Abstract
Solution calorimetry with liquid aluminum as the bath was conducted to measure the enthalpy of a solution of magnesium and palladium as well as the standard formation enthalpies of selected magnesium-palladium alloys. These alloys were synthesized from pure elements, which were melted in a resistance furnace that was placed in a glove box containing high-purity argon and a very low concentration of impurities, such as oxygen and water vapor. A Setaram MHTC 96 Line evo drop calorimeter was used to determine the energetic effects of the solution. The enthalpies of the Mg and Pd solutions in liquid aluminum were measured at 1033 K, and they equaled −8.6 ± 1.1 and −186.8 ± 1.1 kJ/mol, respectively. The values of the standard formation enthalpy of the investigated alloys with concentrations close to the Mg6Pd, ε, Mg5Pd2, and Mg2Pd intermetallic phases were determined as follows: −28.0 ± 1.2 kJ/mol of atoms, −32.6 ± 1.6 kJ/mol of atoms, −46.8 ± 1.4 kJ/mol of atoms, and −56.0 ± 1.6 kJ/mol of atoms, respectively. The latter data were compared with existing experimental and theoretical data from the literature along with data calculated using the Miedema model.
1. Introduction
Energy is a very important commodity in life. Most energy still comes from natural sources, such as coal and oil, but scientists all over the world are constantly searching for an alternative, renewable, and efficient energy source to reduce the climate change caused by the combustion products of natural fuels, which have a negative impact on the climate [1]. Hydrogen is the best-known chemical energy carrier that can be very effectively converted to electricity in Proton-Exchange Membrane Fuel Cells with only water and heat generation. The main problem scientists are trying to solve is finding a suitable material for hydrogen storage with the possibility of the fast absorption and desorption of hydrogen, especially in applications for mobile devices [2,3,4].
Research on solid-state hydrogen storage materials has been conducted for many years. Some of these materials are metals and their alloys and are capable of reversibly absorbing large amounts of hydrogen. Magnesium has been studied extensively for applications as a hydrogen storage material because magnesium hydride, which Mg creates as it reacts with hydrogen, has a high gravimetric and volumetric density of hydrogen storage (7.6 mass % and 110 g H/L, respectively) [5,6,7]. However, its high enthalpy of decomposition requires high operating temperatures for the desorption of hydrogen, while the slow diffusion kinetics of hydrogen by mass, for example, poses challenges for its large-scale deployment. To overcome these difficulties, small amounts of additives are added to magnesium to create magnesium compounds, which, in relation to pure Mg, improves the unfavorable thermodynamics and sometimes the kinetics of the reaction [8]. Significant improvements were made in this field in order to modify the thermodynamics of Mg-based systems starting more than 50 years ago [9,10], but the research is in this area is continuing, including alloying with transition [11,12,13,14,15,16,17] catalysts [18,19], complex hydride additives [20], and even mechanical processing [21,22,23]. It has been indicated that the addition of noble metals, such as palladium or silver, can enhance the storage properties of magnesium [16,24,25,26,27]. Despite this, the thermodynamics and phase diagrams for magnesium systems such as Mg-Pd and Mg-Pt (and others) are limited and sometimes incomplete; this knowledge is necessary for designing and producing proper materials.
The phase diagram of the Mg-Pd system was estimated for the first time by Nayeb-Hashemi and Clark [28]. It was based on limited data presented by [29,30,31] and contained five uncertain intermetallic phases (Mg6Pd, Mg4Pd, Mg5Pd2, MgPd, and Mg0.9Pd1.1).
Next, based on their own experimental data from differential thermal analysis (DTA) for Pd alloys for the composition range between 0 and 56 at.%, Makongo et al. [32] presented a new variant of the binary system which was quite different from what Nayeb-Hashemi and Clark [28] proposed. The last version of the Mg-Pd system was published by Okamoto [33] and is reproduced in Figure 1.
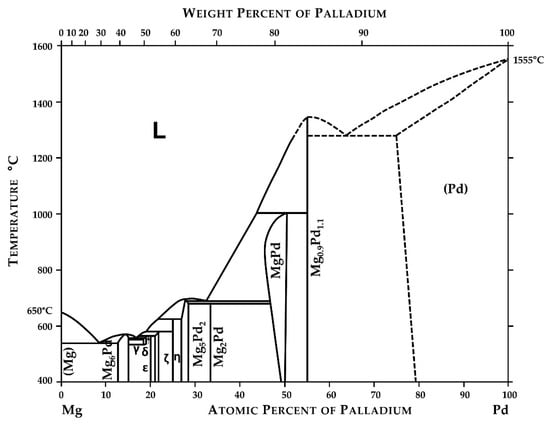
Figure 1.
Phase diagram of the Mg-Pd system. Reprinted with permission from ref. [33]. 2010, Springer Nature.
The calculations of the formation energy for the Mg6Pd, Mg57Pd13, Mg3Pd, Mg5Pd2, and MgPd intermetallic phases were conducted and published by Fernandez et al. [34,35]. The first experimental values of the formation enthalpy of the Mg6Pd and Mg5Pd2 intermetallic compounds were measured by Delsante et al. [36] using direct drop calorimetry, and they were equal to −28.2 ± 1.0 and −39.5 ± 2.8 kJ/mol of atoms, respectively. The formation enthalpies of six Mg-rich alloys corresponding to intermetallic phases from the Mg-Pd system were also presented in our previous work [37]. They were investigated using solution calorimetry in a liquid tin bath, and the determined formation energies equaled −27.0 ± 0.8, −34.4 ± 0.9, −35.2 ± 1.4, −44.2 ± 0.9, −46.0 ± 0.7, and −54.3 ± 2.3 kJ/mol of atoms for alloys containing 14.6 at.% Pd, 19.4 at.% Pd, 20.1 at.% Pd, 27.7 at.% Pd, 29.3 at.% Pd, and 35.5 at.% Pd, respectively. Moreover, the ab initio calculations of the formation energies of all existing intermetallic phases shown in Figure 1 were also reported in our other work [38].
This work is a continuation of research on the thermodynamic properties of the Mg-Pd system initiated by our group. This paper presents an extension of the results of the formation enthalpies of Mg-rich alloys which correspond to intermetallic phases. During the calorimetric measurements, different types of metallic baths were used. The choice of bath is determined by its ability to dissolve the test components forming the alloy during the test. In addition, the bath should have a low melting point, negligible evaporation pressure in the temperature range chosen for tests, and a lower density (as compared to the tested specimen) to prevent the sample from floating on the surface of the liquid bath. For many years, molten tin was used as the main solvent in the calorimetric measurements. However, liquid tin was not always is the best possible solvent for dissolving transition metals, as discussed by Colinet in [39]. In the case of the mentioned research group, liquid aluminum is often used. Despite the fact that the formation enthalpy is a physical value and theoretically should not be affected by the bath type, in practice the measured value is affected by measurement conditions. For these reasons, the presented investigations were conducted by solution calorimetry using a liquid aluminum bath in order to compare the obtained results with previous measurements.
2. Materials and Methods
Table 1 contains a list of the materials that were applied to determine the standard enthalpies of the formation of the investigated alloys. These alloys were prepared in an glove box (Labmaster, MBraun, Garching, Germany) in a high-purity argon atmosphere (H2O < 0.5 ppm, O2 < 0.1 ppm, N2 was not monitored and was absorbed by Ti at 1100 K). Calculated and weighted (0.1 mg precision) amounts of metals (Pd and Mg) were melted in a resistance furnace in stainless steel crucibles (AISI 304L, Accelor Mittal, Luxembourg). After melting and careful stirring, the liquid alloys were poured into a specially designed steel ingot mold. Finally, the obtained alloys were annealed at 663 K for 72 and 84 h (Table 2) in the furnace that was placed in the glove box containing the protective atmosphere characterized above.

Table 1.
Specifications of the applied materials.

Table 2.
Homogenization conditions of the prepared alloys.
The structural studies of the presented Mg-Pd alloys were conducted after the homogenization process with the use of X-ray diffraction (Ultima IV; Rigaku, Tokyo, Japan; Co Kα radiation source; 1.79026 Å) and SEM/EDS (FEI Quanta 3D SEM). A full description of these results was presented in our previous work [37], and both the results of phase analyses and SEM observations are shown in the Supplementary Materials, Figures S1–S4.
The calorimetric studies were performed in a protective argon atmosphere with the use of a Setaram MHTC 96 line evo drop calorimeter using alumina crucibles. The conducted calorimetric studies were similar to our previous calorimetric measurements presented in [40,41,42]. Before each experiment began, the workspace of the calorimeter was purified by evacuation with a vacuum pump and flushed with high-purity argon. Next, the calibration constant was determined using six pieces of Al.
The enthalpy of the formation (ΔfH) values of the measured phases at 298 K were calculated from the difference in the heat effects, which corresponded to heating the samples from room temperature (298 K) to the measurement temperature (1033 K) and observing the dissolution of the studied phases and their components in the aluminum bath. The ΔfH values were computed using the following equation:
where ΔfH is the enthalpy of the formation of the measured phase; xMg and xPd are the mole fractions of the components, respectively; and , , and are the heat effects accompanying the dissolution of one mole of the components (Mg and Pd) and phases in the aluminum bath, respectively. The and values are the sums of the limiting partial enthalpy of the solution of liquid Mg and Pd in a liquid Al bath and the enthalpy change of the pure Mg and Pd from room temperature to measurement temperature:
In this study, the heat effects of the dissolution of the binary alloys as well as metals were measured.
3. Results and Discussion
The limiting partial enthalpy of the solution of Mg and Pd in liquid aluminum was measured at the first stage of the calorimetric investigations. The necessary thermochemical data of metals were calculated using Pandat 2013 [43] (Pan SGTE database based on the original SGTE v4.4 database [44]). The experimental results of the limiting partial enthalpy of the solution of Mg and Pd in liquid aluminum are presented in Table 3 and Table 4, respectively.

Table 3.
Values of the limiting partial enthalpy of the solution of liquid Mg in liquid Al. Atmosphere: argon at a pressure p = 0.1 MPa; calibration constant K = 0.000003207 kJ/μVs; enthalpy of the pure Mg = 30.0048 kJ/mol; temperature of the Al bath TM = 1033 K; and drop temperature TD = 298 K.

Table 4.
Values of the limiting partial enthalpy of the solution of liquid Pd in liquid Al. Atmosphere: argon at a pressure p = 0.1 MPa; a calibration constant K = 0.000003207 kJ/μVs; enthalpy of the pure Pd = 32.3062 kJ/mol; temperature of the Al bath TM = 1033 K; and drop temperature TD = 298 K.
The standard enthalpies of the formation of the Mg-Pd alloys were determined by employing solution calorimetry. The obtained results are presented in Table 5 together with the standard errors.

Table 5.
Heat effects ΔHef of the solution and formation enthalpies ΔfH of the intermetallic phases from the Mg-Pd system. The temperature of the Al bath was 1033 K.
The comparison of the formation enthalpies of the investigated alloys obtained in this study is presented in Figure 2, together with the experimental data obtained from the direct reaction method [36], as well as the results from the ab initio method [34,38] and calculations using the Miedema model [45,46].
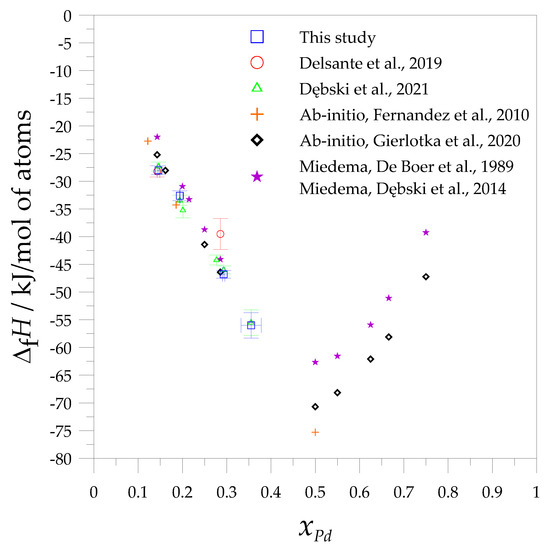
Figure 2.
Comparison of the experimental and calculated values of the standard formation enthalpies of the Mg-Pd intermetallic phases and alloys (solution method: in liquid aluminum—this study, in liquid tin (Dębski et al., 2021 [37]), direct reaction method (Delsante et al., 2019 [36]), with ab initio calculations (Fernandez et al., 2010 [34]; Gierlotka et al., 2020 [38]) and the Miedema model (De Boer et al., 1989 [45]; Dębski et al., 2014 [46]).
As seen in Figure 2, the addition of palladium affects the lowering of the enthalpy of the formation values of the studied alloys. This trend is observed to xPd = 0.5, which is also documented by the Miedema model and ab initio calculations. Moreover, the obtained formation enthalpy of the alloy close to the composition of the Mg6Pd intermetallic phase is in very good agreement with the data measured by the direct reaction method [36], as well as the ab initio calculations [38]. A large discrepancy is observed between the values calculated using the Miedema model [45,46] and the experimental measurements, which reach ~6 kJ/mol of atoms. In the case of the enthalpy of the formation of the alloy in which the concentration is close to the ε- intermetallic phase, the results obtained from the calculations are similar to those obtained from the measurements. In regard to alloys close to the Mg5Pd2 phase, the greatest differences in values are observed between those measured by the direct reaction method and those obtained by the solution method (in Al and Sn), and these calculated values fluctuate between 5 and 7 kJ/mol of atoms. One can suppose that several reasons influence the discrepancy between the results from the direct synthesis method and those obtained from the solution method. In the direct reaction method, the reason for this may be the partial reaction of the sample during the preparation of the powders, the oxidation of the powders, and the fact that the reaction in the calorimeter may not be complete during the measurement. Moreover, for the direct method, the XRD studies were performed after the sample had cooled down together with the calorimeter, which allowed the sample to have a longer reaction time. Taking these factors into consideration for the discrepancies obtained with the Mg5Pd2 phase, it seems that the dissolution method appears to be more accurate for measuring the remaining palladium-rich phases.
Similar observations have been reported by Rzyman et al. [47], who compared the enthalpies of the formation of intermetallic phases from the Al-Ti system obtained by the direct reaction and solution calorimetric methods. Only in the case of the Al3Ti phase were the results obtained from both calorimetric methods in good agreement, while for the remaining phases from the Al-Ti system the differences were about 5 kJ/mol of atoms for the AlTi phase and about 10 kJ/mol of atoms for the AlTi3 phase. Moreover, it was proven that, during the reaction of titanium and aluminum powders, the first obtained product was the Al3Ti phase, regardless of the applied proportion. For this reason, the data for the enthalpy of formation for the Al3Ti phase obtained from both methods are consistent. In the case of the AlTi and AlTi3 phases, the observed differences in the enthalpy of formation values indicate that the reaction of phase formation was not completed in the calorimeter, and this is the reason for the differences in the results obtained from the two methods.
4. Conclusions
This paper presents experimental data of the limiting partial enthalpy of a solution of magnesium and palladium in liquid aluminum at 1033 K, as well as the standard formation enthalpy values of four alloys with chemical compositions close to the Mg6Pd, ε, Mg5Pd2, and Mg2Pd intermetallic phases that were measured by solution calorimetry a liquid aluminum bath. The obtained data for the limiting partial enthalpy of a solution of Pd and Mg in liquid aluminum can be used in future studies of phases and alloys containing these metals in their composition.
The obtained value for the formation enthalpy of the alloy close to the Mg6Pd intermetallic phase agrees well with both the values obtained by the solution calorimetry in liquid Sn and direct reaction methods.
In the case of an alloy with a composition very close to the Mg5Pd2 intermetallic phase, the ΔfH values determined by both solution calorimetry methods are similar and more exothermic than the data obtained by the direct reaction method.
Moreover, data on the standard enthalpies of formation of the Mg-Pd solid phases measured by solution calorimetry showed a slightly better correlation with those obtained by the ab initio calculations than those calculated by the Miedema model.
The calculated formation enthalpies of the Mg-Pd phases and alloys by the ab initio method were more exothermic in comparison to those calculated by the Miedema model, and the observed differences varied between 5 and 15 kJ/mol of atoms.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1944/14/3/680/s1, Figure S1. X-Ray diffraction pattern (Co anode ʎ = 1.78 Å) and SEM (BSE) image of Alloy 1. Figure S2. X-Ray diffraction pattern (Co anode ʎ = 1.78 Å) and SEM (BSE) image of Alloy 2. Figure S3. X-Ray diffraction pattern (Co anode ʎ = 1.78 Å) and SEM (BSE) image of Alloy 3. Figure S4. X-Ray diffraction pattern (Co anode ʎ = 1.78 Å) and SEM (BSE) image of Alloy 4.
Author Contributions
A.D.: Conceptualization, investigation, writing, supervision. S.T.: Investigation. W.G. (Władysław Gąsior): Supervision. W.G. (Wojciech Gierlotka): Investigation, Methodology. M.P. (Magda Pęska): discussion, visualization. J.D.-W.: verification, discussion. M.P. (Marek Polański): supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Science Centre, Poland, for funding Project No. 2018/31/B/ST8/01371 in the years 2019–2022, as a continuation of research on materials for hydrogen storage initiated at the National Center for Research and Development, Poland by research, carried out in the ZAMAT project POIG 01.01.02-00-015/09. The support of the statutory research funds of Department of Functional Materials and Hydrogen Technology, Military University of Technology is appreciated.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [A.D.], upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schlapbach, L.; Zuttel, A. Hydrogen-Storage Materials for Mobile Applications. Nature 2002, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Moradi, R.; Groth, K.M. Hydrogen Storage and Delivery: Review of the State of the Art Technologies and Risk and Reliability Analysis. Int. J. Hydrog. Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Hadjixenophontos, E.; Dematteis, E.M.; Berti, N.; Wołczyk, A.R.; Huen, P.; Brighi, M.; Le, T.T.; Santoru, A.; Payandeh, S.; Peru, F. A Review of the MSCA ITN ECOSTORE—Novel Complex Metal Hydrides for Efficient and Compact Storage of Renewable Energy as Hydrogen and Electricity. Inorganics 2020, 8, 17. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Dam, B.; Denys, R.; Dornheim, M.; Grant, D.; Huot, J.; Jensen, T.R.; De Jongh, P.; Latroche, M.; Milanese, C. Review of Magnesium Hydride-Based Materials: Development and Optimisation. Appl. Phys. A 2016, 122, 97. [Google Scholar] [CrossRef]
- Prabhukhot, P.R.; Wagh Mahesh, M.; Gangal Aneesh, C. A Review on Solid State Hydrogen Storage Material. Adv. Energy Power 2016, 11–22. [Google Scholar] [CrossRef]
- Baran, A.; Polański, M. Magnesium-Based Materials for Hydrogen Storage—A Scope Review. Materials 2020, 13, 3993. [Google Scholar] [CrossRef]
- Webb, C.J. A Review of Catalyst-Enhanced Magnesium Hydride as a Hydrogen Storage Material. J. Phys. Chem. Solids 2015, 84, 96–106. [Google Scholar] [CrossRef]
- Reilly, J.J., Jr.; Wiswall, R.H., Jr. The Reaction of Hydrogen with Alloys of Magnesium and Copper1. Inorg. Chem. 1967, 6, 2220–2223. [Google Scholar] [CrossRef]
- Reilly, J.J., Jr.; Wiswall, R.H., Jr. Reaction of Hydrogen with Alloys of Magnesium and Nickel and the Formation of Mg2NiH4. Inorg. Chem. 1968, 7, 2254–2256. [Google Scholar] [CrossRef]
- Fadonougbo, J.O.; Jung, J.-Y.; Suh, J.-Y.; Lee, Y.-S.; Shim, J.-H.; Cho, Y.W. Low Temperature Formation of Mg2FeH6 by Hydrogenation of Ball-Milled Nano-Crystalline Powder Mixture of Mg and Fe. Mater. Des. 2017, 135, 239–245. [Google Scholar] [CrossRef]
- Fadonougbo, J.O.; Jung, J.Y.; Suh, J.-Y.; Lee, Y.-S.; Shim, J.-H.; Fleury, E.; Cho, Y.W. The Role of Fe Particle Size and Oxide Distribution on the Hydrogenation Properties of Ball-Milled Nano-Crystalline Powder Mixtures of Fe and Mg. J. Alloys Compd. 2019, 806, 1039–1046. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, X.; Nyahuma, F.M.; Yan, N.; Xiao, J.; Su, S.; Zhang, L. Enhancing Hydrogen Storage Properties of MgH2 by Transition Metals and Carbon Materials: A Brief Review. Front. Chem. 2020, 8, 552. [Google Scholar] [CrossRef]
- Dufour, J.; Huot, J. Rapid Activation, Enhanced Hydrogen Sorption Kinetics and Air Resistance in Laminated Mg–Pd 2.5 at.%. J. Alloys Compd. 2007, 439, 5–7. [Google Scholar] [CrossRef]
- Huot, J.; Enoki, H.; Akiba, E. Synthesis, Phase Transformation, and Hydrogen Storage Properties of Ball-Milled TiV0.9Mn1.1. J. Alloys Compd. 2008, 453, 203–209. [Google Scholar] [CrossRef]
- Huot, J.; Yonkeub, A.; Dufour, J. Rietveld Analysis of Neutron Powder Diffraction of Mg6Pd Alloy at Various Hydriding Stages. J. Alloys Compd. 2009, 475, 168–172. [Google Scholar] [CrossRef]
- Fadonougbo, J.O.; Kim, H.-J.; Suh, B.-C.; Suh, J.-Y.; Lee, Y.-S.; Shim, J.-H.; Yim, C.D.; Cho, Y.W. Kinetics and Thermodynamics of Near Eutectic Mg-Mg2Ni Composites Produced by Casting Process. Int. J. Hydrogen Energy 2020, 45, 29009–29022. [Google Scholar] [CrossRef]
- Ouyang, L.; Liu, F.; Wang, H.; Liu, J.; Yang, X.-S.; Sun, L.; Zhu, M. Magnesium-Based Hydrogen Storage Compounds: A Review. J. Alloys Compd. 2020, 832. [Google Scholar] [CrossRef]
- Crivello, J.C.; Denys, R.V.; Dornheim, M.; Felderhoff, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Walker, G.S.; et al. Mg-Based Compounds for Hydrogen and Energy Storage. Appl. Phys. A 2016, 122. [Google Scholar] [CrossRef]
- Pistidda, C. Metals in Hydrogen Technology. Metals 2020, 10, 456. [Google Scholar] [CrossRef]
- Floriano, R.; Leiva, D.R.; Melo, G.C.; Ishikawa, T.T.; Huot, J.; Kaufman, M.; Figueroa, S.J.A.; Mendoza-Zélis, L.A.; Damonte, L.C.; Botta, W.J. Low Temperature Rolling of AZ91 Alloy for Hydrogen Storage. Int. J. Hydrogen Energy 2017, 42, 29394–29405. [Google Scholar] [CrossRef]
- Skryabina, N.; Aptukov, V.; Romanov, P.; Fruchart, D.; de Rango, P.; Girard, G.; Grandini, C.; Sandim, H.; Huot, J.; Lang, J.; et al. Microstructure Optimization of Mg-Alloys by the ECAP Process Including Numerical Simulation, SPD Treatments, Characterization, and Hydrogen Sorption Properties. Molecules 2018, 24, 89. [Google Scholar] [CrossRef]
- Huot, J.; Tousignant, M. Effect of Cold Rolling on Metal Hydrides. Mater. Trans. 2019, 60, 1571–1576. [Google Scholar] [CrossRef]
- Xin, G.; Yang, J.; Fu, H.; Li, W.; Zheng, J.; Li, X. Excellent Hydrogen Sorption Kinetics of Thick Mg–Pd Films under Mild Conditions by Tailoring Their Structures. R. Soc. Chem. 2013, 3, 4167–4170. [Google Scholar] [CrossRef]
- Urretavizcaya, G.; Sarmiento Chávez, A.C.; Castro, F.J. Hydrogen Absorption and Desorption in the Mg-Ag System. J. Alloys Compd. 2014, 611, 202–209. [Google Scholar] [CrossRef]
- Si, T.Z.; Zhang, J.B.; Liu, D.M.; Zhang, Q.A. A New Reversible Mg3Ag-H2 System for Hydrogen Storage. J. Alloys Compd. 2013, 581, 246–249. [Google Scholar] [CrossRef]
- Dufour, J.; Huot, J. Study of Mg6Pd Alloy Synthesized by Cold Rolling. J. Alloys Compd. 2007, 446–447, 147–151. [Google Scholar] [CrossRef]
- Nayeb-Hashemi, A.A.; Clark, J.B. The Mg-Pd (Magnesium-Palladium) System. Bull. Alloy Phase Diagr. 1985, 6, 164–167. [Google Scholar] [CrossRef]
- Savitsky, E.M.; Terekhova, V.F.; Birun, N.A. Equilibrium Diagram of the Mg−Pd System. Russ. J. Inorg. Chem. 1962, 7, 1228–1231. [Google Scholar]
- Ferro, R. Research on the Alloys of Noble Metals with the More Electropositive Elements: III. Micrographic and X-ray Examination of Some Magnesium-Platinum Alloys. J. Less Common Met. 1959, 1, 424–438. [Google Scholar] [CrossRef]
- Kripyakevich, P.I.; Gladyshevskii, E.I. Crystal Structures of Some Compounds of Palladium with Magnesium. Sov. Phys. Crystallogr. 1960, 5, 552–554. [Google Scholar]
- Makongo, J.P.A.; Prots, Y.; Burkhardt, U.; Niewa, R.; Kudla, C.; Kreiner, G. A Case Study of Complex Metallic Alloy Phases: Structure and Disorder Phenomena of Mg–Pd Compounds. Philos. Mag. 2006, 86, 427–433. [Google Scholar] [CrossRef]
- Okamoto, H. Mg-Pd (Magnesium-Palladium). J. Phase Equilibria Diffus. 2010, 31, 407–408. [Google Scholar] [CrossRef]
- Fernandez, J.F.; Ares, J.R.; Cuevas, F.; Bodega, J.; Leardini, F.; Sanchez, C. A Thermodynamic Study of the Hydrogenation of the Pseudo-Binary Mg6Pd0.5Ni0.5 Intermetallic Compound. Intermetallics 2010, 18, 233–241. [Google Scholar] [CrossRef]
- Fernandez, J.F.; Widomb, M.; Cuevas, F.; Ares, J.R.; Bodega, J.; Leardini, F.; Mihalkovi, M.; Sánchez, C. First-Principles Phase Stability Calculations and Estimation of Finite Temperature Effects on Pseudo-Binary Mg6(PdxNi1−x) Compounds. Intermetallics 2011, 19, 502–510. [Google Scholar] [CrossRef]
- Delsante, S.; Novakovic, R.; Gagliolo, A.; Borzone, G. Thermodynamic Investigation on the Mg–Pd Intermetallic Phases. J. Chem. Thermodyn. 2019, 139, 1–8. [Google Scholar] [CrossRef]
- Dębski, A.; Pęska, M.; Dworecka-Wójcik, J.; Terlicka, S.; Gąsior, W.; Gierlotka, W.; Polański, M. Structural and Calorimetric Studies of Magnesium-Rich Mg-PD alloys. J. Alloys Compd. 2021, 858. [Google Scholar] [CrossRef]
- Gierlotka, W.; Dębski, A.; Terlicka, S.; Gąsior, W.; Pęska, M.; Polański, M. Insight into Phase Stability in the Mg-Pd System: The Ab Initio Calculations. J. Phase Equilibria Diffus. 2020, 41, 681–686. [Google Scholar] [CrossRef]
- Colinet, C. High Temperature Calorimetry: Recent Developments. J. Alloys Compd. 1995, 220, 76–87. [Google Scholar] [CrossRef]
- Dȩbski, A.; Dȩbski, R.; Ga̧sior, W.; Góral, A. Formation Enthalpy of Intermetallic Phases from Ag-Ca System. Experiment vs. Modeling. J. Alloys Compd. 2014, 610, 701–705. [Google Scholar] [CrossRef]
- Dębski, A.; Terlicka, S.; Budziak, A.; Gąsior, W. Calorimetric and XRD Studies of Ag-Rich Alloys from Ag-Li System. J. Alloys Compd. 2018, 732, 210–217. [Google Scholar] [CrossRef]
- Dębski, A.; Braga, M.H.; Terlicka, S.; Gąsior, W.; Góral, A. Formation Enthalpy of Ga-Li Intermetallic Phases. Experiment vs. Calculations. J. Chem. Thermodyn. 2018, 124, 101–106. [Google Scholar] [CrossRef]
- Chen, S.L.; Daniel, S.; Zhang, F.; Chang, Y.A.; Yan, X.-Y.; Xie, F.-Y.; Schmid-Fetzer, R.; Oates, W.A. The PANDAT Software Package and Its Applications. Calphad Comput. Coupling Phase Diagr. Thermochem. 2002, 26, 175–188. [Google Scholar] [CrossRef]
- Dinsdale, A.T. SGTE Data for Pure Elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
- De Boer, F.R.; Boom, R.; Mattens, W.C.M.; Miedema, A.R.; Niessen, A.K. Cohesion in Metals: Transition Metal Alloys (Cohesion and Structure); Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Dębski, A.; Dębski, R.; Gąsior, W. New Features of ENTALL Database: Comparison of Experimental and Model Formation Enthalpies. Arch. Metall. Mater. 2014, 59, 1337–1343. [Google Scholar] [CrossRef]
- Rzyman, K.; Moser, Z.; Gachon, J.C. Calorimetric Studies of the Enthalpies of Formation of Al3Ti, AlTi, AlTi3 and Al2Ti Compounds. Arch. Metall. Materials 2004, 49, 545–563. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).