A Photoluminescence Study of Eu3+, Tb3+, Ce3+ Emission in Doped Crystals of Strontium-Barium Fluoride Borate Solid Solution Ba4−xSr3+x(BO3)4−yF2+3y (BSBF)
Abstract
1. Introduction
2. Materials and Experimental Methods
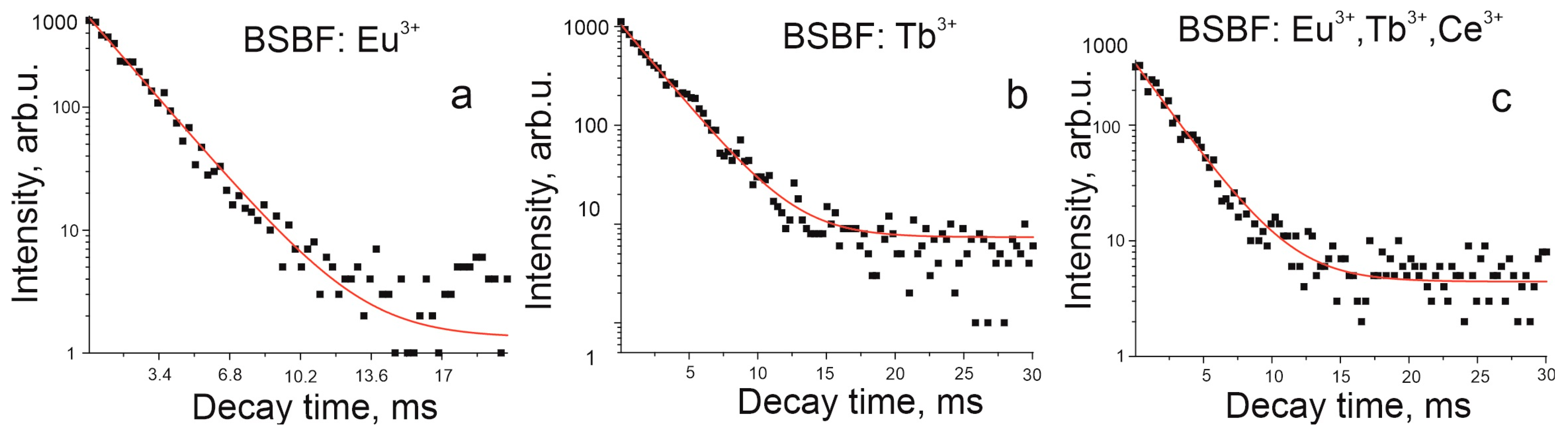
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mutailipu, M.; Poeppelmeier, K.R.; Pan, S. Borates: A Rich Source for Optical Materials. Chem. Rev. 2021, 121, 1130–1202. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Keszler, D.A. Eu2+ luminescence in the borates X2Z (BO3)2 (X = Ba, Sr; Z = Mg, Ca). Chem. Mater. 1997, 9, 2071–2077. [Google Scholar] [CrossRef]
- Spassky, D.A.; Levushkina, V.S.; Mikhailin, V.V.; Zadneprovski, B.I.; Tret’yakova, M.S. Luminescence of borates with yttrium and lutetium cations. Phys. Solid State 2013, 55, 150–159. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Y.; Chen, T.; Jiang, W.; Liu, J.; Zhang, X.; Liang, W.; Wang, L. A red phosphor LaSc3(BO3)4:Eu3+ with zero-thermal-quenching and high quantum efficiency for LEDs. Chem. Eng. J. 2021, 404, 125912. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhang, X.; Luo, Y.; Lu, S.; Ren, X.; Wang, X.; Sun, L.; Yan, C. Luminescent Properties in Relation to Controllable Phase and Morphology of LuBO3:Eu3+ Nano/Microcrystals Synthesized by Hydrothermal Approach. Chem. Mater. 2009, 21, 468–475. [Google Scholar] [CrossRef]
- Gaffuri, P.; Salaün, M.; Gautier-Luneau, I.; Chadeyron, G.; Potdevin, A.; Rapenne, L.; Ibanez, A. Rare-earth-free zinc aluminium borate white phosphors for LED lighting. J. Mater. Chem. C 2020, 8, 11839–11849. [Google Scholar] [CrossRef]
- Khan, Z.S.; Ingale, N.B.; Omanwar, S.K. Synthesis and thermoluminescence properties of rare earth-doped NaMgBO3 phosphor. Environ. Sci. Pollut. Res. 2016, 23, 9295–9302. [Google Scholar] [CrossRef]
- Nagpure, P.A.; Omanwar, S.K. Synthesis and luminescence characteristics of terbium (III) activated NaSrBO3. J. Rare Earths 2012, 30, 856–859. [Google Scholar] [CrossRef]
- Xu, S.; Li, P.; Wang, Z.; Li, T.; Bai, Q.; Sun, J.; Yang, Z. Luminescence and energy transfer of Eu2+/Tb3+/Eu3+ in LiBaBO3 phosphors with tunable-color emission. J. Mater. Chem. C 2015, 3, 9112–9121. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, L.; Pang, Q.; Gong, M. A broadband-excited and narrow-line GdBO3: Ce3+, Tb3+, Eu3+ red phosphor with efficient Ce3+→(Tb3+) n→ Eu3+ energy transfer for NUV LEDs. Opt. Mater. 2014, 36, 1112–1118. [Google Scholar] [CrossRef]
- Bekker, T.B.; Ryadun, A.A.; Davydov, A.V.; Solntsev, V.P.; Grigorieva, V.D. Luminescence properties of rare-earth-doped fluoride borate crystals. J. Alloys Compd. 2022, 900, 163343. [Google Scholar] [CrossRef]
- Rashchenko, S.V.; Bekker, T.B.; Bakakin, V.V.; Seryotkin, Y.V.; Shevchenko, V.S.; Kokh, A.E.; Stonoga, S.Y. New Fluoride Borate Solid-solution Series Ba4−xSr3+x(BO3)4−yF2+3y. Cryst. Growth Des. 2012, 12, 2955–2960. [Google Scholar] [CrossRef]
- Bekker, T.B.; Solntsev, V.P.; Yelisseyev, A.P.; Rashchenko, S.V. Fluoride borates with [(BO3)F]4−↔ [F4]4− anionic isomorphism and X-ray sensitivity. Cryst. Growth Des. 2016, 16, 4493–4499. [Google Scholar] [CrossRef]
- Rothkirch, A.; Gatta, G.D.; Meyer, M.; Merkel, S.; Merlini, M.; Liermann, H.-P. Single-crystal diffraction at the extreme conditions beamline P02.2: Procedure for collecting and analyzing high-pressure single-crystal data. J. Synchrotron Radiat. 2013, 20, 711–720. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP–a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Krist.—Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-Valence Parameters for Solids. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Blasse, G. The Influence of Charge-Transfer and Rydberg States on the Luminescence Properties of Lanthanides and Actinides, Spectra and Chemical Interactions; Braterman, P.S., Blasse, G., Müller, A., Baran, E.J., Carter, R.O., Eds.; Springer: Berlin/Heidelberg, Germany, 1976; pp. 43–79. [Google Scholar]
- Putaj, P.; De Haas, J.T.M.; Kramer, K.W.; Dorenbos, P. Optical and Scintillation Properties of the Thermal Neutron Scintillator Li3YCl6:Ce. IEEE Trans. Nucl. Sci. 2010, 57, 1675–1681. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, X.; Wu, L.; Zhang, H.; Seo, H.J. On the photoluminescence of multi-sites Ce3+ in T-phase orthosilicate and energy transfer from Ce3+ to Tb3+. J. Alloys Compd. 2018, 748, 871–875. [Google Scholar] [CrossRef]
- Xiao, Y.; Hao, Z.; Zhang, L.; Xiao, W.; Wu, D.; Zhang, X.; Zhang, J. Highly efficient green-emitting phosphors Ba2Y5B5O17 with low thermal quenching due to fast energy transfer from Ce3+ to Tb3+. Inorg. Chem. 2017, 56, 4538–4544. [Google Scholar] [CrossRef]
- Lazarowska, A.; Lesniewski, T.; Mahlik, S.; Grinberg, M.; Liu, R.S. Thermal quenching of Ce3+ luminescence in the cuspidine-type oxide nitride compounds Y4Si2−xAlxO7+xN2−x. J. Lumin. 2018, 193, 125–132. [Google Scholar] [CrossRef]
- Linganna, K.; Sreedhar, V.B.; Jayasankar, C.K. Luminescence properties of Tb3+ ions in zinc fluorophosphate glasses for green laser applications. Mater. Res. Bull. 2015, 67, 196–200. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium (III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Porcher, P.; Caro, P. Influence of J-mixing on the phenomenological interpretation of the Eu3+ ion spectroscopic properties. J. Lumin. 1980, 21, 207–216. [Google Scholar] [CrossRef]
- Tanaka, M.; Kushida, T. Effects of static crystal field on the homogeneous width of the 5D0–7F0 line of Eu3+ and Sm2+ in solids. Phys. Rev. B 1995, 52, 4171–4178. [Google Scholar] [CrossRef]
- Malta, O.L.; Azevedo, W.M.; Gouveia, E.A.; de Sa, G.F. On the 5D0→ 7F0 transition of the Eu3+ ion in the {(C4H9)4N}3Y(NCS)6 host. J. Lumin. 1982, 26, 337–343. [Google Scholar] [CrossRef]
- Chen, X.Y.; Liu, G.K. The standard and anomalous crystal-field spectra of Eu3+. J. Solid State Chem. 2005, 178, 419–428. [Google Scholar] [CrossRef]
- Holsa, J.; Porcher, P. Crystal field effects in REOBr:Eu3+. J. Chem. Phys. 1982, 76, 2790. [Google Scholar] [CrossRef]
- Wright, A.O.; Seltzer, M.D.; Gruber, J.B.; Chai, B.H.T. Site-selective spectroscopy and determination of energy levels in Eu3+-doped strontium fluorophosphates. J. Appl. Phys. 1995, 78, 2456. [Google Scholar] [CrossRef]
- Ternane, R.; Panczer, G.; Cohen-Adad, M.T.; Goutaudier, C.; Boulon, G.; Kbir-Ariguib, N.; Trabelsi-Ayedi, M. Relationships between structural and luminescence properties in Eu3+-doped new calcium borohydroxyapatite. Opt. Mater. 2001, 16, 291. [Google Scholar] [CrossRef]
- Binnemans, K.; Görller-Walrand, C. Application of the Eu3+ ion for site symmetry determination. J. Rare Earths 1996, 14, 173–180. [Google Scholar]
- Bunzli, J.C.G.; Pradervand, G.O. The Eu(III) ion as luminescent probe: Laser-spectroscopic investigation of the metal ion sites in an 18-crown-6 complex. J. Chem. Phys. 1986, 85, 2489–2497. [Google Scholar] [CrossRef]
- Tanner, P.A. Some misconceptions concerning the electronic spectra of tri-positive europium and cerium. Chem. Soc. Rev. 2013, 42, 5090–5101. [Google Scholar] [CrossRef]
- Gupta, S.K.; Reghukumar, C.; Kadam, R.M. Eu3+ local site analysis and emission characteristics of novel Nd2Zr2O7:Eu phosphor: Insight into the effect of europium concentration on its photoluminescence properties. RSC Adv. 2016, 6, 53614. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, Z. Multi-color emission evolution and energy transfer behavior of La3GaGe5O16: Tb3+, Eu3+ phosphors. J. Mater. Chem. C. 2014, 2, 6978–6984. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Z.; Wong, H.T.; Zhou, L.; Wong, K.L.; Shiu, K.K.; Tanner, P.A. Energy transfer between Tb3+ and Eu3+ in LaPO4: Pulsed versus switched-off continuous wave excitation. Adv. Sci. 2019, 6, 1900487. [Google Scholar] [CrossRef]
- Carneiro Neto, A.N.; Moura, R.T.; Andrii, S.; Paterlini, V.; Piccinelli, F.; Bettinelli, M.G.; Malta, O.L. Theoretical and experimental investigation of the Tb3+-Eu3+ energy transfer mechanisms in cubic A3Tb0.90Eu0.10({PO}4)3 (A = Sr, Ba) materials. J. Phys. Chem. C 2020, 124, 10105–10116. [Google Scholar] [CrossRef]
- Rastogi, C.K.; Sharma, S.K.; Sasmal, S.; Pala, R.G.S.; Kumar, J.; Sivakumar, S. Terbium ion-mediated energy transfer in WO3: Tb3+ and Eu3+ phosphors for UV-sensitized white light emission. J. Phys. Chem. C 2021, 125, 6163–6175. [Google Scholar] [CrossRef]
- Bekker, T.B.; Ryadun, A.A.; Davydov, A.V.; Rashchenko, S.V. LiBa12(BO3)7F4 (LBBF) crystals doped with Eu3+, Tb3+, Ce3+: Structure and luminescence properties. Dalton Trans. 2023, 52, 8402–8413. [Google Scholar] [CrossRef]

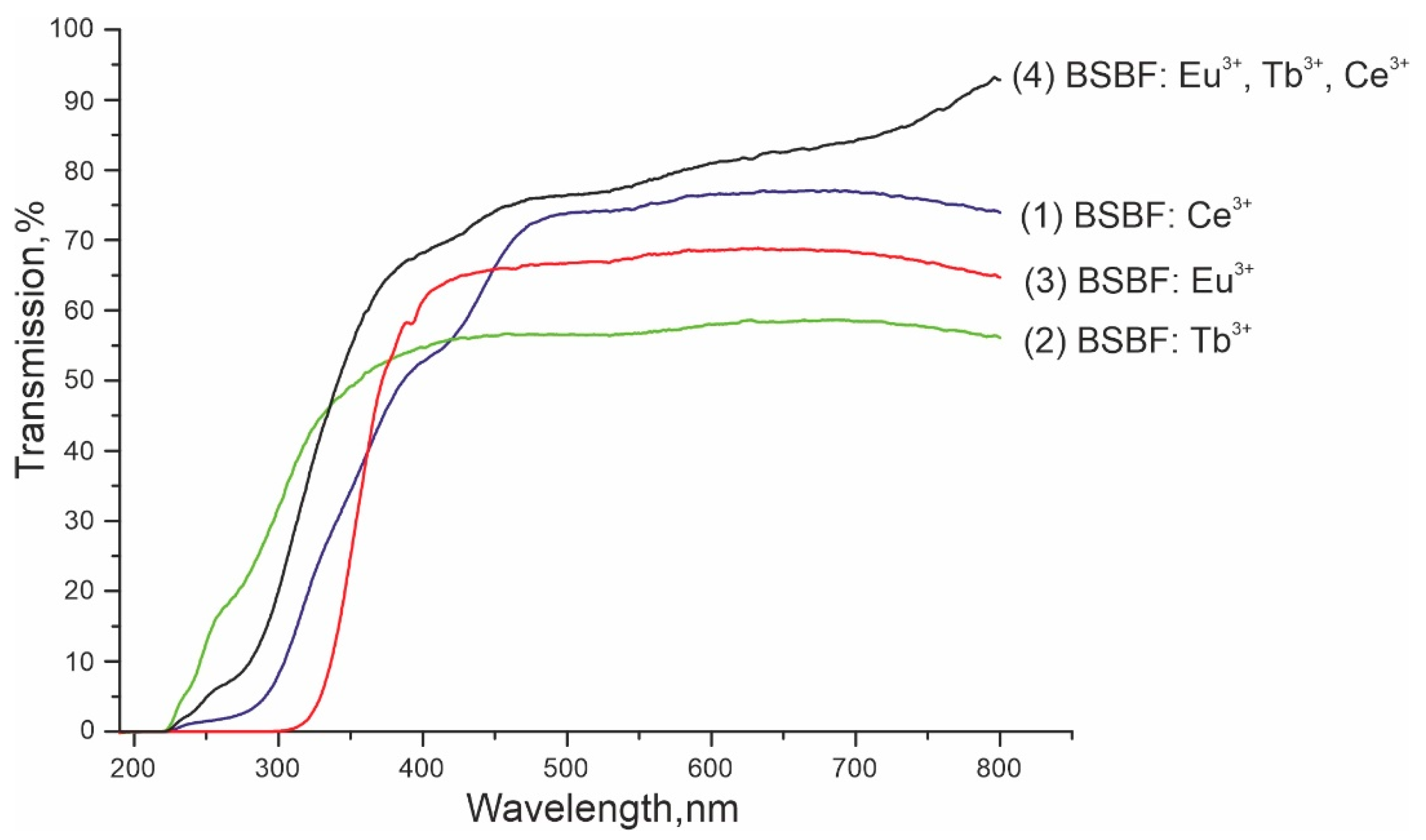

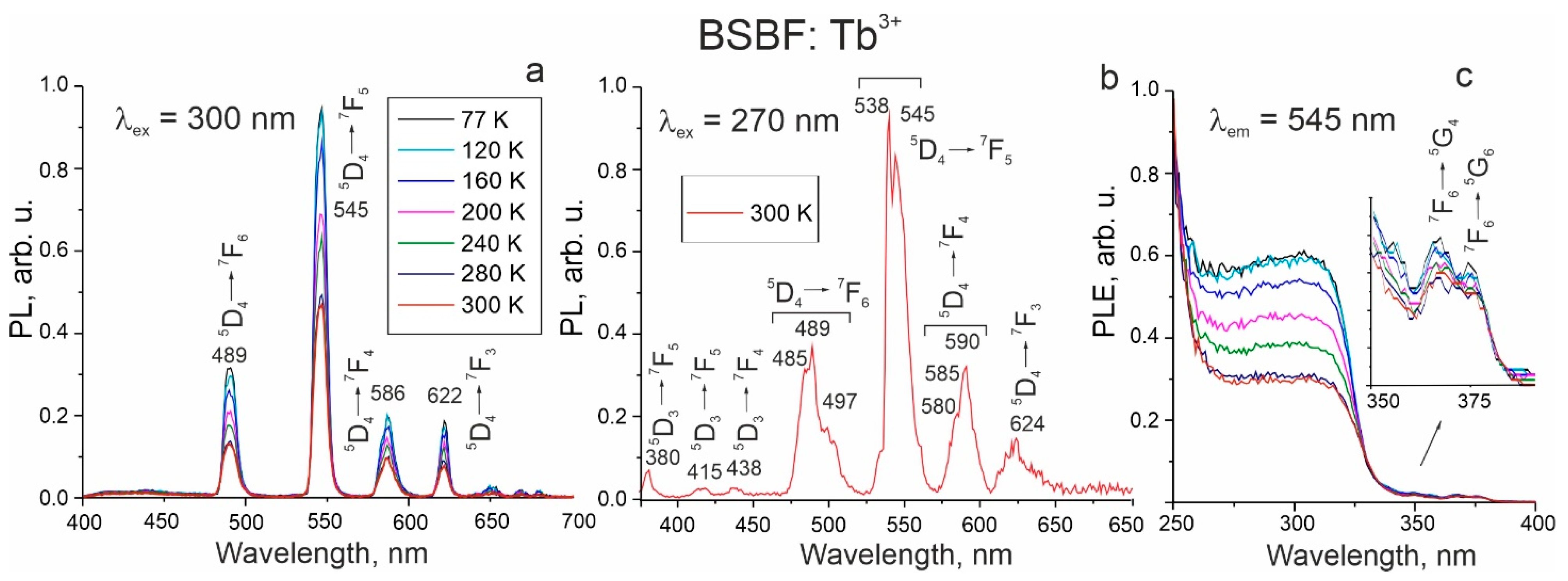
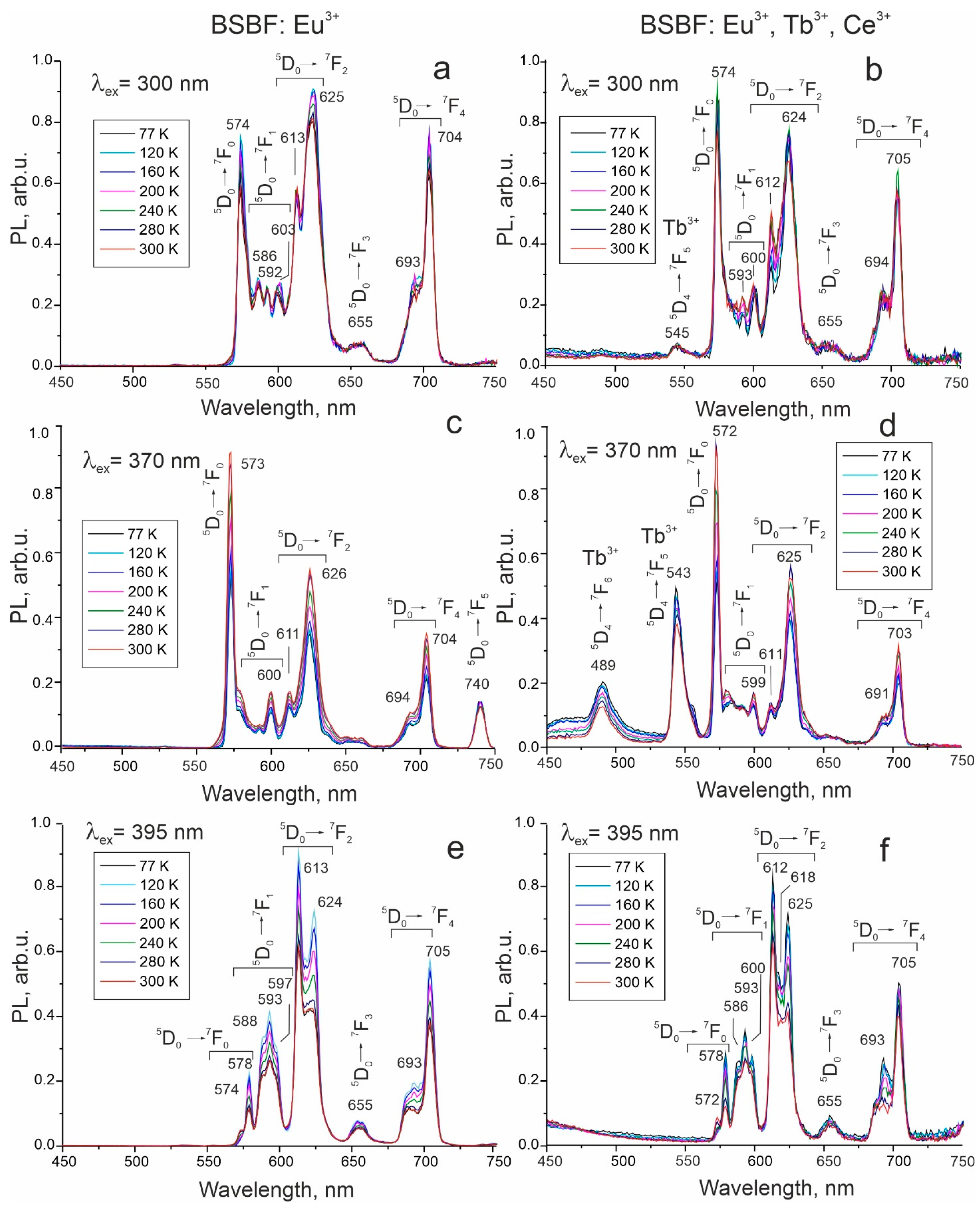
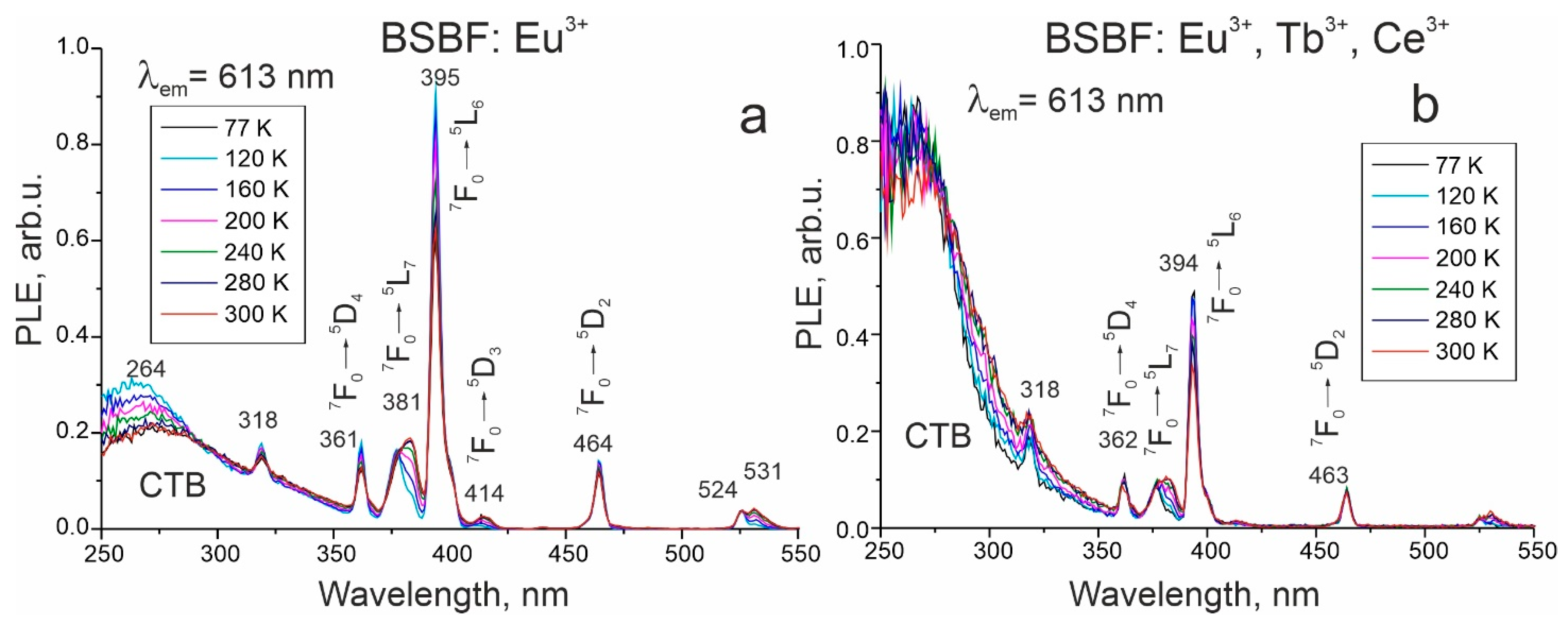
| Composition Crystal | Ba3Sr4(BO3)4F2 (mol%) | NaF (mol%) | Eu3+ (wt%) | Tb3+ (wt%) | Ce3+ (wt%) | Tliq (°C) | Plate Made of Grown Crystal |
|---|---|---|---|---|---|---|---|
| BSBF: Ce3+ | 75 | 25 | − | − | 0.4 | 998 |  |
| BSBF: Tb3+ | 75 | 25 | − | 0.25 | − | 992 |  |
| BSBF: Eu3+ | 75 | 25 | 3.5 | − | − | 999 |  |
| BSBF: Eu3+, Tb3+, Ce3+ | 75 | 25 | 0.1 | 0.25 | 0.4 | 998 |  |
| Band Maximum, nm | ||||||
|---|---|---|---|---|---|---|
| BSBF: Eu3+ | BSBF: Eu3+, Tb3+, Ce3+ | |||||
| Excitation Wavelength, nm | Excitation Wavelength, nm | |||||
| 300 | 370 | 395 | 300 | 370 | 395 | |
| 5D4→7F6 Tb3+ | − | − | − | − | 489 | − |
| 5D4→7F5 Tb3+ | − | − | − | 545 | 543 | − |
| 5D0→7F0 Eu3+ | 574 | 573 * | 574, 578 | 574 | 572 | 572, 578 |
| 5D0→7F1 Eu3+ | 586, 592, 603 | 600 | 588, 593, 597 | 593, 600 | 599 | 586, 593, 600 |
| 5D0→7F2 Eu3+ | 613, 625 | 611, 626 | 613, 624 | 612, 624 | 611, 625 | 612, 618, 625 |
| 5D0→7F3 Eu3+ | 655 | − | 655 | 655 | − | 655 |
| 5D0→7F4 Eu3+ | 693, 704 | 694, 704 | 693, 705 | 694, 705 | 691, 703 | 693, 705 |
| 5D0→7F5 Eu3+ | − | 750 | − | − | − | − |
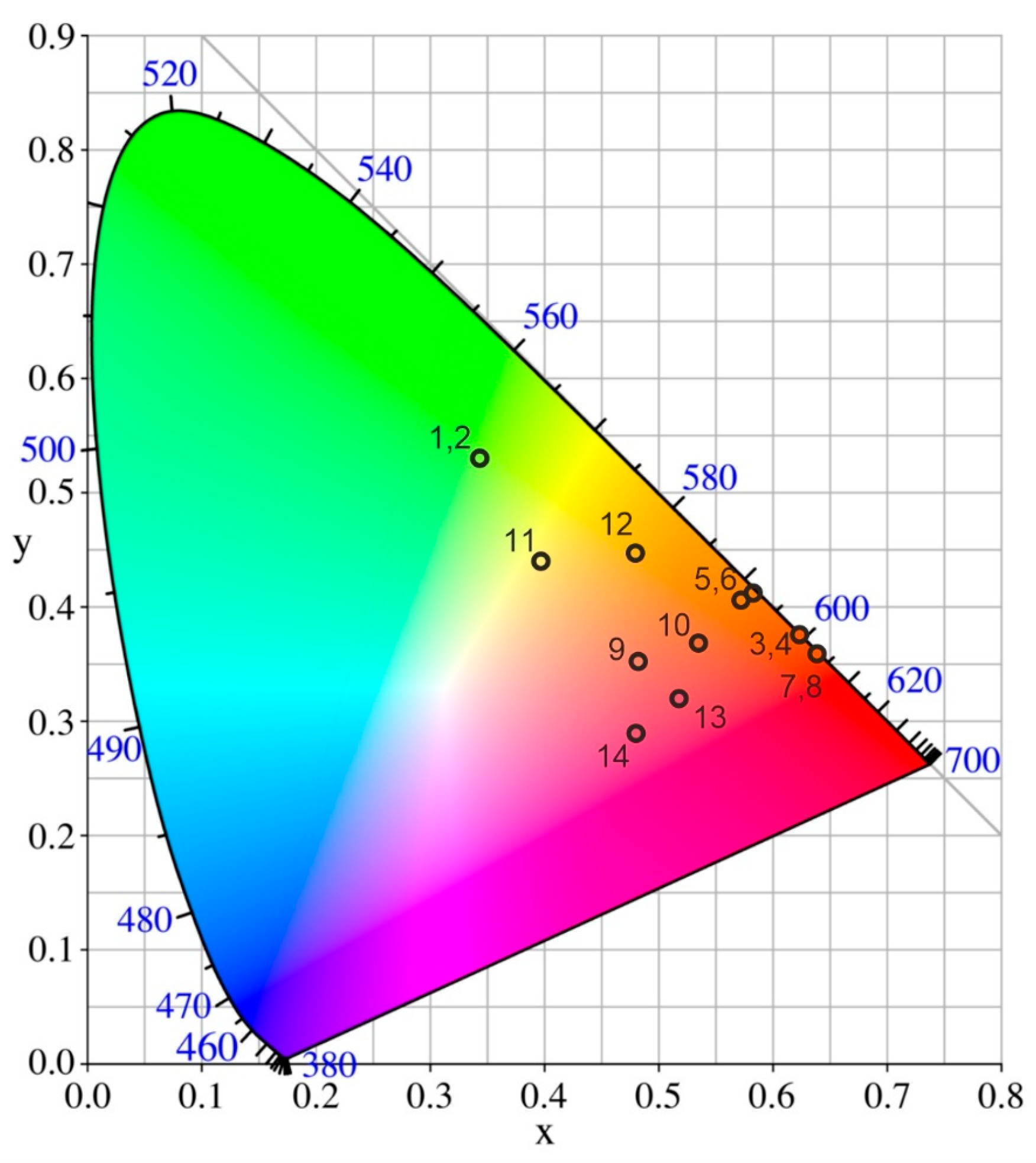
| λex, nm | CIE Coordinates | CCT, K | ||
|---|---|---|---|---|
| 77 K | 300 K | 77 K | 300 K | |
| Ba3Sr4(BO3)4F2: Tb3 + | ||||
| 300 | (1) (0.345; 0.530) | (2) (0.344; 0.524) | 5263 | 5278 |
| Ba3Sr4(BO3)4F2: Eu3 + | ||||
| 300 | (3) (0.617; 0.378) | (4) (0.617; 0.378) | 1836 | 1831 |
| 370 | (5) (0.570; 0.406) | (6) (0.585; 0.410) | 1756 | 1732 |
| 395 | (7) (0.634; 0.359) | (8) (0.633; 0.359) | 2185 | 2176 |
| Ba3Sr4(BO3)4F2: Eu3+, Tb3+, Ce3+ | ||||
| 300 | (9) (0.484; 0.349) | (10) (0.538; 0.373) | 1822 | 1851 |
| 370 | (11) (0.399; 0.439) | (12) (0.481; 0.452) | 3946 | 2728 |
| 395 | (13) (0.513; 0.327) | (14) (0.477;0.291) | 6665 | 4849 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekker, T.B.; Ryadun, A.A.; Rashchenko, S.V.; Davydov, A.V.; Baykalova, E.B.; Solntsev, V.P. A Photoluminescence Study of Eu3+, Tb3+, Ce3+ Emission in Doped Crystals of Strontium-Barium Fluoride Borate Solid Solution Ba4−xSr3+x(BO3)4−yF2+3y (BSBF). Materials 2023, 16, 5344. https://doi.org/10.3390/ma16155344
Bekker TB, Ryadun AA, Rashchenko SV, Davydov AV, Baykalova EB, Solntsev VP. A Photoluminescence Study of Eu3+, Tb3+, Ce3+ Emission in Doped Crystals of Strontium-Barium Fluoride Borate Solid Solution Ba4−xSr3+x(BO3)4−yF2+3y (BSBF). Materials. 2023; 16(15):5344. https://doi.org/10.3390/ma16155344
Chicago/Turabian StyleBekker, Tatyana B., Alexey A. Ryadun, Sergey V. Rashchenko, Alexey V. Davydov, Elena B. Baykalova, and Vladimir P. Solntsev. 2023. "A Photoluminescence Study of Eu3+, Tb3+, Ce3+ Emission in Doped Crystals of Strontium-Barium Fluoride Borate Solid Solution Ba4−xSr3+x(BO3)4−yF2+3y (BSBF)" Materials 16, no. 15: 5344. https://doi.org/10.3390/ma16155344
APA StyleBekker, T. B., Ryadun, A. A., Rashchenko, S. V., Davydov, A. V., Baykalova, E. B., & Solntsev, V. P. (2023). A Photoluminescence Study of Eu3+, Tb3+, Ce3+ Emission in Doped Crystals of Strontium-Barium Fluoride Borate Solid Solution Ba4−xSr3+x(BO3)4−yF2+3y (BSBF). Materials, 16(15), 5344. https://doi.org/10.3390/ma16155344





