Effect of Polyethylene Glycol on Preparation of Magnesium Hydroxide by Electrodeposition
Abstract
:1. Introduction
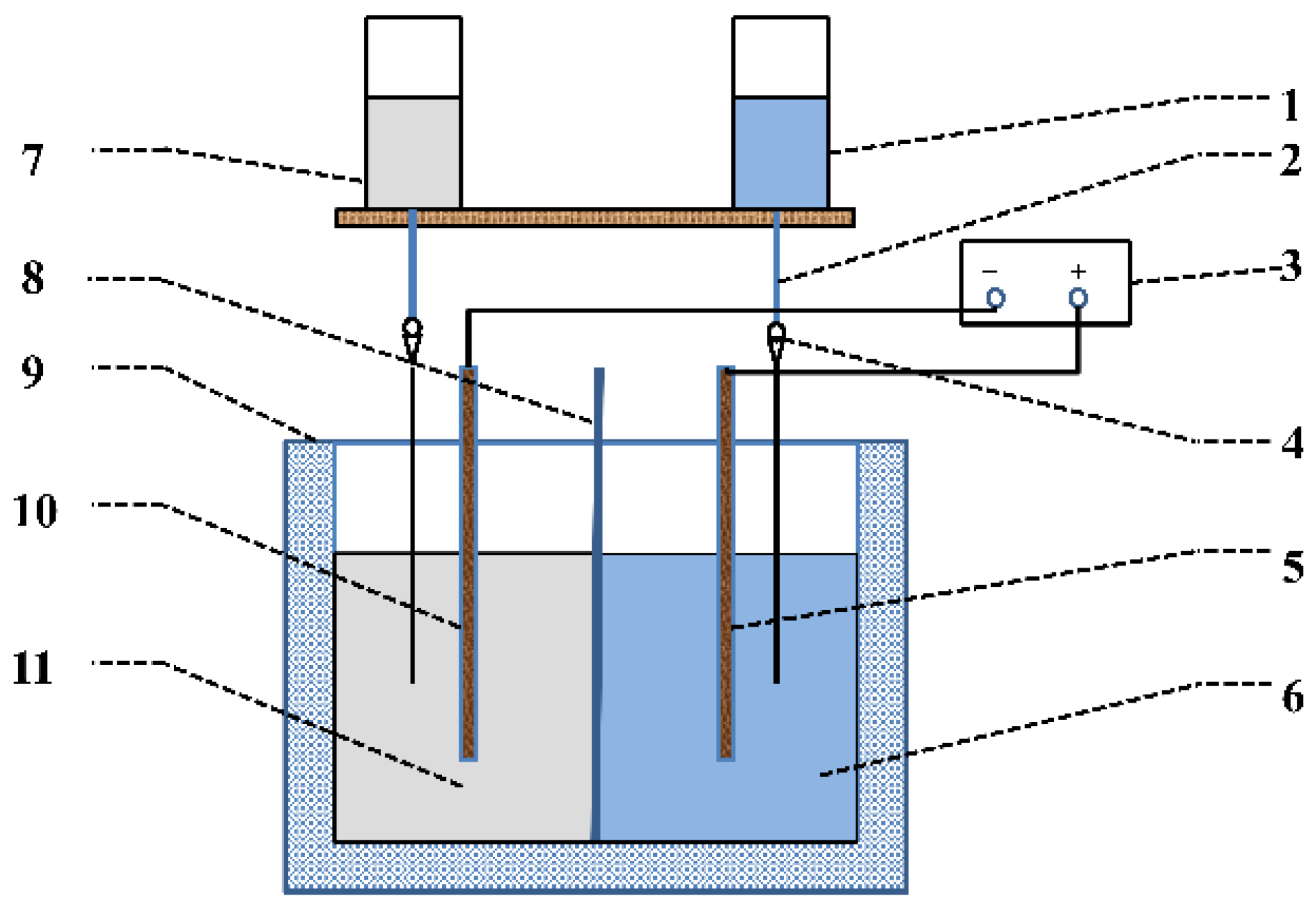
2. Materials and Methods
2.1. Materials
2.2. Preparation of Mg(OH)2
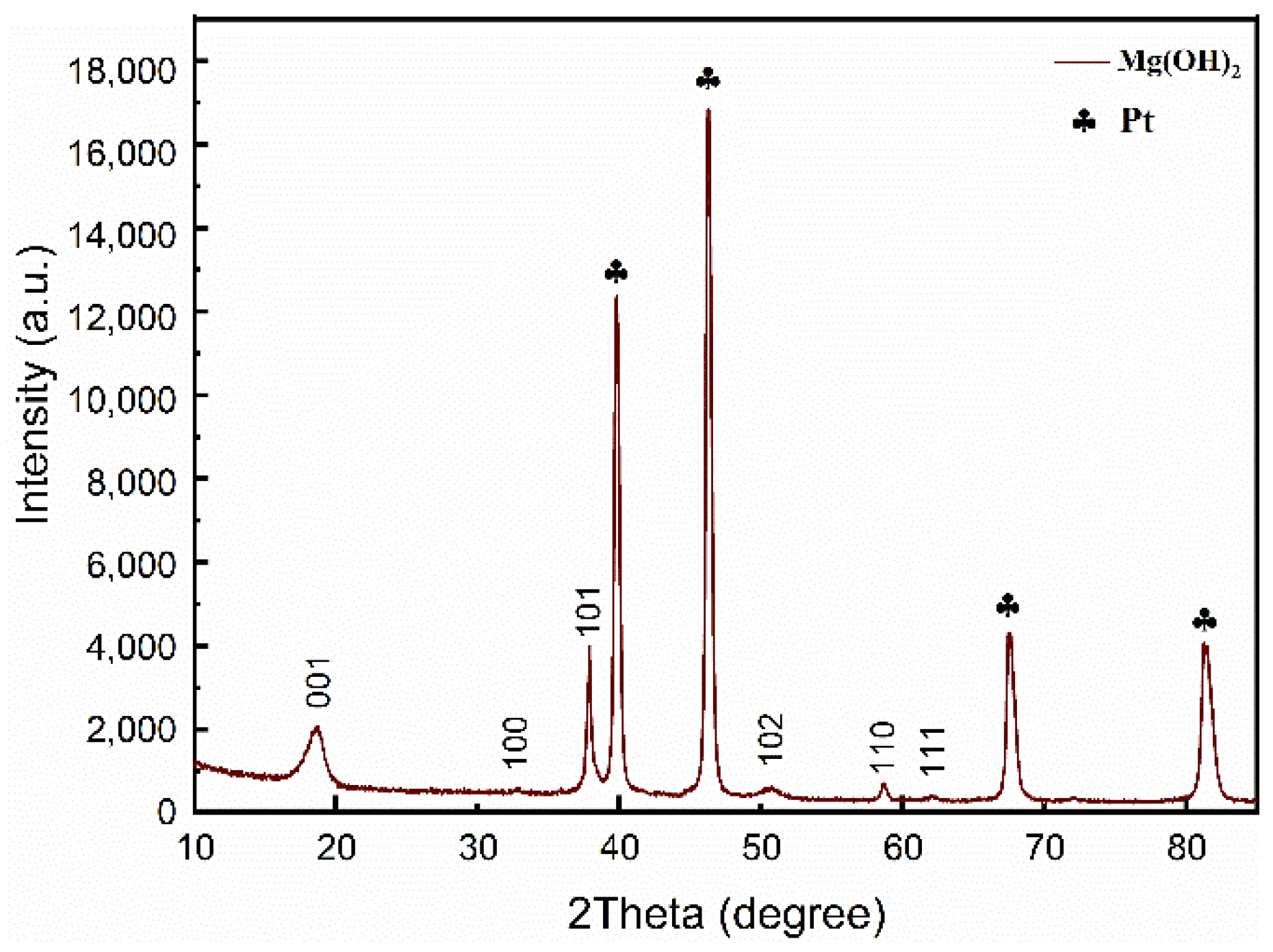
2.3. Characterization of Mg(OH)2
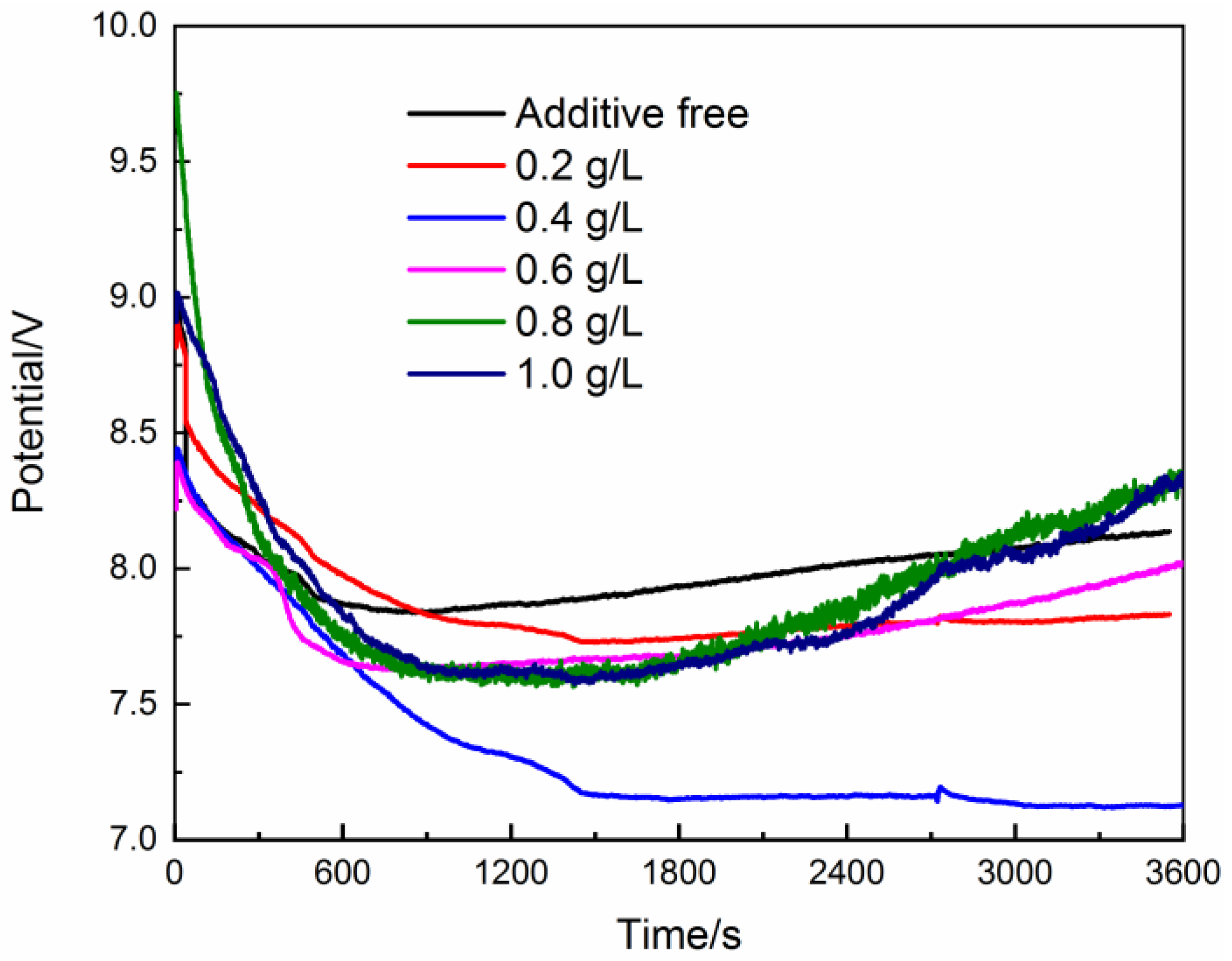
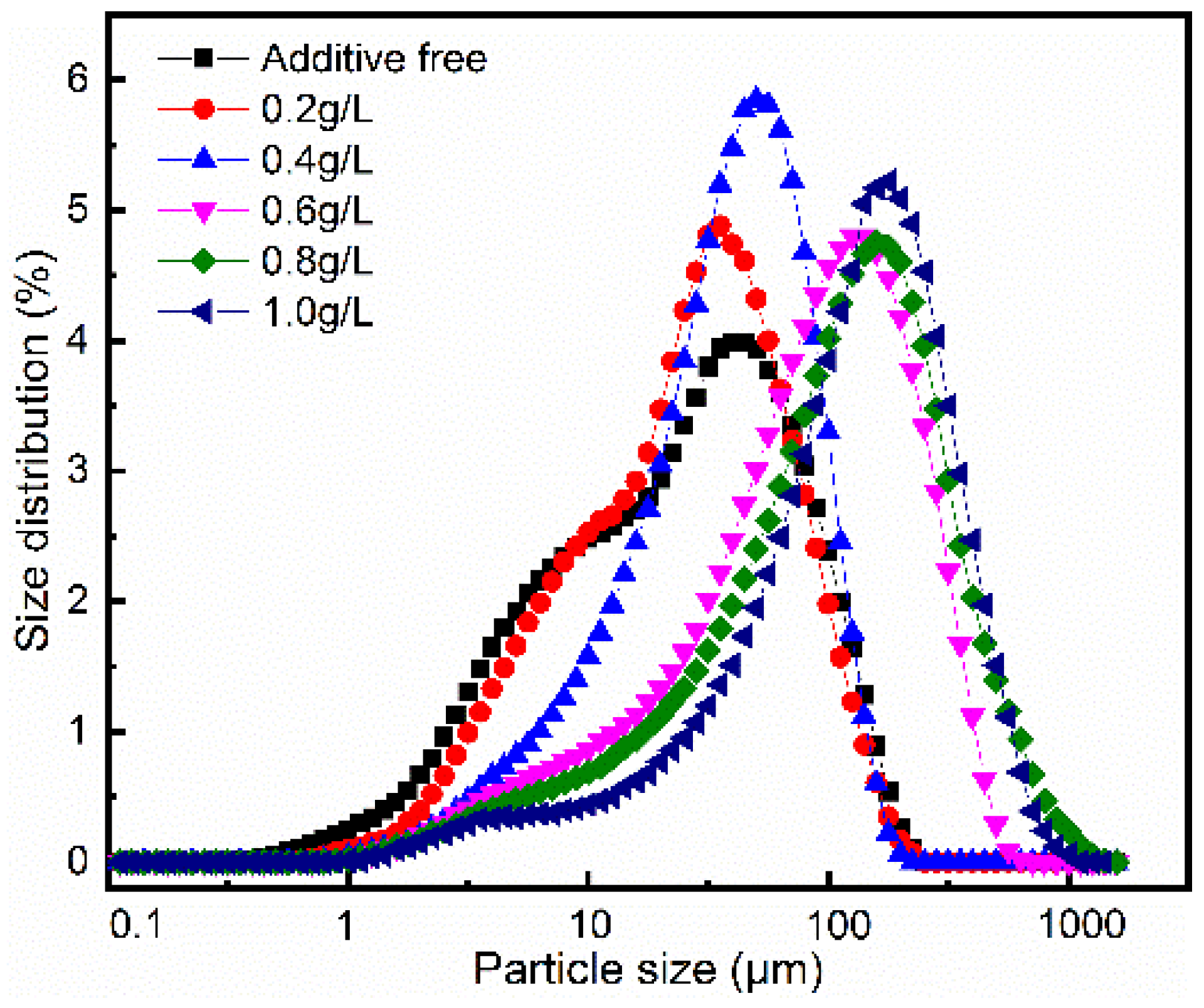
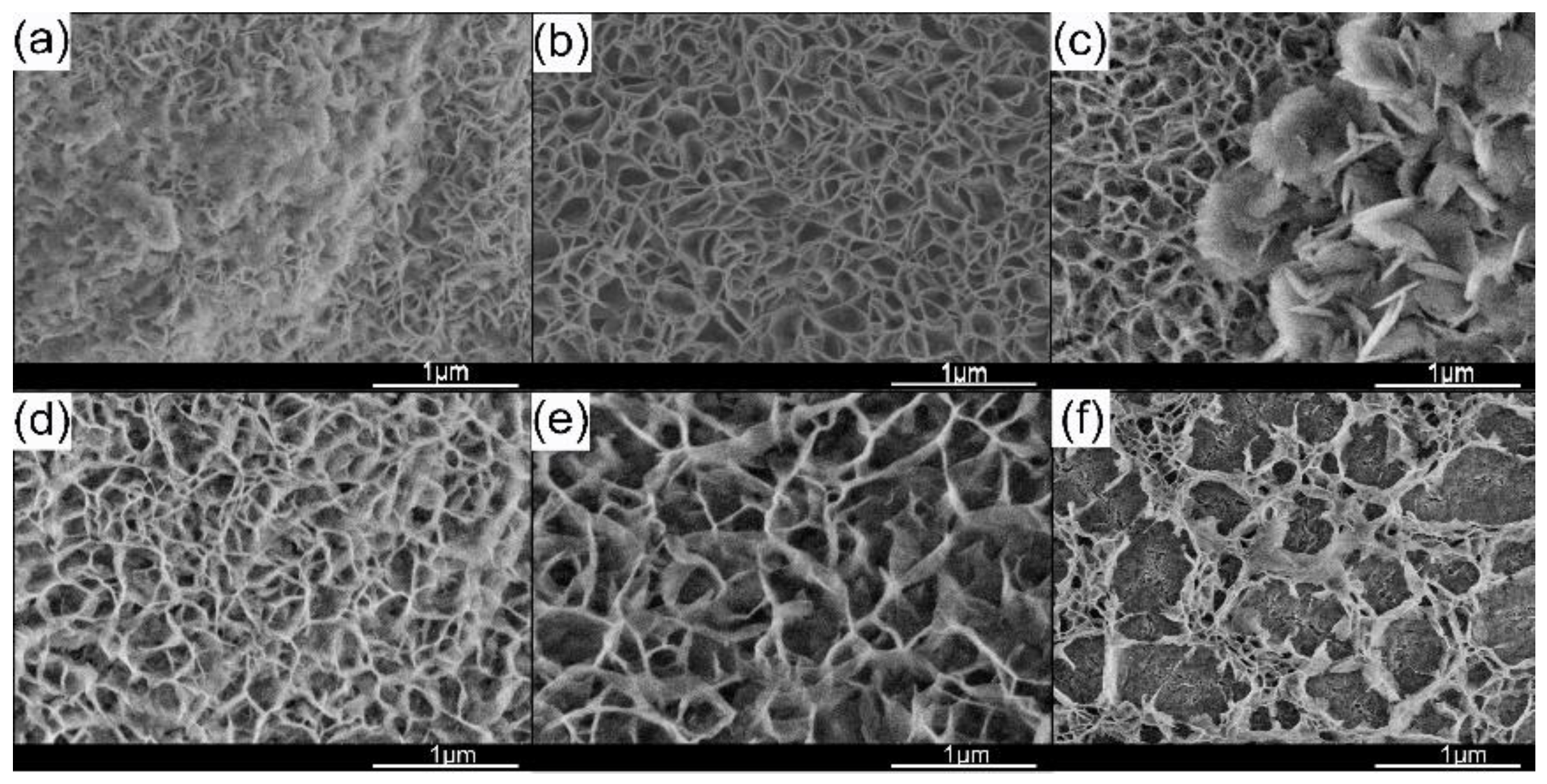
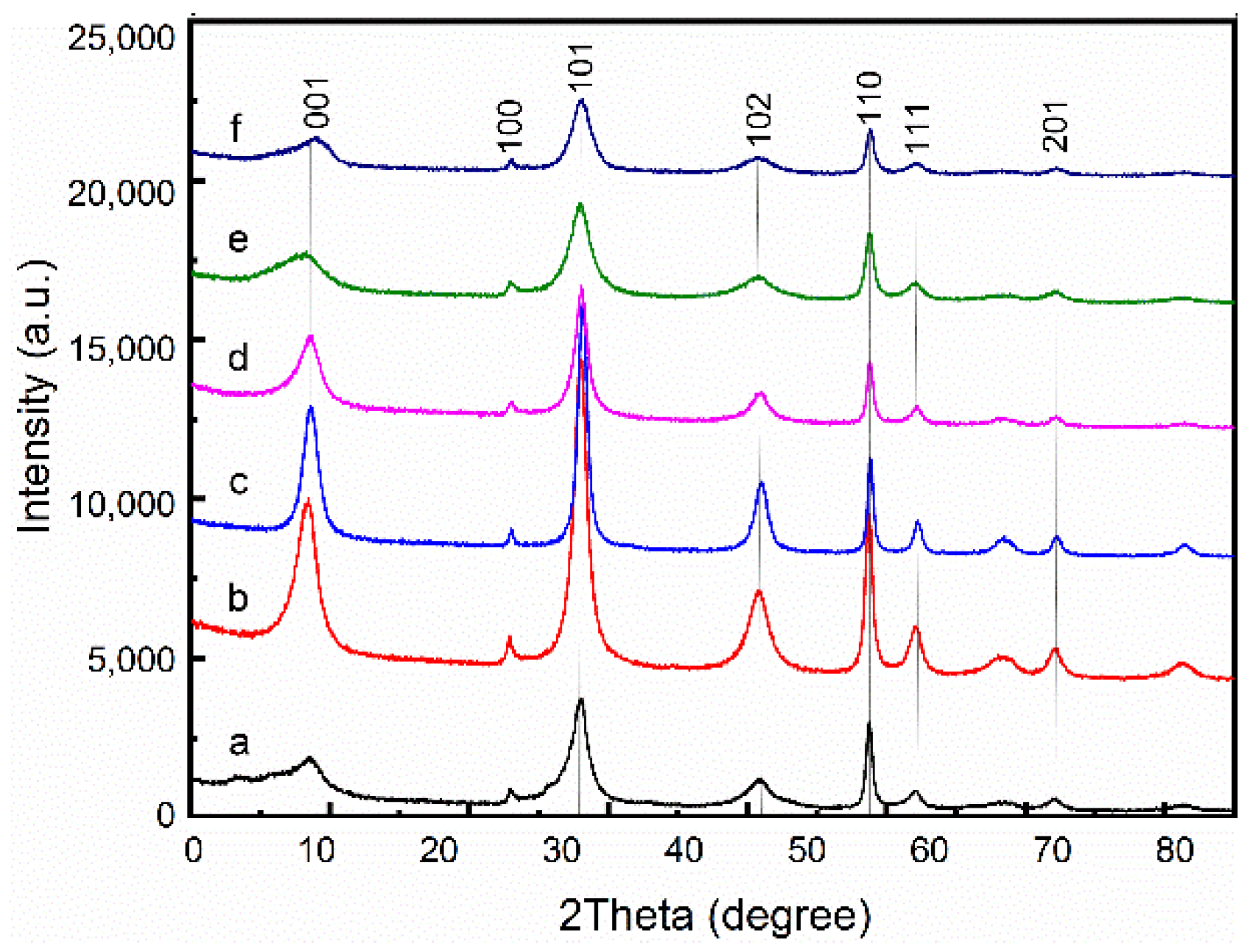
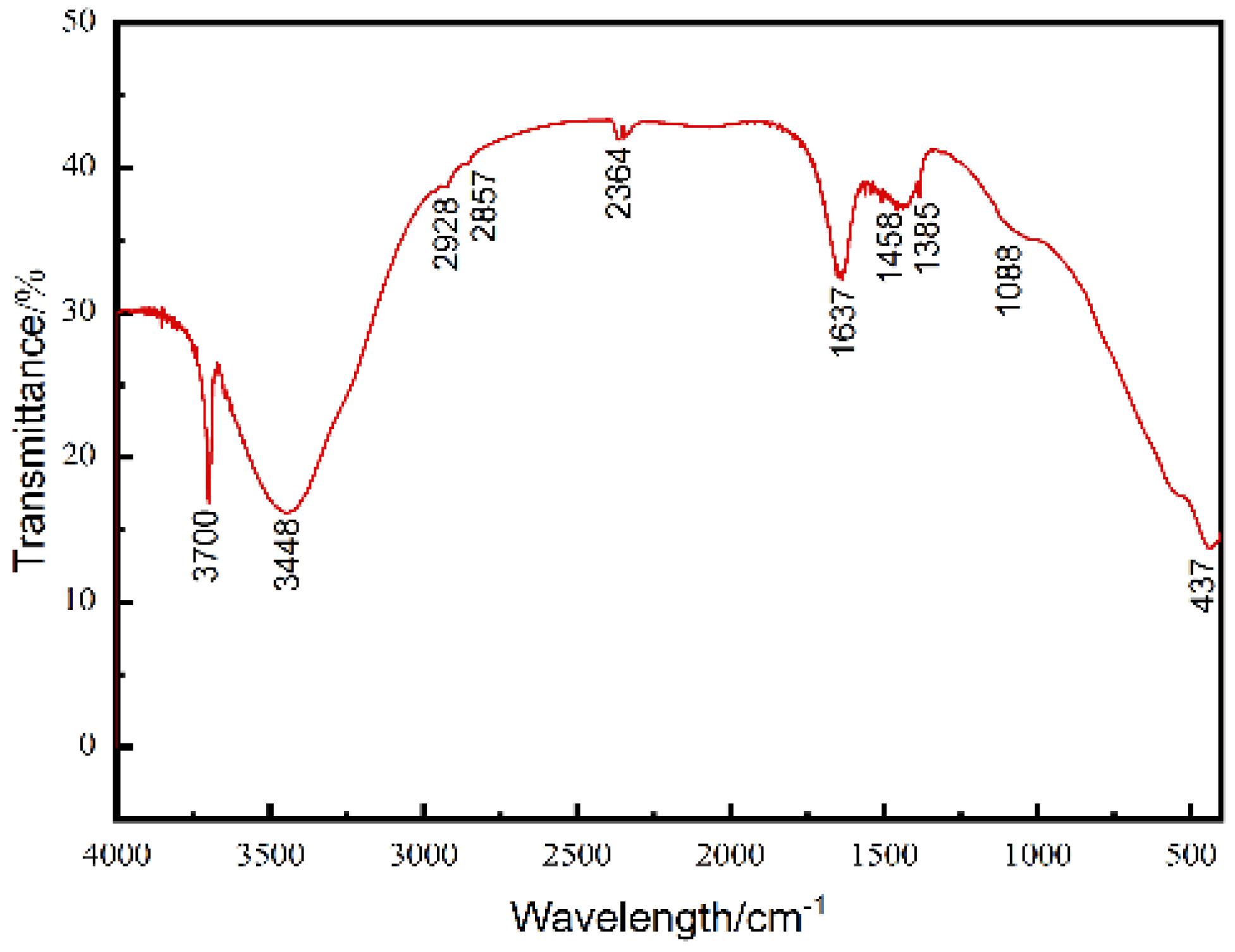
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lozano, J.A.F. Recovery of Potassium Magnesium Sulfate Double Salt from Seawater Bittern. Ind. Eng. Chem. Process Des. Dev. 1976, 15, 445–449. [Google Scholar] [CrossRef]
- Huang, Q.Z.; Lu, G.M.; Yu, J.G. Effect of LiCl·H2O on Sintering Properties of MgO from Bischofite. Adv. Mater. Res. 2013, 813, 364–371. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, X. Hydrochemistry of Salt Lakes of the Qinghai-Tibet Plateau, China. Aquat. Geochem. 2009, 15, 293–320. [Google Scholar] [CrossRef]
- Jiang, Y.Z.; Han, Y.X.; Yin, W.Z.; Li, Y.B. Study on Process and Mecahnism for Preparation of Magnesium Hydroxide Whiskers. Adv. Mater. Res. 2010, 92, 247–254. [Google Scholar] [CrossRef]
- Tong, Z.; Li, L.; Li, Y.; Wang, Q.; Cheng, X. The Effect of In Situ Synthesis of MgO Nanoparticles on the Thermal Properties of Ternary Nitrate. Materials 2021, 14, 5737. [Google Scholar] [CrossRef]
- Senevirathna, H.L.; Wu, S.; Lee, W.P.C.; Wu, P. Morphology Design and Fabrication of Bio-Inspired Nano-MgO–Mg(OH)2 via Vapor Steaming to Enable Bulk CO2 Diffusion and Capture. Materials 2022, 15, 680. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, L.; Hao, Z.; Xia, X. Chemistry of borate in salt lake brine (XXXIV). Sci. China Ser. B-Chem. 2002, 45, 541–550. [Google Scholar] [CrossRef]
- Sugimoto, K.; Dinnebier, R.E.; Hanson, J.C. Structures of three dehydration products of bischofite from in situ synchrotron powder diffraction data (MgCl2·nH2O; n = 1, 2, 4). Acta Crystallogr. Sect. B Struct. Sci. 2010, 63, 235–242. [Google Scholar] [CrossRef]
- Zou, G.; Liu, R.; Chen, W. Highly textural lamellar mesostructured magnesium hydroxide via a cathodic electrodeposition process. Mater. Lett. 2007, 61, 1990–1993. [Google Scholar] [CrossRef]
- Deng, X.Z.; Wang, Y.W.; Peng, J.P.; Liu, K.J.; Feng, N.X.; Di, Y.Z. Surface area control of nanocomposites Mg(OH)2/graphene using a cathodic electrodeposition process: High adsorption capability of methyl orange. RSC Adv. 2016, 6, 88315–88320. [Google Scholar] [CrossRef]
- Deng, X.Z.; Wang, Y.W.; Peng, J.P.; Liu, K.; Feng, N.; Di, Y. Mg(OH)2/Graphene Nanocomposites Prepared by Cathodic Electrodeposition for the Adsorption of Congo Red. Nano 2017, 12, 1750017–1750028. [Google Scholar] [CrossRef]
- Therese, G.; Kamath, P.V. Electrochemical Synthesis of Metal Oxides and Hydroxides. Chem. Mater. 2000, 12, 1195–1204. [Google Scholar] [CrossRef]
- Therese, G.H.A.; Kamath, P.V. Cathodic reduction of different metal salt solutions Part I: Synthesis of metal hydroxides by electrogeneration of base. J. Appl. Electrochem. 1998, 28, 539–543. [Google Scholar] [CrossRef]
- Dinamani, M.; Kamath, P.V. Electrosynthesis of Mg(OH)2 Coatings on Stainless Steel Substrates. J. Appl. Electrochem. 2004, 34, 899–902. [Google Scholar] [CrossRef]
- Li, C.F.; Ho, W.H.; Yen, S.K. Effects of Applied Voltage on Morphology and Crystal Orientation of Mg(OH)2 Coating on Pt by Electrochemical Synthesis. J. Electrochem. Soc. 2009, 156, E29–E34. [Google Scholar] [CrossRef]
- Rabbani, M.; Dincer, I.; Naterer, G.F. Efficiency assessment of a photo electrochemical chloralkali process for hydrogen and sodium hydroxide production. Int. J. Hydrog. Energy 2014, 39, 1941–1956. [Google Scholar] [CrossRef]
- Dolder, H. Process analyzers in the chloralkali industry. Anal. Chim. Acta 1986, 190, 25–31. [Google Scholar] [CrossRef]
- Zou, G.L.; Chen, W.X.; Fu, W.J. Effects of Ionic Liquid Additive on Morphogenesis of Mg(OH)2 Film via Electrodeposition. Mater. Rev. 2010, 24, 71–73. [Google Scholar]
- Cvetkovi, V.S.; Vukievi, N.M.; Nikoli, N.D.; Branković, G.; Barudžija, T.; Jovićević, J.N. Formation of needle-like and honeycomb-like magnesium oxide/hydroxide structures by electrodeposition from magnesium nitrate melts. Electrochim. Acta 2018, 268, 494–502. [Google Scholar] [CrossRef]
- Feng, N.X.; Wang, Y.W.; Deng, X.Z.; Peng, J.P.; Di, Y.Z. Device and method for preparing magnesium hydroxide, hydrogen gas and chlorine gas through magnesium chloride solution. China 2015, 10, 21. [Google Scholar]
- Chen, J.M.; Zhang, Z.; Song, Y.H. Influence of Hydmthermal Conditions on the Microscopic Internal Strain of Magnesium Hydroxide. J. Synth. Cryst. 2011, 40, 396–404. (In Chinese) [Google Scholar]
- Cherevko, S.; Xing, X.; Chung, C.-H. Hydrogen template assisted electrodeposition of sub-micrometer wires composing honeycomb-like porous pb films. Appl. Surf. Sci. 2011, 257, 8054–8061. [Google Scholar] [CrossRef]
- Cvetković, V.S.; Vukićević, N.M.; Nikolić, N.D.; Baščarević, Z.; Barudžija, T.; Jovićević, J.N. A possible mechanism of formation of flower-like MgO/Mg(OH)2 structures by galvanostatic molten salt electrolysis: The concept of local diffusion fields. J. Electroanal. Chem. 2019, 842, 168. [Google Scholar] [CrossRef]
- Henrist, C.; Mathieu, J.P.; Vogels, C.; Rulmont, A.; Cloots, R. Morphological study of magnesium hydroxide nanoparticles precipitated in dilute aqueous solution. J. Cryst. Growth 2003, 249, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Santhiya, D.; Subramanian, S.; Natarajan, K.; Malghan, S. Surface Chemical Studies on the Competitive Adsorption of Poly(acrylic acid) and Poly(vinyl alcohol) onto Alumina. J. Colloid Interface Sci. 1999, 216, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.W.; Guo, F.; Tian, W.; Zhao, Z.; Yang, Z. Synergistic Effect of Chloride Ion and Copper Phthalocyanine in Acid Copper Plating. Mater. Prot. 2002, 35, 22–24. [Google Scholar]
- Yu, J.C.; Xu, A.; Zhang, L.; Song, R.; Wu, L. Synthesis and Characterization of Porous Magnesium Hydroxide and Oxide Nanoplates. J. Phys. Chem. B 2004, 108, 64–70. [Google Scholar] [CrossRef]


| Composition | Concentration (g/L) | Composition | Concentration (g/L) |
|---|---|---|---|
| Mg2+ | 54.40 | BO32− | 2.81 × 10−3 |
| Ca2+ | 0.46 | Cu2+ | 8.15 × 10−5 |
| Na+ | 1.21 | K+ | 0.63 |
| SO42− | 1.20 | Li+ | 0.14 |
| Cl− | 177.48 | Mn2+ | 5.24 × 10−5 |
| Fe3+ | 1.42 × 10−4 | Ni2+ | 1.41 × 10−5 |
| Al3+ | 2.13 × 10−4 | PO43− | 4.76 × 10−5 |
| Si4+ | 3.45 × 10−4 | Pb2+ | 7.49 × 10−5 |
| PEG Content (g/L) | Additive Free | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 |
|---|---|---|---|---|---|---|
| D50 1 | 25.5 | 21.7 | 20.0 | 28.8 | 37.5 | 52.2 |
| D90 1 | 58.0 | 59.0 | 60.7 | 68.6 | 93.0 | 121.0 |
| PEG Content (g/L) | Additive Free | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 |
|---|---|---|---|---|---|---|
| I(101)/(001) | 1.972 | 2.041 | 2.061 | 2.058 | 2.030 | 2.033 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Z.; Di, Y.; Peng, J.; Wang, Y.; Feng, N. Effect of Polyethylene Glycol on Preparation of Magnesium Hydroxide by Electrodeposition. Materials 2022, 15, 3278. https://doi.org/10.3390/ma15093278
Cui Z, Di Y, Peng J, Wang Y, Feng N. Effect of Polyethylene Glycol on Preparation of Magnesium Hydroxide by Electrodeposition. Materials. 2022; 15(9):3278. https://doi.org/10.3390/ma15093278
Chicago/Turabian StyleCui, Zhichun, Yuezhong Di, Jianping Peng, Yaowu Wang, and Naixiang Feng. 2022. "Effect of Polyethylene Glycol on Preparation of Magnesium Hydroxide by Electrodeposition" Materials 15, no. 9: 3278. https://doi.org/10.3390/ma15093278
APA StyleCui, Z., Di, Y., Peng, J., Wang, Y., & Feng, N. (2022). Effect of Polyethylene Glycol on Preparation of Magnesium Hydroxide by Electrodeposition. Materials, 15(9), 3278. https://doi.org/10.3390/ma15093278





