Abstract
The relationship between slag structure and viscosity is studied, employing Raman spectroscopy for the five-component slag system of MnO-SiO2-CaO-Al2O3-MgO and its subsystems. This study aims to investigate the influence of variations in slag composition on viscosity, which is crucial for optimizing industrial processes. Based on industrial slag compositions produced in a silicomanganese submerged arc furnace, 17 slags with a fixed content of MnO of 10 wt% are synthesized with varying contents of SiO2 of 33 to 65 wt%; CaO within the range of 14 to 40 wt%; and fixed contents of Al2O3 and MgO of 17 and 6 wt%, respectively. The slag compositions are divided into four groups, ranging from low basicity (0.38) to high basicity (0.80), with each group containing the four slag systems of MnO-SiO2-CaO, MnO-SiO2-CaO-Al2O3, MnO-SiO2-CaO-MgO, and MnO-SiO2-CaO-Al2O3-MgO, with fixed basicity. Additionally, a five-component composition with the lowest basicity of 0.28 is considered. Raman spectroscopy measurements are performed in the wavenumber range of 200 to 1200 using a green source laser with a 532 nm wavelength. The high-wavenumber region of the Raman spectra (800 to 1200 ) is deconvoluted to quantitatively investigate the effect of each oxide on the slag structure and the degree of polymerization (DOP) of the silicate network. Results indicate that measured NBO/T increases with increasing basicity, demonstrating a reduction in DOP of the silicate structure. This depolymerization effect is more pronounced in slags containing Al2O3 compared to those without it. In a group of slags with similar basicity, the substitution of SiO2 with Al2O3 leads to further depolymerization. In contrast, substituting CaO with MgO has little effect on the silicate structure in slags without Al2O3 but causes depolymerization in slags containing Al2O3. To study the relationship between structure and viscosity, viscosity data obtained from FactSage are used as reference values. The predictions of slag viscosity using the Raman-structure model and the NBO/T viscosity model are then compared to the FactSage results. The adjustable parameters of the Raman-structure model are re-determined using the FactSage data for the studied slag compositions. The NBO/T viscosity model employs both calculated NBO/T values from the slag compositions and measured NBO/T values from the deconvolution results. The findings of this study reveal good agreement between the predictions of the Raman-structure model and the FactSage viscosity data.
1. Introduction
Manganese ferroalloys are categorized as ferromanganese (FeMn) with varying carbon content (high, medium, or low) and silicomanganese (SiMn) [1]. High-carbon FeMn and SiMn are mostly produced through the carbothermic reduction of manganese ores with the addition of a carbon source in electric submerged arc furnaces. In manganese ferroalloys, the main element, manganese, is present withing the range of 60 to 80 wt%, while the content of silicon is typically less than 1 wt% for FeMn and between 16 and 30 wt% for SiMn [2]. In addition to manganese and silicon, iron and carbon are also present in manganese ferroalloys. The slag system produced in manganese ferroalloy processes is mainly composed of five oxides, namely MnO, SiO2, CaO, Al2O3, and MgO. Based on industrial slag compositions, the MnO content is between 15 and 40 wt% in FeMn slags, while for SiMn slags, the MnO and SiO2 contents are typically within 5 to 20 wt% and 40 wt%, respectively [1,2]. Knowledge of slag systems related to the primary production of FeMn or SiMn in submerged arc furnaces was reviewed extensively in a recent paper [2].
In high-temperature metallurgical processes such as the production of manganese ferroalloys, slag properties are of crucial importance because of their key roles in determining the performance of industrial operations [3,4]. Among these properties, viscosity has a significant impact on metal yield by affecting metal–slag separation efficiency [5] and the tapping process [6]. Slag viscosity is dependent on both temperature and slag composition; the viscosity decreases with increasing temperature. To better understand the relationship between viscosity and slag composition, studying the structure of slag is necessary [7,8,9,10,11,12]. Therefore, gaining insight into the atomic structure of molten slags is of fundamental importance in understanding and controlling metallurgical operations [9,10,11,12,13,14]. Among various spectroscopic techniques, the Raman spectroscopy method has been widely used to obtain valuable knowledge on the structural properties of numerous silicate melts and glasses [15,16,17,18,19,20,21,22,23]. The analysis of Raman data reveals the types of vibration species and their relative distribution through the examination of the peak shift and intensity of Raman spectra [24]. Silicate glasses are widely used as structural models for their corresponding melts due to the challenge of studying the structural properties of molten materials. It has been shown that silicate glasses and their molten counterparts possess similar structural properties when the glass is produced by rapidly cooling the molten slag [7,25].
Viscosity has been measured for slag systems containing MnO, including MnO-SiO2-Al2O3-CaO-MgO slag system [26,27,28,29] and its subsystems, for example, binary MnO-SiO2 [30,31], ternary systems of MnO-SiO2-CaO [30,32,33] and MnO-SiO2-Al2O3 [31], and quaternary systems of MnO-SiO2-CaO-MgO [34] and MnO-SiO2-CaO-Al2O3 [26,35]. Many Raman spectroscopy investigations of silicate melts and glasses have also reported data for slag systems containing MnO, such as the ternary systems of CaO-SiO2-MnO [36,37,38] and MnO-SiO2-Al2O3 [39], as well as the quaternary systems of CaO-SiO2-MnO-xCaF2 [x = 0.0 to 14.5 wt%] [38], TiO2-MnO (30 wt%)-SiO2-Al2O3 [40], MO-SiO2-MnO-yCaF2 [M(=Ca or Ba)O, y = 0 to 15 mol%] [41], and MnO-SiO2-Al2O3-zCe2O3 [z = 0.0 to 5.6 mol%] [39]. However, the structure of the five-component slag system in manganese ferroalloy production, namely MnO-SiO2-CaO-MgO-Al2O3, has not been investigated yet.
In this paper, slag structure is studied using the Raman spectroscopy technique for a group of 17 synthetic slags in the five-component slag system of MnO-SiO2-CaO-Al2O3-MgO and its subsystems, including MnO-SiO2-CaO, MnO-SiO2-CaO-Al2O3, and MnO-SiO2-CaO-MgO slag systems. In order to simulate industrial slags in silicomanganese production, fixed contents of MnO, Al2O3, and MgO oxides of 10, 17, and 6 wt%, respectively, are used, while the contents of SiO2 and CaO are varied from 33 to 65 wt% and 14 to 40 wt%, respectively. The slag compositions are divided into four groups, ranging from low basicity (0.38) to high basicity (0.80), with each group containing four slag systems with a fixed basicity. As in previous research [23,42,43,44], the Raman parameter (R) is calculated as the ratio of low-to-high-wavenumber vibrational bands within the range of 200 to 1200 and is used in correlation with basicity (CaO + MgO/(SiO2 + Al2O3)), optical basicity [45], and non-bridging oxygen per tetrahedral cation (NBO/T) [9,45]. In this study, a strong correlation is found between R and the chemical parameters for slags containing Al2O3, where the values of R decrease as basicity, optical basicity, and NBO/T increase. However, for slags without Al2O3, R decreases slightly, particularly in correlation with NBO/T. The effects of basicity and various oxides on slag structure are studied by analyzing the Raman spectra in the high-wavenumber region (800 to 1200 ) using deconvolution techniques. The results are discussed in detail. The predictions of viscosity models, such as the Raman-structure model and the NBO/T viscosity model, are compared to reference values obtained from FactSage 7.3 [46,47]. In the NBO/T viscosity model, both calculated NBO/T values from slag compositions and measured NBO/T values from deconvolution results are used to predict viscosity values. By comparing the predictions of viscosity models with FactSage results, the Raman-structure model predictions are found to be closer to the FactSage viscosity data. The results of this study can help in optimizing slag composition and improving the performance of metallurgical processes, such as silicomanganese production. Additionally, the findings can provide insights into the behavior of slags in various industrial settings.
This work is organized as follows. Section 2 describes sample preparation, characterization techniques, and Raman spectral analysis. In Section 3, the Raman spectroscopy results for the (10 wt%) MnO-SiO2-CaO-Al2O3-MgO slag system and its subsystems are presented, and the relationship between slag structure and viscosity is discussed. Finally, in Section 4, the key findings are summarized and conclusions are drawn.
2. Materials and Methods
2.1. Sample Preparation
This study investigated slag compositions containing 10 wt% MnO through Raman spectroscopy. The slags were selected based on the slag systems produced in a silicomanganese submerged arc furnace, composed of MnO, SiO2, CaO, Al2O3, and MgO. These compositions were designed to study the effects of SiO2, CaO, MgO, and Al2O3 on the polymerization of the silicate network, as SiO2 acts as a network-former oxide, while CaO, MgO, and MnO are network-breaker oxides. The effect of Al2O3 is more complex, as it can act as either an acidic or basic oxide depending on the amount of other oxides present in the slag system.
Table 1 presents the designed compositions with SiO2 contents ranging from 33 to 70 wt%; CaO contents between 14 and 40 wt%; and fixed contents of Al2O3 and MgO of 17 and 6 wt%, respectively. To study the effect of basicity on slag structure, the slag compositions were categorized into groups based on their basicity, which is calculated as the ratio of the sum of CaO and MgO (C + M) to the sum of SiO2 and Al2O3 (S + A), all in wt%. The S + A and C + M contents were fixed in each group to investigate the variation in slag structure by substituting 17 wt% of SiO2 with Al2O3 and 6 wt% of CaO with MgO. The S + A values were 70, 65, 60, 55, and 50 wt%, while C + M values were 20, 25, 30, 35, and 40 wt% for slag groups A, B, C, D, and E, respectively. The groups, classified based on their basicity, range from the lowest basicity of 0.28 in group A to the highest basicity of 0.80 in group E. Each group contains 4 different slag systems, including the MnO-SiO2-CaO ternary system, MnO-SiO2-CaO-Al2O3 and MnO-SiO2-CaO-MgO quaternary systems, and the MnO-SiO2-CaO-Al2O3-MgO five-oxide system, with a constant basicity.

Table 1.
Chemical compositions in weight percent of the MnO-SiO2-CaO-Al2O3-MgO slag system and its subsystems as designed in this study.
The synthetic slags were produced in an induction furnace with a rating of 30 kW (custom-made at NTNU, Trondheim, Norway) using the analytical reagent oxides of MnO (99.00% purity, Alfa Aesar, Kandel, Germany), SiO2 (99.50% purity, Alfa Aesar, Kandel, Germany), CaO (99.95% purity, Alfa Aesar, Kandel, Germany), Al2O3 (99.00% purity, Alfa Aesar, Kandel, Germany), and MgO (99.00% purity, Alfa Aesar, Kandel, Germany). To ensure the accuracy of experiments, the CaO and MgO oxides were calcined at 1273 K for 2 h in a muffle furnace to decompose any hydroxides. The powders were then precisely weighted according to the designed compositions listed in Table 1 and mixed to achieve homogeneous mixtures. A high-purity molybdenum crucible was securely positioned within a high-purity graphite crucible using graphite felt. Then 40 g of powder mixtures was carefully placed inside the molybdenum crucible. The assembly process involved inserting a mica sheet and appropriately sized graphite felt into the copper coil of the induction furnace. Subsequently, the graphite crucible was carefully placed within the copper coil. During the experiments, temperature monitoring was conducted using a type-C thermocouple. The thermocouple was positioned inside a molybdenum thermowell tube with one end closed and fixed to the wall of the graphite crucible. Before initiating the heating process, the induction furnace was vacuumed to achieve a pressure of mbar. It was then filled with Ar gas (99.999% purity) until the pressure reached 1000 mbar. This process was repeated twice. Finally, the furnace was filled with He gas (99.9996% purity) until the pressure reached 1040 mbar. To create the desired experimental conditions, the furnace was heated at a rate of approximately 30 to 50 K/min, reaching temperatures ranging from 1973 to 2043 K, depending on the composition. The temperature was maintained for 2 h to ensure the homogenization of the slag melts. After 2 h, the molten slags were quickly quenched into a copper mold inside the furnace, which was cooled by water. The quenching process was performed quickly to ensure that the temperature of the molten slags was still well above their melting points, resulting in a glassy state. The quenched slag masses varied depending on the composition, within a range of 10 to 30 g. A portion of the quenched samples (approximately 10 g) was then ground for further characterization using techniques described in Section 2.2.
2.2. Characterization Techniques
In this study, the prepared slag samples were analyzed using X-ray fluorescence (XRF), X-ray diffraction (XRD), and Raman spectroscopy techniques. The XRF method (Thermo Fisher Scientific, Degerfors, Sweden) was employed to measure the chemical compositions, with sample preparation conducted using the flux fusion method.
Phase analysis of the slag samples was performed by XRD using a Bruker D8 A25 DaVinciTM instrument (Bruker, Karlsruhe, Germany) equipped with CuK radiation (wavelength of 1.54 Å). The measurement range was defined from 10 to 80∘ with a step size of 0.03.
A WITec Alpha 300R laser confocal Raman spectrometer (WITec GmbH, Ulm, Germany) equipped with a green laser (532 nm) was used to obtain the Raman spectra of the slag samples. The spectra were recorded in the wavenumber range of 200 to 1200 at room temperature to study the relationship between the slag structure and viscosity. The raw Raman data were processed using the cubic baseline B procedure to remove the baseline (or background) [42]. This method defines the baseline between two boundaries where there is no signal, and it passes through one or two invariant domains in the region of 600 to 850 [42]. Baseline correction was carried out using Fityk software (https://fityk.nieto.pl/, accessed on 24 July 2024), which is open-source software for data analysis and nonlinear curve fitting [48].
2.3. Viscosity Calculations
In this study, FactSage 7.3 [46,47], a thermochemical software and database package developed by Thermfact/CRCT (Montreal, QC, Canada) and GTT-Technologies (Aachen, Germany), was employed for the calculation of liquidus temperature and slag viscosity. The FToxid and FactPS databases, which contain extensive thermodynamic data for various oxides and pure substances, respectively, were utilized for these calculations.
3. Results and Discussion
3.1. Characterization Results
The XRF analysis results for the chemical compositions of the 17 slag samples in wt%, along with their liquidus temperatures (Tl (K)) calculated by FactSage are presented in Table 2. The XRF compositions for slags A1 to A3 are unavailable due to the challenges in producing glassy slags using the current furnace and quenching process. The produced slags were partially crystalline despite several attempts, which can be attributed to their high viscosity. As a result, only slag A4 was considered as an example for the five-component slag system of MnO-SiO2-CaO-Al2O3-MgO with the highest SiO2 content of 53 wt% in group A. The compositions obtained from XRF analysis are normalized to 100% and utilized in the following sections. Note that the presence of Al2O3 and MgO in XRF analysis of samples without the planned addition of these oxides can result from contamination during sample preparation and analysis. Furthermore, even high-purity analytical reagent oxides, typically with a purity of around 99%, can contribute to the detection of Al2O3 and MgO in XRF analysis of samples without these oxides.

Table 2.
Measured chemical compositions, in weight percent, of the MnO-SiO2-CaO-Al2O3-MgO slag system and its subsystems.
The results of the XRD analysis are presented in Figure 1; those for slags A4, B1 to B4, and C1 to C4 are shown in Figure 1a, and those for slags D1 to D4 and E1 to E4 are shown in Figure 1b. The XRD analysis shows that there are no crystalline peaks in the patterns, indicating that the quenched samples are in the glassy phase. The glassy state denotes the amorphous, non-crystalline structure that occurs when the molten slag is rapidly quenched. In contrast to crystalline materials, which possess a long-range ordered atomic structure, glassy materials are specified by a lack of periodic atomic arrangement, preserving the high-temperature structure of the slag [7,25].
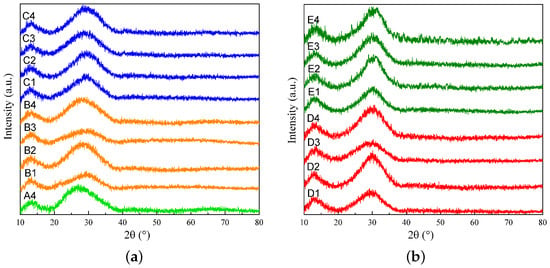
Figure 1.
XRD results for (a) slags A4, B1 to B4, and C1 to C4 and (b) slags D1 to D4 and E1 to E4.
Analysis of Raman Spectra
The normalized Raman spectra for slags A4, B1 to B4, and C1 to C4 are presented in Figure 2a, and the normalized Raman spectra for slags D1 to D4 and E1 to E4 are shown in Figure 2b. As seen in Figure 2, the Raman spectra of silicate glasses typically consist of two broad and asymmetric bands in the frequency range of 200 to 1200 . The first band, known as the low-wavenumber (LW) band, is located between ∼200 and ∼700 . As the degree of depolymerization of the silicate network increases due to higher basicity, this band experiences a shift towards higher wavenumbers and a decrease in intensity. The second band, also known as the high-wavenumber (HW) band, is located between ∼800 and 1200 and has a center around 960 . With an increase in depolymerization of the silicate network, the HW band intensifies and shifts to lower wavenumbers. Additionally, a lower-intensity band can be detected at intermediate wavenumbers between 700 and 800 , with its peak located at around 750 . This is known as the medium-wavenumber (MW) band. The T–O0–T bending vibrations primarily produce the LW band, where T denotes Si or Al and O0 represents bridging oxygen. This region is typically assigned to the D1 (500 ) and D2 (600 ) bands, which are referred to as the breathing vibrations of four and three-membered rings of tetrahedral cations in the aluminosilicate network, respectively [49]. The MW band is generated by the stretching vibration of the T–O0 bond, where its intensity is related to the SiO2 content. In slags with Al2O3 and low basicity, the MW band is intensified [50]. The HW band is made by the stretching vibrations of T–O− bonds, where O− denotes non-bridging oxygen. The HW region results from the combination of aluminosilicate tetrahedral units known as Qn species (), where Q represents a tetrahedron and n is the number of O0 per tetrahedron [51]. These units are denoted as monomer structure (Q0), dimer structure (Q1), chain structure (Q2), sheet structure (Q3), and a three-dimensional structure (Q4) [52]. The deconvolution of Raman spectra in the HW region allows for a quantitative study of these various silicate species. According to the literature, the bands related to Q0, Q1, Q2, Q3, and Q4 are centered at 850 to 880 , 900 to 920 , 950 to 1000 , 1050 to 1100 , and ∼1200 , respectively. However, the Q4 band is difficult to detect due to its low intensity. Hence, four Gaussian functions are allocated to the Qn units () to fit the Raman spectra in the range of 800 to 1200 . Deconvolution of Raman spectra was performed using OriginLab software 2018 [53].
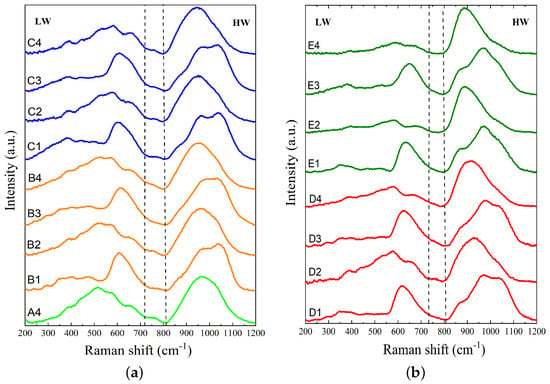
Figure 2.
The normalized Raman spectra after baseline subtraction for (a) slags A4, B1 to B4, and C1 to C4 and (b) slags D1 to D4 and E1 to E4.
3.2. Structure-Related Raman Parameter
Table 3 presents chemical composition parameters, including basicity (), optical basicity (OB), and non-bridging oxygen per tetrahedral cation (NBO/T) calculated using XRF compositions in mol%. The NBO/T is expressed as follows [9,45]:
where denotes the mole fraction of oxide (). Additionally, Raman parameters extracted from Raman spectroscopy measurements, as shown in Figure 2, are given in Table 3. The center of the Raman peaks in the low-wavenumber (LW) and high-wavenumber (HW) regions are represented by and , respectively. The Raman parameter, (R), is calculated as the ratio of the intensity (I) of the two main bands of LW and HW, i.e., R = / [43,44]. Previous research has shown that R values are inversely and non-linearly related to the chemical parameters, with R decreasing as B, OB, and NBO/T increase [23,43,44]. Thus, R serves as a representative parameter for the degree of polymerization of the silicate network in melts and glasses. In the presence of Al2O3, the intensity of the LW band decreases, while the intensity of the HW band increases, as observed in this study. A comparison between slags with and without Al2O3 shows that the addition of Al2O3 results in a reduction in the R values, indicating their role as network modifiers in slag systems. The shifts in and with varying slag compositions indicate changes in the polymerization or depolymerization of the silicate network in the slag structure. A shift in () towards the left (right), i.e., lower (higher) wavenumbers, indicates the depolymerization of the silicate network, while a shift towards the right (left), i.e., higher (lower) wavenumbers, indicates the polymerization of the silicate network in the slag structure.

Table 3.
The values of basicity (B), optical basicity (OB), and NBO/T, as well as the centers of the Raman peaks in LW and HW ranges ( and ) in and the R parameter (ratios of intensity heights (I) in LW and HW regions, /) for the studied slag systems.
Variations in the R parameter (/) against basicity, optical basicity, and calculated NBO/T are illustrated in Figure 3a–c. The slags containing and not containing Al2O3 are represented by solid circles and open circles, respectively. As seen in Figure 3, the R parameter decreases as basicity, optical basicity, and NBO/T increase for slags that contain Al2O3, which is consistent with previous studies [23,43,44]. For slags without Al2O3, such as the MnO-SiO2-CaO and MnO-SiO2-CaO-MgO systems, the R parameter decreases slightly with increasing basicity, optical basicity, and NBO/T.
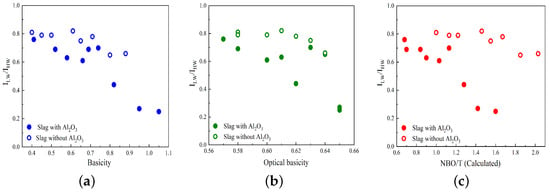
Figure 3.
Variation in the Raman R parameter (/) versus (a) basicity, (b) optical basicity, and (c) NBO/T. The slags with and without Al2O3 are represented by solid circles and open circles, respectively.
3.3. Quantitative Analysis of Raman Spectra
In this section, the results of analyzing the different Qn species () obtained using the deconvolution of Raman spectra in the high-wavenumber range of 800 to 1200 are presented. Figure 4 and Figure 5 display the fitting results for slags B1 to B4 and C1 to C4 and for slags D1 to D4 and E1 to E4, respectively. The bands of Q2 and Q3 species are predominant in slags without Al2O3 in groups B, C, and D, while the substitution of 17 wt% SiO2 by Al2O3 leads to a decline in Q3 and an increase in the Q1 and Q2 bands. In group E, the dominant band is Q2 for slags without Al2O3, while the predominant bands are Q0 and Q1 for slags containing Al2O3. In general, the study shows that the Raman spectra of slags containing SiO2 and slags containing both SiO2 and Al2O3 behave differently, while the Raman spectra of slags with CaO and slags with both CaO and MgO are relatively similar. Additionally, as the contents of SiO2 and Al2O3 are reduced, the Raman spectra in the high-wavenumber range shift to the left-hand side, and Qn species with less bridging oxygen become predominant.
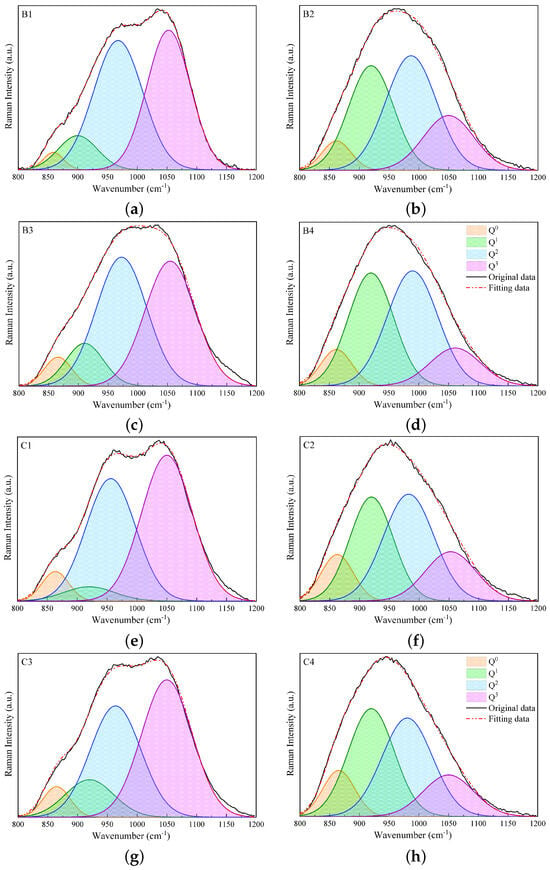
Figure 4.
Fitting results of Raman spectra in the high-wavenumber region, i.e., 800–1200 , using Gaussian functions for slags B1 to B4 (a–d) and C1 to C4 (e–h).
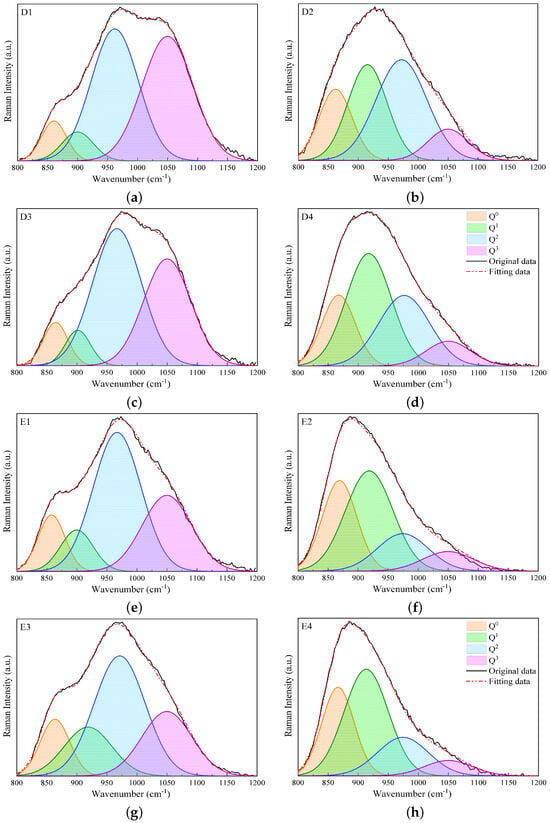
Figure 5.
Fitting results of Raman spectra in the high-wavenumber region, i.e., 800–1200 , using Gaussian functions for slags D1 to D4 (a–d) and E1 to E4 (e–h).
Table 4 presents the data extracted from the Raman spectral deconvolutions shown in Figure 4 and Figure 5, including the relative abundances (area; A0, A1, A2, and A3), and band centers (Cn) of different Qn species. For slags in groups B and C, the variations in An are similar, with A2 changing slightly and A0 having the lowest contribution. A3 and A1 are the predominant relative areas for slags without and with Al2O3, respectively, and their contributions switch when substituting Al2O3 with SiO2. For slags without Al2O3 in group D, namely slags D1 and D3, A2 and A3 are predominant, while A0 and A1 make fewer contributions. When Al2O3 is substituted for SiO2 in slags D2 and D4, A3 decreases, while A0 and A1 increase. For slags in group E, A3 makes fewer contributions, especially for slags with Al2O3 (slags E2 and E4), compared to the other groups. While A2 makes the main contribution for slags E1 and E3, A0 and A1 are predominant for slags E2 and E4.

Table 4.
Deconvolution results of the Raman spectra, including the relative abundance (An) in percent and the band center (Cn) in , for each curve (Qn). The measured NBO/T values were calculated by Equation (3) using the deconvolution results.
The mole fractions of Qn species, namely Xn, are calculated using the An values presented in Table 4 according to the following equation [54]:
where Sn represents the Raman scattering coefficient for Qn with values of , , , and equal to 1, 0.514, 0.242, and 0.09, respectively [55,56].
The relationship between slag structure and viscosity can be quantitatively analyzed by the measured NBO/T value (), which is obtained using Xn calculated according to Equation (2).
The values are inversely proportional to the degree of polymerization (DOP) of the silicate network. This means that a higher NBO/T value corresponds to a lower DOP of the silicate network, typically resulting in a lower slag viscosity. The values calculated by Equation (3) using deconvolution results are presented in Table 4.
Figure 6a–d illustrate the variations in mole fractions for Qn species (X0, X1, X2, and X3) and the values for slags in groups B to E. The figures first present the variations in mole fractions and NBO/T for slags without Al2O3 (slags 1 and 3), where these variations are nearly identical. Subsequently, the variations for slags with Al2O3 (slags 2 and 4) are shown. Slags with similar basicity in each group show wide variations in both Xn and values, with the variations being more pronounced between slags with and without Al2O3. In general, X2 and X3 have higher values, while X0 and X1 make fewer contributions to slag compositions in groups B to E, except for slags containing Al2O3 in group E, where X1 is more prominent. The substitution of 17 wt% SiO2 with Al2O3 leads to a decrease in X3, indicating the depolymerization of the silicate network. Substituting 6 wt% CaO with MgO has little effect on the silicate structure in slags without Al2O3. However, the substitution of 6 wt% CaO with MgO in slags containing Al2O3 causes the depolymerization of the silicate network, as seen by the reduction in X3 and the increase in X1.
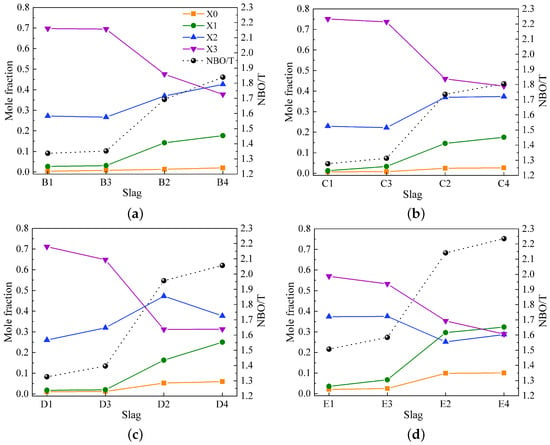
Figure 6.
Variations in mole fractions for Qn species (X0, X1, X2, and X3) and values obtained from the deconvolution of Raman spectra for (a) slags in group B, (b) slags in group C, (c) slags in group D, and (d) slags in group E.
Figure 7 presents the Raman spectra as a function of wavenumbers, the variations in Xn species, and the values for the studied slag systems. For the MnO-SiO2-CaO slag system, the Raman spectra are displayed in Figure 7a. In the high-wavenumber range of 800 to 1200 , the Raman spectra for slags B1 and C1 exhibit two peaks at ∼960 and ∼1050 , corresponding to Q2 and Q3 bands, respectively, and a shoulder at 870 related to the vibrations of Q0 and Q1. As basicity increases, the intensity of the 1050 band decreases and the intensity of the 870 band increases, as seen in slag E1. Figure 7b shows that the mole fraction of X3 decreases and that of X2 increases to compensate for X3. This indicates that the silicate network is depolymerized by increasing the basicity at a fixed MnO content. These findings are consistent with Park’s research on the MnO-SiO2-CaO slag system [36]. For the MnO-SiO2-CaO-Al2O3 slag system, Figure 7c illustrates that the broad band in the high-wavenumber range shifts to the left-hand side as the basicity increases from slag B2 to slag E2. Therefore, the mole fraction of X3 decreases, while X1 and X0 increase, as seen in Figure 7d. The Raman spectral analysis of the MnO-SiO2-CaO-MgO slag system shown in Figure 7e,f is similar to that of the MnO-SiO2-CaO system. The results suggest that the substitution of 6 wt% CaO with MgO causes slight depolymerization in the silicate structure. For the MnO-SiO2-CaO-Al2O3-MgO slag system, the broad band in the high-wavenumber region shifts to the left-hand side with increasing basicity, as presented in Figure 7g. Figure 7f illustrates that the mole fractions of X1 and X0 continuously increase as the basicity increases. The substitution of 6 wt% CaO with MgO leads to depolymerization of the silicate network, as indicated by a comparison of Figure 7d,h. Figure 7 indicates that increases with increasing basicity, demonstrating a reduction in the degree of polymerization (DOP) of the silicate structure. The effect of basicity on the depolymerization is more pronounced in slags containing Al2O3, as indicated by higher values compared to slags without Al2O3.
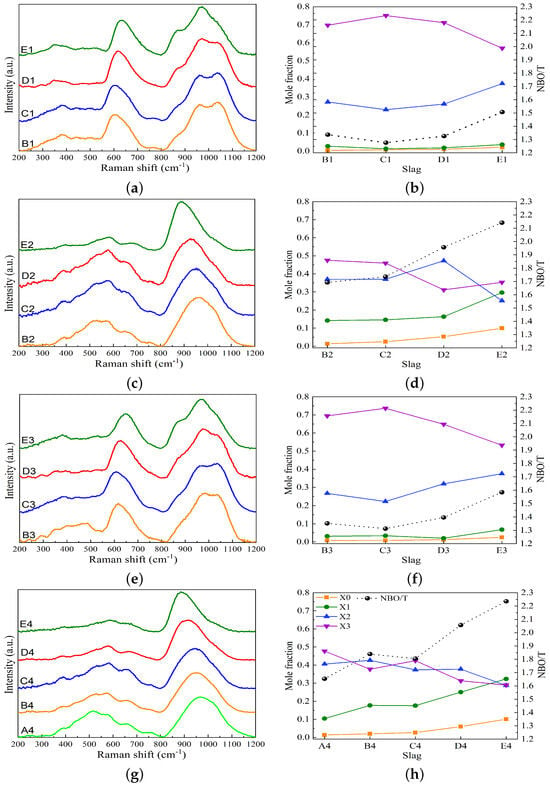
Figure 7.
Raman spectra, the variations in Xn species, and the values plotted against the basicity for the studied slag systems. (a,b) MnO-SiO2-CaO, (c,d) MnO-SiO2-CaO-Al2O3, (e,f) MnO-SiO2-CaO-MgO, and (g,h) MnO-SiO2-CaO-Al2O3-MgO. NBO/T values obtained using deconvolution of Raman spectra (dotted lines).
Figure 8 compares the calculated and measured NBO/T values, represented by and , respectively. Slags with and without Al2O3 are represented by solid circles and open circles, respectively. The acidic (basic) region is identified where values are higher (lower) than the values [36]. This study expands Park’s research [36] by including slags containing Al2O3. Park utilized area fractions to determine measured NBO/T values for a CaO-SiO2-MnO slag system and found that the calculated NBO/T was significantly overestimated for values greater than 2.5 [36]. In this study, mole fractions calculated according to Equation (2) were used to calculate values. For slags with Al2O3, the values are significantly underestimated compared to the measured NBO/T, which may indicate an underestimation of the Al2O3 effect on the depolymerization of the silicate network. In comparison, for slags without Al2O3, the values are relatively close to the measured NBO/T. However, for slags in groups D and E with calculated NBO/T values greater than 1.5, is overestimated, while for slags in group B with calculated NBO/T values around 1, is underestimated. As mentioned by Park, overestimation indicates that the silicate network is not as depolymerized as the calculated NBO/T suggests. Underestimation in the acidic region for slags without Al2O3 is the result of an underestimation of bridging oxygen, corresponding to Q4. This fully polymerized silicate unit was not determined in the deconvolution process due to the low intensity of the 1200 bands in the Raman spectra. For slags containing Al2O3, the values are significantly underestimated compared to the measured NBO/T, especially in slags with lower basicity. Note that the calculation of NBO/T assumes that all Al2O3 act as a network former in the presence of sufficient charge-balancing cations. However, research has revealed that a fraction of Al2O3 exists, even in peralkaline compositions, without being associated with a charge-balancing cations [57]. This fraction of Al2O3 acts as a network modifier, influencing the depolymerization of the silicate network and leading to larger measured NBO/T values compared to the calculated ones. In addition, the presence of aluminosilicate chains (Q2) or cation-deficient regions can contribute to the observed discrepancy between the measured and calculated NBO/T values in slags containing Al2O3 [50,58].
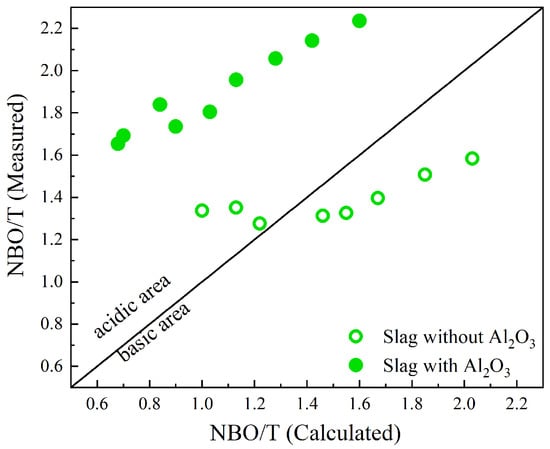
Figure 8.
The relationship between calculated NBO/T values from slag compositions and measured NBO/T values from the deconvolution of Raman spectra, i.e., Equation (3). The slags with and without Al2O3 are represented by solid circles and open circles, respectively.
3.4. Relation between Structure and Viscosity
In this section, the correlation between slag viscosity and structure is presented using various parameters, such as the R parameter (/), calculated NBO/T from slag compositions (), and measured NBO/T from the deconvolution of Raman spectra (). Table 5 presents the viscosity values, denoted as , for temperatures in the range of 1423 to 1873 K for all slag compositions, as obtained by FactSage [46,47]. These viscosity values from the FactSage software are used as reference data for comparing predicted viscosity values from different viscosity models. This choice is due to the unavailability of experimental viscosity values for all slag compositions from a single reference. Note that the Raman-structure model, the NBO/T model, and FactSage predict viscosity based on distinct slag structural parameters and/or viscosity–temperature equations.

Table 5.
The viscosity values () for temperatures in the range of 1423 to 1873 K for all slags, taken from FactSage.
For slags in groups B to E, the variations in log are plotted as a function of temperature in Figure 9a–d. The viscosity decreases as the temperature increases for all slag compositions. Furthermore, slags in group B, characterized by the highest SiO2 content, have higher viscosity values compared to the corresponding slag compositions in other groups.
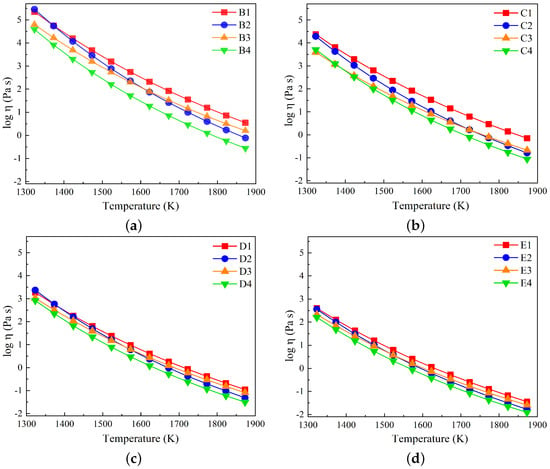
Figure 9.
Variations in log taken from FactSage against temperatures in the range of 1327 to 1873 K for (a) slags in group B, (b) slags in group C, (c) slags in group D, and (d) slags in group E.
In Figure 10a–e, the variations in log are displayed against basicity, optical basicity, , , and the Raman R parameter (/), respectively, for two temperatures of 1673 and 1773 K. The slags with and without Al2O3 are indicated by solid circles and open circles, respectively. In general, as observed previously, the log values decrease as the basicity, optical basicity, and NBO/T values increase. However, in contrast to this pattern, the log values exhibit an increase with an increasing Raman R parameter, as depicted in Figure 10e. The R parameter serves as a representative measure of the polymerization degree within the silicate network, suggesting that an increased degree of polymerization corresponds to higher viscosity values. As shown in Figure 10c, the values for slags containing Al2O3 are smaller compared to those for slags without Al2O3. However, the viscosity values for slags without Al2O3 are larger than the viscosity values for slags with Al2O3, contrary to the expected trend. This deviation can be attributed to the fact that the calculation of NBO/T assumes that all Al2O3 acts as a network former in the presence of sufficient charge-balancing cations. However, the results of this study indicate that a fraction of Al2O3 acts as a network modifier. This network modifier leads to the depolymerization of the silicate network, resulting in a decrease in viscosity, as expected. The variations in the R parameter and log have similar patterns when plotted against basicity, optical basicity, and NBO/T parameters, particularly for slags containing Al2O3, as seen by comparing Figure 3 and Figure 10. This demonstrates the correlation between the Raman R parameter, structural chemical parameters, and viscosity, as reported in previous studies [23,43,44].
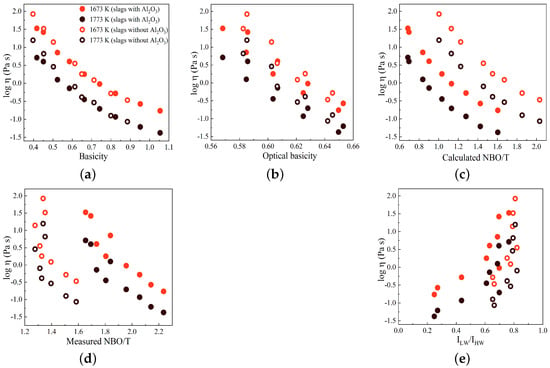
Figure 10.
Variations in log plotted against (a) basicity, (b) optical basicity, (c) calculated NBO/T, (d) measured NBO/T, and (e) Raman R parameter (/) at two temperatures of 1673 and 1773 K. Slags with and without Al2O3 are represented by solid and open circles, respectively.
In Figure 11, the predicted viscosity values obtained using the Raman-structure model and the NBO/T model proposed by Giordano [59] are compared to the viscosity values taken from FactSage. In a previous study [23], the Raman-structure model was presented using the Arrhenius equation with four adjustable parameters for the SiO2-CaO-Al2O3 slag system. In this work, these parameters were re-determined using the viscosity data from FactSage (given in Table 5). By incorporating these new parameters, the viscosity model as a function of temperature and the Raman R parameter can be represented by the following equation:
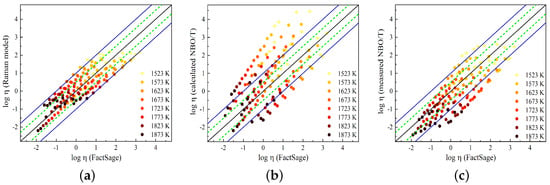
Figure 11.
Comparison of viscosity values obtained from FactSage with viscosity values predicted by (a) the Raman-structure model, (b) the NBO/T model using values calculated from XRF composition, and (c) the NBO/T model using values obtained by Raman deconvolution analysis. The dashed lines represent a range of log units, while the solid lines represent a range of log units.
Viscosity values at low temperatures, where FactSage calculations may be less precise, are included. These values could be corrected to account for partial solidification.
The NBO/T model, proposed by Giordano [59], employs the Tammann–Vogel–Fulcher (TVF) equation to express the temperature dependence of viscosity. This model is given by the following equation:
The adjustable parameters of , , and are temperature-dependent [59]. Figure 11a shows a comparison between the Raman-structure model results and FactSage, while in Figure 11b,c, the results of the viscosity model using and , respectively, are compared to the FactSage results. Good agreement is observed between the Raman-structure model and FactSage viscosities, where the model predicts the FactSage viscosity within log units for most of the slags. The Giordano model overestimates the viscosity values for most of the slags when using the calculated NBO/T, while the model reproduces viscosity values closer to the FactSage values by using the measured NBO/T. In this study, the presence of Al2O3 in the slag compositions was found to result in a decrease in viscosity, indicating its network-modifying effect. While the calculated NBO/T considers to Al2O3 to act as a network-forming component, our measurements suggest that Al2O3 behaves as a network modifier. This discrepancy highlights the difference between viscosity values predicted based on values and viscosity values obtained from values, as displayed in Figure 11b,c. Figure 11 demonstrates that the Raman-structure model predicts the FactSage slag viscosity with good accuracy over a wide range of slag compositions and temperatures.
4. Conclusions
In this study, the relationship between slag structure and viscosity is investigated for a set of 17 synthetic slags that correspond to industrial slags in silicomanganese production. The slag viscosity is an important parameter when considering, for instance, metal–slag separation and tapping, thereby influencing metal yield. Raman spectroscopy is employed to analyze the slags, which include various combinations of MnO, SiO2, CaO, Al2O3, and MgO. The contents of SiO2 and CaO vary from 33 to 65 wt% and 14 to 40 wt%, respectively, while the contents of MnO, Al2O3, and MgO are fixed at 10, 17, and 6 wt%, respectively. The slags are classified into four groups based on their basicity from low basicity (0.38) to high basicity (0.80). The effects of basicity and the different oxides on slag structure are analyzed quantitatively using the results of the deconvolution of Raman spectra in the high-wavenumber region, i.e., 800 to 1200 .
The relationship between slag structure and viscosity is investigated by comparing the predicted viscosity values obtained from the Raman-structure model and the NBO/T viscosity model to the FactSage results. The adjustable parameters for the Raman-structure model are re-calculated for the studied slag set using FactSage data. Both calculated NBO/T values from slag compositions and measured NBO/T values from deconvolution results are used to predict viscosity in the NBO/T model. The results show good agreement between the Raman-structure model and FactSage. Among the results obtained using the NBO/T model, the predictions using measured NBO/T values are closest to the FactSage results. The findings of this study can be useful in optimizing slag composition and improving the efficiency of various metallurgical processes, such as silicomanganese production, as well as to gain a better understanding of the behavior of slags in different industrial systems.
Author Contributions
Conceptualization, S.H., M.T. and K.E.E.; methodology, S.H. and K.E.E.; software, S.H.; validation, S.H. and K.E.E.; data curation, S.H.; writing—original draft preparation, S.H.; writing—review and editing, S.H., K.E.E. and M.T.; supervision, K.E.E. and M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work is funded by SFI Metal Production, Centre for Research-based Innovation, 237738.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Financial support from the Research Council of Norway and the partners of SFI Metal Production is gratefully acknowledged. We would like to thank Wojciech Polkowski for his assistance in calculating the liquidus temperature using FactSage.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FeMn | Ferromanganese |
| SiMn | Silicomanganese |
| R | Raman parameter |
| NBO/T | Non-bridging oxygen per tetrahedral cation |
| XRF | X-ray fluorescence |
| XRD | X-ray diffraction |
| LW | Low-wavenumber |
| MW | Medium-wavenumber |
| HW | High-wavenumber |
| B | Basicity |
| OB | Optical basicity |
| I | Intensity |
| A | Relative abundance |
| C | Band left |
| X | Mole fraction |
References
- Olsen, S.E.; Tangstad, M.; Lindstad, T. Production of Manganese Ferroalloys; Tapir Akademisk Forlag: Trondheim, Norway, 2007. [Google Scholar]
- Tangstad, M.; Bublik, S.; Haghdani, S.; Einarsrud, K.E.; Tang, K. Slag properties in the primary production process of Mn-ferroalloys. Metall. Mater. Trans. B 2021, 52, 3688–3707. [Google Scholar] [CrossRef]
- Mills, K.; Sridhar, S. Viscosities of Ironmaking and Steelmaking Slags. Ironmak. Steelmak. 1999, 26, 262–268. [Google Scholar] [CrossRef]
- Kim, W.H.; Sohn, I.; Min, D.J. A Study on the Viscous Behaviour with K2O Additions in the CaO-SiO2-Al2O3-MgO-K2O Quinary Slag System. Steel Res. Int. 2010, 81, 735–741. [Google Scholar] [CrossRef]
- Eidem, P.A.; Solheim, I.; Ringdalen, E.; Tang, K.; Ravary, B. Laboratory study of slag metal separation for HCFeMn. In Proceedings of the Fourteenth International Ferroalloys Congress (INFACON XIV), Kiev, Ukraine, 31 May–4 June 2015; pp. 190–201. [Google Scholar]
- Folstad, M.B. Slag and Its Effect on Si and FeSi Production. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2023. [Google Scholar]
- Mysen, B.O.; Virgo, D.; Scarfe, C.M. Relations Between the Anionic Structure and Viscosity of Silicate Melts—A Raman Spectroscopic Study. Am. Mineral. 1980, 65, 690–710. [Google Scholar]
- Mysen, B.O. Relationships Between Properties and Structure of Aluminosilicate Melts. Am. Mineral. 1985, 70, 88–105. [Google Scholar]
- Mills, K. Slag Atlas, 2nd ed.; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1995. [Google Scholar]
- Waseda, Y.; Toguri, J. The Structure and Properties of Oxide Melts; World Scientific Publishing: Singapore, 1998. [Google Scholar]
- Sohn, I.; Min, D.J. A review of the relationship between viscosity and the structure of calcium-silicate-based slags in ironmaking. Steel Res. Int. 2012, 83, 611–630. [Google Scholar] [CrossRef]
- Min, D.J.; Tsukihashi, F. Recent Advances in Understanding Physical Properties of Metallurgical Slags. Met. Mater. Int. 2017, 23, 1–19. [Google Scholar] [CrossRef]
- Kim, T.; Heo, J.; Kang, J.; Han, J.; Park, J. Understanding Viscosity-Structure Relationship of Slags and Its Influence on Metallurgical Processes. In Extraction 2018; Davis, B., Ed.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018; pp. 1121–1127. [Google Scholar] [CrossRef]
- Ma, S.; Li, K.; Zhang, J.; Jiang, C.; Bi, Z.; Sun, M.; Wang, Z. Effect of MnO content on slag structure and properties under different basicity conditions: A molecular dynamics study. J. Mol. Liq. 2021, 336, 116304. [Google Scholar] [CrossRef]
- Rossano, S.; Mysen, B. Raman Spectroscopy of silicate glasses and melts in geological systems. In Raman Spectroscopy Applied to Earth Sciences and Cultural Heritage; Dubessy, J., Caumon, M.C., Rull, F., Eds.; Mineralogical Society of Great Britain and Ireland: Twickenham, UK, 2012; Volume 12, pp. 319–364. [Google Scholar] [CrossRef]
- Neuville, D.R.; de Ligny, D.; Henderson, G.S. Advances in Raman spectroscopy applied to Earth and material sciences. Rev. Mineral. Geochem. 2014, 78, 509–541. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, P. A Review of the Structures of Oxide Glasses by Raman Spectroscopy. RSC Adv. 2015, 5, 67583–67609. [Google Scholar] [CrossRef]
- Moulton, B.J.A.; Henderson, G.S.; Fukui, H.; Hiraoka, N.; de Ligny, D.; Sonneville, C.; Kanzaki, M. In situ structural changes of amorphous diopside (CaMgSi2O6) up to 20 GPa: A Raman and O K-edge X-ray Raman spectroscopic study. Geochim. Cosmochim. Acta 2016, 178, 41–61. [Google Scholar] [CrossRef]
- Malfait, W.J. Chapter 8—Vibrational properties of glasses and melts. In Magmas under Pressure; Kono, Y., Sanloup, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 211–236. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Sun, Y.; Liu, L.; Wang, X. Insight into the relationship between viscosity and structure of CaO-SiO2-MgO-Al2O3 molten slags. Metall. Mater. Trans. B 2019, 50, 2930–2941. [Google Scholar] [CrossRef]
- Giordano, D.; Russell, J.K.; González-García, D.; Bersani, D.; Dingwell, D.B.; Del Negro, C. Raman spectroscopy from laboratory and proximal to remote sensing: A tool for the volcanological sciences. Remote Sens. 2020, 12, 805. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, G.H.; Chou, K.C.; Fan, D. Mixed alkali effect in viscosity of CaO-SiO2-Al2O3-R2O melts. Metall. Mater. Trans. B 2020, 51, 985–1002. [Google Scholar] [CrossRef]
- Haghdani, S.; Tangstad, M.; Einarsrud, K.E. A Raman-structure model for the viscosity of SiO2-CaO-Al2O3 system. Metall. Mater. Trans. B 2022, 53, 1733–1746. [Google Scholar] [CrossRef]
- Han, C. Viscosity Studies of High-Temperature Metallurgical Slags Relevant to Ironmaking Process. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2017. [Google Scholar] [CrossRef]
- Mysen, B.O.; Richet, P. Silicate Glasses and Melts: Properties and Structure; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar] [CrossRef]
- Tanabe, I.; Oku, K.; Honda, T. Effects of Mangnesia on the Viscosity of High Carbon Ferro-Manganese slag. J. Electrochem. Soc. Jpn. 1960, 28, 262–266. [Google Scholar] [CrossRef]
- Woollacott, L.C.; Howat, D.D.; Jochens, P.R. The Viscosities and Electrical Conductivities of Slags Associated with the Production of High-Carbon Ferromanganese Alloys; South African Institute of Mining and Metallurgy: Johannesburg, South Africa, 1974; pp. 227–232. [Google Scholar]
- Persson, M. Investigations of Slag Properties and Reactions. Ph.D. Thesis, Royal Institute of Technology, Stockholm, Sweden, 2007. [Google Scholar]
- Yan, B.; Liu, Y.; Shu, Q.; Deng, T.; Glaser, B. Measurements and Model Estimations of Viscosities of the MnO-CaO-SiO2-MgO-Al2O3 Melts. Metall. Mater. Trans. B 2019, 50, 376–384. [Google Scholar] [CrossRef]
- Segers, L.; Fontana, A.; Winand, R. Viscosite de melanges de silicates fondus du systeme CaO-SiO2-MnO. Electrochim. Acta 1979, 24, 213–218. [Google Scholar] [CrossRef]
- Urbain, G.; Bottinga, Y.; Richet, P. Viscosity of Liquid Silica, Silicates and Alumino-Silicates. Geochim. Cosmochim. Acta 1982, 46, 1061–1072. [Google Scholar] [CrossRef]
- Sridhar, S.; Sichen, D.; Seetharaman, S.; Mills, K.C. Viscosity Estimation Models for Ternary Slags. Steel Res. 2001, 72, 3–10. [Google Scholar] [CrossRef]
- Ji, F.Z. Experimental Studies of the Viscosities in CaO-MnO-SiO2 and CaO-FenO-MnO-SiO2 Slags. Metall. Mater. Trans. B 2001, 32, 181–186. [Google Scholar] [CrossRef]
- Ji, F.Z.; Sichen, D.; Seetharaman, S. Viscosities of Multicomponent Silicate Melts at High Temperatures. Int. J. Thermophys. 1999, 20, 309–323. [Google Scholar] [CrossRef]
- Kato, M.; Minowa, S. Viscosity Measurements of Molten Slag-Properties of Slag at Elevated Temperature (Part I). Trans. Iron Steel Inst. Jpn. 1969, 9, 31–38. [Google Scholar] [CrossRef]
- Park, J.H. Structure-property correlations of CaO-SiO2-MnO slag derived from Raman spectroscopy. ISIJ Int. 2012, 52, 1627–1636. [Google Scholar] [CrossRef]
- Park, J.H. Composition–Structure–Property Relationships of CaO–MO–SiO2 (M=Mg2+, Mn2+) Systems Derived from Micro-Raman Spectroscopy. J. Non-Cryst. Solids 2012, 358, 3096–3102. [Google Scholar] [CrossRef]
- Park, J.H.; Ko, K.Y.; Kim, T.S. Influence of CaF2 on the Viscosity and Structure of Manganese Ferroalloys Smelting Slags. Metall. Mater. Trans. B 2015, 46, 741–748. [Google Scholar] [CrossRef]
- Kim, T.S.; Jeong, S.J.; Park, J.H. Structural understanding of MnO–SiO2–Al2O3–Ce2O3 slag via Raman, 27Al NMR and X-ray photoelectron spectroscopies. Met. Mater. Int. 2020, 26, 1872–1880. [Google Scholar] [CrossRef]
- Kim, J.B.; Sohn, I. Effect of SiO2/Al2O3 and TiO2/SiO2 ratios on the viscosity and structure of the TiO2–MnO–SiO2–Al2O3 welding flux system. ISIJ Int. 2014, 54, 2050–2058. [Google Scholar] [CrossRef]
- Kim, T.S.; Park, J.H. Viscosity-structure Relationship of Alkaline Earth Silicate Melts Containing Manganese Oxide and Calcium Fluoride. J. Am. Ceram. Soc. 2019, 102, 4943–4955. [Google Scholar] [CrossRef]
- Mercier, M.; Muro, A.D.; Giordano, D.; Me’trich, N.; Lesne, P.; Pichavant, M.; Scaillet, B.; Clocchiatti, R.; Montagnac, G. Influence of glass polymerisation and oxidation on micro-Raman water analysis in alumino-silicate glasses. Geochim. Cosmochim. Acta 2009, 73, 197–217. [Google Scholar] [CrossRef]
- Giordano, D.; Russell, J.K. Towards a structural model for the viscosity of geological melts. Earth Planet. Sci. Lett. 2018, 501, 202–212. [Google Scholar] [CrossRef]
- Giordano, D.; González-García1, D.; Russell, J.K.; Raneri, S.; Bersani, D.; Fornasini, L.; Genova, D.D.; Ferrando, S.; Kaliwoda, M.; Lottici, P.P.; et al. A calibrated database of Raman spectra for natural silicate glasses: Implications for modelling melt physical properties. J. Raman Spectrosc. 2020, 51, 1822–1838. [Google Scholar] [CrossRef]
- Mills, K.C. The influence of structure on the physico-chemical properties of slags. ISIJ Int. 1993, 33, 148–155. [Google Scholar] [CrossRef]
- Bale, C.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; Pelton, A.; et al. FactSage Thermochemical Software and Databases - Recent Developments. Calphad 2009, 33, 295–311. [Google Scholar] [CrossRef]
- Bale, C.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.; Kang, Y.; Melançon, J.; et al. FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Cryst. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Neuville, D.R.; Mysen, B.O. Role of aluminium in the silicate network: In situ, high-temperature study of glasses and melts on the join SiO2-NaAlO2. Geochim. Cosmochim. Acta 1996, 60, 1727–1737. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, T.J.; Min, D.J. Structure–Property Relationship Amphoteric Oxide Systems via Phase Stability and Ionic Structural Analysis. J. Am. Ceram. Soc. 2021, 104, 140–156. [Google Scholar] [CrossRef]
- McMillan, P. Structural studies of silicate glasses and melts-applications and limitations of Raman spectroscopy. Am. Mineral. 1984, 69, 622–644. [Google Scholar]
- Mysen, B.; Richet, P. Structure of Metal Oxide-Silica Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–262. [Google Scholar] [CrossRef]
- Origin(Pro), Version 2018; OriginLab Corporation: Northampton, MA, USA, 2018.
- Sun, Y.; Wang, H.; Zhang, Z. Understanding the relationship between structure and thermophysical properties of CaO-SiO2-MgO-Al2O3 molten slags. Metall. Mater. Trans. B 2018, 49, 677–687. [Google Scholar] [CrossRef]
- Frantza, J.D.; Mysen, B.O. Raman Spectra and Structure of BaO-SiO2 SrO-SiO2 and CaO-SiO2 Melts to 1600 °C. Chem. Geol. 1995, 121, 155–176. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Jiang, G.C.; You, J.L.; Hou, H.Y.; Chen, H. Raman scattering coefficients of symmetrical stretching modes of microstructural units in sodium silicate melts. Acta Phys. Sin. 2005, 54, 961–966. [Google Scholar] [CrossRef]
- Mysen, B.O.; Toplis, M.J. Structural Behavior of Al3+ in Peralkaline, Metaluminous, and Peraluminous Silicate Melts and Glasses at Ambient Pressure. Am. Mineral. 2007, 92, 933–946. [Google Scholar] [CrossRef]
- Mysen, B.O.; Virgo, D.; Kushiro, I. The Structural Role of Aluminum in Silicate Melts—A Raman Spectroscopic Study at 1 Atmosphere. Am. Mineral. 1981, 66, 678–701. [Google Scholar]
- Giordano, D.; Dingwell, D.B. Non-Arrhenian multicomponent melt Viscosity: A model. Earth Planet. Sci. Lett. 2003, 208, 337–349. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).