Abstract
In this study, borophene and nickel phthalocyanine (NiPc): borophene nanocomposites were prepared using the sonication method. The NiPc: borophene nanocomposite was uniformly obtained as a 10–80 nm-sized spherically shaped particle. Electrical conductivities (s) were measured as 3 × 10−13 Scm−1 and 9.5 × 10−9 Scm−1 for NiPc and the NiPc: borophene nanocomposite, respectively. The SEM image showed that borophene was homogeneously distributed in the NiPc matrix and increased the charge transport pathways. This is the main reason for a 106-fold increase in electrical conductivity. An indium tin oxide (ITO)/NiPc: borophene nanocomposite-based thermoelectric generator (TEG) was prepared and characterized. The Seebeck coefficients (S) were calculated to be 5 μVK−1 and 30 μVK−1 for NiPc and the NiPc: borophene nanocomposite, respectively. A positive Seebeck coefficient value for the NiPc: borophene showed the p-type nature of the nanocomposite. The power factors (PF = sS2) were calculated as 7.5 × 10−16 μW m−1 K−2 and 8.6 × 10−10 μW m−1 K−2 for NiPc and the NiPc: borophene nanocomposite, respectively. Compositing NiPc with borophene increased the power factor by ~106-fold. It has been concluded that the electrical conductivity and Seebeck coefficient of the NiPc: borophene material increases due to energy band convergence because of combining p-type NiPc with p-type borophene. Therefore, the NiPc: borophene nanocomposite is a promising material for TEG.
1. Introduction
Thermal energy is often an overlooked resource in energy economics. Instead of being a solely consumable by-product of an irreversible labor cycle, it can function as an energy source in its own right. Thermal energy is essential and should not be overshadowed by considerable funding efforts to improve the growth rates of various forms of energy, such as solar energy (photovoltaic), electrical energy storage (batteries and supercapacitors), and chemical energy (low- and high-temperature batteries, electrocatalysis, redox-flow systems, and CO2 conversion). Thermoelectric (TE) generators represent solid-state electronic power sources that directly convert thermal energy into electrical energy, providing a crucial resource for future energy economies. Technological advancements in the fields of physics, chemistry, electrical and electronic engineering, and materials science, along with the evolving nature of the energy economy, underscore the crucial role that thermoelectric generators (TEGs) play in harnessing distributed, low-temperature heat sources in the twenty-first century. It is an ideal situation for capturing and transforming energy. In the context of global energy uncertainty and increasing energy demands, improving energy conversion efficiency provides a driving force for energy technologies. TE generators can meet electrical energy requirements as solid-state electronics power sources by converting waste heat into fixed and direct electrical energy using the Seebeck effect [1,2,3,4,5]. Materials must have several specific properties to be thermoelectrically efficient. For efficient thermoelectric (TE) materials, the Seebeck coefficient (S) represents the conversion of a temperature difference in a material into electrical voltage. Mobile charge carriers are distributed along the temperature gradient in the material and play a role in the accumulation of interfacial charge, thus creating a potential difference (ΔV) (Equation (1)). A high S value provides greater electrical energy production. Thermal conductivity (κ) determines a material’s ability to conduct heat. A low κ value increases efficiency by maintaining the thermal gradient. The electrical conductivity (σ) of the material must be high.
S = ΔV/ΔT
The figure of merit (ZT) of the material is calculated using (Equation (2))
where σ represents the proportionality factor, κ represents the thermal conductivity, T represents the temperature, V represents generated voltage, S represents the Seebeck coefficient, and PF represents the power factor. For an efficient thermoelectric material, ZT > 1 [6,7,8,9].
ZT = σS2TκZT = κσS2T
In energy-efficient technologies, TE materials are selected for their high electrical conductivity, high thermoelectric performance, high Seebeck coefficient, and low thermal conductivity properties. As is known, to obtain a high-efficiency thermoelectric (TE) material, a material with low thermal conductivity, high electrical conductivity, and a high Seebeck coefficient is required; however, it is difficult to find a material that combines these properties. Electrical conductivity is directly correlated with thermal conductivity. Recent evidence suggests that an ideal TE material would have a high Seebeck coefficient and high electrical conductivity [10,11].
In the 1950s, the best thermoelectric (TE) materials were inorganic compounds, such as Bi2Te3, and the first thermoelectric generators (TEGs) were prepared based on Bi2Te3 [12,13]. However, the efficiency of the initial Bi2Te3-based thermoelectric generators (TEGs) was low, leading to the development of TEGs using Bi2Te3 alloys. Bi2Te3 alloy-based thermoelectric generators (TEGs) have been reported to operate optimally near room temperature, exhibiting higher efficiency than Bi2Te3-based TEGs [14]. Despite their improved performance, the use of these materials is limited due to their toxicity. Moreover, the rare earth metal Te increases the cost of the alloys, and its preparation as a TE material requires complex vacuum processes [7,15,16]. Since the 1990s, theoretical calculations on thermoelectric (TE) materials have suggested that TE efficiency can be enhanced by incorporating nanostructures, driving extensive studies in this field [17]. Numerous publications have reported that nanostructures in thermoelectric (TE) materials can improve electrical conductivity and reduce thermal conductivity [18,19,20,21,22,23,24].
Semiconductor polymers exhibit high electrical conductivity, a high Seebeck coefficient, and low thermal conductivity, all of which are essential for good thermoelectric (TE) performance [25,26,27]. Among various TEGs, metal–organic complexes have been extensively developed due to their controllable morphology, processability, long-term operational stability, high reliability, and flexibility [28,29,30,31,32]. Metal–organic polymer-based materials hold great potential for improving the performance of TEGs in waste heat recovery systems due to their high repeatability and carrier mobility [33,34,35,36]. Among them, metal–organic polymers and small molecules are two major categories. While metal–organic polymers have been extensively studied for their TE properties, research on metal–organic small molecules is still in its early stages. However, metal–organic small molecules have several advantages over their polymer counterparts. For instance, they can crystallize more easily while maintaining high carrier mobility, which could be beneficial for TE applications. Due to these advantages, the thermoelectric (TE) properties of metal–organic small molecules are currently being investigated. Metallic phthalocyanines (MPcs), as metal–organic small molecules, are a highly versatile class of organic semiconducting materials renowned for their remarkable chemical and thermal stability. They are composed of a central metal ion surrounded by four isoindole-based ligands, which form a planar macrocycle. MPcs find applications in organic photovoltaics, field-effect transistors, and sensors because they exhibit excellent charge transport and optical properties. Most theoretical studies in the field of metal phthalocyanines have suggested that the Seebeck coefficient of these materials ranges from 0.6 to 1.8 mV/K.
It has been reported in the literature that when a semiconductor organic material is combined with the same type of semiconductor inorganic material, the electrical conductivity and Seebeck coefficient of the newly formed organic–inorganic hybrid material tends to increase [9]. Nickel phthalocyanine (NiPc) is a p-type semiconductor [37,38]. It is a metal–organic small molecule with unique chemical and thermal stability properties [28,39,40,41]. Borophene is a p-type semiconductor, and its electrical conductivity falls within the 10−6–10−7 S/cm range at room temperature [42,43]. Currently, there is a lack of research on the utilization of borophene as an additive in nanocomposite-based thermoelectric generators (TEGs). The NiPc: borophene combination is an organic–inorganic hybrid material. The aim of this study is to investigate the effect of combining p-type organic NiPc with p-type inorganic borophene on the thermoelectric properties of the NiPc: borophene material, with a focus on energy band convergence.
2. Experimental
Boron microparticles (>99.95 purity) were purchased from Nanografi (Ankara, Türkiye). ITO-coated glasses were purchased from Sigma-Aldrich (Taufkirchen, Germany). Borophene was prepared as a p-type material using the sonication method described in our previous publication [44]. NiPc was synthesized following the procedure outlined in our earlier work [45] (Figure 1). Borophene and NiPc were mixed in a 1:1 ratio in chloroform and sonicated in an ultrasound bath for 5 min.
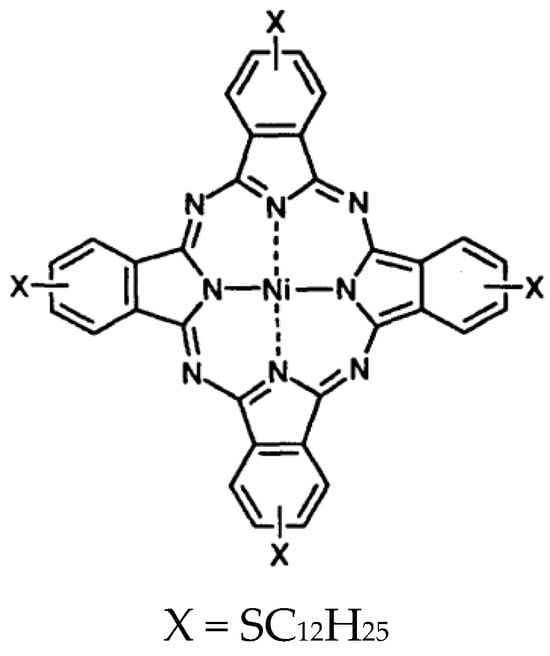
Figure 1.
Structure of the synthesized NiPc.
The ITO-coated glass substrate was covered with chemical isolation tape in a patterned manner. Six tapes, each measuring 1 mm in width and 4 cm in length, were glued to the ITO-coated glass, and this sample was placed in a 20:4 HCl:deionized water solution in a horizontal position. After the sample was kept in this solution for 10 h, it was washed with deionized water and dried using nitrogen. When the tapes were removed, the desired ITO pattern for use as an electrode was obtained.
Then, the prepared NiPc: borophene nanocomposite was coated on the patterned ITO-coated glass surface by dropping and drying at room temperature for 2 min. Subsequently, a NiPc: borophene nanocomposite coating was patterned using thin cotton sticks, resulting in six NiPc: borophene nanocomposite strips on the glass. The schematic illustration of the prepared TEG (6-pair n-p material) on a glass substrate is provided in Figure 2. Serial connections were made between the ITO and the NiPc: borophene nanocomposite electrode strips using silver paste. At the end strips on both sides of the TEG, silver paste was also used for the voltage measurement.
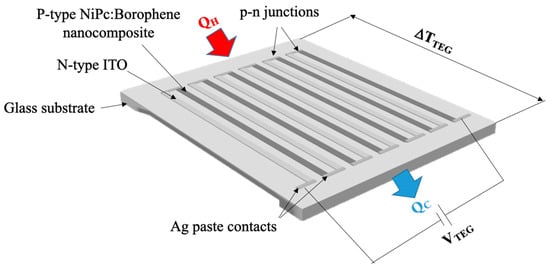
Figure 2.
Schematic illustration of the prepared TEG.
Thermoelectric measurements of the prepared thermoelectric generator (TEG) were conducted by suspending it in an air gap between two temperature-controlled blocks—a Peltier module and a block with the exact dimensions. The temperature of the Peltier module (hot side) was adjusted using a heater. The temperature between the hot and cold sides was monitored using Pt100 thermocouples. The thermovoltage between the hot and cold sides of the TEG was measured across the silver paste leads with a GW Instek GDM83-51 (Good Will Instrument Co., Ltd., Taipei, Taiwan). The schema of the electrical circuit of the TEG and the TEG measurement setup are given in Figure 3.
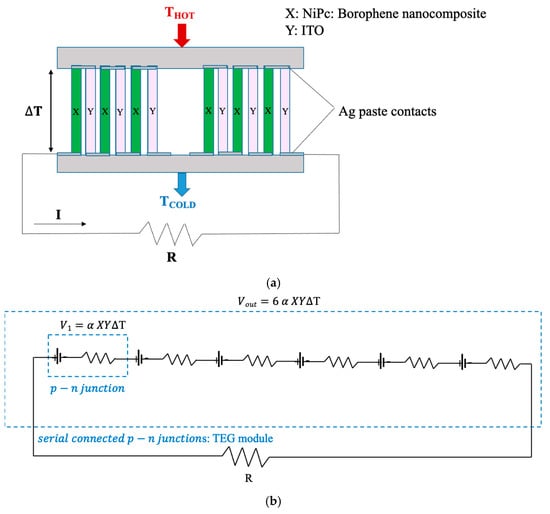
Figure 3.
Schematic illustration of (a) the electrical circuit of the TEG, and (b) the TEG measurement setup.
3. Results and Discussion
3.1. Structural and Chemical Analysis of the NiPc: Borophene Nanocomposite
Borophene was analyzed by X-ray diffraction (XRD) using Cu Kα radiation at 40 kV and 15 mA, with the Highscore Plus XRD program (Version 5.3a) employed. Additionally, high-resolution transmission electron microscopy (HRTEM) and ultraviolet–visible (UV–Vis) diffuse reflectance spectroscopy (using a Shimadzu UV-2501PC spectrometer, Shimadzu Corporation (Kyoto, Japan)) were employed (Figure 4). HRTEM images with low magnification are presented in Figure 4a and those with high magnification in Figure 4b, both in DMF medium. The photos indicate that the obtained borophene is in the form of nanosheets, exhibiting a crystal structure with a d-spacing of 0.41 nm. This aligns with the characteristics of a β-rhombohedral boron structure. Furthermore, an individual borophene nanolayer was examined using fast Fourier transform (FFT), as shown in the inset of Figure 4b. The XRD peaks, analyzed in Figure 4c, are consistent with the (0001) plane of a β-rhombohedral boron (PDF31-0207). UV–Vis diffuse reflectance spectroscopy was utilized to analyze band gap energies, with the Kubelka–Munk function employed to process the obtained data using the formula
where represents the diffuse reflectance value derived from the measurements. The Tauc plot of the Kubelka–Munk function was then applied, revealing both direct and indirect band gap transitions of the borophene sample, as illustrated in Figure 4d. The calculated band gap energies for the synthesized borophene are 0.94 eV and 1.74 eV for indirect and direct transitions, respectively.
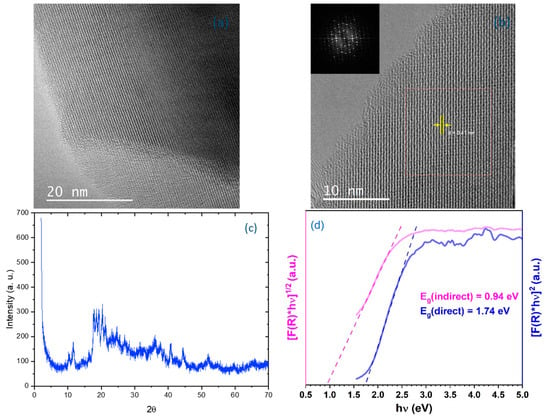
Figure 4.
(a) Low-magnification, (b) high-magnification HRTEM images of the freestanding borophene nanosheets, (c) XRD analysis of the borophene nanosheets, and (d) the Tauc plots of the Kubelka–Munk function display both direct and indirect bandgaps of the prepared borophene sample.
The NiPc: borophene nanocomposite was characterized by scanning electron microscopy (SEM) (FEI Quanta 450 model, Hillsboro, Oregon, USA, conditions: a 6–10 mm working distance, 0–130 Pa pressure, and voltage of 7–10 kV under a low-vacuum medium) and Fourier transform infrared (FTIR) spectroscopy (Perkin Elmer, Waltham, MA, USA, Spectrum Two model, in the 4000–400 cm−1 frequency range) (Figure 5). As shown in Figure 5a,b, the SEM image reveals that borophene is homogeneously distributed in the NiPc matrix, thereby increasing the charge transport pathways. This is the main reason for the 106-fold increase in electrical conductivity. The NiPc: borophene nanocomposite was uniformly obtained as a 10–80 nm spherical-shaped particle due to the borophene nanosheet arrangement with the NiPc. In Figure 5c, the FTIR spectra of the borophene, NiPc, and NiPc: borophene are shown, highlighting the functional groups of all samples. In our previous study, the characteristic peaks of borophene were reported. These results showed that the distinctive peaks of borophene were observed at 3479 cm−1 (O-H), 2929 cm−1 (B-B), 2861 cm−1 (B-H), 1653 cm−1 (C=O), 1496 cm−1 (B-H), 1385 cm−1 (B-O), 1255 cm−1 (B-O), and 1091 cm−1 (B-O-B vibrations). The peaks of the prepared NiPc were observed at 3504 cm−1 (N–H stretching), 2920 cm−1 (C–H stretching), and 1680 cm−1 (C=N/C=C stretching). Additionally, the characteristic peaks of the prepared NiPc were observed at 1090 cm−1, 845 cm−1, and 756 cm−1 and are attributed to phthalocyanine skeletal vibrations. The characteristic peaks of the prepared NiPc: borophene were found at 2922 cm−1 (C–H), 1462 cm−1 (–CN group), and 748 cm−1 (bending modes of vibration of nickel) [45]. Consequently, the appearance of the –CN group at 1462 cm−1 and the disappearance of the –NH peak at 3150 cm−1 in the structure confirmed the formation of NiPc: borophene.

Figure 5.
SEM images of the prepared (a) NiPc: borophene (1300×) and (b) NiPc: borophene (5700×), FTIR spectras of (c) borophene, NiPc, and NiPc: borophene.
3.2. Thermoelectric Performance of the Prepared TEGs
Measurements of electrical conductivity and the Seebeck coefficient for NiPc and NiPc: borophene were taken (Figure 6). Electrical conductivities (s) were measured to be 3 × 10−13 Scm−1 and 9.5 × 10−9 Scm−1 for NiPc and the NiPc: borophene nanocomposite, respectively (Figure 6a). The Seebeck coefficients (S) of the NiPc and the NiPc: borophene nanocomposite were measured as 5 mVK−1 and 30 mVK−1, respectively (Figure 6b). Borophene can enhance charge transport in the NiPc matrix without disrupting the crystal structure. NiPc is a planar, π-conjugated organic semiconductor. When composited with borophene, efficient charge injection can occur at the interfaces. NiPc molecules stack in columnar structures with π–π interactions along the stacking axis (a/b plane). Because borophene is a two-dimensional structure, it can align parallel to the π system of NiPc, facilitating lateral charge delocalization without disrupting the molecular packing. Because borophene is a p-type semiconductor, it can increase hole mobility by attracting charge from the Ni atoms of NiPc [46]. Borophene increased the electrical conductivity of the hybrid material while only causing a slight increase in the thermal conductivity of the NiPc: borophene hybrid material.
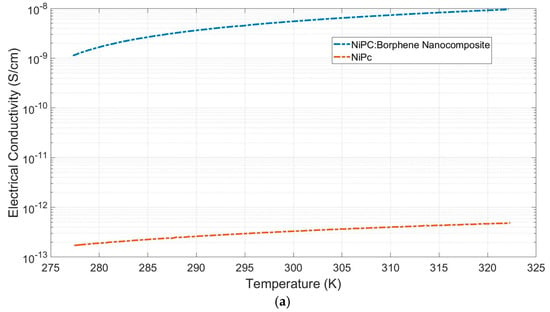
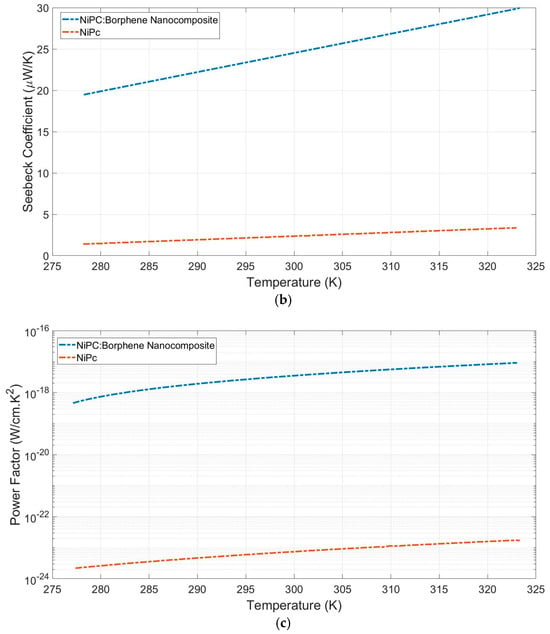
Figure 6.
(a) Electrical conductivity, (b) Seebeck coefficient, and (c) power factor versus temperature of NiPc and the NiPc: borophene nanocomposite.
These results reveal that both NiPc and the NiPc: borophene nanocomposite were p-type semiconductors. The power factors (PF = σS2) were calculated as 7.5 × 10−16 μW m−1 K−2 and 8.6 × 10−10 μW m−1 K−2 for NiPc and the NiPc: borophene nanocomposite, respectively (Figure 6c). Compositing NiPc with borophene increased the power factor by ~106-fold. It has been concluded that the electrical conductivity and Seebeck coefficient of the NiPc: borophene material increases due to energy band convergence due to combining p-type NiPc with p-type borophene. A comparison of the power factor of the prepared TEGs with that of previous studies is presented in Table 1. This study is the first to investigate the thermoelectric properties of NiPc: borophene. It provides a basis for demonstrating the potential of borophene to increase the thermoelectric performance of organic or polymer materials and contributes to the literature. Although the obtained PF values fall short of those of some high-performance materials, such as those listed in Table 1, it may be possible to increase the PF values in the future through electrical conductivity or structural optimizations.

Table 1.
Comparison of the power factor of the prepared TEGs with previous studies.
4. Conclusions
In this study, borophene and nickel phthalocyanine (NiPc)-borophene nanocomposites were prepared using the sonication method. The NiPc: borophene nanocomposite was uniformly obtained as 10–80 nm-sized spherical particles. An ITO/NiPc: borophene nanocomposite-based TEG was prepared and characterized. These results indicate that both NiPc and NiPc-borophene nanocomposites exhibit p-type semiconductor properties. The power factors (PF = σS2) were calculated as 7.5 × 10−8 mW m−1 K−2 and 8.6 × 10−2 mW m−1 K−2 for NiPc and the NiPc: borophene nanocomposite, respectively. Because borophene enhances the electrical conductivity of NiPc, the NiPc: borophene nanocomposite exhibits a higher power factor. This increase in conductivity suggests that borophene doping would lead to a significant improvement in TE efficiency due to its high electrical conductivity. The production of thermoelectric materials at a low cost and through a simple process that can be achieved from a solution is expected to reduce energy costs and enhance welfare, offering a solution to significant challenges in sustainable energy production.
Author Contributions
N.T.: Conceptualization, Methodology, Supervision, Writing—Reviewing and Editing. İ.G.: Writing—Original draft preparation, Analysis. C.T.: Methodology. S.K.: Writing—Original draft preparation. B.B.A.: Analysis, Original draft preparation. A.G.: Analysis, Original draft preparation. M.Y.: Supervision, Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific and Technological Research Council of Turkey (TUBITAK), Project 122N962.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors gratefully acknowledge the Scientific and Technological Research Council of Turkey (TUBITAK) for the financial support.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Russ, B.; Glaudell, A.; Urban, J.J.; Chabinyc, M.L.; Segalman, R.A. Organic thermoelectric materials for energy harvesting and temperature control. Nat. Rev. Mater. 2016, 1, 1–14. [Google Scholar] [CrossRef]
- Urban, J.J.; Menon, A.K.; Tian, Z.; Jain, A.; Hippalgaonkar, K.J. New horizons in thermoelectric materials: Correlated electrons, organic transport, machine learning, and more. Appl. Phys. 2019, 125, 180902. [Google Scholar] [CrossRef]
- Wang, H.; Yu, C. Organic Thermoelectrics: Materials Preparation, Performance Optimization, and Device Integration. Joule 2019, 3, 53–80. [Google Scholar] [CrossRef]
- Nandihalli, N.; Liu, C.J.; Mori, T. Polymer based thermoelectric nanocomposite materials and devices: Fabrication and characteristics. Nano Energy 2020, 78, 105186. [Google Scholar] [CrossRef]
- Bell, L.E. Cooling, heating, generating power, and recovering waste heat with thermoelectric systems. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef]
- Yang, B.; Ahuja, H.; Tran, T.N. Review Article: Thermoelectric Technology Assessment: Application to Air Conditioning and Refrigeration. Hvac&R Res. 2008, 14, 635–653. [Google Scholar]
- Vineis, C.J.; Shakouri, A.; Majumdar, A.; Kanatzidis, M.G. Nanostructured Thermoelectrics: Big Efficiency Gains from Small Features. Adv. Mater. 2010, 22, 3970–3980. [Google Scholar] [CrossRef]
- Lee, J.; Chen, H.F.; Batagoda, T.; Coburn, C.; Djurovich, P.I.; Thompson, M.E.; Forrest, S.R. Deep blue phosphorescent organic light-emitting diodes with very high brightness and efficiency. In Electrophosphorescent Materials and Devices; Jenny Stanford Publishing: Singapore; pp. 877–899.
- Verma, A.K.; Johari, K.K.; Dubey, P.; Sharma, D.K.; Kumar, S.; Dhakate, S.R.; Candolfi, C.; Lenoir, B.; Gahtori, B. Realization of band convergence in p-Type TiCoSb half-heusler alloys significantly enhances the thermoelectric performance. ACS Appl. Mater. Interfaces 2022, 15, 942–952. [Google Scholar] [CrossRef]
- Sirringhaus, H. 25th Anniversary Article: Organic Field-Effect Transistors: The Path Beyond Amorphous Silicon. Adv. Mater. 2014, 26, 1319–1335. [Google Scholar] [CrossRef]
- Cahill, D.G.; Braun, P.V.; Chen, G.; Clarke, D.R.; Fan, S.; Goodson, K.E.; Keblinski, P.; King, W.P.; Mahan, G.D.; Majumdar, A.; et al. Nanoscale thermal transport. II. 2003–2012. Appl. Phys. Rev. 2014, 1, 011305. [Google Scholar] [CrossRef]
- Tan, M.; Wang, Y.; Deng, Y.; Zhang, Z.; Luo, B.; Yang, J.; Xu, Y. Oriented growth of A2Te3 (A = Sb, Bi) films and their devices with enhanced thermoelectric performance. Sens. Actuators A Phys. 2011, 171, 252–259. [Google Scholar] [CrossRef]
- Goldsmid, H.J. The Electrical Conductivity and Thermoelectric Power of Bismuth Telluride. Proc. Phys. Soc. 1958, 71, 633. [Google Scholar] [CrossRef]
- Majumdar, A. Thermoelectricity in Semiconductor Nanostructures. Science 2004, 303, 777–778. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Venkatasubramanian, R.; Siivola, E.; Colpitts, T.; O’Quinn, B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 2001, 413, 597–602. [Google Scholar] [CrossRef]
- Hicks, L.D.; Dresselhaus, M.S. The Effect of Quantum Well Structures on the Thermoelectric Figure of Merit. Phys. Rev. B 1993, 47, 12727. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Liu, W.S.; Zhao, L.D.; Zhou, M. High-performance nanostructured thermoelectric materials. NPG Asia Mater. 2010, 2, 152–158. [Google Scholar] [CrossRef]
- Girard, S.N.; He, J.; Li, C.; Moses, S.; Wang, G.; Uher, C.; Dravid, V.P.; Kanatzidis, M.G. In situ nanostructure generation and evolution within a bulk thermoelectric material to reduce lattice thermal conductivity. Nano Lett. 2010, 10, 2825. [Google Scholar] [CrossRef]
- Zhao, H.; Pokheral, M.; Zhu, G.; Chen, S.; Lukas, K.; Jie, Q.; Opeil, C.; Chen, G.; Ren, Z. Dramatic Thermal Conductivity Reduction by Nanostructures for Large Increase in Thermoelectric Figure-of-merit of FeSb2. Appl. Phys. Lett. 2011, 99, 163101. [Google Scholar] [CrossRef]
- Morelli, D.T.; Meisner, G.P. Low temperature properties of the filled skutterudite CeFe4Sb12. J. Appl. Phys. 1995, 77, 3777–3781. [Google Scholar] [CrossRef]
- Uher, C.; Yang, J.; Hu, S.; Morelli, D.T.; Meisner, G.P. Transport properties of pure and doped MNiSn (M=Zr, Hf). Phys. Rev. B 1999, 59, 8615. [Google Scholar] [CrossRef]
- Nolas, G.S.; Cohn, J.L.; Slack, G.A.; Schujman, S.B. Semiconducting Ge clathrates: Promising candidates for thermoelectric applications. Appl. Phys. Lett. 1998, 73, 178–180. [Google Scholar] [CrossRef]
- Sakurada, S.; Shutoh, N. Effect of Ti substitution on the thermoelectric properties of (Zr,Hf)NiSn half-Heusler compounds. Appl. Phys. Lett. 2005, 86, 082105. [Google Scholar] [CrossRef]
- Sun, Y.; Sheng, P.; Di, C.; Jiao, F.; Xu, W.; Qiu, D.; Zhu, D. Organic Thermoelectric Materials and Devices Based on p- and n-Type Poly(metal 1,1,2,2-ethenetetrathiolate)s. Adv. Mater. 2012, 24, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Chen, S.; Liu, C.; Lu, B.; Xu, J.; Wang, J.; Liu, G. Synthesis, characterization, and thermoelectric properties of a conducting copolymer of 1, 12-bis (carbazolyl) dodecane and thieno [3, 2-b] thiophene. J. Solid State Electrochem. 2012, 16, 117. [Google Scholar] [CrossRef]
- Søndergaard, R.R.; Hösel, M.; Espinosa, N.; Jørgensen, M.; Krebs, F.C. Practical evaluation of organic polymer thermoelectrics by large-area R2R processing on flexible substrates. Energy Sci. Eng. 2013, 1, 81–88. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, W.; Di, C.A.; Zhu, D. Metal-organic complexes-towards promising organic thermoelectric materials. Synth. Met. 2017, 225, 22–30. [Google Scholar] [CrossRef]
- Wang, L.; Dong, B.; Ge, R.; Jiang, F.; Xu, J. Fluorene-Based Two-Dimensional Covalent Organic Framework with Thermoelectric Properties through Doping. ACS Appl. Mater. Interfaces 2017, 9, 7108–7114. [Google Scholar] [CrossRef]
- Bertram, J.R.; Penn, A.; Nee, M.J.; Rathnayake, H. A Novel N-Type OrganosilaneMetal Ion Hybrid of Rhodamine B and Copper Cation for Low-Temperature Thermoelectric Materials. ACS Appl. Mater. Interfaces 2017, 9, 10946–10954. [Google Scholar] [CrossRef]
- Sun, L.; Liao, B.; Sheberla, D.; Kraemer, D.; Zhou, J.; Stach, E.A.; Zakharov, D.; Stavila, V.; Talin, A.A.; Ge, Y.; et al. A Microporous and Naturally Nanostructured Thermoelectric Metal-Organic Framework with Ultralow Thermal Conductivity. Joule 2017, 1, 168–177. [Google Scholar] [CrossRef]
- Shakeel, M.; Rehman, K.; Ahmad, S.; Amin, M.; Iqbal, N.; Khan, A. A low-cost printed organic thermoelectric generator for low-temperature energy harvesting. Renew. Energy 2021, 167, 853–860. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Mahmoud, M.S.; Elsaid, K.; Sayed, E.T.; Wilberforce, T.; Al-Murisi, M.; Maghrabie, H.M.; Olabi, A.G. Prospects of Thermoelectric Generators with Nanofluid. Therm. Sci. Eng. Prog. 2022, 29, 101207. [Google Scholar] [CrossRef]
- Jaziri, N.; Boughamoura, A.; Müller, J.; Mezghani, B.; Tounsi, F.; Ismail, M. A comprehensive review of Thermoelectric Generators: Technologies and common applications. Energy Rep. 2020, 6, 264–287. [Google Scholar] [CrossRef]
- Masoumi, S.; O’Shaughnessy, S.; Pakdel, A. Organic-based flexible thermoelectric generators: From materials to devices. Nano Energy 2022, 92, 106774. [Google Scholar] [CrossRef]
- Doraghi, Q.; Khordehgah, N.; Żabnieńska-Góra, A.; Ahmad, L.; Norman, L.; Ahmad, D.; Jouhara, H. Investigation and Computational Modelling of Variable TEG Leg Geometries. ChemEngineering 2021, 5, 45. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Abd-El-Rahman, K.F.; Farag, A.A.M.; Darwish, A.A.A. Photovoltaic properties of NiPc/p-Si (organic/inorganic) heterojunctions. Org. Electron. 2005, 6, 129–136. [Google Scholar] [CrossRef]
- Nasir, E.M.; Hussein, M.T.; Al-Aarajiy, A.H. Investigation of nickel phthalocyanine thin films for solar cell applications. Adv. Mater. Phys. Chem. 2019, 9, 158–173. [Google Scholar] [CrossRef]
- Wang, D.; Shi, W.; Chen, J.; Xi, J.; Shuai, Z. Modeling thermoelectric transport in organic materials. Phys. Chem. Chem. Phys. 2012, 14, 16505–16520. [Google Scholar] [CrossRef]
- Xing, W.; Chen, J.; Liang, Y.; Zou, Y.; Sun, Y.; Xu, W.; Zhu, D. Optimization of the thermoelectric performance of layer-by-layer structured copper-phthalocyanine (CuPc) thin films doped with hexacyano-trimethylene-cyclopropane (CN6-CP). RSC Adv. 2019, 9, 31840–31845. [Google Scholar] [CrossRef]
- Chen, Y.; Qu, S.; Shi, W.; Yao, Q.; Chen, L. Enhanced thermoelectric properties of copper phthalocyanine/single-walled carbon nanotubes hybrids. Carbon 2020, 159, 471–477. [Google Scholar] [CrossRef]
- Hou, C.; Tai, G.; Liu, Y.; Liu, X. Borophene gas sensor. Nano Res. 2022, 15, 2537–2544. [Google Scholar] [CrossRef]
- Najiya, K.P.P.; Konnola, R.; Sreena, T.S.; Solomon, S.; Gopchandran, K.G. Liquid phase exfoliation of few-layer borophene with high hole mobility for low-power electronic devices. Inorg. Chem. Commun. 2024, 168, 112962. [Google Scholar] [CrossRef]
- Güngör, S.; Taşaltın, C.; Gürol, İ.; Baytemir, G.; Karakuş, S.; Taşaltın, N. Copper phthalocyanine-borophene nanocomposite-based non-enzymatic electrochemical urea biosensor. Appl. Phys. A 2022, 128, 89. [Google Scholar] [CrossRef]
- Gürol, I.; Ahsena, V.; Bekarǒlu, Ö.J. Synthesis of tetraalkylthio-substituted phthalocyanines and their complexation with AgI and PdII. Chem. Soc. Dalton Trans. 1994, 4, 497–500. [Google Scholar] [CrossRef]
- Chen, S.; Ma, J. Charge transport in stacking metal and metal-free phthalocyanine iodides. Effects of packing, dopants, external electric field, central metals, core modification, and substitutions. J. Comput. Chem. 2009, 30, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.P.; Schlettwein, D.; Wohrle, D.; Jaeger, N.I. Charge transport in thin films of molecular semiconductors as investigated by measurements of thermoelectric power and electrical conductivity. Thin Solid Films 1995, 258, 317–324. [Google Scholar] [CrossRef]
- Yamakado, H.; Ida, T.; Ugawa, A.; Yakushi, K.; Awaga, K.; Maruyama, Y.; Imaeda, K.; Inokuchi, H. Structure and solid-state properties of the stable ring-oxidized conductor CoPc(AsF6)0.5: Interaction between ring π-electrons and cobalt d-electrons. Synth. Met. 1994, 62, 169–178. [Google Scholar] [CrossRef]
- Yonehara, Y.; Yakushi, K. High-Pressure Study of One-dimensional Phthalocyanine Conductor, NiPc(AsF6)0.5. Synth. Met. 1998, 94, 149–155. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, Q.; Qu, S.; Shi, W.; Li, H.; Chen, L. Enhanced thermoelectric performance of phthalocyanine complexes/single-walled carbon nanotube hybrids by tuning the types of metal coordination ions. Compos. Commun. 2021, 27, 100891. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).