Time-Resolved Four-Channel Jones Matrix Measurement of Birefringent Materials Using an Ultrafast Laser
Abstract
1. Introduction
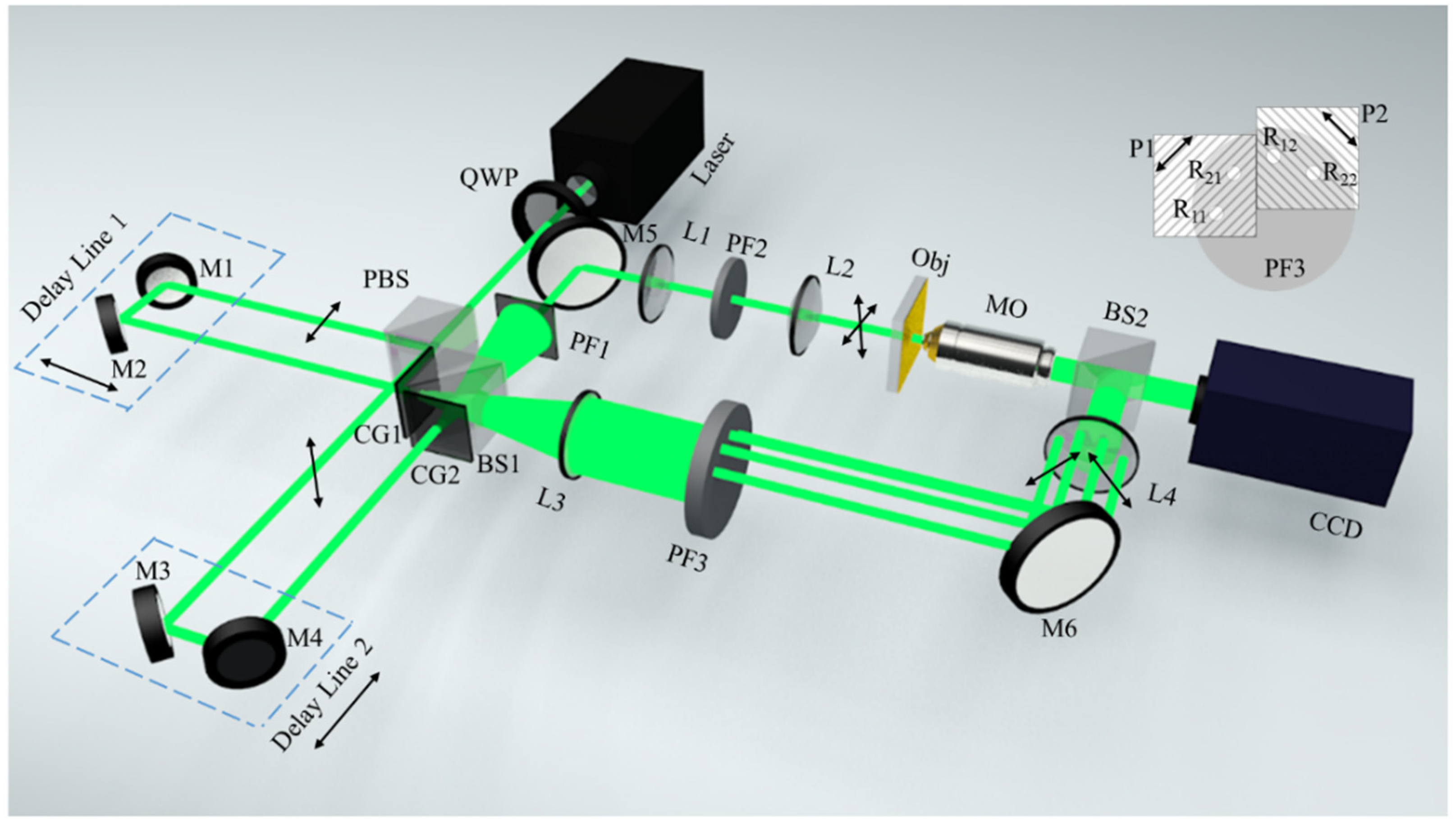
2. Materials and Methods
3. Results and Discussion
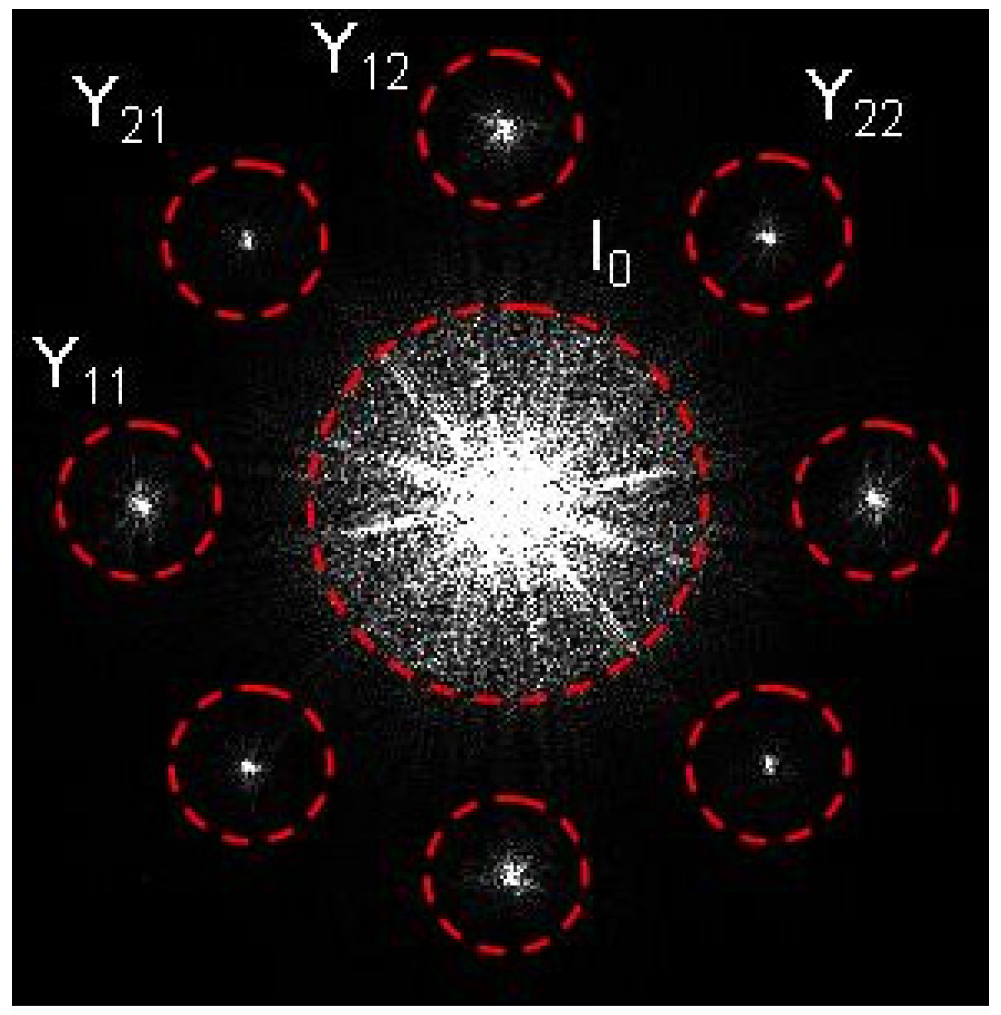
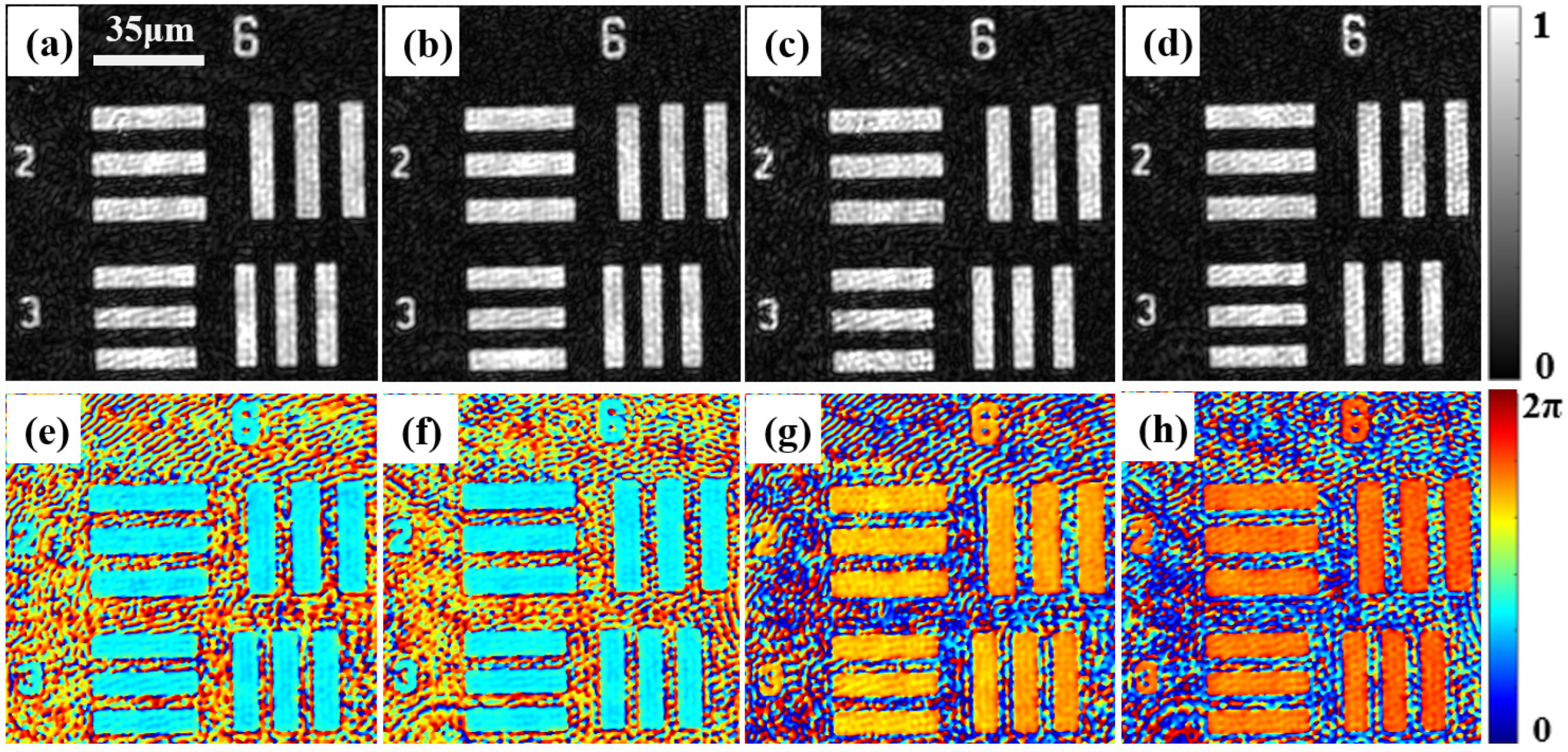
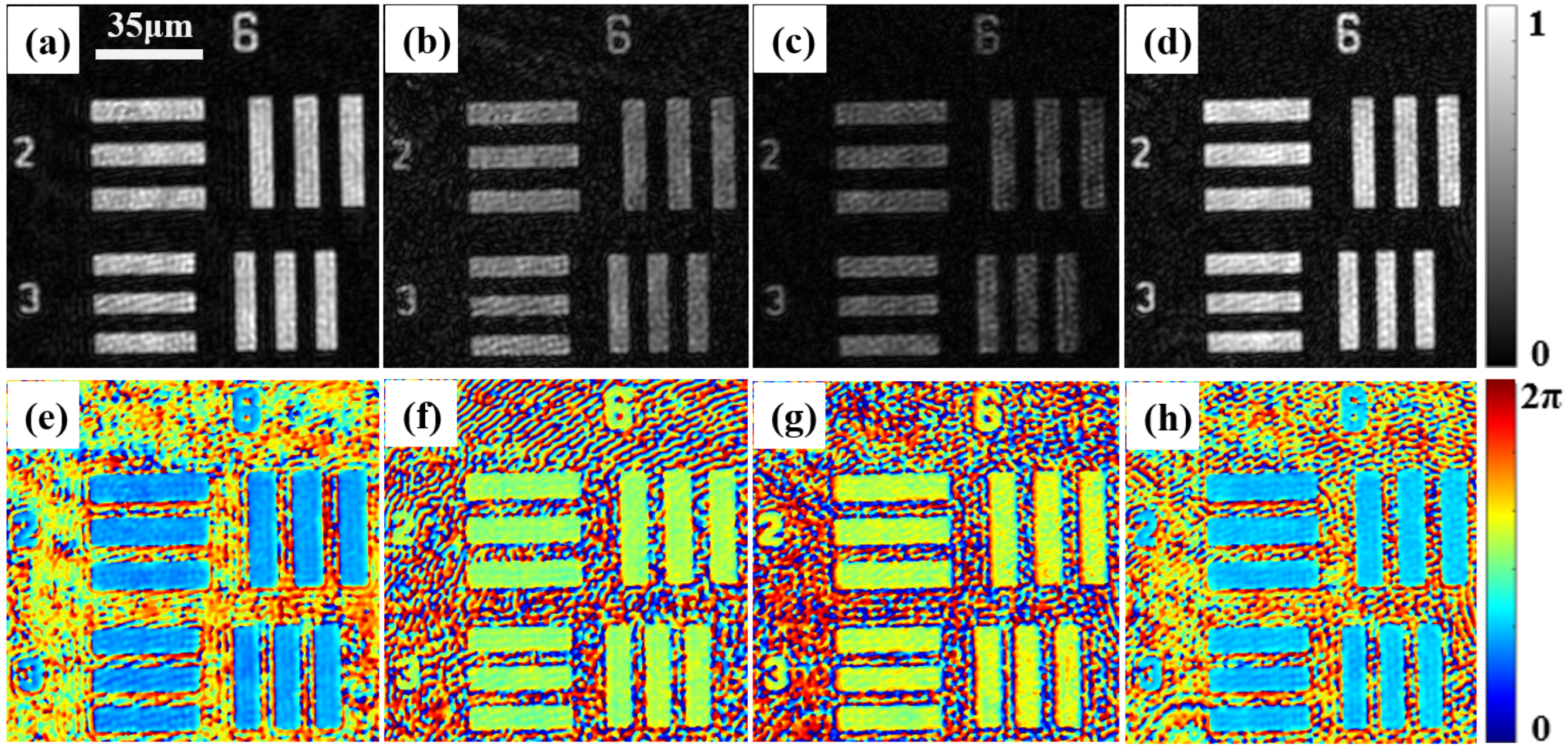
3.1. Characterization of the Spatial Resolution and Demonstration of the Consistency
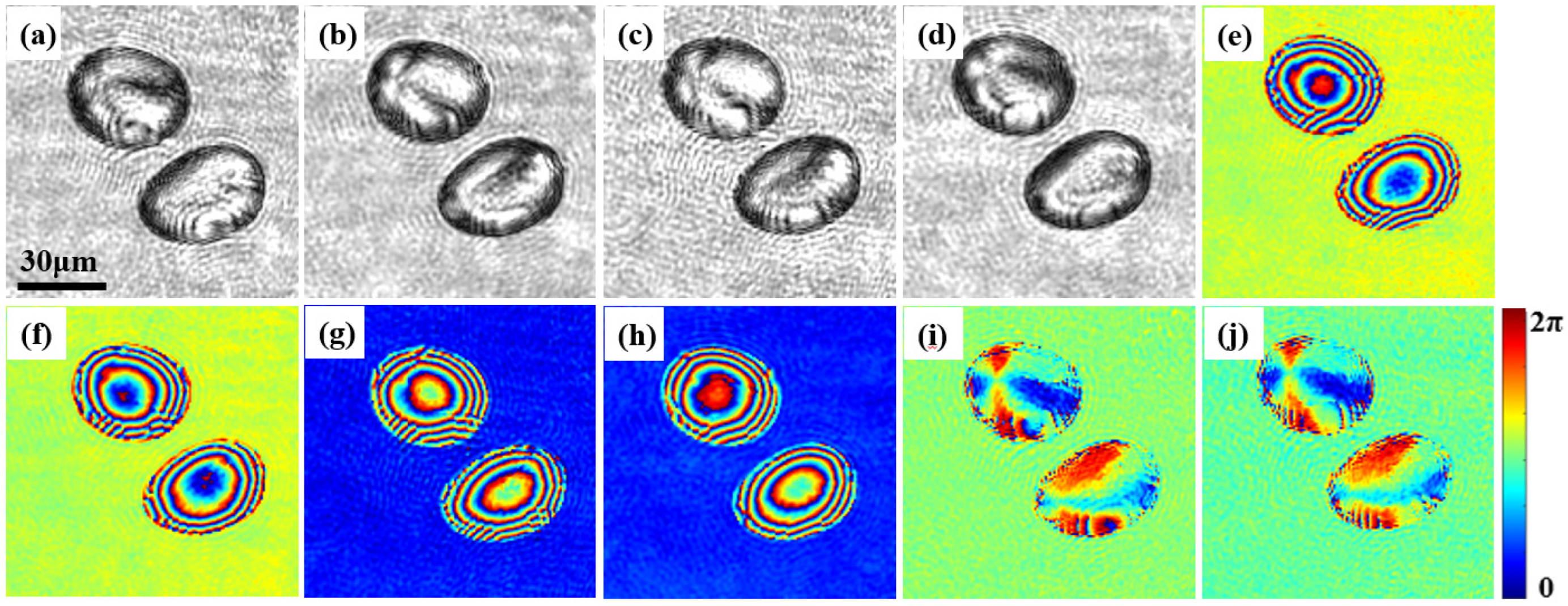
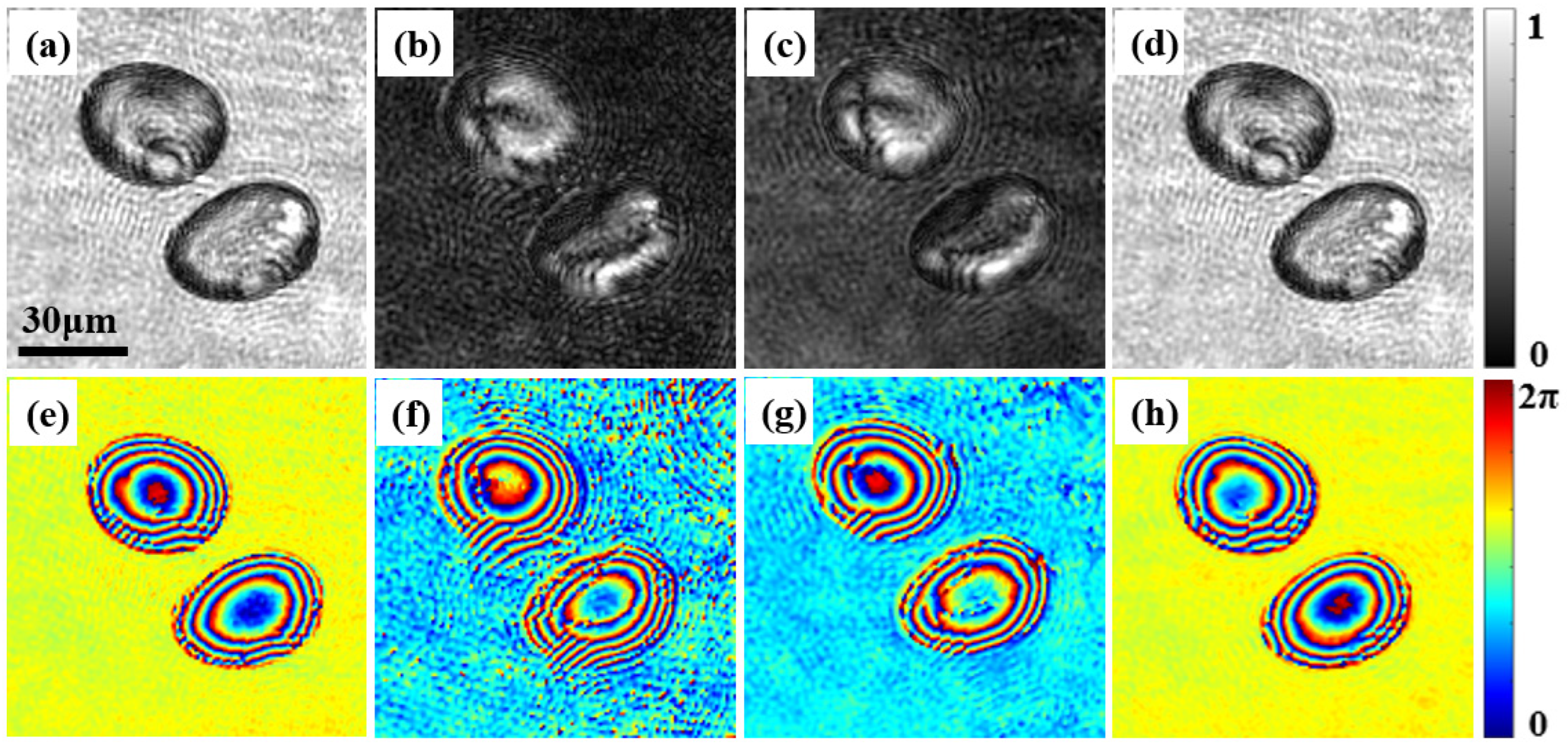
3.2. Jones Matrix Measurement of Potato Starch Granules
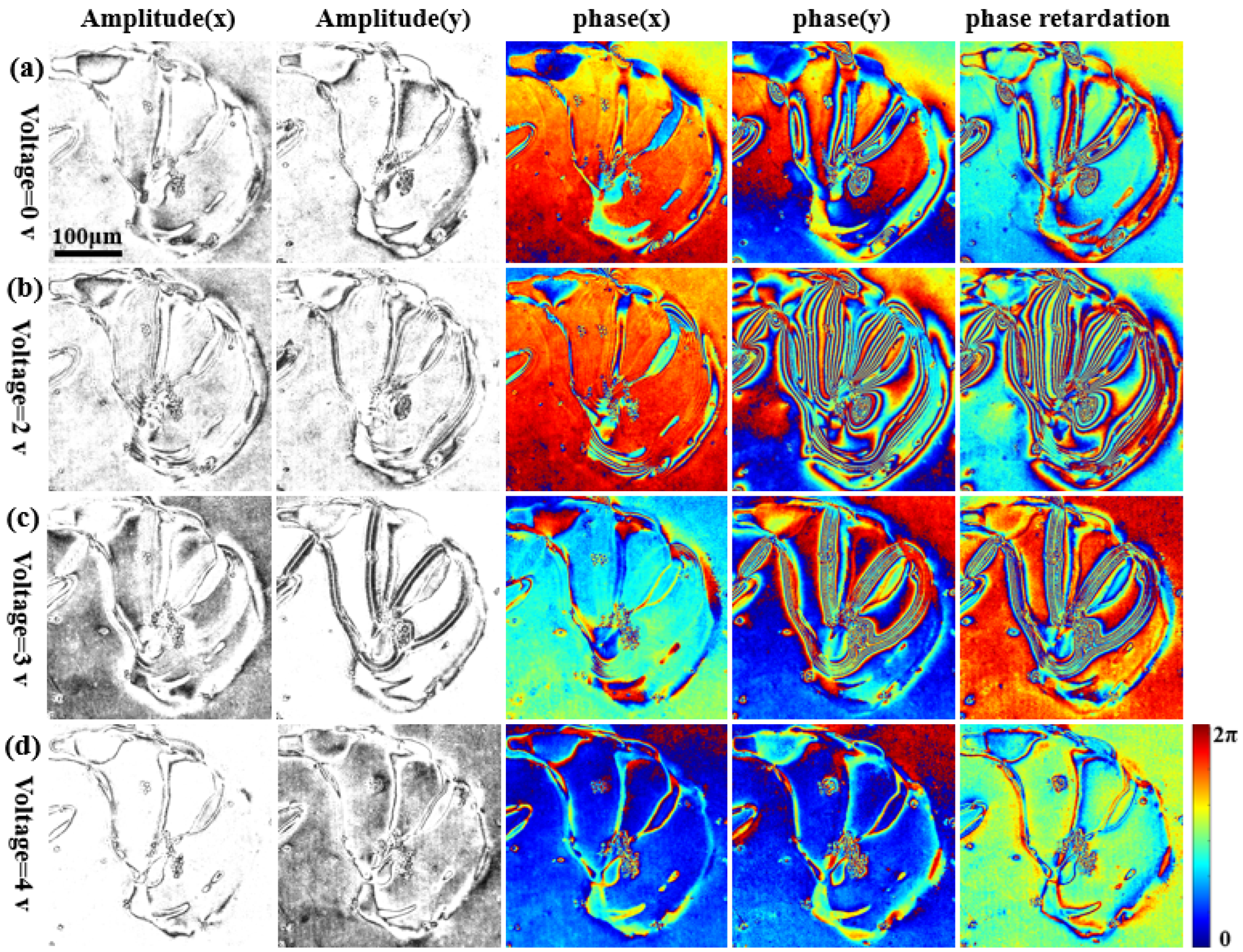
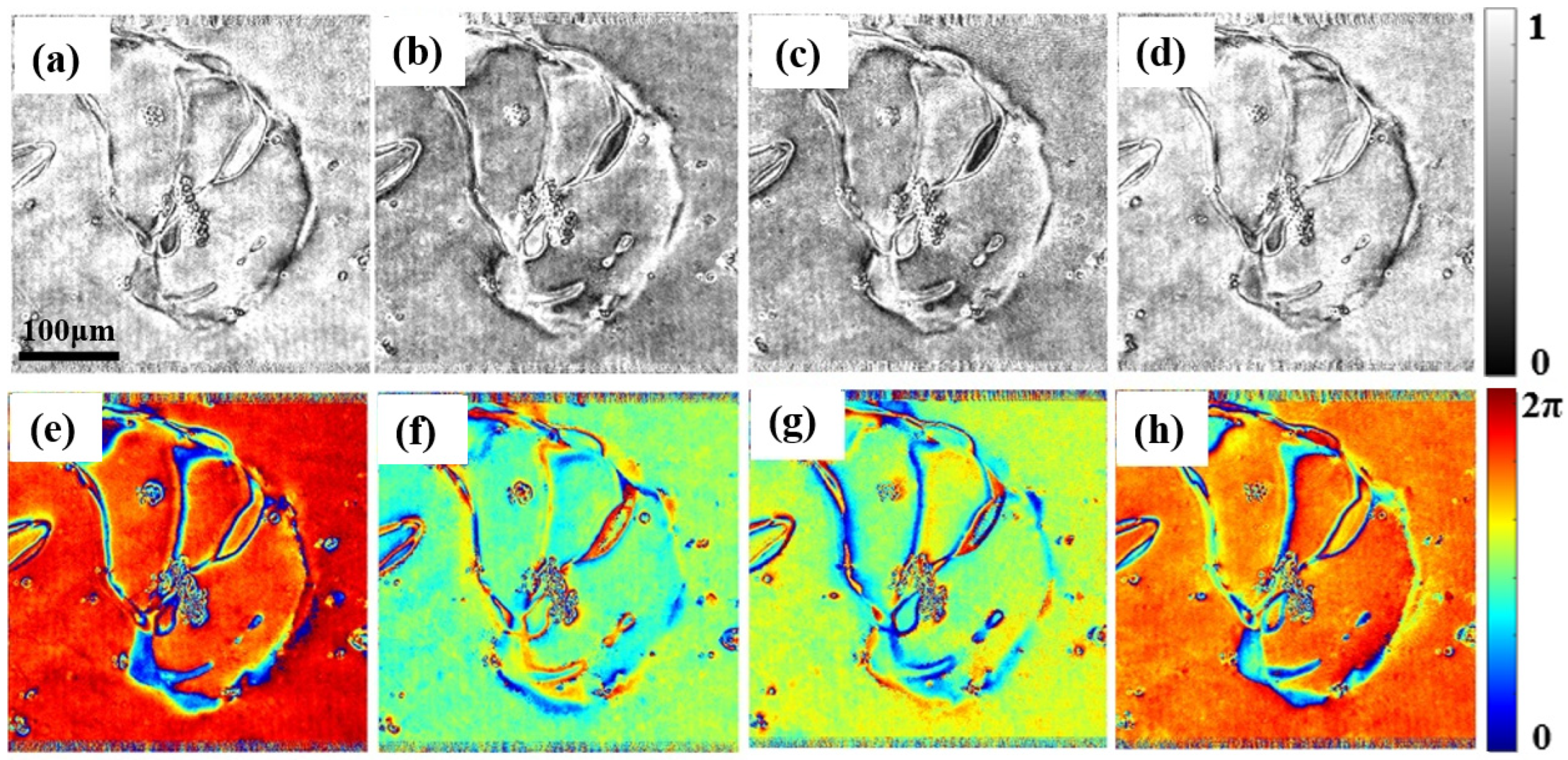
3.3. Measurement of Liquid Crystal Cell
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons: New York, NY, USA, 1983. [Google Scholar] [CrossRef]
- Park, Y.K.; Best, C.A.; Auth, T.; Gov, N.S.; Safran, S.A.; Popescu, G.; Suresh, S.; Feld, M.S. Metabolic remodeling of the human red blood cell membrane. Proc. Natl. Acad. Sci. USA 2010, 107, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Best, C.A.; Badizadegan, K.; Dasari, R.R.; Feld, M.S.; Kuriabova, T.; Henle, M.L.; Levine, A.J.; Popescu, G. Measurement of red blood cell mechanics during morphological changes. Proc. Natl. Acad. Sci. USA 2010, 107, 6731–6736. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Tangella, K.; Popescu, G. Blood testing at the single cell level using quantitative phase and amplitude microscopy. Biomed. Opt. Express 2011, 2, 3259–3266. [Google Scholar] [CrossRef]
- Mir, M.; Wang, Z.; Shen, Z.; Bednarz, M.; Bashir, R.; Golding, I.; Prasanth, S.G.; Popescu, G. Optical measurement of cycle-dependent cell growth. Proc. Natl. Acad. Sci. USA 2011, 108, 13124–13129. [Google Scholar] [CrossRef]
- Park, Y.; Best, C.A.; Kuriabova, T.; Henle, M.L.; Feld, M.S.; Levine, A.J.; Popescu, A.J. Measurement of the nonlinear elasticity of red blood cell membranes. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2011, 83, 051925. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.C.; Xiong, X.; Hu, Y.S.; Hu, Y.H.; Ma, G.B.; Peng, R.W.; Sun, C.; Wang, M. Controlling the polarization state of light with a dispersion-free metastructure. Phys. Rev. X 2014, 4, 021026. [Google Scholar] [CrossRef]
- Gansel, J.K.; Wegener, M.; Burger, S.; Linden, S. Gold helix photonic metamaterials: A numerical parameter study. Opt. Express 2010, 18, 1059–1069. [Google Scholar] [CrossRef]
- Goldberg, D.; Weissman, Z. Compact, high-resolution, self-referenced, optical activity polarimeter for high-pressure liquid chromatography systems. Appl. Opt. 2014, 53, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, P.U.; Møller, U.; Merbold, H. Investigation of aqueous alcohol and sugar solutions with reflection terahertz time-domain spectroscopy. Opt. Express 2007, 15, 14717–14737. [Google Scholar] [CrossRef]
- Yamamoto, A.; Kodama, S.; Matsunaga, A.; Hayakawa, K.; Yasui, Y.; Kitaoka, M. Multi-beam polarized photometric detector for high-performance liquid chromatography. J. Chromatogr. A 2001, 910, 217–222. [Google Scholar] [CrossRef]
- Chen, K.H.; Chu, Y.C.; Chen, J.H. Applying the phase difference property of polarization angle for measuring the concentration of solutions. Opt. Laser Technol. 2012, 44, 251–254. [Google Scholar] [CrossRef]
- Zimm, B.H. Apparatus and methods for measurement and interpretation of the angular variation of light scattering; preliminary results on polystyrene solutions. J. Chem. Phys. 1948, 16, 1099–1116. [Google Scholar] [CrossRef]
- Young, C.Y.; Pindak, R.; Clark, N.A.; Meyer, R.B. Light-scattering study of two-dimensional molecular- orientation fluctuations in a freely suspended ferroelectric liquid-crystal film. Phys. Rev. Lett. 1978, 40, 773–776. [Google Scholar] [CrossRef]
- Pine, D.J.; Weitz, D.; Zhu, J.; Herbolzheimer, E. Diffusing-wave spectroscopy: Dynamic light scattering in the multiple scattering limit. J. Phys. France 1990, 51, 2101–2127. [Google Scholar] [CrossRef]
- Fung, J.; Manoharan, V.N. Holographic measurements of anisotropic three-dimensional diffusion of colloidal clusters. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2013, 88, 020302. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Garmann, R.F.; Manoharan, V.N. Tracking E. coli runs and tumbles with scattering solutions and digital holographic microscopy. Opt. Express 2016, 24, 23719–23725. [Google Scholar] [CrossRef]
- Tuchin, V.V.; Wang, L.V.; Zimnyakov, D.A. Optical Polarization in Biomedical Applications; Springer: Berlin, Germany, 2006. [Google Scholar] [CrossRef]
- Mir, M.; Bhaduri, B.; Wang, R.; Zhu, R.; Popescu, G. Chapter 3: Quantitative Phase Imaging. In Progress in Optics; Wolf, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 57, pp. 133–217. [Google Scholar] [CrossRef]
- Lee, K.; Kim, K.; Jung, J.; Heo, J.H.; Cho, S.; Lee, S.; Chang, G.; Jo, Y.J.; Park, H.; Park, Y.K. Quantitative phase imaging techniques for the study of cell pathophysiology: From principles to applications. Sensors 2013, 13, 4170–4191. [Google Scholar] [CrossRef]
- Kim, Y.; Jeong, J.; Jang, J.; Kim, M.W.; Park, Y. Polarization holographic microscopy for extracting spatio-temporally resolved Jones matrix. Opt. Express 2012, 20, 9948–9955. [Google Scholar] [CrossRef]
- Wang, Z.; Millet, L.J.; Gillette, M.U.; Popescu, G. Jones phase microscopy of transparent and anisotropic samples. Opt. Lett. 2008, 33, 1270–1272. [Google Scholar] [CrossRef]
- Han, L.; Cheng, Z.J.; Yang, Y.; Wang, B.Y.; Yue, Q.Y.; Guo, C.S. Double-channel angular-multiplexing polarization holography with common-path and off-axis configuration. Opt. Express 2017, 25, 21877–21886. [Google Scholar] [CrossRef]
- Lemus-Alonso, G.P.; Fabian, C.M.; Montiel, R.K. One-shot carrier fringe polarimeter in a double-aperture common-path interferometer. Opt. Express 2018, 26, 17624–17634. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, B.Y.; Guo, C.S. One-step Jones matrix polarization holography for extraction of spatially resolved Jones matrix of polarization-sensitive materials. Opt. Lett. 2014, 39, 6170–6173. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Han, L.; Guo, C.S. Fiber-based lensless polarization holography for measuring Jones matrix parameters of polarization-sensitive materials. Opt. Express 2017, 25, 7288–7299. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.R.; Wu, Z.; Poenie, M. Modulated polarization microscopy: A promising new approach to visualizing cytoskeletal dynamics in living cells. Biophys. J. 2001, 80, 972–985. [Google Scholar] [CrossRef][Green Version]
- Shin, I.H.; Shin, S.M.; Kim, D.Y. New, simple theory-based, accurate polarization microscope for birefringence imaging of biological cells. J. Biomed. Opt. 2010, 15, 016028. [Google Scholar] [CrossRef][Green Version]
- Dragomir, N.M.; Goh, X.M.; Curl, C.L.; Delbridge, L.M.; Roberts, A. Quantitative polarized phase microscopy for birefringence imaging. Opt. Express 2007, 15, 17690–17698. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.D.; Park, K.; Kang, Y.G.; Lee, K.J.; Kim, B.-M.; Choi, Y. Single-shot digital holographic microscopy for quantifying a spatially resolved Jones matrix of biological specimens. Opt. Express 2016, 24, 29302–29311. [Google Scholar] [CrossRef]
- Jung, J.H.; Kim, J.; Seo, M.-K.; Park, Y.K. Measurements of polarization-dependent angle-resolved light scattering from individual microscopic samples using Fourier transform light scattering. Opt. Express 2018, 26, 7701–7711. [Google Scholar] [CrossRef]
- Colomb, T.; Dahlgren, P.; Beghuin, D.; Cuche, E.; Marquet, P.; Depeursinge, C. Polarization imaging by use of digital holography. Appl. Opt. 2002, 41, 27–37. [Google Scholar] [CrossRef]
- Bellouard, Y.; Colomb, T.; Depeursinge, C.; Dugan, M.; Said, A.A.; Bado, P. Nanoindentation and birefringence measurements on fused silica specimen exposed to low-energy femtosecond pulses. Opt. Express 2006, 14, 8360–8366. [Google Scholar] [CrossRef]
- Yue, Q.Y.; Cheng, Z.J.; Han, L.; Yang, Y.; Guo, C.S. One-shot time-resolved holographic polarization microscopy for imaging laser-induced ultrafast phenomena. Opt. Express 2017, 25, 14182–14191. [Google Scholar] [CrossRef] [PubMed]
- Cuche, E.; Marquet, P.; Depeursinge, C. Spatial filtering for zero-order and twin-image elimination in digital off-axis holography. Appl. Opt. 2000, 39, 4070–4075. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ortiga, E.; Doblas, A.; Saavedra, G.; Martínez-Corral, M.; Garcia-Sucerquia, J. Off-axis digital holographic microscopy: Practical design parameters for operating at diffraction limit. Appl. Opt. 2014, 53, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Doblas, A.; Sánchez-Ortiga, E.; Martínez-Corral, M.; Garcia-Sucerquia, J. Study of spatial lateral resolution in off-axis digital holographic microscopy. Opt. Commun. 2015, 352, 63–69. [Google Scholar] [CrossRef]
- De Gennes, P.G.; Prost, J. The Physics of Liquid Crystals, 2nd ed.; Oxford University Press: Oxford, UK, 1993. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Zhang, Y.; Liu, X.; Guo, C.; He, C.; Liu, G.; Song, H. Time-Resolved Four-Channel Jones Matrix Measurement of Birefringent Materials Using an Ultrafast Laser. Materials 2022, 15, 7813. https://doi.org/10.3390/ma15217813
Cheng Z, Zhang Y, Liu X, Guo C, He C, Liu G, Song H. Time-Resolved Four-Channel Jones Matrix Measurement of Birefringent Materials Using an Ultrafast Laser. Materials. 2022; 15(21):7813. https://doi.org/10.3390/ma15217813
Chicago/Turabian StyleCheng, Zhenjia, Yuqin Zhang, Xuan Liu, Chengshan Guo, Changwei He, Guiyuan Liu, and Hongsheng Song. 2022. "Time-Resolved Four-Channel Jones Matrix Measurement of Birefringent Materials Using an Ultrafast Laser" Materials 15, no. 21: 7813. https://doi.org/10.3390/ma15217813
APA StyleCheng, Z., Zhang, Y., Liu, X., Guo, C., He, C., Liu, G., & Song, H. (2022). Time-Resolved Four-Channel Jones Matrix Measurement of Birefringent Materials Using an Ultrafast Laser. Materials, 15(21), 7813. https://doi.org/10.3390/ma15217813




