High-k Fluoropolymers Dielectrics for Low-Bias Ambipolar Organic Light Emitting Transistors (OLETs)
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
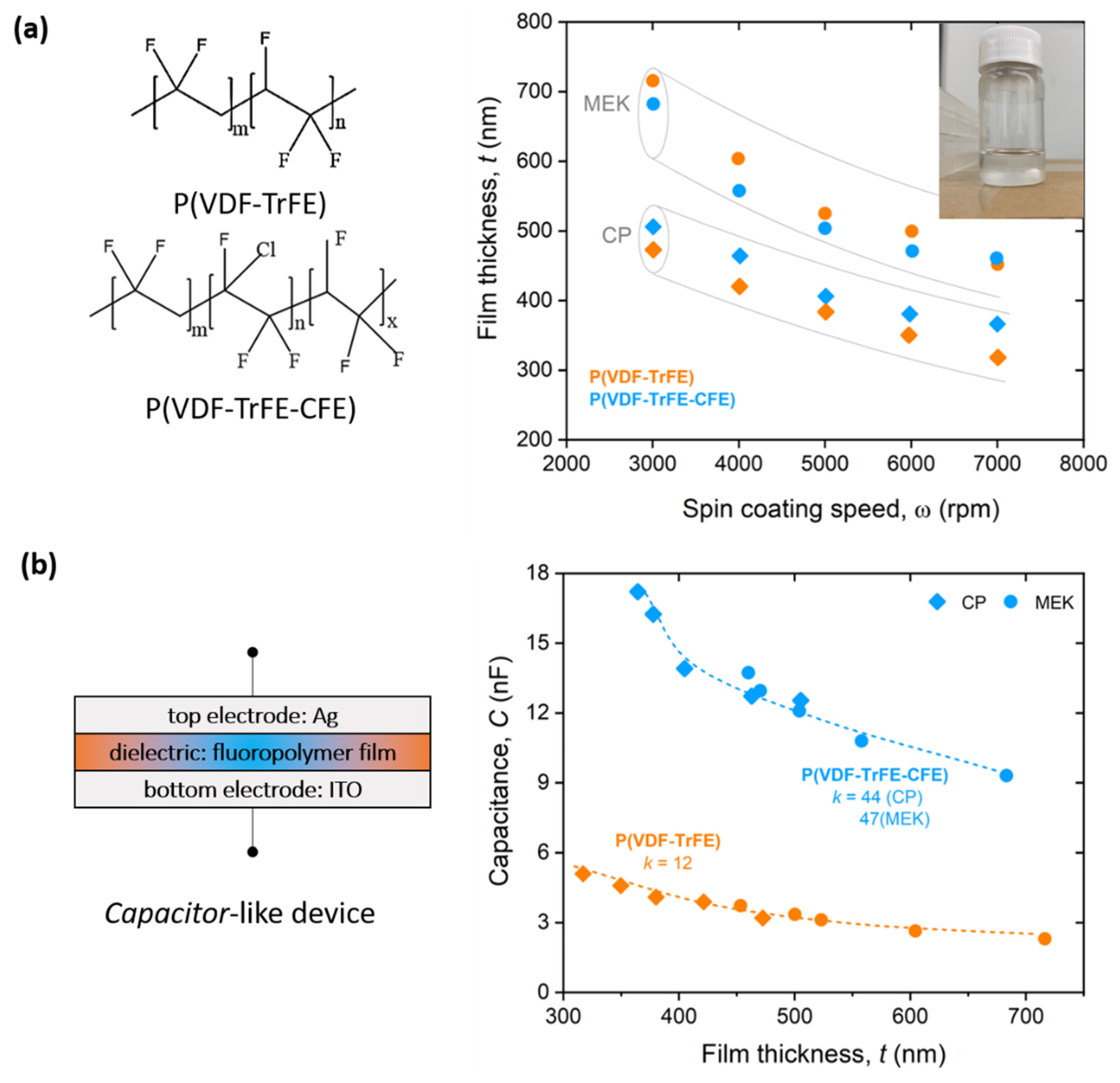
3.1. P(VDF)-Based Films Fabrication and Characterization
3.2. Dielectric Characterization of P(VDF)-Based Films
- (a)
- copolymer: C-E dependence shows the characteristic butterfly shape as a result of the polarization reversal in the film, indicating the ferroelectric nature of the film(s). This shape indicates a local domain switching behavior of P(VDF-TrFE), mostly occurring on the nanoscale level, with a strong polarization dependence from the direction of the applied field. Polarization reversal occurs in correspondence to the peaks in the C-E sweep, with this peak known as the coercive field, EC; it depends, among many factors, on solvents and the monomer content [42]. We found values of the coercive field of approximately ~ 0.47–0.5 MV/cm, consistent with reported values in the literature for our copolymer formulation [43]. We also note that in the limit of higher electric fields (>0.5 MV/cm), the capacitance becomes almost independent of the direction of the sweep in the case of cyclopentanone. This effect is smaller in the case of methylethylketone and likely connected to the solvent intrinsic dipole moment; in fact, a smaller dipole moment leading to shorter bond length (as in the case of MEK), might increase the number of paraelectric defects in P(VDF-TrFE), thus affecting the overall film dielectric constant and coercive field [21].
- (b)
- terpolymer: incorporation of the CFE monomer disturbs the tight packing in the crystal phase, producing a weaker coupling and larger inter-chain spacing, with the overall behavior transitioning from a ferroelectric into a ferroelectric relaxor. In this case, C-E sweeps show no polarization reversal, with a bell-like shape with hysteresis behavior depending on the direction of the sweep, thus suggesting remnant polarization in the materials. In the limit of small fields, the two curves show very similar behavior, and where the offset value is likely the contribution of the solvent dielectric constant [32,44].
- (c)
- PMMA (included here as a benchmark): capacitance shows no field dependence, consistently with the nature and the composition of PMMA. Value of capacitance is overall smaller in this case, because of the low dielectric constant (~3) and remains unchanged under the external applied field.
3.3. P(VDF)-Based Films as Gate Dielectrics in Organic Light Emitting Transistors
- (i)
- for our gate fields, terpolymer and copolymer exhibit values of drain-source current and light output within the same range, with slightly better performances for the terpolymer (higher IDS and EL) and lower threshold fields (expected for larger dielectric constants);
- (ii)
- larger hysteresis for copolymer reproduced the ferroelectric nature of the P(VDF-TrFE) film with the induced polarization upon biasing is expected to affect the local (gate) field applied at the various interfaces in the device. We found that devices using films with methylethylketone show similar performances (and efficiency), but at lower fields, feature as highly desirable when it comes to developing low-power consumption devices. This is likely affected by the physical properties of the solvents (i.e., polarity and dielectric constant, which might change the local field at the interface) and their effect on the OSC packing and film formation leading to improved conduction (improved semiconductor crystallinity or more favorable interfaces [50]). On the other hand, negligible differences are observed when using different solvents for P(VDF-TrFE-CFE)-based films, suggesting that in the case of terpolymers, solvent contribution is reduced. However, considering the complex nature of the multilayer structure here used, it is currently beyond our experimental capabilities to distinguish individual contribution(s).
- -
- the first one, where only the p-type OTFT is operating (right side of the “V” curve in Figure 5a,b),
- -
- the second one, where both OTFTs are in their ON-state, with balanced charge carrier densities (in the vicinity of the apex of the transfer curve) and with increasing number of minority charge carriers from the n-type semiconductor layer toward the recombination area.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, Z.; Locklin, J. (Eds.) Organic Field-Effect Transistors; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Mullen, K.; Scherf, U. (Eds.) Organic Light Emitting Devices: Synthesis, Properties and Applications; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Hatton, R.A. Organic Photovoltaics. In A Comprehensive Guide to Solar Energy Systems; Academic Press: Cambridge, MA, USA, 2018; pp. 255–277. [Google Scholar]
- Muccini, M. A bright future for organic field-effect transistors. Nat. Mater. 2006, 5, 605–613. [Google Scholar] [CrossRef]
- Muccini, M.; Koopman, W.; Toffanin, S. The photonic perspective of organic light-emitting transistors. Laser Photonics Rev. 2012, 6, 258–275. [Google Scholar] [CrossRef]
- Capelli, R.; Toffanin, S.; Generali, G.; Usta, H.; Facchetti, A.; Muccini, M. Organic light-emitting transistors with an efficiency that outperforms the equivalent light-emitting diodes. Nat. Mater. 2010, 9, 496–503. [Google Scholar] [CrossRef]
- Muccini, M.; Toffanin, S. Organic Light-Emitting Transistors: Towards the Next Generation Display Technology; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Hsieh, H.-H.; Chen, W.-C.; Generali, G.; Soldano, C.; D’Alpaos, R.; Turatti, G.; Biondo, V.; Muccini, M.; Huitema, H.; Facchetti, A. Flexible Active-Matrix OLET Display on a Plastic Substrate. Society for Information Display. Dig. Tech. Pap. 2016, 47, 739–742. [Google Scholar] [CrossRef]
- Bronstein, H.; Nielsen, C.B.; Schroeder, B.C.; McCulloch, I. The role of chemical design in the performance of organic semi-conductors. Nat. Rev. Chem. 2020, 4, 66–77. [Google Scholar] [CrossRef]
- Okamoto, T.; Kumagai, S.; Fukuzaki, E.; Ishii, H.; Watanabe, G.; Niitsu, N.; Annaka, T.; Yamagishi, M.; Tani, Y.; Sugiura, H.; et al. Robust, high-performance n-type organic semiconductors. Sci. Adv. 2020, 6, eaaz0632. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Ren, Z.; Yan, S.; Bryce, M.R. All-organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Nat. Rev. Mater. 2018, 3, 18020. [Google Scholar] [CrossRef]
- Facchetti, A.; Yoon, M.; Marks, T.J. Gate Dielectrics for Organic Field-Effect Transistors: New Opportunities for Organic Electronics. Adv. Mater. 2005, 17, 1705–1725. [Google Scholar] [CrossRef]
- Schulz, M. The end of the road for silicon? Nature 1999, 399, 729–730. [Google Scholar] [CrossRef]
- Maity, N.P.; Maity, R.; Baishya, S. (Eds.) High-k Gate Dielectric Materials: Applications with Advanced Metal Oxide Semiconductor Field Effect Transistors (MOSFETs); CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Ortiz, R.P.; Facchetti, A.; Marks, T.J. High-k Organic, Inorganic, and Hybrid Dielectrics for Low-Voltage Organic Field-Effect Transistors. Chem. Rev. 2009, 110, 205–239. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Choi, S.H.; Cho, N.G.; Chang, Y.; Kim, H.G.; Hong, J.M.; Kim, I.D. High stability InGaZnO4 thin-film transistors using sputter-deposited PMMA gate insulators and PMMA passivation layers. Electrochem. Solid State Lett. 2009, 12, H296. [Google Scholar] [CrossRef]
- Zaumseil, J.; Kline, R.; Sirringhaus, H. Electroluminescence imaging and microstructure of organic light-emitting field-effect transistors. Appl. Phys. Lett. 2008, 92, 073304. [Google Scholar] [CrossRef]
- Park, H.; Yoo, S.; Ahn, H.; Bang, J.; Jeong, Y.; Yi, M.H.; Won, J.C.; Jung, S.; Kim, Y.H. Low-Temperature Solution-Processed Soluble Polyimide Gate Dielectrics: From Molecular-Level Design to Electrically Stable and Flexible Organic Transistors. ACS Appl. Mater. Interfaces 2019, 11, 45949–45958. [Google Scholar] [CrossRef]
- Tetzner, K.; Bose, I.R.; Bock, K. Organic Field-Effect Transistors Based on a Liquid-Crystalline Polymeric Semi-conductor using SU-8 Gate Dielectrics on Flexible Substrates. Materials 2014, 7, 7226–7242. [Google Scholar] [CrossRef] [Green Version]
- Raghuwanshi, V.; Bharti, D.; Mahato, A.K.; Varun, I.; Tiwari, S.P. UV cured PVP gate dielectric for Flexible Organic Field Effect Transistors. In Proceedings of the IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Glasgow, UK, 8–10 July 2019; pp. 1–3. [Google Scholar]
- Kong, D.S.; Lee, T.K.; Ko, Y.J.; Jung, J.H. Dielectric and Ferroelectric Properties of P (VDF-TrFE) Films with Different Polar Solvents. J. Korean Phys. Soc. 2019, 74, 78–81. [Google Scholar] [CrossRef]
- Wu, S.; Shao, M.; Burlingame, Q.; Chen, X.; Lin, M.; Xiao, K.; Zhang, Q.M. A high-K ferroelectric relaxor terpolymer as a gate dielectric for organic thin film transistors. Appl. Phys. Lett. 2013, 102, 013301. [Google Scholar] [CrossRef]
- Ullah, A.; Rahman, A.U.; Ahn, C.W.; Rahman, M.-U.; Ullah, A.; Rehman, Z.-U.; Iqbal, M.J.; Kim, I.W. Enhancement of dielectric and energy density properties in the PVDF-based copolymer/terpolymer blends. Polym. Eng. Sci. 2015, 55, 1396–1402. [Google Scholar] [CrossRef]
- Zapsas, G.; Patil, Y.; Gnanou, Y.; Ameduri, B.; Hadjichristidis, N. Poly(vinylidene fluoride)-based complex macromo-lecular architectures: From synthesis to properties and applications. Prog. Polym. Sci. 2020, 104, 101231. [Google Scholar] [CrossRef]
- Barique, M.; Ohigashi, H. Annealing effects on the Curie transition temperature and melting temperature of poly(vinylidene fluoride/trifluoroethylene) single crystalline films. Polymer 2001, 42, 4981–4987. [Google Scholar] [CrossRef]
- Lill, A.T.; Eftaiha, A.A.F.; Huang, J.; Yang, H.; Seifrid, M.; Wang, M.; Bazan, G.C.; Nguyen, T.Q. High-k fluoro-polymer gate dielectric in electrically stable organic field-effect transistors. ACS Appl. Mater. Interfaces 2019, 11, 15821–15828. [Google Scholar] [CrossRef]
- Bao, H.M.; Song, J.F.; Zhang, J.; Shen, Q.D.; Yang, C.Z.; Zhang, Q.M. Phase transitions and ferroelectric relaxor behavior in P (VDF–TrFE–CFE) terpolymers. Macromolecules 2007, 40, 2371–2379. [Google Scholar] [CrossRef]
- Soldano, C. Engineering dielectric materials for high-performance organic light emitting transistors (OLETs). Materials 2021, 14, 3756. [Google Scholar] [CrossRef] [PubMed]
- Soldano, C.; Generali, G.; Cianci, E.; Tallarida, G.; Fanciulli, M.; Muccini, M. Engineering organic/inorganic alumina-based films as dielectrics for red organic light emitting transistors. Thin Solid Film. 2016, 616, 408–414. [Google Scholar] [CrossRef]
- Kang, S.J.; Park, Y.J.; Sung, J.; Jo, P.S.; Park, C.; Kim, K.J.; Cho, B.O. Spin cast ferroelectric beta poly(vinylidene fluoride) thin films via rapid thermal annealing. Appl. Phys. Lett. 2008, 92, 012921. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, H.; Zhang, L.; Chen, X.; Guo, Y.; Sun, H.; Li, Y. Field-effect transistor memories based on ferro-electric polymers. J. Semicond. 2017, 38, 111001. [Google Scholar] [CrossRef]
- Knotts, G.; Bhaumik, A.; Ghosh, K.; Guha, S. Enhanced performance of ferroelectric-based all organic capacitors and transistors through choice of solvent. Appl. Phys. Lett. 2014, 104, 233301. [Google Scholar] [CrossRef]
- Mishra, A.; Bhatt, N.; Bajpai, A. Nanostructured superhydrophobic coatings for solar panel applications. In Nanomaterials-Based Coatings; Elsevier: Amsterdam, The Netherlands, 2019; pp. 397–424. [Google Scholar]
- Kim, H.; Bae, J.-H.; Horowitz, G.; Kim, W.Y.; Choi, Y. Effects of the solvent polarity of a polymeric insulator on field-effect mobility in an organic thin-film transistor. Solid-State Electron. 2013, 81, 140–143. [Google Scholar] [CrossRef]
- Reig, M.; Bagdziunas, G.; Ramanavicius, A.; Puigdollers, J.; Velasco, D. Interface engineering and solid-state organization for triindole-based p-type organic thin-film transistors. Phys. Chem. Chem. Phys. 2018, 20, 17889–17898. [Google Scholar] [CrossRef] [PubMed]
- Vallée, R.; Damman, P.; Dosière, M.; Toussaere, E.; Zyss, J. Nonlinear Optical Properties and Crystalline Orientation of 2-Methyl-4-nitroaniline Layers Grown on Nanostructured Poly(tetrafluoroethylene) Substrates. J. Am. Chem. Soc. 2000, 122, 6701–6709. [Google Scholar] [CrossRef]
- Tanaka, T.; Ishitobi, M. Atomic Groove Effect of Aligned Poly(tetrafluoroethylene) Surfaces upon Oriented Growth of Linear Dye Molecules. J. Phys. Chem. B 2002, 106, 564–569. [Google Scholar] [CrossRef]
- Damman, P.; Coppée, S.; Geskin, V.M.; Lazzaroni, R. What Is the Mechanism of Oriented Crystal Growth on Rubbed Polymer Substrates? Topography vs Epitaxy. J. Am. Chem. Soc. 2002, 124, 15166–15167. [Google Scholar] [CrossRef]
- Moulin, J.-F.; Brinkmann, M.; Thierry, A.; Wittmann, J.-C. Oriented Crystalline Films of Tris(8-hydroxyquinoline) Aluminum(III): Growth of the Alpha Polymorph onto an Ultra-Oriented Poly(tetrafluoroethylene) Substrate. Adv. Mater. 2002, 14, 436–439. [Google Scholar] [CrossRef]
- Dinelli, F.; Murgia, M.; Levy, P.; Cavallini, M.; Biscarini, F.; De Leeuw, D.M. Spatially Correlated Charge Transport in Organic Thin Film Transistors. Phys. Rev. Lett. 2004, 92, 116802. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.B.; Gan, W.C.; Velayutham, T.S.; Goh, B.T.; Hashim, R. Structural control of the dielectric, pyroelectric and ferroelectric properties of poly (vinylidene fluoride-co-trifluoroethylene) thin films. Phys. Chem. Chem. Phys. 2020, 22, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Lohse, O.; Grossmann, M.; Boettger, U.; Bolten, D.; Waser, R. Relaxation mechanism of ferroelectric switching in Pb(Zr,Ti)O3 thin films. J. Appl. Phys. 2001, 89, 2332–2336. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xu, H.; Zhang, Y. Temperature dependence of coercive field and fatigue in poly (vinylidene fluoride-trifluoroethylene) copolymer ultra-thin films. J. Phys. D Appl. Phys. 2011, 44, 155501. [Google Scholar] [CrossRef]
- Ukah, N.B.; Granstrom, J.; Gari, R.R.S.; King, G.M.; Guha, S. Low-operating voltage and stable organic field-effect transistors with poly (methyl methacrylate) gate dielectric solution deposited from a high dipole moment solvent. Appl. Phys. Lett. 2011, 99, 243302. [Google Scholar] [CrossRef]
- Meng, N.; Ren, X.; Zhu, X.; Wu, J.; Yang, B.; Gao, F.; Zhang, H.; Liao, Y.; Bilotti, E.; Reece, M.J.; et al. Multiscale understanding of electric polarization in poly(vinylidene fluoride)-based ferroelectric polymers. J. Mater. Chem. C 2020, 8, 16436–16442. [Google Scholar] [CrossRef]
- Yang, L.; Ho, J.; Allahyarov, E.; Mu, R.; Zhu, L. Semicrystalline Structure–Dielectric Property Relationship and Electrical Conduction in a Biaxially Oriented Poly(vinylidene fluoride) Film under High Electric Fields and High Temperatures. ACS Appl. Mater. Interfaces 2015, 7, 19894–19905. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Liu, S.Z.; Cui, Y.S.; Chen, X.Z.; Ge, H.X.; Shen, Q.D. P(VDF-TrFE-CFE) terpolymer thin-film for high performance nonvolatile memory. Appl. Phys. Lett. 2013, 102, 063103. [Google Scholar] [CrossRef]
- Soldano, C.; D’Alpaos, R.; Generali, G. Highly Efficient Red Organic Light-Emitting Transistors (OLETs) on High-k Dielectric. ACS Photonics 2017, 4, 800–805. [Google Scholar] [CrossRef]
- Soldano, C.; Stefani, A.; Turatti, G.; Generali, G.; Biondo, V.; Basiricò, L.; Ortolani, L.; Morandi, V.; Rizzoli, R.; Veronese, G.P.; et al. ITO-free Organic Light Emitting Transistor Using Graphene Gate Electrode. ACS Photonics 2014, 1, 1082–1088. [Google Scholar] [CrossRef]
- Ukah, N.B.; Senanayak, S.P.; Adil, D.; Knotts, G.; Granstrom, J.; Narayan, K.S.; Guha, S. Enhanced mobility and environmental stability in all organic field-effect transistors: The role of high dipole moment solvent. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1533–1542. [Google Scholar] [CrossRef]





| PMMA | P(VDF-TrFE) | P(VDF-TrFE-CFE) | ||||
|---|---|---|---|---|---|---|
| MEK | CP | MEK | CP | |||
| film thickness, t (nm) | 440 | 450 | 350 | 460 | 380 | |
| capacitance/unit area, Ci (nF/cm2) | ~6.6 | ~24 | ~31 | ~91 | ~110 | |
| dielectric constant, k | ~3 | ~12 | ~12 | ~47 | ~44 | |
| mobility, µ (cm2/Vs) | h+ | 0.81 | 0.72 | 0.54 | 0.5 | 0.5 |
| e− | 5.1 × 10−3 | 3.1 × 10−2 | 7.5 × 10−2 | 1.25 × 10−2 | 1.75 × 10−2 | |
| threshold, Vth (V) | h+ | −41 | −6.7 | −19.3 | −11 | −10.6 |
| e− | 51 | 18.5 | 18. 8 | 15.4 | 17.3 | |
| drain-source maximum current, IDS-max (µA) | h+ | 380 | 860 | 450 | 840 | 850 |
| e− | 2.5 | 25 | 63 | 33 | 52 | |
| gate current, IG @VDS,max (nA) | - | ~0.25 | ~34 | ~24 | ~290 | ~290 |
| optical power, EL (µW) | max EL | 0.23 | 1.36 | 1.11 | 2.2 | 3.3 |
| @ Emax | 0.89 | 1.23 | 1.54 | |||
| external quantum efficiency, EQE (%) | max EQE | 0.024 | 0.083 | 0.15 | 0.08 | 0.22 |
| @ Emax | 0.1 | 0.15 | 0.1 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albeltagi, A.; Gallegos-Rosas, K.; Soldano, C. High-k Fluoropolymers Dielectrics for Low-Bias Ambipolar Organic Light Emitting Transistors (OLETs). Materials 2021, 14, 7635. https://doi.org/10.3390/ma14247635
Albeltagi A, Gallegos-Rosas K, Soldano C. High-k Fluoropolymers Dielectrics for Low-Bias Ambipolar Organic Light Emitting Transistors (OLETs). Materials. 2021; 14(24):7635. https://doi.org/10.3390/ma14247635
Chicago/Turabian StyleAlbeltagi, Ahmed, Katherine Gallegos-Rosas, and Caterina Soldano. 2021. "High-k Fluoropolymers Dielectrics for Low-Bias Ambipolar Organic Light Emitting Transistors (OLETs)" Materials 14, no. 24: 7635. https://doi.org/10.3390/ma14247635
APA StyleAlbeltagi, A., Gallegos-Rosas, K., & Soldano, C. (2021). High-k Fluoropolymers Dielectrics for Low-Bias Ambipolar Organic Light Emitting Transistors (OLETs). Materials, 14(24), 7635. https://doi.org/10.3390/ma14247635







