Abstract
Emerging energy storage systems have received significant attention along with the development of renewable energy, thereby creating a green energy platform for humans. Lithium-ion batteries (LIBs) are commonly used, such as in smartphones, tablets, earphones, and electric vehicles. However, lithium has certain limitations including safety, cost-effectiveness, and environmental issues. Sodium is believed to be an ideal replacement for lithium owing to its infinite abundance, safety, low cost, environmental friendliness, and energy storage behavior similar to that of lithium. Inhered in the achievement in the development of LIBs, sodium-ion batteries (SIBs) have rapidly evolved to be commercialized. Among the cathode, anode, and electrolyte, the cathode remains a significant challenge for achieving a stable, high-rate, and high-capacity device. In this review, recent advances in the development and optimization of cathode materials, including inorganic, organometallic, and organic materials, are discussed for SIBs. In addition, the challenges and strategies for enhancing the stability and performance of SIBs are highlighted.
1. Introduction
The invention of batteries has played a key role in the development of miniaturized electrical devices. In particular, the use of lithium-ion batteries (LIBs) allows portable devices to continuously operate with no, or rarely occurring, disruptions [1]. LIBs are currently used in smartphones, tablets, notebooks, and vehicles. The significant achievement of LIBs is owing to the strong activity of lithium-ion insertion and desertion in storage materials with a high specific capacity (approximately 3860 mAh g−1) [2,3,4,5]. However, with an increase in capacity, various issues associated with LIBs need to be overcome, including safety, toxicity, and cost-effectiveness [6,7,8,9,10]. Meanwhile, sodium is abundantly available on Earth and has similar properties to lithium in storage devices, which is why it is receiving notable attention [11]. The use of sodium-ion batteries (SIBs) reduces the danger of lithium owing to its strong activation; furthermore, the cost and environmental issues can also be resolved [9,12,13,14,15,16,17,18,19,20,21,22]. Considering the development of LIBs, SIBs have become a promising alternative to LIBs. The working mechanisms of LIBs and SIBs are based on the storage of Li and Na ions in two materials with different potentials separated by an electrolyte, as shown in Figure 1. The insertion and desertion of Na ions in the anode and cathode through the electrolyte create and reduce the potential between the two electrodes, corresponding to charge and discharge processes, respectively. Anode materials can also undergo conversion reactions that react with Na ions, forming alloy states that allow high capacities, such as in expanded graphite (284 mAh g−1), TiO2-based anodes (200–300 mAh g−1), antimony sulfides (Sb2S3) (730 mAh g−1), Sn4P3 (>1100 mAh g−1), and phosphorous with a theoretical capacity of ~2596 mAh g−1, among others [23,24,25,26,27,28,29,30]. However, the development of a sodium cathode continues to present limitations such as an unstable and low capacity of 100–200 mAh g−1. SIB cathode materials include a variety of inorganic compounds (metal oxides, phosphates, pyrophosphates, etc.) and organic or organometallic materials [31,32]. Although achievements have been reported for SIBs and they are being commercialized, the current cathode material has been significantly improved and developed to have better electrochemical properties [33,34,35].
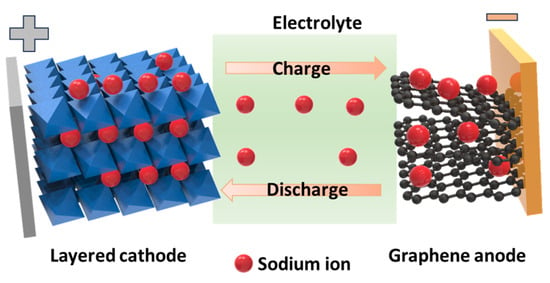
Figure 1.
Schematic of the simple operation of a sodium-ion battery employing a layered cathode and graphene anode.
In this review, we provide an overview of the current state of development of SIB cathode materials, including inorganic, organic, and organometallic materials. Recent advances in the development and optimization of these materials have been extensively discussed. In addition, the challenges and strategies related to enhancing the stability and performance of SIBs are highlighted.
2. Review of SIB Cathode Materials
2.1. Inorganic Compounds
2.1.1. Layered Oxide Materials (NaxMO2)
The layered oxide materials used for SIBs mostly consist of transition-metal oxides [36]. There are two common phases of NaMO2, which are the O3 and P2 phases, classified based on the different stacking of the oxygen ion frameworks as ABCABCABC (O3) or ABBAABBA (P2), as shown in Figure 2a,b [37,38]. In addition, the O2 phase and birnessite are the layered structures with the tightest and loosest packing, respectively, as shown in Figure 2c,d [39,40,41]. Among these phases, O3 phase can provide a high Na content and high specific capacity, which enables its application in full cells. However, the degradation of structure during cycling limits its application. To maintain structure, foreign metals with a large ionic radius such as Fe, Cr, Ti, and V can be used introduced [42]. On the other hand, P2 phase has a lower Na content but a wider layer spacing, which leads to faster diffusion of Na+ ions and improves structural stability during cycling. Similar to LIBs, compounds of Na with Co, Ni, and Mn oxides have layered structures, such as NaxCoO2, NaxNiO2, and NaxMnO2 [43,44,45,46]. However, owing to the large size of Na ions, the behavior of CoO6 or NiO6 in the lattice with the intercalation of Na varies from that of Li [47]. NaxCoO2 and NaxNiO2 compounds have exhibited low capacities below or near 100 mAh g−1 [48,49]. Reddy et al. fabricated P2-NaxCoO2 using the sol–gel method, capable of delivering a capacity of approximately 121 mAh g−1 at a rate of 0.1 C [50]. Similarly, NaNiO2 exhibits a capacity of only approximately 80 mAh g−1 [51]. Meanwhile, NaxMnO2 is a more promising cathode material owing to the multiple oxidation states of the Mn ions in the zigzag layers of the edge-sharing MnO6; therefore, this cathode exhibits a high theoretical capacity of approximately 240 mAh g−1 [52,53,54]. NaxMnO2 can be synthesized from either NaOH and Mn salt or MnO2. Ma et al. used monoclinic NaMnO2 as a cathode for SIBs and demonstrated a high first discharge capacity of approximately 185 mAh g−1 in the 2–3.8 V range [55]. Billaud et al. synthesized β-NaMnO2 which achieved a high capacity of approximately 190 mAh g−1 and retained a capacity of 100 mAh g−1 after 100 cycles at 2 C [56]. Kubota et al. investigated the effect of the voltage change on distorted O3-phase (O’3) NaMnO2 and found that a phase transition of NaMnO2 occurs above 3.52 V, leading to a decrease in crystallinity, thereby rapidly degrading the capacity during the cycling test [57].
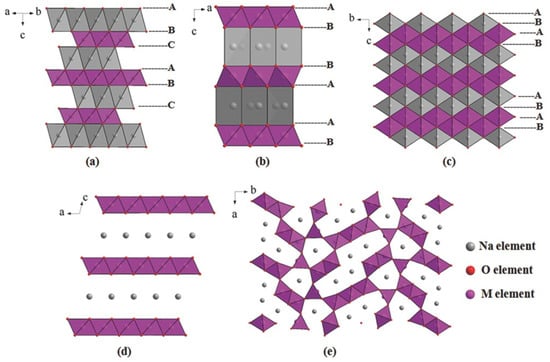
Figure 2.
Layered metal oxide: (a) O3-phase, (b) P2-phase, (c) O2-phase, (d) birnessite-type layered oxides and (e) tunnel oxides. The letters A, B, C are packing patterns of oxygen ions in an abc coordinate system. Reproduced from ref. [37]; Copyright 2015 Wiley-CH.
The drawback of layered materials is their unstable structure in air storage and during cycling; therefore, their capacities can be rapidly or irreversibly degraded [58,59]. Due to its hygroscopic nature, NaMO2 is unstable in air and in moist environments; therefore, its applications are limited. To improve the performance of NaMnO2, the partial replacement of Mn with other metals, such as Li, Ni, Co, Al, Fe, and Zn, has been investigated [60,61,62,63,64,65,66]. Kwon et al. proposed the use of a P2-NaLiMnO2 cathode material that exhibited a high reversible capacity of approximately 160 mAh g−1 [60]. The insertion of Li ions as dopants led to an inhomogeneous electrostatic repulsion between the Mn and Na ions, thereby enhancing the stability of β-Na0.7[Mn1-xLix]O2+y, which exhibited a stable cycling capacity for over 120 cycles without a faded capacity. Liu et al. investigated the use of P2-Na2/3Ni1/3Mn2/3O2 as a cathode material for SIBs simply synthesized via a novel sol–gel method (NSG) by employing polystyrene as an additive, as shown in Figure 3a [67]. The main active metal is Ni with Ni2+/Ni4+ states that contribute to the redox-pair peaks at a voltage between 3.0–4.0 V and a minor Mn3+/Mn4+ redox potential between at 2.0–3.0 V as shown in Figure 3b. Meanwhile, Mn4+ effectively maintains the structure of NaNiMnO2, thereby significantly improving its stability. At voltages below 2.0 V, the Mn4+ ions were activated and reduced to Mn3+, suffered a disproportional reaction, and dispersed into the electrolyte (Mn3+ solid → Mn4+ solid → Mn2+ electrolyte), and the redox at ~4.0–4.5 V was related to the phase transition from P2 to O2 phase due to the stacking faults, as shown in Figure 3c. Therefore, the material can be rapidly degraded below 2 V. The NSG Na2/3Ni1/3Mn2/3O2 cathode exhibited a reversible capacity of approximately 100 mAh g−1 and an excellent rate performance even at rates of 5 C and 10 C, as shown in Figure 3d–f. Nanthagopal et al. used NaFe0.5Mn0.5O2 as a cost-effective SIB cathode material which exhibited a specific capacity of approximately 170 mAh g−1 and retained a capacity of approximately 114 mAh g−1 after 100 cycles [61]. Liu et al. doped Al ions into NaMnO2 to form P2-Na0.67Al0.1Mn0.9O2 as a SIB cathode material [62]. The strong bonding of Al–O leads to enhanced Na spacing; therefore, Na ions can easily insert and desert into the cathode material. Hence, the presence of Al also reduces the Jahn–Teller effect of the phase transition between P2-P2′, which could cause structural defects and collapse during cycling [68]. Therefore, P2-NaAl0.1Mn0.9O2 can deliver a high capacity of 175 mAh g−1 with high stability and rate performance. Replacement with metals such as Ni, Co, Al, and Fe with higher redox states increases the average oxidation state of Mn ions (>3+), which mitigates the structural deterioration resulting from the Jahn–Teller effect and partially increases the redox potential [69]. For example, the redox potential of Mn3+/4+ is below 3.0 V, and the partial reduction of Mn3+ to Mn2+ leads to the dissolution of Mn2+ into electrolyte, resulting in structural degradation and reduced capacity. Introducing Fe ions into the structure causes a Fe3+/4+ redox between 3.0–4.0 V, increasing the average oxidation state of Mn ions and improving the stability. The higher redox potential of Fe3+/4+ also contributes to the working potential of the cathode material. Moreover, Mn and Fe are Earth-abundant elements that promise low-cost and environmentally friendly production. Similar to lithium-based layered metal oxides, the O3 phase of NaTMnOx (T = Ni, Co, Fe) has a high sodium content and provides a more stable layered oxide, making it applicable to full cells [70,71].
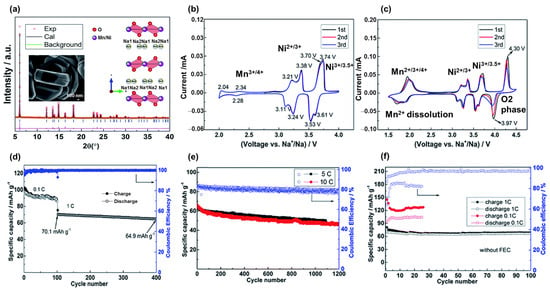
Figure 3.
(a) X-ray diffraction pattern and inset scanning electron microscopy image of the Na2/3Ni1/3Mn2/3O2 material. Cyclic voltammograms (CVs) at (b) 2.0–4.0 V and (c) 1.5–4.5 V. Cycling performances at (d) 0.1 C and 1 C for 400 cycles, (e) 5 C and 10 C for 1200 cycles, and (f) of the electrolyte without the additive (FEC). Reproduced with permission from ref. [67]; Copyright 2019 Royal Chemical Society.
A combination of more than three metals was also investigated, including NaLiNiMnCoO2, NaLiNiMnO2, NaFeMnTiVO2, and NaMnNiCuMgTiO2 [72,73,74,75]. Kataoka et al. prepared a multi-metal complex of NaLiNiMnCoO2 via co-precipitation and electrochemical ion-exchange methods [72]. The produced Na0.95Li0.15(Ni0.15Mn0.55Co)O2 was then employed as a highly stable cathode which delivered a capacity of greater than 200 mAh g−1 for over 40 cycles. Xu et al. investigated the effect of Li ions on NaLiNiMnO2 cathodes in SIBs and determined the importance of each element as follows [74]: The Ni metal was fully oxidized to Ni4+ to balance the overall charge of the cell, which also prevented the Jahn–Teller distortion owing to the active Mn3+. Moreover, Ni ions also contributed to the high-voltage redox state of the cathode, widening the range of the working potential from 2.0 to 4.4 V. Li ions were found surrounding Ni4+ through NMR resonance methods, which indicated that Li could easily migrate to this material. The remaining Li during cycling enhanced the capacity retention; therefore, this cathode delivered a high reversible capacity of 140 mAh g−1 in the 2.0–4.4 V range. Other elements have also been doped to improve the performance of Mn-based cathodes, such as boron-doped NaLiNiFeMnO2, Y-doped P2-type NaNiMnO2, and Mg-doped NaMnMgO2 [76,77,78].
In addition to Co-, Ni-, and Mn-based metal oxide cathodes, Cr-, Cu-, and Fe-based oxides have also received significant attention [79,80,81,82,83,84]. Yu et al. developed carbon-coated NaCrO2 as a SIB cathode via an emulsion-drying method that exhibited an excellent performance at a high rate of 50 C with a capacity of approximately 100 mAh g−1 [79]. The NaCrO2 cathode also demonstrated significant thermal stability up to 400 °C. At temperatures above 290 °C, instead of oxygen evolution owing to the thermal decomposition, NaCrO2 decomposed to Na0.5CrO2 and CrO2 phases. Moreover, Na0.5CrO2 continued to exhibit a stable layered structure from the insertion and desertion of the Na ions. NaxCuO2 and NaxFeO2 also have layered structures and deliver a capacity of approximately 100–200 mAh g−1 [80,81,85,86]. Lee et al. found that Fe3+/Fe4+ in NaxFeO2 was unstable during the redox process, leading to the formation of an octahedral structure, preventing the diffusion of Na ions and degrading the capacity [86]. A typical issue in layered metal oxide materials is the collapse of the structure during the insertion and desertion of sodium ions [37].
2.1.2. Tunnel Oxides
The NaxMO2 tunnel oxide consists of M4+ and M3+ ions at the MO6 and MO5 sites, respectively, as illustrated in Figure 2e [87,88,89,90]. The mixing of MO6 and MO5 creates a tunnel structure that allows Na+ ions to easily diffuse along the tunnels. This structure was first discovered by Parant et al. (1971) for NaxMnO2 (x < 1) [91]. It is worth noting that this structure was simply synthesized using various approaches, such as sol–gel, hydrothermal, spray pyrolysis, and microwave-assisted methods [92,93,94,95]. Na0.44MnO2 is the most noteworthy tunnel oxide owing to its large tunnels, high theoretical capacity of approximately 121 mAh g−1, and high stability [96,97]. He et al. used a polymer-pyrolysis method to fabricate Na0.44MnO2 nanoplates, which exhibited an outstanding capacity of approximately 96 mAh g−1 at a rate of 10 C [98]. However, the capacity of this material could not be improved owing to the fully charged and discharged states of the Na0.22MnO2 and Na0.66MnO2 phases, respectively [96]. Therefore, methods were developed to solve this problem, including cation/anion substitution and surface coating. In cation substitution, Mn4+ can be replaced by Ti, Fe, or Zr or by the partial replacement of Na with Li ions [99,100]. Shi et al. doped Zr ions in Na0.44MNO2 as a high-performance SIB cathode, which exhibited a high capacity of approximately 117 mAh g−1; at a high rate of 5 C, the capacity was reversible at approximately 97 mAh g−1, as shown in Figure 4 [101]. Defects at the Na, MnO6, and MnO5 sites create Na1 and Na2 in the S-shaped tunnels and Na3 in the smaller pentagonal tunnel, which allows the insertion and desertion of Na, thereby increasing the cycling performance, as illustrated in Figure 4a. As shown in Figure 4b, the insertion and desertion of the Na ions demonstrate six pairs of redox peaks with a small gap between each peak, allowing the rapid diffusion of Na ions. Therefore, the current rate and cycling performances were excellent at 10 C for over 100 cycles, as shown in Figure 4c,d. Zheng et al. used the composition of layered Na2Mn3O7 and Na0.44MnO2 as a SIB cathode, which delivered a high specific capacity of approximately 135 mAh g−1 and retained a capacity of 88% of the initial state after 100 cycles at 0.2 A g−1 [102]. Further improvement remains a challenge for scientists because of the change in structure owing to doping or the substitution of ions such as Co and Al [96]. Zhou et al. used Co-substituted Na0.44Mn1-xCoxO2 and found that the structure of the tunnel oxide changed to a layered structure [103]. The substitution of Al can form a mixture of the tunnel and layered phases in NaAl0.1Mn0.9O2 SIB cathodes [104]. In comparison to layered oxides, the tunnel oxides provide large channels for diffusion of Na ions, improving conductivity and stability. However, the low content of Na ions in the structure results in low specific capacity (~100 mAh g−1) and energy density. These drawbacks limit commercialization.
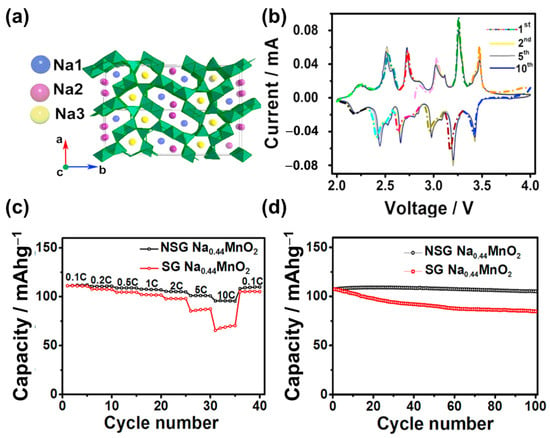
Figure 4.
(a) Illustration of orthorhombic Na0.44MnO2, (b) CV of (novel sol–gel-synthesized) NSG-Na0.44MnO2, (c) rate performance at current rates between 0.1–10 C, and (d) cycling performances of NSG-Na0.44MnO2 and (conventional sol–gel-synthesized) SG-Na0.44MnO2 at 0.5 C. Reproduced with permission from ref. [98]; Copyright 2016 Elsevier.
2.1.3. Polyanionic Compounds
Phosphate-Based Compound
Polyanionic compounds are generally constructed by a tetrahedral XO4 group with Na and Me (Fe, V, Co, or Mn) or MeOx [105,106,107,108]. Basically, olivine NaFePO4 consists of tetrahedral PO4 and octahedral FeO6 sites, forming a framework that holds Na ions in the lattice or allows the diffusion of Na ions [109]. NaFePO4 is a cost-effective material owing to its abundance of elements and high theoretical capacity of approximately 154 mAh g−1. NaFePO4 exists in two phases: maricite and olivine. The maricite phase is a stable structure with cavities that trap Na ions, preventing their diffusion [105,110]. Meanwhile, the less stable olivine phase has a one-dimensional channel, allowing the diffusion of Na ions through this pathway. Therefore, the olivine phase is more attractive, and improving the stability of this structure with various types of doping has also been investigated [58]. Wang et al. used the DFT simulation method to predict the effect of doping Li into NaFePO4 in both maricite and olivine phases [110]. The results demonstrated that when the Li:Na ratio was above 25%, the olivine phase was more stable than maricite, whereas the presence of Li destabilized the maricite structure. Ali et al. synthesized olivine NaFePO4 via an ion-exchange method from LiFePO4 for SIBs, which was then wrapped with polythiophene (PTh) to enhance the stability of the material [111]. NaFePO4 with PTh experiences the expansion of the insertion and restoration of its structure, which is indicated by the minuscule difference in the unit cell volume (from 320.6 to 320.3 Å3). Therefore, the NaFePO4/PTh cathode exhibits a high reversible capacity of approximately 142 mAh g−1. Altundag et al. used an electrochemical process to exchange Li with Na from LiFePO4 to NaFePO4, which delivered a capacity of approximately 74 mAh g−1. Olivine NaFePO4 structures are promising for SIBs; however, their fabrication remains a major obstacle that requires further investigation. Maricite NaCoPO4 (red phase) offered a high redox potential of Co2+/3+ at 4.0–4.6 V; however, the reversible capacity in a SIB was low at ~35 mAh g−1 [107]. Similarly, for NaMnPO4, it was also reported that the specific capacity of maricite phase exhibited a low capacity of ~40 mAh g−1 [108]. Priyanka et al. reported a different precursor (Mn) effect for the quality of NaMnPO4. A cathode prepared with a precursor from manganese acetate showed outstanding performance with an initial capacity over 100 mAh g−1 [112]. The high performance resulting from the acetate-based Mn precursor could be due to the decomposition of acetate creating a carbon source to enhance the conductivity and porosity of NaMnPO4. Venkatachalam et al. synthesized maricite NaMnPO4 with poly-ethylene glycol (PEG) and diethylene glycol (DEG), which can increase the specific capacity from 50 to ~100 mAh g−1 at 0.1 C [113]. The high contribution of carbon sources from PEG and DEG significantly enhanced the conductivity and protected the structure of this material. Phosphate compounds possess a high thermal stability (~600 °C); however, their major drawbacks are low electronic conductivity and low specific capacity, limiting their application in full-cell SIBs [114].
NASICON
A Na super-ionic conductor (NASICON) can be used as an electrolyte and electrode material owing to its 3D-open framework of NaxM2(PO4)3 (M = V, Fe, Ti, Nb, Zr) [115]. NASICON comprises MO6 and PO4 polyhedral sites in a framework that creates large channels for Na diffusion. This structure was first proposed by Hong and Goodenough in a Na1+xZr2P3-xSixO12 compound (P can be replaced by Si, S, Mo, and As) [116,117]. Owing to its high stability, high Na conductivity, and wide electrochemical windows (1.85–4.9 V vs. Na/Na+), NASICON is also applied as a solid electrolyte in SIBs [118]. The ion exchange of Zr4+ with Li+, K+, and Ag+ was first performed, while Si4+ was stabilized in the structure. As a complete NASICON with three full Na ions, Na3V2(PO4)3 (NVP) quickly received significant attention as a promising candidate material for providing a high probability of sodium insertion and desertion [119,120]. NVP has a theoretical capacity of ~117.6 mAh g−1 and a high redox voltage range of 3.3–3.4 V [121]. Therefore, with the modification process including the addition of conductive carbonaceous materials, NVP conductivity can be enhanced, exhibiting a notable rate performance [122]. Song et al. used a carbothermal reduction method to fabricate NVP, which exhibited a high capacity of approximately 117 mAh g−1 [123]. Cao et al. synthesized high-crystalline M-NVP/C nanoparticles using MIL-101 as the V source combined with an amorphous carbon layer, as shown in Figure 5a–c [124]. The M-NVP/C cathode delivered a high capacity of approximately 136 mAh g−1 and an excellent performance at high current rates of 1 C, 5 C, and 10 C for 1000 cycles, as shown in Figure 5d–h. In addition, the substitutions of Na, V, and P were found to be effective in improving its stability [125,126,127]. Lim et al. used the sol–gel method to produce Na3−xKxV2(PO4)3/C [125]. The use of K ions helped increase the Na-ion diffusion pathway and improved the stability and rate performance of NVP. Meanwhile, Mg replaced the V ions and improved the conductivity of the material; Mg0.07-NVP delivered a reversible capacity of approximately 113 mAh g−1 at 0.1 C and a high reversible capacity of 95 mAh g−1 at 10 C [127]. Pal et al. used Si-doped NVP as a replacement for P to form Na3.1V2(PO4)2.9(SiO4)0.1, which delivered an initial capacity of approximately 112 mAh g−1 and enhanced the capacity in comparison to NVP at high rates [126]. Fluorine is also favorable for substitution in the PO4 group owing to the larger ionicity of the metal when bonded with F compared to O. Moreover, F atoms were found to be compatible with the PO4 group in polyanionic compounds; therefore, the fluorine phosphate compound is believed to enhance the diffusion of Na+ ions [128]. Song et al. fabricated Na3V2(PO4)2F3 via a carbothermal reduction method as a SIB cathode material [129]. The existence of F in NVP changes the electrochemical behavior from a single redox couple at 3.06/3.72 V to a double redox couple at 3.24/3.91 and 3.83/4.26 V; therefore, the specific power density is improved.
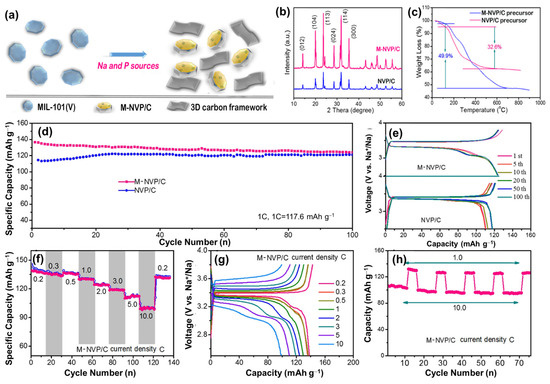
Figure 5.
(a) M-NVP/C synthesis scheme, (b) XRD, (c) thermogravimetric analysis plots, (d) cycling performance at 1 C, (e) voltage profiles of M-NVP and NVP/C; (f) rate performance, (g) voltage profiles at different C rates ranging from 0.2 C–10 C, and (h) rate performance at 1 C and 10 C of the M-NVP cathode. Reproduced with permission from ref. [124]; Copyright 2019 Elsevier.
2.1.4. Pyrophosphates
Pyrophosphate NaxMP2O7 consists of MO6 (M = V, Fe, Mn, Co, Ni) sites and a P2O7 group (interconnected PO4–PO4) that forms a framework with Na ions [130,131,132,133,134,135]. This framework allows the diffusion of Na ions; therefore, it is also a stable cathode material for SIBs. Barpanda et al. revealed that Na2FeP2O7 was constructed by corner-sharing FeO6–FeO6 to form Fe2O11, which combines with the P2O7 group to form a triclinic structure [136]. After calcination at temperatures above 560 °C, the triclinic Na2PeP2O7 transformed into a monoclinic phase, which improved the stability of this material during cycling. Kim et al. used the defect engineering of Na in Na2CoP2O7 to produce a high-voltage cathode for SIBs [137]. The deficiency of the Na-stabilized structure of Na2-xCoP2O7 (x > 0.2) was also found in Fe, Ni, and Mg pyrophosphates, such as Na1.66Fe1.17P2O7, Na1.82Ni1.09P2O7, and Na1.82Mg1.09P2O7 [138,139,140,141]. Specifically, Na2-xCo2P2O7 (x > 0.2) achieved a high average voltage of approximately 4.3 V versus Na/Na+ with a specific capacity of approximately 80 mAh g−1. Owing to the similar roles of the V, Fe, Mn, Co, and Ni transition metals in the structure, the replacement of a cheaper metal such as Fe and the improvement of the voltage by using Co and Ni in other pyrophosphate materials were investigated. Liu et al. investigated the use of Na2Mn3-xFex(P2O7)2 as a SIB cathode and revealed that the diffusion coefficient of Na ions improved by two orders of magnitude with x > 0.5 [142]; however, the capacity remained low at approximately 86 mAh g−1. Deng et al. fabricated a high-Na-content Na7V3(P2O7)4, which was also used as a high-voltage SIB cathode at an average voltage of approximately 4.0 V with a capacity of approximately 80 mAh g−1. Kumar et al. proposed the use of Mo2P2O11 as a SIB cathode with a high capacity of approximately 90 mAh g−1 [143]. The structure of Mo2P2O11 is also a framework of MoO6 sites with PO4 and P2O7 sites. Therefore, incomplete or shared atoms can form a tunnel phase in phosphates. Pyrophosphate-based cathode materials remain limited owing to their low capacity because the P2O7 group is large; therefore, the replacement of this group with PO4 and F could be an efficient method to enhance their capacity and stability [131,144,145]. Pu et al. fabricated a Na4Fe3(PO4)2P2O7/C nanosphere for SIB cathodes, which delivered a high capacity of approximately 128 mAh g−1 at 0.2 C and a remarkable rate performance at 100 C with a capacity of >70 mAh g−1 [144]. Kundu et al. used fluorine to modify pyrophosphates and produced Na4NiP2O7F2 as a high-voltage cathode for SIBs [145]. The presence of strong electronegative F− groups incorporated with P–O moieties increased the redox of Ni2+/Ni4+ owing to the high-charge region between 4.7 and 5.2 V. However, this material was not sufficiently stable to retain the capacity of the SIBs.
2.1.5. Silicates
Silicate compounds, such as lithium orthosilicate Li2FeSiO4 with a theoretical capacity of approximately 300 mAh g−1, generally have a higher theoretical capacity than other polyanions owing to their low molecular weight [146]. Similar to Li2FeSiO4, the sodium silicate Na2MSiO4 compound consists of MO4 (M = Fe, Ni, Mn, Co) and SiO4 sites, forming a framework that allows the diffusion of Na ions [147,148,149]. Silicates were previously popular in the glass industry owing to their high thermal and physical stabilities [150]. Co/Fe-compound sodium silicates were predicted to exhibit anti-site-exchange behavior, promising to be stable electrode materials [151,152]. Na2FeSiO4 is the most promising silicate compound, having a high theoretical capacity of approximately 276 mAh g−1 [153]. Kee et al. fabricated Na2FeSiO4 using a solvothermal method as a SIB cathode material, which exhibited a high initial capacity of approximately 126 mAh g−1 [154]. However, the material’s capacity quickly degrades owing to the collapse of the crystal into an amorphous structure. Meanwhile, Na2CoSiO4 demonstrated a better stability; however, its capacity was approximately 100–120 mAh g−1 [148,149,155]. Guan et al. resolved the stability issue of Na2FeSiO4 by introducing a fluorine dopant, which delivered a high capacity of approximately 270 mAh g−1 as a SIB cathode [156]. The presence of fluorine with strong electronegativity reduced the strain of the Na-hosting cathode, and the volume change during the charge and discharge process was only approximately 1.38%. Law et al. prepared Na2MnSiO4 that delivered a high capacity of approximately 210 mAh g−1 at 0.1 C and a reversible capacity of ~100 mAh g−1 at 5 C [157]. The excellent performance of Na2MnSiO4 was achieved owing to the additional vinylene carbonate in the electrolyte, which allowed the formation of a passivated layer during cycling, as shown in Figure 6. Therefore, the drawback of an unstable structure can be resolved by using a protective layer or surface passivation.
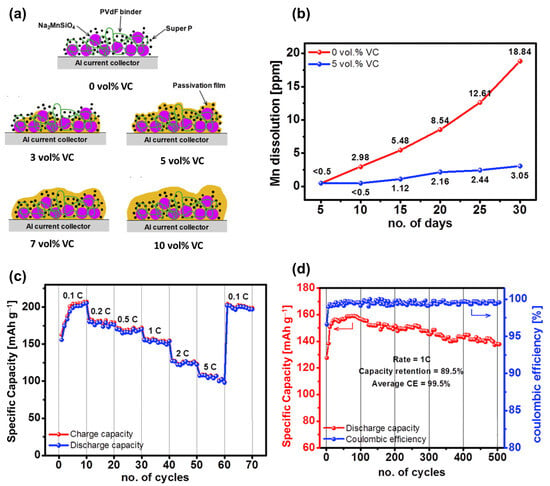
Figure 6.
(a) Illustration of the passivated layer on the Na2MnSiO4 cathode with additive vinylene carbonate (VC). (b) Mn dissolution of the Na2MnSiO4 electrode in electrolytes at room temperature. (c) Rate and (d) cycling performances of Na2MnSiO4 with 5 vol% VC at 1 C. Reproduced from ref. [157]; Copyright 2017 Elsevier.
2.2. Organic Compounds
The development of flexible devices and environmentally friendly materials has encouraged the application of organic compounds as cathode materials in energy storage systems, such as LIBs and SIBs [158]. Ranging from small molecules to high-molecular polymers, organic materials are promising for applications in green renewable energy in the future. For example, the molecular structure of Na4C8H2O6 (2,5-dihydroxyterephthalic acid, NaDTA) was investigated as a SIB cathode material at working potential windows of approximately 1.6–2.8 V versus Na/Na+ and delivered a high capacity of approximately 180 mAh g−1 [159]. NaDTA can also be used as an anode material with a capacity greater than 200 mAh g−1 owing to it binding up to six Na ions [160]. Kim et al. demonstrated the use of C6Cl4O2 (tetrachloro-1,4-benzoquinone) in a porous carbon template as a cathode of SIBs, as illustrated in Figure 7a,b [161]. The carbon skeleton-supported C6Cl4O2 cathode exhibited a high initial capacity of approximately 160 mAh g−1 and an average voltage of approximately 2.72 V, as shown in Figure 7c. Wang et al. produced a polymer from perylene 3,4,9,10-tetracarboxylic dianhydride, pyromellitic dianhydride (PMDA), and 1,4,5,8-naphthalenetetracarboxylic dianhydride, which contained C=O bindings, providing interactions with Na+ ions as a cathode for SIBs, as illustrated in Figure 7d [159]. This polymer demonstrated a high reversible capacity of approximately 150 mAh g−1 at a working voltage of 1.5–3.5 V and a long lifetime of over 5000 cycles, retaining 87.5% of the capacity in comparison to the initial cycle, as shown in Figure 7e,f. Shen et al. fabricated poly(diphenylaminesulfonic acid sodium) as a SIB cathode material, which delivered a reversible capacity of approximately 100 mAh g−1 at a high potential of 3.6 V versus Na/Na+ [162]. Wang et al. proposed an extended π-conjugated structure in sodium 4,4′-stilbene-dicarboxylate (SSDC) to provide an increased charge transport, which could easily enhance the rate performance of this organic cathode material in SIB by up to 10 A g−1 [163]. Organic cathodes can also be based on the insertion of anions such as ClO4−. Han et al. employed non-crystalline oligopyrene as a SIB cathode material, which is based on the insertion of ClO4− [164]. During cycling, pyrene was oxidized and reduced, allowing the interaction with charged ClO4−; therefore, each pyrene unit could store ClO4− anions. As a SIB cathode, oligopyrene exhibits a high theoretical reversible capacity of approximately 134 mAh g−1. Sakaushi et al. fabricated a dicyanobenzene-based aromatic porous honeycomb (APH) cathode with two types of storage mechanisms [165]. APH can store Na+ ions at voltages below 2.8 V. When the voltage is higher than 2.8 V, the APH cathode exhibits a p-doped region, which allows the insertion of ClO4− anions [165]. Therefore, APH has a wide working potential of 1.3–4.1 V versus Na/Na+ and a high reversible capacity greater than 120 mAh g−1 at 0.1 A g−1. Due to the electronic insulative nature of organic materials, they show a low conductivity, limiting their high rate performance. To overcome this limitation, cathode materials should contain high amount of carbon materials (30–60 wt%) to achieve a high rate performance [159,160,162,163,165].
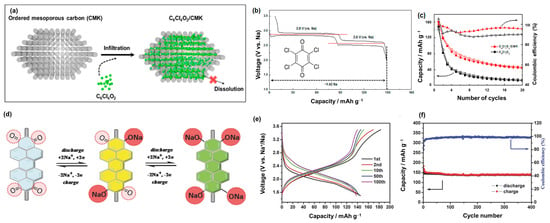
Figure 7.
(a) C6Cl4O2/CMK synthesis scheme, (b) voltage profiles of C6Cl4O2 at 10 mA g−1, and (c) cycling performance of C6Cl4O2 with and without CMK. Reproduced with permission from ref. [161]; Copyright 2015 American Chemical Society. (d) Schematic diagram of the redox mechanism, (e) voltage profiles, and (f) cycling performance of PTCDA-based PIs at 0.1 C. Reproduced with permission from ref. [159]; Copyright 2014 Wiley-CH.
2.3. Metal–Organic Compounds: Prussian Blue Analogs
The combination of inorganic and organic structures has received considerable attention owing to the advantages of both material types [166]. Inorganic materials have a stable structure and high conductivity, whereas organic materials are eco-friendly, easy to process, and safe to use. Recently, the development of organometallic materials in framework structures has introduced an advanced technique for material design, enabling the discovery of new composite properties for metals and organics. Metal–organic frameworks (MOF) can form a tremendous structure from various metal–organic compounds, providing large channels that allow the capture of ions or molecules; therefore, they have been used in various applications, including drug delivery, catalysis, and energy storage [167,168,169]. Simple and famous MOFs used for energy storage are Prussian blue analogs (PBAs), which are alkaline metal ferrocyanides AxMFe(CN)6 (A = Na, K; M = Fe, Mn, Co, Ni, Cu) [170]. The CN, Fe, and M matrices create a cage-like structure, holding the Na and K ions. PBAs generally exhibit a face-centered cubic structure (Fm3-m) [171,172,173]. The performance of PBAs in SIBs is based on the redox reactions of Fe2+/Fe3+ and the metal M, believed to have a high theoretical capacity of approximately 170 mAh g−1 for SIBs [174]. The basic PBA, which is Na4Fe(CN)6, contains the highest number of Na ions; however, it is a soluble compound that is easily degraded during cycling [175,176]. Therefore, Yang et al. demonstrated a solid solution of Na4Fe(CN)6/NaCl in a SIB that exhibited a capacity of approximately 75 mAh g−1 [177]. Qian et al. prepared a composition of Na4Fe(CN)6 with carbon, which also functioned as a SIB cathode [175]. By replacing Na ions with transition metals, the PBA structures were stabilized and widely used in SIBs [178]. Sun et al. used Fe3+ to form Fe4[Fe(CN)6]3 for a SIB cathode, which delivered a high capacity of approximately 146 mAh g−1 at 20 mA g−1 [179]. However, the rate performance and stability remained low; therefore, various types of transition metals, such as Ni, Cu, Mn, and V, have been used to create more stable and high-conductivity cathodes [180,181,182]. Song et al. fabricated Na2MnFe(CN)6 (Mn-PBA) and found that the removal of water molecules from the material significantly improved its performance, as shown in Figure 7 [183]. Mn-PBA and other PBAs naturally have monoclinic (Na-rich) or cubic structures that are partially supported by water molecules in their crystals. After removing the water molecules, Mn-PBA changed into a distorted framework or rhombohedral structure. Owing to the irreversibility of the monoclinic phase to the cubic phase, the Na per unit was reduced. Hence, the Jahn–Teller distortion of Mn3+ led to the degradation of the structure, thereby reducing the capacity, as illustrated in Figure 8a. It is worth noting that the rhombohedral phase allows the Na+ ions to be captured more efficiently; therefore, the water-removed Mn-PBA cathode enhanced the electrochemical performance, delivering a high capacity of approximately 150 mAh g−1 and a high rate performance even at 20 C, as shown in Figure 8b–d. However, dried Mn-PBA quickly absorbs water molecules; therefore, its fabrication and application remain limited. Hu et al. investigated the effect of Ni replacement in Mn-PBA as a SIB cathode [184]. Ni with a 10% replacement of Mn in Mn-PBA can enhance stability, delivering a capacity of approximately 110 mAh g−1 and a high rate performance. Xu et al. proposed a scalable preparation of Mn/Ni-PBA that delivered a capacity of approximately 100 mAh g−1 and an excellent rate performance, even at 100 C [185]. In particular, they reported that the activation in the first cycle at 4.8 V could significantly stabilize the structure, improving the cycling performance of the cathode. The presence of V, Co, and Ti increased the working potential of the PBA [186,187,188,189]. Takachi et al. used NaxCo[Fe(CN)6]0.9 as a SIB cathode, which delivered a capacity of approximately 135 mAh g−1 and demonstrated high-voltage redox couples of approximately 3.4 and 3.8 V versus Na/Na+ [190]. Baster reported that the replacement of Fe2+ with V2+ ions to form vanadium hexacyanoferrate (NaVHCF) exhibited a redox potential of ~2.3/3.6 V versus Na/Na+ [191]. Meanwhile, the presence of V–O binding in sodium vanadium hexacyanoferrate (NaVHCF) as a SIB cathode demonstrated only one redox potential [187]. Nguyen et al. reported that the presence of V–O passivated the high-spin Fe related to the low redox potential, supporting the structure of the low-spin Fe ion; therefore, NaVHCF provided a single redox potential of approximately 3.26/3.65 V versus Na/Na+ [192]. However, the capacity was only approximately 70–80 mAh g−1.
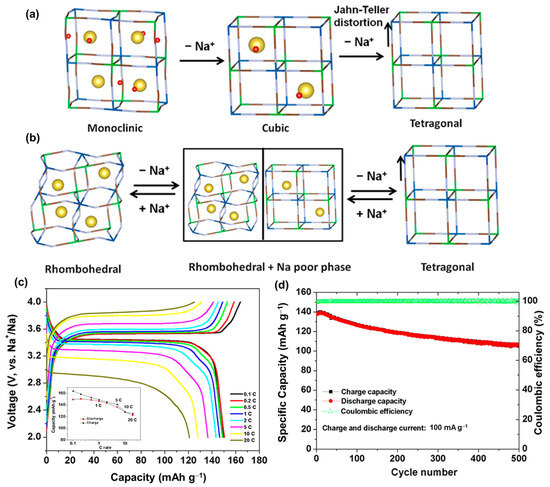
Figure 8.
(a) Schematic of the monoclinic, cubic M-PBA and (b) rhombohedral M-PBA with the insertion and desertion of Na+ ions. (c) Voltage profiles at different rates from 0.1–20 C and the (d) cycling performance of the rhombohedral M-PBA. Reproduced with permission from ref. [183]; Copyright 2015 American Chemical Society.
Other MOFs have received considerable attention as anode materials owing to their new structures and stable redox potentials [193]. However, the application to SIBs as cathode materials remains limited, which may be owing to the fact that a high-voltage cathode is difficult to obtain, and the selection of large organic groups can lead to a decrease in conductivity [194]. In addition, the MOF structure can be used as a template to form oxide, nitride, carbide, and sulfide materials as anode materials in SIBs and for the design of cathode materials [195]. Li et al. used MIL-53(Al) to dope Al into Na2FePO4/C as a SIB cathode, where MIL-53(Al) was used as a template to form a porous structure with a carbon cover [196]. It delivered a high capacity of approximately 115 mAh g−1 [196] with stable cyclability.
3. Discussion
LIBs have become popular in portable devices, vehicles, and energy storage systems for renewable energy. With the development of LIBs, a variety of cathode materials for SIBs have also recently evolved as listed in Table 1. Owing to the abundance of Na, SIBs are believed to be an ideal replacement for LIBs. As shown in Figure 9, each type of cathode material has its advantages and disadvantages. For instance, layered metal oxides have a high capacity and low cost but are sensitive to moisture and structural degradation. Prussian blue is more stable but the effect of water molecules in the structure affects its performance. Organic cathode materials have a good flexibility and stable redox potential but their lower conductivity, thermal stability, and dissolvability in the electrolyte should be resolved. Therefore, the advantages and disadvantages of each practical condition should be carefully considered. To improve their performance, the approach methods were also varied for each type of material. Due to an instability in structure of layered metal oxide cathodes, they were fast degraded during cycling. To stabilize structural stability, inactive metals such as V, Mg, Zn, and Ca can be doped to the lattice, or anions like F can be added [197,198]. Considering a tunnel metal oxide, control of the tunnel size optimizes its capacity. Meanwhile, for polyanionic compounds such as NASICON or other phosphate-based compounds, defect engineering can be considered, including metal- and F-doping methods [199]. Silicate compounds are low-cost and eco-friendly metal sources, and their high capacity needs to improve the structural stability before commercialization [154]. The surfaces of inorganic compounds can be passivated using a carbon-coating method that not only enhances their conductivity but also protects against the effects of humidity or expansion during the insertion of Na ions. The stability of Prussian blue and other organometallic compounds can be enhanced by using a host material such as Ni foam or a porous carbon skeleton [200]. Organic materials can be designed to have a good structure to enhance capacity and conductivity but they remain in the activation group with C=O, C=C, or C=N. Sulfurization and other cross-linking methods can also be considered to yield better combinations [201]. In addition, the use of additives in the electrolyte is another approach to enhance stability, in which the solid electrolyte interface from cycling can be used as a protective layer [202]. Along with the development of electrode materials and electrolytes, SIBs have been commercialized with layered oxides, polyanions, and Prussian blue types [32]. These materials are simple to manufacture (hydrothermal, co-precipitation method, etc.) and inexpensive, and they mainly use Mn and Fe metals and add Ni, Zn, or Mg, to increase stability, and conductive carbon is introduced for air stability and structural protection. Organic materials with low thermal stability and conductivity are utilized for some specific purposes that require biocompatible and/or specified applications. Therefore, it is considered that most of the developed materials have the potential to be commercialized if SIBs can solve current issues such as cost-effectiveness, high capacity, high stability, and high rate performance.

Table 1.
Summary of SIB cathode materials.
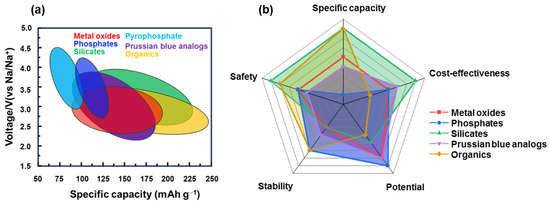
Figure 9.
Comparison of SIB cathode materials’ (a) specific capacity and working potential; (b) specific capacity, cost-effectiveness, potential, stability, and safety issues.
In summary, this review reveals the current developments in SIB cathode materials, such as layered metal oxides, tunnel metal oxides, phosphate-based compounds, organic compounds, Prussian blue analogs, and organometallic compounds. Many methods including anion/cation doping, composition, defect engineering, and structural design of SIB cathodes demonstrate their significant development to be comparable with LIBs. However, the development of SIBs can be further investigated to optimize the process or engineer the structure and design of cathode materials with high capacity, high voltage potential, and long life.
Author Contributions
T.P.N.: conceptualization, visualization, writing, review, and editing. I.T.K.: Project administration, funding acquisition, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2022R1F1A1062928). This research was also supported by the Basic Science Research Capacity Enhancement Project through a Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Ministry of Education (2019R1A6C1010016).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mahmud, S.; Rahman, M.; Kamruzzaman, M.; Ali, M.O.; Emon, M.S.A.; Khatun, H.; Ali, M.R. Recent advances in lithium-ion battery materials for improved electrochemical performance: A review. Results Eng. 2022, 15, 100472. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Z.N.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.Y.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Boebinger, M.G.; Yarema, O.; Yarema, M.; Unocic, K.A.; Unocic, R.R.; Wood, V.; McDowell, M.T. Spontaneous and reversible hollowing of alloy anode nanocrystals for stable battery cycling. Nat. Nanotechnol. 2020, 15, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and Challenges of Lithium Ion Batteries in Automotive Applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Walter, M.; Kovalenko, M.V.; Kravchyk, K.V. Challenges and benefits of post-lithium-ion batteries. New J. Chem. 2020, 44, 1677–1683. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Ming, F.; Alshareef, H.N. Sodium-ion battery anodes: Status and future trends. EnergyChem 2019, 1, 100012. [Google Scholar] [CrossRef]
- Li, X.F.; Dhanabalan, A.; Gu, L.; Wang, C.L. Three-Dimensional Porous Core-Shell Sn@Carbon Composite Anodes for High-Performance Lithium-Ion Battery Applications. Adv. Energy Mater. 2012, 2, 238–244. [Google Scholar] [CrossRef]
- Hatzell, K.B.; Chen, X.C.; Cobb, C.L.; Dasgupta, N.P.; Dixit, M.B.; Marbella, L.E.; McDowell, M.T.; Mukherjee, P.P.; Verma, A.; Viswanathan, V.; et al. Challenges in Lithium Metal Anodes for Solid-State Batteries. ACS Energy Lett. 2020, 5, 922–934. [Google Scholar] [CrossRef]
- Velumani, D.; Bansal, A. Thermal Behavior of Lithium- and Sodium-Ion Batteries: A Review on Heat Generation, Battery Degradation, Thermal Runway—Perspective and Future Directions. Energy Fuels 2022, 36, 14000–14029. [Google Scholar] [CrossRef]
- Ponnada, S.; Kiai, M.S.; Krishnapriya, R.; Singhal, R.; Sharma, R.K. Lithium-Free Batteries: Needs and Challenges. Energy Fuels 2022, 36, 6013–6026. [Google Scholar] [CrossRef]
- Abraham, K.M. How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Mosallanejad, B.; Malek, S.S.; Ershadi, M.; Daryakenari, A.A.; Cao, Q.; Boorboor Ajdari, F.; Ramakrishna, S. Cycling degradation and safety issues in sodium-ion batteries: Promises of electrolyte additives. J. Electroanal. Chem. 2021, 895, 115505. [Google Scholar] [CrossRef]
- Ellis, B.L.; Nazar, L.F. Sodium and sodium-ion energy storage batteries. Curr. Opin. Solid. State Mater. Sci. 2012, 16, 168–177. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, X.; Yang, Q.; Hou, L.; Mu, D.; Tan, G.; Li, L.; Chen, R.; Wu, F. The Progress in the Electrolytes for Solid State Sodium-Ion Battery. Adv. Mater. Technol. 2023, 8, 2200822. [Google Scholar] [CrossRef]
- Åvall, G.; Mindemark, J.; Brandell, D.; Johansson, P. Sodium-Ion Battery Electrolytes: Modeling and Simulations. Adv. Energy Mater. 2018, 8, 1703036. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Liang, C.; Yan, M.; Jiang, Y. An All-Prussian-Blue-Based Aqueous Sodium-Ion Battery. ChemElectroChem 2019, 6, 4848–4853. [Google Scholar] [CrossRef]
- Palomares, V.; Casas-Cabanas, M.; Castillo-Martínez, E.; Han, M.H.; Rojo, T. Update on Na-based battery materials: A growing research path. Energy Environ. Sci. 2013, 6, 2312–2337. [Google Scholar] [CrossRef]
- Li, Y.; Wu, F.; Li, Y.; Liu, M.; Feng, X.; Bai, Y.; Wu, C. Ether-based electrolytes for sodium ion batteries. Chem. Soc. Rev. 2022, 51, 4484–4536. [Google Scholar] [CrossRef]
- Wang, X.; Roy, S.; Shi, Q.; Li, Y.; Zhao, Y.; Zhang, J. Progress in and application prospects of advanced and cost-effective iron (Fe)-based cathode materials for sodium-ion batteries. J. Mater. Chem. A 2021, 9, 1938–1969. [Google Scholar] [CrossRef]
- Lee, J.M.; Singh, G.; Cha, W.; Kim, S.; Yi, J.; Hwang, S.-J.; Vinu, A. Recent Advances in Developing Hybrid Materials for Sodium-Ion Battery Anodes. ACS Energy Lett. 2020, 5, 1939–1966. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-Ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Wen, Y.; He, K.; Zhu, Y.; Han, F.; Xu, Y.; Matsuda, I.; Ishii, Y.; Cumings, J.; Wang, C. Expanded graphite as superior anode for sodium-ion batteries. Nat. Commun. 2014, 5, 4033. [Google Scholar] [CrossRef]
- He, J.; Wei, Y.; Zhai, T.; Li, H. Antimony-based materials as promising anodes for rechargeable lithium-ion and sodium-ion batteries. Mater. Chem. Front. 2018, 2, 437–455. [Google Scholar] [CrossRef]
- Yu, D.Y.W.; Prikhodchenko, P.V.; Mason, C.W.; Batabyal, S.K.; Gun, J.; Sladkevich, S.; Medvedev, A.G.; Lev, O. High-capacity antimony sulphide nanoparticle-decorated graphene composite as anode for sodium-ion batteries. Nat. Commun. 2013, 4, 2922. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Du, H.-L.; Yun, B.-N.; Jeong, M.-G.; Kim, J.-S.; Kim, H.; Jung, H.-G.; Sun, Y.-K. Carbon-Free TiO2 Microspheres as Anode Materials for Sodium Ion Batteries. ACS Energy Lett. 2019, 4, 494–501. [Google Scholar] [CrossRef]
- Ni, J.; Li, L.; Lu, J. Phosphorus: An Anode of Choice for Sodium-Ion Batteries. ACS Energy Lett. 2018, 3, 1137–1144. [Google Scholar] [CrossRef]
- Durai, L.; Gopalakrishnan, A.; Badhulika, S. Solid-state synthesis of β-NaAlO2 nanoflakes as an anode material for high-performance sodium-ion batteries. Mater. Chem. Front. 2022, 6, 2913–2920. [Google Scholar] [CrossRef]
- Nam, K.-H.; Hwa, Y.; Park, C.-M. Zinc Phosphides as Outstanding Sodium-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2020, 12, 15053–15062. [Google Scholar] [CrossRef]
- Xu, H.; Chen, H.; Gao, C. Advanced Graphene Materials for Sodium/Potassium/Aluminum-Ion Batteries. ACS Mater. Lett. 2021, 3, 1221–1237. [Google Scholar] [CrossRef]
- He, M.; Davis, R.; Chartouni, D.; Johnson, M.; Abplanalp, M.; Troendle, P.; Suetterlin, R.-P. Assessment of the first commercial Prussian blue based sodium-ion battery. J. Power Sources 2022, 548, 232036. [Google Scholar] [CrossRef]
- Yadav, P.; Shelke, V.; Patrike, A.; Shelke, M. Sodium-based batteries: Development, commercialization journey and new emerging chemistries. Oxf. Open Mater. Sci. 2022, 3, itac019. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Q.; Ding, X.; Wang, Y.; Xin, Y.; Singh, P.; Wu, F.; Gao, H. The prospect and challenges of sodium-ion batteries for low-temperature conditions. Interdiscip. Mater. 2022, 1, 373–395. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, T.; Li, W.; Li, T.; Zhang, L.; Zhang, X.; Wang, Z. Engineering of Sodium-Ion Batteries: Opportunities and Challenges. Engineering, 2022; in press. [Google Scholar] [CrossRef]
- Xie, J.; Gu, P.; Zhang, Q. Nanostructured Conjugated Polymers: Toward High-Performance Organic Electrodes for Rechargeable Batteries. ACS Energy Lett. 2017, 2, 1985–1996. [Google Scholar] [CrossRef]
- Zuo, W.; Innocenti, A.; Zarrabeitia, M.; Bresser, D.; Yang, Y.; Passerini, S. Layered Oxide Cathodes for Sodium-Ion Batteries: Storage Mechanism, Electrochemistry, and Techno-economics. Acc. Chem. Res. 2023, 56, 284–296. [Google Scholar] [CrossRef]
- Xiang, X.; Zhang, K.; Chen, J. Recent Advances and Prospects of Cathode Materials for Sodium-Ion Batteries. Adv. Mater. 2015, 27, 5343–5364. [Google Scholar] [CrossRef]
- Stansby, J.H.; Sharma, N.; Goonetilleke, D. Probing the charged state of layered positive electrodes in sodium-ion batteries: Reaction pathways, stability and opportunities. J. Mater. Chem. A 2020, 8, 24833–24867. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, M.; Dou, Y.; Chen, S.; Liu, P.; Yin, H.; Zhao, H. Manganese oxides transformed from orthorhombic phase to birnessite with enhanced electrochemical performance as supercapacitor electrodes. J. Mater. Chem. A 2020, 8, 3746–3753. [Google Scholar] [CrossRef]
- Luo, J.; Huang, A.; Park, S.H.; Suib, S.L.; O’Young, C.-L. Crystallization of Sodium-Birnessite and Accompanied Phase Transformation. Chem. Mater. 1998, 10, 1561–1568. [Google Scholar] [CrossRef]
- Chen, S.; Liao, Z.; Kang, J.; Zhang, Y.; Zhi, S.; Cai, X.; Yang, W.; Zou, H.; Yang, W. Enhanced cyclic performance of O2-type Mn-based layered oxide via Al doping for lithium-ion battery. J. Alloys Compd. 2022, 910, 164793. [Google Scholar] [CrossRef]
- Song, T.; Chen, L.; Gastol, D.; Dong, B.; Marco, J.F.; Berry, F.; Slater, P.; Reed, D.; Kendrick, E. High-Voltage Stabilization of O3-Type Layered Oxide for Sodium-Ion Batteries by Simultaneous Tin Dual Modification. Chem. Mater. 2022, 34, 4153–4165. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Wang, C.; Ahn, H.-J.; Wang, G. Single Crystalline Na0.7MnO2 Nanoplates as Cathode Materials for Sodium-Ion Batteries with Enhanced Performance. Chem. Eur. J. 2013, 19, 10884–10889. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Fukuzumi, Y.; Kobayashi, W.; Moritomo, Y. Fast discharge process of layered cobalt oxides due to high Na+ diffusion. Sci. Rep. 2015, 5, 9006. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, W.; Liu, W. First-principles calculations of the Ti-doping effects on layered NaNiO2 cathode materials for advanced Na-ion batteries. J. Indian. Chem. Soc. 2022, 99, 100424. [Google Scholar] [CrossRef]
- Kanwade, A.; Gupta, S.; Kankane, A.; Tiwari, M.K.; Srivastava, A.; Kumar Satrughna, J.A.; Chand Yadav, S.; Shirage, P.M. Transition metal oxides as a cathode for indispensable Na-Ion batteries. RSC Adv. 2022, 12, 23284–23310. [Google Scholar] [CrossRef] [PubMed]
- Heubner, C.; Matthey, B.; Lein, T.; Wolke, F.; Liebmann, T.; Lämmel, C.; Schneider, M.; Herrmann, M.; Michaelis, A. Insights into the electrochemical Li/Na-exchange in layered LiCoO2 cathode material. Energy Stor. Mater. 2020, 27, 377–386. [Google Scholar] [CrossRef]
- Rai, A.K.; Anh, L.T.; Gim, J.; Mathew, V.; Kim, J. Electrochemical properties of NaxCoO2 (x~0.71) cathode for rechargeable sodium-ion batteries. Ceram. Int. 2014, 40, 2411–2417. [Google Scholar] [CrossRef]
- Vassilaras, P.; Ma, X.; Li, X.; Ceder, G. Electrochemical Properties of Monoclinic NaNiO2. J. Electrochem. Soc. 2013, 160, A207. [Google Scholar] [CrossRef]
- Rami Reddy, B.V.; Ravikumar, R.; Nithya, C.; Gopukumar, S. High performance NaxCoO2 as a cathode material for rechargeable sodium batteries. J. Mater. Chem. A 2015, 3, 18059–18063. [Google Scholar] [CrossRef]
- Park, K.; Yu, B.-C.; Goodenough, J.B. Electrochemical and Chemical Properties of Na2NiO2 as a Cathode Additive for a Rechargeable Sodium Battery. Chem. Mater. 2015, 27, 6682–6688. [Google Scholar] [CrossRef]
- Chen, T.; Ouyang, B.; Fan, X.; Zhou, W.; Liu, W.; Liu, K. Oxide cathodes for sodium-ion batteries: Designs, challenges, and perspectives. Carbon. Energy 2022, 4, 170–199. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, Z.; Yang, Y.; Shi, W. First-principles investigation of the monoclinic NaMnO2 cathode material for rechargeable Na-ion batteries. Curr. Appl. Phys. 2018, 18, 1431–1435. [Google Scholar] [CrossRef]
- Palluzzi, M.; Silvestri, L.; Celeste, A.; Tuccillo, M.; Latini, A.; Brutti, S. Structural Degradation of O3-NaMnO2 Positive Electrodes in Sodium-Ion Batteries. Crystals 2022, 12, 885. [Google Scholar] [CrossRef]
- Ma, X.; Chen, H.; Ceder, G. Electrochemical Properties of Monoclinic NaMnO2. J. Electrochem. Soc. 2011, 158, A1307. [Google Scholar] [CrossRef]
- Billaud, J.; Clément, R.J.; Armstrong, A.R.; Canales-Vázquez, J.; Rozier, P.; Grey, C.P.; Bruce, P.G. β-NaMnO2: A High-Performance Cathode for Sodium-Ion Batteries. J. Am. Chem. Soc. 2014, 136, 17243–17248. [Google Scholar] [CrossRef]
- Kubota, K.; Miyazaki, M.; Kim, E.J.; Yoshida, H.; Barpanda, P.; Komaba, S. Structural change induced by electrochemical sodium extraction from layered O′3-NaMnO2. J. Mater. Chem. A 2021, 9, 26810–26819. [Google Scholar] [CrossRef]
- Xiao, J.; Li, X.; Tang, K.; Wang, D.; Long, M.; Gao, H.; Chen, W.; Liu, C.; Liu, H.; Wang, G. Recent progress of emerging cathode materials for sodium ion batteries. Mater. Chem. Front. 2021, 5, 3735–3764. [Google Scholar] [CrossRef]
- Gupta, P.; Pushpakanth, S.; Haider, M.A.; Basu, S. Understanding the Design of Cathode Materials for Na-Ion Batteries. ACS Omega 2022, 7, 5605–5614. [Google Scholar] [CrossRef]
- Kwon, M.-S.; Lim, S.G.; Park, Y.; Lee, S.-M.; Chung, K.Y.; Shin, T.J.; Lee, K.T. P2 Orthorhombic Na0.7[Mn1−xLix]O2+y as Cathode Materials for Na-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 14758–14768. [Google Scholar] [CrossRef]
- Nanthagopal, M.; Ho, C.W.; Shaji, N.; Sim, G.S.; Varun Karthik, M.; Kim, H.K.; Lee, C.W. Enhanced NaFe0.5Mn0.5O2/C Nanocomposite as a Cathode for Sodium-Ion Batteries. Nanomaterials 2022, 12, 984. [Google Scholar] [CrossRef]
- Liu, X.; Zuo, W.; Zheng, B.; Xiang, Y.; Zhou, K.; Xiao, Z.; Shan, P.; Shi, J.; Li, Q.; Zhong, G.; et al. P2-Na0.67AlxMn1−xO2: Cost-Effective, Stable and High-Rate Sodium Electrodes by Suppressing Phase Transitions and Enhancing Sodium Cation Mobility. Angew. Chem. Int. Ed. 2019, 58, 18086–18095. [Google Scholar] [CrossRef]
- Abou-Rjeily, J.; Bezza, I.; Laziz, N.A.; Neacsa, D.; Autret-Lambert, C.; Ghamouss, F. P2-Na0.67Mn0.85Al0.15O2 and NaMn2O4 Blend as Cathode Materials for Sodium-Ion Batteries Using a Natural β-MnO2 Precursor. ACS Omega 2021, 6, 1064–1072. [Google Scholar] [CrossRef]
- Shi, Q.; Qi, R.; Feng, X.; Wang, J.; Li, Y.; Yao, Z.; Wang, X.; Li, Q.; Lu, X.; Zhang, J.; et al. Niobium-doped layered cathode material for high-power and low-temperature sodium-ion batteries. Nat. Commun. 2022, 13, 3205. [Google Scholar] [CrossRef]
- Zuo, W.; Liu, X.; Qiu, J.; Zhang, D.; Xiao, Z.; Xie, J.; Ren, F.; Wang, J.; Li, Y.; Ortiz, G.F.; et al. Engineering Na+-layer spacings to stabilize Mn-based layered cathodes for sodium-ion batteries. Nat. Commun. 2021, 12, 4903. [Google Scholar] [CrossRef] [PubMed]
- Clément, R.J.; Bruce, P.G.; Grey, C.P. Review—Manganese-Based P2-Type Transition Metal Oxides as Sodium-Ion Battery Cathode Materials. J. Electrochem. Soc. 2015, 162, A2589. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Chen, M.; Zou, C.; Jin, H.; Wang, S.; Gu, Q.; Chou, S. P2-type Na2/3Ni1/3Mn2/3O2 as a cathode material with high-rate and long-life for sodium ion storage. J. Mater. Chem. A 2019, 7, 9215–9221. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Z.; Wu, M.; Xu, B.; Ouyang, C. Jahn–Teller type small polaron assisted Na diffusion in NaMnO2 as a cathode material for Na-ion batteries. J. Mater. Chem. A 2019, 7, 6053–6061. [Google Scholar] [CrossRef]
- Nathan, M.G.T.; Yu, H.; Kim, G.-T.; Kim, J.-H.; Cho, J.S.; Kim, J.; Kim, J.-K. Recent Advances in Layered Metal-Oxide Cathodes for Application in Potassium-Ion Batteries. Adv. Sci. 2022, 9, 2105882. [Google Scholar] [CrossRef]
- Xu, J.; Liu, H.; Meng, Y.S. Exploring Li substituted O3-structured layered oxides NaLixNi1/3−xMn1/3+xCo1/3−xO2 (x = 0.07, 0.13, and 0.2) as promising cathode materials for rechargeable Na batteries. Electrochem. Commun. 2015, 60, 13–16. [Google Scholar] [CrossRef]
- Liu, H.; Xu, J.; Ma, C.; Meng, Y.S. A new O3-type layered oxide cathode with high energy/power density for rechargeable Na batteries. Chem. Commun. 2015, 51, 4693–4696. [Google Scholar] [CrossRef]
- Kataoka, R.; Mukai, T.; Yoshizawa, A.; Sakai, T. Development of High Capacity Cathode Material for Sodium Ion Batteries Na0.95Li0.15(Ni0.15Mn0.55Co0.1)O2. J. Electrochem. Soc. 2013, 160, A933–A939. [Google Scholar] [CrossRef]
- Banik, T.; Bhattacharya, I. Novel P2-Type Na0.66Fe0.5-2xMn0.5TixVxO2 Cathode for High-Capacity. In Electrochemical Society Meeting Abstracts 240; MA2021-02; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2021; p. 238. [Google Scholar] [CrossRef]
- Xu, J.; Lee, D.H.; Clément, R.J.; Yu, X.; Leskes, M.; Pell, A.J.; Pintacuda, G.; Yang, X.-Q.; Grey, C.P.; Meng, Y.S. Identifying the Critical Role of Li Substitution in P2–Nax[LiyNizMn1−y−z]O2 (0 < x, y, z < 1) Intercalation Cathode Materials for High-Energy Na-Ion Batteries. Chem. Mater. 2014, 26, 1260–1269. [Google Scholar] [CrossRef]
- Fu, F.; Liu, X.; Fu, X.; Chen, H.; Huang, L.; Fan, J.; Le, J.; Wang, Q.; Yang, W.; Ren, Y.; et al. Entropy and crystal-facet modulation of P2-type layered cathodes for long-lasting sodium-based batteries. Nat. Commun. 2022, 13, 2826. [Google Scholar] [CrossRef]
- Guo, Y.-J.; Wang, P.-F.; Niu, Y.-B.; Zhang, X.-D.; Li, Q.; Yu, X.; Fan, M.; Chen, W.-P.; Yu, Y.; Liu, X.; et al. Boron-doped sodium layered oxide for reversible oxygen redox reaction in Na-Ion battery cathodes. Nat. Commun. 2021, 12, 5267. [Google Scholar] [CrossRef]
- Kim, S.; Min, K.; Park, K. Y-doped P2-type Na0.67Ni0.33Mn0.67O2: A sodium-ion battery cathode with fast charging and enhanced cyclic performance. J. Alloys Compd. 2021, 874, 160027. [Google Scholar] [CrossRef]
- Clément, R.J.; Billaud, J.; Robert Armstrong, A.; Singh, G.; Rojo, T.; Bruce, P.G.; Grey, C.P. Structurally stable Mg-doped P2-Na2/3Mn1−yMgyO2 sodium-ion battery cathodes with high rate performance: Insights from electrochemical, NMR and diffraction studies. Energy Environ. Sci. 2016, 9, 3240–3251. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Park, J.-S.; Jung, H.-G.; Chung, K.-Y.; Aurbach, D.; Sun, Y.-K.; Myung, S.-T. NaCrO2 cathode for high-rate sodium-ion batteries. Energy Environ. Sci. 2015, 8, 2019–2026. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Yoshida, H.; Komaba, S. Crystal Structures and Electrode Performance of Alpha-NaFeO2 for Rechargeable Sodium Batteries. Electrochemistry 2012, 80, 716–719. [Google Scholar] [CrossRef]
- Ono, Y.; Yui, Y.; Hayashi, M.; Asakura, K.; Kitabayashi, H.; Takahashi, K.I. Electrochemical Properties of NaCuO2 for Sodium-Ion Secondary Batteries. ECS Trans. 2014, 58, 33–39. [Google Scholar] [CrossRef]
- Liang, J.; Liu, L.; Liu, X.; Meng, X.; Zeng, L.; Liu, J.; Li, J.; Shi, Z.; Yang, Y. O3-Type NaCrO2 as a Superior Cathode Material for Sodium/Potassium-Ion Batteries Ensured by High Structural Reversibility. ACS Appl. Mater. Interfaces 2021, 13, 22635–22645. [Google Scholar] [CrossRef]
- Myung, S.-T.; Park, J.s.; Jung, H.-G.; Chung, K.Y.; Aurbach, D.; Yu, C.-y.; Sun, Y.-K. NaCrO2 Cathode for High-Rate Sodium-Ionbatteries. In Electrochemical Society Meeting Abstracts 230; MA2016-02; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2016; p. 664. [Google Scholar] [CrossRef]
- Wang, Z.; Shaw, L. Doping of NaCrO2 Cathode Material to Enhance Electrochemical Performance for Sodium-Ion Batteries. In Electrochemical Society Meeting Abstracts 239; MA2021-01; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2021; p. 356. [Google Scholar] [CrossRef]
- Ono, Y. Structural Analysis of NaCuO2 Cathode at Various Charged/Discharged Stages and Its Reaction Mechanism. Electrochemistry 2018, 86, 309–314. [Google Scholar] [CrossRef]
- Lee, E.; Brown, D.E.; Alp, E.E.; Ren, Y.; Lu, J.; Woo, J.-J.; Johnson, C.S. New Insights into the Performance Degradation of Fe-Based Layered Oxides in Sodium-Ion Batteries: Instability of Fe3+/Fe4+ Redox in α-NaFeO2. Chem. Mater. 2015, 27, 6755–6764. [Google Scholar] [CrossRef]
- Feng, J.; Luo, S.; Cai, K.; Yan, S.; Wang, Q.; Zhang, Y.; Liu, X. Research progress of tunnel-type sodium manganese oxide cathodes for SIBs. Chin. Chem. Lett. 2022, 33, 2316–2326. [Google Scholar] [CrossRef]
- Byles, B.; Pomerantseva, E. Stabilization of Tunnel Manganese Oxide Electrodes in Li-Ion and Na-Ion Batteries. In Electrochemical Society Meeting Abstracts 233; MA2018-01; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2018; p. 2581. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Lee, B.; Qiao, R.; Yang, Z.; Xu, S.; Yu, X.; Gu, L.; Hu, Y.-S.; Yang, W.; et al. Ti-substituted tunnel-type Na0.44MnO2 oxide as a negative electrode for aqueous sodium-ion batteries. Nat. Commun. 2015, 6, 6401. [Google Scholar] [CrossRef]
- Oz, E.; Altin, S.; Avci, S. Tunnel/Layer Composite Na0.44MnO2 Cathode Material with Enhanced Structural Stability via Cobalt Doping for Sodium-Ion Batteries. ACS Omega 2023, 8, 27170–27178. [Google Scholar] [CrossRef]
- Parant, J.-P.; Olazcuaga, R.; Devalette, M.; Fouassier, C.; Hagenmuller, P. Sur quelques nouvelles phases de formule NaxMnO2 (x ≤ 1). J. Solid. State Chem. 1971, 3, 1–11. [Google Scholar] [CrossRef]
- Hosono, E.; Matsuda, H.; Honma, I.; Fujihara, S.; Ichihara, M.; Zhou, H. Synthesis of single crystalline electro-conductive Na0.44MnO2 nanowires with high aspect ratio for the fast charge–discharge Li ion battery. J. Power Sources 2008, 182, 349–352. [Google Scholar] [CrossRef]
- Zhou, X.; Guduru, R.K.; Mohanty, P. Synthesis and characterization of Na0.44MnO2 from solution precursors. J. Mater. Chem. A 2013, 1, 2757–2761. [Google Scholar] [CrossRef]
- Shen, K.-Y.; Lengyel, M.; Wang, L.; Axelbaum, R.L. Spray pyrolysis and electrochemical performance of Na0.44MnO2 for sodium-ion battery cathodes. MRS Commun. 2017, 7, 74–77. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, H.; Huang, Y.; Kan, S.; Wu, Y.; Bu, M.; Liu, Y.; He, P.; Liu, H. Engineering sodium-rich manganese oxide with robust tunnel structure for high-performance sodium-ion battery cathode application. Chem. Eng. J. 2021, 417, 128097. [Google Scholar] [CrossRef]
- Chae, M.S.; Elias, Y.; Aurbach, D. Tunnel-Type Sodium Manganese Oxide Cathodes for Sodium-Ion Batteries. ChemElectroChem 2021, 8, 798–811. [Google Scholar] [CrossRef]
- Kim, D.J.; Ponraj, R.; Kannan, A.G.; Lee, H.-W.; Fathi, R.; Ruffo, R.; Mari, C.M.; Kim, D.K. Diffusion behavior of sodium ions in Na0.44MnO2 in aqueous and non-aqueous electrolytes. J. Power Sources 2013, 244, 758–763. [Google Scholar] [CrossRef]
- He, X.; Wang, J.; Qiu, B.; Paillard, E.; Ma, C.; Cao, X.; Liu, H.; Stan, M.C.; Liu, H.; Gallash, T.; et al. Durable high-rate capability Na0.44MnO2 cathode material for sodium-ion batteries. Nano Energy 2016, 27, 602–610. [Google Scholar] [CrossRef]
- Guo, S.; Yu, H.; Liu, D.; Tian, W.; Liu, X.; Hanada, N.; Ishida, M.; Zhou, H. A novel tunnel Na0.61Ti0.48Mn0.52O2 cathode material for sodium-ion batteries. Chem. Commun. 2014, 50, 7998–8001. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Ben, L.; Lyu, Y.; Song, N.; Yang, Z.; Li, Y.; Mu, L.; Yang, H.-T.; Gu, L.; et al. Fe-Based Tunnel-Type Na0.61[Mn0.27Fe0.34Ti0.39]O2 Designed by a New Strategy as a Cathode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1501156. [Google Scholar] [CrossRef]
- Shi, W.-J.; Zheng, Y.-M.; Meng, X.-M.; Liu, S.-B.; Xu, S.-D.; Chen, L.; Wang, X.-M.; Zhang, D. Designing Sodium Manganese Oxide with 4 d-Cation Zr Doping as a High-Rate-Performance Cathode for Sodium-Ion Batteries. ChemElectroChem 2020, 7, 2545–2552. [Google Scholar] [CrossRef]
- Zheng, P.; Su, J.; Wang, Y.; Zhou, W.; Song, J.; Su, Q.; Reeves-McLaren, N.; Guo, S. A High-Performance Primary Nanosheet Heterojunction Cathode Composed of Na0.44MnO2 Tunnels and Layered Na2Mn3O7 for Na-Ion Batteries. ChemSusChem 2020, 13, 1793–1799. [Google Scholar] [CrossRef]
- Zhou, Y.-T.; Sun, X.; Zou, B.-K.; Liao, J.-Y.; Wen, Z.-Y.; Chen, C.-H. Cobalt-substituted Na0.44Mn1-xCoxO2: Phase evolution and a high capacity positive electrode for sodium-ion batteries. Electrochim. Acta 2016, 213, 496–503. [Google Scholar] [CrossRef]
- Han, D.-W.; Ku, J.-H.; Kim, R.-H.; Yun, D.-J.; Lee, S.-S.; Doo, S.-G. Aluminum Manganese Oxides with Mixed Crystal Structure: High-Energy-Density Cathodes for Rechargeable Sodium Batteries. ChemSusChem 2014, 7, 1870–1875. [Google Scholar] [CrossRef]
- Tang, W.; Song, X.; Du, Y.; Peng, C.; Lin, M.; Xi, S.; Tian, B.; Zheng, J.; Wu, Y.; Pan, F.; et al. High-performance NaFePO4 formed by aqueous ion-exchange and its mechanism for advanced sodium ion batteries. J. Mater. Chem. A 2016, 4, 4882–4892. [Google Scholar] [CrossRef]
- Ling, M.; Lv, Z.; Li, F.; Zhao, J.; Zhang, H.; Hou, G.; Zheng, Q.; Li, X. Revisiting of Tetragonal NaVPO4F: A High Energy Density Cathode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 30510–30519. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.; Kim, S.; Fister, T.T.; Johnson, C.S. Microwave-Assisted Synthesis of NaCoPO4 Red-Phase and Initial Characterization as High Voltage Cathode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 4391–4396. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, V.; Savithiri, G.; Subadevi, R.; Sivakumar, M. An emerging electrochemically active maricite NaMnPO4 as cathode material at elevated temperature for sodium-ion batteries. Appl. Nanosci. 2020, 10, 3945–3951. [Google Scholar] [CrossRef]
- Mathew, V.; Kim, S.; Kang, J.; Gim, J.; Song, J.; Baboo, J.P.; Park, W.; Ahn, D.; Han, J.; Gu, L.; et al. Amorphous iron phosphate: Potential host for various charge carrier ions. NPG Asia Mater. 2014, 6, e138. [Google Scholar] [CrossRef]
- Wang, R.; Wu, S.; Zhang, F.; Zhao, X.; Lin, Z.; Wang, C.-Z.; Ho, K.-M. Stabilizing the crystal structures of NaFePO4 with Li substitutions. Phys. Chem. Chem. Phys. 2020, 22, 13975–13980. [Google Scholar] [CrossRef]
- Ali, G.; Lee, J.-H.; Susanto, D.; Choi, S.-W.; Cho, B.W.; Nam, K.-W.; Chung, K.Y. Polythiophene-Wrapped Olivine NaFePO4 as a Cathode for Na-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 15422–15429. [Google Scholar] [CrossRef]
- Priyanka, V.; Savithiri, G.; Rajkumar, P.; Meenatchi, T.; Subadevi, R.; Sivakumar, M. Tweaking the electrochemical activity of maricite NaMnPO4 in sodium batteries using different manganese precursors via polyol method. J. Solid. State Chem. 2020, 290, 121551. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Ganesan, S.; Rengapillai, S.; Marimuthu, S. Gradual Development of Maricite NaMnPO4 with the Influence of Diol Chain Length on the Polyol Process of Surpassed Sodium Intercalation. Ind. Eng. Chem. Res. 2021, 60, 5861–5868. [Google Scholar] [CrossRef]
- Kosova, N.V.; Podugolnikov, V.R.; Devyatkina, E.T.; Slobodyuk, A.B. Structure and electrochemistry of NaFePO4 and Na2FePO4F cathode materials prepared via mechanochemical route. Mater. Res. Bull. 2014, 60, 849–857. [Google Scholar] [CrossRef]
- Jian, Z.; Yuan, C.; Han, W.; Lu, X.; Gu, L.; Xi, X.; Hu, Y.-S.; Li, H.; Chen, W.; Chen, D.; et al. Atomic Structure and Kinetics of NASICON NaxV2(PO4)3 Cathode for Sodium-Ion Batteries. Adv. Funct. Mater. 2014, 24, 4265–4272. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.Y.P.; Kafalas, J.A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Hong, H.Y.P. Crystal structures and crystal chemistry in the system Na1+xZr2SixP3−xO12. Mater. Res. Bull. 1976, 11, 173–182. [Google Scholar] [CrossRef]
- Oh, J.A.S.; He, L.; Plewa, A.; Morita, M.; Zhao, Y.; Sakamoto, T.; Song, X.; Zhai, W.; Zeng, K.; Lu, L. Composite NASICON (Na3Zr2Si2PO12) Solid-State Electrolyte with Enhanced Na+ Ionic Conductivity: Effect of Liquid Phase Sintering. ACS Appl. Mater. Interfaces 2019, 11, 40125–40133. [Google Scholar] [CrossRef]
- Gopalakrishnan, J.; Rangan, K.K. Vanadium phosphate (V2(PO4)3): A novel NASICO N-type vanadium phosphate synthesized by oxidative deintercalation of sodium from sodium vanadium phosphate (Na3V2(PO4)3). Chem. Mater. 1992, 4, 745–747. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, H.; Ma, J.; Chen, P.; Chen, Y. The recent advances of NASICON-Na3V2(PO4)3 cathode materials for sodium-ion batteries. J. Solid. State Chem. 2023, 317, 123669. [Google Scholar] [CrossRef]
- Pandit, B.; Sougrati, M.T.; Fraisse, B.; Monconduit, L. Exploration of a Na3V2(PO4)3/C-Pb full cell Na-ion prototype. Nano Energy 2022, 95, 107010. [Google Scholar] [CrossRef]
- Zhang, X.; Rui, X.; Chen, D.; Tan, H.; Yang, D.; Huang, S.; Yu, Y. Na3V2(PO4)3: An advanced cathode for sodium-ion batteries. Nanoscale 2019, 11, 2556–2576. [Google Scholar] [CrossRef]
- Song, W.; Cao, X.; Wu, Z.; Chen, J.; Huangfu, K.; Wang, X.; Huang, Y.; Ji, X. A study into the extracted ion number for NASICON structured Na3V2(PO4)3 in sodium-ion batteries. Phys. Chem. Chem. Phys. 2014, 16, 17681–17687. [Google Scholar] [CrossRef]
- Cao, X.; Sun, Q.; Zhu, L.; Xie, L. Na3V2(PO4)3 nanoparticles confined in functional carbon framework towards high-rate and ultralong-life sodium storage. J. Alloys Compd. 2019, 791, 296–306. [Google Scholar] [CrossRef]
- Lim, S.-J.; Han, D.-W.; Nam, D.-H.; Hong, K.-S.; Eom, J.-Y.; Ryu, W.-H.; Kwon, H.-S. Structural enhancement of Na3V2(PO4)3/C composite cathode materials by pillar ion doping for high power and long cycle life sodium-ion batteries. J. Mater. Chem. A 2014, 2, 19623–19632. [Google Scholar] [CrossRef]
- Pal, S.K.; Thirupathi, R.; Chakrabarty, S.; Omar, S. Improving the Electrochemical Performance of Na3V2(PO4)3 Cathode in Na-Ion Batteries by Si-Doping. ACS Appl. Energy Mater. 2020, 3, 12054–12065. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.; Wang, Y.; Wang, C.; He, Z.; Li, D.; Guo, L. Insights into the elevated electrochemical performance and kinetic characteristics of magnesium-substituted Na3V2−xMgx(PO4)3/C with superior rate capability and long lifespan. J. Mater. Sci. 2020, 55, 13141–13156. [Google Scholar] [CrossRef]
- Song, W.; Liu, S. A sodium vanadium three-fluorophosphate cathode for rechargeable batteries synthesized by carbothermal reduction. Solid. State Sci. 2013, 15, 1–6. [Google Scholar] [CrossRef]
- Song, W.; Wu, Z.; Chen, J.; Lan, Q.; Zhu, Y.; Yang, Y.; Pan, C.; Hou, H.; Jing, M.; Ji, X. High-voltage NASICON Sodium Ion Batteries: Merits of Fluorine Insertion. Electrochim. Acta 2014, 146, 142–150. [Google Scholar] [CrossRef]
- Wei, P.; Chen, W.; Jing, Q.; Lee, M.-H.; Chen, Z. Effects of P2O7 clusters arrangement on second harmonic generation responses of pyrophosphates. J. Alloys Compd. 2020, 827, 153922. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, Y.; Xu, M. A review on pyrophosphate framework cathode materials for sodium-ion batteries. J. Mater. Chem. A 2019, 7, 15006–15025. [Google Scholar] [CrossRef]
- Uebou, Y.; Okada, S.; Yamaki, J.-I. Electrochemical insertion of lithium and sodium into (MoO2)2P2O7. J. Power Sources 2003, 115, 119–124. [Google Scholar] [CrossRef]
- Gabelica-Robert, M.; Goreaud, M.; Labbe, P.; Raveau, B. The pyrophosphate NaFeP2O7: A cage structure. J. Solid. State Chem. 1982, 45, 389–395. [Google Scholar] [CrossRef]
- Barpanda, P.; Lu, J.; Ye, T.; Kajiyama, M.; Chung, S.-C.; Yabuuchi, N.; Komaba, S.; Yamada, A. A layer-structured Na2CoP2O7 pyrophosphate cathode for sodium-ion batteries. RSC Adv. 2013, 3, 3857–3860. [Google Scholar] [CrossRef]
- Barpanda, P.; Ye, T.; Avdeev, M.; Chung, S.-C.; Yamada, A. A new polymorph of Na2MnP2O7 as a 3.6 V cathode material for sodium-ion batteries. J. Mater. Chem. A 2013, 1, 4194–4197. [Google Scholar] [CrossRef]
- Barpanda, P.; Liu, G.; Ling, C.D.; Tamaru, M.; Avdeev, M.; Chung, S.-C.; Yamada, Y.; Yamada, A. Na2FeP2O7: A Safe Cathode for Rechargeable Sodium-ion Batteries. Chem. Mater. 2013, 25, 3480–3487. [Google Scholar] [CrossRef]
- Kim, H.; Park, C.S.; Choi, J.W.; Jung, Y. Defect-Controlled Formation of Triclinic Na2CoP2O7 for 4 V Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 55, 6662–6666. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.-H.; Woo, S.H.; Mok, D.; Choi, N.-S.; Park, Y.; Oh, S.M.; Kim, Y.; Kim, J.; Lee, J.; Nazar, L.F.; et al. Na4−αM2+α/2(P2O7)2 (2/3 ≤ α ≤ 7/8, M = Fe, Fe0.5Mn0.5, Mn): A Promising Sodium Ion Cathode for Na-ion Batteries. Adv. Energy Mater. 2013, 3, 770–776. [Google Scholar] [CrossRef]
- Erragh, F.; Boukhari, A.; Abraham, F.; Elouadi, B. Study of the Crystal Structures of Sodium Magnesium and Sodium Nickel Diphosphates. J. Solid. State Chem. 2000, 152, 323–331. [Google Scholar] [CrossRef]
- Liu, G.; Nishimura, S.-I.; Chung, S.C.; Fujii, K.; Yashima, M.; Yamada, A. Defect induced sodium disorder and ionic conduction mechanism in Na1.82Mg1.09P2O7. J. Mater. Chem. A 2014, 2, 18353–18359. [Google Scholar] [CrossRef]
- Kim, H.; Shakoor, R.A.; Park, C.; Lim, S.Y.; Kim, J.-S.; Jo, Y.N.; Cho, W.; Miyasaka, K.; Kahraman, R.; Jung, Y.; et al. Na2FeP2O7 as a Promising Iron-Based Pyrophosphate Cathode for Sodium Rechargeable Batteries: A Combined Experimental and Theoretical Study. Adv. Funct. Mater. 2013, 23, 1147–1155. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Zhang, H.; Lian, X.; Dong, Y.; Kuang, Q. Structural and electrochemical properties of Fe-doped Na2Mn3−xFex(P2O7)2 cathode material for sodium ion batteries. J. Power Sources 2017, 370, 114–121. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, M.; Mondal, R.; Kumar, M.; Prakash, R.; Singh, P. Mo2P2O11: A Potential Cathode Material for Rechargeable Sodium-Ion Batteries. Energy Fuels 2023, 37, 1288–1296. [Google Scholar] [CrossRef]
- Pu, X.; Wang, H.; Yuan, T.; Cao, S.; Liu, S.; Xu, L.; Yang, H.; Ai, X.; Chen, Z.; Cao, Y. Na4Fe3(PO4)2P2O7/C nanospheres as low-cost, high-performance cathode material for sodium-ion batteries. Energy Stor. Mater. 2019, 22, 330–336. [Google Scholar] [CrossRef]
- Kundu, D.; Tripathi, R.; Popov, G.; Makahnouk, W.R.M.; Nazar, L.F. Synthesis, Structure, and Na-Ion Migration in Na4NiP2O7F2: A Prospective High Voltage Positive Electrode Material for the Na-Ion Battery. Chem. Mater. 2015, 27, 885–891. [Google Scholar] [CrossRef]
- Masese, T.; Orikasa, Y.; Tassel, C.; Kim, J.; Minato, T.; Arai, H.; Mori, T.; Yamamoto, K.; Kobayashi, Y.; Kageyama, H.; et al. Relationship between Phase Transition Involving Cationic Exchange and Charge-Discharge Rate in Li2FeSiO4. Chem. Mater. 2014, 26, 1380–1384. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, J.; Zhao, Y.; Wu, Y.; Zhang, X.; Wang, L.; Liu, X.; Rui, Y.; Xu, J. Na2CoSiO4 as a novel positive electrode material for sodium-ion capacitors. Mater. Lett. 2015, 158, 300–303. [Google Scholar] [CrossRef]
- Treacher, J.C.; Wood, S.M.; Islam, M.S.; Kendrick, E. Na2CoSiO4 as a cathode material for sodium-ion batteries: Structure, electrochemistry and diffusion pathways. Phys. Chem. Chem. Phys. 2016, 18, 32744–32752. [Google Scholar] [CrossRef]
- Wang, J.; Hoteling, G.; Shepard, R.; Wahila, M.; Wang, F.; Smeu, M.; Liu, H. Reaction Mechanism of Na-Ion Deintercalation in Na2CoSiO4. J. Phys. Chem. C 2022, 126, 16983–16992. [Google Scholar] [CrossRef]
- Pedone, A.; Malavasi, G.; Menziani, M.C.; Cormack, A.N.; Segre, U. A New Self-Consistent Empirical Interatomic Potential Model for Oxides, Silicates, and Silica-Based Glasses. J. Phys. Chem. B 2006, 110, 11780–11795. [Google Scholar] [CrossRef]
- Wu, P.; Wu, S.Q.; Lv, X.; Zhao, X.; Ye, Z.; Lin, Z.; Wang, C.Z.; Ho, K.M. Fe–Si networks in Na2FeSiO4 cathode materials. Phys. Chem. Chem. Phys. 2016, 18, 23916–23922. [Google Scholar] [CrossRef]
- Bianchini, F.; Fjellvåg, H.; Vajeeston, P. First-principles study of the structural stability and electrochemical properties of Na2MSiO4 (M = Mn, Fe, Co and Ni) polymorphs. Phys. Chem. Chem. Phys. 2017, 19, 14462–14470. [Google Scholar] [CrossRef]
- Jin, T.; Li, H.; Zhu, K.; Wang, P.-F.; Liu, P.; Jiao, L. Polyanion-type cathode materials for sodium-ion batteries. Chem. Soc. Rev. 2020, 49, 2342–2377. [Google Scholar] [CrossRef]
- Kee, Y.; Dimov, N.; Staykov, A.; Okada, S. Investigation of metastable Na2FeSiO4 as a cathode material for Na-ion secondary battery. Mater. Chem. Phys. 2016, 171, 45–49. [Google Scholar] [CrossRef]
- Rangasamy, V.S.; Thayumanasundaram, S.; Locquet, J.-P. Solvothermal synthesis and electrochemical properties of Na2CoSiO4 and Na2CoSiO4/carbon nanotube cathode materials for sodium-ion batteries. Electrochim. Acta 2018, 276, 102–110. [Google Scholar] [CrossRef]
- Guan, W.H.; Lin, Q.Y.; Lan, Z.Y.; Pan, W.L.; Wei, X.; Sun, W.P.; Zheng, R.T.; Lu, Y.H.; Shu, J.; Pan, H.G.; et al. Approaching the theoretical capacity limit of Na2FeSiO4-based cathodes with fully reversible two-electron redox reaction for sodium-ion battery. Mater. Today Nano 2020, 12, 100098. [Google Scholar] [CrossRef]
- Law, M.; Ramar, V.; Balaya, P. Na2MnSiO4 as an attractive high capacity cathode material for sodium-ion battery. J. Power Sources 2017, 359, 277–284. [Google Scholar] [CrossRef]
- Schon, T.B.; McAllister, B.T.; Li, P.-F.; Seferos, D.S. The rise of organic electrode materials for energy storage. Chem. Soc. Rev. 2016, 45, 6345–6404. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-G.; Yuan, S.; Ma, D.-L.; Huang, X.-L.; Meng, F.-L.; Zhang, X.-B. Tailored Aromatic Carbonyl Derivative Polyimides for High-Power and Long-Cycle Sodium-Organic Batteries. Adv. Energy Mater. 2014, 4, 1301651. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Zhu, Z.; Hu, Z.; Zhao, Q.; Chen, J. All Organic Sodium-Ion Batteries with Na4C8H2O6. Angew. Chem. 2014, 126, 6002–6006. [Google Scholar] [CrossRef]
- Kim, H.; Kwon, J.E.; Lee, B.; Hong, J.; Lee, M.; Park, S.Y.; Kang, K. High Energy Organic Cathode for Sodium Rechargeable Batteries. Chem. Mater. 2015, 27, 7258–7264. [Google Scholar] [CrossRef]
- Shen, Y.F.; Yuan, D.D.; Ai, X.P.; Yang, H.X.; Zhou, M. Poly(diphenylaminesulfonic acid sodium) as a cation-exchanging organic cathode for sodium batteries. Electrochem. Commun. 2014, 49, 5–8. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Y.; Fang, Y.; Zhou, M.; Liang, L.; Singh, S.; Zhao, H.; Schober, A.; Lei, Y. Extended π-Conjugated System for Fast-Charge and -Discharge Sodium-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 3124–3130. [Google Scholar] [CrossRef]
- Han, S.C.; Bae, E.G.; Lim, H.; Pyo, M. Non-crystalline oligopyrene as a cathode material with a high-voltage plateau for sodium ion batteries. J. Power Sources 2014, 254, 73–79. [Google Scholar] [CrossRef]
- Sakaushi, K.; Hosono, E.; Nickerl, G.; Gemming, T.; Zhou, H.; Kaskel, S.; Eckert, J. Aromatic porous-honeycomb electrodes for a sodium-organic energy storage device. Nat. Commun. 2013, 4, 1485. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Liu, R.; Guo, W.; Li, G.; Fu, Y. Recent advances of organometallic complexes for rechargeable batteries. Coord. Chem. Rev. 2021, 429, 213650. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, Z.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Batteries. Joule 2018, 2, 2235–2259. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Xue, H.; Pang, H.; Xu, Q. Metal–organic frameworks as a platform for clean energy applications. EnergyChem 2020, 2, 100027. [Google Scholar] [CrossRef]
- Du, M.; Li, Q.; Zhao, Y.; Liu, C.-S.; Pang, H. A review of electrochemical energy storage behaviors based on pristine metal–organic frameworks and their composites. Coord. Chem. Rev. 2020, 416, 213341. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544–6546. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Qin, R.; Ding, S.; Wang, Y.; Li, S.; Zhao, Q.; Pan, F. Structure and Properties of Prussian Blue Analogues in Energy Storage and Conversion Applications. Adv. Funct. Mater. 2020, 31, 2006970. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, J.; Yin, S.; Xue, L.; Yan, Y. Acid-Assisted Ball Mill Synthesis of Carboxyl-Functional-Group-Modified Prussian Blue as Sodium-Ion Battery Cathode. Nanomaterials 2022, 12, 1290. [Google Scholar] [CrossRef]
- Xie, B.X.; Sun, B.Y.; Gao, T.Y.; Ma, Y.L.; Yin, G.P.; Zuo, P.J. Recent progress of Prussian blue analogues as cathode materials for nonaqueous sodium-ion batteries. Coord. Chem. Rev. 2022, 460, 214478. [Google Scholar] [CrossRef]
- Fu, H.; Liu, C.; Zhang, C.; Ma, W.; Wang, K.; Li, Z.; Lu, X.; Cao, G. Enhanced storage of sodium ions in Prussian blue cathode material through nickel doping. J. Mater. Chem. A 2017, 5, 9604–9610. [Google Scholar] [CrossRef]
- Qian, J.F.; Zhou, M.; Cao, Y.L.; Ai, X.P.; Yang, H.X. Nanosized Na4Fe(CN)6/C Composite as a Low-Cost and High-Rate Cathode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2012, 2, 410–414. [Google Scholar] [CrossRef]
- Li, W.-J.; Chou, S.-L.; Wang, J.-Z.; Kang, Y.-M.; Wang, J.-L.; Liu, Y.; Gu, Q.-F.; Liu, H.-K.; Dou, S.-X. Facile Method To Synthesize Na-Enriched Na1+xFeFe(CN)6 Frameworks as Cathode with Superior Electrochemical Performance for Sodium-Ion Batteries. Chem. Mater. 2015, 27, 1997–2003. [Google Scholar] [CrossRef]
- Yang, D.; Liao, X.-Z.; Huang, B.; Shen, J.; He, Y.-S.; Ma, Z.-F. A Na4Fe(CN)6/NaCl solid solution cathode material with an enhanced electrochemical performance for sodium ion batteries. J. Mater. Chem. A 2013, 1, 13417–13421. [Google Scholar] [CrossRef]
- Jiao, S.; Tuo, J.; Xie, H.; Cai, Z.; Wang, S.; Zhu, J. The electrochemical performance of Cu3[Fe(CN)6]2 as a cathode material for sodium-ion batteries. Mater. Res. Bull. 2017, 86, 194–200. [Google Scholar] [CrossRef]
- Sun, H.; Sun, H.; Wang, W.; Jiao, H.; Jiao, S. Fe4[Fe(CN)6]3: A cathode material for sodium-ion batteries. RSC Adv. 2014, 4, 42991–42995. [Google Scholar] [CrossRef]
- Matsuda, T.; Takachi, M.; Moritomo, Y. A sodium manganese ferrocyanide thin film for Na-ion batteries. Chem. Commun. 2013, 49, 2750–2752. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Y.; Liu, J.; Xu, M.; Cheng, J.; Zhang, D.; Goodenough, J.B. A Superior Low-Cost Cathode for a Na-Ion Battery. Angew. Chem. Int. Ed. 2013, 52, 1964–1967. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Fu, X.-Y.; Zhang, L.-L.; Yan, B.; Yang, X.-L. High-Performance Fe-Based Prussian Blue Cathode Material for Enhancing the Activity of Low-Spin Fe by Cu Doping. ACS Appl. Mater. Interfaces 2022, 14, 5506–5513. [Google Scholar] [CrossRef]
- Song, J.; Wang, L.; Lu, Y.; Liu, J.; Guo, B.; Xiao, P.; Lee, J.J.; Yang, X.Q.; Henkelman, G.; Goodenough, J.B. Removal of interstitial H2O in hexacyanometallates for a superior cathode of a sodium-ion battery. J. Am. Chem. Soc. 2015, 137, 2658–2664. [Google Scholar] [CrossRef]
- Hu, P.; Peng, W.; Wang, B.; Xiao, D.; Ahuja, U.; Réthoré, J.; Aifantis, K.E. Concentration-Gradient Prussian Blue Cathodes for Na-Ion Batteries. ACS Energy Lett. 2020, 5, 100–108. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Y.; Xie, J.; Nie, Y.; Xu, X.W.; Tu, J.; Zhang, J.; Qiu, L.C.; Zhu, T.J.; Zhao, X.B. Scalable Preparation of Mn/Ni Binary Prussian Blue as Sustainable Cathode for Harsh-Condition-Tolerant Sodium-Ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 13277–13287. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. Vanadium Ferrocyanides as a Highly Stable Cathode for Lithium-Ion Batteries. Molecules 2023, 28, 461. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.T.; He, Z.H.; Hou, J.F.; Kong, L.B. Sodium vanadium hexacyanoferrate as a high-rate capability and long-life cathode material for Na-ion batteries. J. Energy Storage 2022, 53, 105165. [Google Scholar] [CrossRef]
- Noor, N.; Parkin, I.P. Enhanced transparent-conducting fluorine-doped tin oxide films formed by Aerosol-Assisted Chemical Vapour Deposition. J. Mater. Chem. C 2013, 1, 984–996. [Google Scholar] [CrossRef]
- Wu, X.; Wu, C.; Wei, C.; Hu, L.; Qian, J.; Cao, Y.; Ai, X.; Wang, J.; Yang, H. Highly Crystallized Na2CoFe(CN)6 with Suppressed Lattice Defects as Superior Cathode Material for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 5393–5399. [Google Scholar] [CrossRef]
- Takachi, M.; Matsuda, T.; Moritomo, Y. Cobalt Hexacyanoferrate as Cathode Material for Na+ Secondary Battery. Appl. Phys. Express 2013, 6, 025802. [Google Scholar] [CrossRef]
- Baster, D.; Kondracki, L.; Oveisi, E.; Trabesinger, S.; Girault, H.H. Prussian Blue Analogue-Sodium-Vanadium Hexacyanoferrate as a Cathode Material for Na-Ion Batteries. Acs Appl. Energy Mater. 2021, 4, 9758–9765. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. Iron-Vanadium Incorporated Ferrocyanides as Potential Cathode Materials for Application in Sodium-Ion Batteries. Micromachines 2023, 14, 521. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Chang, X.; Guo, H.; Li, Y.; Wang, M.; Li, W.; Jiao, L.; Wang, Y. MOF based on a longer linear ligand: Electrochemical performance, reaction kinetics, and use as a novel anode material for sodium-ion batteries. Chem. Commun. 2018, 54, 11793–11796. [Google Scholar] [CrossRef]
- Férey, G.; Millange, F.; Morcrette, M.; Serre, C.; Doublet, M.-L.; Grenèche, J.-M.; Tarascon, J.-M. Mixed-Valence Li/Fe-Based Metal–Organic Frameworks with Both Reversible Redox and Sorption Properties. Angew. Chem. Int. Ed. 2007, 46, 3259–3263. [Google Scholar] [CrossRef]
- Chen, T.; Liu, X.; Niu, L.; Gong, Y.; Li, C.; Xu, S.; Pan, L. Recent progress on metal–organic framework-derived materials for sodium-ion battery anodes. Inorg. Chem. Front. 2020, 7, 567–582. [Google Scholar] [CrossRef]
- Li, H.; Wang, T.; Wang, X.; Li, G.; Shen, J.; Chai, J. MOF-derived Al-doped Na2FePO4F/mesoporous carbon nanonetwork composites as high-performance cathode material for sodium-ion batteries. Electrochim. Acta 2021, 373, 137905. [Google Scholar] [CrossRef]
- Yu, T.-Y.; Sun, Y.-K. A fluorinated O3-type layered cathode for long-life sodium-ion batteries. J. Mater. Chem. A 2022, 10, 23639–23648. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, L.; Li, W.; Zhao, K.; Liu, M.; Wu, Q.; Yang, Y.; He, G.; Parkin, I.P.; Shearing, P.R.; et al. Sodium Superionic Conductors (NASICONs) as Cathode Materials for Sodium-Ion Batteries. Electrochem. Energy Rev. 2021, 4, 793–823. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, X.; You, H.; Min, H.; Xu, X.; Hao, J.; Liu, X.; Yang, H. Template-directed Prussian blue nanocubes supported on Ni foam as the binder-free anode of lithium-ion batteries. Appl. Surf. Sci. 2022, 571, 151194. [Google Scholar] [CrossRef]
- Kim, H.; Sadan, M.K.; Kim, C.; Jo, J.; Seong, M.; Cho, K.-K.; Kim, K.-W.; Ahn, J.-H.; Ahn, H.-J. Enhanced reversible capacity of sulfurized polyacrylonitrile cathode for room-temperature Na/S batteries by electrochemical activation. Chem. Eng. J. 2021, 426, 130787. [Google Scholar] [CrossRef]
- Huang, Z.-X.; Zhang, X.-L.; Zhao, X.-X.; Zhao, Y.-Y.; Aravindan, V.; Liu, Y.-H.; Geng, H.; Wu, X.-L. Electrode/electrolyte additives for practical sodium-ion batteries: A mini review. Inorg. Chem. Front. 2023, 10, 37–48. [Google Scholar] [CrossRef]
- Hou, Y.; Jin, J.; Huo, C.; Liu, Y.; Deng, S.; Chen, J. New insights into the critical role of inactive element substitution in improving the rate performance of sodium oxide cathode material. Energy Stor. Mater. 2023, 56, 87–95. [Google Scholar] [CrossRef]
- Nanba, Y.; Iwao, T.; Boisse, B.M.D.; Zhao, W.; Hosono, E.; Asakura, D.; Niwa, H.; Kiuchi, H.; Miyawaki, J.; Harada, Y.; et al. Redox Potential Paradox in NaxMO2 for Sodium-Ion Battery Cathodes. Chem. Mater. 2016, 28, 1058–1065. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).