered or seven-membered rings, are particularly useful for enhancing catalytic activity in electrochemical reactions by providing a high density of reactive sites. Surface functional groups, like -COOH or -OH, also play a crucial role in tuning the hydrophilicity of the material, which can influence the adsorption behavior of nitrate and improve mass transport [38,39].
Huang et al. explored amorphous graphene with structural strains and disorders synthesized by laser induction for nitrate reduction, revealing that its unique carbon lattice and oxygen-containing groups enhanced catalytic activity for nitrate-to-ammonia conversion (Figure 2d–g) [40]. The amorphous graphene exhibited a Faradaic efficiency of approximately 100% and an ammonia production rate of 2859 μg cm−2 h−1 at −0.93 V vs. the RHE. The researchers used X-ray pair-distribution function analysis and electron microscopy to uncover the material’s molecular features that facilitate nitrate reduction. In situ Fourier transform infrared spectroscopy and theoretical calculations highlighted the role of these features in stabilizing reaction intermediates through structural relaxation. This work demonstrated the potential of defective carbon materials in electrochemical nitrate reduction for applications like wastewater remediation and sustainable agricultural practices. Du et al. developed a range of carbon-based catalysts with tailored quaternary-N and N vacancies, emphasizing the importance of dual defect sites in improving the NO3RR process [15]. The NHC-1000 catalyst, with precisely engineered active sites, achieved a high NH3 Faradaic efficiency of 91.2% and a NH3 yield rate of 2.6 mmol h−1 g−1 at −0.5 V (vs. RHE). Structural characterization and theoretical studies revealed that the quaternary N played a key role in the rate-determining step of *NO protonation to *NHO and aided in the formation of *NH2 intermediates through the combined action of N vacancies.
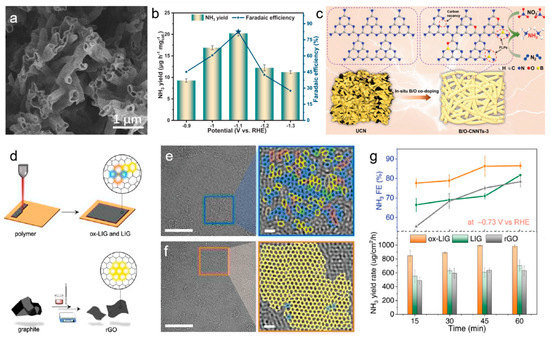

Figure 2.
(a) SEM image of B/O-CNNTs. (b) The NH3 production yield and FE for NO3RR. (c) Schematic illustration for the transformation of carbon nitride to B/O-CNNTs for NO3RR reactions. Reproduced from ref. [37] copyright (2024), with permission from Wiley-VCH. (d) Schematic of the preparation of GO-based catalysts. HRTEM images of ox-LIG (e) and rGO (f). (g) Time-dependent FE and yield rate of NH3 at –0.73 V vs. RHE. Reproduced from ref. [40] copyright (2023), with permission from Wiley-VCH.
3.2.3. Carbon-Supported Metal Catalysts
The incorporation of metal-based nanoparticles or single atoms into carbon supports significantly improves both the catalytic activity and stability of electrocatalysts. The metal active sites, which are well-dispersed on the carbon support, benefit from the high surface area, tunable electronic properties, and conductive nature of carbon materials [41,42,43]. This synergistic interaction not only facilitates efficient electron transfer during the nitrate reduction reaction, but also ensures the long-term durability of the catalyst. Moreover, the carbon support can effectively stabilize the metal species, preventing aggregation and enhancing the overall performance. This approach not only boosts the efficiency of nitrate reduction, but also provides opportunities for precise control over the reaction pathways, improving selectivity and minimizing unwanted side reactions.
For instance, this is demonstrated in an Fe single-atom catalyst coordinated with N and P on a hollow carbon polyhedron [44]. The introduction of P atoms disrupted the intrinsic charge symmetry surrounding the Fe-atom catalyst, improving the adsorption of NO3− and promoting the accumulation of crucial reaction intermediates. The metal atoms provided highly active sites that facilitate electron transfer and stabilize reaction intermediates, thus boosting the efficiency of the reduction reaction. This catalyst achieved 90.3% NH3 Faradaic efficiency and a yield rate of 17,980 μg h−1 mgcat−1 (Figure 3a–d). Moreover, a conductive polymer protection approach was used by modifying polypyrrole onto Cu nanoparticles (Cu-PPy), resulting in enhanced NO3− reduction efficiency under strongly acidic electrolyte [45]. In 0.5 M H2SO4, the Cu-PPy catalyst delivered an NH3 yield rate of 0.55 mmol h−1 cm−2 with 99.99% NH3 selectivity and 96.0% Faradaic efficiency at high NO3− conversion. The PPy layer protected Cu from corrosion, promoted NO3− and NO2− adsorption, stabilized Cu in an intermediate valence state, and accelerated electron transfer (Figure 3e–h). Furthermore, the study constructed a co-production system of NH3 and adipic acid, achieving a high combined electron efficiency.
Carbon materials are also crucial in supporting metal alloys and their oxides for NO3RR. The combination of carbon with metal alloys or oxides offers a unique synergy that enhances both catalytic performance and stability. The high surface area and electrical conductivity of carbon provide an ideal environment for the efficient dispersion of metal species, ensuring improved electron transfer during the reaction. Metal alloys, with their tunable electronic properties, can effectively modulate the adsorption and activation of NO3−, while metal oxides can offer enhanced stability and resistance to corrosion. For instance, RuCo alloy nanosheets on pinewood-derived three-dimensional porous carbon (RuCo@TDC) were developed via electrodeposition [46]. This catalyst showed superior electrocatalytic performance, achieving an ammonia yield of 2.02 ± 0.11 mmol h−1 cm−2 at −0.6 V and a Faradaic efficiency of 95.7 ± 0.8% at −0.2 V in 0.1 M KOH and 0.1 M KNO3 electrolyte. The outstanding performance of RuCo@TDC was due to the synergistic effect between Ru and Co, as well as the favorable three-dimensional porous nanostructure of carbon support. In addition, Kani et al. reported CoO nanoclusters modified on graphene as an effective catalyst for NO3RR [47]. The catalyst showed exceptional performance with over 98% NH3 Faradaic efficiency and a high NH3 current density of approximately 400 mA cm−2. The high mass activity and selectivity of the CoO nanoclusters on graphene made it a promising catalyst for sustainable ammonia production with reduced energy consumption and enhanced efficiency. Furthermore, graphene served as an excellent support material due to its high electrical conductivity and large surface area, which facilitated electron transfer and provided ample space for the active CoO nanoclusters (Figure 3i–l).
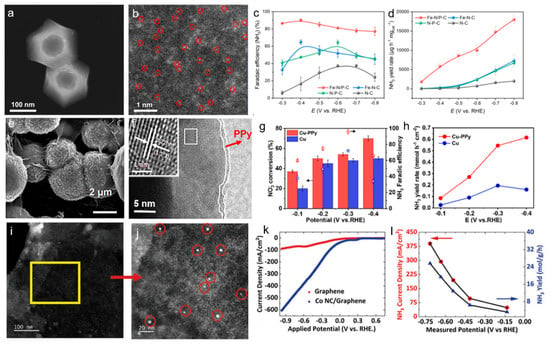

Figure 3.
(a) HAADF-STEM image and (b) aberration-corrected HAADF-STEM of Fe-N/P-C. (c) Potential-dependent FE of NH3 and (d) the corresponding NH3 production rate over four catalysts for 0.5 h. Reproduced from ref. [44] Copyright (2023), with permission from Wiley-VCH. (e) SEM image of Cu-PPy. (f) HRTEM images of Cu-PPy. (g) NO3− conversion and NH3 FE of Cu-PPy and Cu at different applied potentials over 2 h. (h) NH3 yield rate for Cu-PPy and Cu at various applied potentials for 2 h. Reproduced from ref. [45] copyright (2025), with permission from Elsevier. (i) Characterization of Co NC/graphene catalyst, (j) STEM images of CoO NC/graphene at increasing magnifications. (k) LSV curves for graphene and CoO NC/graphene in 1 m KOH + 1 m KNO3 electrolyte. (l) NH3 current densities, yield, and measured potentials during chronopotentiometry at constant current densities −400, −300, −200, −100, and −50 mA cm−2. Reproduced from ref. [47] copyright (2023), with permission from Wiley-VCH.
Table 1 presents a critical trade-off analysis of various carbon material platforms for NO3RR. It compares key factors such as structural characteristics, electrochemical performance, stability, and selectivity across different carbon-based materials. This table serves as a comprehensive overview of the strengths and limitations of each material, providing valuable insights for optimizing catalyst design and improving the efficiency of the NO3RR process.

Table 1.
Critical trade-off analysis of carbon material platforms for NO3RR.
4. Mechanistic Insights
4.1. Adsorption and Activation of Reactive Species
The porous structure of carbon materials provides a large surface area, promoting better contact between the nitrate ions and the catalyst surface. Surface modifications, such as doping with heteroatoms (e.g., nitrogen, sulfur) or functional group introduction, can improve the adsorption affinity and the electron transfer efficiency. Additionally, tailoring the electronic structure of carbon materials can optimize the activation of reaction intermediates, facilitating the reduction process. These strategies collectively enhance the catalytic activity, selectivity, and stability of carbon-based catalysts for nitrate electroreduction to ammonia.
The porous structure of carbon facilitates mass transfer and the adsorption of reactive species, providing essential conditions for further activation [48,49,50]. Chen et al. present a novel Fe/N active site-functionalized porous carbon nanofiber (CNF) catalyst with encapsulated FeCo nanoparticles (FeCo@CNFs-Fe/N) for NO3RR [51]. The FeCo@CNFs-Fe/N electrocatalyst demonstrated excellent performance, attaining an NH3 yield of 498.18 μmol/(h gcat) and a notable reduction in overpotential by 0.565 V. This performance surpassed that of both comparable CNF-based catalysts by 3 to 10 times (Figure 4a–d). The enhanced catalytic efficiency was largely due to the porous carbon structure, which improved mass transfer and facilitated efficient adsorption of reactive species. Furthermore, the adjusted spatial arrangement of active sites promoted faster electron transfer and lowered the reaction energy barrier. Another study focused on an atomically dispersed RuCu dual-atom catalyst (RuCu DAs/NGA) supported on nitrogen-doped graphene aerogels (NGA), synthesized via a pulsed discharge strategy [52]. This catalyst achieved high Faraday efficiency and ammonia yield, with in situ studies revealing the dynamic evolution of asymmetric RuN2-CuN3 active sites during the nitrate reduction process. Density functional theory calculations demonstrated that the Ru atom played a crucial role in the RuN2-CuN3/C structure during electrocatalytic NO3RR. In the early stages, Ru and Cu atoms work together to reduce *NO3 to *NO, while in the later stages, the Ru atom primarily reduces *NO to *NH3. The synergistic interaction between Ru and Cu atoms lowers the reaction free energy and optimizes the adsorption and desorption of intermediates, thus enhancing the NO3RR process. The nitrogen-doped graphene support also played a crucial role in dispersing and stabilizing the metal atoms, preventing their aggregation and thus maintaining the catalyst’s activity and selectivity.
Furthermore, a recent study demonstrated that doping carbon materials with nitrogen has been shown to effectively regulate the charge distribution, thereby facilitating the binding and reduction of NO3− [53,54,55]. The Fe@N10-C catalyst, which incorporated nitrogen-doped carbon, exhibited remarkable performance in nitrate removal and ammonia production [56]. The nitrogen doping not only activated neighboring carbon atoms, but also modulated the electronic structure and chemical environment of the catalyst, enhancing the adsorption of nitrate and the overall reaction process. The heterointerface formed between the Fe nanoparticles and nitrogen-doped carbon resulted in charge rearrangement at the interface, which affected the adsorption of reactant species and boosted reaction kinetics. The results of DFT simulations indicated that the optimal nitrogen-doped catalyst (Fe@N10-C) had a more favorable adsorption of nitrate ions compared to the undoped catalyst, making it more efficient for the nitrate reduction reaction.
In addition, carbon with well-designed defects can create additional active sites, enhancing the efficiency of NO3RR. An example of this is the hierarchical carbon-based metal-free electrocatalyst (C-MFEC) formed by winged carbon coaxial nanocables (W-CCNs), which demonstrated functional separation properties [57]. The pristine CNTs’ inner core enabled efficient charge transfer, while the outer shell, made up of in situ-generated graphene nanosheets and carbon layers with enriched topological defects, significantly contributed to enhancing NO3− adsorption, water dissociation, and N-H bond formation. The W-CCNs exhibited outstanding performance for NO3RR, operating efficiently across a wide pH range. Theoretical calculations revealed that the C577 sites of W-CCNs were more favorable for adsorbing NO3−, while the adjacent C557 sites contributed to generating active hydrogen from water dissociation (Figure 4e–g). The adsorption of *NO3 was identified as the rate-determining step (RDS) for both p-CNTs and W-CCNs. W-CCNs exhibited a smaller ΔG of 2.05 eV compared to p-CNTs (+2.22 eV), indicating that topological defects in W-CCNs enhance *NO3 adsorption. Moreover, significant hydrogenation steps occur on *NO and *NOH for p-CNTs, with ΔG values of 1.27 and 0.64 eV, respectively, which are much higher than those for W-CCNs (0.16 and 0.02 eV). This suggests that W-CCNs reduce the energy barriers for these hydrogenation steps (Figure 4h,i). This synergy between different defect sites created an environment conducive to multi-step NO3RR. The unique structure of W-CCNs, with pristine CNTs as the core and defect-doped outer layers, not only enhanced the adsorption of NO3−, but also facilitated the generation and utilization of *H, leading to improved NH3 production. Additionally, the study emphasized that the integration of multiple defect sites within a hierarchical structure introduced a novel operational principle, enabling C-MFECs to excel in various complex reaction systems.
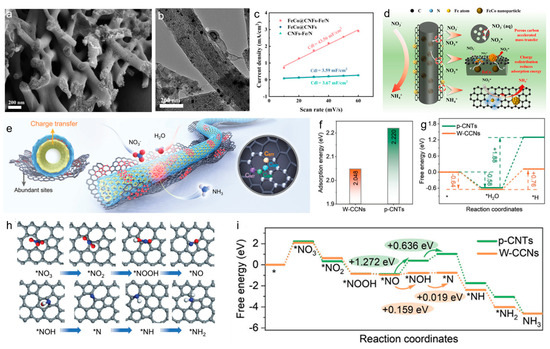

Figure 4.
(a) SEM and (b) TEM images of FeCo@CNFs-Fe/N. (c) The Cdl of different prepared catalysts. (d) Mechanism illustration of electrocatalytic NO3RR on FeCo@CNFs-Fe/N. Reproduced from ref. [51] copyright (2025), with permission from Elsevier. (e) Schematic diagram of W-CCNs and the potential active sites for NO3RR. (f) The comparison of adsorption energy of *NO3 on W-CCNs and p-CNTs. (g) The reaction energies of *H formation over W-CCNs and p-CNTs through H2O electrolysis. (h) Adsorption configurations of NO3RR intermediates on W-CCNs (C: gray, N: blue, O: red, H: light white). (i) Free energy diagrams for NH3 formation from NO3RR on p-CNTs and W-CCNs without pH correction. Reproduced from ref. [57] copyright (2025), with permission from Wiley-VCH.
4.2. Active Hydrogen Regulation
Active atomic hydrogen (H*) plays a pivotal role in the NO3RR process, as it directly participates in the hydrogenation of nitrate species, significantly enhancing the catalytic efficiency and selectivity towards ammonia production. Carbon-based materials, due to their tunable structure and surface properties, can be engineered to effectively modulate the generation and distribution of active atomic hydrogen. Strategies such as defect engineering, surface functionalization, and the introduction of metal nanoparticles on carbon substrates have been explored to optimize atomic hydrogen activation.
One study found that the high NH3 yield and Faradaic efficiency were primarily driven by the dynamic balance between the production of H* on CoP and its rapid consumption by nitrogen intermediates (Figure 5a–c) [58]. The CoP-CNS catalyst, with its unique structure, effectively balanced the production and consumption of H*, leading to superior electrocatalytic performance. This equilibrium ensured that H* was efficiently utilized by nitrogen-containing intermediates, promoting the conversion of NO3− to NH3. Theoretical calculations revealed that the conversion from NO* to HNO* was the rate-determining step for CoP, with a smaller ΔG uphill of 1.57 eV, while for Co, the RDS was NO2* to HNO2* with a significantly higher ΔG of 3.09 eV. This was consistent with experimental observations and underscored the critical role of phosphatization in enhancing NO3RR kinetics. Additionally, the reaction pathway on the CoP surface was much smoother than on Co, indicating superior NO3RR activity on CoP-CNS. The research highlighted that maintaining this H* equilibrium was more effective for improving the NO3RR performance than merely suppressing water splitting. Likewise, the iron phosphide nanoparticles confined on a phosphorus-doped carbon substrates (FeP-PNC) catalyst, with its unique structure, effectively balanced the production and consumption of H*, leading to superior electrocatalytic performance [59]. FeP (011) improved the adsorption of NO3− and NO2−, while the adjacent P-doped C sites facilitated water dissociation more efficiently than N-doped C reactive sites, supplying enough H* for the FeP (011) surface, where various hydrogenation steps of NO3− take place. The synergy between P-doped C and FeP enabled the efficient use of H* by nitrogen-containing intermediates, driving the conversion of NO3− to NH3.
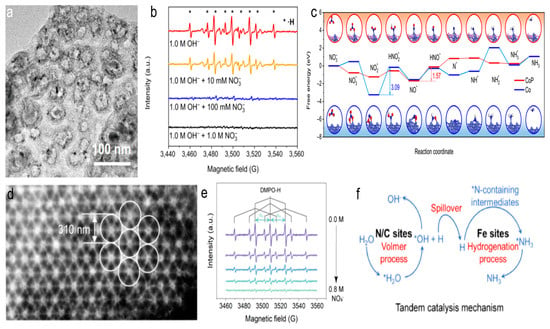

Figure 5.
(a) STEM image of CoP-CNS. (b) ESR spectra of the CoP-CNS-catalyzed NO3RR solutions with different c NO3− using DMPO as the radical trapping reagent. (c) Gibbs free energy diagram along the minimum energy pathway, along with the adsorption configurations of various intermediates formed during NO3RR. Reproduced from ref. [58] copyright (2022), with permission from Springer Nature. (d) HAADF-STEM images of the high-density metal/PNC. (e) ESR spectra of high-density Fe1/PNC with the NO3− concentration increased from 0 to 0.8 M. (f) Schematic diagram of the tandem catalysis mechanism. Reproduced from ref. [60] copyright (2024), with permission from the American Chemical Society.
Additionally, it has been observed that the high-density, atomically dispersed metal sites in metal-N-C catalysts can effectively activate nearby N/C sites, thereby enhancing water adsorption and dissociation [60,61]. This provided sufficient H* to the metal sites for nitrate ammoniation. The research highlighted that the high-density Fe in Fe1/PNC could more effectively activate the adjacent N/C sites compared to low-density counterparts. This activation improved water adsorption/dissociation and supplied sufficient active hydrogen to the Fe sites, thereby promoting the conversion of NO3− to NH3 (Figure 5d–f). The findings provided valuable insights into the design of carbon-based catalysts for efficient electrochemical NO3RR, emphasizing the importance of H* management in catalytic systems.
4.3. Suppressing Competing Hydrogen Evolution Reaction
In the process of NO3RR, the efficient generation and control of H* is essential. However, the HER often competes with NO3RR, leading to reduced ammonia production. To enhance nitrate reduction efficiency, it is essential to suppress HER. One effective strategy involves the design of hydrophobic carbon surfaces [62,63]. By modifying the carbon material to enhance its hydrophobicity, the availability of H+ for the HER can be minimized, thus promoting selective nitrate reduction. For instance, a study by Liu et al. showed that trimethyltetradecylammonium bromide, with its moderate alkyl chain length, formed a highly hydrophobic interface on CNTs, resulting in about 87% selectivity for ammonia production [64]. This hydrophobic surface effectively suppressed the HER, which competed with nitrate reduction. In-depth mechanistic studies using operando Fourier transform infrared spectroscopy and online differential electrochemical mass spectrometry showed that the hydrophobic modification of CNTs enhanced the direct electron transfer pathway, promoting the reduction of nitrate to ammonia. The hydrophobic interface reduced the accessibility of protons at the electrode surface, thereby inhibiting HER, while the positively charged surfactant layer on CNTs favored the adsorption and enrichment of nitrate anions through electrostatic attraction (Figure 6a–c).
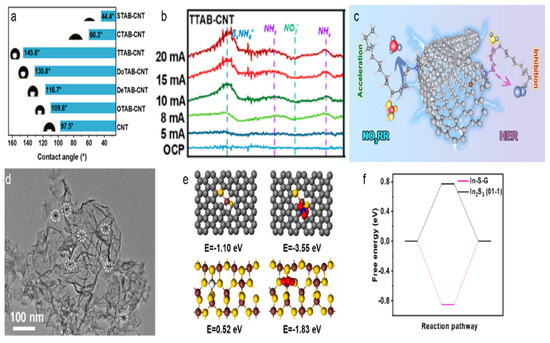

Figure 6.
(a) Contact angle of the CNT modified by different quaternary ammonium surfactants. (b) Potential-dependent operando FTIR spectra of TTAB-CNT. (c) The proposed NO3RR and HER process on TTAB-CNT. Reproduced from ref. [64] copyright (2024), with permission from the American Chemical Society. (d) TEM image of the as prepared In-S-G. (e) The adsorption energy of H and NO3− on the surface of In-S-G and In2S3(01-1). (f) The calculated Gibbs free energy of H* adsorption on the surface of In-S-G and In2S3(01-1). Reproduced from ref. [65] copyright (2021), with permission from Elsevier.
Another effective strategy to suppress HER is the incorporation of metal nanoparticles or doping heteroatom in carbon materials, which can influence molecular adsorption, thereby improving the Faradaic efficiency and selectivity towards ammonia. Lei et al. reported that indium incorporated in a sulfur-doped graphene (I-S-G) catalyst could achieve an ammonia production rate of 220 mmol gcat−1 h−1 and a Faradaic efficiency of about 75% at −0.5 V vs. RHE [65]. The abundant active sites and the hybrid electronic structure of the In species in the catalyst were crucial for promoting NO3RR and suppressing HER. The In-S-G catalyst showed a higher overpotential for HER, indicating efficient suppression of hydrogen production. DFT calculations revealed that the unique electronic structure of In-S-G could enable nitrate adsorption and activation while inhibiting H2 production, thus boosting the selective electroreduction of nitrate to NH3 (Figure 6d–f).
4.4. Tuning the Reaction Pathway
Carbon-based catalysts play a pivotal role in regulating reaction pathways in the NO3RR. These catalysts not only enhance the efficiency of the reduction process, but also play a crucial role in directing the formation of desired products. By modulating the electronic structure and surface characteristics of the catalyst, they can promote selective nitrate reduction, ensuring optimal reaction conditions and minimizing side reactions.
One study demonstrated that zinc phthalocyanines (ZnPc) supported on carbon nanotube (CNT) substrates could effectively suppress the further reduction of hydroxylamine (NH2OH) [66]. By tuning the intrinsic activity of ZnPc and covering the CNT surface with ZnPc molecules to block side reaction sites, the catalyst achieved a Faradaic efficiency of 53 ± 1.7% for NH2OH with a partial current density exceeding 270 mA cm−2 and a turnover frequency of 7.5 ± 0.2 s−1. The free energy diagrams revealed that the NH2OH reduction reaction process encounters an initial energy barrier during NH2OH adsorption, after which the process proceeds through several downhill steps. CoPc displayed a low NH2OH adsorption energy barrier of 0.09 eV, promoting further NH2OH reduction. In contrast, ZnPc and FePc exhibited higher barriers, with ZnPc showing the highest at 0.49 eV, indicating that it had lower activity for NH2OH reduction and was thus more selective for NH2OH production in NO3RR (Figure 7a,b). This shows how carbon-based catalysts can be designed to selectively produce high-value intermediates like NH2OH by controlling reaction pathways and suppressing competing reactions.
Another study emphasized the critical role of carbon-based catalysts in steering reaction pathways towards the selective production of NH3 or N2. A novel Fe single-atom catalyst coordinated with N on an ordered mesoporous carbon framework (Meso-Fe-N-C) was developed, demonstrating excellent electrocatalytic denitrification performance [67]. The N coordination and mesoporous structure tuned the electronic structure of the Fe site, leading to a modest adsorption binding strength with key intermediates. Theoretical calculations indicated that the Gibbs free energy for *N2O generation (ΔG(*N2O) = −0.30 eV) was significantly lower than that for *NOH formation (ΔG(*NOH) = 0.41 eV), suggesting that the 5-electron transfer leading to N2 production was more thermodynamically favorable than the 8-electron transfer required for NH3/NH4+ formation. Moreover, the mesoporous carbon not only enhanced the adsorption and transfer of NO3− but also confined reaction intermediates, promoting N2 formation. (Figure 7c–h) The research underscored how precise control of catalyst architecture, from microscopic electron distribution to macroscopic physical structure, could effectively steer reaction pathways toward desired products.
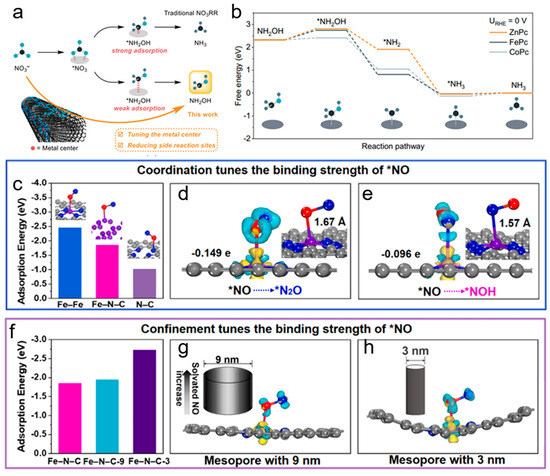

Figure 7.
(a) Selective NH2OH production from nitrate using metal phthalocyanine electrocatalysts. (b) Free energy diagram for the NH2OHRR on different MPcs at 0 V vs. RHE. Reproduced from ref. [66] copyright (2024), with permission from Springer Nature. (c) Reaction energies of *NO adsorption on Fe bulk, Fe-N, and N-C in Fe-N-C. Charge density difference diagrams of *NO on Fe-N-C during the (d) N2 generation pathway (*NO to *N2O) and (e) NH3 formation pathway (*NO to *NOH), with insets showing the corresponding elongation of the N-O bond. (f) Reaction energies and charge density difference diagrams of *NO adsorption on Fe-N-C9 (Me-so-FeN-C with a 9 nm pore size) and Fe-N-C-3 with a 3 nm pore size (g,h). Molecular model: N in blue, O in red, Fe in purple, and C in gray. Reproduced from ref. [67] copyright (2023), with permission from Elsevier.
4.5. Enhancing Reaction Durability
Enhancing the durability of carbon-based catalysts is crucial for the efficient and sustainable NO3RR. The long-term stability of the catalyst ensures consistent performance, especially under the harsh electrochemical conditions of the reaction. Carbon materials can improve catalyst longevity through strategies such as the incorporation of robust metal–carbon interactions, the optimization of surface functionalization, and defect engineering. These modifications help prevent catalyst deactivation due to corrosion or leaching, thereby maintaining high catalytic activity and selectivity over extended reaction cycles. By enhancing the stability of carbon-based catalysts, the overall efficiency and practicality of NO3RR are significantly improved.
The enhancement of catalyst stability and NO3RR performance through metal–carbon interactions in carbon-based catalysts has been highlighted [68,69,70,71]. The Cu-GO@NF sample, fabricated via a low-temperature calcination method, leveraged the synergistic effects of Cu and Ni metals with GO [68]. Crucially, the hydrated cation–π interactions between GO and the metal components (Cu and Ni) significantly improved the stability of the Cu-GO@NF electrocatalyst. During a 150 h continuous reaction, the Cu and Ni contents in the Cu-GO@NF decreased by just 3.3% and 4.5%, respectively, in contrast to the substantial reduction of 34.0% and 51.9% in the Cu@NF sample without GO modification. This indicated that the introduction of GO effectively shielded the metal components from loss and degradation, thereby maintaining the catalyst’s stability and performance (Figure 8a,b). Overall, the metal–carbon interactions in the Cu-GO@NF catalyst played a vital role in enhancing both stability and activity for efficient electrochemical nitrate reduction to ammonia.
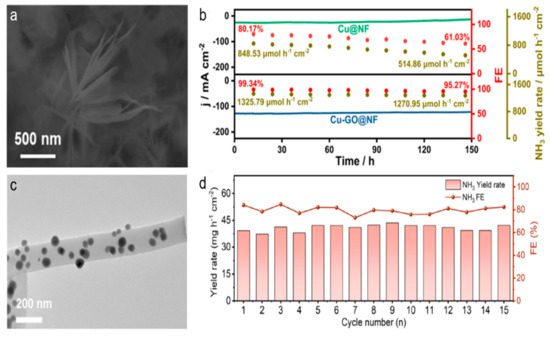

Figure 8.
(a) SEM image of Cu-GO@NF. (b) The catalytic activity and stability of Cu-GO@NF and Cu@NF catalysts were evaluated in a flow cell with a 1 M KOH solution containing 200 ppm NO3−-N at −0.13 V. Reproduced from ref. [68] copyright (2025), with permission from Elsevier. (c) TEM image of CoSn-CNF. (d) The stability test at −0.6 V on a CoSn-CNF catalyst. Reproduced from ref. [69] copyright (2024), with permission from Elsevier.
Additionally, the confined spaces within nanostructured carbon materials, such as carbon nanotubes (CNTs), have been shown to further promote the stability of nitrate reduction [72,73,74]. For example, the carbon nanofiber (CNF) served as a support that prevented the leaching and agglomeration of metal particles, ensuring exceptional cycling performance [69]. The CoSn-CNF catalyst, in particular, demonstrated remarkable stability and high activity. After reaction, CoSn-CNF retained its fibrous architecture and the CoSn alloy particles remained evenly distributed without aggregation or leaching. The CNF structure provided a robust environment for the CoSn alloy nanoparticles (Figure 8c,d). The combination of the CNF support and the electronic structure modulation of the bimetallic alloy leads to a highly efficient and stable electrocatalyst for ammonia production from nitrate reduction.
As illustrated above, the NO3RR can be divided into three key steps: reactant adsorption/activation, proton-coupled electron transfer, and product desorption. Based on these three key steps, we have summarized the structural features and properties of carbon materials, along with their catalytic roles in facilitating these major stages of the reaction process. The summary is provided in the table below (Table 2).

Table 2.
Carbon material design for key steps in NO3RR.
Moreover, Table 3 provides a direct comparison of the performance of various carbon-based catalysts in the reduction of NO3RR to NH3. The table summarizes key performance metrics such as ammonia yield, Faradaic efficiency, and selectivity for each catalyst, offering a clear overview of their comparative effectiveness in the NO3RR process. This comparison highlights the strengths and potential areas for improvement of each material in ammonia production.

Table 3.
Summary of performance of various catalysts for reduction of electrochemical NO3RR to NH3.
5. Conclusions, Challenges, and Perspectives
The electrochemical NO3RR using carbon-based materials has emerged as a promising and sustainable technology for environmental remediation and nitrogen recovery. Carbon materials offer several key advantages in this field, particularly their structural diversity and sustainability. Their unique properties can be fine-tuned through strategies such as doping, functionalization, and defect engineering, allowing the design of catalysts with enhanced selectivity, high surface area, and improved conductivity. This adaptability makes carbon-based materials highly effective for nitrate reduction reactions. Additionally, their sustainability—especially when derived from renewable sources like biomass—provides significant environmental benefits compared to traditional metal-based catalysts.
The integration of material design, mechanism analysis, and engineering application is crucial for advancing the performance and practical deployment of carbon-based catalysts for NO3RR. Innovative material design must be coupled with a deep understanding of the reaction mechanisms at the atomic and molecular levels to optimize the catalytic activity and selectivity. Mechanism analysis helps to identify key intermediates and the roles of different active sites, which can inform the rational design of catalysts with higher efficiency and stability. Finally, translating these advances into engineering applications requires addressing challenges such as scalability, catalyst stability under operational conditions, and integration with existing industrial systems. This full-chain innovation approach is essential to realize the potential of carbon-based catalysts for nitrate reduction in real-world applications. Although carbon-based materials show promising potential in NO3RR, several challenges persist; however, certain perspectives offer hope for overcoming them.
(1) Toxicity of chloride and organic contaminants in real wastewater. One of the major challenges in utilizing carbon catalysts for nitrate reduction in real-world wastewater is the presence of chloride ions and organic contaminants. These impurities can poison the catalyst by blocking active sites or altering the electronic properties of the carbon material, thus reducing its efficiency and lifetime. Addressing this issue requires the development of robust carbon catalysts that can tolerate complex wastewater compositions without significant degradation in performance.
(2) Corrosion and structural collapse at high current densities. At higher current densities, carbon materials are susceptible to corrosion and structural collapse. This phenomenon occurs due to the electrochemical environment under high current loads, which can lead to the degradation of the carbon structure and loss of catalytic activity. Therefore, it is critical to design carbon materials that can withstand the harsh conditions of high current densities without compromising their structural integrity and catalytic performance.
(3) Integrating multi-scale characterization. While ex situ analysis and DFT calculations have provided valuable mechanistic insights into carbon-catalyzed nitrate reduction, real-time monitoring of dynamic processes plays a critical role in connecting these predictions to actual catalytic behavior. These in situ approaches offer unique advantages, such as resolving carbon-specific processes like dopant transformation under applied potentials, intermediate transport within confined pores, and electric double-layer restructuring. Establishing standardized protocols for such real-time analyses will not only enhance mechanistic understanding, but also accelerate the rational design of next-generation catalysts.
(4) Machine learning-guided rational design. The application of machine learning techniques for the rational design of carbon materials offers the potential to accelerate the discovery of new catalysts with optimized properties. By using computational models and high-throughput screening, machine learning can help identify key descriptors, such as doping types and defect densities, that are critical for enhancing nitrate reduction performance. This approach can lead to more efficient and cost-effective materials design.
(5) Carbon neutrality through biomass-derived catalysts. The drive towards carbon neutrality presents a significant opportunity for the development of biomass-derived carbon catalysts. These materials, derived from renewable organic sources, can be synthesized with controlled properties, such as varying levels of doping or defect density, to optimize catalytic activity. Biomass-derived catalysts could play a crucial role in achieving a sustainable and circular carbon economy by providing an alternative to traditional fossil fuel-based catalysts.
(6) Integrated systems development. A promising direction for the future is the development of integrated systems that combine NO3RR with other technologies, such as ammonia fuel cells. By coupling nitrate reduction with ammonia electrolysis or fuel cells, it is possible to create systems that not only produce green ammonia, but also generate electricity or store energy. These integrated systems could significantly improve the overall efficiency and sustainability of nitrogen waste treatment, green ammonia production, and hydrogen energy storage.
Author Contributions
Conceptualization, Y.W. and S.L.; methodology, Q.C.; investigation, L.D. and Y.Z.; resources, L.Z.; writing—original draft preparation, Q.C. and L.D.; writing—review and editing, J.Z. and S.L.; visualization, Y.W. and S.L.; supervision, Y.W. and S.L.; funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Fund from the Research Foundation for Advanced Talents of Xuzhou College of Industrial Technology (Grant XGY2023ZXJB01), and National Natural Science Foundation of China (NSFC Grant number 12404086).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schulte-Uebbing, L.F.; Beusen, A.H.W.; Bouwman, A.F.; de Vries, W. From planetary to regional boundaries for agricultural nitrogen pollution. Nature 2022, 610, 507–512. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, J.; Dieckhöfer, S.; Varhade, S.; Brix, A.C.; Lielpetere, A.; Seisel, S.; Junqueira, J.R.C.; Schuhmann, W. Splicing the active phases of copper/cobalt-based catalysts achieves high-rate tandem electroreduction of nitrate to ammonia. Nat. Commun. 2022, 13, 1129. [Google Scholar] [CrossRef]
- Fang, L.; Wang, S.; Lu, S.; Yin, F.; Dai, Y.; Chang, L.; Liu, H. Efficient electroreduction of nitrate via enriched active phases on copper-cobalt oxides. Chin. Chem. Lett. 2024, 35, 108864. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, Y.; Xu, C.; Ma, W.; Han, H.; Zheng, M.; Zhu, H.; Ma, W. Microbial nitrate removal in biologically enhanced treated coal gasification wastewater of low COD to nitrate ratio by coupling biological denitrification with iron and carbon micro-electrolysis. Bioresour. Technol. 2018, 262, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhu, L.; Yan, Z.; Lin, Z.; Lei, Z.; Zhang, Y.; Xu, H.; Dang, Z.; Wei, C.; Feng, C. Self-Activated Ni Cathode for Electrocatalytic Nitrate Reduction to Ammonia: From Fundamentals to Scale-Up for Treatment of Industrial Wastewater. Environ. Sci. Technol. 2021, 55, 13231–13243. [Google Scholar] [CrossRef]
- Chen, F.Y.; Wu, Z.Y.; Gupta, S.; Rivera, D.J.; Lambeets, S.V.; Pecaut, S.; Kim, J.Y.T.; Zhu, P.; Finfrock, Y.Z.; Meira, D.M.; et al. Efficient conversion of low-concentration nitrate sources into ammonia on a Ru-dispersed Cu nanowire electrocatalyst. Nat. Nanotechnol. 2022, 17, 759–767. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Z.; Lin, Z.; Liang, Y.; Wang, H. Direct electrosynthesis of methylamine from carbon dioxide and nitrate. Nat. Sustain. 2021, 4, 725–730. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, Y.; Wang, M.; Liu, S.; Wang, Z.; Qian, T.; Yan, C. Optimizing Intermediate Adsorption via Heteroatom Ensemble Effect over RuFe Bimetallic Alloy for Enhanced Nitrate Electroreduction to Ammonia. Adv. Energy Mater. 2023, 13, 2301409. [Google Scholar] [CrossRef]
- Fang, L.; Wang, S.; Song, C.; Yang, X.; Li, Y.; Liu, H. Enhanced nitrate reduction reaction via efficient intermediate nitrite conversion on tunable CuxNiy/NC electrocatalysts. J. Hazard. Mater. 2022, 421, 126628. [Google Scholar] [CrossRef]
- Liao, G.; Smith, R.L., Jr.; Guo, H.; Qi, X. Review of carbon-based catalysts for electrochemical nitrate reduction and green ammonia synthesis. Green Chem. 2024, 26, 11797–11831. [Google Scholar] [CrossRef]
- Xue, Y.; Yu, Q.; Ma, Q.; Chen, Y.; Zhang, C.; Teng, W.; Fan, J.; Zhang, W.-x. Electrocatalytic Hydrogenation Boosts Reduction of Nitrate to Ammonia over Single-Atom Cu with Cu(I)-N3C1 Sites. Environ. Sci. Technol. 2022, 56, 14797–14807. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Bai, Y.; Liu, M.; Li, Y.; Yang, H.; Wang, X.; Wu, C. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy Environ. Sci. 2021, 14, 2036–2089. [Google Scholar] [CrossRef]
- Wang, G.; Yu, M.; Feng, X. Carbon materials for ion-intercalation involved rechargeable battery technologies. Chem. Soc. Rev. 2021, 50, 2388–2443. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Kan, M.; Song, D.; Jia, J.; Zhao, Y.; Qian, X. Binderless and Oxygen Vacancies Rich FeNi/Graphitized Mesoporous Carbon/Ni Foam for Electrocatalytic Reduction of Nitrate. Environ. Sci. Technol. 2020, 54, 13344–13353. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Fang, J.; Guo, M.; Yang, G.; Zhang, Q.; Liu, Z.; Peng, F. Nitrogen Defective Engineering of a Metal-Free Carbon Catalyst for Ammonia Electrosynthesis from Nitrate. ACS Sustain. Chem. Eng. 2024, 12, 16320–16328. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Y.; Chen, W.; Xu, X.; Zhang, R.; Ren, X.; Liu, X.; Wang, W.; Qi, J.; Wang, G.; et al. Carbon-Extraction-Triggered Phase Engineering of Rhodium Nanomaterials for Efficient Electrocatalytic Nitrate Reduction Reaction. Angew. Chem. Int. Ed. 2025, 64, e202500985. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Hu, J.; Gao, G.; Zhang, Y.; Shi, G.; Yang, X.; Zhang, L.; Fang, L.; Li, Y. A non-metal doped VTe2 monolayer: Theoretical insights into the enhanced mechanism for the hydrogen evolution reaction. Phys. Chem. Chem. Phys. 2025, 27, 9970–9979. [Google Scholar] [CrossRef]
- Lu, S. Precise Design of Nanoclusters for Efficient Nitrate-to-Ammonia Conversion. Precis. Chem. 2025. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Barcaro, G.; Budnyak, T.M.; Rokicińska, A.; Dronskowski, R.; Budnyk, S.; Kuśtrowski, P.; Monti, S.; Slabon, A. Reaction pathways on N-substituted carbon catalysts during the electrochemical reduction of nitrate to ammonia. Catal. Sci. Technol. 2022, 12, 3582–3593. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, X.; Yao, X.; Chu, J.; Bai, F.; Sun, C.; Wang, Y.-Q. Iron and nickel based alloy nanoparticles anchored on phosphorus-modified carbon-nitrogen plane enhances electrochemical nitrate reduction to ammonia. J. Colloid. Interface Sci. 2025, 680, 632–642. [Google Scholar] [CrossRef]
- Huang, J.; Yu, J.; Lu, X.; Wei, Y.; Song, H.; Cao, A.; Cai, J.; Zang, S.-Q.; Lu, S. Regulating the spin density of CoIII using boron-doped carbon dots for enhanced electrocatalytic nitrate reduction. Inorg. Chem. Front. 2023, 10, 3955–3962. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, Q.; Gao, G.; Zhang, Y.; Zhang, L.; Lu, S.; Fang, L. Theoretical Advances in MBenes for Hydrogen Evolution Electrocatalysis. Energies 2024, 17, 5492. [Google Scholar] [CrossRef]
- Shang, H.; Zhang, Y.; Dong, Y.; Zhu, Y.; Pan, C. Recent Progress in the Electrocatalytic Synthesis of H2O2 from Graphite-Based Materials. ChemCatChem 2025, 17, e202402062. [Google Scholar] [CrossRef]
- Mohamed, N.B.; El-Kady, M.F.; Kaner, R.B. Macroporous Graphene Frameworks for Sensing and Supercapacitor Applications. Adv. Funct. Mater. 2022, 32, 2203101. [Google Scholar] [CrossRef]
- Park, S.; Ji, S.; Yoon, Y.; Kim, S.K.; Song, W.; Myung, S.; Lim, J.; Jung, H.-K.; Lee, S.S.; An, K.-S. Fabrication of fanlike L-shaped graphene nanostructures with enhanced thermal/electrochemical properties via laser irradiation. Carbon 2021, 182, 691–699. [Google Scholar] [CrossRef]
- Harmon, N.J.; Li, J.; Wang, B.T.; Gao, Y.; Wang, H. Influence of Carbon Nanotube Support on Electrochemical Nitrate Reduction Catalyzed by Cobalt Phthalocyanine Molecules. ACS Catal. 2024, 14, 3575–3581. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Liang, M.; Lu, Y.; He, Q.; Chen, F. Efficient electrochemical nitrate reduction by iron phthalocyanine nanorods decorated on multi-walled carbon nanotubes. J. Clean. Prod. 2024, 472, 143514. [Google Scholar] [CrossRef]
- Harmon, N.J.; Rooney, C.L.; Tao, Z.; Shang, B.; Raychaudhuri, N.; Choi, C.; Li, H.; Wang, H. Intrinsic Catalytic Activity of Carbon Nanotubes for Electrochemical Nitrate Reduction. ACS Catal. 2022, 12, 9135–9142. [Google Scholar] [CrossRef]
- Chen, J.; Zuo, K.; Li, Y.; Huang, X.; Hu, J.; Yang, Y.; Wang, W.; Chen, L.; Jain, A.; Verduzco, R.; et al. Eggshell membrane derived nitrogen rich porous carbon for selective electrosorption of nitrate from water. Water Res. 2022, 216, 118351. [Google Scholar] [CrossRef]
- Li, R.; Gao, T.; Wang, P.; Qiu, W.; Liu, K.; Liu, Y.; Jin, Z.; Li, P. The origin of selective nitrate-to-ammonia electroreduction on metal-free nitrogen-doped carbon aerogel catalysts. Appl. Catal. B Environ. 2023, 331, 122677. [Google Scholar] [CrossRef]
- Yu, J.; Gao, R.-T.; Guo, X.; Truong Nguyen, N.; Wu, L.; Wang, L. Electrochemical Nitrate Reduction to Ammonia on AuCu Single-Atom Alloy Aerogels under Wide Potential Window. Angew. Chem. Int. Ed. 2025, 64, e202415975. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, K.; Quan, X.; Chen, S.; Yu, H.; Zhang, Z.; Niu, J.; Zhang, S. Efficient electrochemical nitrate removal on Cu and nitrogen doped carbon. Chem. Eng. J. 2021, 415, 128958. [Google Scholar] [CrossRef]
- Chen, W.; Yang, X.; Chen, Z.; Ou, Z.; Hu, J.; Xu, Y.; Li, Y.; Ren, X.; Ye, S.; Qiu, J.; et al. Emerging Applications, Developments, Prospects, and Challenges of Electrochemical Nitrate-to-Ammonia Conversion. Adv. Funct. Mater. 2023, 33, 2300512. [Google Scholar] [CrossRef]
- Kuramochi, S.; Fiorani, A.; Einaga, Y. Ammonia Synthesis from Electrochemical Reduction of Nitrate Using Boron-Doped Diamond Electrodes. ACS Sustain. Chem. Eng. 2024, 12, 12643–12651. [Google Scholar] [CrossRef]
- Kuang, P.; Natsui, K.; Feng, C.; Einaga, Y. Electrochemical reduction of nitrate on boron-doped diamond electrodes: Effects of surface termination and boron-doping level. Chemosphere 2020, 251, 126364. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Yang, J.; Maeng, J.; Kim, W.B.; Yun, Y. Ammonia production via electrocatalytic nitrate reduction on phosphorus-doped coconut shell-based activated carbons. Appl. Surf. Sci. 2025, 686, 162089. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, Y.; Jia, Z.; Zhong, X.; Sun, Q.; Wang, Y.; Yao, J. Boron and Oxygen Dual-Doped Carbon Nitride Nanotubes with Frustrated Lewis Pairs for Efficient Electrocatalytic Ammonia Synthesis. Small Methods 2024, 9, 2401672. [Google Scholar] [CrossRef]
- Fang, L.; Lu, S.; Wang, S.; Yang, X.; Song, C.; Yin, F.; Liu, H. Defect engineering on electrocatalysts for sustainable nitrate reduction to ammonia: Fundamentals and regulations. Chem. A Eur. J. 2024, 30, e202303249. [Google Scholar] [CrossRef]
- Cheng, L.; Ma, T.; Zhang, B.; Huang, L.; Guo, W.; Hu, F.; Zhu, H.; Wang, Z.; Zheng, T.; Yang, D.-T.; et al. Steering the Topological Defects in Amorphous Laser-Induced Graphene for Direct Nitrate-to-Ammonia Electroreduction. ACS Catal. 2022, 12, 11639–11650. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, L.; Ma, T.; Zhang, J.-J.; Wu, H.; Su, J.; Song, Y.; Zhu, H.; Liu, Q.; Zhu, M.; et al. Direct Synthesis of Ammonia from Nitrate on Amorphous Graphene with Near 100% Efficiency. Adv. Mater. 2023, 35, 2211856. [Google Scholar] [CrossRef]
- Zhu, G.; Bao, W.; Xie, M.; Qi, C.; Xu, F.; Jiang, Y.; Chen, B.; Fan, Y.; Liu, B.; Wang, L.; et al. Accelerating Tandem Electroreduction of Nitrate to Ammonia via Multi-Site Synergy in Mesoporous Carbon-Supported High-Entropy Intermetallics. Adv. Mater. 2025, 37, 2413560. [Google Scholar] [CrossRef]
- Tan, S.F.; Roslie, H.; Salim, T.; Han, Z.; Wu, D.; Liang, C.; Teo, L.F.; Lam, Y.M. Operando Electrodeposition of Nonprecious Metal Copper Nanocatalysts on Low-Dimensional Support Materials for Nitrate Reduction Reactions. ACS Nano 2024, 18, 19220–19231. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, A.; Hong, C.; Zhang, R.; Zheng, H.; Teng, W. Medium entropy alloy nanoparticles incorporated in ordered mesoporous carbons for enhanced electrocatalytic nitrate-to-ammonia conversion. J. Environ. Chem. Eng. 2025, 13, 115047. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, S.; Liu, H.; Liu, S.; Yuan, Y.; Meng, Y.; Wang, M.; Shen, C.; Peng, Q.; Chen, J.; et al. Breaking Local Charge Symmetry of Iron Single Atoms for Efficient Electrocatalytic Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2023, 62, e202308044. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Z.; Li, Y.; Yu, L.; Chen, F.; Li, L. Conductive polymer protection strategy to promote electrochemical nitrate reduction to ammonia in highly acidic condition over Cu-based catalyst. Chem. Eng. J. 2024, 481, 148596. [Google Scholar] [CrossRef]
- Wu, S.; Yan, J.; Zhao, D.; Cai, Z.; Yu, J.; Li, R.; Li, Q.; Fan, G. Three-dimensional RuCo alloy nanosheets arrays integrated pinewood-derived porous carbon for high-efficiency electrocatalytic nitrate reduction to ammonia. J. Colloid. Interface Sci. 2024, 668, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Kani, N.C.; Nguyen, N.H.L.; Markel, K.; Bhawnani, R.R.; Shindel, B.; Sharma, K.; Kim, S.; Dravid, V.P.; Berry, V.; Gauthier, J.A.; et al. Electrochemical Reduction of Nitrates on CoO Nanoclusters-Functionalized Graphene with Highest Mass Activity and Nearly 100% Selectivity to Ammonia. Adv. Energy Mater. 2023, 13, 2204236. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Yao, J.; Dong, K.; Hu, L.; Chen, J.; Zhang, L.; Fan, X.; Cai, Z.; Sun, S.; et al. High-Efficiency Electroreduction of Nitrite to Ammonia on Ni Nanoparticles Strutted 3D Honeycomb-Like Porous Carbon Framework. ChemSusChem 2023, 16, e202300505. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, H.; Duan, W.; Lin, Z.; Feng, C. Metal-encapsulated carbon nanotube arrays for enhancing electrocatalytic nitrate reduction in wastewater: Importance of lying-down to standing-up structure transition. Environ. Sci. Nano 2022, 9, 2841–2853. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Lou, Y.-Y.; Wu, Z.; Huang, X.J.; Sun, S.-G. Spatially Separated Cu/Ru on Ordered Mesoporous Carbon for Superior Ammonia Electrosynthesis from Nitrate over a Wide Potential Window. J. Am. Chem. Soc. 2024, 146, 24966–24977. [Google Scholar] [CrossRef]
- Chen, J.; Wu, A.; Li, J.; Hong, C.; Tang, W.; Zheng, H.; Teng, W. Fe/N modified porous carbon nanofibers with encapsulated FeCo nanoparticles for efficient electrocatalytic nitrate reduction to ammonia. J. Environ. Sci. 2024, 157, 90–99. [Google Scholar] [CrossRef]
- Liu, K.; Sun, Z.; Peng, X.; Liu, X.; Zhang, X.; Zhou, B.; Yu, K.; Chen, Z.; Zhou, Q.; Zhang, F.; et al. Tailoring asymmetric RuCu dual-atom electrocatalyst toward ammonia synthesis from nitrate. Nat. Commun. 2025, 16, 2167. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; An, N.; Zhang, S.; Song, Q.; Yang, Y.; Liu, X. Atomically dispersed Fe atoms anchored on S and N–codoped carbon for efficient electrochemical denitrification. Proc. Natl. Acad. Sci. USA 2021, 118, e2105628118. [Google Scholar] [CrossRef]
- Wang, H.; Yao, Y.; Zhan, J.; Jia, Y.; Yao, T.; Zhang, L.-H.; Yu, F. P-Modified Single-Atom Cu Catalyst Boosting Electrocatalytic Performance of NO3− Reduction to NH3. ChemCatChem 2023, 15, e202201633. [Google Scholar] [CrossRef]
- Su, C.; Soyol-Erdene, T.-O.; Bayanjargal, O.; Jiang, K.; Jiang, G.; Lv, X.; Tang, W. Facile synthesis of coral-like nitrogen and sulfur co-doped carbon-encapsulated FeS2 for efficient electroreduction of nitrate to ammonia. Sep. Purif. Technol. 2024, 348, 127813. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Li, J.; Song, Q.; Liu, X. N-doped carbon–iron heterointerfaces for boosted electrocatalytic active and selective ammonia production. Proc. Natl. Acad. Sci. USA 2023, 120, e2207080119. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, L.; Shen, Y.; Peng, W.; Mao, B.; Hou, J.; Wang, D.; Chen, X.; Dai, Y.; Zhang, C.; et al. Hierarchical Carbon-Based Electrocatalyst with Functional Separation Properties for Efficient pH Universal Nitrate Reduction. Adv. Mater. 2025, 37, 2417623. [Google Scholar] [CrossRef]
- Fan, K.; Xie, W.; Li, J.; Sun, Y.; Xu, P.; Tang, Y.; Li, Z.; Shao, M. Active hydrogen boosts electrochemical nitrate reduction to ammonia. Nat. Commun. 2022, 13, 7958. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Pang, B.; Chen, W.; Cui, F.; Qi, X.; Wu, X.; Wang, N.; Yu, W.; He, G. Synergistic modulation of iron phosphide and phosphorus-doped carbon to boost ammonia electrosynthesis from nitrate. Chem. Eng. J. 2025, 507, 160088. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, J.; Liu, M.; Shi, L.; Chen, M.; Wu, L. High-Density Atomically Dispersed Metals Activate Adjacent Nitrogen/Carbon Sites for Efficient Ammonia Electrosynthesis from Nitrate. ACS Nano 2024, 18, 26722–26732. [Google Scholar] [CrossRef]
- Luo, H.; Li, S.; Wu, Z.; Liu, Y.; Luo, W.; Li, W.; Zhang, D.; Chen, J.; Yang, J. Modulating the Active Hydrogen Adsorption on Fe─N Interface for Boosted Electrocatalytic Nitrate Reduction with Ultra-Long Stability. Adv. Mater. 2023, 35, 2304695. [Google Scholar] [CrossRef] [PubMed]
- Choong, C.E.; Yoon, S.Y.; Wong, K.T.; Kim, M.; Lee, G.; Kim, S.-H.; Jeon, B.-H.; Choi, J.; Yoon, Y.; Choi, E.H.; et al. Hydrophobic sulfur core–shell layered metallic iron for nitrate reduction with nearly 100% dinitrogen selectivity: Mechanism and field studies. Chem. Eng. J. 2023, 454, 140083. [Google Scholar] [CrossRef]
- Gao, J.; Ma, Q.; Zhang, Y.; Xue, S.; Young, J.; Zhao, M.; Ren, Z.J.; Kim, J.-H.; Zhang, W. Coupling Curvature and Hydrophobicity: A Counterintuitive Strategy for Efficient Electroreduction of Nitrate into Ammonia. ACS Nano 2024, 18, 10302–10311. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, Y.; Ren, Y.; Wang, Y.; You, S.; Liu, M. Selective Nitrate Electroreduction to Ammonia on CNT Electrodes with Controllable Interfacial Wettability. Environ. Sci. Technol. 2024, 58, 7228–7236. [Google Scholar] [CrossRef]
- Lei, F.; Xu, W.; Yu, J.; Li, K.; Xie, J.; Hao, P.; Cui, G.; Tang, B. Electrochemical synthesis of ammonia by nitrate reduction on indium incorporated in sulfur doped graphene. Chem. Eng. J. 2021, 426, 131317. [Google Scholar] [CrossRef]
- Tang, Y.; Jiang, Z.; Yuan, Y.; Xu, L.; Jin, C.; Chen, B.; Lin, Z.; Zao, J.; Du, J.; Zhang, X.; et al. Selective electrosynthesis of hydroxylamine from aqueous nitrate/nitrite by suppressing further reduction. Nat. Commun. 2024, 15, 9800. [Google Scholar] [CrossRef]
- Fan, J.; Chen, Y.; Chen, X.; Wu, Z.; Teng, W.; Zhang, W.-x. Atomically dispersed iron enables high-efficiency electrocatalytic conversion of nitrate to dinitrogen on a N-coordinated mesoporous carbon architecture. Appl. Catal. B Environ. 2023, 320, 121983. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Z.; Yang, H.; Ding, T.; Zhang, Z.; Yue, D.; Shi, G. Efficient electrocatalytic nitrate reduction to ammonia at low voltage through copper and graphene oxide co-modified nickel foam. J. Power Sources 2025, 640, 236748. [Google Scholar] [CrossRef]
- Qi, R.; Jiang, Q.; Zhong, M.; Li, W.; Ren, S.; Wang, Y.; Feng, M.; Lu, X. Manipulating d-band center of bimetallic Sn-alloy coupling with carbon nanofibers for high-performance electrocatalytic production of ammonia from nitrate. Chem. Eng. J. 2024, 496, 154094. [Google Scholar] [CrossRef]
- Tu, Y.; Li, P.; Sun, J.; Jiang, J.; Dai, F.; Li, C.; Wu, Y.; Chen, L.; Shi, G.; Tan, Y.; et al. Remarkable Antibacterial Activity of Reduced Graphene Oxide Functionalized by Copper Ions. Adv. Funct. Mater. 2021, 31, 2008018. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, P.; Wang, H.; Li, C.; Peng, W.; Liu, J. Multi-Dimensional Ni@TiN/CNT Heterostructure with Tandem Catalysis for Efficient Electrochemical Nitrite Reduction to Ammonia. Angew. Chem. Int. Ed. 2025, 64, e202501578. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Deng, Z.; Feng, T.; Fan, J.; Zhang, W.X. Nanoscale zero-valent iron (nZVI) encapsulated within tubular nitride carbon for highly selective and stable electrocatalytic denitrification. Chem. Eng. J. 2021, 417, 129160. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, J.; Chen, J.; Luo, H.; Jiang, M.; Yang, C.; Zhang, H.; Chen, J.; Dong, A.; Yang, J. Interfacial Assembly of Nanocrystals on Nanofibers with Strong Interaction for Electrocatalytic Nitrate Reduction. Angew. Chem. Int. Ed. 2023, 62, e202310383. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, J.; Zhang, Z.; Hung, C.-T.; Yang, J.; Li, W. In Situ Confinement of Ultrasmall Metal Nanoparticles in Short Mesochannels for Durable Electrocatalytic Nitrate Reduction with High Efficiency and Selectivity. Adv. Mater. 2023, 35, 2207522. [Google Scholar] [CrossRef]
- Li, M.; Qi, C.; Xu, J.; Zou, R.; Wang, L.; Jiang, W.; Fan, Y.; Qiu, P.; Luo, W. Integrated Three-in-one to Boost Nitrate Electroreduction to Ammonia Utilizing a 1D Mesoporous Carbon Cascade Nanoreactor. ACS Nano 2025, 19, 11309–11322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).