Abstract
Solid-state reaction was used for Li7La3Zr2O12 material synthesis from Li2CO3, La2O3 and ZrO2 powders. Phase investigation of Li7La3Zr2O12 was carried out by x-ray diffraction (XRD), scanning electron microscopy (SEM) and energy-dispersive x-ray spectroscopy (EDS) methods. The thermodynamic characteristics were investigated by calorimetry measurements. The molar heat capacity (Cp,m), the standard enthalpy of formation from binary compounds (ΔoxHLLZO) and from elements (ΔfHLLZO), entropy (S0298), the Gibbs free energy of the Li7La3Zr2O12 formation (∆f G0298) and the Gibbs free energy of the LLZO reaction with metallic Li (∆rGLLZO/Li) were determined. The corresponding values are Cp,m = 518.135 + 0.599 × T − 8.339 × T−2, (temperature range is 298–800 K), ΔoxHLLZO = −186.4 kJ·mol−1, ΔfHLLZO = −9327.65 ± 7.9 kJ·mol−1, S0298 = 362.3 J·mol−1·K−1, ∆f G0298 = −9435.6 kJ·mol−1, and ∆rGLLZO/Li = 8.2 kJ·mol−1, respectively. Thermodynamic performance shows the possibility of Li7La3Zr2O12 usage in lithium-ion batteries.
1. Introduction
The commercial history of the lithium-ion battery was started in 1991 by Sony [1]. Since then, a lot of effort has been directed to improving the electrochemical performance of lithium-ion batteries [2]. One of the perspective methods of stabilizing lithium-ion battery electrochemical characteristics and safety is to apply solid-state inorganic electrolyte instead of liquid organic electrolyte as the traditional electrolyte for commercial lithium-ion batteries [3,4,5,6,7]. Some solid-state electrolytes have high ionic conductivity in an order of magnitude of ~10−3 S·cm−1 [8] in comparison to liquid electrolyte [9].
Between all types of the solid-state electrolytes (perovskite, NASICON- and LISICON-type, LATP- and LAGP-type, garnet, sulfide and halide electrolytes, etc. [8]) garnet-type electrolytes have the most attractive electrochemical performance in combination with manufacturing costs and simplicity in commercial application. Garnet-type Li7La3Zr2O12 (LLZO) solid-state electrolyte has two modifications: cubic and tetragonal. The ionic conductivities are ~10−4–10−3 S·cm−1 and ~10−7–10−6 S·cm−1, respectively [10].
LLZO solid-state electrolyte attracts high attention due to its relatively high electrochemical properties. Though LLZO has lower ionic conductivity in comparison with organic liquid electrolyte (~10−4 versus ~10−2 S·cm−1, respectively [9]), it provides high safety performance, high chemical stability against metallic lithium, a wide electrochemical potential window, low electronic conductivity, and high stability with moisture in the air; LLZO prevents lithium dendrite growth due to high mechanical strength [11,12,13,14,15].
Since as LLZO was first synthesized by Murugan et al. [16], it was investigated to improve its chemical and structural stability, long life cycle, electrode/solid electrolyte interface interactions, and high energy density at room temperature. Thus, heterovalent substitution/doping with Al3+ from alumina crucible (or intentional incorporation) during the synthesis process allows for the enhancement of ionic conductivity up to ~10−3 S·cm−1, but it causes higher activation energy in lithium ion conduction, which limits Li+ mobility [17,18,19,20,21,22,23,24]. Doped with Ga3+ also as Al3+ stabilize structure of LLZO [25,26,27,28,29,30,31,32]. The substitution of Zr4+ with Ta5+ ions allowed for an increase of the ionic conductivity, stabilization of the cubic structure, improved lithium-ion transport, lithium dendrite growth prevention, and the current density [33,34,35,36,37,38]. Ultimately, the above-mentioned elements improve electrochemical and structural stability, increase the ionic conductivity, and prevent lithium dendrite growth and penetration at the solid electrolyte structure.
In this work, synthesis, structure studies and thermodynamics calculations of tetragonal Li7La3Zr2O12 were performed.
2. Materials and Methods
Tetragonal LLZO electrolyte was produced by solid-state synthesis as one of the commonly used synthesis methods for investigation and mass manufacture [39,40,41,42,43,44,45,46,47]. Initial materials Li2CO3 (Xilong Sci., 99%), La2O3 (ReLAB, 99.99%), and ZrO2 (Sinopharm, 99.9%) in stoichiometric ratio were used as sources for Li, La, and Zr, respectively. Excess of 10 wt.% of lithium was initially added to precursor to avoid lithium loss during the synthesis process at high temperatures. Lanthanum oxide was preliminarily dried at 900 °C for 24 h. The mentioned materials were mechanically milled in an agate mortar and then dissolved in acetic acid with subsequent magnetic stirring at 90 °C for 12 h to provide a homogeneous solution. Excess acetic acid was evaporated at 110 °C to get dry precursor powder. Dried precursor was then mechanically milled in an agate mortar and put into an alumina crucible for heat treatment. A muffle furnace (Nabertherm, Lilienthal, Germany) was used for solid-state reaction at air atmosphere. First, the precursor was slowly heated (heat rate was 0.5 °C/min) to 130 °C for 3 h to evaporate the remaining acetic acid. Then, the precursor was heated (heat rate was 2 °C/min) to 900 °C for 8 h to provide solid-state reaction.
The solid-state reaction proceeds according to next formula:
4ZrO2 + 3La2O3 + 7Li2CO3 = 2Li7La3Zr2O12 + 7CO2
X-ray diffraction structural analysis (XRD) was performed by Bruker D8 Advance (Bruker, Karlsruhe, Germany) equipment (diffraction angle step was 0.02°, Cu Kα-radiation). The Rietveld method was used for structure refinement. Diffraction angles for synthesized LLZO powder were set from 15° to 60° (2Ѳ).
Images of the microstructure performance of LLZO powder were taken with a scanning electron microscope (SEM) Tescan MAIA3 (Tescan, Brno, Czech Republic) with secondary electron detection. Bruker XFlash 6–10 (Bruker, Karlsruhe, Germany) was used for energy-dispersive X-ray spectroscopy (EDS).
TAM IV Microcalorimeter (TA Instruments, Shanghai, China) was used for calorimetric investigation. Measurement parameters were as follows: temperature is 298 K, volume of the cell is 20 mL. An aqueous solution of 1 mol·dm−3 HCl was filled in the ampoule at calorimetric cell. The dissolution process of the LLZO powder was started after thermal equilibrium was established. Dissolution enthalpy value was obtained from thermoelectromotive force data during the dissolution process, providing the heat dissolution curve.
3. Results
The XRD pattern of synthesized LLZO is shown at Figure 1. According to diffraction data, LLZO has a I41/acd space group. The vertical lines at the bottom are related to PDF #00-064-0140. The peak indexes and interplanar distances are shown in the Supplementary Materials (Table S1). Synthesized material contains 4 wt.% of La2O3 impurity after solid-state reaction.
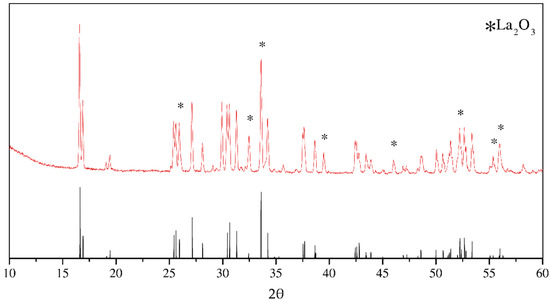
Figure 1.
X-ray diffraction pattern of the synthesized tetragonal Li7La3Zr2O12 by solid-state reaction. Bottom vertical lines belong to PDF #00-064-0140.
SEM images of LLZO powder are shown in Figure 2, made at 2×, 3.5×, 10× and 11.5× magnification, respectively. All images were performed at 10 keV landing energy.
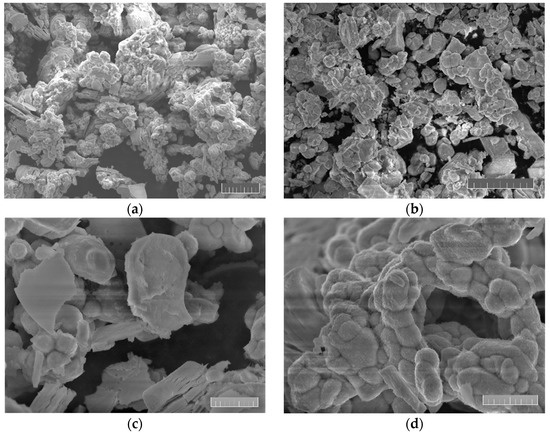
Figure 2.
SEM images of synthesized LLZO powder at different magnification. The scale bar is (a,b) 20 μm and (c,d) 5 μm long.
EDS spectra images are shown at Figure 3. The scale bar is 80 μm long for all images at Figure 3a–d. The green frame in Figure 3a shows the EDS analyzing field. Figure 3b–d show the element distributions for the La, Zr, O and C at Figure 3b; La at Figure 3c; and Zr at Figure 3d elements, respectively. The elements in Figure 3 are evenly distributed. The carbon in Figure 3b is electrically conductive carbon tape for sample holder. Elemental analysis of EDS spectra is shown at Table 1.
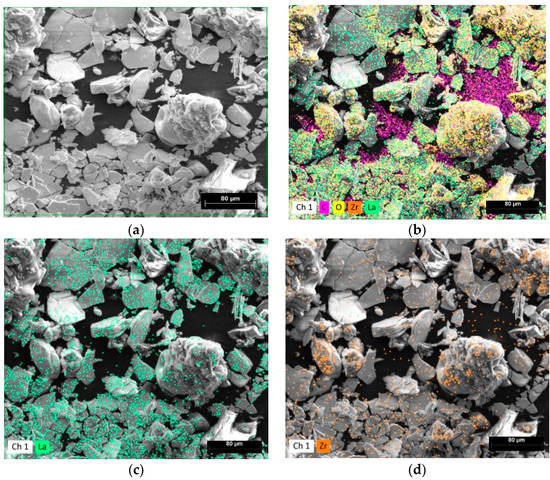
Figure 3.
SEM images of the synthesized LLZO powder at different magnifications. The scale bar is (a,b) 20 μm, and (c,d) 5 μm long.

Table 1.
Elemental EDS analysis of Li7La3Zr2O12 powder.
EDS elemental analysis of the LLZO powder shows lanthanum excess in the solid electrolyte powder, expressed in terms of Li7La3Zr2O12 and La2O3 compounds. Elemental analysis based on Table 1 shows an excess of 3.1 wt.% of lanthanum oxide (III).
4. Discussion
4.1. The Standard Formation Enthalpy
The formation enthalpy of Li7La3Zr2O12 (ΔoxHLLZO) from Li2CO3, La2O3, and ZrO2 is calculated according to Equation (1) from the Experimental Section. The subscript ox means “oxides”, which relates to the initial compounds from Equation (1).
The following thermodynamic cycle was used for enthalpy calculation, Figure 4:
the subscript (aq) indicates “aqueous”. The calorimeter was used for the standard enthalpy (ΔdHLLZO) measurement. The received value after calorimetry measurement was equal to −1911 ± 37 J·g−1, Table 2.
Li7La3Zr2O12 + 24HCl(aq) → 7LiCl(aq) + 3LaCl3(aq) + 2ZrCl4(aq) + 12H2↑+ 6O2↑,
Li2CO3+ 2HCl(aq) → 2LiCl + CO2 + H2O,
La2O3 + 6HCl(aq) → 2LaCl3 + 3H2O,
ZrO2 + 4HCl(aq) → ZrCl4 +2H2O,
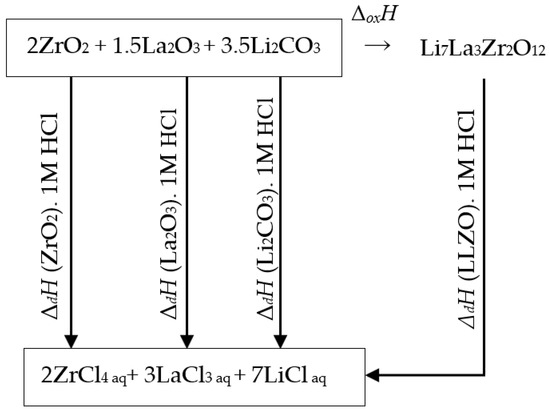
Figure 4.
Diagram of the thermochemical dissolution cycle of Li7La3Zr2O12 in HCl.

Table 2.
The dissolution enthalpies values of the initial components and the Li7La3Zr2O12 compound (p = 101 kPa, T = 298 K, 1 mol·dm−3 HCl(aq)).
It was shown in the Experimental Section that LLZO has 3.1 wt.% of unreacted La2O3 impurity. Thereby, measured ΔdHLLZO should be recalculated considering the amount of La2O3:
where ω is the mass fraction of La2O3. It should be noted that enthalpies, mentioned in Equation (6), are supposed to be specific, not molar. The recalculated value of the dissolution enthalpy of LLZO (with 3.1 wt.% of La2O3) is equal to −1917.7 J·g−1 or −1607.75 kJ·mol−1, Table 2.
The formation enthalpy value of ΔoxHLLZO is estimated by the next formula:
The calorimetry-measured values of ΔdHZrO2, Δd, and Δd are shown in Table 2. The recalculated value of the enthalpy of dissolution of Li7La3Zr2O12 was used for ΔoxHLLZO evaluation. The value of ΔoxHLLZO given by Equation (7) is equal to −186.4 kJ mol−1. The negative value of the enthalpy of Li7La3Zr2O12 formation indicates that Li7La3Zr2O12 is a stable phase; the chemical reaction of Li2CO3, La2O3, and ZrO2 is energetically favorable for Li7La3Zr2O12 synthesis. The values for various lithium zirconates were added to Table 2 to compare with the measured and calculated values in this work. The value of the formation enthalpy from binary oxides ΔoxHLLZO has the same order as corresponding values for lithium zirconate compounds and complex oxides (Table 3), thus it can be concluded that the measurements are correct.

Table 3.
Standard enthalpies of formation of complex oxides from binary oxides (ΔoxH0).
Finally, the enthalpy of Li7La3Zr2O12 formation from elements can be calculated by the following formula:
The corresponding handbook’s materials were used to define the standard enthalpies [53], Table 4.

Table 4.
Standard enthalpies of formation from elements (ΔfH0).
The formation enthalpy value of the Li7La3Zr2O12 compound, calculated by formula (8) is −9327.65 ± 7.9 kJ·mol−1, Table 4. The enthalpy of formation value, rated by Equation (8), can be recommended for use in further thermodynamic calculations of Li7La3Zr2O12 reactivity.
4.2. The Isobaric Heat Capacity
Figure 5 shows the isobaric heat capacity of the Li7La3Zr2O12 as a function of temperature (Cp = f(T)). Pay attention to the certain amount of La2O3 (Figure 1 and Table 1) in LLZO synthesized powder material, the measured isobaric heat capacity for the two-phase system must be recalculated by the following additive rule:
where Cp is a specific heat capacity (p = const), and m is mass. In our case, the two-phase system consists of the solid electrolyte compound (LLZO) and La2O3. Thus, the heat capacity of Li7La3Zr2O12 is expressed from Equation (9) as:
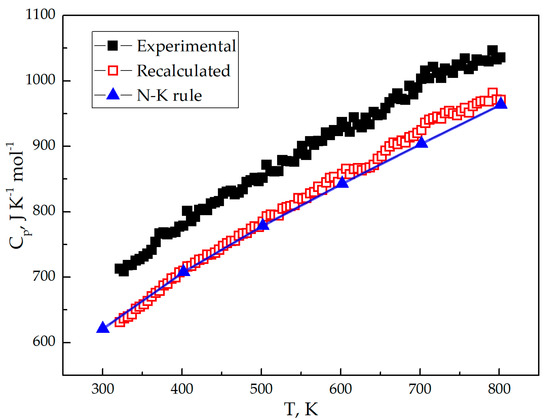
Figure 5.
The experimental (filled square), recalculated (unfilled square) and Neumann-Kopp rule (line-connected triangles) heat capacities of Li7La3Zr2O12.
The impurity compound weight can be recalculated from the total weight of the sample, with the known mass fraction of lanthanum oxide, :
and
Considering Equations (11) and (12), Equation (13) can be expressed as follows:
Equation (13) shows, that the Li7La3Zr2O12 heat capacity can be evaluated by the measured heat capacity (Cp), tabulated heat capacity of La2O3 and La2O3 mass fraction . The dependence of La2O3 specific heat capacity from temperature is required for Equation (13) calculation. For this, tabular data is required to define temperature dependence for the lanthanum oxide heat capacity [53]. The heat capacity polynomial, commonly used for the low temperature range (for 300–800 K in our case) can be expressed as follows:
where a, b, and c are empirical coefficients, and T is the absolute temperature. The La2O3 received coefficients are a = 119.604 J·mol−1·K−1, b = 14.514 × 10−3 J·mol−1·K−2, and c = 13.452 × 105 J·mol−1·K. Considering the La2O3 impurity presence, the LLZO heat capacity can be recalculated via Equations (13) and (14) for the 300–800 K temperature interval. According to XRD and EDS data (Figure 1 and Table 1, respectively) LLZO contains about 3.1 ± 0.12 wt.% La2O3. Figure 5 and Table 5 shows measured and recalculated LLZO heat capacity temperature dependence. The Neumann-Kopp (N-K) rule was used for the empirical value calculation of the heat capacity. The N-K rule approves “that the molecular heat capacity of a solid compound is the sum of the atomic heat capacities of the elements composing it; the elements having atomic heat capacities lower than those required by the Dulong–Petit law retain these lower values in their compounds.” [54]. This rule commonly gives reproducible results for room temperatures, not for high temperatures. To achieve more accurate results, binary materials were used instead of single elements (accurate results are usually obtained for the same aggregate state of materials):
Cp is a molar heat capacity (p = const), n is a stoichiometric coefficient, and CO and BO are complex and binary oxide, respectively. Equation (15), considering Equation (1), can be expressed for LLZO as follows:
Cp = a + bT − cT−2
The calculated from tabular data [53] heat capacity from Equation (16) is shown on Figure 5 and Table 5.

Table 5.
The Li7La3Zr2O12 (s) heat capacities of experimental Cp(exp.), recalculated by Equation (15) Cp(rec.) and calculated by the (N-K) rule Cp(N-K) values as a function of temperature.
Table 5.
The Li7La3Zr2O12 (s) heat capacities of experimental Cp(exp.), recalculated by Equation (15) Cp(rec.) and calculated by the (N-K) rule Cp(N-K) values as a function of temperature.
| T, K | Cp(exp.), J·K−1·mol−1 | Cp(rec.), J·K−1·mol−1 | Cp(N-K), J·K−1·mol−1 |
|---|---|---|---|
| 300 | - | - | 621.1 |
| 400 | 778.6 | 709.7 | 708.1 |
| 500 | 851.8 | 784.7 | 778.8 |
| 600 | 936.7 | 857.4 | 843.1 |
| 700 | 1002.8 | 925.0 | 904.3 |
| 800 | 1035.4 | 971.1 | 964.0 |
The Neumann-Kopp rule and recalculated heat capacity of Li7La3Zr2O12 are in good correlation. Experimental data is for the LLZO compound with La2O3 impurity. The heat capacity temperature dependence (Equation (16)) was calculated using tabular data [53,55]. XRD and EDS quantitative analysis gives accurate enough results to define a small quantity of impurity compounds in the material.
4.3. Entropy
The Third Law of Thermodynamics states, “The entropy of a perfect crystal is zero when the temperature of the crystal is equal to absolute zero (0 K).” Thus, the entropy absolute value can be valued by the equation:
where S is entropy, Tk is temperature of the k-th phase transition (0 < Tk < T), and ΔHk is enthalpy of the k-th phase transition. The Neumann-Kopp rule for entropy calculation can be expressed as follows (considering absence of phase transition at calculating temperature range):
where BO is the binary oxide compound (see Equation (15)). Equation (18) can be rewritten taking into account Equations (15) and (16):
S (LLZO) = 3.5S(Li2CO3) + 1.5S(La2O3) + 2S(ZrO2)
The Li7La3Zr2O12 entropy is equal to 607.18 J·mol−1·K−1 (T = 298 K) by calculating Equation (19) using tabulated data [53,55]. The additive rule for entropy calculation can be used if the following term is met: the complex compound molar volume slightly differs of the molar volumes sum of binary compounds [55]. Thus, the molar volume for Li2CO3 is 35.0 cm3·mol−1 (density is ρ = 2.11 g·cm−3 [56]), for La2O3 is 50.1 cm3·mol−1 (density is ρ = 6.51 g·cm−3 [57]), for ZrO2 is 21.2 cm3·mol−1 (density is ρ = 5.56 g·cm−3 [57]), and for Li7La3Zr2O12 is 165.0 cm3·mol−1 (density is ρ = 5.09 g·cm−3 [58]). The sum of the molar volumes of Li2CO3, La2O3, and ZrO2 with their corresponding stoichiometric coefficients is 240.05 cm3·mol−1 and differs about 45.5% of the LLZO molar volume, which does not allow one to apply the additive rule.
Excepting the additive calculation rule, the W. Herz rule can be used for the LLZO entropy calculation [59]:
where KH is the Herz constant, M is molar mass, Cp,298 is isobaric heat capacity, and m is atoms per formula.
The Herz constant KH has a good correlation with average values of anion molar mass [60]:
where x = 42.4/, and MA is an anion (La3Zr2O127−) molar mass. For Li7La3Zr2O12, anion molar mass is 791.154 g·mol−1. Thus, KH constant is equal to 33.5.
Considering Cp,298 from Table 5 and Equation (21), calculated by Equation (20) the LLZO entropy is equal to 362.3 J mol−1·K−1. The calculated value of LLZO entropy by the W. Herz rule is in good correlation with Ref. [60]. Hence, the N-K rule cannot be used for the entropy calculations, as follows from molar masses principle.
4.4. The Standard Gibbs Free Energy
Calculated formation enthalpy and entropy allows one to rate the standard Gibbs free energy () of LLZO formation (T = 298 K):
For Equation (22), the value of LLZO is equal to −9435.6 kJ·mol−1.
The stability against metallic lithium can be estimated by the Gibbs free energy calculation of the following reaction at room temperature:
The Gibbs free energy of reaction (∆rGLLZO/Li) can be expressed as the difference between the and the Gibbs energy values of reactants and resultants of the reaction. The for single elements is equal to zero, for Li2O is −561.2 kJ·mol−1, and for La2O3 is −1706.7 kJ·mol−1 [53]. The Li7La3Zr2O12 Gibbs free energy has been calculated above. Thus, the Gibbs free energy for reaction (23) is ∆rGLLZO/Li = 8.2 kJ·mol−1; this means that the reaction is thermodynamically impossible. Finally, Li7La3Zr2O12 is stable against metallic lithium at room temperature.
5. Conclusions
The thermodynamic characteristics were determined for Li7La3Zr2O12 solid-state electrolyte material for lithium-ion battery. Solid-state reaction was used as the synthesis method of Li7La3Zr2O12 from Li2CO3, La2O3, and ZrO2. The synthesized material had 3.1 wt.% of the lanthanum oxide (La2O3) impurity according to XRD and EDS data. Probably, this amount of La2O3 is unreacted oxide from the synthesis process. The enthalpy of Li7La3Zr2O12 formation from binary oxides (and from Li2CO3) ΔoxHLLZO and from the elements ΔfHLLZO were calculated according to the measured enthalpy of dissolution of reagents and the products of the Li7La3Zr2O12 formation reaction. The obtained values are equal to −186.4 ± 7.3 kJ·mol−1 and −9327.65 ± 7.9, respectively. The formation enthalpy from binary oxides ΔoxHLLZO is in good correlation with similar zirconate compounds, which confirms the correctness of the measurements.
The recalculated LLZO heat capacity considering La2O3 presence is in good correlation with that calculated by the Neumann-Kopp rule. Finally, the temperature dependence of the LLZO heat capacity can be expressed by the formula Cp(T) = 518.135 + 0.599 × T − 8.339 × T−2 (T is absolute temperature). The LLZO entropy is S0298 = 362.3 J·mol−1·K−1, the Gibbs free energy of formation of Li7La3Zr2O12 is −9435.6 kJ mol−1. Li7La3Zr2O12 material is stable against metallic lithium, according to the Gibbs free energy of the LLZO reaction with metallic Li. All thermodynamic values and functions measured and calculated for Li7La3Zr2O12 can be used for modelling and further calculations of all-solid-state batteries.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma15010281/s1, Table S1: HKL indexes for XRD pattern (corresponding to Figure 1).
Author Contributions
Conceptualization, A.P. and P.N.; methodology, D.A.; software, D.A.; validation, D.A. and Q.W.; formal analysis, A.P.; investigation, D.A.; resources, Q.W.; data curation, P.N.; writing—original draft preparation, D.A.; writing—review and editing, P.N.; visualization, D.A.; supervision, A.P.; project administration, Q.W.; funding acquisition, Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
The research is partially funded by the Ministry of Science and Higher Education of the Russian Federation: Advanced Digital Technologies (contract No. 075-15-2020-934 dated from 17.11.2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nishi, Y. Lithium-ion secondary batteries; past 10 years and the future. J. Power Sources 2001, 100, 101–106. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Grundish, N.S.; Goodenough, J.B.; Chen, Y.; Guo, L.; Peng, Z.; Qi, X.; Yang, F.; Qie, L.; et al. The 2021 battery technology roadmap. J. Phys. D Appl. Phys. 2021, 54, 183001. [Google Scholar] [CrossRef]
- Horowitz, Y.; Schmidt, C.; Yoon, D.H.; Riegger, L.M.; Katzenmeier, L.; Bosch, G.M.; Noked, M.; Ein-Eli, Y.; Janek, J.; Zeier, W.G.; et al. Between Liquid and All Solid: A Prospect on Electrolyte Future in Lithium-Ion Batteries for Electric Vehicles. Energy Technol. 2020, 8, 2000580. [Google Scholar] [CrossRef]
- Han, L.; Lehmann, M.L.; Zhu, J.; Liu, T.; Zhou, Z.; Tang, X.; Heish, C.T.; Sokolov, A.P.; Cao, P.; Chen, X.C.; et al. Recent Developments and Challenges in Hybrid Solid Electrolytes for Lithium-Ion Batteries. Front. Energy Res. 2020, 8, 202. [Google Scholar] [CrossRef]
- Tan, D.H.; Chen, Y.T.; Yang, H.; Bao, W.; Sreenarayanan, B.; Doux, J.M.; Li, W.; Lu, B.; Ham, S.Y.; Sayahpour, B.; et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science 2021, 373, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.W.; Kim, H. Review on Interface and Interphase Issues in Sulfide Solid-State Electrolytes for All-Solid-State Li-Metal Batteries. Electrochem 2021, 2, 30. [Google Scholar] [CrossRef]
- Tripathi, A.K. Ionic liquid based solid electrolytes (ionogels) for application in rechargeable lithium battery. Mater. Today Energy 2021, 20, 100643. [Google Scholar] [CrossRef]
- Yu, T.; Yang, X.; Yang, R.; Bai, X.; Xu, G.; Zhao, S.; Duan, Y.; Wu, Y.; Wang, J. Progress and perspectives on typical inorganic solid-state electrolytes. J. Alloys Compd. 2021, 885, 161013. [Google Scholar] [CrossRef]
- Chan, C.K.; Yang, T.; Weller, J.M. Nanostructured garnet-type Li7La3Zr2O12: Synthesis, properties, and opportunities as electrolytes for Li-ion batteries. Electrochim. Acta 2017, 253, 268–280. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, W.; Zhu, G.; Huang, Y.; Feng, Q.; Lu, Y. Design Principles of the Anode–Electrolyte Interface for All Solid-State Lithium Metal Batteries. Small Methods 2020, 4, 1900592. [Google Scholar] [CrossRef]
- Tikekar, M.D.; Choudhury, S.; Tu, Z.; Archer, L.A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 2016, 1, 16114. [Google Scholar] [CrossRef]
- Wang, H.; Yu, D.; Kuang, C.; Cheng, L.; Li, W.; Feng, X.; Zhang, Z.; Zhang, X.; Zhang, Y. Alkali metal anodes for rechargeable batteries. Chem 2019, 5, 313–338. [Google Scholar] [CrossRef] [Green Version]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shao, Y.; Lotsch, B.; Hu, Y.S.; Li, H.; Janek, J.; Nazar, L.F.; Nan, C.W.; Maier, J.; Armand, M.; et al. New horizons for inorganic solid state ion conductors. Energy Environ. Sci. 2018, 11, 1945–1976. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Yu, B.C.; Jung, J.W.; Li, Y.; Zhou, W.; Gao, H.; Son, S.; Goodenough, J.B. Electrochemical nature of the cathode interface for a solid-state lithium-ion battery: Interface between LiCoO2 and garnet-Li7La3Zr2O12. Chem. Mater. 2016, 28, 8051–8059. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angewandte Chemie. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Bai, Y.X.; Zhang, J.; Yang, Y.B.; Yang, R.; Yan, Y.L.; Wang, J. Enhance electrochemical performance of LiFePO4 cathode material by Al-doped Li7La3Zr2O12 and carbon co-coating surface modification. J. Alloy. Compd. 2020, 843, 154915. [Google Scholar] [CrossRef]
- Matsui, M.; Takahashi, K.; Sakamoto, K.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Imanishi, N. Phase stability of a garnet-type lithium ion conductor Li7La3Zr2O12. Dalton Trans. 2014, 43, 1019–1024. [Google Scholar] [CrossRef]
- Dermenci, K.B.; Çekiç, E.; Turan, S. Al stabilized Li7La3Zr2O12 solid electrolytes for all-solid state Li-ion batteries. Int. J. Hydrog. Energy 2016, 41, 9860–9867. [Google Scholar] [CrossRef]
- Kotobuki, M.; Hanc, E.; Yan, B.; Molenda, J.; Lu, L. Stabilization of cubic Li7La3Zr2O12 by Al substitution in various atmospheres. Solid State Ion. 2020, 350, 115323. [Google Scholar] [CrossRef]
- Polizos, G.; Sharma, J.; Jafta, C.J.; Muralidharan, N.; Veith, G.M.; Keum, J.K.; Kukay, A.; Sahore, R.; Wood, D.L., III. Nanostructured ligament and fiber Al–doped Li7La3Zr2O12 scaffolds to mediate cathode-electrolyte interface chemistry. J. Power Sources 2021, 513, 230551. [Google Scholar] [CrossRef]
- Liu, X.; Gao, M.; Liu, Y.; Xiong, L.; Chen, J. Improving the room temperature ionic conductivity of Al-Li7La3Zr2O12 ceramics by Ba and Y or Ba and W co-doping. Ceram. Int. 2019, 45, 13488–13495. [Google Scholar] [CrossRef]
- PPosch, P.; Lunghammer, S.; Berendts, S.; Ganschow, S.; Redhammer, G.J.; Wilkening, A.; Lerch, M.; Gadermaier, B.; Rettenwander, D.; Wilkening, H.M.R. Ion dynamics in Al-stabilized Li7La3Zr2O12 single crystals–Macroscopic transport and the elementary steps of ion hopping. Energy Storage Mater. 2020, 24, 220–228. [Google Scholar] [CrossRef]
- Matsuki, Y.; Noi, K.; Suzuki, K.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. Microstructure and conductivity of Al-substituted Li7La3Zr2O12 ceramics with different grain sizes. Solid State Ion. 2019, 342, 115047. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Ratchford, J.; Rangasamy, E.; Sakamoto, J.; Allen, J.L. Synthesis and high Li-ion conductivity of Ga-stabilized cubic Li7La3Zr2O12. Mater. Chem. Phys. 2012, 134, 571–575. [Google Scholar] [CrossRef]
- Jalem, R.; Rushton, M.J.D.; Manalastas, W., Jr.; Nakayama, M.; Kasuga, T.; Kilner, J.A.; Grimes, R.W. Effects of gallium doping in garnet-type Li7La3Zr2O12 solid electrolytes. Chem. Mater. 2015, 27, 2821–2831. [Google Scholar] [CrossRef] [Green Version]
- Bernuy-Lopez, C.; Manalastas, W., Jr.; Lopez del Amo, J.M.; Aguadero, A.; Aguesse, F.; Kilner, J.A. Atmosphere controlled processing of Ga-substituted garnets for high Li-ion conductivity ceramics. Chem. Mater. 2014, 26, 3610–3617. [Google Scholar] [CrossRef]
- El Shinawi, H.; Janek, J. Stabilization of cubic lithium-stuffed garnets of the type “Li7La3Zr2O12” by addition of gallium. J. Power Sources 2013, 225, 13–19. [Google Scholar] [CrossRef]
- Huang, X.; Su, J.; Song, Z.; Xiu, T.; Jin, J.; Badding, M.E.; Wen, Z. Synthesis of Ga-doped Li7La3Zr2O12 solid electrolyte with high Li+ ion conductivity. Ceram. Int. 2021, 47, 2123–2130. [Google Scholar] [CrossRef]
- Su, J.; Huang, X.; Song, Z.; Xiu, T.; Badding, M.E.; Jin, J.; Wen, Z. Overcoming the abnormal grain growth in Ga-doped Li7La3Zr2O12 to enhance the electrochemical stability against Li metal. Ceram. Int. 2019, 45, 14991–14996. [Google Scholar] [CrossRef]
- Shen, L.; Wang, L.; Wang, Z.; Jin, C.; Peng, L.; Pan, X.; Sun, J.; Yang, R. Preparation and characterization of Ga and Sr co-doped Li7La3Zr2O12 garnet-type solid electrolyte. Solid State Ion. 2019, 339, 114992. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, Y.; Wang, W.; Zhou, Y. Effects of Ga–Ba Co-doping on the morphology and conductivity of Li7La3Zr2O12 electrolyte synthesized by sol-gel method. Ceram. Int. 2021, 48, 963670. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, J.; Hu, D.; Chen, F.; Shen, Q.; Zhang, L.; Dong, S. Synergistic regulation of garnet-type Ta-doped Li7La3Zr2O12 solid electrolyte by Li+ concentration and Li+ transport channel size. Electrochim. Acta 2019, 296, 823–829. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, W. Phase transition in lithium garnet oxide ionic conductors Li7La3Zr2O12: The role of Ta substitution and H2O/CO2 exposure. J. Power Sources 2015, 275, 612–620. [Google Scholar] [CrossRef]
- Chen, X.; Wang, T.; Lu, W.; Cao, T.; Xue, M.; Li, B.; Zhang, C. Synthesis of Ta and Ca doped Li7La3Zr2O12 solid-state electrolyte via simple solution method and its application in suppressing shuttle effect of Li-S battery. J. Alloy. Compd. 2018, 744, 386–394. [Google Scholar] [CrossRef]
- Chen, X.; Cao, T.; Xue, M.; Lv, H.; Li, B.; Zhang, C. Improved room temperature ionic conductivity of Ta and Ca doped Li7La3Zr2O12 via a modified solution method. Solid State Ion. 2018, 314, 92–97. [Google Scholar] [CrossRef]
- Guo, H.; Su, J.; Zha, W.; Xiu, T.; Song, Z.; Badding, M.E.; Jin, J.; Wen, Z. Achieving high critical current density in Ta-doped Li7La3Zr2O12/MgO composite electrolytes. J. Alloy. Compd. 2021, 856, 157222. [Google Scholar] [CrossRef]
- Hosokawa, H.; Takeda, A.; Inada, R.; Sakurai, Y. Tolerance for Li dendrite penetration in Ta-doped Li7La3Zr2O12 solid electrolytes sintered with Li2.3C0.7B0.3O3 additive. Mater. Lett. 2020, 279, 128481. [Google Scholar]
- Huang, X.; Xiu, T.; Badding, M.E.; Wen, Z. Two-step sintering strategy to prepare dense Li-Garnet electrolyte ceramics with high Li+ conductivity. Ceram. Int. 2018, 44, 5660–5667. [Google Scholar] [CrossRef]
- He, M.; Cui, Z.; Chen, C.; Li, Y.; Guo, X. Formation of self-limited, stable and conductive interfaces between garnet electrolytes and lithium anodes for reversible lithium cycling in solid-state batteries. J. Mater. Chem. A 2018, 6, 11463–11470. [Google Scholar] [CrossRef]
- Xue, W.; Yang, Y.; Yang, Q.; Liu, Y.; Wang, L.; Chen, C.; Cheng, R. The effect of sintering process on lithium ionic conductivity of Li6.4Al0.2La3Zr2O12 garnet produced by solid-state synthesis. RSC Adv. 2018, 8, 13083–13088. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Song, Z.; Xiu, T.; Badding, M.E.; Wen, Z. Sintering, micro-structure and Li+ conductivity of Li7−xLa3Zr2-xNbxO12/MgO (x = 0.2−0.7) Li-Garnet composite ceramics. Ceram. Int. 2019, 45, 56–63. [Google Scholar] [CrossRef]
- Yang, T.; Li, Y.; Wu, W.; Cao, Z.; He, W.; Gao, Y.; Liu, J.; Li, G. The synergistic effect of dual substitution of Al and Sb on structure and ionic conductivity of Li7La3Zr2O12 ceramic. Ceram. Int. 2018, 44, 1538–1544. [Google Scholar] [CrossRef]
- Xiang, X.; Chen, F.; Shen, Q.; Zhang, L.; Chen, C. Effect of the lithium ion concentration on the lithium ion conductivity of Ga-doped LLZO. Mater. Res. Express 2019, 6, 085546. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Hu, Q.; Cao, S.; Song, S.; Lu, X.; Shen, Q. S/MWCNt/LLZO Composite Electrode with e-/S/Li+ Conductive Network for All-Solid-State Lithium–Sulfur Batteries. J. Solid State Chem. 2021, 301, 122341. [Google Scholar] [CrossRef]
- Goswami, N.; Indu, M.S.; Murugan, R.; Kant, R. Experimental corroboration of theory for impedance response of solid electrolytes: Doped cubic garnet LLZO. J. Electroanal. Chem. 2021, 897, 115611. [Google Scholar] [CrossRef]
- Aravinth, K.; Ramasamy, P.; Sen, S.; Arumugam, R. Tunable photoluminescence properties of Dy3+ doped LLZO phosphors for WLED and dosimetry applications. Ceram. Int. 2021, 48, 1402–1407. [Google Scholar]
- Samui, P.; Modi, K.B.; Phapale, S.; Parida, S.C.; Mishra, R. Calorimetric investigations on lithium based ceramics. J. Chem. Thermodyn. 2021, 163, 106590. [Google Scholar] [CrossRef]
- Wyers, G.P.; Cordfunke, E.H.P.; Ouweltjes, W. The standard molar enthalpies of formation of the lithium zirconates. J. Chem. Thermodyn. 1989, 21, 1095–1100. [Google Scholar] [CrossRef]
- Bolech, M.; Cordfunke, E.H.P.; van Genderen, A.C.G.; van Der Laan, R.R.; Janssen, F.J.J.G.; Van Miltenburg, J.C. The heat capacity and derived thermodynamic functions of La2Zr2O7 and Ce2Zr2O7 from 4 to 1000 K. J. Phys. Chem. Solids 1997, 58, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Navrotsky, A.; Venkatraman, S.; Manthiram, A. Enthalpy of Formation of LixCoO2 (0.5 ≤ x ≤ 1.0). J. Electrochem. Soc. 2005, 152, J82. [Google Scholar] [CrossRef]
- Huntelaar, M.E.; Booij, A.S.; Cordfunke, E.H.P. The standard molar enthalpies of formation of BaZrO3 (s) and SrZrO3 (s). J. Chem. Thermodyn. 1994, 26, 1095–1101. [Google Scholar] [CrossRef]
- Glushko, V.P.; Gurvich, L.V.; Bergman, G.A.; Veits, I.V.; Medvedev, V.A.; Khachkuruzov, G.A.; Yungman, V.S. Thermodinamicheskie Svoitsva Individual’nykh Veshchestv; Nauka: Moscow, Russia, 1978. [Google Scholar]
- Millard, E.B. Physical Chemistry for Colleges; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1921; ISBN-10: 1146961987. [Google Scholar]
- Pankratz, L.B. Thermodynamic Properties of Carbides, Nitrides, and Other Selected Substances; Bureau of Mines: Washington, DC, USA, 1995; ISBN-10: 9995679329. [Google Scholar]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 86th ed.; 2005–2006; CRC Press: Boca Raton, FL, USA, 2005; pp. 4–70. ISBN 0849304865 9780849304866. [Google Scholar]
- Samsonov, G.V. The Oxide Handbook; Springer: Boston, MA, USA, 1973; ISBN 978-1-4615-9597-7. [Google Scholar] [CrossRef]
- Awaka, J.; Kijima, N.; Hayakawa, H.; Akimoto, J. Synthesis and structure analysis of tetragonal Li7La3Zr2O12 with the garnet-related type structure. J. Solid State Chem. 2009, 182, 2046–2052. [Google Scholar] [CrossRef]
- Morachevskiy, A.G.; Sladkov, I.B.; Firsova, Y.G. Termodinamicheskiye Raschety v Khimii i Metallurgii; Lan’: St. Petersburg, Russia, 2018; ISBN 978-5-8114-3023-9. [Google Scholar]
- Il’ina, E.A.; Raskovalov, A.A.; Reznitskikh, O.G. Thermodynamic properties of solid electrolyte Li7La3Zr2O12. J. Chem. Thermodyn. 2019, 128, 68–73. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).