Abstract
The separation of titanium, vanadium and chromium in vanadium slag (VS) is a difficult problem restricting the comprehensive utilization of VS. This paper presents the first study on the separation of titanium, vanadium and chromium from oxalic acid leachate of VS. Firstly, the separation of titanium from the leachate by hydrothermal method was studied. The results show that more than 99% of titanium in the leachate was precipitated in the form of spherical anatase TiO2 with the purity of 95.7%. Then, the extraction separation of vanadium and chromium from the titanium-free filtrate by three-stage extraction of acidified N235 extractant and four-stage stripping of HCl solution was investigated. The extraction mechanism was identified as the anion exchange reaction between acidified N235 extractant and vanadium and chromium complex anions, which were further stripped by HCl solution in the stripping process. After obtaining the concentrated and purified stripping solution containing vanadium and chromium, the separation of vanadium and chromium from the stripping solution by hydrothermal method was studied, and the product was mainly composed of VO2 and Cr2O3. This process provides an idea for the comprehensive utilization of titanium, vanadium and chromium in oxalic acid system.
1. Introduction
Vanadium, titanium and chromium are significant strategic metals which are widely used in aerospace, energy and alloy fields, etc. [1]. Vanadium slag (VS) is a by-product of smelting vanadium titanomagnetite, which is mainly composed of vanadium, titanium, chromium, iron, manganese and silicon [2,3]. However, as a high-quality raw material containing vanadium, titanium and chromium, only the vanadium bearing in VS can be extracted through traditional processes, namely a sodium salt roasting–water leaching process and calcification roasting–acid leaching process [4,5]. As for the principle of these processes, it can be summarized as follows. During the roasting step, vanadium (III) occurred in VS is oxidized and reacts with sodium salt or calcium salt to form soluble vanadate. Then, vanadium pentoxide (V2O5) is produced by leaching, purification, precipitation and calcination processes [6]. Unfortunately, the valuable titanium and chromium are not extracted and left in tailings, resulting in a waste of resources and environmental pollution [7]. In view of the above problems, the sub-molten salt method with the characteristics of co-extraction of vanadium and chromium was proposed. Insoluble vanadium (III) and chromium (III) in VS are oxidized and leached in a high-concentration sodium hydroxide medium under oxygen pressure, but the titanium is still not extracted [8]. To solve the above problems, in our previous research [9], a new method for co-extracting vanadium, titanium and chromium from vanadium slag by oxalic acid hydrothermal leaching was proposed, by which the vanadium, titanium and chromium were extracted from VS directly and simultaneously under hydrothermal conditions by oxalic acid solution. The leaching extents of vanadium, titanium and chromium can reach 97.9, 98.4 and 93.3%. However, confronting this pregnant oxalic acid leachate, how to separate the vanadium, titanium and chromium becomes an urgent and important problem due to scarce attention paid to this complex organic acid solution system and thus, few studies carried out on it.
Based on our previous study and other published results [10,11,12], it can be found that under appropriate hydrothermal conditions, the vanadium complex ions that occurred in oxalic acid solution began to decompose and precipitate as vanadium oxide. Thus, an attempt of sequential hydrothermal separation of vanadium, titanium and chromium from the pregnant oxalic acid leachate was made in the present study. Fortunately, the results show that titanium oxide precipitates firstly from the pregnant leachate at a relatively low hydrothermal temperature, while the vanadium and chromium complex ions can remain in the solution. After filtering out the titanium oxide, a titanium-free filtrate (TFF) containing vanadium, chromium and impurities was obtained. Then, the next problem is how to separate vanadium and chromium from the TFF.
Considering the TFF after titanium separation, it is characterized by relatively low concentrations of vanadium and chromium and high contents of impurities. Therefore, it is better to purify the TFF and upgrade the concentrations of vanadium and chromium before further treatment. As for the purification and enrichment, solvent extraction and stripping are commonly adopted operations. Some investigations about the extraction of vanadium in oxalic acid leachate have been reported. For example, it was reported that about 99% of vanadium in oxalic acid leachate of waste catalyst can be extracted with Alamine-336 extractant by two-stage extraction and about 99.64% of vanadium in the oxalic acid leachate of shale can be extracted with Aliquat-336 extractant by five-stage extraction. The extracting mechanism was assumed to be the replacement of Cl− ion in Aliquat-336 extractant by VO(C2O4)22− complex anion in leachate [13,14]. Comparably, the vanadium (III and IV) in the present obtained TFF was identified to occur as V(C2O4)33− and VO(C2O4)22−, and chromium (III) mainly exists in the form of Cr(C2O4)33−. Therefore, an acidified anionic N235 extractant was selected as the co-extractant of vanadium and chromium from the filtrate.
Based on the above analysis, a process for separating titanium, vanadium and chromium from the oxalic acid leachate of VS was proposed and investigated systematically in the present study. In summary, this new process consists of the following steps: firstly, the titanium is precipitated from the oxalic acid leachate by hydrothermal method; next, the vanadium and chromium left in the TFF are extracted by acidified N235 extractant and separated from the impurities; then, the vanadium and chromium are stripped by HCl solution; finally, the vanadium and chromium in the stripping solution are coprecipitated by a hydrothermal method.
2. Materials and Methods
2.1. Materials
The titanium-, vanadium- and chromium-bearing oxalic acid leachate of VS was prepared by hydrothermal leaching, and the detailed preparation procedure can be found in our previous publication [9]. For brevity, only the main leaching parameters are provided here. The VS from HBIS Group ChengSteel of Chengde, China was hydrothermally leached at 125 °C for 90 min, with oxalic acid concentration of 25 wt% (Beijing Honghu United Chemical Products Co., Ltd., Beijing, China), liquid–solid mass ratio of 8:1 and iron powder of 3.2 wt% (Sinopharm chemical reagent Beijing Co., Ltd., Beijing, China). The components of the leachate analyzed by ICP Optical Emission Spectrometer (ICP-OES, OPTIMA 7000DV, Waltham, MA, USA) are listed in Table 1. It can be seen that, in addition to the principal component V, Ti and Cr, a certain amount of impurities of Fe, Mn, Si, Ca, Mg and Al exist in the leachate. The initial pH of the leachate was measured to be about 0.7.

Table 1.
Chemical components of the leachate (g/L).
In addition, the N235 extractant (AR) and 2-Octanol phase modifier (AR) produced by Beijing Honghu United Chemical Products Co., Ltd., Beijing, China and the kerosene diluent (AR) produced by Beijing Tongguang Fine Chemical Co., Ltd., Beijing, China. were used.
2.2. Procedure
The flow diagram of separating titanium, vanadium and chromium from the oxalic acid leachate of VS was proposed, as shown in Figure 1, including the following three segments. The first is the hydrothermal precipitation of titanium from the leachate to obtain TiO2 directly. The second is the extraction separation of vanadium and chromium from the TFF by solvent extraction, and the stripping solution enriching vanadium and chromium was obtained. The third is the hydrothermal precipitation of vanadium and chromium from the stripping solution. The product was mainly composed of VO2 and Cr2O3, which can be used to produce V-Cr based alloy.
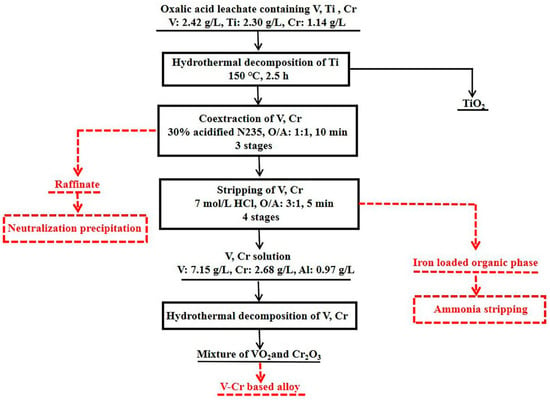
Figure 1.
Flow diagram of separating titanium, vanadium and chromium from the oxalic acid leachate of vanadium slag.
2.2.1. Hydrothermal Precipitation of Titanium
The first step is to separate the component titanium from the oxalic acid leachate by hydrothermal precipitation in a 50 mL autoclave (Jieang Instrument Co., Ltd., Shanghai, China), and the influences of reaction temperature and reaction time on the precipitation process were investigated. Specifically, 15 mL leachate was loaded into an autoclave, and the experiment was performed after setting the reaction temperature and reaction time. After the experiment, the autoclave was cooled to room temperature. Then, the solid and liquid were separated by filtration and washing. The solid product was dried in an oven at 60 °C for 5 h for X-ray diffraction analysis (XRD, MAC Science Co. Ltd., Kanagawa, Japan), Field Emission SEM analysis (SEM, JSM-6701F, Beijing, China), Fourier transform infrared spectroscopy analysis (FTIR, Thermo Fisher Nicolet iS50 spectrometer, Shanghai, China), and the liquid was analyzed by ICP Optical Emission Spectrometer (ICP-OES, OPTIMA 7000DV, Waltham, MA, USA) to determine the concentrations of ions. The precipitation extent (η) was calculated by Equation (1).
where, η is the precipitation extent (%); Cr and Cf represent the ion concentration in liquid before and after hydrothermal experiment (g/L), respectively; Vr and Vf represent the volume of liquid before and after hydrothermal experiment (L), respectively.
2.2.2. Extraction Separation of Vanadium and Chromium
After the component titanium was hydrothermally precipitated from the oxalic acid leachate, the components vanadium and chromium left in TFF were separated by the solvent extraction method. The organic solvent N235 (Beijing Honghu United Chemical Products Co., Ltd., Beijing, China) acidified with HCl solution was used as the extractant, and the acidification was performed by stirring the mixture of N235 and equal volume 1 mol/L HCl solution with stirring speed of 600 r/min for 30 min. Then, the extractant was mixed with the filtrate and stirred in a beaker at the speed of 500 r/min. Thereafter, the loaded organic phase (LOP) and the raffinate were separated in a separation funnel. Finally, the concentrations of ions in the raffinate were measured by ICP, and the extraction extent (E) was calculated by Equation (2).
where, E is the extraction extent (%); C0 is the ion concentration in extract (g/L); C1 is the ion concentration in raffinate (g/L).
To further recover the components vanadium and chromium from the loaded organic phase, a stripping step was performed. The LOP was mixed with stripping agent HCl in a beaker and stirred with a speed of 500 r/min. After the reaction, the lean organic phase and the stripping solution were separated in a separation funnel. The stripping extent (S) was calculated by Equation (3).
where, S is the stripping extent (%); V′Aq. and VOrg. represent the volumes of stripping solution and LOP (L), respectively; C′Aq. and COrg. represent the ion concentration in stripping solution and in organic phase (g/L), respectively.
2.2.3. Hydrothermal Precipitation of Vanadium and Chromium from Stripping Solution
Firstly, the pH value of stripping solution was adjusted to about 0.7 using sodium hydroxide. Then, 20 mL stripping solution was loaded into a 50 mL autoclave, and the experiment was performed after setting the reaction temperature and reaction time. After solid–liquid separation, the concentrations of ions in liquid and the phase of the product were analyzed by ICP and XRD. The η was calculated by Equation (1).
3. Results and Discussion
3.1. Hydrothermal Precipitation of Titanium from the Pregnant Leachate
3.1.1. Influence of Reaction Temperature on Titanium Precipitation
The reaction temperature is an important factor in hydrothermal experiments. Therefore, the influence of reaction temperature on the ηs was studied preferentially. The experiments were carried out at the reaction temperature of 140–170 °C and the reaction time of 1.5 h.
As shown in Figure 2, with the temperature varied from 140 to 170 °C, the η of titanium increased from 68.1 to 96.7%. However, when the temperature exceeded 150 °C, the ηs of impurities also increased significantly. The η was calculated by Equation (1). This led to the low purity of titanium dioxide and the loss of high-value vanadium. Therefore, the preferred reaction temperature was 150 °C.
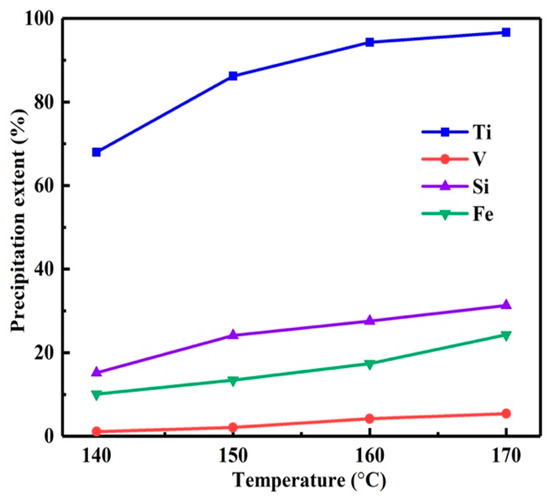
Figure 2.
Influence of reaction temperature on precipitation extents.
In order to clarify the hydrothermal decomposition process of leachate, the phase transition of products at different temperatures was characterized by XRD. As shown in Figure 3, the hydrothermal decomposition product of titanium was anatase titanium dioxide. However, with the increase in temperature, the characteristic peak intensity of titanium dioxide became weaker and the half peak width became wider. This may be caused by the production of amorphous silica. Therefore, in order to further understand the changes of impurities in hydrothermal decomposition process, the FTIR spectra of products at different temperatures were studied.
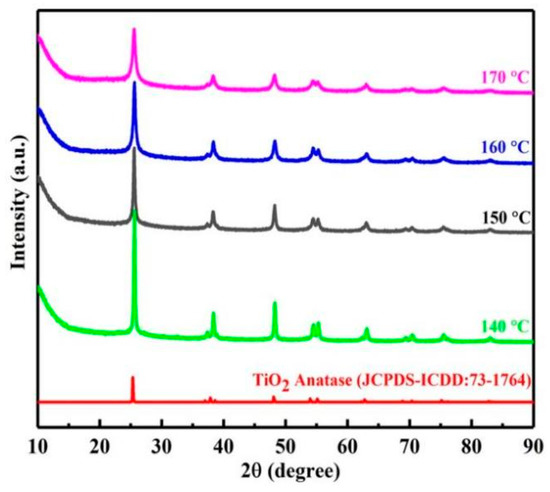
Figure 3.
XRD of products at different temperatures.
As can be seen from Figure 4, compared with the FTIR spectra of pure titanium dioxide, the characteristic peaks of impurities in the product were detected. Specifically, for impurity silicon, the peak at 1000–1250 cm−1 with large strength and a wide shape was attributed to the characteristic peak of Si-O-Si asymmetric stretching vibration, and the peak at 965 cm−1 belonged to the characteristic peak of Si-OH stretching vibration, indicating the formation of amorphous silicon dioxide. As the temperature rose from 140 °C to 170 °C, the intensity of the characteristic peak at 1000–1250 cm−1 increased, and 1062 cm−1 at the low wavenumber was shifted to 1097 cm−1 at the high wavenumber due to the increase in amorphous silica content [15].
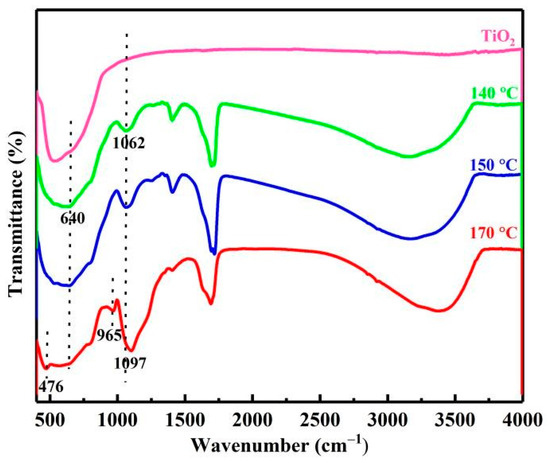
Figure 4.
FTIR of products at different temperatures.
For the impurity vanadium, the characteristic peaks of vanadium oxide should be concentrated in 400–1000 cm−1, most of which may be overlapped by the peaks of titanium dioxide. However, the V=O characteristic peak of vanadium dioxide (VO2) at 640 cm−1 was detected [16], which proved that vanadium dioxide (VO2) was a decomposition product of vanadium. Similarly, for impurity iron, the Fe-O characteristic peak of α-Fe2O3 at 476 cm−1 was detected at 170 °C, indicating that iron will precipitate in the form of Fe2O3 with the increase in temperature [17].
3.1.2. Influence of Reaction Time on Titanium Precipitation
Reaction time is another important factor in hydrothermal experiments, which affects the η and the micromorphology of the product. Therefore, the influence of reaction time on the η was investigated with the reaction temperature of 150 °C and the reaction time of 1.5 h–3 h. As depicted in Figure 5, prolonging the reaction time can improve the η of titanium, but it will also lead to the coprecipitation of impurities such as silicon, iron and vanadium.
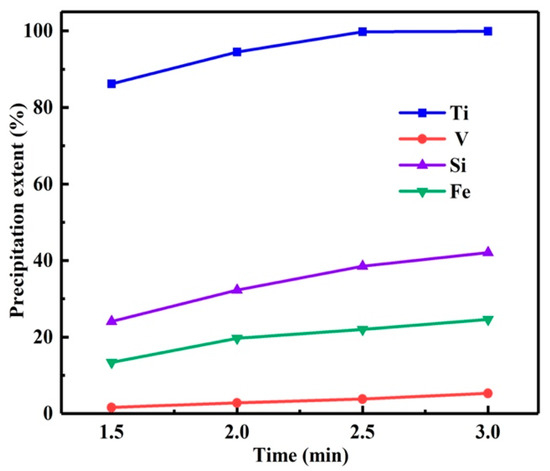
Figure 5.
Influence of reaction time on precipitation extents.
In order to further clarify the micromorphology change of titanium dioxide during hydrothermal decomposition, SEM analysis was performed. As shown in Figure 6, when the reaction time was 1.5 h, the particle size of titanium dioxide was uniformly about 200 nm, while when the reaction time was 2.5 h, the titanium dioxide particles aggregated and grew about 600 nm. Therefore, considering the η of titanium and the morphology of titanium dioxide, the reaction time was selected as 2.5 h.

Figure 6.
The micromorphology of products at 1.5 h (a) and 2.5 h (b).
3.1.3. Characteristics of the Precipitated Titanium Oxide
The decomposition product was prepared at 150 °C for 2.5 h. The valence state of titanium in the product was characterized by XPS, as shown in Figure 7, the binding energy peaks at 458.3 and 464.3 eV were assigned to Ti4+ 2p3/2 and Ti4+ 2p1/2 [18]. Therefore, titanium in the product exists in the form of anatase titanium dioxide with +4 valence. In addition, the purity of the product was analyzed by ICP, as shown in Table 2; the purity of titanium dioxide was 95.7%.
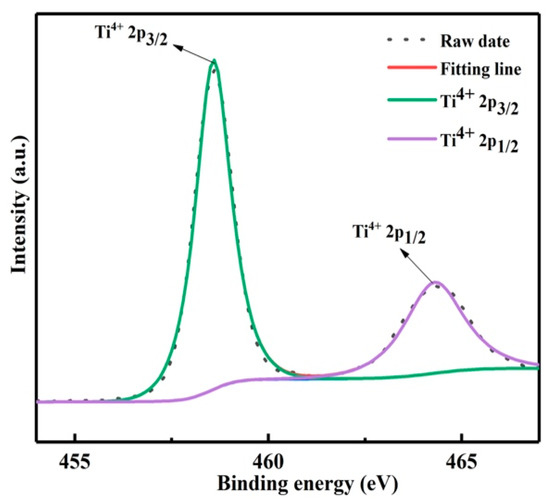
Figure 7.
XPS survey spectrum of Ti 2p.

Table 2.
Chemical composition of the product (wt%).
3.2. Solvent Extraction of Vanadium and Chromium from the TFF
3.2.1. Mechanism Analysis of Extraction Process
In order to clarify the extraction mechanism, the occurrence states of vanadium, chromium, iron and aluminum ions in the extract were studied. Firstly, the valence states of vanadium and chromium ions were analyzed by XPS, as shown in Figure 8a, the binding energy peaks at 515.29 and 522.61 eV were attributed to V3+ 2p3/2 and V3+ 2p1/2, and the binding energy peaks at 516.2 and 523.4 eV were assigned to V4+ 2p3/2 and V4+ 2p1/2. Therefore, the vanadium ions in the extract are composed of V3+ and V4+ [19]. The XPS results of chromium ions are shown in Figure 8b, the binding energies of 577.1 and 586.5 eV belonged to 2p3/2 and 2p1/2 of Cr3+, and the binding energies of 579.2 and 588.5 eV belonged to 2p3/2 and 2p3/2 of Cr6+. Therefore, chromium ions in the extract mainly exist in the form of Cr3+ and a small amount of Cr6+ [18,20].
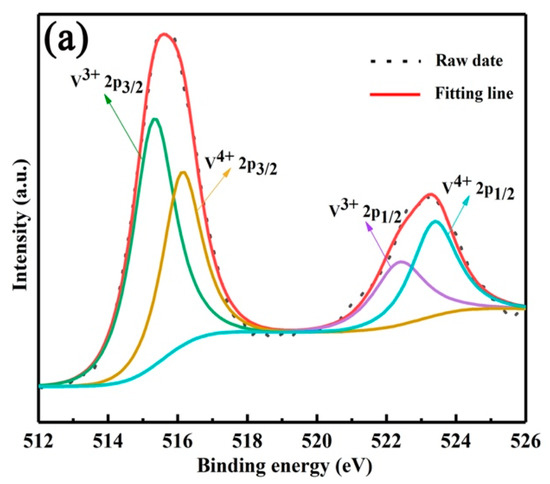
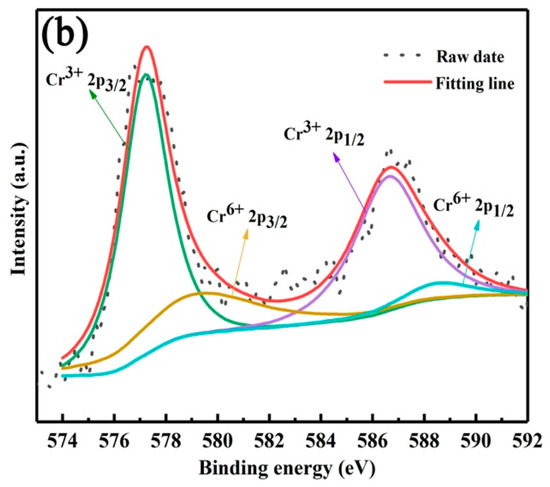
Figure 8.
XPS survey spectrum of V 2p (a) and Cr 2p (b).
Then, the complexing states of vanadium, chromium, iron and aluminum ions in the extract were analyzed by FTIR. As shown in Figure 9A the peak at 3417 cm−1 belonged to the O−H stretching vibrations of crystal water [21]. The peak at 1675 cm−1 was identified as the asymmetrical deformation vibrations νas(C=O). The peak at 1390 cm−1 was assigned to the symmetrical deformation vibrations νs(C−O) + ν(C−C) stretching band. The peak at 1253 cm−1 was assigned to the symmetrical deformation vibrations νs(C−O) + δ(O−C=O) bending vibration. The peak at about 810 cm−1 was attributed to the bending vibration δ(O−C=O) + ν(M−O) stretching band, M represent elements V, Cr, Fe, Al. The peaks at 986 and 547 cm−1 belonged to the V=O and V−O. The peak at about 485 cm−1 was attributed to the stretching vibration ν(Al−O) and ν(Fe−O). The peak at about 415 cm−1 was attributed to the stretching vibration ν(Cr−O). [22,23,24]. Therefore, complex ions of vanadium, chromium, iron and aluminum in the extract should exist in the form of V(C2O4)33−, VO(C2O4)22−, Cr(C2O4)33−, Fe(C2O4)33− and Al(C2O4)33−.
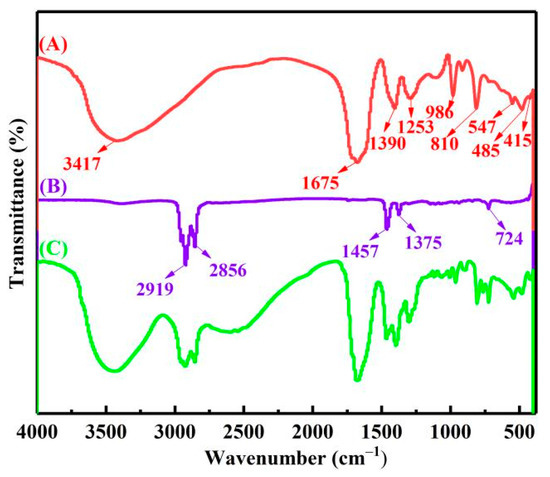
Figure 9.
The FTIR of extract (A) 30% N235 + 10% 2−octanol + 60% kerosene organic phase (B) loaded organic phase (C).
Figure 9B shows the FTIR spectra of the organic phase, with characteristic peaks of 2919, 2856, 1457, 1375 and 724 cm−1. The FTIR spectra of the LOP is shown in Figure 9C. After extraction, the complex anions of vanadium, chromium, iron and aluminum in the extract were anion exchanged with Cl− in the acidified N235 extractant, and transferred to the organic phase. Therefore, the extraction process can be represented by Equation (4)–(9).
(R3N)org + HCl =(R3NHCl)org
3(R3NHCl)org + V(C2O4)33− = ((R3NH)3·V(C2O4)3)org + 3Cl−
2(R3NHCl)org + VO(C2O4)22− = ((R3NH)2·VO(C2O4)2)org + 2Cl−
3(R3NHCl)org + Cr(C2O4)33− = ((R3NH)3·Cr(C2O4)3)org + 3Cl−
3(R3NHCl)org + Fe(C2O4)33− = ((R3NH)3·Fe(C2O4)3)org + 3Cl−
3(R3NHCl)org + Al(C2O4)33− = ((R3NH)3·Al(C2O4)3)org + 3Cl−
3.2.2. Influence Factors of Extracting Process
With the O/A of 3:1, extraction time of 10 min, the influence of N235 extractant concentration on Es was investigated as shown in Figure 10. With the N235 concentration from 20 to 35%, the Es of vanadium and chromium increased from 89.2, 55.3 to 97.4, 63.9%. Meanwhile, the impurities iron and aluminum were also co-extracted with the Es from 51.3, 49.9 to 70.2, 58.3%. The E is calculated by Equation (2). However, with the increase in N235 concentration, the viscosity of organic phase was increased, which led to the slow separation of organic phase and aqueous phase. Moreover, a third phase was found in the organic phase during the extraction process. Therefore, the concentration of N235 extractant was selected as 30%.
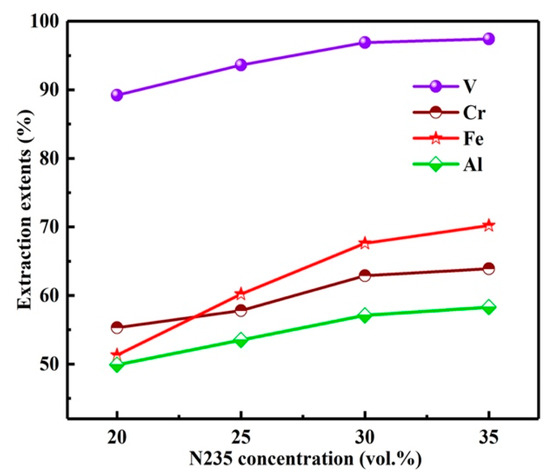
Figure 10.
Influence of N235 extractant concentration on extraction extents.
In order to improve the performance of the organic phase, phase modifier 2-Octanol was added. The influence of 2-octanol concentration on the extraction process was studied under the conditions of N235 extractant concentration of 30%, O/A of 3:1, extraction time of 10 min. The results are shown in Figure 11.
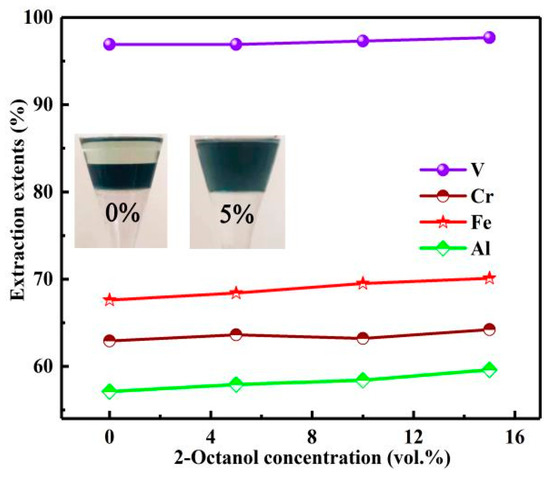
Figure 11.
Influence of 2-octanol concentration on extraction extents.
Although the phase modifier 2-octanol did not significantly improve the Es of vanadium and chromium, it could evidently improve the performance of organic phase. When the addition amount of 2-Octanol was 5%, the stratification of organic phase disappeared and the phase separation rate was accelerated. Therefore, in order to further improve the phase separation rate, the 2-Octanol concentration was selected as 10%.
Based on the above research, the composition of organic phase was determined as 30% N235 + 10% 2-Octanol + 60% sulfonated kerosene. With the fixed O/A of 3:1, the influence of extraction time on Es was studied.
As shown in Figure 12, with the extension of extraction time from 4 to 10 min, the Es of vanadium and chromium gradually increased, especially the E of chromium. In the extraction process, with the increase in extraction time, the concentrations of vanadium ions and chromium ions in aqueous phase decreased continuously, while the concentrations of vanadium ions and chromium ions in organic phase increased gradually to equilibrium, resulting in the slow growth of Es after 8 min. When the extraction time was 10 min, the Es of vanadium and chromium were 97.3, 63.2%. Further increasing the extraction time cannot significantly improve the Es of vanadium and chromium, but will increase the Es of impurities. Therefore, the extraction time was 10 min.
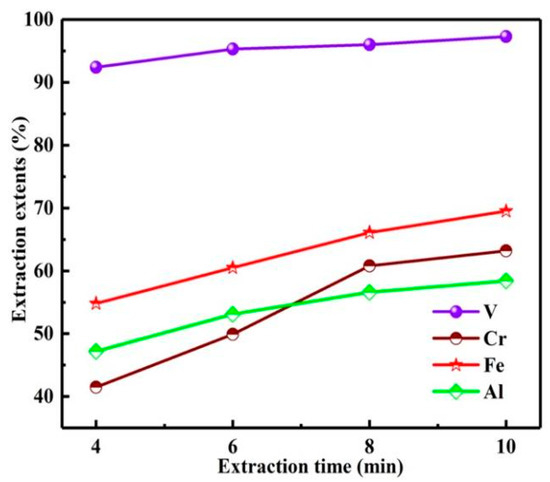
Figure 12.
Influence of extraction time on extraction extents.
The O/A is an important factor in the extraction process, which affects the Es and enrichment extents of vanadium and chromium. With organic phase of 30% N235 + 10% 2-octanol + 60% sulfonated kerosene, extraction time of 10 min, and the influence of O/A on the extraction extents was investigated.
As can be seen from the results in Figure 13, with the O/A increase from 1:1 to 4:1, the Es of vanadium and chromium increased from 88.2, 43.6 to 97.8, 64.8%. However, higher O/A is not conducive to the enrichment of vanadium and chromium. Therefore, multi-stage extraction was considered to enrich and extract vanadium and chromium at relatively low O/A. The O/A ratio was determined as 1:1 in this experiment.
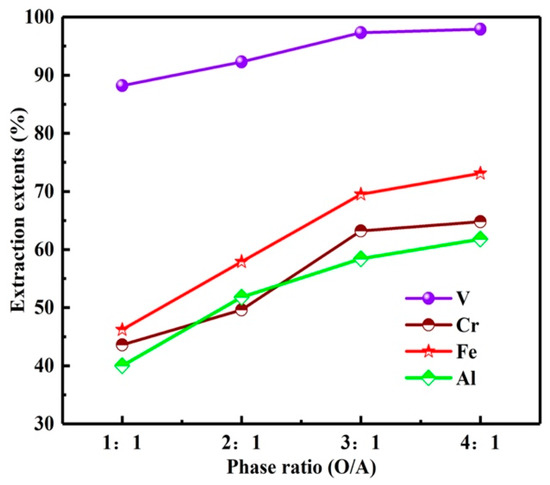
Figure 13.
Influence of O/A on extraction extents.
3.2.3. Extraction McCabe–Thiele Graph of Vanadium
In order to determine the number of extraction stages, the extraction McCabe–Thiele graph of vanadium was made according to different phase ratios, as shown in Figure 14. The theoretical stages number of countercurrent extraction of vanadium was two, which was determined by the number of horizontal lines. However, in order to ensure the E, the actual stage was one more stage than the theoretical stages in practice. With the organic phase of 30% N235 + 10% 2-octanol + 60% sulfonated kerosene, O/A of 1:1 and extraction time of 10 min, the experiments of three-stage countercurrent extraction were performed.
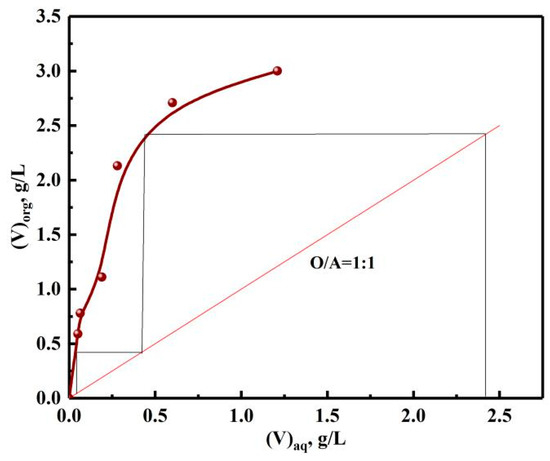
Figure 14.
Extraction McCabe–Thiele graph of vanadium.
As shown in Table 3, the Es of vanadium and chromium were 99.6 and 78.8%. Aluminum and iron were co-extracted with the Es of 91.2 and 95.6%, while other impurity elements were hardly extracted and remained in the raffinate. As for the impurity iron, it was further separated from vanadium and chromium in the stripping step. After extraction, vanadium and chromium were separated from a large number of impurities.

Table 3.
Experimental results of three-stage countercurrent extraction.
3.3. Stripping Vanadium and Chromium from LOP
3.3.1. Mechanism Analysis of Stripping Process
The stripping mechanism of vanadium, chromium, iron and aluminum ions in LOP in HCl solution was studied by FTIR, as shown in Figure 15. As discussed in the extraction mechanism, the peaks at 986, 810, 547, 485, 415 cm−1 belonged to V(C2O4)33−, VO(C2O4)22−, Cr(C2O4)33−, Fe(C2O4)33− and Al(C2O4)33− complex ions in LOP disappeared after stripping, which proved that vanadium and chromium iron and aluminum complex ions were stripped into the stripping solution. Comparing the mechanisms of extraction and stripping, it can be seen that in the extraction process, the Cl− ion in the acidified N235 extractant could be replaced by the complex anions of vanadium, chromium, iron and aluminum in the extract. In the stripping process, the complex anions of vanadium, chromium iron and aluminum loaded in the LOP could be replaced by the Cl− ion in the high-concentration HCl solution. Therefore, the acidity was an important factor affecting the stripping process. However, after stripping, iron ions still existed in the organic phase judged by stripping extent. According to the relevant research [25], Fe(C2O4)33− in the LOP can be stripped by high concentration HCl solution, and further reacts with Cl− ion to form FeCl4− and extracted into the organic phase again. The stripping process can be expressed by Equation (10)–(16).
((R3NH)3·V(C2O4)3)org + 3HCl = 3(R3NHCl)org + V(C2O4)33− + 3H+
((R3NH)2·VO(C2O4)2)org + 2HCl = 2(R3NHCl)org + VO(C2O4)22− + 2H+
((R3NH)3·Cr(C2O4)3)org + 3HCl = 3(R3NHCl)org + Cr(C2O4)33− + 3H+
((R3NH)3·Fe(C2O4)3)org + 3HCl = 3(R3NHCl)org + Fe(C2O4)33− + 3H+
((R3NH)3·Al(C2O4)3)org + 3HCl = 3(R3NHCl)org + Al(C2O4)33− + 3H+
Fe(C2O4)33- + 4Cl− = FeCl4− + 3C2O42−
(R3NHCl)org + FeCl4− = (R3NHFeCl4)org + Cl−
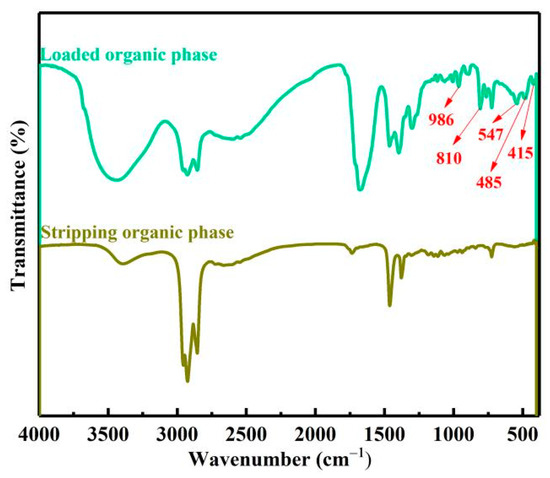
Figure 15.
The FTIR of loaded organic phase and lean organic phase.
3.3.2. Influence Factors of the Stripping Process
In order to strip vanadium and chromium from the LOP, the stripping experiments were carried out. With the O/A of 3:1 and time of 5 min, the influence of HCl concentration on the Ss of vanadium and chromium was studied, as shown in Figure 16.
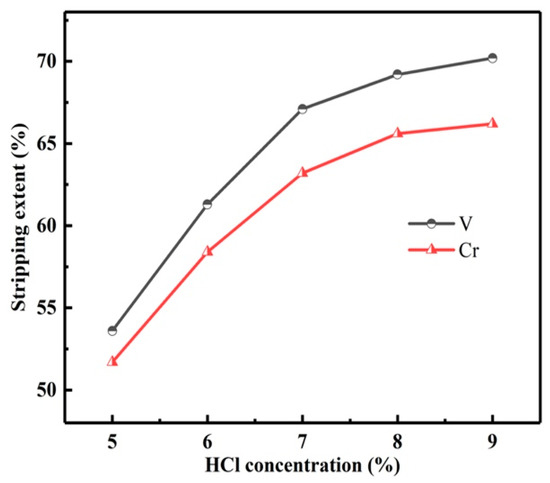
Figure 16.
Influence of HCl concentration on stripping extents of vanadium and chromium.
With the HCl concentration from 5 to 7 mol/L, the Ss of vanadium and chromium increased rapidly. However, with the HCl concentration higher than 7 mol/L, the Ss of vanadium and chromium increased slowly. The S is calculated by Equation (3). Therefore, the HCl concentration was determined to be 7 mol/L.
The reaction time is another important factor affecting the Ss of vanadium and chromium. Therefore, the influence of reaction time on the Ss of vanadium and chromium was studied, as shown in Figure 17.
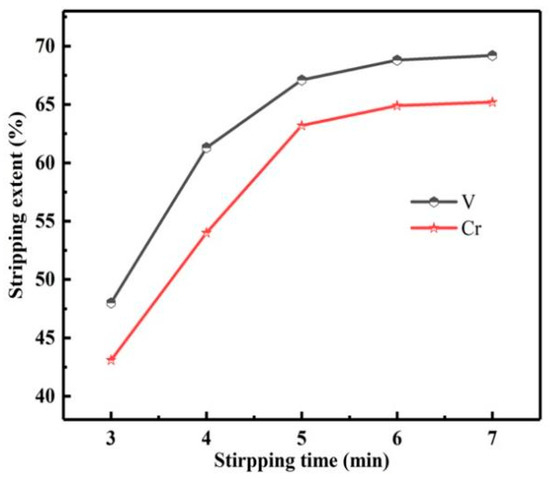
Figure 17.
Influence of stripping time on stripping extents of vanadium and chromium.
Prolonging the stripping time was conducive to the Ss of vanadium and chromium, but the Ss of vanadium and chromium increased slowly with the time more than 5 min. Therefore, multistage stripping could be considered to improve the Ss of vanadium and chromium.
3.3.3. Stripping McCabe–Thiele Graph of Vanadium
In order to determine the number of stripping stages, the stripping McCabe–Thiele graph of vanadium was drawn according to different phase ratios. As depicted in Figure 18, the theoretical stage number of countercurrent stripping of vanadium was three, which was determined by the number of horizontal lines. Similarly, four stages were required to ensure the S of vanadium.
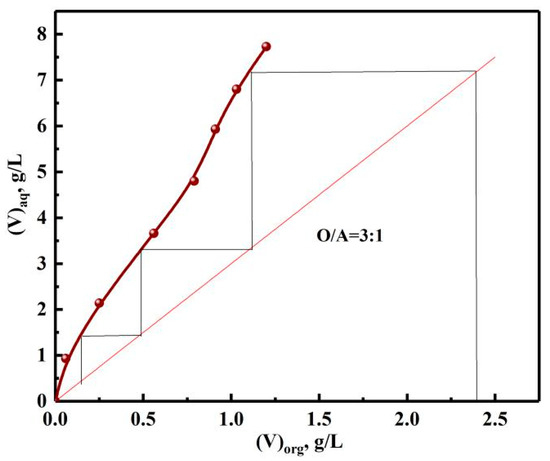
Figure 18.
Stripping McCabe–Thiele graph of vanadium.
With HCl concentration of 7 mol/L, O/A=3:1, and stripping time of 5 min, the Ss of vanadium, chromium were 99.7 and 99.3%. The components of the stripping solution are shown in Table 4. The results show that vanadium and chromium were separated from impurities and enriched from 2.42, 1.14 to 7.15, 2.68 g/L.

Table 4.
Chemical composition of four-stage countercurrent stripping solution.
3.4. Hydrothermal Precipitation of Vanadium and Chromium
With the reaction temperature from 210 to 240 °C, pH about 0.7, reaction time of 12 h, and reaction pressure of 2.9, 3.3, 3.9, and 4.6 Mpa. The influence of reaction temperature on ηs was investigated, as shown in Figure 19. With the increase in temperature, vanadium, chromium and aluminum ions were coprecipitated. When the reaction temperature was 240 °C, the ηs of vanadium, chromium and aluminum were 98.3, 97.6 and 96.5%, respectively. Then, the phase of the product was analyzed by XRD, as shown in Figure 20; the product was composed of VO2 and Cr2O3. The obtained mixture of vanadium, chromium and a small amount of aluminum is a good raw material for the production of V-Cr based alloy.
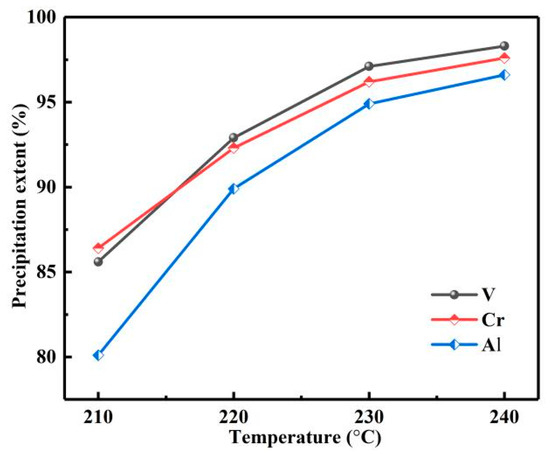
Figure 19.
Influence of reaction temperature on precipitation extents.
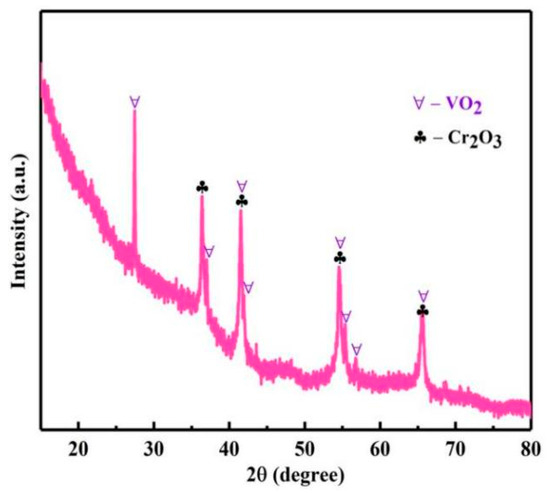
Figure 20.
XRD analysis of the product.
4. Conclusions
The process developed in this study is plausible for separating the titanium, vanadium and chromium sequentially from the oxalic acid leachate of VS. The detailed steps and results of the process are as follows:
1. The titanium in the pregnant leachate can be precipitated by a hydrothermal method. With the reaction temperature of 150 °C and reaction time of 2.5 h, more than 99% titanium was precipitated in the form of spherical anatase TiO2 with the purity of 95.7%.
2. With three-stage extraction of acidified N235 extractant and four-stage stripping of HCl solution, more than 99% vanadium and 78% chromium in the TFF can be transferred and enriched in the stripping solution. The concentration of vanadium was improved from 2.42 to 7.15 g/L, and chromium from 1.14 to 2.68 g/L.
3. The vanadium and chromium in the stripping solution can be coprecipitated by a hydrothermal method. Under the reaction temperature of 240 °C and reaction time of 12 h, the ηs of vanadium, chromium and aluminum were 98.3, 97.6 and 96.5%, respectively. The product is mainly composed of VO2, Cr2O3 and a small amount of aluminum.
Using this process, the titanium, vanadium and chromium in oxalic acid system can be separated in the form of TiO2 and mixtures of VO2 and Cr2O3. Furthermore, the TiO2 can be used to prepare pigment titanium dioxide, and the mixtures of VO2 and Cr2O3 can be used as raw material for the production of V-Cr based alloy.
Author Contributions
Investigation, data curation, writing—original draft preparation, Z.D.; software, formal analysis, resources, supervision, writing—review and editing, J.Z.; conceptualization, methodology, funding acquisition, project administration, B.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant number 52174274 and Fundamental Research Funds for the Central Universities, grant number FRF-MP-19-015 and FRF-MP-20-21.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| VS | vanadium slag |
| TFF | titanium-free filtrate |
| LOP | loaded organic phase |
| η | precipitation extent (%) |
| Cr | ion concentration in liquid before hydrothermal experiment (g/L) |
| Cf | ion concentration in liquid after hydrothermal experiment (g/L) |
| Vr | volume of liquid before hydrothermal experiment (L) |
| Vf | volume of liquid after hydrothermal experiment (L) |
| E | extraction extent (%) |
| C0 | ion concentration in extract (g/L) |
| C1 | ion concentration in raffinate (g/L) |
| S | stripping extent (%) |
| V′Aq. | volume of stripping solution (L) |
| VOrg. | volume of LOP (L) |
| C′Aq. | ion concentration in stripping solution (g/L) |
| COrg. | ion concentration in organic phase (g/L) |
References
- Xue, N.N.; Zhang, Y.M.; Huang, J.; Liu, T.; Wang, L.Y. Separation of impurities aluminum and iron during pressure acid leaching of vanadium from stone coal. J. Clean. Prod. 2017, 166, 1265–1273. [Google Scholar] [CrossRef]
- Fang, H.X.; Li, H.Y.; Xie, B. Effective Chromium Extraction from Chromium-containing Vanadium Slag by Sodium Roasting and Water Leaching. Isij Int. 2012, 52, 1958–1965. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, L.A.; Kushnarev, A.V.; Fomichev, M.S.; Rovnushkin, V.A. Oxygen-converter processing of vanadium-bearing hot metal. Steel Transl. 2013, 43, 587–592. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, T.; Liu, Y.; Xue, X. Extraction Behavior of Vanadium and Chromium by Calcification Roasting-Acid Leaching from High Chromium Vanadium Slag: Optimization Using Response Surface Methodology. Miner. Process. Extr. Metall. Rev. 2018, 40, 56–66. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Xue, Z. Oxidation Kinetics of Vanadium Slag Roasting in the Presence of Calcium Oxide. Miner. Process. Extr. Metall. Rev. 2017, 38, 265–273. [Google Scholar] [CrossRef]
- Lin, X.; Wang, X.; Cao, H. High-efficient extraction of vanadium and its application in the utilization of the chromium-bearing vanadium slag. Chem. Eng. J. 2016, 301, 132–138. [Google Scholar]
- Wen, J.; Jiang, T.; Xu, Y.Z.; Liu, J.Y.; Xue, X.X. Efficient Separation and Extraction of Vanadium and Chromium in High Chromium Vanadium Slag by Selective Two-Stage Roasting-Leaching. Met. Mater Trans B 2018, 49, 1471–1481. [Google Scholar] [CrossRef]
- Liu, B.; Du, H.; Wang, S.N.; Zhang, Y.; Zheng, S.L.; Li, L.J.; Chen, D.H. A novel method to extract vanadium and chromium from vanadium slag using molten NaOH. AICHE J. 2013, 59, 541–552. [Google Scholar] [CrossRef]
- Dong, Z.H.; Zhang, J.; Yan, B.J. Co-extraction of Vanadium Titanium and Chromium from Vanadium Slag by Oxalic Acid Hydrothermal Leaching with Synergy of Fe Powder. Met. Mater Trans B 2021, 52, 3961–3969. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Yan, B. A clean method of precipitation vanadium from the vanadium bearing oxalic acid leaching solution. Miner. Eng. 2021, 165, 106864. [Google Scholar] [CrossRef]
- Kang, Q.; Zhang, Y.; Bao, S. An environmentally friendly hydrothermal method of vanadium precipitation with the application of oxalic acid. Hydrometallurgy 2019, 185, 125–132. [Google Scholar] [CrossRef]
- Kumbour, P.; Sikong, L. Effect of Oxalic Acid and Temperature on Hydrothermal VO2 (B) Transformation to VO2 (M). Adv. Mater. Res. 2013, 785–786, 335–338. [Google Scholar] [CrossRef]
- Kim, H.I.; Lee, K.W.; Mishra, D.; Yi, K.M.; Hong, J.H.; Jun, M.K.; Park, H.K. Separation and recovery of vanadium from leached solution of spent residuehydrodesulfurization (RHDS) catalyst using solvent extraction. J. Ind. Eng. Chem. 2014, 20, 4457–4462. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Zhang, Y.; Liu, T.; Luo, D. Separation and recovery of vanadium and aluminum from oxalic acid leachate of shale by solvent extraction with Aliquat 336. Sep. Purif. Technol. 2020, 249, 116867. [Google Scholar] [CrossRef]
- Liu, H.L.; Hu, J.H.; Wang, H.; Wang, C.; Li, J.Q. Phase transformation in hydrogen reduction of copper slag. Chin. J. Process Eng. 2012, 12, 265. [Google Scholar]
- Botto, I.L.; Vassallo, M.B.; Baran, E.J.; Minelli, G. IR spectra of VO2 and V2O3. Mater. Chem. Phys. 1997, 50, 267–270. [Google Scholar] [CrossRef]
- Tu, S.H.; Luo, Z.C.; Liu, T.; Zhu, X.P.; Du, J. Preparation of different morphologies α-Fe2O3 by hydrothermal method. Mater. Rep. 2016, 30, 5. [Google Scholar]
- Liu, S.; He, X.; Wang, Y.; Wang, L. Cleaner and effective extraction and separation of iron from vanadium slag by carbothermic reduction-chlorination-molten salt electrolysis. J. Clean. Prod. 2020, 284, 124674. [Google Scholar] [CrossRef]
- Liu, X.; Xie, G.; Chi, H.; Qian, X.; Zhang, Y.; Luo, Y. A facile method for preparing VO2 nanobelts. Mater. Lett. 2008, 62, 1878–1880. [Google Scholar] [CrossRef]
- Mittal, J.; Konno, H.; Inagaki, M. Synthesis of graphite intercalation compounds with CrVI compounds using CrO3 and HCl at room temperature. Synth. Met. 1998, 96, 103–108. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y. Mechanism of vanadium selective separation from iron in shale under an environmentally friendly oxalate ligand system. Sep. Purif. Technol. 2021, 276, 119269. [Google Scholar] [CrossRef]
- Fujita, J.; Martell, A.; Nakamoto, K. Infrared Spectra of Metal Chelate Compounds. VI. A Normal Coordinate Treatment of Oxalato Metal Complexes. J. Chem. Phys. 1962, 36, 324–331. [Google Scholar] [CrossRef]
- Selbin, J.; Holmes, L.H.; Mcglynn, S.P. Electronic structure, spectra and magnetic properties of oxycations—IV ligation effects on the infra-red spectrum of the vanadyl ion. J. Inorg. Nucl. Chem. 1963, 25, 1359–1369. [Google Scholar] [CrossRef]
- Kharitonov, Y.Y.; Buslaev, Y.A. Infrared absorption spectra of oxyfluorides of some metals of the fourth and fifth groups of the periodic table. Russ. Chem. Bull. 1962, 11, 365–372. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Zhang, Y.; Liu, T.; Zheng, Q. Separation and recovery of vanadium and iron from oxalic-acid-based shale leachate by coextraction and stepwise stripping. Sep. Purif. Technol. 2020, 244, 116532. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).