Abstract
A new heterocyclic azo dye ligand (L) was synthesized by the combination of 4-amino antipyrine with 4-aminophenol. The new Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), and Cd(II) complexes were synthesized in excellent yields. The metal chelate structures were elucidated using elemental analyses, FT-IR, 1H-NMR, mass, magnetic moment, diffused reflectance spectral and thermal analysis (TG-DTG), and molar conductivity measurement. According to the FT-IR study, the azo dye ligand exhibited neutral tri-dentate behavior, binding to the metal ions with the azo N, carbonyl O, and protonated phenolic OH. The 1H-NMR spectral study of the Zn(II) complex supported the coordination of the zo dye ligand without proton displacement of the phenolic OH. Diffused reflectance and magnetic moment studies revealed the octahedral geometry of the complexes, as well as their good electrolytic nature, excepting the Zn(II) and Cd(II) complexes, which were nonelectrolytes, as deduced from the molar conductivity study. The theoretical calculations of optimized HOMO–LUMO energies, geometrical parameters, electronic spectra, natural atomic charges, 3D-plots of MEP, and vibrational wavenumbers were computed and elucidated using LANL2DZ and 6-311G (d, p) basis sets of density functional theory (DFT) with the approach of B3LYP DFT and TD-DFT methods. The ligand and complexes have been assayed for their antimicrobial activity and compared with the standard drugs. Most of the complexes have manifested excellent antimicrobial activity against various microbial strains. A molecular docking investigation was also performed, to acquire more information about the binding mode and energy of the ligand and its metal complexes to the Escherichia coli receptor using molecular docking. Altogether, the newly created ligand and complexes showed positive antibacterial effects and are worth future study.
1. Introduction
The most popular synthetic dyes used in industry are azo dyes. The latter are aromatic substances that include the diazinyl (N=N) groups [1]. Azo dyes are frequently involved in many industries, including food and textile, paper printing, and cosmetics [2,3]. On the other hand, coordination chemistry plays an important role in enhancing the biomedical application of ligands [4,5,6,7,8,9,10]. The azo group possesses excellent donor properties and is important in coordination chemistry. Therefore, chemists have recently become interested in developing and physicochemical characterization of first-row transition metal chelates with a wide range of azo dye ligands [11,12]. Furthermore, azo dye-based metal chelates have contributed significantly to the modern coordination chemistry revolution, due to their interesting properties and diverse applications [13].
Furthermore, their facile synthetic protocol and structural diversity allows access to a wide range of metal chelates [14,15]. Moreover, simple structural modifications (e.g., incorporation of bioactive heterocycles) and chelation with metal ions are usually associated with characteristic alterations and enhancement of their biological properties, including the antioxidant and antiproliferative activities [16], as well as their DNA binding affinities [17,18]. For instance, coordination is usually associated with bathochromic shift, making the color duller. Besides this, oxidation resistance and light fastness properties are increased, whereas aqueous solubility is lowered.
The current study describes the synthesis of new azo dye ligands derived from 4-amino-antipyrine with 4-aminophenol. The resulting novel azo dye ligand coordination was investigated with metal ions such as CrIII, MnII, FeIII, CoII, NiII, CuII, ZnII, and CdII ions. Their corresponding composition was elucidated using various spectral methods, such as IR and 1H NMR. The latter aided the verification of the coordination manner. Furthermore, DFT calculations supported the structural elucidation. Likewise, the antimicrobial properties of the azo dye ligand and its metal chelates were also assessed. Moreover, the antimicrobial activity was confirmed using molecular docking.
2. Materials and Methods
2.1. Starting Materials, Reagents, and Methods
All chemicals and methods employed in the preparation and characterization of the compounds under inspection are supplied in the supporting information.
2.2. Azo Dye Ligand (L) Preparation
4-Amino-antipyrine (3 g, 14.85 mmol) was dissolved in EtOH (40 mL) and put in a 100 mL flask. HCl solution (4 mL) was added at 0–5 °C while stirring. Sodium nitrite solution (5 g, 72.4 mmol, 10 mL H2O) was added drop-by-drop over 10 min, and the resulting solution was then agitated for further 20 min at 0–5 °C. Stirring was continued for an additional hour, and the mixture was poured into the 2,4-diaminophenol coupling component (1.61 g, 14.85 mmol) in EtOH (25 mL) and an aqueous catalyst solution of 4 g CH3COONa. The produced compound was sterilized through filtration, washed with H2O, and vacuum-dried at 298 K.
Yield 84%; m.p. = 124 °C; brownish-red solid. Analysis Calcd for C17H17N5O2(%):C, 63.16; H, 5.26; N, 21.67. Found (%): C, 63.10; H, 5.02; N, 21.48. IR (cm−1):ν(OH) 3433; carbonyl group ν(C=O) 1639; azo ν(N=N) 1620.1H/ NMR (400 MHz/d6 -DMSO/δ, ppm): 7.37–7.72 (m, 8H, Ar H), 3.43 (s, 3H, CH3-N), 2.54 (s, 3H, CH3), 6.90 (m, 2H, NH2), 10.00(s, 1H, OH).
2.3. Metal Chelates Preparation
In EtOH, azo dye ligand (L) was dissolved (0.4 g, 1.2383 mmol). The identical molar ratio (1:1) of the following ethanolic metal salt solutions was added to the solution of azo ligand: 0.3300 g Cr(III), 0.2004 g Mn(II), 0.3347 g Fe(III), 0.293 g Co(II), 0.294 g Ni(II), 0.211 g Cu(II), 0.169 g Zn(II), and 0.227 g Cd(II). For 2–3 hours, the mixture was reflux heated while stirring. The precipitates were removed by filtering and then dried over dehydrated CaCl2 in a vacuum desiccator after being washed with EtOH and diethyl ether.
[Cr(L)Cl2(H2O)]Cl Yield = 73%; m.p. = 235 °C; solid brown. Analysis Calcd for Cr(C17H19Cl3N5O3) (%): C, 40.84; H, 3.80; N, 14.01; Cr, 10.41; Found (%): C, 40.74; H, 3.70; N, 13.81; Cr, 10.23. μeff /BM 3.81; Λm /Ω−1 mol−1 cm2 56.4. IR /cm−1: carbonyl group ν(C=O) 1628; ν(OH) 3402; azo ν(N=N) 1610; ν(M-O) 551; ν(M-N) 435. λmax (nm): 241 (π–π*), 349 (n–π*). Ref. S.: 17,361, 19,436 and 22,624 cm−1 assignable to 4A2g(F) to 4T1g(F), 4A2g(F) to 4T2g(F) and 4A2g(F) to 4T1g(P) transitions, respectively.
[Mn(L)Cl2(H2O)]2H2O. Yield = 85%; solid yellowish brown; m.p. = 205 °C. Analysis Calcd for Mn (C17H23Cl2N5O5) (%): C, 40.56; H, 4.57; N, 13.92; Mn, 10.93; Found (%): C, 40.13; H, 4.50; N, 13.72; Mn, 10.68. μeff /BM 5.51; Λm/Ω−1 cm2 mol−1 46.4. IR: carbonyl group ν(C=O) 1623; ν(OH) 3420; azo ν(N=N) 1615, ν(M-O) 597; ν(M-N) 436. λmax (nm): 219 (π–π*), 368 (n–π*). Ref. S.: 19,950, 21,260 and 25,478 cm−1 assignable to 6A1g → T2g(F), 6A2g(G) → 5T2g(F) and charge transfer transitions, respectively.
[Fe(L)Cl2(H2O)]Cl.H2O Yield = 88%; solid brown; m.p. = 215 °C. Analysis calculated for Fe(C17H21Cl3N5O4) (%): C, 39.12; H, 4.03; N, 13.42; Fe, 10.74; Found (%): C, 39.02; H, 4.01; N, 13.38; Fe, 11.05. μeff /BM 5.05; Λm/Ω−1 cm2 mol−1 93.6. IR: carbonyl group ν(C=O) 1627; ν(OH) 3407; azo ν(N=N) 1614; ν(M-O) 572; ν(M-N) 433. λmax (nm): 219 (π–π*), 365 (n–π*). Ref. S.: 13,280, 18,580 and 27,625 cm−1 assignable to 3A2g(F) → 3T2g(F), 3A2g(F) → 3T1g(F) and 3A2g(F) → 3T1g(P) transitions, respectively.
[Co(L)Cl(H2O)]Cl.H2O. Yield = 83%; solid faint pink; m.p. = 250 °C. Analysis calculated for Co(C17H21Cl2N5O4) (%): C, 41.72; H, 4.29; N, 14.32; Co, 12.07; Found (%): C, 41.64; H, 4.20; N, 14.25; Co, 12.06. μeff: 5.18; Λm/Ω−1 cm2 mol−1 36.4. IR: carbonyl group ν(C=O) 1634; ν(OH) 3421; azo ν(N=N) 1618; ν(M-O) 513; ν(M-N) 434. λmax (nm): 221 (π–π*), 369 (n–π*). Ref. S.: 10,690, 15,187 and 25,025 cm−1 assignable to 4T1g to 4T2g(F), 4T1g to 4A2g(F) and 4T1g to 4T1g(p) transitions, respectively.
[Ni(L)Cl(H2O)2]Cl. Yield= 83%; solid faint green; m.p. 240 °C. Analysis calculated for Ni(C17H21Cl2N5O4) (%): C, 41.72; H, 4.29; N, 14.32; Ni, 12.07; Found (%): C, 41.66; H, 4.15; N, 14.08; Ni, 12.63. μeff: 3.76; Λm/Ω−1 cm2 mol−1 83.3. IR: carbonyl group ν(C=O) 1626; ν(OH) 3416; azo ν(N=N) 1610; ν(M-O) 529; ν(M-N) 433. λmax (nm): 261 π–π*, 369 n–π*. Ref. S.: 13,455, 16,253 and 22,056 cm−1 assignable to 3A2g to 3T2g,3A2g to 3T1g(F) and 3A2g to 3T1g(P) transitions, respectively.
[Cu(L)Cl(H2O)2]Cl. Yield= 89%; solid yellowish brown; m.p. 240 °C. Analysis calculated for Cu(C17H21Cl2N5O4) (%): C, 39.88; H, 4.50; N, 13.69; Cu, 12.41; Found (%): C, 39.66; H, 4.42; N, 13.30; Cu, 12.32. μeff: 1.79; Λm/Ω−1 cm2 mol−1 88.3. IR: carbonyl group ν(C=O) 1640; ν(OH) 3434; azo ν(N=N) 1614; ν(M-O) 527; ν(M-N) 435. λmax (nm): 269 (π–π*), 368 (n–π*). Ref. S.: 18,950, 21,260, and 24,478 cm−1 assignable to 6A1g→ T2g, 6A1g→ 5T1g(F), and charge transfer transitions, respectively.
[Zn(L)Cl2(H2O)]2H2O. Yield = 89%; solid brownish white; m.p. = 185 °C. Analysis calculated for Zn(C17H23Cl2N5O5) (%): C, 39.77; H, 4.48; N, 13.65; Zn, 12.67; Found (%): C, 39.35; H, 4.33; N, 13.58; Zn, 12.86. μeff. diamagnetic; Λm/Ω−1 cm2 mol−1 39.4. IR: carbonyl group ν(C=O) 1645; ν(OH) 3431; azo ν(N=N) 1622; ν(M-O) 576; ν(M-N) 435 λmax (nm): 221 (π–π*), 369 (n–π*). 1H NMR: 7.29–7.49 (m, 8H, Ar H), 3.41 (s, 3H, CH3-N), 2.52 (s, 3H, CH3), 6.91 (m, 2H, NH2):10.20(s, 1H, OH). λmax (nm): 221 (π–π*), 368 n–π*.
[Cd(L)Cl2(H2O)]. Yield= 83%; solid brownish white; m.p. = 145 °C. Analysis calculated for Cd(C17H19Cl2N5O3) (%): C, 38.93; H, 3.63; N,13.36; Cd, 21.37; Found (%): C, 39.72; H, 3.60; N, 13.25; Cd, 21.43. μeff. diamagnetic; Λm /Ω−1 cm2 mol−1 31.2. IR: carbonyl group ν(C=O) 1629; ν(OH) 3439; azo ν(N=N) 1610; ν(M-O) 577; ν(M-N) 430 λmax (nm): 241 (π–π*), 368 (n–π*). 1H NMR: 7.33–7.72 (m, 8H, Ar H), 3.40 (s, 3H, CH3-N), 2.50 (s, 3H, CH3), 6.91 (m, 2H, NH2), 9.40(s, 1H, OH).
2.4. Spectrophotometric Measurements
To study UV-Vis spectra in the region of 200:700 nm, stock solutions of metal chelates of 1 × 10−4 Mol/L were created by dissolving an exact quantity of the metal chelates in DMF.
2.5. DFT Calculations
The Gaussian 09 was used for all quantum chemistry computations and visualizations [19]. The DFT approach was used to perform geometry optimizations at the B3LYP level, utilizing the 6-311G(d, p) basis set for O, N, C, and H atoms [20]. LANL2DZ was used for the Co, Ni, Cu, Mn, Fe, Cr, Zn, and Cd atoms [19]. Using the optimized structures, frontier molecular orbitals (LUMOs and HOMOs) and MEP have been estimated [21]. Chemical hardness (η) and softness (σ), and other additional chemical descriptors that provide insight into the reactivity of molecules, were calculated [22].
2.6. Pharmacological Studies
2.6.1. Anti-Pathogenic Activity
A filter paper disk (5 mm) was added to 100 mL flasks that comprised 15 mL of the test solution’s working concentration (100 mg/mL). All flasks were autoclaved at 121 °C for 20 min. Four bacterial strains were used, namely: Bacillus subtilis (B. subtilis) and Staphylococcus aureus (S. aureus) Gram(+ve), and Salmonella sp. and Escherichia coli (E. coli) Gram(-ve) bacteria. Furthermore, Aspergillus fumigatus (A. fumigatus) and Candida albicans (C. albicans) fungal strains were also used. All microbes were inoculated on the surfaces of LB agar media utilizing the diffusion agar technique (agar plates). All the chemicals were distributed among four evenly spaced locations in the inoculated Petri plates, each 2 cm from the center. A reference solvent for antipathogenic activity was DMSO. Clear or inhibitory zones were seen around every disk after these incubations at 25 °C for 48 h. The experiment’s control flask was created to run under the same conditions previously explained for each microbe, but only using a DMSO solution. The antibacterial activity was estimated by subtracting the diameter of the inhibition zone created by the DMSO treatment from the diameter obtained in each experiment [23,24,25].
Amikacin and ketoconazole are standard drugs used as references for their antifungal and antibacterial properties, respectively. The data shown are the mean values from all experiments carried out in triplicate.
2.6.2. Docking for the Inspected Molecules
Molecular docking is frequently used to deduce structure-based activity relationships, as it enables the prediction of the confirmation of small-molecule ligands’ binding to the desired binding location. The binding nature of the metal chelates was predicted using MOE software. In addition, a file containing the crystal structure of the E. coli receptor’s active site (PDB ID: 3t88) was retrieved from the data bank of the protein.
3. Results & Discussion
3.1. Structural Inspection of the Azo Dye Ligand
The coupling of 4-amino-antipyrine with 4-aminophenol led to the formation of the free ligand L, as shown in Figure 1. The free ligand L is soluble in ethanol, DMF, and DMSO solvents. The IR spectrum of the L ligand demonstrated in the experimental section (Figure S1) revealed three bands that were assigned to the stretching vibrations of OH, C=O, and N=N functions: a broad band at 3433 cm−1; a medium band at 1639 cm−1; and a faint band at 1620 cm−1. Unfortunately, the NH2 band is not very distinct, due to the overlap with the OH bands in the same area [26].
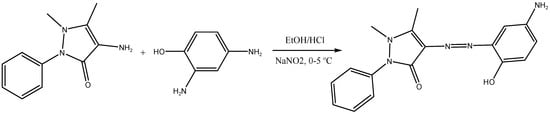
Figure 1.
The proposed structure of azo dye ligand (L).
The ligand’s 1H NMR spectra revealed a single signal at 10 ppm that is associated with the OH proton. The aromatic ring protons appeared multiple times between 7.37 and 7.72 ppm, followed by amino group signals at 6.9 ppm and the two methyl group signals at 3.43 ppm (Figure S2). When deuterated solvent was added, the signals from the OH and NH2 groups vanished from the ligand’s 1H NMR spectra, confirming their positions. The major molecular ion (M+) peak was found at m/z = 323 amu, which matches the elemental analysis results. The rest molecular ion peaks with their respective intensities are provided in Figure 2.
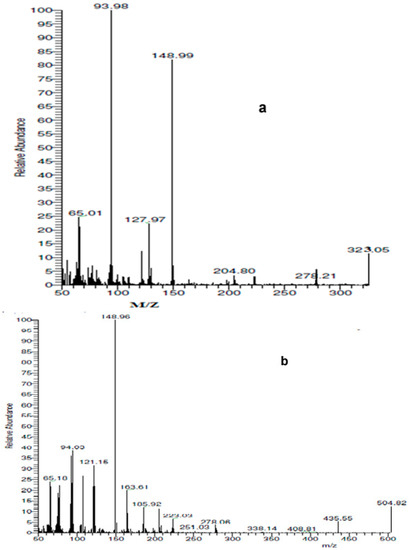
Figure 2.
Mass spectra of (a) azo dye ligand (L), (b) Cd L complex.
3.2. Structural Identification of Metal Chelates under Inspection
3.2.1. C, H, and N percent in the compounds under investigation
The experimental section and the complex’s molecular formulae show the elemental analyses (C, H, and N). The metal-ligand ratio in all the metal chelates examined was found to be 1:1. The metal chelates have high melting (decomposition) points and are stable in the presence of air. All the metal chelates are entirely insoluble in water but readily soluble in EtOH, DMF, and DMSO.
3.2.2. Evaluation of Molar Conductance
The molar conductivities were scanned at 27 °C using 10−3 mol/L of each metal chelate in DMF as solvent. The molar conductivity findings were outlined in the experimental section. The MnII, CoII, ZnII, and CdII complexes had molar conductivities of 36.4, 36, 39.4, and 11.2 Ω−1mol−1cm2, respectively, showing that they were non-electrolytes and non-ionic [27,28]. On the other hand, the molar conductivity values of the chelates of Cr(III), Ni(II), Cu(II), and Fe(III) were found to be 56.3, 83.3, 88.3, and 93.4 Ω−1mol−1cm2, respectively, revealing their ionic character and confirming that they were 1:1 electrolytes.
3.2.3. IR Spectral Studies
The metal chelates’ infrared spectral bands are listed in the experimental section and Supplementary Figure S1. The coordination sites incorporated in chelation were deduced by comparing the IR spectra of the free ligand and its metal chelates. All produced metal complexes’ characteristic IR spectra assignments contrasted with the matching free ligand. Because the metal complexes do not have the same absorption bands as the free azo dye ligand, this suggests that the ligand and metal ion are coordinated.
The band seen in the L spectrum at 3433 cm−1 strongly suggests that OH is present in L [29] while, in the spectra of the metal chelates, it was moved to 3402–3439 cm−1. The absorption band confirmed the presence of the carbonyl group in the free ligand at 1639 cm−1, which was shifted to 1623–1645 cm−1 in the metal chelates IR spectra as a result of the complex formation [30,31]. The band at 1620 cm−1 was attributed to the azo group’s stretching vibration in the free ligand and was shifted to 1610–1622 cm−1 in the metal chelates spectra [32] due to complexation of the ligand. The OH, C=O, and N=N groups’ shifts in band positions confirmed their participation in the bonding to the metal ions.
A new band attributed to the symmetric and asymmetric vibration of coordinated water molecules occurred at 941–975 and 835–863 cm−1. It was determined that v(M-O) and (M-N) were responsible for the weak bands’ frequencies in the ranges of 551–577 cm−1 and 430–436 cm−1, respectively [33,34,35]. These bands did not arise in azo ligand; they were seen in metal complexes. The ligand demonstrates neutral tridentate behavior with NOO donor sites, according to the aforementioned information.
3.2.4. Spectral Studies via 1H NMR
By considering the variations in the 1H NMR spectra of CdII and ZnII chelates compared to the free ligand (Supplementary Figure S1), significant evidence for the structure of complexes was obtained. The phenyl group in the free ligand caused a cluster of multiple signals to appear between 7.37 and 7.72 ppm (m, 8H, Ar H). The phenolic-OH group in the ligand was responsible for the signal at 10 ppm (s, 1H, OH) [36], while the signals at 3.43 ppm (s, 6H, CH3) and 6.9 ppm (s, 2H, NH2) were attributed to the methyl and amino groups, respectively. The 1H NMR spectra of the ZnII and CdII chelates revealed signals for the methyl, aromatic, and phenolic OH groups at 3.41 and 3.40 ppm (s, 6H, CH3), 7.29–7.49 and 7.29–7.72 ppm (m, 8H, Ar-H), and 10.20 and 9.40 ppm (s, 1H, OH), respectively. The ZnII and CdII chelates signals at 6.90 and 6.91 ppm (s, 2H, NH2) belong to the amino group. The change in the location of the OH band demonstrated that phenolic groups’ oxygen atoms were coordinated with the metal ions. Additionally, it showed no proton displacement between the phenolic OH group and the metal ions.
3.2.5. Mass Spectral Investigations
The hypothesized structures in Figure 2 are supported by the mass spectral data of the free ligand and its CdII azo dye complex, which are consistent with the molecular ion fragments. The molecular ion peak at m/z 504.82 amu in the Cd(II) chelate’s recorded mass spectrum was in good agreement with the expected values and corresponded to the molecular weight of the relevant compounds (M-H2O).
3.2.6. UV–Vis Absorption Studies
Several factors affect the UV/Vis spectra of azo dye and its chelates. These include chemical composition, such as chromophores, substituted groups, azo groups number, cations, pH levels, solvents, and more, which frequently affect the UV/Vis spectra of these dyes [37]. The same solvent was used as a blank to measure the UV/Vis spectra of the free azo ligand (L) (1 × 10−4 M) and its chelates (1 × 10−4 M) solutions in ethanol between 700 and 200 nm at 298 K. As seen, the unbound azo ligand has two separate zones of absorption. The first band at 225 nm could be due to the π-π* transition between the antipyrine and benzene rings. The second band, visible at 365 nm, is related to the n-π* electronic transition (Supplementary Figure S3). Specifically, the n-π* transition band was observed at 346–353 nm, while the π-π* transition band was discovered at 271–275 nm. This demonstrates the ligand-metal ion coupling of the azo dye [38,39].
3.2.7. Molecular Electronic Transitions and Magnetic Moment Measurements
For Cr(III) metal chelate as hexa-coordinated, there were three spins permitted for transitions 4A2g(F) to4 T1g(P), 4A2g(F) to 4T2g(F), and 4A2g(F) to 4T2g(F). These spins allowed transitions in the spectrum of diffused reflectance of Cr(III) chelate at 22,624, 19,436, and 17,361 cm−1. The chelate’s stated electronic spectrum is reasonably consistent with those in the literature. The magnetization was determined to be 3.81 B.M. at 298 K, which is in line with what is predicted for octahedral Cr(III) chelate [40].
Three weak bands at 19,950, 21,260, and 25,478 cm−1 in the spectrum of diffused reflectance of the Mn(II) metal chelate can be attributed to the transitions 6A1g to T2g (G), 6A1g to 5T1g, and charge transfer, correspondingly. The μeff suggested an octahedral geometry. value of 5.51 BM for the manganese(II) metal chelate [41,42].
The Fe(III) metal chelate’s μeff. value was determined to be 5.05 BM, as predicted by the octahedral geometry surrounding the Iron(III) ions [43]. Three bands distinguished the spectrum of reflectance for the Ni(II) metal chelate in the areas 3A2g(F to 3T2g(F)(υ1) (13,280 cm−1), 3A2g(F) to 3T1g(F)(υ2) (18,580 cm−1) and 3A2g(F) to 3T1g(P)(υ3) (27,625 cm−1) transition in an idealized octahedral geometry.
The μeff. of the Co(II) metal chelate was 5.18 B.M., which correlates to three single electrons. The diffused reflectance spectrum of the Cobalt (II) metal chelate displayed three bands at 10,690 cm−1, 15,187 cm−1 and 25,051 cm−1. The transitions 4T1g to 4T2g(F)(ν1), 4T1g to 4A2g(F), and 4T1g to T1g(P), correspondingly, may be attributed to these bands. Band positions suggested that the Co(II) complex’s an octahedral shape [44,45].
The high spin Ni(II) metal chelate had a μeff. value of 3.76 B.M. at 298 K, indicating that it was an octahedral geometry [46]. Its reflectance spectrum displayed three bands at (13,455 cm−1): 3A2g to 3T2g; (16,253 cm−1): 3A2g to 3T1g(F); and (22,056 cm−1): 3A2g to 3T1g(P). Additionally, a band at 26,333 cm_1 was detected in the spectrum, which may be related to the charge transfer of ligand to metal.
The reflectance spectrum for Copper(II) metal chelate showed three sholder bands at 18,950, 21,260, and 24,478 cm−1, which could be attributed to 6A1g to T2g (G), 6A1g to 5T1g transitions and charge transfer, correspondingly. The μeff. value of 1.79 BM for Cu(II) metal chelate and the electronic transitions suggested an octahedral shape for the complex [47].
An octahedral shape was suggested for the Zn(II) and Cd(II) metal chelates based on their empirical formulae, which were diamagnetic.
3.2.8. Analysis of Thermal Findings
The thermal stability of the azo dye ligand and its metal chelates was inspected using the TG curve (Supplementary Figure S4). Data in Supplementary Table S1 show the disintegration of the ligand and its metal chelates.
The TG curve of the azo dye ligand exhibited three mass loss steps. The first step has a temperature range from 25 to 305 °C anticipating a mass disposal of 28.48% (computed loss of mass = 28.79%), which corresponds to the disposal of C6H7N. The second step takes place in the range of 305–410 °C with anticipated mass disposal of 9.88% corresponding to C2H6 molecules. Finally, the last step finished at the temperature between 410–1000 °C with anticipated mass disposal of 61.91%, corresponding to C9H4N4O.
The curve of TG for [Cr(L)(H2O)Cl2]Cl metal chelate displayed two instances of weight decrease. The first stage of decomposition occurred in the range of 45 to 495 °C, with a max.of 250 °C, and relates to the disposal of H2O molecules of coordination, C6H6, and chlorine. As in the next step, the mass loss stage (270–800 °C) was due to the ligand’s partial degradation (C11H16N5ClO1/2). The final residue was ½ chromium oxide as the residue of decomposition. The TG curve of the Mn(II) metal chelate with a molecular formula [Mn(L)Cl2(H2O)]2H2O was degraded thermally in three stages. The first step occurred within the range of the temperature from 45 to 235 °C, anticipating mass disposal of 7.11% (computed loss of mass = 7.17%), matched with the disposal of coordinated water molecules. The second step takes place in the range of 235 to 475 °C with anticipated mass disposal of 24.54%, corresponding to the disposal of H2O, C2H6, and Cl2 molecules. The last step occurred at the temperature from 475 to 1000 °C, anticipating mass disposal of45.95%, corresponding to disposal of C15H13N5O and MnO as a residue.
It was noted from the TG-DTG curve of [Fe(L)Cl2(H2O)]Cl.H2O metal chelate that the weight loss percentage happened in three stages at 45–1000 °C. In the first step, with a maximum of DTG at 118 °C, 3.51% (theoretically calculated: 3.45%), there was a weight decrease in the temperature range of 45 to 170 °C, which indicated the disposal of the H2O molecule of hydration [48]. The second decay stage occurs in the vicinity of 400 to 700 °C, corresponding to the disposal of H2O, HCl and Cl2 molecules with anticipated mass disposal of 23.82% (theoretically computed at 23.83%). The azo bonds in the metal chelates under investigation were destroyed when the temperature exceeded 260 °C [49]. The third step of 56.67% (theoretically calculated 56.76%) weight loss was noted in the temperature range of 400 to 1000 °C in the TG curve, which showed the degradation of the C17H15N5O1/2 molecule and remaining ½Fe2O3 as residue [50].
For [Co(L)Cl2(H2O)]H2O metal chelate, the mass disposal of 21.25% (computed 21.72%) within the temperature range of 45 to 215 °C can be assigned to the disposal of two molecules of water and Cl2 gas. At the temperature between 215–1000 °C, the mass disposal of 63.14% (calc. 62.90%) was attributed to the disposal of the C17H17N5O molecule. The decomposition was completed, leading to the formation of the nickel oxide (CoO) residue [51].
TG curve of [Ni(L)Cl2(H2O)] metal chelate exhibited that weight disposal of 18.72% (computed 19.01%) occurred in the temperature range of 45–220 °C is attributable to the mass disposal of H2O and Cl2. The second step indicated that weight disposal of 13.37% (computed 13.03%) occurred in the temperature range of 220–350 °C, with a max. of DTG at 250 °C, and corresponded to the removal of C2H9NO. The third step corresponds to mass disposal of 52.56% (calc. 52.13%). In conjunction with this step, the ligand completely decomposes (C15H8N4O) and forms NiO as a residue.
The TG thermogram of [Cu(L)Cl(H2O)2]Cl metal chelate demonstrated that the decomposition occurred in three stages. The first step within the temperature range of 40 to 250 °C with a maximum of 150 °C may be assigned to the disposal of two H2O molecules with a loss of mass of 7.25% (computed = 7.33%). The second degradation step, at 250–485 °C with a maximum of 370 °C, includes disposal of two molecule Cl2 gas, with a loss of mass of 14.10% (computed = 14.3%). Finally, the third decomposition step occurred within a temperature range of 285 to 1000 °C with a maximum of 670 °C. The ligand completely evaporates during this stage (C17H17N5O2) and produces leftover cupric oxide (CuO).
Zn(II) complex showed TG curves in the temperature range of 45–165 °C disposal of 2H2O hydrated water molecules. The second stage is related to the disposal of HCl and coordination water (165–440 °C) at a maximum of 280 °C due to mass disposal of 10.07% (computed 10.53%). The third stage showed the removal of C17H16N5ClO in the temperature range (440–800 °C) relating to the disposal of 66.88 (computed 66.35%). The remaining ligand’s breakdown, which leaves behind zinc oxide residue, causes final weight losses [52].
The TG curve for [Cd(L)Cl2(H2O)] metal chelate exhibits two separate weight losses. The first step of decomposition occurs within the range of 45 to 520 °C, with a maximum at 250 °C, and corresponds to the disposal of H2O, Cl2, C6H6NO, C6H6, Cl2, corresponding to the removal of 36.91 (computed 37.56%). As in the next step, the loss of mass (520–1000 °C) is caused by the breakdown of a portion of the ligand (C11H11N4). The final residue is CdO.
3.3. Correlation between All Findings for Structural Inspection
From CHN analyses, vibration spectral, molar conductivity, thermal analysis, and mass spectral data, the structures of the metal chelates of the titled ligand with Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), and Cd(II) ions were validated. Therefore, from the vibration spectra, it was established that the azo ligand acts as a tri-dentate neutral ligand coupled to the cations via oxygen of the carbonyl group, oxygen of the phenolic group, and one nitrogen of the azo group. The molar conductivity data revealed that the metal chelates were electrolytes, except for MnII, CoII, ZnII, and CdII. Based on the information mentioned earlier and the magnetic and solid reflectance measurements, an octahedral shape was postulated for the studied metal chelates. In Figure 3, the complexes structural formulas were summed up as follows: [M(L)(H2O)Cl2]Clx.nH2O when M is (Chromium(III), x = 1, n = 0, Manganese(II), x = 0, n = 2, Iron(III), x = n = 1, Cobalt(II), x = 0, n = 1), and Zinc(II), x = 0, n = 2), then [M(L)Cl(H2O)2]Cl.nH2O where M = Nickel(II), n = 0, and Copper(II), n = 1.

Figure 3.
The structure of the L-metal complexes.
3.4. DFT Calculations
The B3LYP and 6–311G (d, p) basis sets were used to optimize the geometry of the ligand and its CrIII, MnII, FeIII, CoII, NiII, CuII, ZnII, and CdII metal chelates in the gas phase. Figure 4 displays the L, CrL, MnL, FeL, CoL, NiL, CuL, ZnL, and CdL geometries in their fully optimized and numbered states.
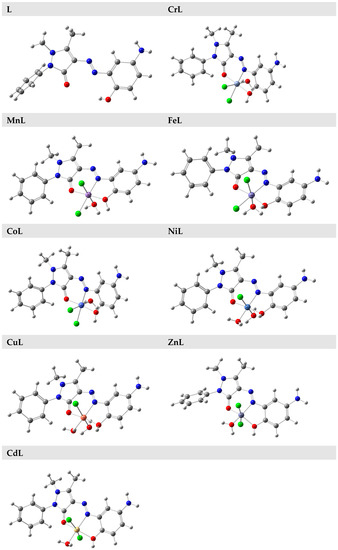
Figure 4.
Optimized geometry of the studied compounds.
The highest occupied molecular orbital (HOMO) describes the ability of a molecule to release electrons, whereas the smallest vacant molecular orbital describes a molecule’s ability to take electrons (LUMO) [53]. The three-dimensional orbitals that resulted from computing the HOMOs and LUMOs of the compounds using the DFT/B3LYP level of theory are presented in Figure 5, together with their energy values in eV. The HOMO/LUMO of the ligand/metal complexes was concentrated throughout the whole molecule, as seen in Figure 5.
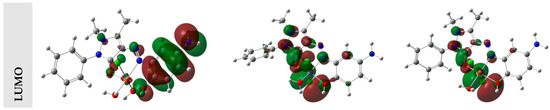
Figure 5.
The HOMO and LUMO of the L, CrL, MnL, FeL, Co, NiL, Cu, ZnL, and CdL compounds.
When describing a molecule’s properties, such as chemical stability, the energy difference between HOMO and LUMO is quite helpful [53]. Any molecule’s electron distribution varies less and exhibits low polarization when the HOMO–LUMO energy difference is high. These molecules are referred to as hard molecules. If there is little change in HOMO–LUMO energy, the polarization is strong, the electron distribution is easily steered, and the molecules are referred to as soft molecules. [54].
The HOMO–LUMO energy gap of the ligand and its metal chelates with CrIII, MnII, FeIII, CoII, NiII, CuII, ZnII, and CdII ions, on the other hand, was calculated to be 3.42, 2.28, 2.01, 1.01, 1.95, 2.42, 2.20, and 2.56 eV., respectively. The ligand’s HOMO–LUMO energy gap was greater than the metal complexes. If the examined compounds were ranked according to their energy gaps, L > CdL > ZnL > NiL > CrL > CuL > MnL > CoL > FeL was shown to be the order. The hardness and softness of molecules can be determined based on various factors.
It is crucial to assess the molecules’ HOMO–LUMO energy gaps to provide information. This led to the conclusion that the most challenging and stable molecule was (L). According to calculations, the molecule that was the softest and most reactive was (FeL). As a result, it was found that, among the neutral molecules, (L) was the hardest and most stable, and (FeL) was the softest and most reactive.
Chemical hardness (η), softness (σ), and other parameters are additional chemical descriptors that provide insight into the chemical reactivity of molecules [55,56]. Typically, the HOMO and LUMO energies are used to calculate the values of chemical hardness (η) and softness (σ). Table 1 contains the computed values.

Table 1.
Chemical descriptors calculated using DFT.
Based on the total electron density surface, the electrostatic potential mapping instantaneously shows the molecular distribution, size, molecular shape, and dipole moments of the molecule’s electrostatic potential (electron + nuclei) [57]. Knowing the relative polarity graphically is useful. The MEP for the ligand and the complexes of CrL, MnL, FeL, CoL, NiL, CuL, ZnL, and CdL are shown in Figure 6. Regions that are electron-rich, electron-deficient, moderately electron-deficient, and neutral are represented on the MEP surface by the colors red, blue, yellow, and green, respectively. In the vicinity of oxygen and chloride, one can find the region with the most negative potential (red) and the most positive charge adjacent to hydrogen atoms.
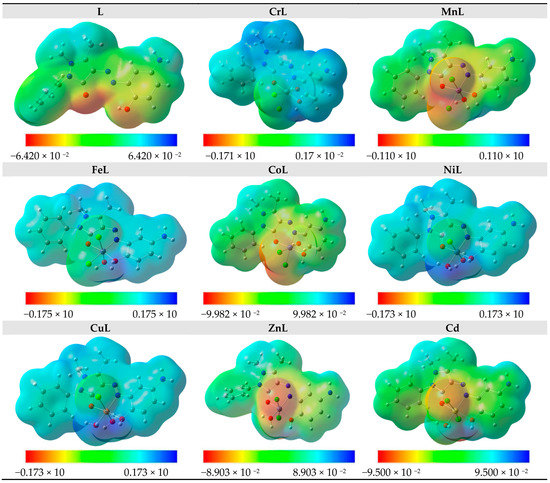
Figure 6.
MEP of the studied compounds.
3.5. Biological Activities
The biological properties of the complexes are affected by the ligand’s chelating nature, the type of the donor atoms, the complexes’ overall charge, the metal ion’s nature, the composition of the opposing ions that balance out the complex, and the complex’s geometrical structure [58,59,60]. The antimicrobial action of azo dye compounds may be significantly enhanced by the presence of an azo group with chelating properties. These properties may be used in metal transport across the bacterial membranes, or to attach to the bacterial cells at a specific site, from which it can interfere with their growth [61].
Supplementary Table S2 summarizes the antimicrobial characteristics of the azo dye ligand and its metal chelates. The common antibiotics ketoconazole and amikacin were used as the standard antifungal and antibacterial agents, respectively. The results displayed in Figure 7 were obtained by comparing the biological properties of the ligand and its metal chelates. Most complexes were more potent than the free ligand against fungi and bacteria. Furthermore, the Fe(III) metal chelate was the most effective against A. fumigatus (lower than ketoconazole standard) and E. coli (higher than amikacin standard).
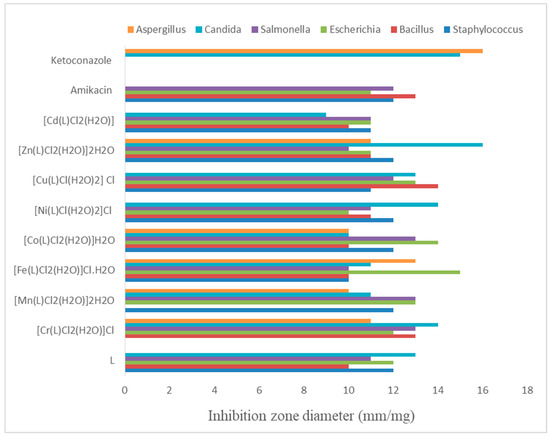
Figure 7.
Biological activity of azo dye ligand (L) and its complexes.
Additionally, the azo dye ligand, Mn(II), Co(II), Ni(II) and Zn(II) metal chelate showed the highest antibacterial potential against S. aureus (similar to amikacin standard). Furthermore, the Zn(II), Ni(II) and Cr(III) complexes showed the higher antifungal activity against C. albicans and almost higher or similar to the ketoconazole standard. Besides that, Co(II) and Cr(III) complexes demonstrated the most promising antibacterial activity against Salmonella sp bacteria and were higher than the antifungal standard. In contrast, the Cu(II) metal chelate showed the highest antibacterial activity against the B. subtilis bacteria. In addition, the azo dye ligand, Ni(II), Cu(II) and Cd(II) complexes showed no antifungal activity against Aspergillus fumigatus, while the remaining complexes showed remarkable antifungal activity, but lower than the standard. The partial sharing of metal ions’ positive charge with donor groups is the basis for the enhanced antipathogenic action [61,62,63].
It was reported previously [64] that all the compounds have antibacterial activity against Gram positive-bacteria when compared with a gentamicin standard in a similar manner to the cited complexes. The data pointed out that the ligand (HL) and its metal complexes (1–5) were found to have no antibacterial activity against Gram-negative bacterium (P. aeruginosa), but have antibacterial activity against E. coli when compared with an ampicillin standard, which is in contrast to our findings here, where the azo dye ligand and complexes showed remarkable activity. The antifungal activity of HL and its complexes (1–5) were reported; they were found to have antifungal activity against A. fumigatus, which is in contrast to our findings reported here. The HL ligand, complexes (2) and (4) have no antifungal activity against C. albicans, while the complexes (1), (3), and (5) have antifungal activity against C. albicans, and the inhibition zone is 19.6, 15.7 and 17.1 mm, respectively, while the azo dye ligand and all metal complexes reported here showed remarkable activity.
The compounds reported previously by researcher [65] exhibited high antimicrobial activity against E. coli than Staphylococcus aureus. This trend is in similar to our reported data (Supplementary Table S2). It was reported by [65] that the complexes showed higher biological activity than the ligands, as is observed in our study. Thus, the metal coordination enhanced antimicrobial activity [65]. This pattern thus supports the possibility of using these complexes as antimicrobial treatments to treat E. coli-related illnesses and/or infections, as surface coating or paint pigments, or as corrosion inhibitors in anti-corrosion paints [65]. The compounds may have the ability to halt or prevent the action of sulphate-reducing bacteria, which starts microbiologically-induced corrosion, based on their antimicrobial activity against anaerobic E. coli [65].
The capacity to consider these new compounds and complexes as prospective antibacterial and antitumor agents, as suggested by [38], is indicated by their significant antimicrobial activity against the Gram-negative bacteria E. coli.
3.6. Molecular Docking
Molecular docking, a crucial technique in the design of the structure, can be used to facilitate and speed up drug discovery by giving researchers information about a virtual screen of the interaction between the target receptor protein and the ligand and predicting the binding affinities and conformations of any species to target proteins.
In the present investigation, molecular docking was used to examine the interactions of a few inhibitors with the E’s active site. coli receptor (PDB ID: 3t88). The gathered information is displayed in Figure 8 and Table 2.
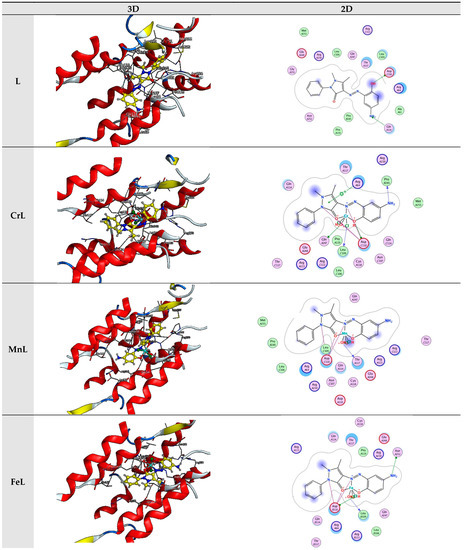
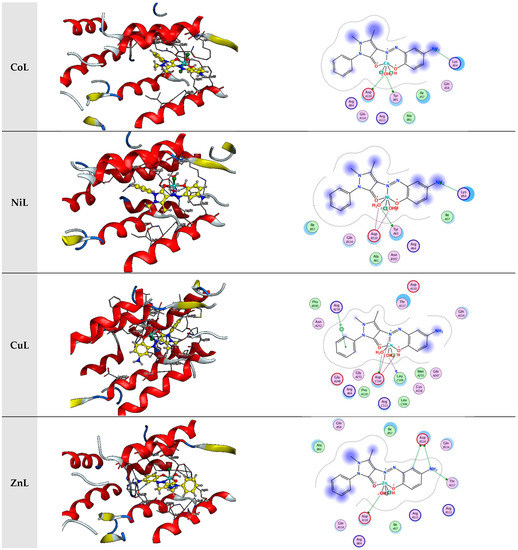
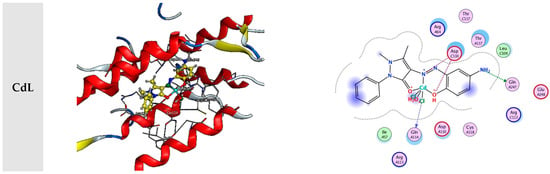
Figure 8.
Interaction between the active site of 3t88 with synthesized complexes.

Table 2.
Docking score obtained and corresponding interaction type.
The incredible ability to dock toward a targeted protein and the decision of the ligand and target protein’s docking outcomes, which were based on the energy value of associated binding, were both represented by the binding energy’s more considerable negative value. The binding of the ligand and its metal-derived complexes to the E coli receptor is mainly mediated by charge and hydrophobic interactions, according to the docking score in Table 2.
The evaluation of the binding scores, resulting from the interaction of all tested compounds with 3t88, revealed that each interaction was exothermic and occurred independently. However, it became apparent that the scores of metal chelates were lower than those of the L ligand when the scores of compounds were compared. This infers that they were more stable than the L-3t88 complex; the complexes formed by binding the complexes to 3t88. L, CrL, MnL, NiL, FeL, CoL, CdL, and ZnL metal complexes’ respective binding scores with 3t88 were −7.82, −7.17, −6.55, −6.50, −6.36, −6.18, −6.11, −5.96, and −5.78 kcal/mol. According to the ratings, the molecule FeL that binds to the 3t88 protein was shown to be the strongest.
Figure 7 depicts the positions the FeL molecule should be in to optimally bind to 3t88 and adhere to the protein’s hydrophobic surface. It was discovered that the ASN 252, LEU 109, and ASP 110 regions of the protein were where the FeL molecule interacted most steadily. Additionally, it was assumed that the protein and shorter hydrogen bond formed this relationship by the FeL complex, as compared to the other compounds, caused the higher binding of the FeL complex to the protein, as opposed to other metal chelates.
4. Conclusions
The azo-coupling reaction of 4-amino-antipyrine and 4-aminophenol afforded the azo dye ligand (L). The azo dye structures are verified using IR, 1H NMR, elemental analysis, and mass spectroscopies. The azo dye ligand was reacted with some metal ions to afford a new batch of octahedral-structured transition metal complexes. The structure of the metal chelates was completely elucidated using elemental analysis, infrared, mass, electronic, and 1HNMR spectra data, as well as magnetic studies. The IR spectra demonstrated that the chelation mode takes place through the OH (oxygen atom of phenolic), oxygen atoms (of CO group), and the nitrogen atom (of N=N groups). Molar conductivity in ethanol indicates that the MnII, CoII, ZnII, and CdII metal chelates are non-electrolytes. The CrIII, NiII, CuII, and FeIII are electrolytes. The proposed structural formulas of the meta chelates are [M(L)(H2O)Cl2]Clx.nH2O where M = (CrIII, x = 1, n = 0, MnII and ZnII, x = 0, n = 2, FeIII, x = n = 1, CoII, x = 0, n = 1) and ZnII, x = 0, n = 2) and [M(L)Cl(H2O)2]Cl.nH2O where M = NiII, n = 0, and CuII, n = 1. Frontier molecular orbitals and optimal geometries were predicted by the DFT with the B3LYP method, using 6-311G (d, p) and LANL2DZ basis sets for the ligand and its metal chelates, respectively. Among all the compounds, FeL complex was the softest and most reactive one, according to the values of the HOMO–LUMO gap. The antimicrobial tests showed that most complexes have higher antifungal activity than the free ligand, and all metal chelates have anti-pathogenic activities behaviors. Additionally, a molecular docking analysis was performed to examine the bonding styles of the molecules produced with the E. coli receptor’s active region (PDB ID: 3t88). FeL complex was found to have the lowest binding energy score of all the compounds tested, coming in at −8.82 kcal/mol.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma16030897/s1, Figure S1: IR spectra of (L) L, (a) L-Cr, (b) L-Mn, (c) L-Fe, (d) L-Co, (e) L-Ni, (f) L-Cu, (g) L-Zn and (h) L-Cd metal complexes; Figure S2: 1H-NMR spectra of (a–i) L in DMSO, (b–i) L-Zn(II) in DMSO and (c–i) L-Cd(II) in DMSO; Figure S3. UV-Vis. spectra of (l) L, (a) L-Cr(III), (b) L-Mn(II), (c) L-Fe(III), (d) L-Co(II), (e) L-Ni(II), (f) L-Cu(II), (g) L-Zn(II) and (h) L-Cd(II) complexes; Figure S4. TG Thermograms of (a) L, (b) L-Cr(III), (c) L-Mn(II), (d) L-Fe(III), (e) L-Co(II), (f) L-Ni(II), (g) L-Cu(II), (h) L-Zn(II) and (i) L-Cd(II) complexes. Table S1: Thermodynamic data of the thermal decomposition of azo dye ligand (L) and its metal complexes; Table S2: Biological activity of azo dye ligand (L2) and its metal complexes.
Author Contributions
Conceptualization, M.A.I.A.-G., A.M.A.-D., G.G.M., A.A. and H.M.A.E.-L.; methodology, A.M.A.-D., A.A. and H.M.A.E.-L.; validation, A.M.A.-D., S.S., G.G.M., M.A.I.A.-G., M.G. and M.S.; investigation, A.M.A.-D., H.M.A.E.-L., M.S. and M.M.K.; writing—original draft preparation, A.M.A.-D., M.G., A.A., S.S., G.G.M. and M.S.; writing—review and editing, A.M.A.-D., G.G.M., M.G., S.S. and M.S., supervision, A.M.A.-D., M.M.K. and G.G.M.; project administration, A.M.A.-D., G.G.M. and H.M.A.E.-L.; funding acquisition, M.A.I.A.-G., H.M.A.E.-L., M.G., S.S. and M.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at King Faisal University, Saudi Arabia (Grant 1979).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw/processed data generated in this work are available upon request from the corresponding author.
Acknowledgments
This work was supported by the Deanship of Scientific Research, Vice Presi-dency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [GRANT1979], through its KFU Research Summer initiative. Additionally, the authors extend their appreciation to the Faculty of Science for funding this work through project No. FC-2202410.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zollinger, H. Color Chemistry, 2nd ed.; VCH Weinheim: Weinheim, Germany, 1991. [Google Scholar]
- Ganesh, R.; Boardman, G.D.; Michelson, D. Fate of azo dyes in sludges. Water Res. 1994, 28, 1367. [Google Scholar] [CrossRef]
- O’Neill, C.; Hawkes, F.R.; Hawkes, D.L.; Lourenco, N.D.; Pinheiro, H.M.; Delee, W. Colour in Textile Effluents—Sources, Measurement, Discharge Consents and Simula-tion: A Review. J. Chem. Tech. Biotechn. 1999, 74, 1009. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Abd El-Lateef, H.M.; Gouda, M.; Sayed, F.N.; Mohamed, G.G.; Abu-Dief, A.M. Design Structural Inspection and Bio-Medicinal Applications of Some Novel Imine Metal Complexes Based on Acetylferrocene. Materials 2022, 15, 4842. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dief, A.M.; El-Khatib, R.M.; Aljohani, F.S.; Al-Abdulkarim, H.A.; Alzahrani, S.; El-Sarrag, G.; Ismael, M. Synthesis, structuralelucidation, DFT calculation, biological studies and DNA inter-action of some aryl hydrazone Cr3+, Fe3+, and Cu2+ chelates. Comput. Biol. Chem. 2022, 97, 107643. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Sagher, H.M.; Shehata, M.R. Fabrication, spectroscopic characterization, calf thymus DNA binding investigation, antioxidant and anticancer activities of some antibiotic azomethine Cu(II), Pd(II), Zn(II) and Cr(III) complexes. Appl. Organomet. Chem. 2019, 33, e49432019. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Díaz-Torres, R.; Sañudo, E.C.; Abdel-Rahman, L.H.; Aliaga-Alcalde, N. Novel sandwich triple-decker dinuclear NdIII-(bis-N, N′-p-bromo-salicylideneamine-1, 2-diaminobenzene) complex. Polyhedron 2013, 64, 203–208. [Google Scholar] [CrossRef]
- Aljohani, E.T.; Shehata, M.R.; Alkhatib, F.; Alzahrani, S.O.; Abu-Dief, A.M. Development and structure elucidation of new VO2+, Mn2+, Zn2+, and Pd2+ complexes based on azomethine ferrocenyl ligand: DNA interaction, antimicrobial, antioxidant, anticancer activities, and molecular docking. Appl. Organomet. Chem. 2021, 35, e61542021. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Rahman, L.H.; Abdel-Mawgoud, A.A.H. A robust in vitro anticancer, antioxidant and antimicrobial agents based on new metal-azomethine chelates incorporating Ag (I), Pd (II) and VO (II) cations: Probing the aspects of DNA interaction. Appl. Organomet. Chem. 2020, 34, e53732020. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, F.; Luo, D.; Huang, J.; Ouyang, J.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.; Peng, Y. Recent advances in Ti-based MOFs in biomedical applications. Dalton Trans. 2022, 51, 14817. [Google Scholar] [CrossRef]
- Al-Khateeb1, Z.T.; Karam, F.F.; Al-Adilee, K. Synthesis and characterization of some metals complexes with new heterocyclic azo dye ligand 2-[2 - - (5-Nitro thiazolyl) azo]-4- methyl -5- nitro phenol and their biological activities. J. Phys. Conf. Ser. 2019, 1294, 052043. [Google Scholar] [CrossRef]
- Abdul Karem, L.K.; Ganim, F.H.; Rahem Al-Shemary, R.K. Synthesis, characterization, structural, thermal, POM studies, antimicrobial and DNA cleavage activity of a new schiff base-Azo Ligand and its complexation with selected metal ions. Biochem. Cell. Arch. 2018, 18, 1437–1448. [Google Scholar]
- Modhavadiya, V.A. Synthesis, Characterization, Spectral Studies, Biocidal Activities of Fe (II) and Cu (II) complexes of Azo dye Ligand Derived from Sulfamethoxazole and Substituted p-Cresol. Orient. J. Chem. 2012, 28, 921–925. [Google Scholar] [CrossRef]
- Chakraborty, P.; Adhikary, J.; Sanyal, R.; Khan, A.; Manna, K.; Dey, S.; Zangrando, E.; Bauz, A.; Frontera, A.; Das, D. Role of ligand backbone of tridentate Schiff-base on complex nuclearity and bio-relevant catalytic activities of zinc(II) complexes: Experimental and theoretical investigations. Inorg. Chim. Acta 2014, 421, 364. [Google Scholar] [CrossRef]
- Salehi, M.; Rahimifar, F.; Kubicki, M.; Asadi, A. Structural, spectroscopic, electrochemical and antibacterial studies of some new nickel (II) Schiff base complexes. Inorg. Chim. Acta 2016, 443, 28. [Google Scholar] [CrossRef]
- Metwally, M.A.; Gouda, M.A.; Harmal, N.A.; Khalil, A.M. 3-Iminobutanenitrile as building block for the synthesis of substituted pyrazolo[1,5-a]pyrimidines with antitumor and antioxidant activities. Int. J. Mod. Og. Chem. 2012, 1, 96. [Google Scholar]
- Raman, N.; Selvan, A.; Manisankar, P. Spectral, magnetic, biocidal screening, DNA binding and photocleavage studies of mononuclear Cu(II) and Zn(II) metal complexes of tricoordinate heterocyclic Schiff base ligands of pyrazolone and semicarbazide/thiosemicarbazide based derivatives. Spectrochim. Acta A 2010, 76, 161. [Google Scholar] [CrossRef]
- Xiaoyi, L.; Wu, Y.; Gu, D.; Fuxi, G. Spectral, thermal and optical properties of metal (II)–azo complexes for optical recording media. Dyes Pigm. 2010, 86, 182. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Casida, M.E.; Jamorski, C.; Casida, K.C.; Salahub, D.R. Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: Characterization and correction of the time-dependent local density approximation ionization threshold. J. Chem. Phys. 1998, 108, 4439–4449. [Google Scholar] [CrossRef]
- Mohamed, I.; Abdou, A.; Abdel-Mawgoud, A.-M. Synthesis, Characterization, Modeling, and Antimicrobial Activity of FeIII, CoII, NiII, CuII, and ZnII Complexes Based on Tri-substituted Imidazole Ligand. Z. Anorg. Allg. Chem. 2018, 644, 1203–1214. [Google Scholar]
- Mahmoud, W.H.; Mohamed, G.G.; El-Dessouky, M.M.I. Coordination modes of bidentate lornoxicam drug with some transition metal ions. Synthesis, characterization and in vitro antimicrobial and antibreastic cancer activity studies. Spectrochim. Acta A 2014, 122, 598. [Google Scholar] [CrossRef]
- Aljohani, E.T.; Shehata, M.R.; Abu-Dief, A.M. Design, synthesis, structural inspection of Pd2+, VO2+, Mn2+, and Zn2+ chelates incorporating ferrocenyl thiophenol ligand: DNA interaction and pharmaceutical studies. Appl. Organomet. Chem. 2021, 35, e61692021. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Khalaf, M.M.; Shehata, M.R.; Abu-Dief, A.M. Fabrication, DFT calculation, and molecular docking of two Fe (III) imine chelates as anti-COVID-19 and pharmaceutical drug candidate. Int. J. Mol. Sci. 2022, 23, 3994. [Google Scholar] [CrossRef] [PubMed]
- Joseyphus, R.S.; Shiju, C.; Joseph, J.; Dhanaraj, C.J.; Arish, D. Synthesis and characterization of metal complexes of Schiff base ligand derived from imidazole-2-carboxaldehyde and 4-aminoantipyrine. Spectrochim. Acta A 2014, 133, 149. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Basha, M.; Hassan Abdel-Mawgoud, A.A. Three novel Ni (II), VO (II) and Cr (III) mononuclear complexes encompassing potentially tridentate imine ligand: Synthesis, structural characterization, DNA interaction, antimicrobial evaluation and anticancer activity. Appl. Organomet. Chem. 2017, 31, e3750. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Metwaly, N.M.; Alzahrani, S.O.; Alkhatib, F.M.; Abualnaja, M.; El-Dabea, T.; Ali, M.A.A. Synthesis and characterization of Fe (III), Pd (II) and Cu (II)-thiazole complexes; DFT, pharmacophore modeling, in-vitro assay and DNA binding studies. J. Mol. Liq. 2021, 326, 115277. [Google Scholar] [CrossRef]
- Al-Saeedi, S.I.; Abdel-Rahman, L.H.; Abu-Dief, A.M.; Abdel-Fatah, S.M.; Alotaibi, T.M.; Alsalme, A.M.; Nafady, A. Catalytic Oxidation of Benzyl Alcohol Using Nanosized Cu/Ni Schiff-Base Complexes and Their Metal Oxide Nanoparticles. Catalysts 2018, 8, 452. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A Microporous 2D Cobalt-Based MOF with Pyridyl Sites and Open Metal Sites for Selective Adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Bouhdada, M.; Amane, M.E.; El Hamzaoui, N. Synthesis, spectroscopic studies, X-ray powder diffraction data and antibacterial activity of mixed transition metal complexes with sulfonate azo dye, sulfamate and caffeine ligands. Inorg. Chem. Commun. 2019, 101, 32–39. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Rahman, L.H.; Abdelhamid, A.A.; Marzouk, A.A.; Shehata, M.R.; Bakheet, M.A.; Almaghrabi, O.A.; Nafady, A. Synthesis and characterization of new Cr (III), Fe (III) and Cu (II) complexes incorporating multi-substituted aryl imidazole ligand: Structural, DFT, DNA binding, and biological implications. Spectrochim. Acta A 2020, 228, 117700. [Google Scholar] [CrossRef] [PubMed]
- Al-Shamry, A.A.; Khalaf, M.M.; El-Lateef, H.M.A.; Yousef, T.A.; Mohamed, G.G.; El-Deen, K.M.K.; Gouda, M.; Abu-Dief, A.M. Development of New Azomethine Metal Chelates Derived from Isatin: DFT and Pharmaceutical Studies. Materials 2023, 16, 83. [Google Scholar] [CrossRef]
- Aljohani, F.S.; Omran, O.A.; Ahmed, E.A.; Al-Farraj, E.S.; Elkady, E.F.; Alharbi, A.; El-Metwaly, N.M.; Barnawi, I.O.; Abu-Dief, A.M. Design, structural inspection of new bis (1H-benzo [d] imidazol-2-yl) methanone complexes: Biomedical applications and theoretical implementations via DFT and docking approaches. Inorg. Chem. Commun. 2023, 128, 110331. [Google Scholar] [CrossRef]
- Subbaraj, P.; Ramub, A.; Raman, N.; Dharmaraja, J. Novel mixed ligand complexes of bioactive Schiff base (E)-4-(phenyl (phenylimino) methyl) benzene-1, 3-diol and 2-aminophenol/2-aminobenzoic acid: Synthesis, spectral characterization, antimicrobial and nuclease studies. J. Spectrochim. Acta A 2014, 117, 65. [Google Scholar] [CrossRef]
- Gur, M.; Kocaokutgen, H.; Tasx, M. Synthesis, spectral, and thermal characterisations of some azo-ester derivatives containing a 4-acryloyloxy group. Dyes Pigm. 2007, 72, 101. [Google Scholar] [CrossRef]
- Mohamed, G.G.; Omar, M.M.; Ibrahim, A.A. Biological activity studies on metal complexes of novel tridentate Schiff base ligand. Spectroscopic and thermal characterization. Eur. J. Med. Chem. 2009, 44, 4801. [Google Scholar] [CrossRef]
- Aljohani, F.S.; Abu-Dief, A.M.; El-Khatib, R.M.; Al-Abdulkarim, H.A.; Alharbi, A.; Mahran, A.; Khalifa, M.E.; El-Metwaly, N.M. Structural inspection for novel Pd (II), VO (II), Zn (II) and Cr (III)-azomethine metal chelates: DNA interaction, biological screening and theoretical treatments. J. Mol. Stru. 2021, 1246, 131139. [Google Scholar] [CrossRef]
- Abd El-Halim, H.F.; Mohamed, G.G.; El-Dessouky, M.M.I.; Mahmoud, W.H. Ligational behaviour of lomefloxacin drug towards Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Th(IV) and UO2(VI) ions: Synthesis, structural characterization and biological activity studies. Spectrochim. Acta A 2011, 82, 8. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.H.; Mohamed, G.G. Preparation, spectroscopic and thermal characterization of new metal complexes of verlipride drug. In vitro biological activity studies. Spectrochim. Acta A 2012, 91, 11. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Aboelez, M.O.; Abdel-Mawgoud, A.A.H. DNA interaction, antimicrobial, anticancer activities and molecular docking study of some new VO (II), Cr (III), Mn (II) and Ni (II) mononuclear chelates encompassing quaridentate imine ligand. J. Photochem. Photobiol. B Biol. 2017, 170, 271–285. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Mohamad, A.D.M.; Shehata, M.R.; Abu-Dief, A.M. Targeted synthesis of two iron (III) tetradentate dibasic chelating Schiff base complexes toward inhibition of acidic induced steel corrosion: Empirical and DFT insights. Appl. Organomet. Chem. 2022, 36, e67182022. [Google Scholar] [CrossRef]
- Alaghaz, A.M.A.; Bayoumi, H.A.; Ammar, Y.A.; Aldhlmani, S.A. Synthesis, characterization, and antipathogenic studies of some transition metal complexes with N,O-chelating Schiff’s base ligand incorporating azo and sulfonamide Moieties. J. Mol. Stru. 2013, 1035, 383. [Google Scholar] [CrossRef]
- Abdel Rahman, L.H.; Abu-Dief, A.M.; El-Khatib, R.M.; Abdel-Fatah, S.M.; Adam, A.M.; Ibrahim, E.M.M. Sonochemical synthesis, structural inspection and semiconductor behavior of three new nano sized Cu (II), Co (II) and Ni (II) chelates based on tri-dentate NOO imine ligand as precursors for metal oxides. Appl. Organomet. Chem. 2018, 32, e41742018. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G.; Murillo, C.A.; Bochmann, M. Advanced Inorganic Chemistry, 6th ed.; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Abdel-Rahman, L.H.; Adam, M.S.S.; Abu-Dief, A.M.; Moustafa, H.; Basha, M.T.; Aboraia, A.S.; Al-Farhan, B.S.; Ahmed, H.E.S. Synthesis, theoretical investigations, biocidal screening, DNA binding, in vitro cytotoxicity and molecular docking of novel Cu (II), Pd (II) and Ag (I) complexes of chlorobenzylidene Schiff base: Promising antibiotic and anticancer agents. Appl. Organomet. Chem. 2018, 32, e45272018. [Google Scholar] [CrossRef]
- Sakai, H.; Matsuyama, T.; Maeda, Y.; Yamaoka, H.J. An 129I Mössbauer spectroscopic study of iodine doped in poly (vinylpyridines). J. Chem. Phys. 1981, 75, 5155. [Google Scholar] [CrossRef]
- El-Sonbati, A.Z.; Diab, M.A.; El-Bindary, A.A.; Eldesoky, A.M.; Morgan, S.M. Correlation between ionic radii of metals and thermal decomposition of supramolecular structure of azodye complexes. Spectrochim. Acta A. 2015, 135, 774. [Google Scholar] [CrossRef]
- Baran, N.Y.; Demir, M.K.; Saçak, M. Synthesis, characterization, conductivity and antimicrobial study of a novel thermally stable polyphenol containing azomethine group. J. Mol. Struct. 2016, 1123, 153. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Alharbi, A.; Abualnaja, M.M.; Hossan, A.; Alhasani, M.; Abu-Dief, A.M.; Khalifa, M.E.; El-Metwaly, N.M. Synthesis and elucidation of binuclear thiazole-based complexes from Co (II) and Cu (II) ions: Conductometry, cytotoxicity and computational implementations for various verifications. J. Mol. Liq. 2022, 349, 118100. [Google Scholar] [CrossRef]
- Shoair, A.F.; El-Shobaky, A.R.; Abo-Yassin, H.R. Synthesis, spectroscopic characterization, catalytic and antibacterial studies of ruthenium (III) Schiff base complexes. J. Mol. Liq. 2015, 211, 217. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Alotaibi, N.; Al-Farraj, E.; Qasem, H.; Alzahrani, S.; Mahfouz, M.; Abdou, A. Fabrication, structural elucidation, theoretical, TD-DFT, vibrational calculation and molecular docking studies of some novel adenine imine chelates for biomedical applications. J. Mol. Liq. 2022, 365, 119961. [Google Scholar] [CrossRef]
- Abdou, A. Synthesis, Structural, Molecular Docking, DFT, Vibrational Spectroscopy, HOMO-LUMO, MEP Exploration, antibacterial and antifungal activity of new Fe(III), Co(II) and Ni(II) hetero-ligand complexes. J. Mol. Struct. 2022, 1262, 132911. [Google Scholar] [CrossRef]
- Abdou, A.; Hassan, M.M.; Abdel-Mawgoud, M. Seven metal-based bi-dentate NO azocoumarine complexes: Synthesis, physicochemical properties, DFT calculations, drug-likeness, in vitro antimicrobial screening and molecular docking analysis. Inorg. Chim. Acta 2022, 539, 121043. [Google Scholar] [CrossRef]
- Ali El-Remaily, M.A.E.A.A.; El-Dabea, T.; Alsawat, M.; Mahmoud, M.H.; Alfi, A.A.; El-Metwaly, N.; Abu-Dief, A.M. Development of new thiazole complexes as powerful catalysts for synthesis of pyrazole-4-carbonitrile derivatives under ultrasonic irradiation condition supported by DFT studies. ACS Omega 2021, 6, 21071–21086. [Google Scholar] [CrossRef] [PubMed]
- Elkanzi, N.A.A.; Ali, A.M.; Hrichi, H.; Abdou, A. New mononuclear Fe(III), Co(II), Ni(II), Cu(II), and Zn(II) complexes incorporating 4-{[(2 hydroxyphenyl)imino]methyl}phenyl-4-methylbenzenesulfonate (HL): Synthesis, characterization, theoretical, anti-inflammatory, and molecular docking investigation. Appl. Organomet. Chem. 2022, 36, e66652022. [Google Scholar]
- Abu-Dief, A.M.; El-Khatib, R.M.; Salah, M.E.; Alzahrani, S.; Alkhatib, F.; El-Sarrag, G.; Ismael, M. Synthesis and intensive characterization for novel Zn (II), Pd (II), Cr (III) and VO (II)-Schiff base complexes; DNA-interaction, DFT, drug-likeness and molecular docking studies. J. Mol. Struct. 2021, 1244, 131017. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Rahman, L.H.; Shehata, M.R.; Abdel-Mawgoud, A.A.H. Novel azomethine Pd (II)-and VO (II)-based metallo-pharmaceuticals as anticancer, antimicrobial, and antioxidant agents: Design, structural inspection, DFT investigation, and DNA interaction. J. Phys. Org. Chem. 2019, 32, e40092019. [Google Scholar] [CrossRef]
- Qasem, H.A.; Aouad, M.R.; Al-Abdulkarim, H.A.; Al-Farraj, E.S.; Attar, R.M.; El-Metwaly, N.M.; Abu-Dief, A.M. Tailoring of some novel bis-hydrazone metal chelates, spectral based characterization and DFT calculations for pharmaceutical applications and in-silico treatments for verification. J. Mol. Struc. 2022, 1264, 133263. [Google Scholar] [CrossRef]
- Panchal, P.K.; Pansuria, P.B.; Patel, M.N. In-vitro biological evaluation of some ONS and NS donor Schiff’s bases and their metal complexes. J. Enzym. Inhib. Med. Chem. 2006, 21, 203. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Moustafa, H.; Abdel-Mawgoud, A.A.H. Design and nonlinear optical properties (NLO) using DFT approach of new Cr (III), VO (II), and Ni (II) chelates incorporating tri-dentate imine ligand for DNA interaction, antimicrobial, anticancer activities and molecular docking studies. Arab. J. Chem. 2020, 13, 649–670. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abdelhamid, A.A.; Abu-Dief, A.M.; Shehata, M.R.; Bakhe, M.A. Facile synthesis, X-ray structure of new multi-substituted aryl imidazole ligand, biological screening and DNA binding of its Cr (III), Fe (III) and Cu (II) coordination compounds as potential antibiotic and anticancer drugs. J. Mol. Struct. 2020, 1200, 127034. [Google Scholar] [CrossRef]
- El-Sonbati, A.Z.; El-Bindary, A.A.; Mohamed, G.G.; Morgan, S.M.; Hassan, W.M.I.; Elkholy, A.K. Geometrical structures, thermal properties and antimicrobial activity studies of azodye complexes. J. Mol. Liq. 2016, 218, 16–34. [Google Scholar] [CrossRef]
- KofiKyei, S.; Akaranta, O.; Darko, G. Synthesis, characterization and antimicrobial activity of peanut skin extract-azo-compounds. Sci. Afr. 2020, 8, e004062020. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).