Abstract
The chemical industry is one of the main fossil fuel consumers, so its reliance on sustainable and renewable resources such as wind and solar energy should be increased to protect the environment. Accordingly, solar-driven thermocatalytic synthesis of octahydroquinazolinone using polyvinylchloride (PVC)-supported aluminum oxide (Al2O3) as a catalyst under natural sunlight is proposed in this work. The Al2O3/PVC catalysts were characterized by FT-IR, SEM, BET, XRD, and XPS techniques. The obtained results indicate that the yield and reaction time can be modified by adjusting the molar ratio of the catalyst. To investigate the stability of the catalyst, the spent catalyst was reused in several reactions. The results indicated that, when a 50% Al2O3 catalyst is employed in an absolute solar heat, it performs exceptionally well in terms of yield (98%) and reaction time (35 min). Furthermore, the reaction times and yield of octahydroquinazolinone derivatives with an aryl moiety were superior to those of heteroaryl. All the synthesized compounds were well characterized by FT-IR, 1H-NMR, and 13C-NMR. The current work introduces a new strategy to use solar heat for energy-efficient chemical reactions using a cost-effective, recyclable environmentally friendly PVC/Al2O3 catalyst that produces a high yield.
1. Introduction
As the chemical industry requires considerable amounts of energy, its reliance on fossil fuels needs to be reduced to protect the environment. As solar energy is readily available and does not generate any harmful byproducts, it is a viable alternative [1]. In extant studies, chemical reactions induced by solar radiation have already been explored [2,3], indicating that different reagents can be successfully activated by specific wavelengths in the solar spectrum. However, the mechanisms currently employed for this purpose are complex, expensive, difficult to monitor, and insufficiently selective [4]. Moreover, in most cases, useful molecules can only be synthesized if the incident radiation can be tuned to be efficiently absorbed by the materials being used. Since many substances do not absorb radiation in the visible part of the solar spectrum, or require specific wavelengths from the UV region, the use of natural light for chemical processes is often impractical. Nonetheless, as sunlight generates considerable heat, it can promote dependable and sustainable chemical synthesis [5] without generating any harmful byproducts [6]. These benefits are already recognized, as organic transformations induced by insolation have already been used in the synthesis of new chemical bonds [7]. Heterogeneous catalysts are of particular interest in this context, as these chemical compounds are physically distinct from the reactants and/or products involved in the catalyzed chemical reaction [8].
In extant research, solid-phase heterogeneous catalysts, such as aluminum oxide, are widely employed to expedite the chemical reaction between two reactants [9,10] due to their beneficial chemical properties and mechanical stability [11]. Given that the acidic properties of aluminum oxide as a single (Al2O3), binary (SiO2-Al2O3, Al2O3-TiO2), and tertiary (TiO2-SiO2-Al2O3) oxide in different organic reactions are well understood [12], its different forms are economically attractive catalysts for a wide variety of organic reactions [13], as they are capable of inducing a wide range of acid-catalyzed reactions [12]. However, metal leaching that typically accompanies this process prevents the reuse of these oxides, making them too expensive for large-scale applications. These issues can be overcome by employing inorganic (calcium carbonate, calcium kaolinites) and organic (such as polystyrene or divinylbenzene) polymers for promoting organic reactions [14,15,16,17,18]. The most optimal solid organic polymers for this purpose are chosen based on their stability and recyclability, as well as whether they bind with the substrate/reagent covalently or non-covalently [16]. Accordingly, polyvinylchloride (PVC) is frequently used for solid-phase synthesis as it is inexpensive and versatile [19], even though its thermal stability and processibility are inferior to that of polyethylene, polypropylene, and polyamide [20]. These shortcomings could be overcome by combining PVC with organic and inorganic fillers such as calcium carbonate and calcium kaolinites [20,21], as well as by chelating it with metal ions such as Co+2, Ti+3, Cr+3, Fe+3, Cu+2, and Zn+2 [22,23]. Thus, Al2O3 could be supported by a PVC matrix to improve its binding with a polymeric partner and enhance the thermocatalytic synthesis selectivity and yield.
Numerous medicinally effective substances such as antimicrobial [24], anti-inflammatory [25], anticancer [26] and antiviral [27] drugs contain octahydroquinazoline and its derivatives, as these are cost-effective substances characterized by high selectivity. In recent years, several multicomponent techniques for the synthesis of octahydroquinazolinone derivatives have been proposed [28], including Biginelli reactions [29] which rely on the condensation of carbonyl compounds and urea in the presence of multiple Lewis acid catalysts to synthesize quinazolines [30,31,32,33,34]. The greatest advantage of this process is that octahydroquinazolinone can be synthesized through simultaneous condensation of dimedone, aldehyde, and urea/thiourea with Lewis acid, protic acid, and solid acid under traditional reflux and solvent-free conditions by exposing the components to microwave and ultrasonic irradiation [35]. However, the high acidic potential of these catalysts makes them difficult to handle, pointing to the need for more environmentally friendly alternatives [36].
Thus, research in this field is increasingly shifting toward solar heat as its use would reduce the number of reaction steps [37] while resulting in more environmentally friendly chemical processes [6]. To date, several photo-assisted mechanistic approaches for the synthesis of quinazoline/octahydroquinazolinone have been proposed, such as photocatalysis, energy transfer, proton-coupled electron-transfer system, and hydrogen atom transfer [38]. We have envisaged with our previous efforts using recyclable catalysts and conventional heating methods for the synthesis of octahydroquinazolinone/quinazoline synthesis [39,40]. To contribute to this ongoing endeavor, in this work, we report on a novel three-component reaction for solar irradiation-induced octahydroquinazolinone synthesis based on aluminum oxide supported by PVC as a thermocatalyst, as shown in Figure 1.
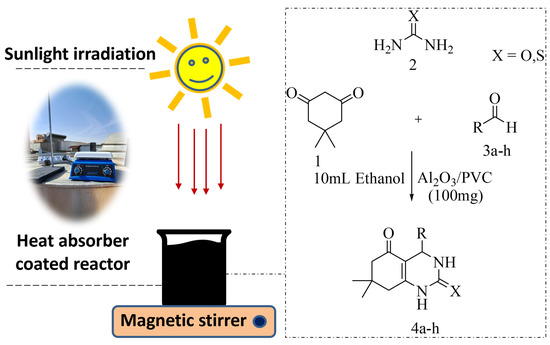
Figure 1.
The setup used in the present study for the thermocatalytic synthesis of octahydroquinazolinone by Al2O3/PVC catalyst.
2. Materials and Methods
2.1. Materials
Aluminum oxide was purchased from Loba Chemie (Mumbai, India), while a fine PVC (Polyvinyl Chloride) powder with a 36 μm particle diameter was supplied from SABIC corporation (Riyadh, Saudi Arabia) as a support material for the catalyst. Dimedone (5,5-Dimethyl-1,3-cyclohexanedione) (CAS No.126-81-8), Urea (CAS No. 57-13-6), Thiourea (CAS No. 62-56-6), and aldehydes such as 4-Chlorobenzaldehyde (CAS No. 104-88-1), 4-Fluorobenzaldehyde (CAS No. 459-57-4), 4-Hydroxy-3-methoxybenzaldehyde (Vanillin) (CAS No. 21-33-5), Furfural (Furan-2-carboxyaldehydes) (CAS No. 98-01-1) were acquired from Sigma Aldrich (St. Louis, MO, USA).
2.2. Catalyst Characterization
Thermo Science’s iD5 ATR diamond Nicolet is 5 FT-IR Spectrometer was used to record the Fourier transform infrared (FT-IR) spectra, while Cu Kα radiation (λ = 1.543 Å) provided by an X-ray diffractometer (Ultima IV, Rigaku, Japan) in the 10°−80° 2θ range was used to capture X-ray diffraction (XRD) spectra to define the phase composition of produced catalysts. The morphological properties of all studied samples were assessed using a field emission scanning electron microscope (FESEM, Model: Quanta FEG 250, Thermo Fisher Scientific, Amsterdam, The Netherland), and their chemical composition was characterized using Thermo scientific K-alpha X-ray photoelectron spectrometer (XPS) Waltham, MA, USA with a characteristic energy of 1486.6 eV generated by a monochromic Al Kα source. Throughout the XPS measurements, a pressure of approximately 10−8 m bar, room temperature (RT), and 400 µm spot size were maintained. XPS survey scans used for elemental identification were obtained at 200 eV pass energy and 1 eV step size, while 50 eV and 0.1 eV were employed for capturing high-resolution XPS images. The catalyst’s BET surface area, pore radius, and pore volume were estimated by N2-physisorption at 77 K using Quantachrome ASiQwin software, version 5.2. Finally, Bruker-Plus (400 MHz) nuclear magnetic resonance (NMR) apparatus was used to record the 1H-NMR and 13C spectra of synthetic octahydroquinazolinones with tetramethylsilane serving as an internal reference.
2.3. Al2O3/PVC Catalyst Preparation
Catalysts were prepared by the wet impregnation method. For the preparation of a 5% Al2O3 catalyst, 50 mL of distilled water was mixed with Al2O3 and PVC (at 5:95 wt. ratio) in a 100 mL beaker and the contents were rapidly agitated at 100 °C for up to one hour. The resulting catalyst was then stored inside a hot air oven overnight at 90 °C. The same process was adopted to obtain Al2O3/PVC catalysts containing 25%, 50%, 60%, and 75% Al2O3 (henceforth denoted as 25% Al2O3, 50% Al2O3, 60% Al2O3, and 75% Al2O3) all of which subjected to XRD, FT-IR, Brunauer–Emmett–Teller (BET), and scanning electron microscopy (SEM) analyses, along with pure PVC and Al2O3 samples to facilitate comparisons.
2.4. Generalized Method for the Synthesis of Octahydrquinazolinone Derivatives
For the synthesis of octahydroquinazolinone derivatives, dimedone, urea/thiourea, and various aldehydes were mixed with an optimized amount of ethanol and 100 mg of Al2O3/PVC catalyst in a 100 mL beaker placed on a magnetic stirrer. Heat (75–80 °C) generated by sunlight was regularly measured a by thermometer and slight solvent evaporation was compensated for during the reaction. Before its use, the beaker was painted black to facilitate the absorption of the heat generated by sunlight. Once the beaker was painted can be reused many times in several reactions. Mixtures in a ratio of 7:3 of acetone and ethyl acetate were used as the solvent, and the reaction progress was regularly monitored. As the aim was to reuse the solid catalyst in subsequent reactions, the organic layer was separated by centrifugation, after which the solid product was purified by evaporation and recrystallization with ethanol. The final compounds were characterized by determining the melting point, as well as by analyzing the FT-IR, 1H NMR, and 13C NMR spectra.
2.5. Spectroscopic Data of the Newly Synthesized Compound
4-(furan-2-yl)-7,7-dimethyl-2-thioxo-1,2,3,4,5,6,7,8-octahydroquinazoline-2-thione. White solid; FT-IR (cm−1, ATR); 3429 and 2952 (NH), 1583 (C=O, ring), 1456 (C=S, thiourea), 1372 (C=C); 1H NMR (DMSO-d6, 400 MHz): 10.66 (s, 1H, NH), 7.39 (s, 1H, NH), 6.27–6.25 (s, 1H, J = 8.16 Hz, Ar–H), 6.27–6.25 (s, 1H, J = 8.16 Hz, Ar–H), 5.85–5.37(2H, m, Ar–H), 5.19 (1H, s, CH), 2.50 (d, 2H, J = 1.44 Hz, CH2), 2.28(s, 2H, CH2), 1.01 (s, 6H, 2 × CH3); 13C NMR (DMSO-d6, 100 MHz): δ 187.50 (C=O), 141.46 (NC=C), 113.42, 110.43, (ArC), 105.43 (OC−C=C), 46.97(1C, CH2), 31.86 (1C, CH2), 28.03 (1C, CH), 26.89 (2CH3).
3. Results and Discussion
3.1. Characterization
3.1.1. X-ray Diffraction (XRD) Measurements
Figure 2 shows the XRD patterns of raw PVC, Al2O3, and prepared Al2O3/PVC catalysts. The XRD pattern of PVC powder displays relatively broad peaks, indicating its amorphous character [41]. However, crystallinity starts to emerge as the Al2O3 content in the Al2O3/PVC catalyst increases from 25% to 75%. The XRD patterns of the prepared samples, Al2O3/PVC catalysts, demonstrate that a mixture comprising 67.3% aluminum oxide, 16.7% bassanite (2CaSO4·H2O), 2.5% calcium sulfate, 10.3% aluminum oxide hydroxide (boehmite), and 3.2% aluminum hydroxide oxide hydrate (nordstrandite) as the raw material reacts with water molecules from the air or the aqueous medium used in sample preparation.
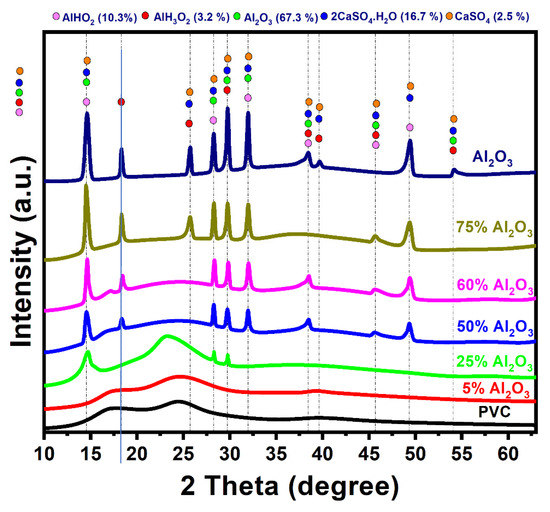
Figure 2.
XRD patterns produced by Al2O3, PVC, and Al2O3/PVC samples containing different aluminum trioxide amounts.
3.1.2. Fourier Transform Infrared (FT-IR) Measurements
The FT-IR spectra of aluminum oxide, polyvinyl chloride, and Al2O3/PVC catalyst with different aluminum oxide ratios are displayed in Figure 3. As can be seen from the graph, the main infrared peaks produced by the aluminum powder are located at ~594 and ~465 cm−1, which corresponds to the Al−O bending vibration of Al–OH groups. In addition, the peak at 652 cm−1 represents Al−O stretching vibration.
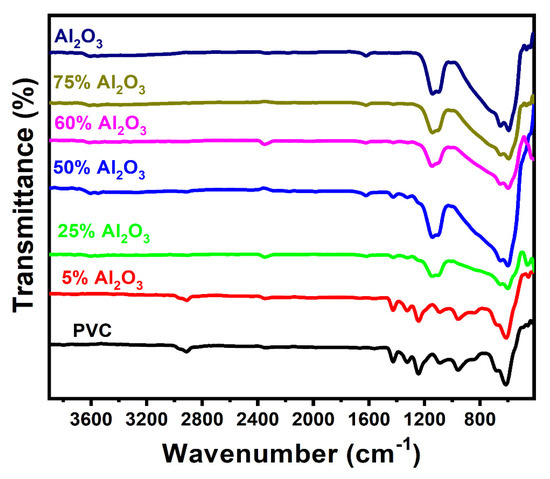
Figure 3.
FT-IR spectra of pure Al2O3, PVC, and Al2O3/PVC samples containing different aluminum trioxide amounts.
Al2O3 FT-IR spectra show bands of absorption at 3606 cm−1 that are corresponding to the stretching vibrations of the O–H groups and water [42]. On the other hand, PVC produces peaks at 2913 cm−1, ~1425 cm−1, 1324 cm−1, 1088 cm−1, 957 cm−1 and 614 cm−1, respectively, reflecting the –CH2- asymmetric stretching vibration, wagging –CH2, CH2 deformation, C−H stretching from CH−Cl, rocking CH2, and C−Cl stretching [43]. The obtained spectra further reveal that, as the Al2O3 content in the sample increases from 5% to 75%, several PVC peaks diminish and can no longer be discerned might be due to Al2O3 concentration overlapping the PVC peaks.
3.1.3. Structural Properties
Table 1 displays the surface area analysis of the pure PVC, Al2O3, and Al2O3/PVC catalysts with different amounts of Al2O3 in the range of 5–75 wt.%. It can be noted that pure PVC has a very low surface area of 3.70 m2/g, whereas pure Al2O3 has a high surface area of 105.40 m2/g. In addition, it can be seen that increasing the amount of Al2O3 in Al2O3/PVC catalyst enhances the surface area of the catalyst and pore volume where they increased from 7.30 up to 76.50 m2/g and from 0.017 up to 0.182 cc/g, respectively. Additionally, the pore radius was observed to increase with increasing the amount of Al2O3 to 50 wt.% and then decreased with 60 and 75 wt.% of Al2O3. The maximum pore radius was noted using a 50 wt.% Al2O3/PVC catalyst where it was 32.70 Å. However, it seems that there is an integration between Al2O3 and PVC that has led to an increase in the surface area, as Al2O3 has a higher surface area than PVC.

Table 1.
Textural properties of the Al2O3/PVC catalysts. Specific surface area pore volume and pore radius.
Figure 4a,b presents the N2 adsorption-desorption isotherms and pore size distributions of the catalysts. According to the IUPAC standard, all catalysts exhibited a type V isotherm with an H3 hysteresis loop as shown in Figure 4a. This type of hysteresis revealed that the catalysts have mesoporous structures. However, type H3 hysteresis is generally found on solid materials that consist of aggregates or agglomerates of particles forming slit-shaped pores (plates or edged particles such as cubes), with irregular size and/or shape [44]. Moreover, it can be seen that the increase in the amount of Al2O3 added to the PVC led to higher N2 adsorbed volume in the p/p° range of 0.49 to 0.95. This finding is consistent with the results shown in Table 1, where the surface area and pore volume increased with increasing the amount of Al2O3. Figure 4b illustrates the pore size distributions. It was reported that solid materials which have pore sizes in the range from 2 to 50 nm classify as mesoporous [44]. From Figure 4b, it can be noted that increasing the Al2O3 content produced pore sizes well within the mesoporous range, where the pore size distribution was with a peak at 40 nm. The pure PVC sample showed a very low number of pores, and they increased as a result of the addition of Al2O3. Therefore, it seems that the Al2O3 content in the samples plays a significant role in changing the textural properties of the Al2O3 PVC catalysts.
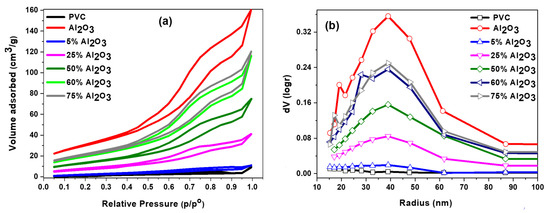
Figure 4.
Nitrogen adsorption/desorption isotherms (a) and BJH pore size distribution (b) of Al2O3/PVC catalyst with different amounts of Al2O3.
3.1.4. Scanning Electron Microscopy (SEM) Analysis
The morphology of Al2O3, PVC, and Al2O3/PVC samples with different molar ratios of Al2O3 was analyzed using SEM at different magnifications and the obtained images are shown in Figure 5. As can be seen from Figure 5a,g, PVC comprises sporadic mushroom cap spores, while the Al2O3 structure is characterized by the agglomeration of fine particles of various shapes (rectangles, oval bars, and cubes) and sizes. It is also apparent that the Al2O3 particle size is smaller than that of pure PVC. Consequently, the Al2O3/PVC catalysts with 5 to 75% Al2O3 content exhibit a gradual shift in morphology, becoming progressively more similar to Al2O3, as shown in Figure 5c−f. As surface area is inversely proportional to the particle size [45], increasing the amount of Al2O3 in the Al2O3/PVC catalyst increases the surface available for chemical reaction. This is well-aligned with the BET surface area results, in Table 1.
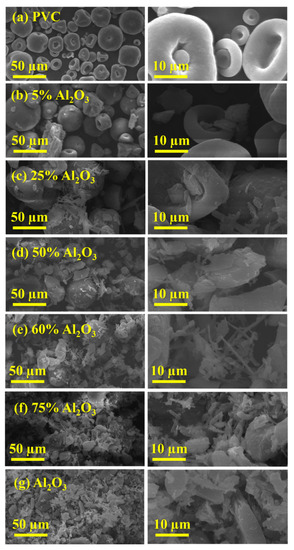
Figure 5.
SEM micrograph at different magnifications of (a) pure Al2O3, (g) PVC, and (b–f) Al2O3/PVC samples containing different aluminum trioxide amounts.
3.1.5. X-ray Photoelectron Spectrometry (XPS) Analysis
XPS survey spectra were captured to study the surface chemistry and the chemical composition of PVC, Al2O3, and Al2O3/PVC catalysts with different Al2O3 amounts, as indicated in Figure 6a. It is evident that both PVC and Al2O3/PVC contain C, Cl, and O. Additionally, the Al2O3/PVC catalysts contain Al, Ca, and S. However, the Al2O3 sample comprises C, O, Al, Ca, and S. The presence of oxygen in the XPS survey spectra of PVC might be ascribed to contamination or polymer chain oxidation [46]. Similarly, the presence of both Ca and S in the Al2O3/PVC spectra is expected, because the Al2O3 sample contains CaSO4·H2O. The high-resolution C 1s spectra produced by PVC, Al2O3/PVC catalysts, and Al2O3 are shown in Figure 6b, respectively. The PVC sample comprises three kinds of carbon, which produce peaks at 287.13 eV, 288.52 eV, and 289.54 eV binding energies that correspond to the C–C and C–H and the C–Cl bonds and O=C−O bonds, respectively [46,47]. Additionally, catalysts containing smaller amounts of Al2O3 exhibit three peaks at around 285.5, 287.5, and 289.6 eV, reflecting the presence of C−C, C−Cl, and O=C−O bonds. However, as the Al2O3 content in the Al2O3/PVC catalyst increases, C–C and C–Cl peaks decrease in amplitude, while the magnitude of O–C=O peaks increases. The C 1s XPS spectra of the 75% Al2O3 catalyst and Al2O3 samples can be deconvoluted into two peak components at around 286.6 and 289.7 eV binding energies, which are attributed to the C−C and O=C−O species, respectively.
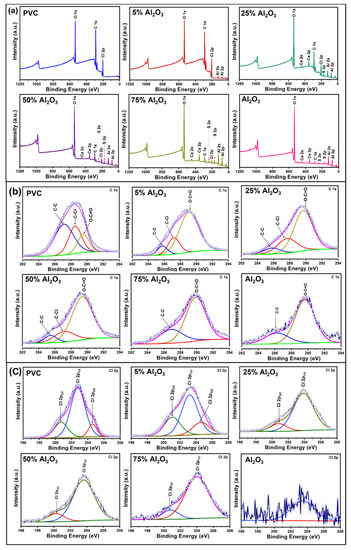
Figure 6.
XPS survey (a), C 1s (b), and Cl 2p (c) spectra of Al2O3, PVC, and Al2O3/PVC samples containing different aluminum trioxide amounts as indicated.
Figure 6c shows high-resolution C1 2p spectra produced by PVC, Al2O3/PVC catalysts, and Al2O3. The PVC spectrum can be decomposed into three peaks, namely Cl 2p1/2 at 204.7 eV and peak Cl 2p3/2 at 202.6 eV representing organic chlorine atoms covalently bounded sp2 carbon [46,47], whereby the latter is assigned to the chloride ion and the hydrogen bonds [48]. On the other hand, Cl 2p spectra produced by most Al2O3/PVC catalysts contain two peaks of high intensity with a maximum of 203.2 eV (Cl 2p1/2) and 200.1 eV (Cl 2p3/2), which are attributed to the C–Cl bonds. The oxygen O 1s spectra of the PVC, Al2O3/PVC catalysts, and Al2O3 sample are also presented in Figure S1a (S = Supplementary) where the two peaks at 533.1 and 535.2 eV, which are attributed to the C–C=O and C–O–H bonds, respectively, characterize the XPS O 1s spectrum produced by PVC [46]. On the other hand, the O 1s peak produced by most of the Al2O3/PVC catalyst samples can be decomposed into three peaks located at around 532 and 534 eV, corresponding to O bound to the Al lattice (Al−O−Al bonds) and OH/COO bonds, respectively [49]. The evidence of OH/COO bonds in the Al2O3 spectra is attributed to the use of H2O as the reaction medium in the present study and is consistent with the findings reported by other authors [50,51]. Similarly, the peak at 536 eV arises due to the adsorption of free water molecules. As noted above, the intensity and number of O 1s peaks increase, while the intensity and number of C 1s and Cl 2p peaks decrease with the increase in Al2O3 content in the Al2O3/PVC catalysts, confirming that adding Al2O3 introduces abundant oxygen atoms into the PVC chain.
Figure S1b shows the Al 2p spectra produced by Al2O3, Al2O3/PVC catalysts, and PVC. The Al2O3/PVC samples contain the two peaks produced by Al 2p of Al2O3 (at around 77 and 79 eV) confirming the presence of Al–O and Al–OH bonds, respectively [52]. Additionally, the peak located at around 75 eV is attributed to the AlO(OH) bond. In the spectrum produced by the 50% Al2O3 catalyst sample, a greater contribution from the OH groups is evident [50,51]. Thus, as demonstrated by the XPS results, which are in good agreement with the XRD and FT-IR data, Al2O3 was successfully mixed and dispersed inside the PVC matrix to create Al2O3/PVC catalysts. Furthermore, Table S1 with the ratios of the electronic state of the elements for the as-prepared Al2O3/PVC catalysts was displayed in the supplementary file.
3.2. Catalytic Activity
To achieve optimized reaction conditions, the catalyst and solvent amounts, as well as the reaction, were modified, and the findings are reported in Table 2, Table 3, Table 4, Table 5 and Table 6.

Table 2.
Effects of the Al2O3/PVC catalyst loaded with different amounts of Al2O3 on the synthesis of model compound (4a) a.

Table 3.
Effect of 50% Al2O3 catalyst loading for the synthesis of the model compound under optimized conditions under solar heat.

Table 4.
Model compound synthesized with different solvents using 100 mg of 50% Al2O3 catalyst under solar heat a.

Table 5.
Effects of different catalyst types on the synthesis duration and yield under solar heat.

Table 6.
The results obtained when using a 50% Al2O3 catalyst for the synthesis of different octahydroquinazolinone derivatives under solar heat.
When ethanol was used without a catalyst, no reaction was observed after 12 h. Moreover, experiments conducted with different Al2O3 amounts revealed that the reaction involving 50% Al2O3 resulted in the highest (98%) yield while requiring only 35 min to complete, which could be attributed to the sufficient number of Brönsted and Lewis acid sites [3], as reflected in a greater pore radius, Table 1. Thus, as this Al2O3 quantity in the PVC matrix may be led to the optimal development of Lewis acid-base interactions between the polar surface group of the Al2O3 and the ionic species of the PVC, it can be adopted to improve the ionic conductivity, thermal conductivity, and mechanical stability of the 50% Al2O3 catalyst. Consequently, in the subsequent experiments, 50% Al2O3 was used as a catalyst, but its quantity was varied in the 20–120 mg range, to assess the influence of these factors on the reaction efficiency. As can be seen from Table 3, 100 mg of catalyst yields the most optimal results.
Next, 100 mg of 50% Al2O3 catalyst was used while varying the solvent type to assess its influence on the reaction efficiency. As can be seen from Table 4, ethanol is most conducive for high yields, as the values obtained for CH2Cl2, DMF, MeOH, EtOH, H2O, and EtOH/H2O are much lower and the reactions took longer time to complete, and also the amount of solvent in mL was also wasted in each type during sunlight irradiation depending upon the boiling point of solvents. Therefore, each solvent was continuously added as required during the course of the reaction.
To ensure that 50% Al2O3 is the most beneficial catalyst, additional experiments were conducted using HCl (Conc.), H2SO4 (Conc.), P-TsOH, Al2O3, and PVC and the results are presented in Table 5.
The reactions based on the 50% Al2O3 catalyst require the shortest time to complete while producing the highest yield, confirming that Al2O3/PVC has superior catalytic potential compared to all other tested compounds, including aluminum oxide and polyvinyl chloride (PVC).
Finally, the optimal conditions established through previous experiments (100 mg of 50% Al2O3 as a catalyst, optimized amount of ethanol) were adopted for the synthesis of 4-(substituted)-7,7-dimethyl-1,2,3,4,5,6,7,8-octahydroquinazoline-2,5-dione to assess the adaptability of the proposed protocol to other processes. Various substituents on aldehyde including Cl, F, OCH3, OH, and Furan moieties were used for this purpose and the obtained results are reported in Table 6.
As evident from the tabulated results, 50% Al2O3 outperformed all considered compounds in terms of both generated yield and reaction time.
3.3. Plausible Octahydroquinazolinone Production Mechanism Using Al2O3/PVC as a Catalyst
As shown in Figure 7, Al2O3/PVC activates the carbonyl group of the aldehyde to produce Intermediate I under the influence of solar heat. This is followed by condensation with urea/thiourea (Intermediate II) and subsequent dehydration, resulting in Intermediate III, which reacts with dimedone to produce Intermediate IV. Finally, cyclization with the removal of water yields the desired octahydroquinazolinones.
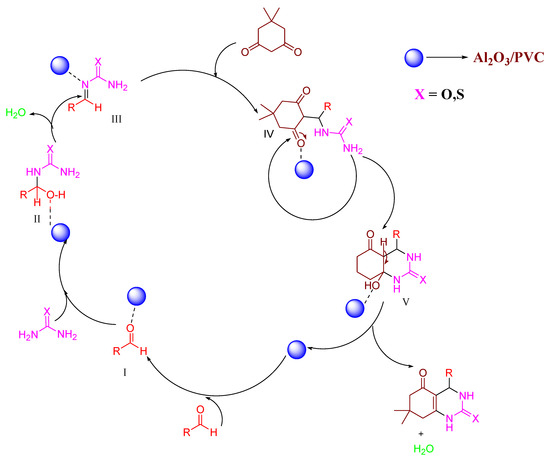
Figure 7.
A proposed mechanism for the synthesis of octahydroquinazolinone derivatives using Al2O3/PVC catalyst.
3.4. Recyclability of the Al2O3/PVC
As sufficient catalyst reusability and recovery are crucial for obtaining sustainable and environmentally friendly synthesis methods that can be adopted in practice, these aspects were also considered in the present study, and the findings are reported in Table 7. The catalyst can be reused up to four times without a significant loss in yield and most of its original mass can be recovered by centrifugation.

Table 7.
Recyclability studies of 50% Al2O3 catalyst a under solar heat.
4. Conclusions
In this work, functionalized 1,2,3,4,5,6,7,8-octahydroquinazolinone derivatives were successfully synthesized through an affordable process that relies on polyvinylchloride-supported aluminum oxide through sunlight exposure as a free source of heat. As the catalyst can be reused, this environmentally friendly method can be adopted in a wide range of processes, given that it produces a higher yield in a shorter time compared to the previously reported conventional thermal technique. The FT-IR and XRD results indicated the formation of the Al2O3/PVC catalysts. Additionally, the obtained results indicate that the yield and reaction time can be modified by adjusting the molar ratio of the catalyst. A total of 50% Al2O3/PVC catalyst performed better than any other alternative under solar heat in terms of yield and reaction time. Additionally, under solar heat, aryl-modified octahydroquinazolinone performed much better than heteroaryl when compared to reaction times and yield. In the context of green chemistry, this study introduces a new strategy towards the use of abundant solar energy for a cost-effective, energy-efficient, and environmentally friendly chemical industry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma16072835/s1, Figure S1. O1s XPS spectra (a) and C 1s (b), and Cl 2p spectra of Al2O3, PVC, and Al2O3/PVC samples containing different aluminum trioxide amounts as indicated. Table S1. the ratios of the electronic state of the elements for the as-prepared Al2O3/PVC catalysts. NMR Spectra of Synthesized Compounds 4a–h.
Author Contributions
Conceptualization, M.A.B., A.I.A. and T.F.Q.; methodology, M.A.B., A.I.A., A.M.A. and T.F.Q.; software, A.I.A. and M.A.A.; validation., M.A.B., A.M.A. and T.F.Q.; formal analysis, I.A., M.A.B. and A.M.A.; investigation, M.A.B.; resources, M.N.A.-S.; data curation, A.I.A. and M.A.A.; writing—original draft preparation, M.A.B., A.I.A. and T.F.Q.; writing—review and editing, M.A.B. and M.N.A.-S.; visualization, M.A.A.; supervision, M.A.B. and T.F.Q.; project administration, M.A.B., A.I.A. and I.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through project number (2022/02/21704).
Acknowledgments
The author extends his appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through project number (2022/02/21704).
Conflicts of Interest
All the authors have no conflict issues related to anything scientific or financial.
References
- Protti, S.; Fagnoni, M. The sunny side of chemistry: Green synthesis by solar light. Photochem. Photobiol. Sci. 2009, 8, 1499–1516. [Google Scholar] [CrossRef]
- Larsen, G. Principles and Practice of Heterogeneous Catalysis By J. M. Thomas (University of Cambridge) and W. J. Thomas (University of Bath). VCH: Weinheim, 1997. xxiii + 669 pp. DM88.00. ISBN 3-527-29239-X. J. Am. Chem. Soc. 1997, 119, 11560. [Google Scholar] [CrossRef]
- Schlögl, R. Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2015, 54, 3465–3520. [Google Scholar] [CrossRef]
- Hoffmann, N. Photochemical Reactions as Key Steps in Organic Synthesis. Chem. Rev. 2008, 108, 1052–1103. [Google Scholar] [CrossRef] [PubMed]
- Crisenza, G.E.M.; Melchiorre, P. Chemistry glows green with photoredox catalysis. Nat. Commun. 2020, 11, 803. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-K.; Yang, D.-Y. Visible-light-mediated reaction: Synthesis of quinazolinones from 1,2-dihydroquinazoline 3-oxides. RSC Adv. 2016, 6, 65988–65994. [Google Scholar] [CrossRef]
- Gentry, E.C.; Knowles, R.R. Synthetic Applications of Proton-Coupled Electron Transfer. Acc. Chem. Res. 2016, 49, 1546–1556. [Google Scholar] [CrossRef]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef]
- Braslavsky, S.E. Glossary of terms used in photochemistry, 3rd edition (IUPAC Recommendations 2006). Pure Appl. Chem. 2007, 79, 293–465. [Google Scholar] [CrossRef]
- Marshall, C.P.; Scholz, G.; Braun, T.; Kemnitz, E. Strong Lewis acidic catalysts for C–F bond activation by fluorination of activated γ-Al2O3. Catal. Sci. Technol. 2020, 10, 391–402. [Google Scholar] [CrossRef]
- Sabu, K.R.P.; Rao, K.V.C.; Nair, C.G.R. A Comparative Study on the Acidic Properties and Catalytic Activities of TiO2, SiO2, Al2O3, SiO2–Al2O3, SiO2–TiO2, Al2O3–TiO2, and TiO2–SiO2–Al2O3. Bull. Chem. Soc. Jpn. 1991, 64, 1920–1925. [Google Scholar] [CrossRef]
- Torres-Olea, B.; Mérida-Morales, S.; García-Sancho, C.; Cecilia, J.A.; Maireles-Torres, P. Catalytic Activity of Mixed Al2O3-ZrO2 Oxides for Glucose Conversion into 5-Hydroxymethylfurfural. Catalysts 2020, 10, 878. [Google Scholar] [CrossRef]
- Sherrington, D.C. Polymer-supported reagents, catalysts, and sorbents: Evolution and exploitation—A personalized view. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2364–2377. [Google Scholar] [CrossRef]
- Basu, B.; Paul, S. Solid-Phase Organic Synthesis and Catalysis: Some Recent Strategies Using Alumina, Silica, and Polyionic Resins. Catalysts 2013, 2013, e614829. [Google Scholar] [CrossRef]
- Sherrington, D.C. Polymer-supported synthesis. In Chemistry of Waste Minimization; Clark, J.H., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 1995; pp. 141–200. [Google Scholar] [CrossRef]
- Gravert, D.J.; Janda, K.D. Organic Synthesis on Soluble Polymer Supports: Liquid-Phase Methodologies. Chem. Rev. 1997, 97, 489–510. [Google Scholar] [CrossRef]
- Hori, M.; Gravert, D.J.; Wentworth, P.; Janda, K.D. Investigating highly crosslinked macroporous resins for solid-phase synthesis. Bioorg. Med. Chem. Lett. 1998, 8, 2363–2368. [Google Scholar] [CrossRef] [PubMed]
- Malenfant, P.R.L.; Fréchet, J.M.J. The first solid-phase synthesis of oligothiophenes. Chem. Commun. 1998, 2657–2658. [Google Scholar] [CrossRef]
- Gong, F.; Feng, M.; Zhao, C.; Zhang, S.; Yang, M. Thermal properties of poly(vinyl chloride)/montmorillonite nanocomposites. Polym. Degrad. Stab. 2004, 84, 289–294. [Google Scholar] [CrossRef]
- Ameer, A.A.; Abdallh, M.S.; Ahmed, A.A.; Yousif, E.A. Synthesis and Characterization of Polyvinyl Chloride Chemically Modified by Amines. Open J. Polym. Chem. 2013, 3, 11–15. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Zhang, Y.; Lang, J.-P. A tricobalt(II) coordination polymer incorporating in situ generated 5-methyltetrazolate ligands. Inorg. Chem. Commun. 2008, 11, 572–575. [Google Scholar] [CrossRef]
- Al-Jibouri, M.N.A.; Al-Ameri, S.A.H.; Al-Jibouri, W.M.; Al-Souz, M.A.K. Spectroscopic study of the effect of a new metal chelate on the stability of PVC. J. Assoc. Arab Univ. Basic Appl. Sci. 2013, 14, 67–74. [Google Scholar] [CrossRef]
- Blazsó, M.; Jakab, E. Effect of metals, metal oxides, and carboxylates on the thermal decomposition processes of poly (vinyl chloride). J. Anal. Appl. Pyrolysis 1999, 49, 125–143. [Google Scholar] [CrossRef]
- Kidwai, M.; Saxena, S.; Khan, M.K.R.; Thukral, S.S. Synthesis of 4-aryl-7,7-dimethyl-1,2,3,4,5,6,7,8-octahydroquinazoline-2-one/thione-5-one derivatives and evaluation as antibacterials. Eur. J. Med. Chem. 2005, 40, 816–819. [Google Scholar] [CrossRef]
- Kawanishi, N.; Sugimoto, T.; Shibata, J.; Nakamura, K.; Masutani, K.; Ikuta, M.; Hirai, H. Structure-based drug design of a highly potent CDK1,2,4,6 inhibitor with novel macrocyclic quinoxalin-2-one structure. Bioorganic. Med. Chem. Lett. 2006, 16, 5122–5126. [Google Scholar] [CrossRef]
- Kidwai, M.; Bhatnagar, D.; Kumar, R.; Luthra, P.M. Synthesis of 2-Oxo/Thioxooctahydroquinazolin-5-one Derivatives and Their Evaluation as Anticancer Agents. Chem. Pharm. Bull. 2010, 58, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Chien, T.-C.; Chen, C.-S.; Yu, F.-H.; Chern, J.-W. Nucleosides XI. Synthesis and Antiviral Evaluation of 5′-Alkylthio-5′-deoxy Quinazolinone Nucleoside Derivatives as S-Adenosyl-<small>L</small>-homocysteine Analogs. Chem. Pharm. Bull. 2004, 52, 1422–1426. [Google Scholar] [CrossRef]
- Arndtsen, B.A. Metal-Catalyzed One-Step Synthesis: Towards Direct Alternatives to Multistep Heterocycle and Amino Acid Derivative Formation. Eur. J. Chem. 2009, 15, 302–313. [Google Scholar] [CrossRef]
- Lu, J.; Bai, Y.; Wang, Z.; Yang, B.; Ma, H. One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using lanthanum chloride as a catalyst. Tetrahedron Lett. 2000, 41, 9075–9078. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, M.; Xiong, L.; Yao, X.; Fan, M.; Chen, D.; Jiang, Q.; Jin, Z.; He, Q. Novel photo-theranostic GdB6 nanoparticles for fluorescence imaging and NIR-photothermal therapy. Chin. Chem. Lett. 2021, 32, 3487–3490. [Google Scholar] [CrossRef]
- Cao, S.-L.; Feng, Y.-P.; Jiang, Y.-Y.; Liu, S.-Y.; Ding, G.-Y.; Li, R.-T. Synthesis and in vitro antitumor activity of 4(3H)-quinazolinone derivatives with dithiocarbamate side chains. Bioorg. Med. Chem. Lett. 2005, 15, 1915–1917. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Catalysis by Heteropoly Acids and Multicomponent Polyoxometalates in Liquid-Phase Reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef]
- Misono, M. Unique acid catalysis of heteropoly compounds(heteropolyoxometalates) in the solid state. Chem. Commun. 2001, 13, 1141–1152. [Google Scholar] [CrossRef]
- Zou, S.; Wang, S.; Xi, C. ROTf-induced annulation of heteroatom reagents and unsaturated substrates leading to cyclic compounds. R. Soc. Open Sci. 2018, 5, 181389. [Google Scholar] [CrossRef]
- Mozafari, R.; Heidarizadeh, F. One Pot Synthesis of Octahydroquinazolinone Derivatives Using (Me (Im)12) H4CuPW11O39 as a Surfactant Type Catalyst. J. Clust. Sci. 2016, 27, 1629–1643. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Lan, J.; Yan, L.; Chen, X.; Li, Q.; Meng, J.; Le, Z. Photocatalyst-free visible-light-promoted quinazolinone synthesis at room temperature utilizing aldehydes generated in situ via CC bond cleavage. Org. Biomol. Chem. 2021, 19, 2436–2441. [Google Scholar] [CrossRef]
- Choi, K.-H.; Park, S.-Y.; Park, B.J.; Jung, J.-S. Recyclable Ag-coated Fe3O4@TiO2 for efficient photocatalytic oxidation of chlorophenol. Surf. Coat. Technol. 2017, 320, 240–245. [Google Scholar] [CrossRef]
- Bakht, M.A.; Alotaibi, M.; Alharthi, A.I.; Ud Din, I.; Ali, A.; Ali, A.; Ahsan, M.J. Pd-HPW/SiO2 Bi-functional Catalyst: Sonochemical Synthesis, Characterization, and Effect on Octahydroquinazolinone Synthesis. Catalysts 2021, 11, 1273. [Google Scholar] [CrossRef]
- Bakht, M.A.; Alotaibi, M.; Alharthi, A.I.; Geesi, M.H.; Alshammari, M.B.; Riadi, Y.; Samad, A.; Khan, M.; Kamal, M. Highly Reduced Graphene Oxide-Phosphomolybdic Acid Catalyzed Synthesis of Quinazoline Derivatives in Deep Eutectic Solvent: An Expeditious Approach. Indian J. Heterocyclic. Chem. 2020, 30, 29–39. [Google Scholar]
- Abdelghany, A.M.; Meikhail, M.S.; Hamdy, R. Enrichment of Poly Vinyl Chloride (PVC) Biological uses Through Sodium Chloride Filler, Density Functional Theory (DFT) Supported Experimental Study. Adv. Phys. 2018, 14, 5682–5692. [Google Scholar] [CrossRef]
- Zykova, A.; Livanova, A.; Minakova, T.; Bugrova, T.; Mamontov, G. Composite Catalysts for Hydrocarbons Dehydrogenation Prepared from Aluminum Nanopowder. AIP Conf. Proc. 2016, 1772, 030018. [Google Scholar] [CrossRef]
- Abdellah Ali, S.F.; Althobaiti, I.O.; El-Rafey, E.; Gad, E.S. Wooden Polymer Composites of Poly(vinyl chloride), Olive Pits Flour, and Precipitated Bio-Calcium Carbonate. ACS Omega 2021, 6, 23924–23933. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catalysis Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Supandi, A.R.; Nunotani, N.; Imanaka, N. Particle size effect of ZrO2 supports on catalytic liquid-phase oxidation of phenol over Pt/CeO2-ZrO2-Bi2O3/ZrO2 catalysts. J. Asian Ceram. Soc. 2020, 8, 745–752. [Google Scholar] [CrossRef]
- Abdel-Fattah, E.; Alharthi, A.I.; Fahmy, T. Spectroscopic, optical and thermal characterization of polyvinyl chloride-based plasma-functionalized MWCNTs composite thin films. Appl. Phys. A 2019, 125, 475. [Google Scholar] [CrossRef]
- Louette, P.; Bodino, F.; Pireaux, J.-J. Poly(vinyl chloride) (PVC) XPS Reference Core Level and Energy Loss Spectra. Surf. Sci. Spectra 2005, 12, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Liang, L.; Hu, E.; Chen, H.; Wang, D.; He, C.; Feng, Q. Dechlorination of polyvinyl chloride by hydrothermal treatment with cupric ion. Process. Saf. Environ. Prot. 2021, 146, 108–117. [Google Scholar] [CrossRef]
- Sun, C.; Zeng, R.; Zhang, J.; Qiu, Z.-J.; Wu, D. Effects of UV-Ozone Treatment on Sensing Behaviours of EGFETs with Al2O3 Sensing Film. Materials 2017, 10, 1432. [Google Scholar] [CrossRef]
- Hirchenhahn, P.; Al-Sayyad, A.; Bardon, J.; Plapper, P.; Houssiau, L. Binding Mechanisms Between Laser-Welded Polyamide-6.6 and Native Aluminum Oxide. ACS Omega 2021, 6, 33482–33497. [Google Scholar] [CrossRef]
- Dinh, C.-T.; Jain, A.; de Arquer, F.P.G.; De Luna, P.; Li, J.; Wang, N.; Zheng, X.; Cai, J.; Gregory, B.Z.; Voznyy, O.; et al. Multi-site electrocatalysts for hydrogen evolution in neutral media by destabilization of water molecules. Nat. Energy 2019, 4, 107–114. [Google Scholar] [CrossRef]
- Liu, F.C.; Dong, P.; Lu, W.; Sun, K. On formation of AlOC bonds at aluminum/polyamide joint interface. Appl. Surf. Sci. 2019, 466, 202–209. [Google Scholar] [CrossRef]
- Arunkumar, R.; Babu, R.S.; Usha Rani, M. Investigation on Al2O3 doped PVC–PBMA blend polymer electrolytes. J. Mater. Sci. Mater. Electron. 2016, 28, 3309–3316. [Google Scholar] [CrossRef]
- Hadigavabar, A.D.; Tabatabaeian, K.; Zanjanchi, M.A.; Mamaghani, M. Molybdenum anchored onto zeolite beta: An efficient catalyst for the one-pot synthesis of octahydroquinazolinone derivatives under solvent-free conditions. React. Kinet. Mech. Catal. 2018, 124, 857–871. [Google Scholar] [CrossRef]
- Kamble, S.B.; Kumbhar, A.S.; Jadhav, S.N.; Salunkhe, R.S. Microwave Assisted Attractive and Rapid Process for Synthesis of Octahydroquinazolinone in Aqueous Hydrotropic Solutions. Procedia Mater. Sci. 2014, 6, 1850–1856. [Google Scholar] [CrossRef]
- Kuraitheerthakumaran, A.; Pazhamalai, S.; Manikandan, H.; Gopalakrishnan, M. Rapid and efficient one-pot synthesis of octahydroquinazolinone derivatives using lanthanum oxide under solvent-free condition. J. Saudi. Chem. Soc. 2014, 18, 920–924. [Google Scholar] [CrossRef]
- Karki, B.S.; Verma, S.; Aggarwal, A.; Kasana, V. One Pot three component organocatalyzed synthesis of octahydroquinazolinones. Int. J. Chem. Stud. 2017, 5, 280–290. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).