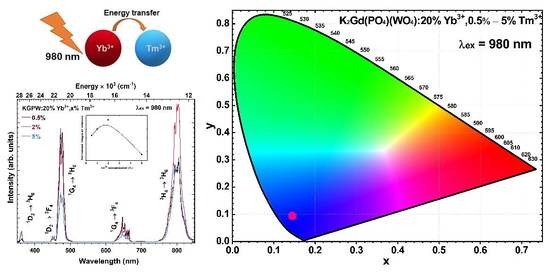
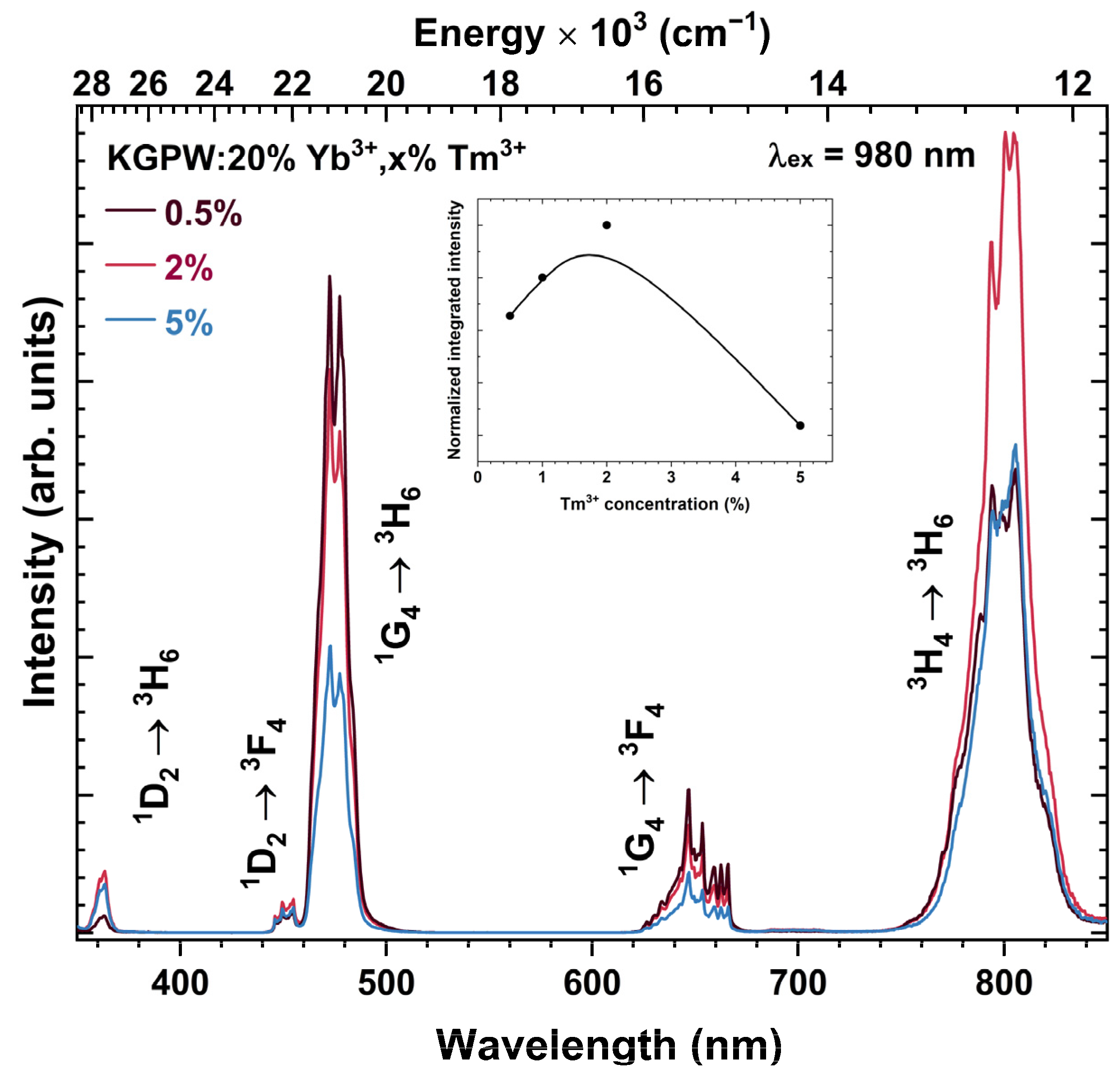
Optical Properties Investigation of Upconverting K2Gd(PO4)(WO4):20%Yb3+,Tm3+ Phosphors
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.H.; Wang, B.; Liu, Y.H.; Bai, G.Y.; Fu, Z.L.; Liu, H. Upconversion luminescence and temperature sensing characteristics of Yb3+/Tm3+:KLa(MoO4)2 phosphors. Dalton Trans. 2021, 50, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Kumar, K. Studies on up/down-conversion emission of Yb3+ sensitized Er3+ doped MLa2(MoO4)4 (M = Ba, Sr and Ca) phosphors for thermometry and optical heating. Opt. Mater. 2018, 75, 770–780. [Google Scholar] [CrossRef]
- Wen, X.; Tang, G.W.; Yang, Q.; Chen, X.D.; Qian, Q.; Zhang, Q.Y.; Yang, Z.M. Highly Tm3+ doped germanate glass and its single mode fiber for 2.0 mu m laser. Sci. Rep. 2016, 6, 20344. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Mondal, M.; Rai, V.K.; Singh, S.N. Photoluminescence study in Ho3+/Tm3+/Yb3+/Li+:Gd2(MoO4)3 nanophosphors for near white light emitting diode and security ink applications. Methods Appl. Fluoresc. 2018, 6, 015003. [Google Scholar] [CrossRef]
- Maurizio, S.L.; Tessitore, G.; Kramer, K.W.; Capobianco, J.A. BaYF5:Yb3+, Tm3+ Upconverting Nanoparticles with Improved Population of the Visible and Near-Infrared Emitting States: Implications for Bioimaging. ACS Appl. Nano Mater. 2021, 4, 5301–5308. [Google Scholar] [CrossRef]
- Li, A.M.; Li, X.B.; Wang, J.L.; Guo, Y.S.; Li, C.G.; Chen, W.G.; Wang, Z.W.; Tang, Y.A. Multifunctional alpha-NaYbF4:Tm3+ nanocrystals with intense ultraviolet self-sensitized upconversion luminescence and highly efficient optical heating. Ceram. Int. 2022, 48, 22961–22966. [Google Scholar] [CrossRef]
- Chen, S.; Weitemier, A.Z.; Zeng, X.; He, L.M.; Wang, X.Y.; Tao, Y.Q.; Huang, A.J.Y.; Hashimotodani, Y.; Kano, M.; Iwasaki, H.; et al. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science 2018, 359, 679–683. [Google Scholar] [CrossRef]
- Liu, Y.J.; Lu, Y.Q.; Yang, X.S.; Zheng, X.L.; Wen, S.H.; Wang, F.; Vidal, X.; Zhao, J.B.; Liu, D.M.; Zhou, Z.G.; et al. Amplified stimulated emission in upconversion nanoparticles for super-resolution nanoscopy. Nature 2017, 543, 229–233. [Google Scholar] [CrossRef]
- Lamon, S.; Wu, Y.; Zhang, Q.; Liu, X.; Gu, M. Nanoscale optical writing through upconversion resonance energy transfer. Sci. Adv. 2021, 7, eabe2209. [Google Scholar] [CrossRef]
- Li, K.; Van Deun, R. Mutual energy transfer luminescent properties in novel CsGd(MoO4)2:Yb3+,Er3+/Ho3+ phosphors for solid-state lighting and solar cells. Phys. Chem. Chem. Phys. 2019, 21, 4746–4754. [Google Scholar] [CrossRef]
- Mahata, M.K.; Koppe, T.; Kumar, K.; Hofsass, H.; Vetter, U. Upconversion photoluminescence of Ho3+-Yb3+ doped barium titanate nanocrystallites: Optical tools for structural phase detection and temperature probing. Sci. Rep. 2020, 10, 8775. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.Y.; Liu, L.; Ma, B.; Li, B.X.; Zhao, X.D.; Tong, W.M.; Wang, X.F. beta-NaYF4: Yb, Tm: Upconversion properties by controlling the transition probabilities at the same energy level. Inorg. Chem. Front. 2016, 3, 1082–1090. [Google Scholar] [CrossRef]
- Yu, H.Q.; Jiang, P.P.; Chen, B.J.; Sun, J.S.; Cheng, L.H.; Li, X.P.; Zhang, J.S.; Xu, S. Electrospinning preparation and upconversion luminescence of Y2Ti2O7:Tm/Yb nanofibers. Appl. Phys. A 2020, 126, 690. [Google Scholar] [CrossRef]
- Fan, G.; Tian, Z.C.; Wang, X.J.; Tang, S.L.; Chen, Y.N. High quantum efficiency red-emitting K2Gd(PO4)(WO4): Sm3+ phosphor: Preparation, characterization and photoluminescence properties. J. Mater. Sci. Mater. Electron. 2018, 29, 17681–17688. [Google Scholar] [CrossRef]
- Han, L.L.; Zhao, L.; Zhang, J.; Wang, Y.Z.; Guo, L.N.; Wang, Y.H. Structure and luminescence properties of the novel multifunctional K2Y(WO4)(PO4):Ln3+ (Ln = Tb, Eu, Yb, Er, Tm and Ho) phosphors. RSC Adv. 2013, 3, 21824–21831. [Google Scholar] [CrossRef]
- Zhang, X.G.; He, P.; Zhou, L.Y.; Shi, J.X.; Gong, M.L. Energy transfer and luminescent properties of a green-to-red color tunable Tb3+, Eu3+ co-doped K2Y(WO4)(PO4) phosphor. Mater. Res. Bull. 2014, 60, 300–307. [Google Scholar] [CrossRef]
- Terebilenko, K.V.; Zatovsky, I.V.; Baumer, V.N.; Slobodyanik, N.S.; Shishkin, O.V. K2Ho(PO4)(WO4). Acta Crystallogr. Sect. E-Crystallogr. Commun. 2008, 64, I75. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Marin, R.; Skripka, A.; Vetrone, F. Small and Bright Lithium-Based Upconverting Nanoparticles. J. Am. Chem. Soc. 2018, 140, 12890–12899. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; He, Q.; Xu, J.X.; Shao, C.Y.; Sun, S.Y.; Fan, S.H.; Hu, L.L. Efficient NIR to NIR up-conversion in LiYE4:Yb3+Tm3+ micro-octahedrons by modified hydrothermal method. J. Lumin. 2020, 227, 117396. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Zatovsky, I.V.; Terebilenko, K.V.; Slobodyanik, N.S.; Baumer, V.N.; Shishkin, O.V. Synthesis, characterization and crystal structure of K2Bi(PO4)(MoO4). J. Solid State Chem. 2006, 179, 3550–3555. [Google Scholar] [CrossRef]
- Carnall, W.T.; Crosswhite, H.; Crosswhite, H.M. Energy Level Structure and Transition Probabilities in the Spectra of the Trivalent Lanthanides in LaF3. In Argonne National Laboratory Report; U.S. Department of Energy Office of Scientific and Technical Information: Washington, DC, USA, 1977. [Google Scholar]
- Han, B.; Liu, B.K.; Zhang, J.; Shi, H.Z. Luminescence properties of novel Ba2MgWO6:Eu3+ and g-C3N4/Ba2MgWO6:Eu3+ phosphors. Optik 2017, 131, 764–768. [Google Scholar] [CrossRef]
- Zheng, Y.; Deng, L.Z.; Li, J.P.; Jia, T.Q.; Qiu, J.R.; Sun, Z.R.; Zhang, S.A. Controlling multiphoton excited energy transfer from Tm3+ to Yb3+ ions by a phase-shaped femtosecond laser field. Photonics Res. 2019, 7, 486–492. [Google Scholar] [CrossRef]
- El-Maaref, A.A.; Wahab, E.A.A.; Shaaban, K.S.; Abdelawwad, M.; Koubisy, M.S.I.; Borcsok, J.; Yousef, E.S. Visible and mid-infrared spectral emissions and radiative rates calculations of Tm3+ doped BBLC glass. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 242, 118774. [Google Scholar] [CrossRef]
- Halubek-Gluchowska, K.; Szymanski, D.; Tran, T.N.L.; Ferrari, M.; Lukowiak, A. Upconversion Luminescence of Silica-Calcia Nanoparticles Co-doped with Tm3+ and Yb3+ Ions. Materials 2021, 14, 937. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Xu, J.; Zhang, Z.G.; Zhang, X.; Wang, L.Z.; Gai, S.L.; He, F.; Niu, N.; Zhang, M.L.; Yang, P.P. Rapid, morphologically controllable, large-scale synthesis of uniform Y(OH)3 and tunable luminescent properties of Y2O3:Yb3+/Ln3+ (Ln = Er, Tm and Ho). J. Mater. Chem. 2012, 22, 16136–16144. [Google Scholar] [CrossRef]
- Yi, Z.G.; Li, X.L.; Xue, Z.L.; Liang, X.; Lu, W.; Peng, H.; Liu, H.R.; Zeng, S.J.; Hao, J.H. Remarkable NIR Enhancement of Multifunctional Nanoprobes for In Vivo Trimodal Bioimaging and Upconversion Optical/T2-Weighted MRI-Guided Small Tumor Diagnosis. Adv. Funct. Mater. 2015, 25, 7119–7129. [Google Scholar] [CrossRef]
- Lahoz, F.; Martin, I.R.; Mendez-Ramos, J.; Nunez, P. Dopant distribution in a Tm3+-Yb3+ codoped silica based glass ceramic: An infrared-laser induced upconversion study. J. Chem. Phys. 2004, 120, 6180–6190. [Google Scholar] [CrossRef] [PubMed]
- Lupei, A.; Lupei, V.; Ikesue, A.; Gheorghe, C.; Hau, S. Nd → Yb energy transfer in (Nd, Yb):Y2O3 transparent ceramics. Opt. Mater. 2010, 32, 1333–1336. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorjevaite, J.; Katelnikovas, A. Optical Properties Investigation of Upconverting K2Gd(PO4)(WO4):20%Yb3+,Tm3+ Phosphors. Materials 2023, 16, 1305. https://doi.org/10.3390/ma16031305
Grigorjevaite J, Katelnikovas A. Optical Properties Investigation of Upconverting K2Gd(PO4)(WO4):20%Yb3+,Tm3+ Phosphors. Materials. 2023; 16(3):1305. https://doi.org/10.3390/ma16031305
Chicago/Turabian StyleGrigorjevaite, Julija, and Arturas Katelnikovas. 2023. "Optical Properties Investigation of Upconverting K2Gd(PO4)(WO4):20%Yb3+,Tm3+ Phosphors" Materials 16, no. 3: 1305. https://doi.org/10.3390/ma16031305
APA StyleGrigorjevaite, J., & Katelnikovas, A. (2023). Optical Properties Investigation of Upconverting K2Gd(PO4)(WO4):20%Yb3+,Tm3+ Phosphors. Materials, 16(3), 1305. https://doi.org/10.3390/ma16031305